Abstract
Oceanospirillaceae, a family within the order Oceanospirillales, currently consists of 17 genera including Amphritea, Balneatrix, Bermanella, Marinomonas, Marinospirillum, Neptuniibacter, Neptunomonas, Nitrincola, Oceaniserpentilla, Oceanobacter, Oceanospirillum (type genus), Oleibacter, Oleispira, Pseudospirillum, Reinekea, Spongiispira, and Thalassolituus, though recent phylogenetic analyses suggest a taxonomic realignment may be necessary as the inclusion of several genera has been shown dependent on the algorithm used to calculate their respective positions. Nearly all species inclusive to this aerobic family are Gram negative, motile rods, or helical shaped with positive oxidase and catalase reactions. All have DNA GC content of 41–63 mol% with the genome size of a member Marinomonas species having been reported at approximately 4.7 Mb through whole-genome sequence analysis. Most species, save those in the genus Balneatrix, are halophilic, requiring sodium ions for growth, and are widely distributed in marine environments, including marine organisms, seaglass, seawater, and sea sediment. The non-halophilic genus Balneatrix inhabits freshwater and has been identified as a human pathogen. Numerous Oceanospirillaceae species have unique characteristics applicable to industrial fields, including the capability for degrading petroleum compounds and secretion of bactericidal compounds or melanin pigment.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Taxonomy, Historical and Current
Short Description of Oceanospirillaceae
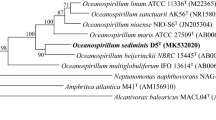
Oceanospirillaceae (O.ce.an.o.spi.ril.la’les. M. L. neut. n. Oceanospirillum type genus of the family;-aceae ending to denote family; M. L. fem pl. n. Oceanospirillaceae the Oceanospirillum family) was established by Garrity et al. (2005) on the basis of phylogenetic analysis of 16S rDNA sequences. The family Oceanospirillaceae belongs to the order Oceanospirillales of the class gammaproteobacteria; the order contains the families Oceanospirillaceae, Alcanivoraceae, Hahellaceae, Halomonadaceae, Oleiphilaceae, and Saccharospirillaceae. Oceanospirillaceae contains the genera Amphritea, Balneatrix, Bermanella, Marinomonas, Marinospirillum, Neptuniibacter, Neptunomonas, Nitrincola, Oceaniserpentilla, Oceanobacter, Oceanospirillum (type genus; Hylemon, Wells, Krieg and Jannasch 1973, 361AL.), Oleibacter, Oleispira, Pseudospirillum, Reinekea, Spongiispira, and Thalassolituus, though this taxonomy must be reevaluated as the inclusion of Balneatrix, Marinospirillum, Nitrincola, Pseudospirillum, and Reinekea is dependent on the algorithm used to calculate their respective phylogenetic positions (e.g., positions vary between NJ and ML methods such that, at minimum, Marinospirillum and Reinekea should be transferred to other families) (Fig. 24.1 ). Prior to the establishment of the family Oceanospirillaceae, many of these genera and bacterial groups were categorized as lesser known members of the γ-Proteobacteria.
Phylogenetic reconstruction of the family Oceanospirillaceae based on 16S rRNA and created using the neighbor-joining algorithm with the Jukes–Cantor correction. The sequence datasets and alignments were used according to the All-Species Living Tree Project (LTP) database (Yarza et al. 2010; http://www.arb-silva.de/projects/living-tree). The tree topology was stabilized with the use of a representative set of nearly 750 high-quality-type strain sequences proportionally distributed among the different bacterial and archaeal phyla. In addition, a 40 % maximum frequency filter was applied in order to remove hypervariable positions and potentially misplaced bases from the alignment. Scale bar indicates estimated sequence divergence
Almost all genera are halotolerant or halophilic marine bacteria, the exception being Balneatrix which has been isolated solely from freshwater and human clinical samples. Cells are primarily rod shaped, though some genera display helical or S-shaped morphologies, and all are motile by polar flagella. Physiologically, members of the Oceanospirillaceae are aerobic and strictly respiratory, save for Neptunomonas, which can perform weak fermentation reactions. All members are oxidase positive. Several species in the genera Bermanella, Neptuniibacter, Neptunomonas, Oceaniserpentilla, Oceanobacter, Oleibacter, Oleispira, Spongiispira, and Thalassolituus have been shown to be involved in petroleum degradation, and some Marinomonas strains have been shown to secrete bactericidal compounds and produce melanin. In most species, the primary isoprenoid quinones are Q8, while the majority of fatty acids are 14:0, 16:1ω7, 16:0, and 17:1ω6. The mol% GC of all Oceanospirillaceae DNA is 41–63. Genome size of a Marinomonas species has been determined to be approximately 4.7 Mb through whole-genome sequence analysis, although it remains to be seen if this is representative of the family as a whole. Summary of phenotypic information in Oceanospirillaceae is shown in Table 24.1 .
Taxonomic History
As stated previously, Oceanospirillaceae was established in 2005 by Garrity et al. Prior to its establishment, many marine genera and bacterial groups were categorized as the lesser known marine genera of the class γ-Proteobacteria; in fact, several marine bacterial groups have been recognized in the literature as “Oceanospirillum and related genera,” e.g., the 3rd edition of the Prokaryotes. The oldest genus of this family, Oceanospirillum, was established officially in 1973 (Hylemon et al. 1973), although several reclassifications and renamings for this bacterial group’s members have since occurred. Presented here is a description of the history for this genus and related bacteria subsequently followed by a description of the second oldest (and largest) genus, Marinomonas, and other selected genera.
The genus Oceanospirillum was originally created to distinguish the marine species of the genus Spirillum from those found in freshwater (Hylemon et al. 1973). The Spirillum genus has a long history, originally encompassing all of the known aerobic and microaerophilic spirilla, including both marine and freshwater species (Ehrenberg 1832; Watanabe 1959; Hylemon et al. 1973). As originally defined, the DNA base composition for the genus ranged from 38 to 65 mol% G+C, unusually broad for a bacterial genus (Krieg 1974). Moreover, three distinct groups were evident within the genus: (a) aerobic, freshwater spirilla unable to tolerate 3 % NaCl (mol% G+C 50–65); (b) aerobic, marine spirilla requiring seawater for growth (mol% G+C 42–48); and (c) large, microaerophilic spirilla that belong to the species S. volutans (mol% G+C =38). To make the genus more consistent with other taxa, Hylemon et al. (1973) divided it into three genera: the genus Aquaspirillum containing the aerobic freshwater spirilla, the genus Oceanospirillum containing the marine species, and the genus Spirillum which was comprised solely of the single species, S. volutans. The Oceanospirillum as described by Hylemon et al. (1973) contained six species: O. linum, O. minutulum, O. beijerinckii, O. maris, O. japonicum, and interestingly an organism known as “Spirillum lunatum” (Williams and Rittenberg 1957), though this last inclusion poses taxonomic problems. The characteristics of the type strain (ATCC 11337 or NCMB 54) of “Spirillum lunatum” did not fit the original description of the species, and Linn and Krieg (1978) found that NCMB strain 54 actually consisted of a mixture of two dissimilar organisms. One was a short, vibroid rod that possessed a single polar flagellum, grew in either the presence or absence of seawater, catabolized sugars, did not form coccoid bodies, and had a mol% G+C of 63–64. The other was a larger, helical organism that possessed bipolar flagellar tufts, required seawater for growth, failed to utilize sugars, formed coccoid bodies, and had a mol% G+C of 45. The smaller organism did not appear to belong to either Oceanospirillum or Aquaspirillum and to date remains unclassified. The larger organism had characteristics more in accord with the original description of “S. lunatum” but differed in certain respects; it has since been classified as a new subspecies of O. maris: O. maris subsp. williamsae. Terasaki later added four more species: O. hiroshimense, O. pelagicum, O. pusillum, and O. multiglobuliferum (Terasaki 1973, 1979), and together these nine species (including one subspecies) were subsequently described in Bergey’s Manual of Systematic Bacteriology (Krieg 1984). In 1984, Bowditch et al. described two new species, Oceanospirillum jannaschii and O. kriegii, as well as transferred two species, Alteromonas communis (currently Marinomonas communis) and A. vaga (currently M. vaga), to Oceanospirillum based on the immunological similarity analysis in marine bacteria, though van Landschoot and De Ley (1983) have proposed the establishment of a new genus for the two based on DNA–rRNA hybridization data. As a result, the genus definition of Oceanospirillum needed to be changed drastically, with the unfortunate loss of most of the readily determinable phenotypic features from the genus definition (Krieg 1984) and the extension of the upper mol% G+C limit for the genus from 51 to 57. By this extension, a considerable overlap of mol% G+C range was introduced between the genera Aquaspirillum (49–65 mol% G+C) and Oceanospirillum (42–51 mol% G+C), confounding one of the most reliable genotypic features discriminating between the genera (Pot et al. 1989, 1992). Although many of these species have since been reclassified into other genera or recognized as subjective synonyms of other species, some of taxonomic discrepancies remained within the genus solely based on their phenotypic characteristics (McElroy and Krieg 1972; Terasaki 1972, 1973; Hylemon et al. 1973; Carney et al. 1975; Krieg and Hylemon 1976). To resolve the problem, chemotaxonomic and genetic analyses were performed for the genus, including fatty acid composition analysis (Sakane and Yokota 1994), isoprenoid quinone profiling (Sakane and Yokota 1994), polyamine composition (Hamana et al. 1994), DNA–DNA hybridization (Pot et al. 1989), DNA–rRNA hybridization (Pot et al. 1989), and 16S rRNA sequence analyses (Woese et al. 1982, 1985). The resulting DNA–rRNA hybridization data (Pot et al. 1989) have indicated that O. communis (currently Marinomonas communis) and O. vaga (currently M. vaga) cannot be regarded as members of the genus Oceanospirillum and that the Oceanospirillum sensu stricto or the “core group” consisted of only five species, including the type species O. linum, O. maris, O. beijerinckii, O. multiglobuliferum, and, more distantly, O. japonicum. O. pelagicum and O. hiroshimense were unified as subspecies into O. beijerinckii and O. maris, respectively, and O. kriegii, O. jannaschii, O. minutulum, and O. pusillum were eliminated from the genus Oceanospirillum altogether (taxonomic positions for these species have yet to be determined). The analysis of the 16S rRNA oligonucleotide data catalogs of the Oceanospirillum species O. linum, O. maris, O. japonicum (currently Pseudospirillum japonicum), and O. minutulum (currently Marinospirillum minutulum) indicated that O. japonicum and O. minutulum were phylogenetically distinct from O. linum and O. maris, respectively (Woese et al. 1982, 1985). Subsequent chemotaxonomic studies (Sakane and Yokota 1994; Hamana et al. 1994) confirmed that O. pusillum (currently Terasakiella pusilla) had different profiles of 3-hydroxy fatty acids and quinine composition (comprised primarily of Q-10) from that of the Oceanospirillum sensu stricto and other pending Oceanospirilla species, whereas phylogenetic analysis indicated it should be assigned in alphaproteobacteria (Kawasaki et al. 1997). More recently, Satomi et al. (1998, 2002) conducted polyphasic taxonomic analyses targeting 16S rRNA and gyrB genes to examine the phylogeny of numerous Oceanospirillum strains. It was demonstrated that O. minutulum clustered on a separate branch together with new isolates from kusaya gravy (Satomi et al. 1998); thusly, a new genus, Marinospirillum, was proposed (Satomi et al. 1998) containing the two species, M. minutulum and M. megaterium (details given in the Marinospirillum description below). As a result, it was suggested that the Oceanospirillum core group consisted of four species: Oceanospirillum linum, O. maris, O. beijerinckii, and O. multiglobuliferum. Four other Oceanospirillum species were demonstrated to occupy taxonomic positions separate from the Oceanospirillum core group: O. jannaschii, O. japonicum, and O. kriegii in the gammaproteobacteria and O. pusillum in the alphaproteobacteria. Subsequently, O. jannaschii was transferred to the genus Marinobacterium as it was demonstrated to cluster with Marinobacterium georgiense (González et al. 1997), Pseudomonas iners (Iizuka and Komagata 1964), and P. stanieri (Baumann et al. 1983). Given that the other three species did not cluster with known genera, Satomi et al. (2002) proposed the creation of three new genera: Pseudospirillum gen. nov. for Oceanospirillum japonicum (Pseudospirillum japonicum comb. nov.), Oceanobacter gen. nov. for Oceanospirillum kriegii (Oceanobacter kriegii comb. nov.), and Terasakiella gen. nov. for Oceanospirillum pusillum (Terasakiella pusilla comb. nov). These reclassifications were further supported by phenotypic characteristics. For instance, O. japonicum differs from other Oceanospirillum species as it grows best at 35–37 °C, it does not form coccoid bodies, and its flagella appear to be crescent shaped with less than one helical turn (Oceanospirillum sp. typically have one or more helical turns). In addition, O. jannaschii and O. kriegii both have a higher mol% G+C (54.8–54.9) as well as other phenotypic characteristics that discriminate these species from the genus Oceanospirillum (Table 24.1 ). Moreover, as was discussed previously, O. pusillum possessed completely different chemotaxonomic features from the genus core group. Utilizing DNA–DNA hybridization, gyrB sequence analysis, and phenotypic characteristics, Satomi et al. (2002) further demonstrated a lack of significant diversity among the subspecies of O. maris and O. beijerinckii, suggesting that they should not be regarded as such. However, as González and Whitman (2006) pointed out, in the absence of a formal ruling by the International Committee on Systematic Bacteriology (ICSB), the subspecies designations are still valid, and these taxa continue to possess standing in the nomenclature. It should be noted that surveys of large collections of strains have never been performed, and thus, these subspecies have not been shown to represent genetic or phenotypic groups that might exist in nature. By assigning these strains as reference strains, the taxonomy does not prejudice the question of whether subspecies exist as biological entities. The current taxonomic status of species that have been assigned at one time or another to Oceanospirillum is summarized in Table 24.2 .
The genus Marinomonas was established in 1984 by van Landschoot and De Ley and represents the second oldest and largest genus in the Oceanospirillaceae, currently encompassing 20 species. As mentioned previously the original two species, M. communis (type species; type strain ATCC 27118) and M. vaga, were initially described as belonging to Alteromonas (Baumann et al. 1972), a genus created to accommodate Gram-negative heterotrophs with a single polar flagellum that, with a lower DNA mol% G+C of 38–50, were distinct from the Pseudomonas (DNA mol% G+C of 55 to 64; Baumann et al. 1972). In 1983, van Landschoot and De Ley demonstrated that A. vaga and A. communis belonged to a different DNA–rRNA hybridization group than other species of Alteromonas and proposed the genus Marinomonas. A year later, Bowditch et al. (1984) independently proposed that these species instead be classified within Oceanospirillum on the basis of immunological studies of the iron-containing superoxide dismutases and glutamine synthetases. They found that these enzymes cross-reacted most strongly with antisera prepared to the enzymes from Oceanospirillum beijerinckii and O. jannaschii. They also further characterized species in the genus Oceanospirillum, in addition to 33 and 17 strains of M. communis and M. vaga, respectively. However, Pot et al. (1989) again demonstrated with DNA–rRNA hybridization that these strains were not closely related to the type species of Oceanospirillum, further supporting their currently classification within Marinomonas, a conclusion later validated by 16S rRNA sequencing (Satomi et al. 1998, 2002). A third species, the melanin-producing Marinomonas mediterranea, was later described by Solano and Sanchez-Amat (1999), and the genus has subsequently expanded to include 20 total species. Almost all species were isolated from the marine environment, including habitats such as seawater, sediment, and seaglass. It should be noted that the genus Marinomonas cannot be clearly differentiated from other groups of marine, Gram-negative bacteria solely on the basis of phenotypic characteristics as numerous major phenotypic characteristics are shared with the genera Alteromonas and Pseudoalteromonas, as well as others (Akagawa-Matsushita et al. 1992; Baumann et al. 1972; González and Whitman 2006), although the use of 16S rRNA sequence analysis or DNA–DNA hybridization readily resolves these taxa.
