Abstract
In the yeast Saccharomyces cerevisiae, eight proteins are encoded by the mitochondrial genome. Seven of them are core catalytic subunits of complexes III and IV of the respiratory chain and the ATP synthase and thus essential for oxidative phosphorylation (OXPHOS), while one protein is soluble and a constituent of the small subunit of mitochondrial ribosomes. The expression of these proteins is mainly controlled posttranscriptionally by so-called translational activators. These nuclear-encoded factors act on the 5′-untranslated region (UTR) of their specific client mRNA and stimulate translation. In addition, translational activators play multiple roles in regulation and organization of mitochondrial protein synthesis. The mitochondrial OXPHOS complexes are assembled from subunits encoded by both the nuclear and the mitochondrial DNA. During the biogenesis of OXPHOS complexes, translational activators help to coordinate cytosolic and mitochondrial translation by adjusting mitochondrial protein synthesis to levels that can successfully be assembled. This chapter summarizes the current knowledge about how mitochondrial protein synthesis in the model organism Saccharomyces cerevisiae is coordinated with OXPHOS complex assembly.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Translational activators
- Mitochondrial protein synthesis
- Feedback regulation
- Mitochondrial ribosomes
- Respiratory complex assembly
5.1 Mechanisms of Protein Synthesis in Yeast Mitochondria
5.1.1 The Mitochondrial Genome in Yeast
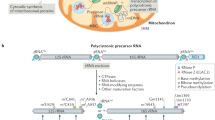
Mitochondria are key organelles of eukaryotic cells that participate in important metabolic processes like the TCA cycle, fatty acid oxidation, and amino acid degradation; they also play important roles in a variety of biosynthetic pathways and contribute to regulation of cellular signaling and apoptosis. Mitochondria evolved 2 billion years ago when an archaeal cell established a symbiotic relationship with aerobic bacteria (Sagan 1967; Gray 1989). In the course of evolution, most of the former bacterial genes were transferred to the nuclear DNA. Concomitantly, the organellar genetic code developed away from the standard genetic code, so that the codon usages differ significantly between both systems. The most obvious alteration is that the universal stop codon TGA is translated into tryptophan (Barrell et al. 1979; Fox 1979). Today, mitochondrial genomes of fungi and higher eukaryotes typically code only for a small number of genes. These include genes for proteins of OXPHOS complexes as well as tRNAs and rRNAs of the mitochondrial translation machinery. In the yeast Saccharomyces cerevisiae, eight proteins are encoded in the mitochondrial DNA (mtDNA), 24 tRNAs as well as the rRNA of the small (15S) and large (21S) subunit of the mitochondrial ribosome (Borst and Grivell 1978). Of the eight mitochondrially encoded proteins, seven represents very hydrophobic core subunits of OXPHOS complexes (cytochrome b of the bc 1 complex, Cox1, Cox2, and Cox3 of cytochrome oxidase and Atp6, Atp8, and Atp9 of the ATP synthase) located in the inner membrane of mitochondria, while one is a soluble protein and a component of the small mitochondrial ribosomal subunit (Fig. 5.1). These proteins are synthesized on mitochondrial ribosomes which are, unlike bacterial ribosomes, permanently associated to the inner membrane (Fiori et al. 2003; Ott et al. 2006; Prestele et al. 2009), thereby allowing co-translational membrane insertion of the hydrophobic OXPHOS subunits (Hell et al. 2001; Jia et al. 2003; Szyrach et al. 2003; Ott and Herrmann 2010).
Translational activators in yeast mitochondria. a General scheme of gene expression in yeast mitochondria. The mRNAs contain 5′- and 3′-untranslated regions (UTRs) flanking the respective open reading frame (ORF). Especially, the 5′-UTR is the target of specific translational activators (X), whose action is required to activate translation of their client mRNA. b Specific expression of genes encoded in the mitochondrial DNA with the help of translational activators. The mitochondrial genome (mtDNA) of S. cerevisiae encodes two ribosomal RNAs (15S and 21S rRNA), eight proteins and 24 tRNAs (not shown). So far, translational activators for six of the eight mRNAs have been identified. The topologies of the mitochondrially encoded proteins in the inner membrane are depicted. Var1 is a component of the small ribosomal subunit. IMM, inner mitochondrial membrane. IMS, intermembrane space
5.1.2 The Mitochondrial Ribosome in Yeast and the Process of Translation
The central components of the translation machinery in mitochondria are mitochondrial ribosomes. Due to the endosymbiotic origin of the organelles, it has long been assumed that mitochondrial ribosomes closely resemble the bacterial particles. However, although this is true for certain aspects like catalytic properties or sensitivity against antibiotics, millions of years of evolution have rendered the organellar ribosomes strikingly different from their ancestors. This is especially evident from their structure, because mitochondrial ribosomes contain typically much more protein and less rRNA. Furthermore, mitochondrial ribosomes greatly differ between species. Whereas the protein–rRNA ratio of bacterial ribosomes is 1:2, mitochondrial ribosomes of S. cerevisiae and S. pombe denote a 1:1 ratio and the relative gain in protein mass is even more pronounced in mitochondrial ribosomes of mammals (2:1). The functional significance of this huge increase in protein content is not entirely clear, but a likely explanation is that these ribosomes require additional stabilization due to the greatly reduced ribosomal RNA sequences and losses in structurally important RNA folds (Mears et al. 2006).
The general process of protein synthesis in mitochondria involves many conserved translation factors and has mainly been analyzed by studying mammalian mitochondria, see also the Chap. 2. Whereas the elongation cycle of translation in mitochondria is assumed to closely resemble the bacterial process, termination of protein synthesis deviates from the ancestral system (Chrzanowska-Lightowlers et al. 2011; Kehrein et al. 2013). The initiation step of mitochondrial translation is only poorly understood. Unlike bacterial mRNAs or transcripts in the cytosol of eukaryotes, mitochondrial mRNAs do not contain Shine–Dalgarno sequences or 5′-cap structures that promote initiation of translation in these systems. Although in yeast nucleotides in the 15S rRNA of the small ribosomal subunit are complementary to sequences in mitochondrial mRNAs (Li et al. 1982), those regions do not fulfill a Shine–Dalgarno-like function as they are dispensable for translation (Costanzo and Fox 1988; Mittelmeier and Dieckmann 1995). Rather, sequences adjacent to the start AUG in the mRNA are implicated in this process. Accordingly, removal of the start AUG did not allow initiation of translation at alternative AUG codons within downstream sequences; changing the start AUG to AUA in COX2 and COX3 mRNAs only modestly reduced synthesis of Cox2 and Cox3 (Folley and Fox 1991; Mulero and Fox 1994; Bonnefoy and Fox 2000). Importantly, the 5′- and 3′-untranslated regions (UTRs) are, at least in yeast, the target of transcript-specific translational activators. These factors are mediating in a yet unknown manner the translation of their specific client mRNA.
5.1.3 The Concept of Translational Activators and Their Possible Functions
Translational activators (TAs) in yeast mitochondria have been studied for more than four decades (Fig. 5.1). The cytochrome oxidase subunits Cox2 and Cox3 were the first examples for which the concept of specific translational activation in yeast mitochondria was introduced. Early studies showed that deletion of the nuclear genes PET111 and PET494 specifically impairs the expression of the mitochondrial COX2 and COX3 gene, respectively (Cabral and Schatz 1978). In subsequent years, genetic screens revealed that mutants lacking one TA (and therefore one mitochondrially encoded protein) could regain respiratory growth by remodeling of sequences within the mitochondrial genome. Those rearrangements led to the generation of fusion genes or the exchange of regulatory regions with the result that affected transcripts acquired 5′-UTRs of other genes, making their expression independent from the authentic, missing TA but dependent on the factor controlling synthesis of the other gene (Muller et al. 1984). In yeast, expression of six of the eight mitochondrially encoded proteins depends on TAs; these factors are described below.
How exactly TAs exert their function in translation is not yet understood. Different molecular functions have been suggested, and because there is experimental evidence for all of them, TAs might not share one universal function but rather exert their roles at different steps of the translation process and in some cases have more than one function. Some TAs like Cbp1, Pet309, Pet111, Aep2, and Atp25 are required for stabilizing the transcripts they act on (Poutre and Fox 1987; Payne et al. 1991; Manthey and McEwen 1995; Ellis et al. 1999; Islas-Osuna et al. 2002; Zeng et al. 2008).
