Abstract
Molecular epidemiology studies have provided convincing evidence of antigenic and sequence variability among respiratory syncytial virus (RSV) isolates. Circulating viruses have been classified into two antigenic groups (A and B) that correlate with well-delineated genetic groups. Most sequence and antigenic differences (both inter- and intra-groups) accumulate in two hypervariable segments of the G-protein gene. Sequences of the G gene have been used for phylogenetic analyses. These studies have shown a worldwide distribution of RSV strains with both local and global replacement of dominant viruses with time. Although data are still limited, there is evidence that strain variation may contribute to differences in pathogenicity. In addition, there is some but limited evidence that RSV variation may be, at least partially, immune (antibody) driven. However, there is the paradox in RSV that, in contrast to other viruses (e.g., influenza viruses) the epitopes recognized by the most effective RSV-neutralizing antibodies are highly conserved. In contrast, antibodies that recognize strain-specific epitopes are poorly neutralizing. It is likely that this apparent contradiction is due to the lack of a comprehensive knowledge of the duration and specificities of the human antibody response against RSV antigens. Since there are some data supporting a group- (or clade-) specific antibody response after a primary infection in humans, it may be wise to consider the incorporation of strains representative of groups A and B (or their antigens) in future RSV vaccine development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory Syncytial Virus
- Respiratory Syncytial Virus Infection
- Human Respiratory Syncytial Virus
- Respiratory Syncytial Virus Disease
- Immune Selection
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
An early study of the seroepidemiology of RSV in Sendai, Japan found that patient sera did not differ in neutralization of a small number of homologous and heterologous RSV strains, as measured by reduction in tissue culture infectious dose (TCID50) in HEp-2 cells (Suto et al. 1965). Using a methylcellulose overlay plaque assay developed in 1966, sera from infected ferrets detected limited strain antigenic variability, reflected in slightly different plaque reduction neutralization (PRN) titers for homologous (Long) versus heterologous (CH18537) strains (Coates et al. 1966). However, it was also found in those early days that children could be naturally infected in consecutive years with RSV strains indistinguishable by cross-PRN, and adults were naturally reinfected despite preexisting neutralizing antibodies (Abs) (Beem 1967).
Despite the previous comments, antigenic groups of RSV strains were definitively identified by enzyme-linked immunosorbent assay (ELISA) using a panel of 10 monoclonal Abs (mAbs) obtained from mice immunized with different RSV strains, such as A2, Long, and CH18537 (Anderson et al. 1985). In a separate study from the same year, RSV isolates from West Virginia were probed with a panel of mAbs generated against RSV Long (Mufson et al. 1985). RSV proteins recognized by the mAbs were identified by radioimmunoprecipitation assay (RIPA) and SDS-PAGE of 35S-labeled infected cell extracts. When these mAbs were tested against RSV field isolates by RIPA, it was revealed that RSV separated into two antigenic groups, A and B, based on eight epitope differences in the attachment glycoprotein (G), one epitope difference in the fusion glycoprotein (F), and one epitope difference in the nucleoprotein (N). The antigenic groups correlated with genetic differences identified by sequencing cDNA clones of the G genes of RSV A2 (A group), Long (A group), and CH18537 (B group) strains. Thus, while the deduced G-protein sequences of A2 and Long strains shared 94 % amino acid identity, those of CH18537 and A2 strains shared only 53 % amino acid identity, with the majority of the diversity residing in the predicted extracellular domain (Johnson et al. 1987b). The classification of RSV isolates into A and B antigenic groups is now more often done via sequencing of variable region(s) of the G extracellular domain, rather than by mAb reactivity. The RSV A and B group designation is also referred to as antigenic “subgroups” in the literature, group A being more prevalent than group B (Hall et al. 1990; Matheson et al. 2006).
Sequence-based molecular epidemiology of RSV led to the identification of genetically distinct, cocirculating genotypic lineages. Evidence of RSV lineages within group A was revealed in isolates from Birmingham, U.K. (1989) using partial sequences of the small hydrophobic (SH) gene and restriction patterns of RSV nucleoprotein (N) gene PCR amplicons (Cane and Pringle 1991). RSV G gene sequences from 27 group A isolates from Montevideo, Uruguay and Madrid, Spain (1987–1993) were aligned with those of A2, Long, and six isolates from Birmingham, UK to analyze the phylogenic relatedness of group A strains, and distinct lineages were evident (Garcia et al. 1994). Similarly, lineages were observed by analyzing sequences of the two variable domains of the G gene from 48 group A RSV isolates collected from 1956 to 1993 in the US, Australia, UK, Norway, Sweden, and Finland (Cane and Pringle 1995). Both studies also found local cocirculation of group A lineages and a high ratio of nonsynonymous to synonymous (dN/dS) mutations in the C-terminal variable region of G, suggesting positive selection. Furthermore, both studies probed isolates with panels of mAbs to the G protein and found that the strength of reactivity roughly paralleled the position on the phylogenetic dendrogram, consistent with contribution of immune selection to RSV G variability (Cane and Pringle 1995; Garcia et al. 1994).
A more detailed picture of RSV genetic lineages emerged with additional sequences. It was determined that the C-terminal 270 nt of the G gene can serve as a proxy for full length G gene variability (Peret et al. 1998). Phylogenetic analysis of G sequences from 204 RSV isolates collected in Rochester, New York from winter 1990/1991 to winter 1994/1995 revealed a number of genetically distinct clusters of genotypes (clades) within A and B groups of RSV (Peret et al. 1998). These clades were designated GA1 to GA5 for group A and GB1 to GB4 for group B. This work provided a clade nomenclature and framework that was consistent with earlier observations of distinct RSV lineages and aided ongoing investigations of RSV molecular epidemiology. Subsequent RSV studies with large sequence datasets of RSV isolates over time from around the globe confirmed these cocirculating clades and identified additional clades (Gaunt et al. 2011; Matheson et al. 2006; Reiche and Schweiger 2009; Shobugawa et al. 2009; Venter et al. 2001; Zlateva et al. 2007; Botosso et al. 2009). Genetic relatedness of RSV group A strains is depicted in Fig. 1 by a phylogenetic tree composited from multiple studies, showing clades and representative isolates. Since the late 1990s, the GA2 and GA5 clades have dominated among group A RSV clades, with season-to-season fluctuation in relative rate of isolation. Group B RSV clades will be discussed in more detail below. In general, there is greater diversity between group A clades than there is between group B clades.