Oceanobacter was created by Satomi et al. in 2002 and to date consists solely of one species, Oceanobacter kriegii. Originally isolated as strain H-1 (Baumann et al. 1972), this rod-shaped species was classified as Oceanospirillum kriegii on the basis of immunological analysis of their superoxide dismutases and glutamine synthetases (Bowditch et al. 1984). Subsequent DNA–rRNA hybridizations between this species and representatives of closely related organisms, along with 16S rRNA gene sequence analysis of the 16S rRNA gene, supported reclassification and the establishment of this genus (Pot et al. 1989; Satomi et al. 2002).
The genus Pseudospirillum was created by Satomi et al. (2002) for Oceanospirillum japonicum (former name), which was isolated from putrid infusions of shellfish and initially described as Spirillum japonicum (Watanabe 1959). Terasaki (1963, 1972) additionally described three similar strains, namely, IF4 (IFO 15447), IF8, and UF3, that based on phenotype and cellular morphology may belong to this or a closely related species. In 1973 Hylemon et al. reclassified S. japonicum, along with the other marine strains of Spirillum, into a new genus, Oceanospirillum. However, numerous independent lines of investigation indicated that this species had low phenotypic similarity to the Oceanospirillum sensu stricto; for example, older cultures did not form cocci or microcysts (Terasaki 1972; Carney et al. 1975), while the fatty acid composition of P. japonicum (current name) significantly differed from that of other members of the Oceanospirillum (Sakane and Yokota 1994). Based on DNA–rRNA hybridizations and 16S rRNA gene sequence analysis, it was ultimately reclassified as the type species of the new genus, Pseudospirillum (Pot et al. 1989; Satomi et al. 1998, 2002).
The genus Marinospirillum currently encompasses five species and was created to accommodate Oceanospirillum minutulum, originally classified as Spirillum minutulum (Watanabe 1959), and a new isolate Marinospirillum megaterium (Satomi et al. 1998). Currently, this genus is classified as a member of Oceanospirillaceae, although the phylogenetic position of this genus has been shown to be significantly closer to the family Halomonadaceae, suggesting that it should be reclassified at the family level. However, in accordance with the current taxonomic classification, this genus will be briefly described here. Originally, Watanabe (1959) isolated two strains, ATCC 19192 and ATCC 19193, and described them as Spirillum halophilum and S. minutulum, respectively. When the genus Spirillum was divided into freshwater and marine species, these strains were moved to Oceanospirillum along with the other marine species. Due to their shared similarities, Hylemon et al. (1973) proposed that the two strains did not warrant classification as two different species and they were thus reclassified together as Oceanospirillum minutulum. Subsequently, DNA–rRNA hybridization studies (Pot et al. 1989) and fatty acid composition analysis (Sakane and Yokota 1994) indicated that O. minutulum was significantly different from O. linum and members of the Oceanospirillum sensu stricto, respectively. Finally, based on 16S rRNA gene sequence analysis, Marinospirillum minutulum (current name) was reclassified as the type species of a new genus, Marinospirillum, along with the new isolate M. megaterium (Satomi et al. 1998), as described previously. Since then, three alkaliphilic species, M. alkaliphilum (Zhang et al. 2002), M. insulare (Satomi et al. 2004), and M. celere (Namsaraev et al. 2009), have additionally been added to the genus.
The genus Marinobacterium was created by Gonzalez et al. (1997) and, in accordance with current classification, is encompassed by the family Alteromonadaceae. However, its affiliation is unclear as based on 16S rRNA gene sequence analysis, the genus clusters with Nitrincola and occupies a position along the Oceanospirillaceae branch. Reclassification at the family level should therefore be considered.
The years of establishment and features for genera belonging to the Oceanospirillaceae are shown in Table 24.3 .
Phylogenetic Structure of the Family and Its Genera
According to the phylogenetic branching of the type strains of Oceanospirillales in the 16S rRNA gene tree of the Living Tree Project (Yarza et al. 2008, 2010), Oceanospirillaceae is moderately related to the families Saccharospirillaceae, Litoricolaceae, Halomonadaceae, and several genera for which taxonomic affiliation at the family level remains unclear (Fig. 24.1 ). Although the genus Marinospirillum and Reinekea are affiliated with the family Oceanospirillaceae, phylogenetically both genera are more closely related to Halomonadaceae and Saccharospirillaceae, respectively. Conversely, based on 16S rRNA sequence analysis, the genera Amphritea, Bermanella, Marinomonas, Neptuniibacter, Neptunomonas, Oceaniserpentilla, Oceanobacter, Oceanospirillum, Oleibacter, Oleispira, Spongiispira, and Thalassolituus comprise one defined Oceanospirillaceae familial cluster. The robustness of this clustering is supported by both neighbor-joining and maximum likelihood algorithms, although the clustering patterns vary for the remaining genera (Balneatrix, Nitrincola, and Pseudospirillum) depending on the sequence analysis method utilized. In the case of NJ method, these three genera cluster with and form loose groups to the Oceanospirillaceae core genera. However, when the ML method is utilized (data not shown), Balneatrix and Pseudospirillum are positioned outside of the Oceanospirillaceae cluster and apart from other described families, while Nitrincola groups within the Neptunomonas cluster along with Marinobacterium (which itself belongs to the family Alteromonadaceae). Thus, more study and consideration is required to adequately describe the family affiliation of Oceanospirillaceae. Of the genera with consistently defined phylogenetic positions, the Marinomonas form a robust cluster with sufficient phylogenetic distance among its 20 species. Neighboring branches to Marinomonas consist of Bermanella, Oceaniserpentilla, Oceanobacter, Oleibacter, Oleispira, Spongiispira, and Thalassolituus, which form robust clusters based on NJ analysis. These bacterial genera each consists of a single species and is physiologically associated with petroleum degradation. The genera Amphritea, Neptuniibacter, Neptunomonas, and Oceanospirillum also form a distinct cluster, although the clustering partners of Oceanospirillum are varied when using ML analysis. On the basis of phylogenetic analysis, it is clear that the genus Marinobacterium should be transferred from Alteromonadaceae to Oceanospirillaceae, while the genera Marinospirillum and Reinekea should be reclassified out of Oceanospirillaceae.
Molecular Analyses
Phylogeny
The widespread adoption of molecular tools such as the polymerase chain reaction (PCR) and DNA sequencing and subsequent phylogenetic studies based on 16S rDNA gene sequences have resulted in a major taxonomic reclassification of bacteria, including the establishment of the family Oceanospirillaceae. Phylogenetic analysis of 16S rRNA sequences provides relatively accurate information for Oceanospirillaceae taxonomy, although it occasionally lacks the required specificity for the differentiation of close relatives (Fox et al. 1992; Stackebrandt and Goebel 1994; Yamamoto and Harayama 1995, 1996, 1998), and thus, higher-resolution molecular identification markers have been required to distinguish between some species. Studies targeting the rapidly evolving gyrB gene, encoding the B subunit of DNA gyrase, have been utilized to elucidate the phylogeny of several taxonomically ambiguous bacterial species (Venkateswaran et al. 1999; Satomi et al. 2003). Satomi et al. (2002) previously demonstrated that gyrB sequence analysis demonstrated good correlation with DNA–DNA hybridization values and provided higher resolution for describing the taxonomy of Oceanospirillum species. Recently, a more precise method for phylogenetic evaluation has been reported. Multilocus sequence typing (MLST) has been shown proficient at analyzing phylogenetic relationships between individual species or strains and in cases has been utilized to verify the validity of subspecies; however, to date this method has not been applied to Oceanospirillum strains.
rRNA–DNA Hybridization
Before widespread adoption of molecular tools, such as PCR and DNA sequencing, and subsequent phylogenetic studies based on 16S rDNA gene sequences, rRNA–DNA hybridization was the powerful tool to prove the phylogenetic relationships between bacterial species based on molecular base study. In fact, rRNA–DNA hybridization resulted in a major reclassification of bacterial taxonomy including the marine bacteria (van Landschoot and De Ley 1983; Pot et al. 1989; De Vos et al. 1989). As was mentioned above, this technique was applied to study for intra-/intergeneric relationships of the genus Oceanospirillum and demonstrated that Oceanospirillum sensu stricto or the “core group” belongs to rRNA superfamily II. O. japonicum also positioned in this group but not making clusters with Oceanospirillum sensu stricto. O. pusillum was also misnamed as it belongs in rRNA superfamily IV (Pot et al. 1989). This study also proves Oceanospirillum vagum and O. communis should be relegated to their real generic positions, Marinomonas vaga and Marinomonas communis, respectively (Pot et al. 1989). However, the methods for rRNA sequence analysis are shifted to the recent PCR-based DNA–RNA sequence methods.
DNA–DNA Hybridization
In accordance with the consensus molecular definition of a species (Wayne et al. 1987), phylogenetic definitions generally include strains with “approximately 70 % or greater DNA–DNA relatedness and with 5 °C or less ΔTm.” Furthermore, “phenotypic characteristics should agree with this definition and are allowed to override the phylogenetic concept of species only in a few exceptional cases.” Thus, DNA–DNA reassociation values derived from DNA–DNA hybridization methods with labeled DNA (Ezaki et al. 1989) or thermal renaturation methods (De ley et al. 1970) should be respected before 16S rRNA sequence analysis in bacterial taxonomy. As mentioned previously, because 16S rRNA gene analysis occasionally lacks the specificity to differentiate close relatives, DNA–DNA relatedness values among closely related microbes are essential information for the determination of species’ taxonomic affiliation. With respect to Oceanospirillaceae, DNA–DNA hybridization is generally performed to describe new species in the case of the genus consisting of multiple species.
Historically, such techniques have been used for the taxonomic study of Oceanospirillum species as it often has been difficult to distinguish each species based solely on morphological and biochemical characteristics. In fact, Krieg (1984) suggested that “Species distinctions are less firmly based within a particular morphological group of strains, especially if they have a similar DNA base composition. Therefore, it is possible that some presently recognized species may not deserve separate species status (for example, O. maris vs. O. beijerinckii). It is likely that DNA/ DNA hybridization experiments could resolve many such questions.” Pot et al. (1989) demonstrated that only five species, including the type species, constituted the genus Oceanospirillum (O. linum, O. maris, O. beijerinckii, O. multiglobuliferum, and, more distantly, O. japonicum) and that the former species O. hiroshimense and O. pelagicum should be assigned as the subspecies, O. maris subsp. hiroshimense and O. beijerinckii subsp. pelagicum, respectively. Although O. japonicum was transferred to the new genus Pseudospirillum based on 16S rRNA gene sequence analysis (Satomi et al. 2002), the proposal by Pot et al. (1989) that the Oceanospirillum sensu stricto or the “core group” constituted O. linum, O. maris, O. beijerinckii, and O. multiglobuliferum was strongly supported. Additionally, as both reports indicated, the DNA–DNA reassociated values were sufficiently high within subspecies of O. maris (three subspecies) and O. beijerinckii (two subspecies) to label them as the same species, respectively. This, however, has yet to be demonstrated with a large-scale phenotypic investigation.
In the cases of the other genera, DNA–DNA hybridization experiments were performed to propose the new species or to study taxonomy within the genus. The phylogenetic study based on 16S rRNA gene sequence analysis within the genus Marinomonas species showed occasionally close and unclear to determine as different species due to lacking of resolution as the molecular identification markers. Thus, DNA–DNA hybridization is necessary to evaluate new taxa. In most of the cases, the DNA–DNA hybridization values showed less than 40 % among Marinomonas species, even though they have close relationships (>99 %) with each other on the 16S rRNA sequence analysis. As well as Marinomonas, DNA–DNA hybridization is available to distinguish species in Marinospirillum, Marinobacterium, and Reinekea, which constituted by multiple species, though their appropriate taxonomic positions are pending.
GC Content
DNA base composition values for Oceanospirillaceae vary from 41 to 63 mol%, and the ranges for individual genus are shown in Table 24.1 . DNA base compositions are analyzed using high-performance liquid chromatography methods (Tamaoka and Komagata 1984), buoyant density method described as Bd (Schildcraut et al. 1962), and thermal denaturation methods described as Tm (Marmur and Doty 1962). These methods can determine GC base composition, although the resulting values may be slightly different. Recent whole-genome sequence analysis is more precise for the determination of GC content. DNA base composition values are good chemotaxonomic marker to distinguish genus, especially for marine bacteria, which have similar phenotypic characteristics. Most Oceanospirillaceae genus show 44–54 GC mol%; hence, Spongiispira norvegica, which is the only species of this genus, have high GC content, 62.6 %. Since the genus Spongiispira have been established based on single strain, further study is necessary based on multiple strains.
Whole-Cell Protein Profile
Whole-cell protein profile is also a common strain or species typing method and has been applied to distinguish some Oceanospirillum strains, including formerly Oceanospirillum (Pot et al. 1989). Protein electropherogram analysis for Oceanospirillum core group demonstrated that the genus distinguished the following three groups of strains: (i) O. maris subsp. maris ATCC 27509T, ATCC 27648, and ATCC 27649; (ii) O. maris subsp. williamsae ATCC 29547T and O. hiroshimense IFO13616T; and (iii) O. linum ATCC 11336T and ATCC 12753. The rest of the species, O. japonicum ATCC 19191T, O. pelagicum IFO 13612T, O. multiglobuliferum IFO13614T, and O. beijerinckii NCMB 52T, occupied a separate position on the dendrogram based on whole-cell protein profiling. It was supported by rRNA–DNA hybridization and DNA–DNA hybridization experiment performed at same time. Thus, the method is available for separating species–subspecies level; it is likely rather than strain typing. As a recent protein-based bacterial identification method, the whole-cell protein fingerprinting technique using matrix-assisted laser desorption–ionization time-of-flight mass spectrometry has been developed (Bohme et al. 2010). However, it has not performed for Oceanospirillaceae strains yet.