Another possibility is that TAs work by supporting initiation of translation by, e.g., assisting to load the mRNA correctly onto the ribosome as Shine–Dalgarno-like sequences are absent in mitochondrial messengers. Interactions of TAs with both the 5′-UTR and mitochondrial ribosomes would support such an idea and have been shown primarily by genetic study (McMullin et al. 1990; Haffter et al. 1991; Haffter and Fox 1992; Fox 1996). A direct binding of TAs to sequences within specific 5′-UTRs has been demonstrated in the cases of Pet111 and Pet122, where substitutions of amino acids in the TA could rescue adverse mutations in the 5′-UTR of COX2 and COX3, respectively (Costanzo and Fox 1993; Mulero and Fox 1993a).
Translational activators participate in the organization of mitochondrial protein synthesis. Many TAs are peripheral or integral membrane proteins, and therefore localize the mRNAs to the matrix face of the inner membrane to facilitate interactions with the permanently membrane-associated translation machinery in mitochondria (McMullin and Fox 1993; Sanchirico et al. 1998). However, the organization of translation by TAs might be even more intricate than this. For example, Pet309 was found to be present in a complex of a molecular mass of about 900 kDa that also contained Cbp1, a protein required to stabilize the cytochrome b mRNA (Krause et al. 2004). Pet309 was independently shown to be in contact with the general mRNA metabolism factor Nam1 (Naithani et al. 2003). A third study revealed a Nam1 interaction with the yeast mitochondrial RNA polymerase (Rodeheffer et al. 2001). From all these findings, a model was suggested that links transcription, mRNA maturation, and protection as well as translation at the inner mitochondrial membrane (Krause et al. 2004). Furthermore, the specific TAs of Cox1, Cox2, and Cox3 interact with each other and thereby organize expression of the three cytochrome oxidase subunits in a way that allows efficient assembly of this respiratory chain complex (Naithani et al. 2003).
The last, and comparably well documented, function of TAs is the regulation of mitochondrial protein synthesis in response to the efficiency of respiratory chain assembly. Respiratory chain complexes and the ATP synthase are composed of subunits produced by two different genetic systems and the assembly of these complex machineries is a highly intricate event. To allow efficient assembly of the respiratory chain, the expression of mitochondrially and nuclear encoded subunits has to be coordinated. Translational activators participate in these regulatory circuits in the case of the bc 1 complex, cytochrome oxidase, and the ATP synthase in yeast. In general, when assembly of an OXPHOS complex is blocked due to missing nuclear-encoded structural subunits or the absence of specific assembly factors, sequestration of a TA in an assembly intermediate that contains a mitochondrially encoded subunit of the OXPHOS complex lowers the amounts of the TA available to stimulate translation (Fig. 5.2). By this, mitochondrial protein synthesis is adjusted to levels that can successfully be assembled into OXPHOS complexes. The coupling of synthesis and assembly is a conserved process as it had first been described for the biogenesis of chloroplast photosystems and there been termed “control of epistatic synthesis” (CES) (Wollman et al. 1999). The detailed mechanisms of how specific TAs act in such regulatory feedback loops in yeast mitochondria are outlined in the last section of this chapter.
The general principle of feedback loops regulating mitochondrial protein synthesis in response to the efficiency of OXPHOS complex assembly. Translation in mitochondria requires specific translational activators (TAs). Some TAs (TA2 in the scheme) have a dual role in activating translation of their client mRNA and in mediating assembly of the encoded protein into a respiratory chain complex. a Under normal circumstances when assembly is not disturbed, the TA2 is only transiently present in an assembly intermediate and released upon further assembly. It can then stimulate further translation of the regulated subunit (dark orange) at the mitochondrial ribosome (+). b If complex assembly is perturbed due to the absence of structural OXPHOS subunits or assembly factors encoded in the nucleus, TA2 cannot be released efficiently and is sequestered in the assembly intermediate. The TA is therefore not available for activating translation, which reduces of synthesis of the mitochondrially encoded OXPHOS subunit. By this, mitochondrial translation is adjusted to level that can be incorporated into OXPHOS complexes. IMM, inner mitochondrial membrane. IMS, intermembrane space
5.1.4 Synthesis of Cytochrome b
Cytochrome b is the only subunit of the bc 1 complex which is encoded in the mitochondrial genome and translation of its mRNA (COB mRNA) is dependent on several factors (Rödel 1997). The COB gene is co-transcribed with an adjacent tRNA and the resulting bi-cistronic precursor has to be processed in a complex way (Christianson et al. 1983; Hollingsworth and Martin 1986; Chen and Martin 1988) The COB gene furthermore contains introns, some of which encode maturases that are required for the excision of introns within the same or other transcripts (Lazowska et al. 1980; Nobrega and Tzagoloff 1980; Dhawale et al. 1981; De La Salle et al. 1982). Both the unprocessed and the mature COB transcripts are shielded from exonucleolytic degradation by the factor Cbp1 (Weber and Dieckmann 1990; Mittelmeier and Dieckmann 1995; Islas-Osuna et al. 2002). This mRNA stabilization involves the interaction of Cbp1 with the 5′-UTR of cytochrome b; a single CCG triplet near the 5′-end is especially important (Chen and Dieckmann 1997). In addition to stabilization, Cbp1 is also directly necessary for translation of the COB mRNA (Islas-Osuna et al. 2002).
Four other TAs are specifically required for the synthesis of cytochrome b. Yeast cells lacking either the product of the CBS1 or the CBS2 gene cannot translate COB mRNA and accumulate the unprocessed pre-COB transcript (Rödel et al. 1985; Rödel 1986). Because Cbs1 and Cbs2 are needed for cytochrome b synthesis also in a strain where the COB gene does not contain any introns, they do not function in processing of pre-COB (Muroff and Tzagoloff 1990). The accumulation of the unprocessed transcript is presumably a secondary effect in Δcbs1 and Δcbs2 cells, because synthesis of the maturases encoded within introns requires translation of the pretranscript. Similar to the case of Cbp1, the COB 5′-UTR dictates the dependence on Cbs1 and Cbs2 (Rödel et al. 1985; Rödel and Fox 1987). The region within the 5′-UTR recognized by the two TAs lies in the sequence −232 to −4 relative to the start AUG at +1 (Mittelmeier and Dieckmann 1995). However, a direct interaction between either Cbs1 or Cbs2 and the 5′-untranslated region of COB mRNA was not yet shown, so it is not clear whether the factors act directly or indirectly through yet unknown components.
Additional factors involved in cytochrome b translation are the proteins Cbp3 and Cbp6, which form a functionally and structurally inseparable complex that binds to mitochondrial ribosomes in close proximity to the tunnel exit (Gruschke et al. 2011). In contrast to Δcbs1 and Δcbs2 cells, the pre-COB transcript is processed and matured similar to the wild type in the absence of Cbp3 or Cbp6 (Dieckmann and Tzagoloff 1985; Gruschke et al. 2011). Despite this, cytochrome b cannot accumulate in the mutants and the phenotype cannot be suppressed by a typical gene rearrangement within the mitochondrial genome (Tzagoloff et al. 1988; Gruschke et al. 2012; Kühl et al. 2012). This can be attributed to the fact that the Cbp3–Cbp6 complex exerts a second function in cytochrome b biogenesis; it is required for the stabilization and assembly of the newly synthesized protein. As soon as cytochrome b is fully synthesized, Cbp3–Cbp6 binds to the protein, the complex is released from the ribosome and after recruitment of the assembly factor Cbp4 cytochrome b is fed into the bc 1 complex assembly line (Gruschke et al. 2011; Gruschke et al. 2012). This dual role of Cbp3–Cbp6 in both translation of the COB mRNA and assembly of cytochrome b enables Cbp3–Cbp6 to act in a regulatory feedback circuit that adjusts the level of cytochrome b synthesis to the assembly efficiency of the bc 1 complex (Gruschke et al. 2012). This regulation is explained in more detail in the last section of the chapter.