Phylogenetic tree for RSV group A isolates. All group A G gene sequences from the following references were obtained from the NCBI database (Garcia et al. 1994; Peret et al. 1998, 2000; Venter et al. 2001, 2002; Botosso et al. 2009; Matheson et al. 2006; Zhang et al. 2007; Gaunt et al. 2011; Reiche and Schweiger 2009; Stokes et al. 2011; Yamaguchi et al. 2011). The isolates span years 1961–2007. Using Geneious Pro (Aukland, New Zealand) software, the sequences were trimmed to 270 nt corresponding to the second variable region of G, and identical, redundant sequences were removed. A total of 496 unique G sequences were aligned using ClustalW. Phylogenetic reconstruction was by the Maximum Likelihood (general time reversible model, 1000 bootstrap replicates) using MEGA5 software (Tamura et al. 2011). The tree was graphed and annotated using FigTree version 1.4. Representative isolates are shown to the right. Clades GA1 through GA7 are indicated, as well as the span of years of isolation for each clade. Branch lengths are measured in the number of substitutions per site (scale bar)
2 Temporal and Geographical Distribution of RSV Strains
Initial analysis of multisequence alignments of RSV G gene sequences showed clustering of strains temporally and not geographically. Phylogenetically related RSV strains could be found at similar times in different continents, whereas isolates collected in the same place from the same epidemic may show greater diversity (Garcia et al. 1994). Additional evidence for temporal and not geographic clustering came from a study comparing 106 group A and 38 group B RSV isolates from New Zealand (collected 1967–2004) with published isolates from around the world. The New Zealand isolates did not cluster with each other, and RSV isolates clustered by clade, not by country (Matheson et al. 2006). However, despite data on wide geographic dissemination of RSV strains, community-based transmission likely plays a role in RSV epidemiology. RSV isolates were collected from the 1994–1995 RSV season in the following cities, states/province: Birmingham, Alabama, Rochester, New York, Houston, Texas, St. Louis, Missouri, and Winnipeg, Mannitoba (Peret et al. 2000). The GA1 clade was most prevalent in Birmingham, Rochester, and St. Louis, whereas the GA5 clade was dominant in Houston, and the GA5 and GA7 clades codominated that season in Winnipeg. RSV isolates from Japan generally clustered with known clades, but some were genotypically unique, suggesting a role for geographic clustering and community-based spread (Kuroiwa et al. 2005).
One way to gauge community-specific RSV distribution is to compare RSV studies reporting RSV isolates from different locations spanning the same time frame. Table 1 compares the prevalence of RSV group A and group B as well as the prevalence of the dominant group A clades (GA2, GA5, and GA7) over time in different regions. Dominant group B clades were omitted in Table 1 because their classification is less consistent. Table 1 shows some patterns of widespread RSV distribution. For example, the relative A to B and relative GA2 to GA5 rates are roughly similar in Belgium, Germany, and Buenos Aries over three RSV seasons, 1998–1999, 1999–2000, and 2000–2001 (Table 1). Yet, site-specific group and clade restrictions are also evident. For example, Belgium and Germany differ in whether A or B is more prevalent in three of the eight overlapping seasons (Table 1). Also, GA5 was the dominant group A clade in Belgium, Germany, and Japan in the 2003–2004 season. However, GA2 was dominant the next season in Belgium and Japan, whereas GA5 remained dominant in Germany (Table 1). In tropical and subtropical regions, the relative prevalence of A and B differed between Buenos Aires and Kenya in two out of three overlapping seasons (Table 1). RSV seasonality in tropical regions is distinct, and epidemics occur during the rainy season from July to November, with a biennial pattern of low and high incidence seasons, as observed in Cambodia (Arnott et al. 2011). In Cambodia and China, the dominance of group A or group B in a particular season was strong (Table 1). In summary, factors determining RSV group and clade compositions of epidemics are complex. Region-specific factors play a role, and, as discussed in more detail below, specific genotypes can also spread globally.
Although RSV groups and clades cocirculate and can appear in successive years, year-to-year changes in the predominance of group A and group B and year-to-year changes in the predominance of clades were observed in a given location (Peret et al. 1998). Subsequent studies confirmed that clades co-circulate locally and alternate in predominance over time, potentially due to immune selection but without evidence of progressive evolution as defined by new strain emergence (Botosso et al. 2009; Gaunt et al. 2011; Matheson et al. 2006; Reiche and Schweiger 2009; Venter et al. 2001). Two key questions about the temporal distribution of RSV strains are: (i) what drives season-to-season changes in clade predominance? and (ii) do RSV clades impact natural infection and reinfection by, for example, providing some degree of immune evasion? A recurring hypothesis has been that alternating clade prevalence is a result of short-lived strain- or clade-specific herd immunity that favors circulation of a heterologous clade. Although it has yet to be shown definitively, there are published data consistent with the hypothesis of selection by herd immunity. Thus, several of the amino acid residues in the C-terminal hypervariable region of the G protein identified as having a high dN/dS ratio map within known Ab epitopes (Botosso et al. 2009; Garcia et al. 1994). Furthermore, a number of positively selected amino acid sites in the C-terminus of G show a reversion (“flip-flop”) pattern of evolution, consistent with rising and waning strain-specific immunity (Botosso et al. 2009). The infant serum Ab response to primary RSV infection contains clade-specific Abs, as measured by ELISA using plates coated with polypeptides corresponding to the C-terminus of G (Scott et al. 2007). More recently, the RSV group and clade of infecting and reinfecting strains were identified in a birth cohort in Kenya. Excluding seven reinfections that occurred during the same epidemic, there were 46 reinfections documented (Agoti et al. 2012). Over 28 (61 %) of those reinfections were group heterologous (A then B, or B then A). Among the 46 reinfections, there were only six instances where group A infection was followed by group A in a subsequent season. Of those six, four were heterologous for GA2/GA5 clade. Although the numbers are small, the authors state that the majority of reinfections are heterologous at the group or clade level (Agoti et al. 2012). It would be more compelling if higher rates of heterologous clade reinfection could be documented when the reinfecting clade is subdominant.
A better understanding of the potential for global spread of RSV strains has been facilitated by the discovery, in 1999, of a novel group B RSV genotype. It was isolated for the first time in Buenos Aires and contained a 60 nt duplicated region in the C-terminal one-third of G, and the clade was named BA (Trento et al. 2003). These initial BA isolates had an exact 60 nt duplication, resulting in two tandem sequences of TERDTSTSQSTVLDTTTSKH from amino acids 240 to 280 in G (Trento et al. 2003). This insertion was then used as a “tag” to analyze the global distribution and evolution of a new RSV genotype since its initial emergence (Trento et al. 2006, 2010). The BA clade rapidly disseminated worldwide and became dominant, and these findings have been confirmed by a number of RSV molecular epidemiology studies with relatively large datasets (Baek et al. 2012; Gaunt et al. 2011; Zhang et al. 2007; Botosso et al. 2009). The BA clade continued to evolve, the two exact 60 nt repeats diverged slightly, and a new BA-IV lineage essentially replaced all other group B RSV G strains. The biological significance of the duplicated region in BA G is not known.
Besides evolutionary studies, analysis of sequence variation in the G gene may have other practical applications as, for instance, tracing the origin of infecting viruses during outbreaks in hospitalized patients (Mazzulli et al. 1999; Taylor et al. 2001).
3 Clinical Differences Between RSV Strains
Early work showed that group A RSV is associated with slightly greater clinical severity than group B RSV (Hall et al. 1990). RSV disease severity was correlated with RSV clades in small cohorts, but these studies are not definitive because clade GA2 was found to be less pathogenic in one study and more pathogenic in others (Gilca et al. 2006; Martinello et al. 2002). Thus, the role of RSV strain differences in disease remains to be elucidated.