Other Molecular Technique-Based Analysis
Nucleotide- or protein-based techniques have been developed to detect specific bacteria from various samples, including food, clinical, and environmental samples. PCR and quantitative PCR are useful and sensitive detection methods for specific genes from bacteria including Oceanospirillaceae. Since most interests for Oceanospirillaceae bacteria is related to diversity and their habitation including main role in ocean environment, most researches were performed to monitor the microbial dynamics in ocean environment using PCR-clone library methods, in which microbial DNA was directly extracted from samples and bacterial species existing in the sample were identified using 16S rRNA gene sequence. The method can give useful information about microbial diversity and assumed bacterial flora in samples without cultural bias, though DNA extraction, PCR condition, and copy number of target gene for identification are effected to quantitative results. For example, Giovannoni and Rappé (2000) studied microbial diversity in the Sargasso Sea by using PCR-clone library sequence method and indicated that rRNA genes closely related to Oceanospirillum species have not been encountered in libraries prepared from marine environmental rDNA, suggesting that the abundance of Oceanospirillum in the marine environment is low. The other biochemical identification methods, immuno-based methods, are additional common methods for the detection of specific bacteria. DeLong et al. (1984) tried to apply bacterial identification–classification based on immunological reaction using the antiserum prepared by the iron-containing superoxide dismutases and glutamine synthetases. The method is suitable to detect the specific bacteria; however, it is difficult to presume genetic relationships among irrelativeness bacteria.
Genome Analysis
Whole-genome sequences for some Oceanospirillaceae species have been determined and reported in public databases: Bermanella marisrubri, Marinomonas mediterranea, M. pontica, and Neptuniibacter caesariensis. The genome size of the species sequenced ranges from 3.53 to 4.68 Mb, and 7–8 copies of the ribosomal RNA operon are present in each genome and no plasmids in sequenced strains. The GC content of each species agrees with data obtained using high-performance liquid chromatography or the Tm method. Whole-genome sequences are expected to provide useful information with which to elucidate metabolic pathways for particular characteristics of Oceanospirillaceae strains, e.g., petroleum degradation, melanin production, and bactericidal compounds secretion. Thus, Marinomonas mediterranea MMB-1T is of interest, and its whole genome is sequenced because it can synthesize melanin pigments, which are mediated by the activity of a tyrosinase and also express other oxidases with biotechnological interest, such as a multicopper oxidase with laccase activity and a novel l-lysine-epsilon-oxidase. As the detailed data of whole-genome sequence in M. mediterranea showed that 4,684,316 bp long genome harbors 4,228 protein-coding genes and 98 RNA genes (Lucas-Elío et al. 2012).
Plasmid and Page
There is less information about phages and plasmids harbored in Oceanospirillaceae species. Full genome sequence of bacteriophage P12026 that can lytically infect bacterial strain IMCC12026, a member of the genus Marinomonas, was reported (Kang et al. 2012). Bacteriophage P12026 was isolated by using enrichment culture followed by plaque assay from a seawater sample collected from the same station. On the basis of transmission electron microscopy observation, the phage P12026 was regarded as a member of the Siphoviridae, since it has isometric heads and long noncontractile tails and double-stranded DNA. The genome sequence of phage P12026 was 31,766 bp in length with G+C content of 46.0 mol% and had 54 open reading frames (ORFs) predicted. The genome of phage P12026 seemed to have modular structure, as shown in many other phages (Krupovick et al. 2011).
Phenotypic Analyses
General Description
O.ce.an.o.spi.ril.la’les. M.L.neut.n.Oceanospirillum type genus of the family;-aceae ending to denote family; M.L.fem pl. n. Oceanospirillaceae the Oceanospirillum family. The main features of members of Oceanospirillaceae are listed in Table 24.1 . Most genera are halotolerant or halophilic and motile. Almost all genera are aerobic or microaerophilic chemoorganotroph. As mentioned above, most genera of this family are consist of single species; moreover, in some cases, the genus established only single strain. Thus, genus characteristics are lesser known.
Type genus: Oceanospirillum Hylemon, Wells, Krieg and Jannasch 1973, 361AL.
Differentiation of the Oceanospirillaceae from Other Families
Major phenotypic characteristics among Oceanospirillaceae are similar to other members of Oceanospirillales and major marine bacteria, mainly Alteromonadales, indicating that based only upon phenotypic characteristics, it is difficult to classify in relation to other Gram-negative marine bacteria. Owing to the diversity of phenotypic characteristics within the family, only a few properties are shared by all of members of this family, thus indicating that some of strains may be misidentified. Moreover, the problem is also compounded by the scarcity of strains in each species. Thus, other methods have contributed greatly to our current understanding of their systematics. These include DNA–DNA hybridization (Pot et al. 1989; Satomi et al. 2002), immunological analysis (Bowditch et al. 1984; DeLong et al. 1984), DNA–rRNA hybridization (Pot et al. 1989), 16S rRNA sequence analyses (Woese et al. 1982; Satomi et al. 1998, 2002), and chemotaxonomy of polyamines, fatty acids, and isoprenoid quinones (Hamana et al. 1994; Sakane and Yokota 1994). Based upon these analyses, the classification of Oceanospirillaceae is accomplished.
Morphology
Oceanospirillaceae species shows that various cell shapes depend on genus or species and most species are curved or rod-shaped form. In the case of Oceanospirillum, which is type genus of this family, all species consist of rigid helical cells, although variants having less curvature may arise after prolonged transfer. Marinospirillum also have rigid helical shape. The cells have a constant and characteristic type of clockwise (right-handed) helix in both genera. On the other hand, Terasakiella pusilla belonging to the alphaproteobacteria, which has been classified as member of Oceanospirillum, has a counterclockwise (left-handed) helix (Terasaki 1972), indicating that helix characteristics also give taxonomic information. Bermanella, Oceaniserpentilla, Oleispira, Pseudospirillum, Spongiispira, and Thalassolituus have thin helical shape. The type strain of Pseudospirillum japonicum consisted initially of long, helical cells with several turns (Watanabe 1959), but now consists of slightly curved or S-shaped cells. An unusual elaboration of the plasma membrane, the “polar membrane,” occurs in all of the Oceanospirillum species so far examined (Krieg 1984). It is attached to the inside of the plasma membrane by bar-like links and is located, most commonly, in the region surrounding the polar flagella (Krieg 1984). Such a membrane has been found mainly in genera of helical bacteria, such as Spirillum, Campylobacter, Aquaspirillum, Ectothiorhodospira, and Rhodospirillum. However, further study related to membrane structure and flagella formation has not done since it has been reported. As a recent study based on transmission electron microscope observation for cells with ultrathin section, Thalassolituus and some thin spiral-shaped species show one to four polar flagella and present a Gram-negative cell wall architecture with an outer membrane (Yakimov et al. 2003, 2004).
As the unique characteristics, Oceanospirillum and other rigid helical bacteria can form coccoid bodies (sometimes termed “microcysts”) in old culture. These bodies have thin walls and resemble spheroplasts; however, they are resistant to lysis in distilled water (Krieg 1984). Whether coccoid bodies are resistant to desiccation is not known. There is still less information about these characteristics due to lacking of study in this field past 20 years. Three main modes of formation of coccoid bodies were described by Williams and Rittenberg (1957) as follows: (a) two cells may entwine and apparently fuse. The cells become shorter and thicker and a protuberance develops at the point of fusion. This gradually enlarges and absorbs the organisms to form the coccoid body. More than one coccoid body may develop from a pair of entwined spirilla; (b) a Spirillum may become shorter and thicker and a protuberance arises from the center of the cell or from each end of the cell. The protuberances enlarge and eventually merge into a single coccoid body as the helical cell is absorbed; (c) a Spirillum may undergo a gradual shortening and rounding to form a coccoid body. The majority of coccoid bodies present in old cultures appear to be viable and can “germinate” when placed into a fresh medium (Williams and Rittenberg 1957). Germination is by unipolar or bipolar growth of a helical cell from the coccoid body, with the latter being absorbed into the developing helical cell. To elucidate details of this character, further studies including molecular sequence and gene expression analysis are needed.
Genus Description
Oceanospirillum
O.ce.an.o.spi.ril’lum. Gr. n. okeanos the ocean; N.L. dim. neut. n. spirillum a small spiral from Gr. n. spira spiral; N.L. Oceanospirillum a small spiral organism from the ocean (seawater). Rigid, helical cells with clockwise helix. Cells 0.4–1.2 μ-m in diameter; length of the helix, 2.0–4.0 μ-m. A polar membrane underlies the cytoplasmic membrane at the cell poles in all species examined so far by electron microscopy. Intracellular poly-b-hydroxybutyrate (PHB) is formed. All species form thin-walled coccoid bodies, which predominate in old cultures. Gram negative. Motile by bipolar tufts of flagella. Chemoorganotrophic, having a strictly respiratory type of metabolism with oxygen as the terminal electron acceptor. Nitrate respiration does not occur; nitrate is not reduced to nitrite or beyond the nitrite stage. Optimum temperature, 25–32 °C. Oxidase positive. Indole negative. Casein, starch, hippurate, and esculin are not hydrolyzed. Seawater required for growth. Carbohydrates are neither fermented nor oxidized. Amino acids or the salts of organic acids serve as carbon sources. Growth factors are not usually required. Isolated from coastal seawater, from decaying seaweed, and from putrid infusions of marine mussels. The G+C content of the genomic DNA ranges from 45 to 50 mol% (as determined by the thermal denaturation method). Type species is Oceanospirillum linum (Williams and Rittenberg 1957) Hylemon et al. 1973 (Approved Lists 1980). The genus is currently encompassing four species. Phenotypic features of this genus are shown in Table 24.4 .
Marinomonas
Ma.ri.no.mo.nas.L. adj. marinus pertaining to the sea; Gr. N. monas a unit, monad; M.L. Marinomonas. Gram negative, straight, or curbed rods. Motile by means of polar flagella at one or both poles. Aerobic, having a strictly respiratory type of metabolism. Oxidase positive or oxidase negative. Na+ is required for growth. Do not accumulate poly-β-hydroxybutyrate. Do not require organic growth factors. Do not produce extracellular amylase. Utilize acetate but not butyrate or valerate. Utilize glutamate, sorbitol, and malate. Commonly isolated from seawater. The mol% G+C of the DNA is 45–50. The genus is currently encompassing 20 species. Type species: Marinomonas communis (Baumann et al. 1972) van Landschoot and De Ley 1983. Phenotypic features of Marinomonas species are shown in Table 24.5 .
Amphritea
Am.phri’tea. N.L. fem. n. Amphritea from Gr. fem. n. Amphrite, a nymph of the ocean in Greek mythology, referring to the habitat of the bacteria. Cells are Gram-negative rods, motile by monopolar flagella. Coccoid bodies may be formed in old cultures. Catalase and oxidase positive and accumulate PHB. Growth range is from 4 °C to 40 °C, from 0.3 % to 9 % salinity, and from pH 4.6 to 9.5. Various sugars and carboxylic acids are oxidized. Predominant fatty acids are C18: 1ω7c, C16: 1ω7c, and C16: 0. 16S rRNA gene sequence analysis positions the genus in close proximity to the genera Oceanospirillum and Neptunomonas within the family Oceanospirillaceae. The type species is Amphritea atlantica. The genus constitute with three species, A. atlantica, A. balenae, and A. japonica. A. atlantica was isolated from a Bathymodiolus sp. specimen collected from the Logatchev hydrothermal vent field at the Mid-Atlantic Ridge at a depth of about 3,000 m (Gärtner et al. 2008). It is not unclear whether barophilic or not. A. balenae and A. japonica were isolated from the sediment adjacent to sperm whale carcasses off Kagoshima, Japan, at a depth of about 230 m (Miyazaki et al. 2008b). The genus Amphritea was regarded as the relatives to a symbiotic bacteria of Osedax (Goffredi et al. 2007), because its phylogenetic position was nearby that of a symbiotic bacterial clone when the first species of the genus isolated. The genus Osedax (Polychaeta: Siboglinidae) has recently been discovered in Osedax specimens host symbiotic bacteria in their ovisac and root systems. In fact, the latter two species, A. balenae and A. japonica, were isolated from the sediment adjacent to sperm whale carcasses, which are closely related to Osedax sp. habitats on the deep-sea floor. It is indicated that Amphritea is associated with Bathymodiolus sp. and Osedax sp. as symbiotic bacteria; however, there is less information to prove it. Phenotypic features are shown in Table 24.6 .
Balneatrix
Bal'ne.a.trix,L. fem. n., ba ther. Gram-negative, straight or curved rods, motile by a single polar flagellum. Strictly aerobic. Growth occurs at a wide range of temperatures (20–46 °C) on nutrient agar. Growth occurs in media containing 0–0.5 % (w/v) NaCl and not in media with more than 1 % NaCI. No growth factors required. Nitrate reduced to nitrite. Carbohydrates utilized with acid production. G+C content of the DNA (one strain determined) is around 54 mol%. The type species is Balneatrix alpica. The genus Balneatrix is consist of a single species, B. alpaca (Dauga et al. 1993), which is the only species are reported as clinical related bacteria in Oceanospirillaceae. B. alpaca have occurred the outbreak of pneumonia and meningitis in a spa therapy center, southern France, in 1987 (Hubert et al. 1991). However, no more outbreaks caused by B. alpaca have been reported. Details are described in pathogenicity and clinical relevance section. Phenotypic features of this genus are shown in Table 24.1 .
Bermanella
Ber.ma.nel’la. N.L. fem. dim. n. Bermanella named after the aquatic microbial ecologist Dr. Tom Berman. Gram-negative, strictly aerobic, chemoorganotrophic bacteria. Oxidase and catalase positive. Cells are motile, thin spirilla. Gas vesicles are not observed. Poly-b-hydroxybutyrate granules are produced. Slightly halophilic; no growth without seawater or the addition of combined marine salts to the medium. Mesophilic. Do not ferment carbohydrates, as determined on anaerobic Hugh and Leifson O/F medium (Difco) with half-strength artificial seawater (Baumann et al. 1972). Stenotrophic; the only carbon sources that serve as single carbon sources are organic acids. The type species is Bermanella marisrubri. The genus Bermanella is consist of a single species, B. marisrubri (Pinhassi et al. 2009), isolated from a surface seawater sample collected from the Gulf of Eilat in the northern Red Sea. The DNA G+C content of the type strain is 44.0 mol%. As the genus was established based on the single strain, the true habitat and biological features of genus are less known. Phenotypic features of this genus are shown in Table 24.1 .
Marinospirillum
Ma. ri. no. spi. ril’lum. L. adj. mar inus of the sea; Gr. n. spira a spiral; M.L. dim. neut. n. spirillum a small spiral; Marinospirillum a small spiral from the sea. Gram-negative, rigidly helical, nonspore-producing, coccoid body-forming, halophilic, aerobic or microaerobic, chemoheterotrophic, and PHB-accumulating bacteria. Motile by means of flagella. Oxidase positive. Catalase reaction is negative or positive. Carbohydrates are not catabolized. Genomic DNA G+C content of 42–45 mol% (as determined by HPLC). The isoprenoid quinone type is Q-8. The type species is Marinospirillum minutulum. The genus Marinospirillum currently encompasses five species and was created to accommodate Oceanospirillum minutulum, originally classified as Spirillum minutulum (Watanabe 1959). Currently, this genus is classified as a member of Oceanospirillaceae, although the phylogenetic position of this genus has been shown to be significantly closer to the family Halomonadaceae, suggesting that it should be reclassified at the family level. Phenotypic features of this genus are shown in Table 24.7 .