5.1.5 Synthesis of Mitochondrially Encoded Cytochrome Oxidase Subunits
Three subunits of cytochrome oxidase (COX, complex IV) are encoded in the mitochondrial genome, namely Cox1, Cox2, and Cox3. The TA for COX2, Pet111, and one of the TAs for COX3, Pet494, served as the first examples for specific translational activation in mitochondria (Cabral and Schatz 1978). Due to the pioneering work of Tom Fox, Pet111 is the best-studied translational activator. Like for other TAs, the phenotype of the PET111 deletion mutant was found to be suppressible by the exchange of COX2 regulatory regions to 5′-UTRs of other mitochondrial genes (Poutre and Fox 1987; Mulero and Fox 1993b). The 5′-UTR of the COX2 mRNA is relatively short (54 bases), making mutagenetic analyses spanning the complete sequence easier than in other cases. By this means, it was shown that the sequence between −16 and −47 (relative to the translation start at +1) is sufficient to confer Pet111-mediated translational activation; within this region lies a predicted stem–loop structure between position −20 and −35 that is especially important (Dunstan et al. 1997). Like in the case of Cbp1 and the COB 5′-UTR, a direct interaction between this structural motif in the COX2 5′-UTR and Pet111 is very likely, because mutations in the protein can rescue base exchanges in the mRNA (Mulero and Fox 1993a). However, the functional role of Pet111 in translational activation of COX2 on the molecular level has, as for the other TAs, not been elucidated yet.
Translational activation of COX3 mRNA depends on three proteins, Pet54, Pet122, and Pet494 (Cabral and Schatz 1978; Muller et al. 1984; Costanzo and Fox 1986, 1988). All of these factors act on the 613 nucleotide long 5′-UTR of COX3 mRNA (Costanzo and Fox 1988). In the case of Pet122, this interaction presumably is directed as a mutation within the protein can restore translation of an mRNA lacking a functionally important part of the 5′-UTR (Costanzo and Fox 1993). Furthermore, Pet54, Pet122, and Pet494 interact with each other at the inner mitochondrial membrane and thereby presumably help localizing synthesis of this cytochrome oxidase subunit to the membrane (McMullin and Fox 1993; Brown et al. 1994). In addition to a function in Cox3 synthesis, Pet54 has been proposed to play a role in maturation of the COX1 mRNA (Valencik and McEwen 1991).
The third cytochrome oxidase subunit encoded in the mitochondrial genome is Cox1. Its synthesis depends on two proteins that are involved in posttranscriptional processes. Pet309 is required for translation of the COX1 transcript, because yeast strains harboring an intronless COX1 gene accumulate the mature mRNA, but fail to synthesize Cox1 (Manthey and McEwen 1995). Pet309 belongs to the class of PPR proteins that contain pentatricopeptide repeats, a motif involved in protein-RNA interactions (Lipinski et al. 2011). All of the seven PPRs of Pet309 are required for supporting translation of the COX1 mRNA, suggesting direct interaction of the protein with the 5′-UTR of the messenger (Tavares-Carreon et al. 2008). Mss51 is the second protein involved in translation of the COX1 transcript. Although initially thought to be required for splicing of the COX1 precursor mRNA, experiments with strains harboring an intronless COX1 gene showed that Mss51 rather functions as a translational activator (Faye and Simon 1983; Decoster et al. 1990). The COX1 5′-UTR again was shown to direct Mss51 dependence; however, the exchange of this regulatory region for that of another mitochondrial gene did not bypass the requirement for Mss51 (Perez-Martinez et al. 2003; Zambrano et al. 2007). The reason for this is a second posttranslational function of Mss51 in Cox1 biogenesis; it interacts with newly synthesized Cox1 and is part of cytochrome oxidase assembly intermediates (Perez-Martinez et al. 2003; Barrientos et al. 2004). Recent studies have shown that the synthesis of Cox1 is regulated in a highly complex manner in response to cytochrome oxidase assembly. In this process, Mss51 plays a key role by mediating both translation of the COX1 mRNA and assembly of this respiratory chain complex (Fontanesi et al. 2008; Mick et al. 2011). It thus represents a second example of a TA mediating feedback modulation of mitochondrial protein synthesis in the context of OXPHOS complex assembly (see below).
5.1.6 Synthesis of Mitochondrially Encoded ATP Synthase Subunits
The mitochondrial genome of S. cerevisiae contributes three subunits to the formation of the ATP synthase, Atp6, Atp8 and Atp9, all of which are part of the membrane-integrated Fo unit. The ATP6 and ATP8 genes are transcribed as one long precursor mRNA together with COX1 (Simon and Faye 1984). After endonucleolytic cleavage of this pretranscript, maturation, and stabilization of the ATP8/ATP6 bi-cistronic and/or the single mRNAs is accomplished by several nuclear encoded factors: Nca2, Nca3, and Nam1 (which is not only specific for Atp6 and Atp8) and Aep3 (Groudinsky et al. 1993; Camougrand et al. 1995; Pelissier et al. 1995; Ellis et al. 2004).
Translation of ATP6 depends on the factor Atp22 (Zeng et al. 2007). Similar to other TAs, the absence of Atp22 can be overcome by a mitochondrial gene rearrangement leading to the generation of a Cox1::ATP6 transcript. Translation of this mRNA is only dependent on Pet309 and Mss51, the TAs of COX1, but not on Atp22 (Zeng et al. 2007). In accordance, efficient synthesis of a mitochondrially encoded reporter gene was strictly dependent on the presence of Atp22 (Rak and Tzagoloff 2009). Although ATP6 and ATP8 are produced from a bi-cistronic transcript, ATP22 deletion mutants specifically lack Atp6 but show normal translation rates of ATP8 (Zeng et al. 2007). This suggests that a translational activator for ATP8 still awaits identification.
The core component of the Fo part of the ATP synthase is an oligomer of Atp9 subunits that forms the proton conducting channel. The ATP9 gene is transcribed together with an adjacent tRNA and the VAR1 gene and the polycistronic transcript is matured by endonucleolytic cleavage (Zassenhaus et al. 1984). The importance of the 5′-UTR of ATP9 was recognized very early, as insertion of bases into this region impaired translation (Ooi et al. 1987). Three proteins influence translation of the ATP9 mRNA: Aep1, which acts as a TA, Aep2 that is either required for the stabilization of the ATP9 transcript or stimulating its translation and Atp25, which has a dual role in translation and assembly of ATP synthase (Payne et al. 1991, 1993; Ellis et al. 1999; Zeng et al. 2008). Atp25 is split into two halves and both portions function in mitochondria. The C-terminal half of the protein is conferring stability to the ATP9 mRNA and expression of this part of the protein is sufficient to allow Atp9 synthesis in the ATP25 deletion mutant (Zeng et al. 2008). In the absence of the N-terminal half of Atp25; however, the translated Atp9 is not stably assembled into the Atp9-oligomer. This suggests that the N-terminal half of Atp25 is not dispensable for the biogenesis of Atp9 and might even mediate assembly of the Atp9 ring. Hence, Atp25 is a protein of dual function with a probability to modulate expression of ATP9 in a feedback loop. However, this hypothesis has not yet been analyzed experimentally. Importantly, it was recently demonstrated that the synthesis of Atp6 and Atp8 is regulated in response to the assembly process of the ATP synthase complex (Rak and Tzagoloff 2009). This is described in detail below.