4 Phenotypic Differences Between RSV Strains In Vitro and in Animal Models
Small changes in viral gene sequences may have a large impact on pathogenesis. For instance, the elevated virulence of 1918 and avian influenza strains hinges on few amino acids differences (Conenello et al. 2007; Tumpey et al. 2005). Passage of viruses in animal hosts can lead to adaptation. The RSV Long strain was adapted to mice (line 19) by serial intracranial inoculation, resulting in higher virus replication presumably due to mutations that have not been characterized (Cavallaro and Maassab 1966). Infection of BALB/c mice with the laboratory RSV strains A2 or Long results in a predominant TH1-type antiviral response (Moore and Peebles 2006). In contrast, the line 19 strain of RSV induces a TH2 type and IL-13-dependent airway hyperreactivity (AHR) and pulmonary mucus in BALB/c mice (Lukacs et al. 2006). The F protein of the line 19 RSV strain has a unique sequence and is a factor that can induce pulmonary IL-13 and mucin expression in RSV infection (Moore et al. 2009). In addition, six RSV isolates have been screened for lung IL-13 levels and airway mucin expression in BALB/c mice. Three of these isolates induced lung IL-13 and gob-5 (a marker of mucin) expression, and were found to be differentially mucogenic in BALB/c mice (Stokes et al. 2011). RSV clinical isolates also infect the airway epithelium of mice to a greater extent than the laboratory adapted A2 strain (Stokes et al. 2011). In differentiated primary pediatric airway epithelial cells cultured at air–liquid interface, a RSV clinical isolate exhibited enhanced infectivity (but not virus yield) and induced greater mucus production than the laboratory A2 strain (Villenave et al. 2012). The molecular bases for these strain-specific phenotypes are largely unknown.
5 Protective Immunity
Animal and human studies have provided a wealth of evidence indicating that protection against RSV infection is afforded mainly by neutralizing antibodies. In early experiments (Prince et al. 1983), it was found that cotton rats infected with RSV developed complete resistance to pulmonary reinfection which lasted at least 18 months. Adaptive transfer studies with the convalescence blood showed that serum antibody, but not circulating lymphocytes, conferred resistance. Immune pregnant cotton rat females transmitted protective antibodies to their young mainly through colostrum and milk (Prince et al. 1983). Similar findings were observed in pregnant ferrets (Prince and Porter 1975) and guinea pigs (Buraphacheep and Sullender 1997). Human convalescent serum and human immunoglobulin (Ig) preparations were also found to confer pulmonary protection against RSV in the cotton rats. Serum neutralizating antibody titres of 1:380 or higher were required for complete protection in the lungs, whereas about 10-fold higher titres were required for protection in the nose (Prince et al. 1985). Therapeutic administration of neutralizing Abs reduced significantly the level of RSV replication in the lungs of cotton rats and owl monkeys but showed only a slight trend of beneficial effects in a limited number of RSV-infected children (Hemming and Prince 1990). In addition to polyclonal antibodies, administration of certain monoclonal antibodies (mAbs) directed against either G or F glycoproteins protected the lungs of mice (Taylor et al. 1984) and cotton rats (Walsh et al. 1984) against a RSV challenge. In one study, it was found that passive protection of mice afforded by a nonneutralizing mAb directed against the G glycoprotein was dependent on both the Fc fragment of the antibody and the host complement (Corbeil et al. 1996), explaining the lack of strict correlation between in vitro neutralization in the absence of complement and in vivo protection (Taylor et al. 1984; Walsh et al. 1984).
In humans, protection of adult volunteers to RSV challenge was correlated with high titres of preexisting serum neutralizing antibodies (Hall et al. 1991). Additionally, an inverse correlation was observed between high titres of RSV-neutralizing serum antibodies and risk of infection in children (Glezen et al. 1986). However, whereas in animal models the quantitative aspects of passive protection to RSV infection by neutralizing antibodies is well established, the situation in human is less certain. For instance, contradicting results were reported by the same group, about correlation (Falsey and Walsh 1998) or lack of correlation (Falsey and Walsh 1992) between neutralizing antibody titers and risk of RSV infection in the elderly. Nevertheless, the consensus inferred from the majority of data is that neutralizing antibodies protect against RSV infection and particularly against RSV-associated pathology. Perhaps the best evidence for this assertion was provided by the clinical studies carried out with a Ig preparation (RS-IVIG, Respigam™), selected for high titers of RSV-neutralizing antibodies which was administered prophylactically to high-risk infants (Groothuis et al. 1993). The beneficial effect of RS-IVIG was noticed in the reduction of hospitalizations (55 %) and days of intensive care (97 %) rather than frequency of RSV infections. These studies led to licensing of Respigam in 1996 for prophylaxis of RSV infections in high-risk infants. Respigam was replaced in 1998 by a humanized neutralizing mAb (MEDI-493, palivizumab) directed against the RSV F glycoprotein (Beeler and Van Wyke 1989) that showed similar efficacy but it was easier to administer than Respigam (see chapter by H.Y. Chu and J.A. Englund, this volume).
Despite the beneficial effects of antibodies in protection against RSV, some caution is needed because:
-
(1)
Passive serum Abs have been shown to inhibit the antibody responses to F and G glycoproteins expressed by recombinant vaccinia viruses (Murphy et al. 1988) or administered as purified antigens adjuvanted with alum (Murphy et al. 1991); however, they did not suppress the T-cell response nor the priming for a strong secondary antibody response (Fisher et al. 1999; Crowe et al. 2001). Therefore, a well-balanced dose of prophylactic neutralizing antibodies may be required to avoid interference with the host immune response to either RSV infection or RSV vaccination.
-
(2)
Weakly neutralizing Abs may have detrimental effects that contribute to the enhanced respiratory disease observed in seronegative children that were vaccinated in the 1960s with a formalin inactivated RSV preparation (Kim et al. 1969). It has been shown that antibodies may lead to formation of immune complexes that correlate with enhanced pathology in mice after an RSV challenge. Immune complex activation of complement was also observed in postmortem lung sections from children with enhanced RSV disease (Polack et al. 2002). Nonreplicating RSV vaccines that fail to promote antibody affinity maturation may prime for immune complex formation upon RSV infection (Delgado et al. 2009).
Although antibodies are important for resistance to infection, T cells are imperative for virus clearance. Thus, individuals with compromised T cell immunity can shed virus for months (Hall et al. 1986). Prolonged virus shedding is also observed in nude or irradiated mice (Cannon et al. 1987) and in mice depleted of both CD4+ and CD8+ lymphocytes (Graham et al. 1991). Furthermore, CD8+ cytotoxic lymphocytes may provide some protection in mice against infection, but this effect is short-lived (Connors et al. 1991, 1992). In infants with severe RSV infection, the peak of activated CD8+ T cell numbers in bronchoalveolar lavage (BAL) samples and in blood correlated with convalescence, consistent with a role for CD8+ T cells in recovery (Heidema et al. 2007).
6 Protective Antigens
Identification of antigens able to induce a neutralizing and protective immune response was achieved initially by immunization of mice or cotton rats with recombinant vaccinia viruses encoding individual RSV gene products (Stott et al. 1986, 1987; Wertz et al. 1987; Olmsted et al. 1986). It was promptly found that only the external F and G glycoproteins were able to confer long-lasting protection against RSV infection and that this protection correlated with induction of neutralizing antibodies. The nucleoprotein (N) and the M2-1 (or 22k) proteins were also able to induce partial and short-lived protection which, at least in the case of the M2-1 was mediated by cytotoxic T lymphocytes (Connors et al. 1992).