Neptuniibacter
Nep.tu.ni.i.bac’ter. L. adj. Neptunius Neptunian, pertaining to Neptune, Roman god of the sea; N.L. masc. n. bacter a rod; N.L. masc. n. Neptuniibacter a Neptunian rod, referring to the habitat of the bacteria.
Gram-negative, strictly aerobic, chemoorganotrophic bacteria. Oxidase and catalase positive. Cells are rod shaped and motile. Gas vesicles not observed. Produce poly-b-hydroxybutyrate granules. Slightly halophilic; no growth can be obtained without seawater or the addition of combined marine salts to the medium. Mesophilic. Do not ferment carbohydrates. Preferred carbon sources are organic acids and amino acids. Possess ubiquinone Q-8 as a respiratory quinone. DNA G+C content is around 47 mol%. The type species is Neptuniibacter caesariensis isolated from a surface seawater sample collected from the eastern Mediterranean Sea, offshore from the historic location of Caesarea. The genus constitutes with a single species and is established based on the single strain; the true habitat and biological features of genus are less known. Phenotypic features of this genus are shown in Table 24.1 .
Neptunomonas
Nep-.tu.no.mo’nas. Rom. myth. Neptune, the Roman god of the sea; Gr. n. monas, unit; M.L. n. Neptunomonas, Neptune’s bacterium. Gram-negative rod-shaped bacteria. Cells of the type species are approximately 0.7–0.9 by 2.0–3.0 mm and are motile by means of a single polar flagellum. Facultatively aerobic. Oxidase and catalase positive. May utilize amino acids, carbohydrates, organic acids, sugar alcohols, and some polycyclic aromatic hydrocarbons (PAHs ) as sole carbon and energy sources. Cells can degrade PHAs and require sodium ions for growth. The DNA G+C content is 46 mol%. The type species of the genus is Neptunomonas naphthovorans, isolated from Eagle Harbor, a creosote-contaminated Environmental Protection Agency superfund site in Puget Sound, Washington. The genus is currently encompassing three species, which is related to petroleum and PAHs degrading. Phenotypic features of this genus are shown in Table 24.8 .
Nitrincola
Nit.rin’co.la. L. neut. n. nitrum soda; L. masc. n.incola inhabitant, dweller; N.L. masc. n. Nitrincola an inhabitant of a soda environment. Alkaliphilic, halotolerant and heterotrophic. Cells are non-pigmented, asporogenous, motile, Gram-negative rods. NO2 and O2 can be used as electron acceptors. Fermentable carbon sources do not support growth. Chemoorganotrophic. Requires sodium for growth. Oxidase and catalase positive. Optimal pH for growth is 9.0. The genus is isolated from a saline, alkaline lake. The DNA G+C content is 47.4 mol% (Tm). The type species is Nitrincola lacisaponensis isolated from decomposing wood taken from the shore of the Soap Lake, a saline, alkaline lake in Grant County, WA, USA. Soap Lake is a closed, evaporative system with neither a significant surface inlet nor outlet. This results in the concentration of salts, mainly sodium carbonate and sodium sulfate (Anderson 1958). As the genus is established based on the single strain, the true habitat and biological features of genus are less known. Phenotypic features of this genus are shown in Table 24.1 .
Oceaniserpentilla
O.ce.a.ni.ser.pen.til’la. L. masc. n. oceanus the ocean; L. fem. n. serpens -tis a snake; N.L. fem. n. serpentilla a small snake; N.L. fem. n. Oceaniserpentilla small snake of the ocean, indicating shape and origin. Gram-negative, motile, obligately aerobic, vibroid to spiral, nonspore-forming cells. Oxidase activity is present, whereas catalase activity is absent. The type species is Oceaniserpentilla haliotis isolated from after filtration of abalone hemolymph serum through a filter with a pore size of 0.2 mm. The hemolymph serum was isolated from the blacklip abalone Haliotis rubra harvested near Hobart, Tasmania. Its habitat can be associated with black abalone; however, details are not known. As the genus is established based on the single strain, the true habitat and biological features of genus are less known. Phenotypic features of this genus are shown in Table 24.1 .
Oceanobacter
Gram-negative, straight rod, nonspore-forming, halophilic, aerobic, chemoheterotrophic, PHB-accumulating and oxidase-positive bacteria. Motility is by means of flagella. Some carbohydrates are catabolized. In addition, some strains utilize simple alcohols and organic acids, such as ethanol and lactate, as well as betaine and aminobutyrate as sole carbon sources. These bacteria reduce nitrate to nitrite. The mol% G+C content of its DNA is 54–56. The ubiquinone type is Q-8 (Sakane and Yokota 1994). The major nonpolar fatty acids in the phospholipids are C16:1 (36 %), C18:1 (27 %), and C16:0 (16 %; Sakane and Yokota 1994). The major 3-hydroxy fatty acids are C12:0 (54 %), C16:0 (27 %), and C10:0 (19 %). Spermidine (0.84 μmol/g of wet cells) and putrescine (0.03 μmol/g of wet cells) were the only detectable polyamines (Hamana et al. 1994). The type and only species of this genus is Oceanobacter kriegii isolated from seawater. Phenotypic features of this genus are shown in Table 24.1 .
Oleibacter
O.le’i.bac’ter. L. n. oleum oil; N.L. masc. n. bacter rod; N.L. masc. n. Oleibacter an oil (−degrading) rod. According to 16S rRNA gene sequence analysis, belongs to the gammaproteobacteria. Cells are Gram-negative, motile, aerobic rods. n-Alkane-degrading activity is observed. Predominant cellular fatty acids are C16: 0, C16: 1ω7, and C18: 1ω9, and hydroxy fatty acids are C12: 0 3-OH and C10: 0 3-OH. The major isoprenoid quinone is Q-9 and minor amounts of Q-8 are present. Polar lipids are phosphatidylglycerol, a ninhydrin-positive phospholipid(s) and glycolipids. The DNA G+C content of known strains of the type species is 53.0–53.1 mol%. The type and only species of this genus is Oleibacter marinus isolated from Indonesian seawater after enrichment with crude oil and a continuous supply of supplemented seawater. The strains exhibited high n-alkane-degrading activity, which indicated that the strains were important degraders of petroleum aliphatic hydrocarbons in tropical marine environments. Phenotypic features of this genus are shown in Table 24.1 .
Oleispira
O.le.i’spi.ra. L. n. oleum oil; Gr. fem. n. spira a spire; N.L. fem. n. Oleispira an oil-degrading, spiral-shaped organism. Gram-negative, vibroid to spiral cells, 2.0–5.0 mm long by 0.4–0.8 mm wide, motile by a single polarly inserted, long (>5 mm) flagellum. Chemoheterotroph with strong preference for aliphatic carbon substrates. Aerobic. Able to grow under anaerobic conditions by nitrate reduction. Oxidase and catalase are present. Ammonia and nitrate may serve as nitrogen sources. The narrow range of growth-supporting substrates is restricted to aliphatic hydrocarbons, Tweens, and volatile fatty acids. Uptake of common carbohydrates or amino acids as sole carbon sources for growth is detected in a very narrow spectrum. Stenohaline requires Na+ ions, exhibiting optimal growth in the presence of 3–5 % (w/v) NaCl. Psychrophilic growth, with optimal growth temperature of 2–4 °C. The major cellular fatty acids are monounsaturated fatty acids. The DNA G+C content of known strains of the type species is 41–42 mol%. The type and only species of the genus is Oleispira antarctica isolated from hydrocarbon-degrading enrichment cultures obtained from Antarctic coastal marine environments (Rod Bay, Ross Sea). Phenotypic features of this genus are shown in Table 24.1 .
Pseudospirillum
Pseudospirillum (Pseu.do.spi.ril«lum. Gr. adj. pseudes false ; N.L. n. Spirillum genus of spiral-shaped bacteria ; N.L. n. Pseudospirillum false Spirillum). Gram-negative, curved, straight or S-shaped, nonspore-forming, halophilic, aerobic, chemoheterotrophic, and PHB-accumulating bacteria. Motile by means of flagella. Oxidase positive. Catalase-negative or catalase-positive reaction. Carbohydrates are not catabolized. Coccoid body is not forming. Genomic DNA G+C content of 45 mol% (as determined by HPLC). Major fatty acids are C16:0, C16:1ω7c, and C18:1ω7c. The isoprenoid quinone type is Q-8. The type and only species of this genus is Pseudospirillum japonicum (basonym Oceanospirillum japonicum). Phenotypic features of this genus are shown in Table 24.1 .
Reinekea
Rei.ne.ke’a. N.L. fem. n. Reinekea derived from Reineke, geographical name of Reineke Island, Peter the Great Bay, Sea of Japan, Russia, the place where the bacterium was first isolated. Gram negative, heterotrophic, oxidase and catalase positive, rod shaped, and motile. Sodium ions are essential for growth. Growth occurs in 0.5–5 % NaCl and between 4 °C and 37 °C. No growth is observed in >5 % NaCl or at 40 °C. Facultatively anaerobic; acid is produced from some carbohydrates under anaerobic and aerobic conditions. Predominant isoprenoid quinone is Q-8. Polar lipids include phosphatidylglycerol, diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylinositol, and an unknown phospholipid. Major fatty acids are C16:0, C16:1ω7c, and C18:1ω7c. The type species is Reinekea marinisedimentorum. The genus is currently encompassing three species. Currently, this genus is classified as a member of Oceanospirillaceae, although the phylogenetic position of this genus has been shown to be significantly closer to the family Saccharospirillaceae, suggesting that it should be reclassified at the family level. Phenotypic features of this genus are shown in Table 24.9 .
Spongiispira
Spon.gi.i.spi9ra. L. fem. n. spongia sponge; L. fem. n. spira curvature, spiral; N.L. fem. n. Spongiispira spiral-shaped bacterium from a sponge. Gram-negative, spiral-shaped cells, motile by a single polar flagellum. Aerobic, chemoheterotrophic, positive for lipase activity, relatively restricted nutritional profile, not able to reduce nitrate, oxidase positive, catalase negative, and mesophilic. Salt is essential for growth. The major cellular fatty acids are C16:1ω7 and C16:0. The type and only species of this genus is Spongiispira norvegica isolated from the cold-water sponge Isops phlegraei (class Demospongiae) collected from a depth of approximately 320 m in the Sula Ridge off the coast of mid-Norway. As the genus is established based on the single strain, the true habitat and biological features of genus are less known. Phenotypic features of this genus are shown in Table 24.1 .
Thalassolituus
Tha.las.so.li.tu9us. Gr. fem. n. thalassa the sea; L. masc. n. lituus a curved rod, crook; N.L. masc. n. Thalassolituus a marine, curve-shaped organism. Gram-negative, vibroid to spiral, motile cells, 1.2–3.5 mm long by 0.6 mm wide. Strictly halophilic: Na+ ions are required for growth. Chemoorganoheterotrophic; strictly aerobic; unable to grow under anaerobic conditions by fermentation, nitrate reduction, or phototrophically. Oxidase positive. Ammonia and nitrate may serve as nitrogen sources. Indole, arginine dihydrolase, and gelatinase negative. Acetate, C7–C20 aliphatic hydrocarbons, and their oxidized derivatives are the only carbon sources that are used for growth. Principal cellular fatty acids are laurate, palmitate, and octadecenoate. Predominant isoprenoid quinone is Q-9 (Teramoto et al. 2011). The type and only species (to date) of the genus is Thalassolituus oleivorans isolated from extinction dilution from an n-tetradecane enrichment culture that was established from seawater–sediment samples collected in the harbor of Milazzo, Italy. As the genus is established based on the single strain, the true habitat and biological features of genus are less known. Phenotypic features of this genus are shown in Table 24.1 .
Marinobacterium
Ma.ri.no.bac.te’ri.um. L. adj. marinus, of the sea; Gr. neut. n. bakterion, a small rod; L. neut. n. Marinobacterium, marine rod. Cells are rod shaped and Gram negative and have numerous vesicles on their surfaces. Strict aerobe. Oxidase and catalase positive. Grows on sugars, fatty acids, aromatic compounds, and amino acids. Requires sea salt-based medium for growth. The type species is Marinobacterium georgiense. The genus Marinobacterium was created by Gonzalez et al. (1997) and, in accordance with current classification, is encompassed by the family Alteromonadaceae. However, its affiliation is unclear as based on 16S rRNA gene sequence analysis, the genus clusters with Nitrincola and occupies a position along the Oceanospirillaceae branch. Reclassification at the family level should therefore be considered. For reference, here, genus description is shown. Details of this genus are described in section Alteromonadaceae.
Chemotaxonomic Characteristics
Fatty Acid Profiles
Fatty acid profile in Oceanospirillaceae is reported for almost all species belonging to the family with description of the new genus or species. Oleispira antarctica can synthesize EPA (C20:5ω3). The most abundant nonpolar fatty acids are C16: 1, C16: 0, and C18: 1 in most species of this family. The fatty acid profiles can be different depending on the analytical method used in each study—for instance, results obtained using a microbial identification system differ from those obtained with traditional methods that analyze extracted cytoplasmic lipids using gas chromatography and gas chromatography–mass spectrometry. In fact, results of fatty acid profile are different among the papers describing the new species of Marinomonas, even though they analyzed the same strain at the same condition, for instance, major fatty acid in M. aquimarina was reported as C16:0 (Espinosa et al. 2010); hence, Gupta et al. (2006) determined isoC16:0 as predominant fatty acid. However, it is obvious that fatty acid profile is a distinctive feature on the bacterial taxonomy. For instance, the thirteen strains of formerly Oceanospirillum that have been investigated for their fatty acid composition by Sakane and Yokota (1994) were divided into three groups. Group I included the ten strains belonging to O. linum, O. maris subsp. hiroshimense, O. maris subsp. williamsae, O. beijerinckii subsp. beijerinckii, O. beijerinckii subsp. pelagicum, O. multiglobuliferum, and O. japonicum (currently Pseudospirillum japonicum), all of which have a low mol% G+C (42.5–48.4). Group II included the two type strains of O. jannaschii (currently Marinobacterium jannaschii) and O. kriegii (currently Oceanobacter kriegii) and had a high mol% G+C content (54.8–54.9). Group III included only O. pusillum (currently Terasakiella pusilla) and could be clearly distinguished from other marine spirilla in having C14:0 3OH as the major 3-hydroxy fatty acid, which is a useful chemical indicator for separating bacterial species (Oyaizu and Komagata 1983), besides Q-10. Bertone et al. (1996) confirmed the separate position of O. japonicum, O. jannaschii, and O. kriegii. Yakimov et al. (2004) analyzed detail fatty acid profile in Thalassolituus oleivorans. The principal fatty acids in total major cellular fatty acids (TLFA), phospholipid fatty acids (PLFA), and glycolipid fatty acids profiles are C12: 0, C16: 0, and C18: 1. The TLFA and PLFA profiles are characterized by an almost equal presence of saturated and monounsaturated fatty acids, with a strong predominance of C14: 0, C16: 1, C16: 0, and C18: 1. They also analyzed the fatty acid profile at the position of the two fatty acids in the glycerol moiety, PE and PG, using CID-MS spectra analysis. They demonstrated that all lipids were possessed with an unsaturated fatty acid at sn-2 of the glycerol moiety, whereas the sn-1 position was mainly occupied by saturated fatty acids, as well as general feature of bacterial phospholipids.