5.2 Nuclear Control of Protein Synthesis in Yeast Mitochondria
Translational activators are encoded in the nucleus. Soon after the discovery of Pet111 and Pet494 and the establishment of the concept of specific translational activation, regulation/control of the expression of these nuclear genes was investigated. Studies using a yeast strain with a chromosomal gene fusion consisting of the COX3-specific PET494 and the E. coli β-galactosidase gene lacZ revealed that Pet494 is expressed at very low levels (Marykwas and Fox 1989). The TAs Pet122 and Pet111 are present in similarly low amounts (Fox 1996). The low abundance of translational activators implies that they are rate limiting for mitochondrial protein synthesis. This was confirmed for Pet494 by investigating diploid yeast strains homo- or heterozygous for the PET494 locus or haploids carrying a high copy plasmid to overexpress the gene. Additionally, these strains harbored a mitochondrial genome that encodes the reporter construct ARG8 m (Steele et al. 1996). ARG8 is a nuclear gene coding for a soluble enzyme, which is normally posttranslationally imported into mitochondria and involved in the biogenesis of arginine. The recoded version of the gene ARG8 m was integrated into the mtDNA of yeast deficient in the nuclear copy of ARG8. In the study of Steele et al., the open reading frame of COX3 was substituted by the ARG8 m gene (cox3::ARG8 m mtDNA), making Arg8 synthesis dependent on COX3-specific translational activation. The available amount of Pet494 clearly correlated with the Arg8 expression rate, while cox3::ARG8 m expression only moderately correlated with Pet122 level (Steele et al. 1996).
The expression of many genes involved in respiration in yeast is modulated over a wide range of growth conditions and TAs seem to be no exception to this. PET494 expression is subject to catabolite repression (Marykwas and Fox 1989). In the presence of glucose, the levels of the TA drop four to sixfold in comparison to cells grown on nonfermentable carbon sources. Furthermore, synthesis of Pet494 is regulated by oxygen, but in contrast to the transcriptional repression by glucose this is rather achieved on a translational level (Marykwas and Fox 1989). Interestingly, expression of PET494 is heme independent. This is opposed to other respiratory genes that are responding to oxygen levels, where transcriptional upregulation under aerobic conditions is mediated by heme (Guarente and Mason 1983; Keng and Guarente 1987). The regulation of the expression of other TAs was not analyzed similarly detailed. However, expression of many genes necessary for respiration and especially subunits of the OXPHOS system is influenced by growth conditions (Guarente and Mason 1983; Lowry et al. 1983; Myers et al. 1987; Forsburg and Guarente 1989). Taken into account the feedback regulatory circles that were revealed in the last years, this regulation of nuclear gene expression can be considered as an example of how mitochondrial gene expression is influenced by carbon source, oxygen levels, or presence of heme .
5.3 Regulation of Mitochondrial Protein Synthesis in Response to Assembly of the Oxphos System
Both the nuclear as well as the mitochondrial protein synthesis machinery contribute subunits to the OXPHOS complexes. To ensure efficient assembly, these expression systems have to be coordinated temporally and spatially in a precise manner. In recent years, different groups have revealed how regulation of mitochondrial protein synthesis is accomplished and how the levels of mitochondrially encoded subunits are adjusted to allow an efficient OXPHOS assembly process (Fig. 5.2). The general principle is that TAs with dual functions are sequestered in OXPHOS assembly intermediates. When assembly proceeds normally, the TA is released to stimulate synthesis of its client protein. In contrast, when further assembly fails, the TA is trapped in the assembly intermediate and not available to activate new rounds of translation.
5.3.1 Regulation of Cytochrome b Synthesis
The yeast bc 1 complex is composed of nine nuclear-encoded subunits that are assembled around the core component cytochrome b, which is produced by the mitochondrial genetic system. Catalytically active are only the three proteins, cytochrome b, cytochrome c 1 (Cyt1), and the Fe/S protein Rip1, whereas the remaining seven subunits are accessory structural subunits. The step-wise assembly process involves four intermediates and has mainly been analyzed by the use of yeast strains lacking individual structural subunits of the bc 1 complex and their analysis by Blue Native polyacrylamide gel electrophoresis (BN PAGE) (Zara et al. 2007, 2009a, b; Gruschke et al. 2012; Smith et al. 2012). Assembly starts with synthesis and membrane insertion of cytochrome b, which is immediately bound by the Cbp3–Cbp6 complex (Fig. 5.3). Recruitment of the assembly factor Cbp4 results in assembly intermediate I that serves as a pool of unassembled cytochrome b even at steady state. Addition of the first two nuclear-encoded structural subunits Qcr7 and Qcr8 induces release of Cbp3–Cbp6, whereas Cbp4 stays attached. The cytochrome b-Cbp4-Qcr7-Qcr8 complex represents the second assembly intermediate and is further joined by the two core proteins Cor1 and Cor2. Addition of Cyt1 and the small acidic accessory subunit Qcr6 to intermediate III forms intermediate IV, which was previously described as the 500 kDa complex (Zara et al. 2009b). The incorporation of two accessory subunits (Qcr9 and Qcr10) and the last catalytic subunit (Rip1) completes formation of the bc 1 complex. When assembly is disturbed before intermediate IV can be generated, the synthesis of cytochrome b is reduced (Gruschke et al. 2012) (Fig. 5.3). This is caused by sequestration of Cbp3–Cbp6 in assembly intermediate I, which accumulates under these conditions and as a result the Cbp3–Cbp6 complex is not available at the mitochondrial ribosome to fulfill its function as a TA for the COB mRNA (Gruschke et al. 2012). This negative feedback can be overcome by overexpression of the Cbp3–Cbp6 complex, demonstrating its key role in this process. Formation of assembly intermediate IV seems to be a critical point in the pathway as disturbance of the last assembly step by either deletion of one of the structural subunits QCR9, QCR10, RIP1 or required assembly factors (MZM1, BCS1) does not lead to reduced cytochrome b translation.
Schematic representation of the regulatory feedback loop modulating cytochrome b synthesis in response to the assembly of the bc 1 complex. The Cbp3–Cbp6 complex exerts a dual role in the biogenesis of cytochrome b: In its ribosome-bound form it acts as a translational activator and together with Cbs1 and Cbs2 stimulates translation of the COB mRNA. It is also present as a nonribosome-bound form in association with cytochrome b and Cbp4, forming the first assembly intermediate of the bc 1 complex assembly line. A: Cytochrome b assembles through four intermediates into a functional bc 1 complex, three of which are depicted in the scheme. When assembly is undisturbed or can proceed at least until intermediate IV is formed, Cbp3–Cbp6 is released from intermediate I upon further assembly, can again activate COB mRNA translation at the ribosome (+) and cytochrome b synthesis is not affected. B: If complex assembly is disturbed before intermediate IV is formed, Cbp3–Cbp6 cannot be released efficiently and is sequestered in the accumulating intermediate I (thick black arrow). The complex is therefore not available for activating translation and consequently cytochrome b synthesis is reduced. IMM, inner mitochondrial membrane. IMS, intermembrane space
5.3.2 Regulation of Cox1 Synthesis
The cytochrome oxidase (COX, complex IV) is composed of 11 subunits in yeast, three of which are encoded in the mitochondrial genome. Two of these three subunits harbor redox-active heme and/or copper co-factors. Electrons flow from the CuA center of Cox2 to the heme a cofactor of Cox1, from where they are passed further to the active site of Cox1 composed of the CuB center and heme a 3 . Heme a 3 binds molecular oxygen which serves as the final electron acceptor. The assembly of this OXPHOS complex is characterized very well and assisted by a considerable number of factors involved in co-factor acquisition, mediation of subunit interaction and feedback regulation (Fontanesi et al. 2006; Mick et al. 2011). Assembly of cytochrome oxidase is initiated from the central subunit of the complex, Cox1. Unassembled Cox1 with its redox-active cofactors is potentially harmful for cells as it may give rise to reactive oxygen species (Khalimonchuk et al. 2007). To ensure integrity of the cell, Cox1 synthesis has to be monitored precisely and adjusted to levels that can successfully be incorporated into COX. In recent years, it was found that Cox1 translation in yeast is subject to a complex feedback regulatory circle that achieves this fine tuning (Mick et al. 2011). The key role in this feedback loop is played by the dually functioning protein Mss51, which acts as a TA for COX1 mRNA as well as a Cox1-assembly factor by binding the newly synthesized protein (Perez-Martinez et al. 2003). Like Cbp3–Cbp6 in the case of the bc 1 complex, Mss51 is sequestered in assembly intermediates that cannot be resolved when further assembly is blocked and thereby is precluded from activating new rounds of COX1 translation (Fig. 5.4). Very recently, it was demonstrated that Mss51 contains two heme binding motifs in its N-terminus, thereby allowing it to act as a heme sensor and coordinate COX assembly with heme availability (Soto et al. 2012).