It was also noticed that: (i) recombinant vaccinia viruses expressing the F protein conferred higher level of protection against RSV than those expressing the G protein (Olmsted et al. 1986) and (ii) the neutralizing immune response against the F protein was cross-protective against viruses of a different antigenic group, whereas the neutralizing and protective response against the G protein was restricted to viruses of the same antigenic group (Johnson et al. 1987a; Stott et al. 1987). These differences reflect the dissimilar structural and antigenic characteristics of the F and G glycoproteins and their differences at the level of antigenic and genetic relatedness between RSV isolates.
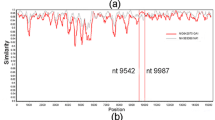
In the case of the F glycoprotein (the viral glycoprotein that mediates fusion of the virus and cell membranes), there is 89 % amino acid sequence identity between the proteins of groups A and B of human RSV (Johnson and Collins 1988). This is reflected in the high level of antigenic relatedness observed with murine mAbs (Garcia-Barreno et al. 1989). Epitopes have been mapped in the F protein primary structure primarily by isolation and sequencing of mutants that grow in the presence of individual mAbs. These escape mutants normally contained single amino acid substitutions that obliterated the epitopes recognized by the antibodies used in their selection (see chapter by J.S. McLellan et al., this volume). Figure 2 summarizes the amino acid changes in escape mutants reported up to date by different groups (Arbiza et al. 1992; Lopez et al. 1998; Crowe et al. 1998; Zhao et al. 2004a, b). In most cases, selection was done with murine mAbs, except in the case of the mutant I266 M that was selected with a recombinant human Fab fragment (Crowe et al. 1998). It is worth stressing that frequently the same mutation could be repeatedly selected with different antibodies (e.g., K272N isolated with mAbs 151, 1200, 47F, and palivizumab). Occasionally, more than one amino acid change was selected at the same position (e.g., N262S or N262Y) and one of the changes (N268I) was also selected after passing the virus in the presence of a polyclonal rabbit antiserum raised against purified F protein (Tome et al. 2012). These findings probably reflect the dominance of certain epitopes and the propensity of the F protein to incorporate certain mutations but not others. The amino acid changes depicted in Fig. 2 and therefore the corresponding antibody epitopes are clustered in three regions (antigenic sites) of the F protein primary structure, named sites II, I, and IV and corresponding to sites A, B, and C as designated by Beeler and Van Wyke (1989). The epitopes of antigenic sites II and IV (Anderson et al. 1985; Beeler and Van Wyke 1989; Garcia-Barreno et al. 1989) are very conserved among RSV strains, while those of antigenic site I are group-specific; i.e., they are conserved only in RSV strains of the same antigenic group (Garcia-Barreno et al. 1989).
Scheme of the F protein primary structure. The sequence length is indicated above the main rectangle. Black boxes denote the hydrophobic signal peptide (SP), fusion peptide (FP), and transmembrane region (TM). Shaded boxes symbolize heptad repeat sequences, HRA, and HRB and the two arrows indicate the location of the proteolytic cleavage sites. Vertical lines denote the location of the indicated amino acid changes selected in escape mutants described in the following articles: Arbiza et al. (1992), Lopez et al. (1998), Crowe et al. (1998), and Zhao et al. (2004a, b). These amino acid changes have been grouped in antigenic sites I, II, and IV as shown above the main rectangle
In contrast to the antigenic and genetic conservation of the F protein, the G glycoproteins of RSV group A and B strains share less than 50 % amino acid sequence identity (Johnson et al. 1987b). This genetic variation is reflected in the high level of antigenic differences detected with anti-G mAbs (Garcia-Barreno et al. 1989). As with the F protein, the epitopes recognized by anti-G MAbs have been mapped in the protein primary structure mainly by selection and sequencing of escape mutants (Fig. 3). Three types of epitopes were identified: (i) epitopes conserved among all RSV strains that mapped in the central unglycosylated segment of the G protein ectodomain, (ii) group - specific epitopes that overlapped partially with the conserved epitopes and that were retained only in RSV strains of the same antigenic group, and (iii) a majority of strain - specific epitopes that were located in the C-terminal third of the G protein ectodomain and that were present only in some RSV strains of the same antigenic group (Martinez et al. 1997). Additionally, and in clear distinction with the escape mutants selected with anti-F MAbs, those selected with anti-G mAbs frequently contained drastic genetic alterations, other than single amino acid changes and which included: (i) frame-shift mutations that altered the C-terminal third of the G molecule (Garcia-Barreno et al. 1990), (ii) premature stop codons that shortened the G polypeptide between 1 and 42 amino acids (Rueda et al. 1991, 1995; Martinez et al. 1997), (iii) multiple A-G transitions (A-G hypermutations) that change several amino acids, including some cysteines in the G protein ectodomain (Rueda et al. 1994; Martinez et al. 1997; Walsh et al. 1998), and (iv) amino acid changes that prevent insertion of G in the viral membrane (Walsh et al. 1998). All these findings emphasize the extreme plasticity of the G molecule to adopt sequence changes, something that might be related to the fact that G is not required for RSV replication in Vero cells, although viruses lacking G are attenuated in HEp-2 cells and in vivo (Karron et al. 1997).
Scheme of the G protein primary structure. The sequence length is indicated above the main rectangle. Two mucin-like variable regions of the G protein ectodomain are indicated. Black dots denote Cys residues, black arrowheads denote N-glycosylation sites, and short vertical lines (below the main rectangle) O-glycosylation sites. The black box delineates the transmembrane region. The gray boxes delineate the location of conserved and group-specific epitopes in the central segment and the strain-specific epitopes in the C-terminal mucin-like region, respectively (Garcia-Barreno et al. 1990; Rueda et al. 1991, 1994, 1995; Martinez et al. 1997; Walsh et al. 1998). The continuous horizontal line denotes the segment of identical sequence (amino acids 164–176) in all RSV isolates and the broken horizontal line the segment of identical sequence (amino acids 163–189) in all RSV group A isolates
Another major difference between mAbs specific for the F and G glycoproteins is their potency and mechanism of neutralization. Whereas certain mAbs reacting with epitopes in sites II or IV of the F glycoprotein are potent neutralizers of RSV infectivity in vitro (Lopez et al. 1998) most mAbs specific of the G glycoprotein neutralize RSV very poorly, even if tested against the homologous strain used in their isolation. However, mixtures of anti-G mAbs show a synergistic effect in neutralization that is not observed with anti-F MAbs (Martinez and Melero 1998; Anderson et al. 1988). It has then been postulated that neutralizing antibodies directed against the F glycoprotein inhibit RSV infectivity by blocking the conformational changes that follow activation of RSV F to initiate the process of virus-cell membrane fusion (Magro et al. 2010). In contrast, results obtained with anti-G mAbs suggest that neutralization is afforded in this case by steric hindrance of G-protein interactions with cell surface components (likely proteoglycans). This steric inhibition requires simultaneous binding of several antibodies to the same G molecule, explaining the synergistic effect found with combinations of these antibodies (Martinez and Melero 1998). Despite these findings, it has been reported that a minority of human antibodies with high affinity for the central conserved region of RSV G are potent neutralizers of virus infectivity (Collarini et al. 2009) and may neutralize RSV infectivity by mechanisms others than steric hindrance.