Quinones
The quinones found in Oceanospirillaceae are mainly ubiquinone-8. Menaquinone-6 is found in some species (Zhang et al. 2002), but its quantities is low. Exceptionally Oleibacter marinus and Thalassolituus oleivorans have Q-9 as major isoprenoid quinone with minor amounts of Q-8 (Teramoto et al. 2011). Details of quinine profile in Oceanospirillum including formerly members of this genus were described by Sakane and Yokota (1994); all species, except T. pusilla, contained ubiquinone-8 as major respiratory quinone (more than 80 %). Like other spirilla from the alphaproteobacteria (see the genus Aquaspirillum in this book), T. pusilla contained over 90 % Q-10.
Lipids
The polar lipids of Oceanospirillaceae species consist almost entirely of phosphatidylethanolamine (PE), phosphatidylglycerol (PG), and diphosphatidylglycerol (DPG) with variable proportions of their lyso derivatives. The predominant phospholipids of Oceanospirillum linum are PE, PG, and trace amount of DPG (Wilkinson 1988). This pattern is typical of Gram-negative bacteria (Wilkinson 1988). As the other reports, Bermanella marisrubri have PG, PE, DPG, amino phospholipid, and glycolipid (Pinhassi et al. 2007). In the case of Marinomonas polaris, PE and PG are predominant (Gupta et al. 2006). Neptuniibacter caesariensis have PE and PG as major components and also have a moderate amount of unknown aminophospholipid and a minor amount of DPG (Arahal et al. 2007). Polar lipids of Oleibacter marinus are PG, a ninhydrin-positive phospholipid(s), and glycolipids (Teramoto et al. 2011). Phospholipids in Thalassolituus oleivorans are also represented by the PE and PG types (Yakimov et al. 2004).
The structure of the lipid A from the LPS of Marinomonas vaga (formerly Alteromonas vaga) ATCC 27119T has been described (Krasikova et al. 2004). Its lipid A shows stoichiometric lack of the phosphate ester group at C4’. Moreover, also in this case, the main form is represented by a penta-acyl species, with a (3 + 2) distribution of fatty acids, and acylation is principally performed by the short-chained 10:0 (3-OH). It has been reported for the first time in this occasion that the 3-hydroxy moiety is also present as the secondary substituent at the amide-linked fatty acid of GlcN II (Krasikova et al. 2004). However, there is less information on the details of structure of LPS in other species of this family. Thus, further studies are necessary to elucidate the structure of LPS in Oceanospirillaceae.
Polyamines
There is little information on the polyamine composition in Oceanospirillaceae species. However, Hamana et al. (1994) demonstrated analysis of polyamine components in Oceanospirilla (formerly members of this genus) and reported that all Oceanospirillum species including O. jannaschii and O. kriegii contain both putrescine and spermidine. The relative content of putrescine is very small when compared with the level found in members of the alphaproteobacteria, e.g., Terasakiella pusilla (formerly O. pusillum). The absence of 2-hydroxy putrescine and homospermidine is a unifying character for the gammaproteobacteria.
Isolation, Enrichment, and Maintenance Procedures
General Isolation Methods
Most Oceanospirillaceae species can be isolated by direct plating of seawater samples on a complex medium such as marine agar (Zobell 1941) without enrichment procedures, though incubation temperature should be carefully considered as many marine species fail to grow at mesophilic temperatures (e.g., the psychrophilic genera Oceaniserpentilla and Oleispira require cultivation temperatures of 2–10 °C). Nevertheless, Marinomonas species, as well as species belonging to neighboring genera, are routinely isolated from seawater by direct plating methods onto marine agar and form colonies at 20–30 °C within 1 week. Suzuki et al. (1997) demonstrated that organisms related to Oceanospirillum could be isolated without enrichment using a modified version of R2A∗∗ medium incubated at 15 °C in the dark (Reasoner and Geldreicht 1985).
Enrichment
Generally, most species of Oceanospirillaceae grow readily in artificial medium and can be isolated using simple procedures, such as direct plating method onto agar plates. However, the isolation of species of Oceanospirillum often requires enrichment techniques utilizing seawater and infusions of marine shellfish (Williams and Rittenberg 1957; Hylemon et al. 1973; Terasaki 1970, 1973, 1979) due to the low predominance of marine spirilla in environmental samples. Briefly, the following is the method used by Williams and Rittenberg (1957) to enrich and isolate O. linum and O. beijerinckii: Seawater was mixed with an equal volume of Giesberger’s medium (Williams and Rittenberg 1957) and incubated at 30–32 °C until the appearance of spirilla in microscopic observation. At this time, a portion of the culture was autoclaved and then mixed with an equal volume of Giesberger’s medium lacking NH4Cl. This medium was then inoculated with the unsterilized portion of the initial culture and again incubated at 30–32 °C. Spirilla were shown to predominate after one to three subcultures in this medium. For isolation, the enrichment was decimally diluted 1: 100 to 1: 100, 000 in sterile seawater. After mixing, the flasks were allowed to stand for 20 min to allow the spirilla to migrate to the surface. Plates with the appropriate medium were then inoculated with surface water. The method of Terasaki (1970) has yielded excellent results for the isolation of Oceanospirilla from putrid infusions. In a typical enrichment, the bodies of one to three pulverized shellfish were submerged in a Petri dish containing 2.5 % NaCl and the suspension incubated for up to 3 days at 20–30 °C. Microscopic examination revealed that spirilla were generally apparent early in the putrefaction. When spirilla became abundant, a loopful of the suspension was removed and touched successively to a sterile glass slide to produce small droplets. The smallest of these droplets was then streaked onto agar medium containing peptone and 2.75 % NaCl. This methodology was utilized to isolate O. multiglobuliferum, O. beijerinckii, and O. maris (Terasaki 1973, 1979). Another enrichment method using a horizontal glass tube for the isolation of spirilla has also been reported (Fujii et al. 1990). The principal of this method was based on high motility of spirilla, which allowed them to migrate rapidly through medium in a horizontal glass tube. Subsequent to repeated enrichments in this manner, pure cultures were isolated via streaking on Petri plates. This methodology was particularly effective in the isolation of Marinospirillum megaterium due to its requirement for microaerobic, reductive, and viscous environments, although the ability to form colonies on agar plates was lost upon subsequent transfers (Fujii et al. 1990). Lastly, isolation of the genus Balneatrix, the only species reported as a causative agent of human infection, can be achieved using TSA or other clinical media as, despite being a member of the Oceanospirillaceae, it is non-halophilic with a low tolerance to salt (lower than 1 % NaCl for growth).
Enrichment for Unique Characteristic Bacteria
For the isolation of species having specific characteristics other than the abovementioned bacteria, several enrichment methods have additionally been reported.
m-Hydroxybenzoate Degrading Bacteria
Some Marinomonas strains degrade m-hydroxybenzoate and can be enriched by amending 500 ml of seawater with 25 ml of a solution of 1 M Tris–HCl (pH 7.5), 0.5 g of NH4Cl, 38 mg of K2HPO4 · 7 H2O, 14 mg of FeSO4 · 7 H2O, and 0.5 g of m-hydroxybenzoate. Cultures are incubated at 20–25 °C for up to 10 days, and isolates are obtained on Basal Medium Agar (BMA) plates containing 0.1 % m-hydroxybenzoate (Baumann et al. 1984). Using a complex medium, Eilers et al. (2000) reported that 6 % of the isolates from a seawater sample taken directly from the North Sea were strains of Marinomonas. However, prefiltration through a 1.2 μm pore size filter favored the isolation of Marinomonas, and five out of nine isolates obtained under these conditions were close relatives of M. communis. Additional isolates of Marinomonas spp. were obtained by Ansede et al. (2001) using media containing 1 mM of the osmolyte dimethylsulfoniopropionate (DMSP). Either basal salts or f/2 media was used with the pH adjusted to 7.2 by adding potassium phosphate to a final concentration of 10 mM. Serial dilutions of seawater or sediment slurries were plated directly on this medium. In some cases, bacteria are first enriched in f/2 medium (Guillard 1975) with 1 mM DMSP prior to plating.
Petroleum-Degrading Bacteria
Some Oceanospirillaceae species have been reported to degrade petroleum compounds (Teramoto et al. 2009). Briefly, methods for enrichment of these bacteria are described here: One liter of non-sterilized seawater was supplemented with 1 g NH4NO3, 0.2 g K2HPO4, and 12 mg FeCl3 (SW medium) and incubated in a glass basin with gentle shaking at room temperature (around 25 °C). Three grams of chocolate-mousse crude oil (comprised of 0.5 g crude oil and 2.5 g seawater) was applied to one side of a sterile pumice stone and floated on the SW medium with the coated side down. SW medium was continuously supplied to the glass basin at a rate of 200 ml per day, while the same amount of the medium was pumped out from the glass basin to maintain the culture volume at 1 L. Continuous flow cultures were conducted in two different modes to isolate hydrocarbon-degrading bacteria of wider diversity. In the first type (culture 1), non-sterilized SW medium prepared with fresh seawater was supplied for the first 10 days, and sterilized SW medium, prepared by autoclaving, was supplied for the next 6 days. In the second type (culture 2), non-sterilized SW medium prepared with fresh seawater was supplied for the first 3 days, and sterilized SW medium was supplied for the next 13 days. On the 16th day after starting the cultivation, the surface of the chocolate-mousse oil and the aqueous phase of the culture were spread onto an SW medium plate (1.5 % (w/v) agar; 9 cm in diameter) covered with 30 ml crude oil. The plates were incubated at 18 °C for up to 6 weeks. Bacterial colonies that appeared on the crude oil-covered SW medium plates were purified at room temperature (around 25 °C) on dMB plates containing 0.5 % (w/v) pyruvate. The dMB plate medium comprised (per liter) 15 g agar, 0.9 L seawater, 0.1 L distilled water, and 3.74 g marine broth 2216 (Difco).
∗Ingredients of Giesberger’s medium (per liter): 1 g of NH4Cl, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7 H2O, with 1 % calcium lactate (Williams and Rittenberg 1957).
∗∗Ingredients of R2A medium in g/l of 75 % seawater: yeast extract, 0.5; proteose peptone, 0.5; casamino acids, 0.5; dextrose, 0.5; soluble starch, 0.5; sodium pyruvate, 0.3; and agar, 15.
∗∗∗Preparation of crude oil and of its chocolate mousse. Arabian light crude oil was treated at 214 °C for 10 h to remove the volatile fraction (30 % in volume) and used in this study. Chocolate-mousse crude oil was prepared by mixing the crude oil and fresh seawater collected at Pari Island in a ratio of 1 : 5 (w/w), followed by vigorous and continuous shaking for 1 day. The resultant chocolate mousse was stable for several weeks.
Phenotypic Test
In general, common and conventional methods can be used to characterize for most Oceanospirillaceae strains, although media containing natural or artificial seawater must be used for all characterization tests. For some of the species, the genus Oceaniserpentilla and Oleispira, low cultural temperature should be employed, e.g., 2-10 °C, due to their psychrophilic property. The capability to ferment carbohydrates is tested most effectively in using oxidation–fermentation medium of Leifson (1963) to which carbohydrates have been added at a concentration of 0.5 % (w/v). This medium is more sensitive than the usual Hugh and Leifson medium because it contains phenol red instead of bromothymol blue. Other biochemical and nutritional traits can be tested using standard procedures carried out under suitable growth conditions, e.g., appropriate incubation temperature and sodium and iron concentration. Commercial identification kits (API, Biolog, and others) are also available for routine biochemical testing. Baumann’s minimal medium (Baumann et al. 1972) is a common basal medium for carbohydrate or amino acid utilization tests. For Oceanospirilla characterization methods have been described in detail by Terasaki (1972, 1979) and Hylemon et al. (1973). The comments given in the Prokaryotes for the genus Aquaspirillum also apply to the genus Oceanospirillum, except that media containing natural or artificial seawater must be used for all characterization tests. For alkaliphilic species, such as Marinospirillum alkaliphilum and M. celere, growth medium should be adjusted to high pH unit (around 9.0 pH unit) with appropriate buffer, for instance, NaHCO3 and Na2CO3 buffer (Namsaraev et al. 2009).
Maintenance Procedures
In general, common and conventional methods can be used to preserve and maintain most of Oceanospirillaceae strains in a carbohydrate-free medium, such as nutrient or marine agar in which they grow well, because these species are relatively robust. Long-term preservation of Marinomonas species and most of Oceanospirillaceae strains can be achieved by lyophilization using 20 % skim milk as a cryoprotectant. A suitable protocol for the preparation of the cells has been described by Gauthier and Breittmayer (1992). The lyophilized cultures are reconstituted by adding 0.5 ml of marine broth. A few drops are streaked onto marine agar, and the remaining is transferred to a tube containing 4 mol of marine broth. It is advisable to avoid high aeration of the culture during the first hours of incubation. Growth is observed after 2–3 days. An alternative method of preservation is freezing. Strains can sustain viability for over 6 months when frozen in liquid nitrogen or cryopreserved at −80 °C in broth containing 20–30 % glycerol or dimethyl sulfoxide. Using this protocol, frozen Marinomonas communis and M. vaga cells have remained viable for more than 5 years. Strains can be maintained by serial transfer on marine agar for routine work in the lab. After 2–3 days of growth at 25 °C, the plates can be preserved for 3–4 weeks at 15 °C. It is not recommended to keep the cultures at 4 °C, because viability is lost much faster than when the cultures are stored at 15 °C.
Also in the case of Oceanospirilla, preservation can be accomplished by suspending a dense suspension of cells in seawater nutrient broth or marine broth containing 10 % (v/v) dimethyl sulfoxide or 20 % (v/v) glycerol, with subsequent freezing in liquid nitrogen (Krieg 1984) or cryopreserved at −80 °C. Freeze-drying can be performed with cells grown on the appropriate medium (Terasaki 1975). Cells may be maintained in semisolid PSS seawater medium (containing 0.15 % agar to give a jelly-like consistency) at 30 °C with weekly transfer (Hylemon et al. 1973). Cultures may also maintain as stabs in seawater nutrient agar at room temperature with monthly transfer (Terasaki 1972).
Peptone–Succinate–Salt (PSS) Medium
Peptone 10 g, succinic acid 1 g, (NH4)2SO4 1 g, MgSO4 · 7 H2O 1 g, FeCl3 · 6 H2O 0.002 g, MnSO4 · H2O 0.002 g, agar 1.5 g, and synthetic seawater 1 l; the pH is adjusted to 7.8.