Schematic representation of the regulatory feedback loop modulating Cox1 synthesis in response to the assembly of the COX complex. a Mss51 has two functions in the biogenesis of this OXPHOS complex; in concert with Pet309 it serves as a translational activator for COX1 mRNA and, in addition, Mss51 is part of assembly intermediates, acting as a Cox1 chaperone. Mss51 is a heme-binding protein, which additionally allows to regulate COX1 synthesis in response to the heme homeostasis of the cell. Hem15 (ferrochelatase) incorporates iron into the protophorphyrin IX ring (PPIX), thereby forming heme b from which subsequently heme a, one of the cofactors present in Cox1, is synthesized through the concerted action of Cox10 and Cox15. Together with hemylated Mss51, Cox14, Coa3/Cox25, and Coa1 are part of early Cox1 assembly intermediates. Presumably, at the step where Cox1 is hemylated and Shy1 enters the pathway, Mss51 is released and can again activate COX1 mRNA translation (+). Incorporation of the remaining structural subunits releases the other assembly factors until formation of cytochrome oxidase is completed. b If heme biosynthesis or COX assembly is disturbed, Mss51 cannot function properly or is sequestered in assembly intermediates and therefore not available for activating translation; Cox1 synthesis consequently is reduced. IMM, inner mitochondrial membrane. IMS, intermembrane space
Besides Mss51, several other factors participate in COX assembly and the feedback regulation mechanism (Fig. 5.4). The presence of Cox14 and Coa3/Cox25 is required to allow efficient interaction of Mss51 with newly synthesized Cox1, and thus they act as negative regulators of COX1 synthesis by ensuring efficient sequestration of Mss51 (Barrientos et al. 2004; Perez-Martinez et al. 2009; Mick et al. 2010; Fontanesi et al. 2011). COX1 feedback regulation depends on the C-terminal region of Cox1 itself. Mutants lacking this part of the protein can synthesize and assemble Cox1 into a functional cytochrome oxidase, but do not exhibit assembly responsive reduction of Cox1 synthesis. It has been speculated that the molecular reason for this lies in the weakened interaction between Mss51 and Cox14 (Shingu-Vazquez et al. 2010). Coa1, Coa2, Shy1, and the mitochondrial Hsp70 chaperone Ssc1 are additional factors participating in COX assembly (Barrientos et al. 2002; Pierrel et al. 2007; Fontanesi et al. 2008; Pierrel et al. 2008; Fontanesi et al. 2010). The exact molecular composition of all COX assembly intermediates is, however, still under debate (McStay et al. 2012). A subcomplex consisting of Mss51, Cox14, Coa3/Cox25, and Coa1 bound to an oxidatively harmless, unhemylated form of Cox1 is stable in wild-type cells and presumably serves as a pool of assembly competent Cox1 (Khalimonchuk et al. 2010). In contrast, the Shy1-containing assembly intermediate comprises hemylated Cox1; however, Shy1 is most likely not required for hemylation per se, but rather stabilizes Cox1 in a conformation allowing the insertion of heme a 3 . Although it is not entirely resolved yet, Mss51 presumably is released from Cox1 when Shy1 enters the assembly pathway. Mss51 can then again act as a TA and induce further Cox1 synthesis.
5.3.3 Regulation of Atp6/8 Synthesis
The ATP synthase is composed of three functionally and structurally distinct parts. The membrane-embedded Fo part comprises Atp9 subunits, which form a ring-like structure, and the two proteins Atp6 and Atp8. These three proteins are encoded in the mitochondrial genome. The hydrophilic F1 part is formed by a hexamer of alternating α and β subunits that mediate ATP synthesis and the central stalk, which is made up of subunits γ, δ, and ε. The stalk is in contact to the Atp9-ring as well as the α3β3 hexamer. The third part of the enzyme is the peripheral stator stalk made up of four subunits, which is attached to both the α3β3 oligomer and Atp6 in the membrane. By this, the α3β3 hexamer, Atp6, and the stator form the stationary part of the enzyme. Driven by the electrochemical gradient across the membrane, protons flow back from the intermembrane space into the matrix at the interface between the Atp9 ring and Atp6, thereby rotating the Atp9 part and the central stalk stepwise and inducing conformational changes at the catalytic sites of the α3β3 hexamer that drive ATP synthesis (Stock et al. 2000). The assembly process of the ATP synthase is not understood in every detail (Ackerman and Tzagoloff 2005; Rak et al. 2009). Early experiments indicated that assembly of the F1 unit is independent from assembly of Fo (Schatz 1968). The current idea is that ATP synthase assembly involves to distinct, but coordinately formed modules which are joined at the end (Rak et al. 2011). The main Fo component, the Atp9 ring, is assembled from Atp9 monomers with the help of the N-terminal part of Atp25 and then interacts with the pre-assembled F1 unit (Zeng et al. 2008; Rak et al. 2011). In parallel, a complex of Atp6, Atp8, and at least two stator stalk subunits is generated. Together with Atp6, the Atp9 ring forms the proton translocating channel of the enzyme complex. The joining of Atp6 with the Atp9 ring seems to occur at a rather late step of assembly and involves the assembly factor Atp10 and the inner membrane protein Oxa1 (Tzagoloff et al. 2004; Jia et al. 2007).
In 2009, Rak and Tzagoloff reported that translation of the ATP8/ATP6 bi-cistronic mRNA is dependent on F1 assembly (Fig. 5.5) (Rak and Tzagoloff 2009). Mutants lacking assembly factors required for the formation of the F1 unit, Atp11 or Atp12, or the two main structural F1 subunits α and β display reduced synthesis rates of Atp6 and Atp8. It was excluded that this was caused by an increased turnover of the newly translated proteins by analyzing expression of the ARG8 m reporter genes (atp6::ARG8 m or atp8::ARG8 m) that revealed impaired Arg8 synthesis. Overexpression of Atp22, the translational activator of ATP6, was able to suppress the phenotype (Rak and Tzagoloff 2009). Currently, it is not clear how exactly this feedback regulation is mediated. It could be achieved either by the sequestration of Atp22 in some form of assembly intermediate (similar to the cases of Cbp3–Cbp6 and Mss51) or it could involve yet uncharacterized components. This system is physiological important, as it prevents the dissipation of the membrane potential in case the Fo part cannot efficiently be coupled to the F1 complex. Although the F1-dependent regulation of Fo biogenesis mechanistically differs from the feedback-regulated expression of COB or COX1, it provides another example of how mitochondrial translation is adjusted to the level of cytoplasmic protein synthesis.
Schematic representation of the regulatory feedback loop modulating Atp6/Atp8 synthesis in response to the assembly of the ATP synthase. A: The mitochondrially encoded ATP synthase subunits Atp6 and Atp8 are translated from a bi-cistronic mRNA with the help of the translational activator for ATP6, Atp22, and a yet unknown translational activator for ATP8. Atp6 and Atp8 are after their synthesis assembled with the stator stalk. The monomeric forms of the F1 subunits α and β are prevented from aggregation and assembled into the α3β3 hexamer by the assembly factors Atp11 and Atp12. After addition of the central stalk subunits, the F1 part is joined to the Atp9 ring and the Atp6/8 module, forming the fully assembled ATP synthase. The successful assembly of the α3β3 hexamer is required for efficient translation of the ATP8/ATP6 mRNA (+). B: If F1 formation is perturbed, synthesis of Atp6 and Atp8 is impaired (−). This regulation presumably involves Atp22, for details see text. IMM, inner mitochondrial membrane. IMS, intermembrane space
5.4 Outlook
Biogenesis of OXPHOS complexes of dual genetic origin requires cross-talk of the two genetic systems involved. This regulation occurs at the level of mitochondrial protein synthesis, which is modulated by TAs that sense efficiency of assembly to down-regulate expression of their client protein when assembly fails. Despite the fact that specific translational activation of mitochondrial protein synthesis by nuclear genes is known since more than 40 years, we do not yet understand which exact molecular functions TAs exert during protein synthesis in the organelle. In addition to a presumably direct role in translation, mitochondrial TAs appear to be implicated in the organization of translation. The organization of cytochrome b biogenesis might serve as a good example to illustrate this: Cytochrome b is only efficiently synthesized when one of its TAs, the Cbp3–Cbp6 complex is present at the ribosomal tunnel exit (Gruschke et al. 2011). Because Cbp3–Cbp6 is also an essential assembly factor for cytochrome b, it is ensured that the newly synthesized protein experiences an optimally tailored environment for further assembly. Indeed, when cytochrome b is synthesized from an mRNA containing the 5′-UTR of another transcript, the proteins fails to accumulate robustly, while rates of synthesis of this ectopically expressed protein are indistinguishable from the authentic protein (Gruschke et al. 2012). Similar observations have also been reported previously for other ectopically expressed proteins (Sanchirico et al. 1998), suggesting that in mitochondria, each mRNA is translated by ribosomes that are specifically designed to optimally support biogenesis of the client protein (Gruschke and Ott 2010). It thus appears that mitochondrial protein synthesis is probably much more sophisticated organized than anticipated and that this system still harbors many exciting previously unidentified features.