In summary, most data indicate that F is more potent than G in inducing neutralizing and protective antibodies. The potent neutralizing antibody response afforded by anti-F antibodies is widely cross-reactive, while the poorer neutralizing anti-G response is rather strain or group-specific. These differences are probably deep-rooted in the level of genetic variability of F and G between RSV isolates and in the mechanism of virus neutralization afforded by different antibodies, particularly in vivo.
7 Human Antibody Response
As noted in previous sections, most studies carried out so far with neutralizing antibodies have been done with murine mAbs. Therefore, two relevant questions are: (i) what are the specificities of human neutralizing Abs? and (ii) are they the same as those of murine Abs?
Not much is known about the specificities of human anti-RSV neutralizing Abs. This gap between human and animal studies is due at least in part to the inherent difficulty of experimenting with humans. The use of technologies to clone and express human antibodies produced by individual B cells should facilitate in the future dissecting the repertoire of specificities represented in the human neutralizing antibody response against RSV.
Nevertheless, it has been reported already that human Ig preparations contain antibodies that compete with most murine anti-F antibodies (Sastre 2004); however, human neutralizing Abs have been identified that recognize F protein fragments laying outside of the antigenic sites demarcated by murine mAbs (Sastre et al. 2004). Additionally, neutralizing Abs specific for the untriggered form of RSV F, not represented in the collections of murine mAbs so far described were recently unveiled in human Ig preparations (Magro et al. 2012). Therefore, although the specificities of murine anti-F Abs are present in human sera, human Abs seem to embrace a broader range of specificities than murine Abs.
Similarly, certain specificities of anti-G mAbs also seem to be relevant in the human antibody response. Thus, human Igs were able to out compete binding of certain anti-G mAbs that recognized epitopes of the unglycosylated central segment of the G protein ectodomain (Sastre 2004). Furthermore, neutralizing antibodies could be purified from human Ig preparations by affinity chromatography with a fragment of the G glycoprotein corresponding to the conserved ectodomain segment (Sastre et al. 2004). In agreement with these results, group-specific serum antibodies directed against the central nonglycosylated G-protein segment have been found after primary infections of children (Murata et al. 2010a, b). These antibodies are likely responsible for the partially group-specific neutralizing antibody response reported in previous studies after primary RSV infections (Hendry et al. 1988; Muelenaer et al. 1991). However, it remains uncertain whether or not human sera contain Abs reacting with strain-specific epitopes of the C-terminal third of the RSV G glycoprotein. Detection of this type of antibodies in convalescent sera is likely dependent on the use of an appropriate G protein (or fragment thereof) that matches the antigenic properties of the infecting virus. Since detailed information about the infecting virus is not available for most sera, detection of strain-specific antibodies has been reported only in a few cases (Palomo et al. 2000; Cane et al. 1996; Jones et al. 2002; Scott et al. 2007)
In summary, it is apparent that the antibody specificities of murine mAbs are also represented in human convalescent sera, but human antibodies seem to recognize a broader range of F and G-protein epitopes than murine Abs. It will be important in the future to elucidate the actual relevance of the different antibody specificities for protection against infection and pathology. This might be particularly relevant in the case of anti-G Abs; for instance, it has been reported that certain murine Abs that block the interaction of the fractalkine-like motif of RSV_G with the CX3CR1 receptor reduced pulmonary inflammation and virus replication in mice (Zhang et al. 2010b). Likewise, other Abs may block additional, but still unrecognized activities of the F or G glycoproteins involved in virus replication and/or pathology.
8 Antigenic Variation, Immune Selection, and RSV Evolution
It has been observed that generally viral antigens accumulate amino acid changes at the sites that are recognized by neutralizing Abs as exemplified by the influenza virus haemaglutinin (HA) (Knossow and Skehel 2006). In this case, most of the sequence changes that are retained in viruses of the same subtype over the years involve residues on the surface of the HA head that are part of the epitopes recognized by neutralizing Abs. In other words, to escape neutralization by preexisting antibodies new influenza strains are positively selected that can infect again the same human population.
The situation described for influenza virus is in apparent contradiction with that found in RSV and outlined in previous sections. Thus, in the case of human RSV, the most effective neutralizing antibodies are those directed against antigenic sites II and IV of the F glycoprotein (Fig. 2), which are highly conserved among RSV isolates. In contrast, the antibodies that recognize epitopes in the C-terminal third of the G glycoprotein are only weakly neutralizing (Fig. 3), although this segment of the G molecule represents an extreme example of sequence variation, compared with antigens of related viruses (e.g., influenza virus). In other words, the paradoxical situation in RSV is that the epitopes recognized by the most potent neutralizing antibodies are highly conserved, whereas those recognized by poorly neutralizing antibodies are extremely variable.
As mentioned before, two types of evidence support the notion that the sequence variation found in the G protein is the result of positive selection: (i) whereas synonymous nucleotide changes have a uniform distribution along the G-protein gene, nonsynonymous changes accumulate in the two variable mucin-like regions of G (Melero et al. 1997), in analogy with the accumulation of nonsynonymous changes at the antigenic sites of influenza HA due to positive Darwinian evolution (Fitch et al. 1991) and (ii) phylogenetic methods have identified several positively selected sites in group A and B of RSV isolates (Woelk and Holmes 2001; Botosso et al. 2009). The fact that some of the positively selected sites coincide with epitopes recognized by anti-G mAbs and that the antigenic changes detected with a panel of anti-G mAbs correlated with the position of RSV isolates in a phylogenetic tree (Garcia et al. 1994) support the notion that positive selection of changes in the G glycoprotein might be immune driven.
Data from human studies offer conflicting interpretations about the relevance of antigenic variation and immune selection in protection against infection and in RSV evolution, respectively. On the one hand, as noted before the epitopes recognized by the most efficient neutralizing anti-F mAbs are highly conserved among RSV isolates and antibodies with the same specificities are present in human Ig preparations (Sastre et al. 2004). These results would argue against immune selection playing any role in the generation of RSV variability and support the idea that RSV vaccines may require only one virus strain or its antigens.
On the other hand, studies conducted with convalescent sera have provided evidence that upon primary RSV infections there was some dominance of group-specific neutralizing antibodies (Muelenaer et al. 1991; Cane et al. 1996; Scott et al. 2007). This group-specific response was also observed by ELISA with sera from convalescent primary infections using segments of the central core of the G glycoprotein as antigens (Murata et al. 2010a; Scott et al. 2007; Cane et al. 1996). However, the group-specificity of the neutralizing and antigen-binding antibodies was blurred upon secondary infections (Murata et al. 2010a). Consequently, there is a critical need for detailed assessment of the antibody specificities (including strain-specific) induced upon RSV infection and reinfection and their actual contribution to the human neutralizing immune response.