Synthetic Seawater
NaCl 27.5 g, MgCl2 5 g, MgSO4 · 7 H2O 2 g, CaCl2 0.5 g, KCl 1 g, FeSO4 0.01 g, and distilled water 1,000 ml.
Energy Metabolism
Oceanospirillaceae is basically aerobic having a strictly respiratory type of metabolism with oxygen as the terminal electron accepter. Neptunomonas is exceptionally facultative aerobic. Oceanospirillaceae strains are able to utilize various carbohydrate and amino acid compounds, as a sole carbon and energy source (Tables 24.1 and 24.4 – 24.9 ). In the case of Marinomonas communis and M. vaga, it was proved that they grow on d-fructose and d-glucose using the Entner–Doudoroff pathway. This pathway may be widespread among members of the large group of marine γ-proteobacteria (Baumann and Baumann 1973; Sawyer et al. 1977). Some petroleum-degrading strains can utilize unusual carbohydrates, e.g., aliphatic, aromatic, and branched-alkane compounds, as a sole carbon and energy source.
Ecology (Habitation)
Oceanospirillaceae species distribute basically marine environment and require sodium ion for growth. Almost all species have been isolated from marine environments, seawater, seawater sediments, putrid shell fish, seaglass surface, and others, strongly indicating that their main habitats are marine or similar environment to marine. The only exceptional case is Balneatrix isolated from freshwater spa, and moreover, the bacterium caused pneumonia and meningitis in human. Here we discuss habitation of each genus separately.
Oceanospirillum
Oceanospirilla have been isolated from coastal seawater (Williams and Rittenberg 1957), decaying seaweed (Jannasch 1963), and putrid infusions of marine mussels (Terasaki 1963, 1970, 1979). Isolation sources of each Oceanospirillum species are shown in Table 24.2 . Because the main isolation source of Oceanospirillum spp. is coastal marine environments, O. linum, O. maris, and O. beijerinckii, or putrid shellfish infusions, O. maris (formerly O. hiroshimense), O. beijerinckii (formerly O. pelagicum), and O. multigloburiferum, interesting question arose as to whether it lives as epibiotic strains in the tissues of various marine animals or as a free-living organism in seawater. By direct microscopic counts of the bacteria present in clear and turbid seawaters near Port Aransas, Texas, Oppenheimer and Jannasch (1962) found that spirilla comprised only 0.1–2.5 % of the total bacteria present. Also, rRNA genes closely related to Oceanospirillum species have not been encountered in libraries prepared from marine environmental rDNA (Giovannoni and Rappé 2000), which suggests that the abundance of Oceanospirillum in the marine environment is low. In chemostat experiments, Jannasch (1963) suggested that the growth of Oceanospirilla might be restricted to environments of higher nutrient concentration than is found in ordinary seawater, such as in zones surrounding decaying particulate matter. Hence, multiple strains have been isolated from putrid infusions of marine mussels, suggesting that this trait appears to be associated with the capability of these species to survive as epibiotic strains in the tissues of various marine animals. However, it can be simply indicated that the source is most likely marine mud adherent to the mussels (Terasaki 1970). The true habitat is still unclear.
Marinomonas
All Marinomonas species appear to be strictly marine in origin, although M. polaris has no requirement of sodium ion for growth (Gupta et al. 2006). At the time of writing, the genus Marinomonas comprise 20 species, mainly originating from seawater from different geographical locations, e.g., Marinomonas communis and M. vaga (Baumann et al. 1972; van Landschoot and De Ley 1983) were isolated from the Pacific Ocean, M. polaris (Gupta et al. 2006) and M. ushuaiensis (Prabagaran et al. 2005) were isolated from subantarctic regions, while M. primoryensis (Romanenko et al. 2003) and M. arctica (Zhang et al. 2008) were isolated from sea ice. The next major isolation source is the seaglass, P. oceanica, which plays an important role in Mediterranean Sea ecosystems. Totally seven Marinomonas species have been isolated from this sea plant, as part of a study to characterize the microbiota associated with P. oceanica (Espinosa et al. 2010; Lucas-Elío et al. 2011). The coral Mussismilia hispida and oyster are also reported as isolation source for Marinomonas species, M. brasiliensis (Chimetto et al. 2011), and M. aquimarina (Macian et al. 2005), respectively. Also in this genus, interesting question arose as to whether it lives as epibiotic strains on the surface of various sea plants and organisms or as a free-living organism in seawater. A high number of M. communis and M. vaga strains have been isolated using enrichment methods with different compounds as carbon sources (Baumann et al. 1972). These isolations led to the view that Marinomonas is a usual component of the bacterial flora in marine waters; however, there are few data about the ecological distribution of each Marinomonas species owing to the scarcity of strains in each species. The true habitat is also unclear, however.
Marinospirillum and Nitrincola
As eluted above, the phylogenetic position of the genus Marinospirillum is clearly far from Oceanospirillaceae, indicating it belongs to other bacterial family. Since Marinospirillum is included in Oceanospirillaceae at the time of writing, its habitation is also shown here. The genus Marinospirillum is encompassing five species and isolated from putrid shellfish infusions, fermented sea food, and in an alkali lakes called a sada Lake. Three species of Marinospirillum, M. minutulum, M. megaterium, and M. insulare, were isolated from environments rich in organic matter. Marinospirillum minutulum was isolated from a putrid infusion of a marine shellfish (Watanabe 1959). Enrichment was carried out in synthetic medium with peptone, and the bacterium was finally isolated in medium with calcium lactate and peptone as carbon sources. M. megaterium and M. insulare were isolated from kusaya gravy, which is rich in nutrients; contains 3 % NaCl, volatile nitrogen compounds, and low oxygen concentrations; and is used to make Japanese dried fish (Fujii 1977, 1978; Fujii et al. 1985, 1990, 1993). Satomi et al. (1997) investigated predominant microbial species using PCR-clone library sequence in kusaya gravy. Although helical or S-shaped cells were obviously recognized on microscopic observation in this gravy sauce, Marinospirillum spp. or putative helical bacteria were not determined, indicating that the abundance of Marinospirillum and its relatives in the gravy sauce can be low. As was mentioned in the section enrichment, the strains should be enriched to isolate on the basis of their high motility, which allowed them to migrate rapidly through medium in a horizontal glass tube. M. alkaliphilum was isolated from Haoji Soda Lake (pH 9.5) in Inner Mongolia Autonomous Region of China as an alkaliphilic helical bacterium (Zhang et al. 2002). Subsequently, M. celere was isolated from combined water – a novel sediment slurry sample taken from a hot spring (40 °C, pH 9.3, salinity 25 g l−1) on Paoha Island on Mono Lake (CA, USA) as a haloalkaliphilic, helical bacterium in Marinospirillum. Both species prefer alkaline environment and require sodium ion for growth, suggesting that their habitats can be ocean or ocean-like environments other than alkali Soda Lakes. As well as two Marinospirillum, M. alkaliphilum and M. celere, a novel alkaliphilic bacterium, Nitrincola lacisaponensis, strain was also isolated from decomposing wood taken from the shore of Soap Lake, a saline, alkaline lake in Grant County, WA, USA (Dimitriu et al. 2005). Although all Marinospirillum species are halophilic alkaliphilic bacteria as a common characteristic, there are few data about the ecological distribution of Marinospirillum. The true habitat in nature is also unclear.
Other Oceanospirillaceae Species
Other Oceanospirillaceae species also appear to be strictly marine in origin (see Table 24.3 ). As describe later, petroleum-degrading bacteria are widely distributed in ocean with no limit to seawater temperature from tropical to polar region. Population of these bacteria in environment are likely to vary always depending on nutritional condition or competition against other microorganisms. These bacteria could dominate in the natural marine environment after an accidental oil spill and would continue to dominate in the environment after biostimulation (Teramoto et al. 2009), despite low occupation under usual environmental condition. Amphritea balenae, A. japonica, and Neptunomonas japonica were isolated from sediment adjacent to sperm whale carcasses. These species are regarded as the most closely related to a symbiotic bacterial clone of the genus Osedax (Hedlund et al. 1999; Arahal et al. 2007; Goffredi et al. 2007). The genus Osedax, composed of siboglinid polychaete, has recently been discovered in whale carcasses on the deep-sea floor (Rouse et al. 2004; Glover et al. 2005; Fujikura et al. 2006; Braby et al. 2007). Members of the genus Osedax host symbiotic bacteria in the ovisac and root systems. These bacterial species may have related to the symbiont-like bacteria clone of Osedax japonicus. As well as two Marinospirillum, M. alkaliphilum and M. celere, a novel alkaliphilic bacterium, Nitrincola lacisaponensis, was also isolated from decomposing wood taken from the shore of Soap Lake, a saline, alkaline lake in Grant County, WA, USA (Dimitriu et al. 2005).
Pathogenicity and Clinical Relevance
Balneatrix alpaca is the only species that is reported as clinical related bacteria in Oceanospirillaceae. The genus Balneatrix is consist of single species and established in 1993 by Dauga et al. for an unknown bacterium occurred during the outbreak of pneumonia and meningitis in a spa therapy center, southern France, in 1987 (Hubert et al. 1991). Thirty-five cases of pneumonia and two cases of meningitis occurred. Isolates from eight patients were recovered from blood, cerebrospinal fluid, and sputum and one from water. Morphology and conventional biochemical characteristics have been described (Casalta et al. 1989). This organism, previously referred to as a “new non-fermentative unknown Gram-negative bacterium,” shared some phenotypic properties with the genus Flavobacterium, although the G+C content of the DNA was 54 mol%. Further studies indicated that nine isolates of this pathogen constituted a tight DNA hybridization group and belonged to the gamma subclass of the proteobacteria with close relationships to Oceanospirillum based on the rRNA–DNA hybridization and 16S rRNA sequencing. The new bacterium differed from the genus Oceanospirillum by lacking the NaCI requirement and by reducing nitrate into nitrite, producing indole from tryptophan and producing acid from carbohydrates. It is obvious that Balneatrix alpaca is a remarkable causative agent of human infection; however, the fact is that also it is a rare case that Oceanospirillaceae strains become an infectious agent. Balneatrix strains are susceptible in vitro to a variety of antimicrobial agents, including ß-lactam, macrolides, and aminoglycoside antibiotics; sulfamethoxazole–trimethoprim; chloramphenicol; doxycycline; minocycline; ofloxacin; and nalidixic acid. They are resistant to clindamycin and vancomycin. To data, Balneatrix strains have been isolated only from thermal water and clinical specimens at a spa therapy center in southern France. According to epidemiological data, the bacteria were present in the hot water spring spa, and favorable growing conditions were found only in vapor baths. After disinfection of water pipes by chlorination, no further cases of infection were observed.
Applications
Applications for Oceanospirillaceae strains as current-generating devices include degradation of chemical pollutants including petroleum-related compounds, production of bactericidal elements, and extraction of useful enzymes, such as unique oxidases related to melanin pigment production.
Degradation of Petroleum-Related Compounds
Unique features of some Oceanospirillaceae strains are known. Some species have been isolated from hydrocarbon-rich environments, crude oil-contaminating temperate seawater, seawater and sediment after oil spill, adjacent area of sperm whale carcasses, and others. They are able to degrade petroleum hydrocarbons, such as aliphatic, aromatic, and branched-alkane compounds (Hedlund et al. 1999; Yakimov et al. 2003, 2004; Teramoto et al. 2009). Yakimov et al. (2003) isolated hydrocarbon-degrading strains from Antarctic coastal marine environments (Rod Bay, Ross Sea) using enrichment method and established new genus Oleispira, which is psychrophilic, halophilic, aerobic, and Gram negative with polar flagella. As unique characteristics, the strains were able to synthesize the polyunsaturated fatty acid eicosapentaenoic acid (20: 5w3) at low temperatures, as well as many psychrophilic marine bacteria, and exhibited a restricted substrate profile, with a preference for aliphatic hydrocarbons, that is typical of marine hydrocarbonoclastic microorganisms such as Alcanivorax, Marinobacter, and Oleiphilus. Also Teramoto et al. (2009) reported that Oleibacter isolated from Indonesian seawater accidentally contaminated by crude oil can degrade n-alkane-hydrocarbons. Although the bacteria did not show degrading activity for branched-alkane degradation, they have high degrading activity for n-alkane-hydrocarbons and become the most dominant in microcosms that simulated a crude oil spill event with Indonesian seawater, thus could be key bacteria for biodegradation in tropical seas. As well as Oleibacter, Thalassolituus oleivorans also has been reported to degrade aliphatic hydrocarbons (Yakimov et al. 2004), and Thalassolituus strains have recently been shown to dominate in n-alkane-containing temperate seawater microcosms (Yakimov et al. 2005; McKew et al. 2007) and in crude oil-containing temperate estuarine seawater microcosms (Harayama et al. 1999; Kasai et al. 2001; Coulon et al. 2007; McKew et al. 2007). On the other hand, Neptunomonas naphthovorans is isolated from creosote-contaminated Puget Sound sediment based on their ability to utilize naphthalene as a sole carbon and energy source and able to degrade polycyclic aromatic hydrocarbon (PAH) compounds, including 2-methylnaphthalene, 1-methylnaphthalene, 2, 6-dimethylnaphthalene, and phenanthrene. Details for mechanisms for degradation of PAHs including related genes were investigated (Hedlund et al. 1999) in N. naphthovorans. The bacteria were not able to degrade acenaphthene as a sole carbon source but degrade with a mixture of seven other PAHs. A naphthalene dioxygenase iron–sulfur protein (ISP) gene was determined in the bacteria, and PAH dioxygenase ISP-deduced amino acid sequences showed close relationships between the genes encoding naphthalene dioxygenases of Pseudomonas and Burkholderia strains. N. japonica was isolated from sediments adjacent to sperm whale carcasses, which may contain rich and unique lipids, suggesting that the bacteria can be related to lipid derogation. However, there is less information that all of Neptunomonas can degrade PAH compounds. Also Neptuniibacter sp. strain CAR-SF can utilize carbazole as its sole carbon and nitrogen sources. The genes related to carbazole degradation pathway, consisting with carAa, carBa, carBb, and carC, were investigated (Nagashima et al. 2010). As mentioned above, some Oceanospirillaceae strains can degrade petroleum and related compounds and are expected to apply their ability to improve environment, such as oil spill and artificial pollution.