References
Ackerman SH, Tzagoloff A (2005) Function, structure, and biogenesis of mitochondrial ATP synthase. Prog Nucleic Acid Res Mol Biol 80:95–133
Barrell BG, Bankier AT, Drouin J (1979) A different genetic code in human mitochondria. Nature 282:189–194
Barrientos A, Korr D, Tzagoloff A (2002) Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh’s syndrome. EMBO J 21:43–52
Barrientos A, Zambrano A, Tzagoloff A (2004) Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J 23:3472–3482
Bonnefoy N, Fox TD (2000) In vivo analysis of mutated initiation codons in the mitochondrial COX2 gene of Saccharomyces cerevisiae fused to the reporter gene ARG8 m reveals lack of downstream reinitiation. Mol Gen Genet 262:1036–1046
Borst P, Grivell LA (1978) The mitochondrial genome of yeast. Cell 15:705–723
Brown NG, Costanzo MC, Fox TD (1994) Interactions among three proteins that specifically activate translation of the mitochondrial COX3 mRNA in Saccharomyces cerevisiae. Mol Cell Biol 14:1045–1053
Cabral F, Schatz G (1978) Identification of cytochrome c oxidase subunits in nuclear yeast mutants lacking the functional enzyme. J Biol Chem 253:4396–4401
Camougrand N, Pelissier P, Velours G, Guerin M (1995) NCA2, a second nuclear gene required for the control of mitochondrial synthesis of subunits 6 and 8 of ATP synthase in Saccharomyces cerevisiae. J Mol Biol 247:588–596
Chen JY, Martin NC (1988) Biosynthesis of tRNA in yeast mitochondria. An endonuclease is responsible for the 3′-processing of tRNA precursors. J Biol Chem 263:13677–13682
Chen W, Dieckmann CL (1997) Genetic evidence for interaction between Cbp1 and specific nucleotides in the 5′ untranslated region of mitochondrial cytochrome b mRNA in Saccharomyces cerevisiae. Mol Cell Biol 17:6203–6211
Christianson T, Edwards JC, Mueller DM, Rabinowitz M (1983) Identification of a single transcriptional initiation site for the glutamic tRNA and COB genes in yeast mitochondria. Proc Natl Acad Sci USA 80:5564–5568
Chrzanowska-Lightowlers ZM, Pajak A, Lightowlers RN (2011) Termination of protein synthesis in mammalian mitochondria. J Biol Chem 286:34479–34485
Costanzo MC, Fox TD (1986) Product of Saccharomyces cerevisiae nuclear gene PET494 activates translation of a specific mitochondrial mRNA. Mol Cell Biol 6:3694–3703
Costanzo MC, Fox TD (1988) Specific translational activation by nuclear gene products occurs in the 5′ untranslated leader of a yeast mitochondrial mRNA. Proc Natl Acad Sci USA 85:2677–2681
Costanzo MC, Fox TD (1993) Suppression of a defect in the 5′ untranslated leader of mitochondrial COX3 mRNA by a mutation affecting an mRNA-specific translational activator protein. Mol Cell Biol 13:4806–4813
De La Salle H, Jacq C, Slonimski PP (1982) Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell 28:721–732
Decoster E, Simon M, Hatat D, Faye G (1990) The MSS51 gene product is required for the translation of the COX1 mRNA in yeast mitochondria. Mol Gen Genet 224:111–118
Dhawale S, Hanson DK, Alexander NJ, Perlman PS, Mahler HR (1981) Regulatory Interactions between Mitochondrial Genes - Interactions between 2 Mosaic Genes. Proc Natl Acad Sci USA 78:1778–1782
Dieckmann CL, Tzagoloff A (1985) Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem 260:1513–1520
Dunstan HM, Green-Willms NS, Fox TD (1997) In vivo analysis of Saccharomyces cerevisiae COX2 mRNA 5′-untranslated leader functions in mitochondrial translation initiation and translational activation. Genetics 147:87–100
Ellis TP, Helfenbein KG, Tzagoloff A, Dieckmann CL (2004) Aep3p stabilizes the mitochondrial bicistronic mRNA encoding subunits 6 and 8 of the H+-translocating ATP synthase of Saccharomyces cerevisiae. J Biol Chem 279:15728–15733
Ellis TP, Lukins HB, Nagley P, Corner BE (1999) Suppression of a nuclear aep2 mutation in Saccharomyces cerevisiae by a base substitution in the 5′-untranslated region of the mitochondrial oli1 gene encoding subunit 9 of ATP synthase. Genetics 151:1353–1363
Faye G, Simon M (1983) Analysis of a yeast nuclear gene involved in the maturation of mitochondrial pre-messenger RNA of the cytochrome oxidase subunit I. Cell 32:77–87
Fiori A, Mason TL, Fox TD (2003) Evidence that synthesis of the Saccharomyces cerevisiae mitochondrially encoded ribosomal protein Var1p may be membrane localized. Eukaryot Cell 2:651–653
Folley LS, Fox TD (1991) Site-directed mutagenesis of a Saccharomyces cerevisiae mitochondrial translation initiation codon. Genetics 129:659–668
Fontanesi F, Clemente P, Barrientos A (2011) Cox25 teams up with Mss51, Ssc1, and Cox14 to regulate mitochondrial cytochrome c oxidase subunit 1 expression and assembly in Saccharomyces cerevisiae. J Biol Chem 286:555–566
Fontanesi F, Soto IC, Barrientos A (2008) Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life 60:557–568
Fontanesi F, Soto IC, Horn D, Barrientos A (2006) Assembly of mitochondrial cytochrome c oxidase, a complicated and highly regulated cellular process. Am J Physiol Cell Physiol 291:C1129–C1147
Fontanesi F, Soto IC, Horn D, Barrientos A (2010) Mss51 and Ssc1 facilitate translational regulation of cytochrome c oxidase biogenesis. Mol Cell Biol 30:245–259
Forsburg SL, Guarente L (1989) Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol 5:153–180
Fox TD (1979) Five TGA “stop” codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc. Natl. Acad. Sci. U S A 76:6534–6538
Fox TD (1996) Translational control of endogenous and recoded nuclear genes in yeast mitochondria: regulation and membrane targeting. Experientia 52:1130–1135
Gray MW (1989) The evolutionary origins of organelles. Trends Genet 5:294–299
Groudinsky O, Bousquet I, Wallis MG, Slonimski PP, Dujardin G (1993) The NAM1/MTF2 nuclear gene product is selectively required for the stability and/or processing of mitochondrial transcripts of the atp6 and of the mosaic, cox1 and cytb genes in Saccharomyces cerevisiae. Mol Gen Genet 240:419–427
Gruschke S, Kehrein K, Römpler K, Gröne K, Israel L, Imhof A et al (2011) Cbp3-Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly. J Cell Biol 193:1101–1114
Gruschke S, Ott M (2010) The polypeptide tunnel exit of the mitochondrial ribosome is tailored to meet the specific requirements of the organelle. BioEssays 32:1050–1057
Gruschke S, Rompler K, Hildenbeutel M, Kehrein K, Kuhl I, Bonnefoy N et al (2012) The Cbp3-Cbp6 complex coordinates cytochrome b synthesis with bc 1 complex assembly in yeast mitochondria. J Cell Biol 199:137–150
Guarente L, Mason T (1983) Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell 32:1279–1286
Haffter P, Fox TD (1992) Suppression of carboxy-terminal truncations of the yeast mitochondrial mRNA-specific translational activator PET122 by mutations in two new genes, MRP17 and PET127. Mol Gen Genet 235:64–73
Haffter P, McMullin TW, Fox TD (1991) Functional interactions among two yeast mitochondrial ribosomal proteins and an mRNA-specific translational activator. Genetics 127:319–326
Hell K, Neupert W, Stuart RA (2001) Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J 20:1281–1288
Hollingsworth MJ, Martin NC (1986) RNase P activity in the mitochondria of Saccharomyces cerevisiae depends on both mitochondrion and nucleus-encoded components. Mol Cell Biol 6:1058–1064
Islas-Osuna MA, Ellis TP, Marnell LL, Mittelmeier TM, Dieckmann CL (2002) Cbp1 is required for translation of the mitochondrial cytochrome b mRNA of Saccharomyces cerevisiae. J Biol Chem 277:37987–37990
Jia L, Dienhart M, Schramp M, McCauley M, Hell K, Stuart RA (2003) Yeast Oxa1 interacts with mitochondrial ribosomes: The importance of the C-terminal hydrophilic region of Oxa1. EMBO J 22:6438–6447
Jia L, Dienhart MK, Stuart RA (2007) Oxa1 directly interacts with Atp9 and mediates its assembly into the mitochondrial F1Fo-ATP synthase complex. Mol Biol Cell 18:1897–1908
Kehrein K, Bonnefoy N, Ott M (2013) Mitochondrial Protein Synthesis: Efficiency and Accuracy. Antioxid Redox Signal. doi:10.1089/ars.2012.4896
Keng T, Guarente L (1987) Constitutive expression of the yeast HEM1 gene is actually a composite of activation and repression. Proc. Natl. Acad. Sci. U S A 84:9113–9117
Khalimonchuk O, Bestwick M, Meunier B, Watts TC, Winge DR (2010) Formation of the redox cofactor centers during Cox1 maturation in yeast cytochrome oxidase. Mol Cell Biol 30:1004–1017
Khalimonchuk O, Bird A, Winge DR (2007) Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase. J Biol Chem 282:17442–17449
Krause K, Lopes de Souza R, Roberts DG, Dieckmann CL (2004) The mitochondrial message-specific mRNA protectors Cbp1 and Pet309 are associated in a high-molecular weight complex. Mol Biol Cell 15:2674–2683
Kühl I, Fox TD, Bonnefoy N (2012) Schizosaccharomyces pombe homologs of the Saccharomyces cerevisiae mitochondrial proteins Cbp6 and Mss51 function at a post-translational step of respiratory complex biogenesis. Mitochondrion 12:381–390
Lazowska J, Jacq C, Slonimski PP (1980) Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell 22:333–348
Li M, Tzagoloff A, Underbrink-Lyon K, Martin NC (1982) Identification of the paromomycin-resistance mutation in the 15 S rRNA gene of yeast mitochondria. J Biol Chem 257:5921–5928
Lipinski KA, Puchta O, Surendranath V, Kudla M, Golik P (2011) Revisiting the yeast PPR proteins–application of an Iterative Hidden Markov Model algorithm reveals new members of the rapidly evolving family. Mol Biol Evol 28:2935–2948
Lowry CV, Weiss JL, Walthall DA, Zitomer RS (1983) Modulator sequences mediate oxygen regulation of CYC1 and a neighboring gene in yeast. Proc. Natl. Acad. Sci. USA 80:151–155
Manthey GM, McEwen JE (1995) The product of the nuclear gene PET309 is required for translation of mature mRNA and stability or production of intron-containing RNAs derived from the mitochondrial COX1 locus of Saccharomyces cerevisiae. EMBO J 14:4031–4043
Marykwas DL, Fox TD (1989) Control of the Saccharomyces cerevisiae regulatory gene PET494: transcriptional repression by glucose and translational induction by oxygen. Mol Cell Biol 9:484–491
McMullin TW, Fox TD (1993) COX3 mRNA-specific translational activator proteins are associated with the inner mitochondrial membrane in Saccharomyces cerevisiae. J Biol Chem 268:11737–11741
McMullin TW, Haffter P, Fox TD (1990) A novel small-subunit ribosomal protein of yeast mitochondria that interacts functionally with an mRNA-specific translational activator. Mol Cell Biol 10:4590–4595
McStay GP, Su CH, and Tzagoloff A (2012) Modular assembly of yeast cytochrome oxidase. Mol. Biol. Cell
Mears JA, Sharma MR, Gutell RR, McCook AS, Richardson PE, Caulfield TR et al (2006) A structural model for the large subunit of the mammalian mitochondrial ribosome. J Mol Biol 358:193–212
Mick DU, Fox TD, Rehling P (2011) Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat Rev Mol Cell Biol 12:14–20
Mick DU, Vukotic M, Piechura H, Meyer HE, Warscheid B, Deckers M et al (2010) Coa3 and Cox14 are essential for negative feedback regulation of COX1 translation in mitochondria. J Cell Biol 191:141–154
Mittelmeier TM, Dieckmann CL (1995) In vivo analysis of sequences required for translation of cytochrome b transcripts in yeast mitochondria. Mol Cell Biol 15:780–789
Mulero JJ, Fox TD (1993a) Alteration of the Saccharomyces cerevisiae COX2 mRNA 5′-untranslated leader by mitochondrial gene replacement and functional interaction with the translational activator protein PET111. Mol Biol Cell 4:1327–1335
Mulero JJ, Fox TD (1993b) PET111 acts in the 5′-leader of the Saccharomyces cerevisiae mitochondrial COX2 mRNA to promote its translation. Genetics 133:509–516
Mulero JJ, Fox TD (1994) Reduced but accurate translation from a mutant AUA initiation codon in the mitochondrial COX2 mRNA of Saccharomyces cerevisiae. Mol Gen Genet 242:383–390
Muller PP, Reif MK, Zonghou S, Sengstag C, Mason TL, Fox TD (1984) A nuclear mutation that post-transcriptionally blocks accumulation of a yeast mitochondrial gene product can be suppressed by a mitochondrial gene rearrangement. J Mol Biol 175:431–452
Muroff I, Tzagoloff A (1990) CBP7 codes for a co-factor required in conjunction with a mitochondrial maturase for splicing of its cognate intervening sequence. EMBO J 9:2765–2773
Myers AM, Crivellone MD, Koerner TJ, Tzagoloff A (1987) Characterization of the yeast HEM2 gene and transcriptional regulation of COX5 and COR1 by heme. J Biol Chem 262:16822–16829
Naithani S, Saracco SA, Butler CA, Fox TD (2003) Interactions among COX1, COX2, and COX3 mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane of Saccharomyces cerevisiae. Mol Biol Cell 14:324–333
Nobrega FG, Tzagoloff A (1980) Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273–10B. J Biol Chem 255:9828–9837
Ooi BG, Lukins HB, Linnane AW, Nagley P (1987) Biogenesis of mitochondria: a mutation in the 5′-untranslated region of yeast mitochondrial oli1 mRNA leading to impairment in translation of subunit 9 of the mitochondrial ATPase complex. Nucleic Acids Res 15:1965–1977
Ott M, Herrmann JM (2010) Co-translational membrane insertion of mitochondrially encoded proteins. Biochim Biophys Acta 1803:767–775
Ott M, Prestele M, Bauerschmitt H, Funes S, Bonnefoy N, Herrmann JM (2006) Mba1, a membrane-associated ribosome receptor in mitochondria. EMBO J 25:1603–1610
Payne MJ, Finnegan PM, Smooker PM, Lukins HB (1993) Characterization of a second nuclear gene, AEP1, required for expression of the mitochondrial OLI1 gene in Saccharomyces cerevisiae. Curr Genet 24:126–135
Payne MJ, Schweizer E, Lukins HB (1991) Properties of two nuclear pet mutants affecting expression of the mitochondrial oli1 gene of Saccharomyces cerevisiae. Curr Genet 19:343–351
Pelissier P, Camougrand N, Velours G, Guerin M (1995) NCA3, a nuclear gene involved in the mitochondrial expression of subunits 6 and 8 of the Fo-F1 ATP synthase of S. cerevisiae. Curr Genet 27:409–416
Perez-Martinez X, Broadley SA, Fox TD (2003) Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J 22:5951–5961
Perez-Martinez X, Butler CA, Shingu-Vazquez M, Fox TD (2009) Dual functions of Mss51 couple synthesis of Cox1 to assembly of cytochrome c oxidase in Saccharomyces cerevisiae mitochondria. Mol Biol Cell 20:4371–4380
Pierrel F, Bestwick ML, Cobine PA, Khalimonchuk O, Cricco JA, Winge DR (2007) Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J 26:4335–4346
Pierrel F, Khalimonchuk O, Cobine PA, Bestwick M, Winge DR (2008) Coa2 is an assembly factor for yeast cytochrome c oxidase biogenesis that facilitates the maturation of Cox1. Mol Cell Biol 28:4927–4939
Poutre CG, Fox TD (1987) PET111, a Saccharomyces cerevisiae nuclear gene required for translation of the mitochondrial mRNA encoding cytochrome c oxidase subunit II. Genetics 115:637–647
Prestele M, Vogel F, Reichert AS, Herrmann JM, Ott M (2009) Mrpl36 is important for generation of assembly competent proteins during mitochondrial translation. Mol Biol Cell 20:2615–2625
Rak M, Gokova S, Tzagoloff A (2011) Modular assembly of yeast mitochondrial ATP synthase. EMBO J 30:920–930
Rak M, Tzagoloff A (2009) F1-dependent translation of mitochondrially encoded Atp6p and Atp8p subunits of yeast ATP synthase. Proc Natl Acad Sci USA 106:18509–18514
Rak M, Zeng X, Briere JJ, Tzagoloff A (2009) Assembly of F0 in Saccharomyces cerevisiae. Biochim Biophys Acta 1793:108–116
Rodeheffer MS, Boone BE, Bryan AC, Shadel GS (2001) Nam1p, a protein involved in RNA processing and translation, is coupled to transcription through an interaction with yeast mitochondrial RNA polymerase. J Biol Chem 276:8616–8622
Rödel G (1997) Translational activator proteins required for cytochrome b synthesis in Saccharomyces cerevisiae. Curr Genet 31:375–379
Rödel G (1986) Two yeast nuclear genes, CBS1 and CBS2, are required for translation of mitochondrial transcripts bearing the 5′-untranslated COB leader. Curr Genet 11:41–45
Rödel G, Fox TD (1987) The yeast nuclear gene CBS1 is required for translation of mitochondrial mRNAs bearing the cob 5′ untranslated leader. Mol Gen Genet 206:45–50
Rödel G, Korte A, Kaudewitz F (1985) Mitochondrial suppression of a yeast nuclear mutation which affects the translation of the mitochondrial apocytochrome b transcript. Curr Genet 9:641–648
Sagan L (1967) On the origin of mitosing cells. J Theor Biol 14:255–274
Sanchirico ME, Fox TD, Mason TL (1998) Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J 17:5796–5804
Schatz G (1968) Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic “petite” mutant of Saccharomyces cerevisiae. J Biol Chem 243:2192–2199
Shingu-Vazquez M, Camacho-Villasana Y, Sandoval-Romero L, Butler CA, Fox TD, Perez-Martinez X (2010) The carboxyl-terminal end of Cox1 is required for feedback-assembly regulation of Cox1 synthesis in Saccharomyces cerevisiae mitochondria. J Biol Chem 285:34382–34389
Simon M, Faye G (1984) Organization and processing of the mitochondrial oxi3/oli2 multigenic transcript in yeast. Mol Gen Genet 196:266–274
Smith PM, Fox JL, Winge DR (2012) Biogenesis of the cytochrome bc 1 complex and role of assembly factors. Biochim Biophys Acta 1817:276–286
Soto IC, Fontanesi F, Myers RS, Hamel P, Barrientos A (2012) A heme-sensing mechanism in the translational regulation of mitochondrial cytochrome c oxidase biogenesis. Cell Metab 16:801–813
Steele DF, Butler CA, Fox TD (1996) Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc Natl Acad Sci USA 93:5253–5257
Stock D, Gibbons C, Arechaga I, Leslie AG, Walker JE (2000) The rotary mechanism of ATP synthase. Curr Opin Struct Biol 10:672–679
Szyrach G, Ott M, Bonnefoy N, Neupert W, Herrmann JM (2003) Ribosome binding to the Oxa1 complex facilitates cotranslational protein insertion in mitochondria. EMBO J 22:6448–6457
Tavares-Carreon F, Camacho-Villasana Y, Zamudio-Ochoa A, Shingu-Vazquez M, Torres-Larios A, Perez-Martinez X (2008) The pentatricopeptide repeats present in Pet309 are necessary for translation but not for stability of the mitochondrial COX1 mRNA in yeast. J Biol Chem 283:1472–1479
Tzagoloff A, Barrientos A, Neupert W, Herrmann JM (2004) Atp10p assists assembly of Atp6p into the F0 unit of the yeast mitochondrial ATPase. J Biol Chem 279:19775–19780
Tzagoloff A, Crivellone MD, Gampel A, Muroff I, Nishikimi M, Wu M (1988) Mutational analysis of the yeast coenzyme QH2-cytochrome c reductase complex. Philos Trans R Soc Lond B Biol Sci 319:107–120
Valencik ML, McEwen JE (1991) Genetic evidence that different functional domains of the PET54 gene product facilitate expression of the mitochondrial genes COX1 and COX3 in Saccharomyces cerevisiae. Mol Cell Biol 11:2399–2405
Weber ER, Dieckmann CL (1990) Identification of the CBP1 polypeptide in mitochondrial extracts from Saccharomyces cerevisiae. J Biol Chem 265:1594–1600
Wollman FA, Minai L, Nechushtai R (1999) The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim Biophys Acta 1411:21–85
Zambrano A, Fontanesi F, Solans A, de Oliveira RL, Fox TD, Tzagoloff A et al (2007) Aberrant translation of cytochrome c oxidase subunit 1 mRNA species in the absence of Mss51p in the yeast Saccharomyces cerevisiae. Mol Biol Cell 18:523–535
Zara V, Conte L, Trumpower BL (2009a) Biogenesis of the yeast cytochrome bc 1 complex. Biochim Biophys Acta 1793:89–96
Zara V, Conte L, Trumpower BL (2009b) Evidence that the assembly of the yeast cytochrome bc 1 complex involves the formation of a large core structure in the inner mitochondrial membrane. FEBS J 276:1900–1914
Zara V, Conte L, Trumpower BL (2007) Identification and characterization of cytochrome bc 1 subcomplexes in mitochondria from yeast with single and double deletions of genes encoding cytochrome bc 1 subunits. FEBS J 274:4526–4539
Zassenhaus HP, Martin NC, Butow RA (1984) Origins of transcripts of the yeast mitochondrial var1 gene. J Biol Chem 259:6019–6027
Zeng X, Barros MH, Shulman T, Tzagoloff A (2008) ATP25, a new nuclear gene of Saccharomyces cerevisiae required for expression and assembly of the Atp9p subunit of mitochondrial ATPase. Mol Biol Cell 19:1366–1377
Zeng X, Hourset A, Tzagoloff A (2007) The Saccharomyces cerevisiae ATP22 gene codes for the mitochondrial ATPase subunit 6-specific translation factor. Genetics 175:55–63
Acknowledgments
We thank all members of our group for stimulating discussions. Our work is supported by the Swedish research council (VR), the Center for Biomembrane Research (CBR) at Stockholm University, the German research council (Research unit 967), Jaensson foundation, and the Carl Tryggers foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Gruschke, S., Ott, M. (2013). Mechanisms and Control of Protein Synthesis in Yeast Mitochondria. In: Duchêne, AM. (eds) Translation in Mitochondria and Other Organelles. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39426-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-39426-3_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39425-6
Online ISBN: 978-3-642-39426-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)