It is possible that the immune selective pressure on RSV is not as strong as that operating in influenza virus; for instance, if the antibody response is short-lived in RSV in comparison with influenza virus, replacement of preexisting RSV strains by new variants may be a slower process than in influenza. Of note, the new variants of RSV group B with a 60-nucleotide duplication in the G-protein gene replaced the preexisting strains of the same antigenic group worldwide but after a 6 to 7-year period (Trento et al. 2010), which is clearly longer than the observed replacement of influenza A viruses by new variants (usually 2–3 years). A combination of immune selection and high plasticity of the G protein may be at the basis of the extreme sequence variation observed for this gene among RSV isolates. In consequence, until new information concerning the specificities of human Ab responses is available, it would be wise to include viruses (or antigens) representing the two RSV antigenic groups in vaccine development.
References
Agoti CN, Mwihuri AG, Sande CJ, Onyango CO, Medley GF, Cane PA, Nokes DJ (2012) Genetic relatedness of infecting and reinfecting respiratory syncytial virus strains identified in a birth cohort from rural Kenya. J Infect Dis 206:1532–1541
Anderson LJ, Hierholzer JC, Tsou C, Hendry RM, Fernie BF, Stone Y, McIntosh K (1985) Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis 151:623–633
Anderson LJ, Bingham P, Hierholzer JC (1988) Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol 62:4232–4238
Arbiza J, Taylor G, Lopez JA, Furze J, Wyld S, Whyte P, Stott EJ, Wertz G, Sullender W, Trudel M, Melero JA (1992) Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol 73(Pt 9):2225–2234
Arnott A, Vong S, Mardy S, Chu S, Naughtin M, Sovann L, Buecher C, Beaute J, Rith S, Borand L, Asgari N, Frutos R, Guillard B, Touch S, Deubel V, Buchy P (2011) A study of the genetic variability of human respiratory syncytial virus (HRSV) in Cambodia reveals the existence of a new HRSV group B genotype. J Clin Microbiol 49:3504–3513
Baek YH, Choi EH, Song MS, Pascua PN, Kwon HI, Park SJ, Lee JH, Woo SI, Ahn BH, Han HS, Hahn YS, Shin KS, Jang HL, Kim SY, Choi YK (2012) Prevalence and genetic characterization of respiratory syncytial virus (RSV) in hospitalized children in Korea. Arch Virol 157:1039–1050
Beeler JA, Van Wyke CK (1989) Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol 63:2941–2950
Beem M (1967) Repeated infections with respiratory syncytial virus. J Immunol 98:1115–1122
Botosso VF, Zanotto PM, Ueda M, Arruda E, Gilio AE, Vieira SE, Stewien KE, Peret TC, Jamal LF, Pardini MI, Pinho JR, Massad E, Sant’anna OA, Holmes EC, Durigon EL (2009) Positive selection results in frequent reversible amino acid replacements in the G protein gene of human respiratory syncytial virus. PLoS Pathog 5:e1000254
Buraphacheep W, Sullender WM (1997) The guinea pig as a model for the study of maternal immunization against respiratory syncytial virus infections in infancy. J Infect Dis 175:935–938
Cane PA, Pringle CR (1991) Respiratory syncytial virus heterogeneity during an epidemic: analysis by limited nucleotide sequencing (SH gene) and restriction mapping (N gene). J Gen Virol 72(Pt 2):349–357
Cane PA, Pringle CR (1995) Evolution of subgroup A respiratory syncytial virus: evidence for progressive accumulation of amino acid changes in the attachment protein. J Virol 69:2918–2925
Cane PA, Thomas HM, Simpson AF, Evans JE, Hart CA, Pringle CR (1996) Analysis of the human serological immune response to a variable region of the attachment (G) protein of respiratory syncytial virus during primary infection. J Med Virol 48:253–261
Cannon MJ, Stott EJ, Taylor G, Askonas BA (1987) Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology 62:133–138
Cavallaro JJ, Maassab HF (1966) Adaptation of respiratory syncytial (RS) virus to brain of suckling mice. Proc Soc Exp Biol Med 121:37–41
Coates HV, Alling DW, Chanock RM (1966) An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol 83:299–313
Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, Harriman WD, Carroll SF, Ellsworth SL, Anderson LJ, Tripp RA, Walsh EE, Keyt BA, Kauvar LM (2009) Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol 183:6338–6345
Conenello GM, Zamarin D, Perrone LA, Tumpey T, Palese P (2007) A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS Pathog 3:1414–1421
Connors M, Collins PL, Firestone CY, Murphy BR (1991) Respiratory syncytial virus (RSV) F, G, M2 (22 K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol 65:1634–1637
Connors M, Kulkarni AB, Collins PL, Firestone CY, Holmes KL, Morse HC III, Murphy BR (1992) Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J Virol 66:1277–1281
Corbeil S, Seguin C, Trudel M (1996) Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus Long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine 14:521–525
Crowe JE, Firestone CY, Crim R, Beeler JA, Coelingh KL, Barbas CF, Burton DR, Chanock RM, Murphy BR (1998) Monoclonal antibody-resistant mutants selected with a respiratory syncytial virus-neutralizing human antibody fab fragment (Fab 19) define a unique epitope on the fusion (F) glycoprotein. Virology 252:373–375
Crowe JE Jr, Firestone CY, Murphy BR (2001) Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol 167:3910–3918
Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP (2009) Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15:34–41
Falsey AR, Walsh EE (1992) Humoral immunity to respiratory syncytial virus infection in the elderly. J Med Virol 36:39–43
Falsey AR, Walsh EE (1998) Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 177:463–466
Fisher RG, Johnson JE, Dillon SB, Parker RA, Graham BS (1999) Prophylaxis with respiratory syncytial virus F-specific humanized monoclonal antibody delays and moderately suppresses the native antibody response but does not impair immunity to late rechallenge. J Infect Dis 180:708–713
Fitch WM, Leiter JM, Li XQ, Palese P (1991) Positive Darwinian evolution in human influenza A viruses. Proc Natl Acad Sci U S A 88:4270–4274
Garcia O, Martin M, Dopazo J, Arbiza J, Frabasile S, Russi J, Hortal M, Perez-Brena P, Martinez I, Garcia-Barreno B (1994) Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J Virol 68:5448–5459
Garcia-Barreno B, Palomo C, Penas C, Delgado T, Perez-Brena P, Melero JA (1989) Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J Virol 63:925–932
Garcia-Barreno B, Portela A, Delgado T, Lopez JA, Melero JA (1990) Frame shift mutations as a novel mechanism for the generation of neutralization resistant mutants of human respiratory syncytial virus. EMBO J 9:4181–4187
Gaunt ER, Jansen RR, Poovorawan Y, Templeton KE, Toms GL, Simmonds P (2011) Molecular epidemiology and evolution of human respiratory syncytial virus and human metapneumovirus. PLoS ONE 6:e17427
Gilca R, De Serres G, Tremblay M, Vachon ML, Leblanc E, Bergeron MG, Dery P, Boivin G (2006) Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis 193:54–58
Glezen WP, Taber LH, Frank AL, Kasel JA (1986) Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140:543–546
Graham BS, Bunton LA, Wright PF, Karzon DT (1991) Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 88:1026–1033
Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC (1993) Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med 329:1524–1530
Hall CB, Powell KR, Macdonald NE, Gala CL, Menegus ME, Suffin SC, Cohen HJ (1986) Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med 315:77–81
Hall CB, Walsh EE, Schnabel KC, Long CE, McConnochie KM, Hildreth SW, Anderson LJ (1990) Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis 162:1283–1290
Hall CB, Walsh EE, Long CE, Schnabel KC (1991) Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698
Heidema J, Lukens MV, van Maren WW, van Dijk ME, Otten HG, van Vught AJ, van der Werff DB, van Gestel SJ, Semple MG, Smyth RL, Kimpen JL, van Bleek GM (2007) CD8 + T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol 179:8410–8417
Hemming VG, Prince GA (1990) Immunoprophylaxis of infections with respiratory syncytial virus: observations and hypothesis. Rev Infect Dis 12:S470–S475
Hendry RM, Burns JC, Walsh EE, Graham BS, Wright PF, Hemming VG, Rodriguez WJ, Kim HW, Prince GA, McIntosh K (1988) Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis 157:640–647
Johnson PR, Collins PL (1988) The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol 69(Pt 10):2623–2628
Johnson PR, Olmsted RA, Prince GA, Murphy BR, Alling DW, Walsh EE, Collins PL (1987a) Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol 61:3163–3166
Johnson PR, Spriggs MK, Olmsted RA, Collins PL (1987b) The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A 84:5625–5629
Jones LP, Zheng HQ, Karron RA, Peret TC, Tsou C, Anderson LJ (2002) Multiplex assay for detection of strain-specific antibodies against the two variable regions of the G protein of respiratory syncytial virus. Clin Diagn Lab Immunol 9:633–638
Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, Harris DO, Randolph VB, Udem SA, Murphy BR, Sidhu MS (1997) Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A 94:13961–13966
Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH (1969) Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434
Knossow M, Skehel JJ (2006) Variation and infectivity neutralization in influenza. Immunology 119:1–7
Kuroiwa Y, Nagai K, Okita L, Yui I, Kase T, Nakayama T, Tsutsumi H (2005) A phylogenetic study of human respiratory syncytial viruses group A and B strains isolated in two cities in Japan from 1980–2002. J Med Virol 76:241–247
Lopez JA, Bustos R, Orvell C, Berois M, Arbiza J, Garcia-Barreno B, Melero JA (1998) Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J Virol 72:6922–6928
Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, Ho SB, Peebles RS Jr (2006) Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol 169:977–986
Magro M, Andreu D, Gomez-Puertas P, Melero JA, Palomo C (2010) Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein. J Virol 84:7970–7982
Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C (2012) Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A 109:3089–3094
Martinello RA, Chen MD, Weibel C, Kahn JS (2002) Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis 186:839–842
Martinez I, Melero JA (1998) Enhanced neutralization of human respiratory syncytial virus by mixtures of monoclonal antibodies to the attachment (G) glycoprotein. J Gen Virol 79(Pt 9):2215–2220
Martinez I, Dopazo J, Melero JA (1997) Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J Gen Virol 78(Pt 10):2419–2429
Matheson JW, Rich FJ, Cohet C, Grimwood K, Huang QS, Penny D, Hendy MD, Kirman JR (2006) Distinct patterns of evolution between respiratory syncytial virus subgroups A and B from New Zealand isolates collected over thirty-seven years. J Med Virol 78:1354–1364
Mazzulli T, Peret TC, McGeer A, Cann D, MacDonald KS, Chua R, Erdman DD, Anderson LJ (1999) Molecular characterization of a nosocomial outbreak of human respiratory syncytial virus on an adult leukemia/lymphoma ward. J Infect Dis 180:1686–1689
Melero JA, Garcia-Barreno B, Martinez I, Pringle CR, Cane PA (1997) Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol 78(Pt 10):2411–2418
Moore ML, Peebles RS Jr (2006) Respiratory syncytial virus disease mechanisms implicated by human, animal model, and in vitro data facilitate vaccine strategies and new therapeutics. Pharmacol Ther 112:405–424
Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE Jr, Goleniewska K, Williams JV, Collins PL, Peebles RS Jr (2009) A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol 83:4185–4194
Muelenaer PM, Henderson FW, Hemming VG, Walsh EE, Anderson LJ, Prince GA, Murphy BR (1991) Group-specific serum antibody responses in children with primary and recurrent respiratory syncytial virus infections. J Infect Dis 164:15–21
Mufson MA, Orvell C, Rafnar B, Norrby E (1985) Two distinct subtypes of human respiratory syncytial virus. J Gen Virol 66(Pt 10):2111–2124
Murata Y, Lightfoote PM, Biear JN, Falsey AR, Walsh EE (2010a) Humoral response to the central unglycosylated region of the respiratory syncytial virus attachment protein. Vaccine 28:6242–6246
Murata Y, Lightfoote PM, Falsey AR, Walsh EE (2010b) Identification of and human serum reactogenicity to neutralizing epitopes within the central unglycosylated region of the respiratory syncytial virus attachment protein. Clin Vaccine Immunol 17:695–697
Murphy BR, Olmsted RA, Collins PL, Chanock RM, Prince GA (1988) Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol 62:3907–3910
Murphy BR, Prince GA, Collins PL, Hildreth SW, Paradiso PR (1991) Effect of passive antibody on the immune response of cotton rats to purified F and G glycoproteins of respiratory syncytial virus (RSV). Vaccine 9:185–189
Olmsted RA, Elango N, Prince GA, Murphy BR, Johnson PR, Moss B, Chanock RM, Collins PL (1986) Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A 83:7462–7466
Palomo C, Cane PA, Melero JA (2000) Evaluation of the antibody specificities of human convalescent-phase sera against the attachment (G) protein of human respiratory syncytial virus: influence of strain variation and carbohydrate side chains. J Med Virol 60:468–474
Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ (1998) Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol 79(Pt 9):2221–2229
Peret TC, Hall CB, Hammond GW, Piedra PA, Storch GA, Sullender WM, Tsou C, Anderson LJ (2000) Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis 181:1891–1896
Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H, Lirman DD, Rabold R, Hoffman SJ, Karp CL, Kleeberger SR, Wills-Karp M, Karron RA (2002) A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med 196:859–865
Prince GA, Porter DD (1975) The pathogenesis of respiratory syncytial virus infection in infant ferrets. Am J Pathol 82:339–352
Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM (1983) Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun 42:81–87
Prince GA, Horswood RL, Chanock RM (1985) Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol 55:517–520
Reiche J, Schweiger B (2009) Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J Clin Microbiol 47:1800–1810
Rueda P, Delgado T, Portela A, Melero JA, Garcia-Barreno B (1991) Premature stop codons in the G glycoprotein of human respiratory syncytial viruses resistant to neutralization by monoclonal antibodies. J Virol 65:3374–3378
Rueda P, Garcia-Barreno B, Melero JA (1994) Loss of conserved cysteine residues in the attachment (G) glycoprotein of two human respiratory syncytial virus escape mutants that contain multiple A-G substitutions (hypermutations). Virology 198:653–662
Rueda P, Palomo C, Garcia-Barreno B, Melero JA (1995) The three C-terminal residues of human respiratory syncytial virus G glycoprotein (Long strain) are essential for integrity of multiple epitopes distinguishable by antiidiotypic antibodies. Viral Immunol 8:37–46
Sastre P (2004) Caracterización de anticuerpos dirigidos frente al virus respiratorio sincitial humano presentes en preparaciones de inmunoglobulinas humanas. PhD Thesis
Sastre P, Melero JA, Garcia-Barreno B, Palomo C (2004) Comparison of antibodies directed against human respiratory syncytial virus antigens present in two commercial preparations of human immunoglobulins with different neutralizing activities. Vaccine 23:435–443
Scott PD, Ochola R, Ngama M, Okiro EA, Nokes DJ, Medley GF, Cane PA (2004) Molecular epidemiology of respiratory syncytial virus in Kilifi district, Kenya. J Med Virol 74:344–354
Scott PD, Ochola R, Sande C, Ngama M, Okiro EA, Medley GF, Nokes DJ, Cane PA (2007) Comparison of strain-specific antibody responses during primary and secondary infections with respiratory syncytial virus. J Med Virol 79:1943–1950
Shobugawa Y, Saito R, Sano Y, Zaraket H, Suzuki Y, Kumaki A, Dapat I, Oguma T, Yamaguchi M, Suzuki H (2009) Emerging genotypes of human respiratory syncytial virus subgroup A among patients in Japan. J Clin Microbiol 47:2475–2482
Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS Jr, Moore ML (2011) Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 85:5782–5793
Stott EJ, Ball LA, Young KK, Furze J, Wertz GW (1986) Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol 60:607–613
Stott EJ, Taylor G, Ball LA, Anderson K, Young KK, King AM, Wertz GW (1987) Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol 61:3855–3861
Suto T, Yano N, Ikeda M, Miyamoto M, Takai S, Shigeta S, Hinuma Y, Ishida N (1965) Respiratory syncytial virus infection and its serologic epidemiology. Am J Epidemiol 82:211–224
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Taylor G, Stott EJ, Bew M, Fernie BF, Cote PJ, Collins AP, Hughes M, Jebbett J (1984) Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology 52:137–142
Taylor GS, Vipond IB, Caul EO (2001) Molecular epidemiology of outbreak of respiratory syncytial virus within bone marrow transplantation unit. J Clin Microbiol 39:801–803
Tome L, Frabasile S, Candia C, Pittini A, Farina N, Melero JA, Arbiza J (2012) Selection and characterization of human respiratory syncytial virus escape mutants resistant to a polyclonal antiserum raised against the F protein. Arch Virol 157(6):1071–1080
Trento A, Galiano M, Videla C, Carballal G, Garcia-Barreno B, Melero JA, Palomo C (2003) Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J Gen Virol 84:3115–3120
Trento A, Viegas M, Galiano M, Videla C, Carballal G, Mistchenko AS, Melero JA (2006) Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J Virol 80:975–984
Trento A, Casas I, Calderon A, Garcia-Garcia ML, Calvo C, Perez-Brena P, Melero JA (2010) Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol 84:7500–7512
Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, Garcia-Sastre A (2005) Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77–80
Venter M, Madhi SA, Tiemessen CT, Schoub BD (2001) Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J Gen Virol 82:2117–2124
Venter M, Collinson M, Schoub BD (2002) Molecular epidemiological analysis of community circulating respiratory syncytial virus in rural South Africa: comparison of viruses and genotypes responsible for different disease manifestations. J Med Virol 68:452–461
Viegas M, Mistchenko AS (2005) Molecular epidemiology of human respiratory syncytial virus subgroup A over a six-year period (1999–2004) in Argentina. J Med Virol 77:302–310
Villenave R, Thavagnanam S, Sarlang S, Parker J, Douglas I, Skibinski G, Heaney LG, McKaigue JP, Coyle PV, Shields MD, Power UF (2012) In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci U S A 109:5040–5045
Walsh EE, Schlesinger JJ, Brandriss MW (1984) Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun 43:756–758
Walsh EE, Falsey AR, Sullender WM (1998) Monoclonal antibody neutralization escape mutants of respiratory syncytial virus with unique alterations in the attachment (G) protein. J Gen Virol 79(Pt 3):479–487
Wertz GW, Stott EJ, Young KK, Anderson K, Ball LA (1987) Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol 61:293–301
Woelk CH, Holmes EC (2001) Variable immune-driven natural selection in the attachment (G) glycoprotein of respiratory syncytial virus (RSV). J Mol Evol 52:182–192
Yamaguchi M, Sano Y, Dapat IC, Saito R, Suzuki Y, Kumaki A, Shobugawa Y, Dapat C, Uchiyama M, Suzuki H (2011) High frequency of repeated infections due to emerging genotypes of human respiratory syncytial viruses among children during eight successive epidemic seasons in Japan. J Clin Microbiol 49:1034–1040
Zhang Y, Xu W, Shen K, Xie Z, Sun L, Lu Q, Liu C, Liang G, Beeler JA, Anderson LJ (2007) Genetic variability of group A and B human respiratory syncytial viruses isolated from 3 provinces in China. Arch Virol 152:1425–1434
Zhang RF, Jin Y, Xie ZP, Liu N, Yan KL, Gao HC, Song JR, Yuan XH, Xiao NG, Guo MW, Zhou QH, Hou YD, Duan Z (2010a) Human respiratory syncytial virus in children with acute respiratory tract infections in China. J Clin Microbiol 48:4193–4199
Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA (2010b) Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157
Zhang ZY, Du LN, Chen X, Zhao Y, Liu EM, Yang XQ, Zhao XD (2010c) Genetic variability of respiratory syncytial viruses (RSV) prevalent in Southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J Clin Microbiol 48:1201–1207
Zhao X, Chen FP, Megaw AG, Sullender WM (2004a) Variable resistance to palivizumab in cotton rats by respiratory syncytial virus mutants. J Infect Dis 190:1941–1946
Zhao X, Chen FP, Sullender WM (2004b) Respiratory syncytial virus escape mutant derived in vitro resists palivizumab prophylaxis in cotton rats. Virology 318:608–612
Zlateva KT, Vijgen L, Dekeersmaeker N, Naranjo C, Van Ranst M (2007) Subgroup prevalence and genotype circulation patterns of human respiratory syncytial virus in Belgium during ten successive epidemic seasons. J Clin Microbiol 45:3022–3030
Acknowledgments
Work in the Madrid laboratory is currently funded by grants GR09/0039 from Instituto de Salud Carlos III and SAF2009-11632 and SAF2012-31217 from Plan Nacional de I+D+i. Work in the Atlanta laboratory is supported by the following grants: NIH 1R01AI087798 and NIH 1U19AI095227.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Melero, J.A., Moore, M.L. (2013). Influence of Respiratory Syncytial Virus Strain Differences on Pathogenesis and Immunity. In: Anderson, L., Graham, B. (eds) Challenges and Opportunities for Respiratory Syncytial Virus Vaccines. Current Topics in Microbiology and Immunology, vol 372. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38919-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-38919-1_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38918-4
Online ISBN: 978-3-642-38919-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)