Marinomonas mediterranea is melanogenic (Solano et al. 1997; Solano and Sanchez-Amat 1999; Solano et al. 2000; Sanchez-Amat et al. 2001, 2010) and produces polyphenol oxidase, an enzyme involved in melanin synthesis. Melanin pigments are made from l-tyrosine as precursor and by the involvement of the enzyme tyrosinase (EC 1.14.18.1) (Solano et al. 1997), which is a copper protein belonging to the group of polyphenol oxidases (PPOs). The other important copper enzyme in this group is laccase (EC 1.10.3.2). M. mediterranea also shows this activity, due to a multipotent enzyme showing both tyrosinase and laccase activities (Solano et al. 1997). These enzymes are commonly isolated from fungi and are of interest because of their potential biotechnological applications in polymerization of phenols, oxidation of xenobiotics, pulp bleaching, and oxidation of lignin substrates. Marinomonas mediterranea strain MMB-1 is one of the few bacterial isolates where laccase activity (one of the enzymes of the family of polyphenol oxidases) has been detected. Marinomonas mediterranea synthesizes a novel antimicrobial protein (LodA) with lysine-epsilon-oxidase activity (EC 1.4.3.20). As mentioned above, production of useful enzymes have been investigated in M. mediterranea deeply. Since main habitation of most Marinomonas species is similar to that of M. mediterranea, they also may have unique and useful properties as well as M. mediterranea. Further investigation including whole-genome analysis in these bacteria may lead to developing new technology.
References
Akagawa-Matsushita M, Itoh T, Katayama Y, Kuraishi H, Yamasato K (1992) Isoprenoid quinone composition of some marine Alteromonas, Marinomonas, Deleya, Pseudomonas and Shewanella species. J Gen Microbiol 138:2275–2281
Anderson GC (1958) Seasonal characteristics of two saline lakes in Washington. Limnol Oceanogr 3:51–68
Ansede JH, Friedman R, Yoch DC (2001) Phylogenetic analysis of culturable dimethyl sulfide-producing bacteria from a Spartina-dominated salt marsh and estuarine water. Appl Environ Microbiol 67:1210–1217
Arahal DR, Lekunberri I, González JM, Pascual J, Pujalte MJ, Pedrós-Alió C, Pinhassi J (2007) Neptuniibacter caesariensis gen. nov., sp. nov., a novel marine genome-sequenced gammaproteobacterium. Int J Syst Evol Microbiol 57:1000–1006
Baumann L, Baumann P (1973) Enzymes of glucose catabolism in cell-free extracts of non-fermentative marine eubacteria. Can J Microbiol 19:302–304
Baumann L, Baumann P, Mandel M, Allen RD (1972) Taxonomy of aerobic marine eubacteria. J Bacteriol 110:402–429
Baumann P, Bowditch RD, Baumann L, Beaman B (1983) Taxonomy of marine Pseudomonas species: P. stanieri sp. nov.; P. perfectomarina sp. nov., nom. rev.; P. nautica; and P. doudorofii. Int J Syst Bacteriol 33:857–865
Baumann P, Gauthier MJ, Baumann L (1984) Genus Alteromonas Baumann, Baumann, Mandel and Allen 1972. In: Krieg NR, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore, pp 343–352
Bertone S, Giacomini M, Ruggiero C, Piccarolo C, Calegari L (1996) Automated systems for identification of heterotrophic marine bacteria on the basis of their Fatty Acid composition. Appl Environ Microbiol 62:2122–2132
Bohme K, Fernandez-No IC, Barros-Velazquez J, Gallardo JM, Calo-Mata P, Canas B (2010) Species differentiation of seafood spoilage and pathogenic Gram-negative bacteria by MALDI-TOF mass fingerprinting. J Proteome Res 9:3169–3183
Bowditch RD, Baumann L, Baumann P (1984) Description of Oceanospirillum kriegii sp. nov. and O. jannaschii sp. nov. and assignment of two species of Alteromonas to this genus as O. commune comb. nov. and O. vagum comb. nov. Curr Microbiol 10:221–230
Braby CE, Rouse GW, Johnson SB, Jones WJ, Vrijenhoek RC (2007) Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey Bay, California. Deep Sea Res Part I Oceanogr Res Pap 54:1773–1791
Carney JF, Wan L, Lovelace TE, Colwell RR (1975) Numerical taxonomy study of Vibrio and Spirillum spp. Int J Syst Bacteriol 25:38–46
Casalta JP, Peloux Y, Raoult D, Brunet P, Gallais H (1989) Pneumonia and meningitis caused by a new nonfermentative unknown gram-negative bacterium. J Clin Microbiol 1989(27):1446–1448
Chimetto LA, Cleenwerck I, Brocchi M, Willems A, Vos PD, Thompson FL (2011) Marinomonas brasiliensis sp. nov., isolated from the coral Mussismilia hispida, and reclassification of Marinomonas basaltis as a later heterotypic synonym of Marinomonas communis. Int J Syst Evol Microbiol 61:1170–1175
Choi A, Cho J-C (2010) Reinekea aestuarii sp. nov., isolated from tidal flat sediment. Int J Syst Evol Microbiol 60:2813–2817
Coulon F, McKew BA, Osborn AM, McGenity TJ, Timmis KN (2007) Effects of temperature and biostimulation on oil degrading microbial communities in temperate estuarine waters. Environ Microbiol 9:177–186
Dauga C, Gillis M, Vandamme P, Ageron E, Grimont F, Kersters K, de Mahenge C, Peloux Y, Grimont PA (1993) Balneatrix alpica gen. nov., sp. nov., a bacterium associated with pneumonia and meningitis in a spa therapy center. Res Microbiol 144:35–46
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridisation from renaturation rates. Eur J Biochem 12:133–142
De Vos P, van Landschoot A, Segers P, Tytgat R, Gillis M, Bauwens M, Rossau R, Goor M, Pot B, Kersters K, Lizzaraga P, De Ley J (1989) Genotypic relationships and taxonomic localization of unclassified Pseudomonas and Pseudomonas-like strains by deoxyribonucleic acid: ribosomal ribonucleic acid hybridizations. Int J Syst Bacteriol 39:35–49
DeLong EF, Baumann L, Bowditch RD, Baumann P (1984) Evolutionary relationships of superoxide dismutases and glutamine synthetases from marine species of Alteromonas, Oceanospirillum, Pseudomonas and Deleya. Arch Microbiol 138:170–178
Dimitriu PA, Shukla SK, Conradt J, Márquez MC, Ventosa A, Maglia A, Peyton BM, Pinkart HC, Mormile MR (2005) Nitrincola lacisaponensis gen. nov., sp. nov., a novel alkaliphilic bacterium isolated from an alkaline, saline lake. Int J Syst Evol Microbiol 55:2273–2278
Ehrenberg CG (1832) Beiträge zur Kenntnis der Organization der Infusorien und ihrer geographischen Verbreitung; besonders in Sibirien. Abhandlung der König, Akademie der Wissenschafft zu Berlin, Berlin, pp 1–88
Eilers H, Pernthaler J, Glöckner FO, Amann R (2000) Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl Environ Microbiol 66:3044–3051
Espinosa E, Marco-Noales E, Gómez D, Lucas-Elío P, Ordax M, Garcías-Bonet N, Duarte CM, Sanchez-Amat A (2010) Taxonomic study of Marinomonas strains isolated from the seagrass Posidonia oceanica, with descriptions of Marinomonas balearica sp. nov. and Marinomonas pollencensis sp. nov. Int J Syst Evol Microbiol 60:93–98
Ezaki T, Hashimoto Y, Yabuuchi E (1989) Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol 39:224–229
Fox GE, Wisotzkey JD, Jurtshuk P Jr (1992) How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol 42:166–170
Fujii T (1977) Studies on ‘Kusaya’-I. Comparison of composition of ‘Kusaya’ brine at Niijima and Oshima islands. Nippon Suisan Gakkaishi 43:517–521
Fujii T (1978) Microbial flora of ‘Kusaya’ brine of Niijima and Oshima islands. Nippon Suisan Gakkaishi 44:45–48
Fujii T, Sugita H, Deguchi Y (1985) Obligately anaerobic bacteria isolated from ‘Kusaya’ gravy. Nippon Suisan Gakkaishi 51:473–478
Fujii T, Hayashi M, Okuzumi M (1990) New device for isolation of spiral bacteria from ‘Kusaya’ gravy. Nippon Suisan Gakkaishi 56:1661
Fujii T, Kuda T, Okuzumi M (1993) Anaerobic microflora of ‘Kusaya’ gravy. Nippon Suisan Gakkaishi 59:185
Fujikura K, Fujiwara Y, Kawato M (2006) A new species of Osedax (Annelida: Siboglinidae) associated with whale carcasses off Kyushu, Japan. Zoolog Sci 23:733–740
Garrity GM, Bell JA, Lilburn T (2005) Order VIII. Oceanospirillales ord. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey’s manual of systematic bacteriology, vol 2B, 2nd edn. Springer, New York, p 270
Gärtner A, Wiese J, Imhoff JF (2008) Amphritea atlantica gen. nov., sp. nov., a gammaproteobacterium from the Logatchev hydrothermal vent field. Int J Syst Evol Microbiol 58:34–39
Gauthier MJ, Breittmayer VA (1992) The genera Alteromonas and Marinomonas. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, New York, pp 3046–3070
Giovannoni SJ, Rappé M (2000) Evolution, diversity, and molecular ecology of marine prokaryotes. In: Kirchman D (ed) Microbial ecology of the oceans. Wiley, New York, pp 47–84
Glover AG, Källström B, Smith CR, Dahlgren TG (2005) World-wide whale worms? A new species of Osedax from the shallow north Atlantic. Proc Biol Sci 272:2587–2592
Goffredi SK, Johnson SB, Vrijenhoek RC (2007) Genetic diversity and potential function of microbial symbionts associated with newly discovered species of Osedax polychaete worms. Appl Environ Microbiol 73:2314–2323
González JM, Whitman WB (2006) Oceanospirillum and related genera. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, vol 6, 3rd edn. Springer, New York, pp 887–915
González JM, Mayer F, Moran MA, Hodson RE, Whitman WB (1997) Microbulbifer hydrolyticus gen. nov., sp. nov., and Marinobacterium georgiense gen. nov., sp. nov., two marine bacteria from a lignin-rich pulp mill waste enrichment community. Int J Syst Bacteriol 47:369–376
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 29–60
Gupta P, Chaturvedi P, Pradhan S, Delille D, Shivaji S (2006) Marinomonas polaris sp. nov., a psychrohalotolerant strain isolated from coastal sea water off the subantarctic Kerguelen islands. Int J Syst Evol Microbiol 56:361–364
Hamana K, Sakane T, Yokota A (1994) Polyamine analysis of the genera Aquaspirillum, Magnetospirillum, Oceanospirillum and Spirillum. J Gen Appl Microbiol 40:75–82
Harayama S, Kishira H, Kasai Y, Shutsubo K (1999) Petroleum biodegradation in marine environments. J Mol Microbiol Biotechnol 1:63–70
Hedlund BP, Geiselbrecht AD, Bair TJ, Staley JT (1999) Polycyclic aromatic hydrocarbon degradation by a new marine bacterium, Neptunomonas naphthovorans gen. nov., sp. nov. Appl Environ Microbiol 65:251–259
Howey RT, Lock CM, Moore LVH (1990) Subspecies names automatically created by Rule 46. Int J Syst Bacteriol 40:317–319
Hubert B, de Mahenge A, Grimont F, Richard C, Peloux Y, de Mahenge C, Fleurette J, Grimont PA (1991) An outbreak of pneumonia and meningitis caused by a previously undescribed gram-negative bacterium in a hot spring spa. Epidemiol Infect 107:373–381
Hylemon PB, Wells JS Jr, Krieg NR, Jannasch HW (1973) The genus Spirillum: a taxonomic study. Int J Syst Bacteriol 23:340–380
Iizuka H, Komagata K (1964) Microbiological studies on petroleum and natural gas. II: determination of pseudomonads isolated from oil-brines and related materials. J Gen Appl Microbiol 10:223–231
Ivanova EP, Onyshchenko OM, Christen R, Lysenko AM, Zhukova NV, Shevchenko LS, Kiprianova EA (2005) Marinomonas pontica sp. nov., isolated from the Black Sea. Int J Syst Evol Microbiol 55:275–279
Jannasch HW (1963) Studies on the ecology of a marine spirillum in the chemostat. In: 1st international symposium on marine microbiology. C. C. Thomas, Springfield, pp 558–566
Kaesler I, Graeber I, Borchert MS, Pape T, Dieckmann R, von Döhren H, Nielsen P, Lurz R, Michaelis W, Szewzyk U (2008) Spongiispira norvegica gen. nov., sp. nov., a marine bacterium isolated from the boreal sponge Isops phlegraei. Int J Syst Evol Microbiol 58:1815–1820
Kang I, Jang H, Oh HM, Cho JC (2012) Complete genome sequence of Marinomonas bacteriophage P12026. J Virol 86:8909–8910
Kasai Y, Kishira H, Syutsubo K, Harayama S (2001) Molecular detection of marine bacterial populations on beaches contaminated by the Nakhodka tanker oil-spill accident. Environ Microbiol 3:246–255
Kawasaki H, Yamasato K, Sugiyama J (1997) Phylogenetic relationships of the helical-shaped bacteria in the alpha Proteobacteria inferred from 16S rDNA sequences. J Gen Appl Microbiol 43:89–95
Krasikova IN, Kapustina NV, Isakov VV, Dmitrenok AS, Dmitrenok PS, Gorshkova MM, Solov'eva TF (2004) Detailed structure of lipid A isolated from lipopolysaccharide from the marine proteobacterium Marinomonas vaga ATCC 27119 T. Eur J Biochem 271:2895–2904
Krieg NR (1974) The genus Spirillum. In: Buchanan RE, Gibbons NE (eds) Bergey’s manual of determinative bacteriology, 8th edn. Williams and Wilkins, Baltimore, pp 196–207
Krieg NR (1984) Aerobic/microaerophillic, motile, helical/ vibroid Gram-negative bacteria. In: Krieg NR, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore, pp 71–93
Krieg NR, Hylemon PB (1976) The taxonomy of the chemoheterotrophic spirilla. Annu Rev Microbiol 30:303–325
Krupovick M, Prangishvili D, Hendrix RW, Bamford DH (2011) Genomics of bacterial and archaeal viruses: dynamics within the prokaryotic virosphere. Microbiol Mol Biol Rev 75:610–635
Lau KWK, Ren J, Wai NLM, Lau SCL, Qian P-Y, Wong P-K, Wu M (2006) Marinomonas ostreistagni sp. nov., isolated from a pearl-oyster culture pond in Sanya, Hainan Province, China. Int J Syst Evol Microbiol 56:2271–2275
Leifson E (1963) Determination of carbohydrate metabolism of marine bacteria. J Bacteriol 85:1183–1184
Linn DM, Krieg NR (1978) Occurrence of two organisms in cultures of the type strain of Spirillum lunatum: Proposal for rejection of the name Spirillum lunatum and characterization of Oceanospirillum maris subsp. williamsae and an unclassified vibroid bacterium. Int J Syst Bacteriol 28:132–138
Lucas-Elío P, Marco-Noales E, Espinosa E, Ordax M, López MM, Garcías-Bonet N, Marbà N, Duarte CM, Sanchez-Amat A (2011) Marinomonas alcarazii sp. nov., M. rhizomae sp. nov., M. foliarum sp. nov., M. posidonica sp. nov. and M. aquiplantarum sp. nov., isolated from the microbiota of the seagrass Posidonia oceanic. Int J Syst Evol Microbiol 61:2191–2196
Lucas-Elío P, Goodwin L, Woyke T, Pitluck S, Nolan M, Kyrpides NC, Detter JC, Copeland A, Teshima H, Bruce D, Detter C, Tapia R, Han S, Land ML, Ivanova N, Mikhailova N, Johnston AW, Sanchez-Amat A (2012) Complete genome sequence of the melanogenic marine bacterium Marinomonas mediterranea MMB-1T. Stand Genomic Sci 6:63–73
Macian MC, Arahal DR, Garay E, Pujalte MJ (2005) Marinomonas aquamarina sp. nov., isolated from oysters and seawater. Syst Appl Microbiol 28:145–150
Marmur J, Doty P (1962) Determination of deoxyribonucleic acid from thermal denaturation temperature. J Mol Biol 5:109–118
McElroy LJ, Krieg NR (1972) A serological method for the identification of Spirilla. Can J Microbiol 18:57–64
McKew BA, Coulon F, Yakimov MM, Denaro R, Genovese M, Smith CJ, Osborn AM, Timmis KN, McGenity TJ (2007) Efficacy of intervention strategies for bioremediation of crude oil in marine systems and effects on indigenous hydrocarbonoclastic bacteria. Environ Microbiol 9:1562–1571
Miyazaki M, Nogi Y, Fujiwara Y, Kawato M, Kubokawa K, Horikoshi K (2008a) Neptunomonas japonica sp. nov., an Osedax japonicus symbiont-like bacterium isolated from sediment adjacent to sperm whale carcasses off Kagoshima, Japan. Int J Syst Evol Microbiol 58:866–871
Miyazaki M, Nogi Y, Fujiwara Y, Kawato M, Nagahama T, Kubokawa K, Horikoshi K (2008b) Amphritea japonica sp. nov. and Amphritea balenae sp. nov., isolated from the sediment adjacent to sperm whale carcasses off Kagoshima, Japan. Int J Syst Evol Microbiol 58:2815–2820
Nagashima H, Zulkharnain AB, Maeda R, Fuse H, Iwata K, Omori T (2010) Cloning and nucleotide sequences of carbazole degradation genes from marine bacterium Neptuniibacter sp. strain CAR-SF. Curr Microbiol 61:50–66
Namsaraev Z, Akimov V, Tsapin A, Barinova E, Nealson K, Gorlenko V (2009) Marinospirillum celere sp. nov., a novel haloalkaliphilic, helical bacterium isolated from Mono Lake. Int J Syst Evol Microbiol 59:2329–2332
Oppenheimer CH, Jannasch HW (1962) Some bacterial populations in turbid and clear sea water near Port Aransas, Texas, vol 8. Institute of Marine Science, Port Aransas, pp 56–60
Oyaizu H, Komagata K (1983) Grouping of Pseudomonas species on the basis of cellular fatty acid composition and quinone system with special reference to the existence of 3-hydroxy fatty acids. J Gen Appl Microbiol 29:17–40
Pinhassi J, Pujalte MJ, Macián MC, Lekunberri I, González JM, Pedrós-Alió C, Arahal DR (2007) Reinekea blandensis sp. nov., a marine, genome-sequenced gammaproteobacterium. Int J Syst Evol Microbiol 57:2370–2375
Pinhassi J, Pujalte MJ, Pascual J, González JM, Lekunberri I, Pedrós-Alió C, Arahal DR (2009) Bermanella marisrubri gen. nov., sp. nov., a genome-sequenced gammaproteobacterium from the Red Sea. Int J Syst Evol Microbiol 59:373–377
Pot B, Gillis M, De Ley J (1992) The genus Oceanospirillum. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, Berlin, pp 3230–3236
Pot B, Gillis M, Hoste B, van De Velde A, Bekaert F, Kersters K, De Ley J (1989) Intra- and intergeneric relationships of the genus Oceanospirillum. Int J Syst Bacteriol 39:23–34
Prabagaran SR, Suresh K, Manorama R, Delille D, Shivaji S (2005) Marinomonas ushuaiensis sp. nov., isolated from coastal sea water in Ushuaia, Argentina, sub-Antarctica. Int J Syst Evol Microbiol 55:309–313
Reasoner DJ, Geldreicht EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Romanenko LA, Uchino M, Mikhailov VV, Zhukova NV, Uchimura T (2003) Marinomonas primoryensis sp. nov., a novel psychrophile isolated from coastal sea-ice in the Sea of Japan. Int J Syst Evol Microbiol 53:829–832
Romanenko LA, Schumann P, Rohde M, Mikhailov VV, Stackebrandt E (2004) Reinekea marinisedimentorum gen. nov., sp. nov., a novel gammaproteobacterium from marine coastal sediments. Int J Syst Evol Microbiol 54:669–673
Romanenko LA, Tanaka N, Frolova GM (2009) Marinomonas arenicola sp. nov., isolated from marine sediment. Int J Syst Evol Microbiol 59:2834–2838
Rouse GW, Goffredi SK, Vrijenhoek RC (2004) Osedax: bone-eating marine worms with dwarf males. Science 305:668–671
Sakane T, Yokota A (1994) Chemotaxonomic investigation of heterotrophic, aerobic and microaerophilic spirilla, the genera Aquaspirillum, Magnetospirillum and Oceanospirillum. Syst Appl Microbiol 17:128–134
Sanchez-Amat A, Lucas-Elio P, Fernández E, Garcia-Borrón JC, Solano F (2001) Molecular cloning and functional characterization of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. Biochim Biophys Acta 1547:104–116
Sanchez-Amat AF, Solano F, Lucas-Elío P (2010) Finding new enzymes from bacterial physiology: a successful approach illustrated by the detection of novel oxidases in Marinomonas mediterranea. Mar Drugs 8:519–541
Satomi M, Kimura B, Takahashi G, Fujii T (1997) Microbial diversity of kusaya gravy. Fish Sci (Tokyo) 63:1019–1023
Satomi M, Kimura B, Hayashi M, Shouzen Y, Okuzumi M, Fujii T (1998) Marinospirillum gen. nov., with descriptions of Marinospirillum megaterium sp. nov., isolated from kusaya gravy, and transfer of Oceanospirillum minutulum to Marinospirillum minutulum comb. nov. Int J Syst Bacteriol 48:1341–1348
Satomi M, Kimura B, Hamada T, Harayama S, Fujii T (2002) Phylogenetic study of the genus Oceanospirillum based on 16S rRNA and gyrB genes: Emended description of the genus Oceanospirillum and Pseudospirillum gen. nov., Oceanobacter gen. nov., and Terasakiella gen. nov.; transfer of Oceanospirillum jannaschii, and Pseudomonas stanieri to Marinobacterium with Marinobacterium jannaschii comb. nov., and Marinobacterium stanieri comb. nov. Int J Syst Evol Microbiol 52:739–747
Satomi M, Oikawa H, Yano Y (2003) Shewanella marinintestina sp. nov., Shewanella schlegeliana sp. nov. and Shewanella sairae sp. nov., novel eicosapentaenoic-acid-producing marine bacteria isolated from sea-animal intestines. Int J Syst Evol Microbiol 53:491–499
Satomi M, Kimura B, Hayashi M, Okuzumi M, Fujii T (2004) Marinospirillum insulare sp. nov., a novel halophilic helical bacterium isolated from kusaya gravy. Int J Syst Evol Microbiol 54:163–167
Sawyer MH, Baumann P, Baumann L (1977) Pathways of d-fructose and d-glucose catabolism in marine species of Alcaligenes, Pseudomonas marina, and Alteromonas communis. Arch Microbiol 112:169–172
Schildcraut CL, Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. Mol Biol 4:430–443
Schlösser A, Lipski A, Schmalfuß J, Kugler F, Beckmann G (2008) Oceaniserpentilla haliotis gen. nov., sp. nov., a marine bacterium isolated from haemolymph serum of blacklip abalone. Int J Syst Evol Microbiol 58:2122–2125
Solano F, Sanchez-Amat A (1999) Studies of the phylogenetic relationships of melanogenic marine bacteria: proposal of Marinomonas mediterranea sp. nov. Int J Syst Bacteriol 49:1241–1246
Solano F, García E, Pérez de Egea E, Sanchez-Amat A (1997) Isolation and characterization of strain MMB-1 (CECT 4803), a novel melanogenic marine bacterium. Appl Environ Microbiol 63:3499–3506
Solano F, Lucas-Elío P, Fernández E, Sanchez-Amat A (2000) Marinomonas mediterranea MMB-1 transposon mutagenesis: isolation of a multipotent polyphenol oxidase mutant. J Bacteriol 182:3754–3760
Stackebrandt E, Goebel BM (1994) Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44:846–849
Suzuki MT, Rappé MS, Haimberger ZW, Winfield H, Adair N, Ströbel J, Giovannoni SJ (1997) Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl Environ Microbiol 63:983–989
Tamaoka J, Komagata K (1984) Determination of DNA base composition by reversed-phase high performance liquid chromatography. FEMS Microbiol Lett 25:125–128
Teramoto M, Suzuki M, Okazaki F, Hatmanti A, Harayama S (2009) Oceanobacter-related bacteria are important for the degradation of petroleum aliphatic hydrocarbons in the tropical marine environment. Microbiology 155:3362–3370
Teramoto M, Ohuchi M, Hatmanti A, Darmayati Y, Widyastuti Y, Harayama S, Fukunaga Y (2011) Oleibacter marinus gen. nov., sp. nov., a bacterium that degrades petroleum aliphatic hydrocarbons in a tropical marine environment. Int J Syst Evol Microbiol 61:375–380
Terasaki Y (1963) On the isolation of Spirillum. Bull. Suzugamine Women’s College. Nat Sci 10:1–10
Terasaki Y (1970) Some observations on the life history of Spirillum serpens. Bull. Suzugamine Women’s College. Nat Sci 15:1–7
Terasaki Y (1972) Studies on the genus Spirillum Ehrenberg. I: morphological, physiological, and biochemical characteristics of water spirilla. Bull. Suzugamine Women’s College. Nat Sci 16:1–146
Terasaki Y (1973) Studies on the genus Spirillum Ehrenberg. II: Comments on type and reference strains of Spirillum and description of new species and new subspecies. Bull. Suzugamine Women’s College. Nat Sci 17:1–71
Terasaki Y (1975) Freeze-dried cultures of water spirilla made on experimental basis. Bull. Suzugamine Women’s College. Nat Sci 19:1–10
Terasaki Y (1979) Transfer of five species and two subspecies of Spirillum to other genera (Aquaspirillum and Oceanospirillum), with emended descriptions of the species and subspecies. Int J Syst Bacteriol 29:130–144
van Landschoot A, De Ley J (1983) Intra- and intergeneric similarities of the rRNA cistrons of Alteromonas, Marinomonas (gen. nov.) and some other Gram-negative bacteria. J Gen Microbiol 129:3057–3074
Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealsonl KH (1999) Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. Nov. Int J Syst Bacteriol 49:705–724
Watanabe N (1959) On four new halophilic species of Spirillum. Bot Mag (Tokyo) 72:77–86
Wayne LG, Brenner DJ, Colwell RR et al (1987) Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Wilkinson SG (1988) Gram-negative bacteria. In: Ratledge C, Wilkinson SG (eds) Microbial lipids, vol 1. Academic, London, pp 299–488
Williams MA, Rittenberg SC (1957) A taxonomic study of the genus Spirillum Ehrenberg. Int Bull Bacteriol Nomencl Taxon 7:49–111
Woese CR, Blanz P, Hespell RB, Hahn CM (1982) Phylogenetic relationships among various helical bacteria. Curr Microbiol 7:119–124
Woese CR, Weisburg WG, Hahn CM, Paster BJ, Zablen LB, Lewis BJ, Macke TJ, Ludwig W, Stackebrandt E (1985) The phylogeny of purple bacteria: the gamma subdivision. Syst Appl Microbiol 6:25–33
Yakimov MM, Giuliano L, Gentile G, Crisafi E, Chernikova TN, Abraham W-R, Lünsdorf H, Timmis KN, Golyshin PN (2003) Oleispira antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal sea water. Int J Syst Evol Microbiol 53:779–785
Yakimov MM, Giuliano L, Denaro R, Crisafi E, Chernikova TN, Abraham W-R, Luensdorf H, Timmis KN, Golyshin PN (2004) Thalassolituus oleivorans gen. nov., sp. nov., a novel marine bacterium that obligately utilizes hydrocarbons. Int J Syst Evol Microbiol 54:141–148
Yakimov MM, Denaro R, Genovese M, Cappello S, D’Auria G, Chernikova TN, Timmis KN, Golyshin PN, Giluliano L (2005) Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily) and community successions during microcosm enrichment with various hydrocarbons. Environ Microbiol 7:1426–1441
Yamamoto S, Harayama S (1995) PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol 61:1104–1109
Yamamoto S, Harayama S (1996) Phylogenetic analysis of Acinetobacter strains based on the nucleotide sequences of gyrB genes and on the amino acid sequences of their products. Int J Syst Bacteriol 46:506–511
Yamamoto S, Harayama S (1998) Phylogenetic relationships of Pseudomonas putida strains deduced from the nucleotide sequences of gyrB, rpoD and 16S rRNA genes. Int J Syst Bacteriol 48:813–819
Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer KH, Ludwig W, Glöckner FO, Rossello-Mora R (2008) The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol 31:241–250
Yarza P, Ludwig W, Euzéby J, Amann R, Schleifer K-H, Glöckner FO, Rosselló-Móra R (2010) Update of the All-Species Living-Tree Project based on 16S and 23S rRNA sequence analyses. Syst Appl Microbiol 33:291–299
Yoon J-H, Kang S-J, T-K O (2005) Marinomonas dokdonensis sp. nov., isolated from sea water. Int J Syst Evol Microbiol 55:2303–2307
Zhang W, Xue Y, Ma Y, Grant WD, Ventosa A, Zhou P (2002) Marinospirillum alkaliphilum sp. nov., a new alkaliphilic helical bacterium from Haoji soda lake in Inner Mongolia Autonomous Region of China. Extremophiles 6:33–37
Zhang D-C, Li H-R, Xin Y-H, Liu H-C, Chen B, Chi Z-M, Zhou P-J, Yu Y (2008) Marinomonas arctica sp. nov., a psychrotolerant bacterium isolated from the Arctic. Int J Syst Evol Microbiol 58:1715–1718
Zhang X-Y, Zhang Y-J, Yu Y, Li H-J, Gao Z-M, Chen X-L, Chen B, Zhang Y-Z (2010) Neptunomonas antarctica sp. nov., isolated from marine sediment. Int J Syst Evol Microbiol 60:1958–1961
Zobell CE (1941) Studies on marine bacteria. I. The cultural requirements of heterotrophic aerobes. J Mar Res 4:139–143
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Satomi, M., Fujii, T. (2014). The Family Oceanospirillaceae . In: Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F. (eds) The Prokaryotes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38922-1_286
Download citation
DOI: https://doi.org/10.1007/978-3-642-38922-1_286
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38921-4
Online ISBN: 978-3-642-38922-1
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences