Abstract
Orthodontic treatment using a multibracket appliance is the most popular and precise method for correcting misaligned dentition. We have been working to replace a conventional orthodontic metal wire with a fiber reinforced thermo-plastic (FRP) wire and have developed an esthetic transparent FRP wire containing biocompatible glass fibers and poly (methyl methacrylate) (PMMA). However, loss of mechanical properties carried by failure related with glass fibers has not yet been resolved. Meanwhile, we are working on developing a super-fiber (high-strength and high-modulus fibers) reinforced thermo-plastic wire; for this we will have to focus on improving the stiffness of PMMA without causing any deterioration in its flexibility. Transparent PMMA/layered silicate nanocomposites were fabricated by a solution intercalation method. Montmorillonite, organically modified with quaternary alkylammonium ions, was selected as the filler for reinforcement. The nanocomposites are transparent enough for their use as esthetic orthodontic wires. The flexural modulus of them was favorably improved. Those results encourage us to make them applicable for other dental use. To the best of our knowledge, there has been little or no research on polymer/layered silicate nanocomposites in the field of dentistry. A novelty and an application potentiality of our challenge are beneficial for evolving dentistry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Esthetic preference of dental patients is one of the most important factors necessary for developing dental materials, and orthodontic appliances are no exception. A multibracket appliance is the most popular and precise method for correcting misaligned dentition (Fig. 15.1). Brackets are bonded to teeth and wires are fastened to them, and then, forces exerted from the wire for tooth movement transmit to each tooth through the intervention of brackets. Until recently, both brackets and wires were made of metals. To make these fixed appliances more esthetically acceptable, brackets made from either polymers or ceramics have been introduced. However, wires are still metallic. Consequently, a somewhat unnatural facial appearance caused due to the metal wires discourages many potential patients from opting for this treatment.
As for an overview of the present status of orthodontic material usage, as stated above, commercialized esthetic products exclusively are plastic and ceramic brackets. Major raw materials of the currently available plastic brackets are mostly polycarbonate, and the others are polyurethane, polyoxymethylene, polyamide, or something else [1]. Such preference for bracket materials is probably based on their izod impact strength. Poly(methyl methacrylate) (PMMA), with very low izod impact strength, is not preferred as a bracket material for this reason. However, brackets, if being fabricated only by those raw materials, result in excessive creep deformation when subjected to torsional loads generated by wires. Currently available plastic brackets are improved by reinforcing with ceramic or glass-fiber fillers and/or metallic insertion onto bases of slots for wire setting [2]. Nevertheless, they still have not sufficient strength and wear resistance and have problems due to intraoral plasticization and softening. Moreover, polyoxymethylene has another anxiety about possible formaldehyde release associated with degradation [1]. Meanwhile, ceramic brackets manufactured from polycrystalline alumina, single-crystal alumina, and polycrystalline zirconia provide higher strength and wear resistance than those of plastic brackets. They also have superior esthetics and better color stability. Recently, in addition to less esthetic appearances due to opaque color, no significant advantage of zirconia brackets over polycrystalline alumina brackets in regard to their frictional properties has been reported [2]. Therefore, zirconia brackets have become obsolete. Although ceramic brackets are more attractive than plastic brackets, they have some disadvantages such as low toughness and severe iatrogenic enamel wear of the opposing teeth when they are placed in the lower dentition.
As mentioned above, durability and toughness are emphasized in case of brackets, whereas mechanical properties such as stiffness and elasticity play a much more prominent role in orthodontic wires as well as durability. This is not only due to technical requirements but also related to duration of use. The standard period of time which is normally required to complete an orthodontic treatment using a multibracket appliance is at least 2–3 years. Each bracket is fixed to individual tooth all the while as an intermediate device of orthodontic force generated by wires. In contrast, each wire is ordinarily supposed to be replaced with new one once a month. Thus, PMMA was inevitably selected for our study to develop an esthetic orthodontic wire because of its superior intrinsic flexural modulus, surface hardness, chemical stability, and formability among engineering plastics. Polycarbonate, which has comparable modulus and transparency to PMMA, is a possible candidate material; however, it was not selected by reason of some disadvantages such as inferior formability and a risk of a dioxin emission by incineration.
The intrinsic Young’s modulus of PMMA, about 2–3 GPa, is too low to use as an orthodontic wire when it is thermoformed with PMMA alone. We have been working to replace conventional orthodontic metal wires with transparent or translucent wires and have already developed an esthetic transparent glass-fiber reinforced plastic (GFRP) wire containing biocompatible continuous glass fibers oriented in the direction of the long axis and poly(methyl methacrylate) [3–7]. They are 0.5 mm in diameter and their Young’s modulus is adjustable in the range from 20 to 40 GPa by changing the volume fraction of glass fibers and is adequate for the middle and latter stages of orthodontic treatment. The form of dentition should have been well arranged to a considerable degree as seen in Fig. 15.2a until these stages; any excessive bend is not needed for the wires. In the initial stage of orthodontic treatment, wires should be warped or bent with small radius of curvature due to varying degrees of tooth crowding (Fig. 15.2b). Therefore, if the GFRP wire is used in the initial treatment stage, loss of mechanical properties carried by failure related with glass fibers occurs.
A desired load range to ensure the movement of individual tooth at the initial stage is around 1 N or less. In case of conventional metal wires, a thinner wire can be chosen depending on the degree of teeth misalignment to make it exert adequate load level. Unfortunately, to make our GFRP wires thinner than present products should not be recommended from the aspect of possible breakage risks. For these reasons, we are working on developing a super-fiber (high-strength and high-modulus fibers) reinforced plastic wire; for this we will have to take note of the improvement of the stiffness of PMMA without causing any deterioration in its flexibility. For this idea to work, we focused on nanocomposites. Polymer/layered silicate nanocomposites have attracted considerable interest in the past decade due to their enhanced mechanical and thermal properties as compared to homopolymer or other fillers. However, to the best of our knowledge, there has been little or no research on polymer/layered silicate nanocomposites in the field of dentistry [8–10].
In recent years, montmorillonite (MMT), which is a typical swelling clay mineral, has been the most commonly used reinforcement in polymer nanocomposites [11, 12]. The chemical structure of MMT consists of an edge-shaped octahedral sheet of aluminum hydroxide or magnesium hydroxide sandwiched between two tetrahedral silica sheets. Several studies on PMMA/MMT nanocomposites have been conducted to date [13–16]. However, to the best of our knowledge, there are no previous reports on PMMA/MMT nanocomposites prepared by a solution intercalation method [17–20]. In this chapter, we introduce an outline of the preparation and some characterization of PMMA/organically modified MMT (OMMT) nanocomposites.
2 Preparation and Property Test of the PMMA/OMMT Nanocomposites
Some chemical and mechanical methods, mainly polymerization of an intercalated monomer or melt intercalation, have been utilized to enhance expansion of the d-spacing of layered silicates and to achieve the optimum dispersion quality [10, 13–16, 21–23]. However, it is not always possible for some research institutes to conduct such methods needing large-scale equipments. On the other hand, since a simplified and efficient method at low cost is more and more expected, a solution intercalation method is thought to be preferable for experimental stages.
2.1 Preparation of the Nanocomposites
Organically modified montmorillonite (OMMT) was prepared by an ion exchange reaction between MMT and quaternary alkylammonium ion using dimethyldistearylammonium salt. PMMA (ACRYPETTM MD) was purchased from Mitsubishi Rayon (Tokyo, Japan). The grade MD has adequate mechanical properties and a good melt flow rate (6.0 g/10 min at the temperature 503.15 K, 37.7 N) for our purpose.
PMMA not only has desirable physical and mechanical properties but also has superior properties such as esthetic merits, durability, and usability. It is one of the most widely used polymer materials in dental practices, e.g., dental prostheses such as dentures and temporary crowns, adhesives, and removable orthodontic appliances. PMMA is conveniently highly soluble in acetone, an aprotic polar solvent. More importantly, the viscosity of a mixture of dissolved PMMA does not increase easily even if MMT is added. Therefore, the relatively low shear force applied to the mixture was reasonably sufficient to enable good dispersion of OMMT in the solution. The entire fabricating process of the PMMA/OMMT nanocomposites is outlined in Fig. 15.3.
2.2 Property Tests of the Nanocomposite Specimens
Both X-ray diffraction (XRD) analysis and transmission electron microscopy (TEM) observation are essential in the microstructural investigation of the nanocomposites.
Transparency is one of the most important properties for the nanocomposites to be applied as some dental materials. We use a haze meter to measure total light transmittance (Tt), parallel light transmittance (Tp), and haze and usually employ an illuminant C as a light source. Haze is defined as the proportion of diffuse transmittance to Tt. Here, the diffusion light is defined as refracting light that deviates more than 2.5° from the parallel light.
Coordination between strength and flexibility of polymer materials is also one of the most important concerns for dental use. Three-point transverse tests (span, 20 mm; crosshead speed, 1 mm/min) are necessary to evaluate the mechanical properties.
3 Characterization of the PMMA/OMMT Nanocomposites
3.1 Intercalation of OMMT with Polymer Molecules
Recently, studies using the solution intercalation method are occasionally reported as a valid method for intercalation. As we know, montmorillonite (MMT), hydrophilic in nature, should be organically modified before being blended with polymer in order to increase its layer spaces and to promote hydrophobicity especially when a solution intercalation method is used. The swelling power of the organically modified MMT (OMMT) we used is 35.0 ml/2 g, which is comparable to that of natural hydrophilic bentonite.
As seen in Fig. 15.4, the XRD pattern of the original OMMT showed a peak at 2θ = 7.6°, whereas the PMMA/OMMT samples showed a peak at 2θ = 5.4°. The spacing of 1.63 nm, as calculated by Bragg’s formula, obtained from the nanocomposite samples is inadequate to conclude that the peak is really (001) d-spacing [24]. The shift of 2θ value of the peaks toward lower implies a diffusion of polymer chains into the interlayer regions of OMMT. A small angle scattering should be analyzed after this.
The TEM micrograph revealed well-intercalated and partially exfoliated structures of the OMMT (Fig. 15.5). The expansion is probably due to solvation of acetone with long chains or benzene rings of quaternary alkylammonium ion. The stacked silicate layers due to their cohesive force dispersed randomly in the PMMA matrix. These features are mostly affected by both the hydrogen bond as an interaction at edge surfaces and the perpendicular bonding owing to a rearrangement of negatively charged basal plane surfaces and positively charged edge surfaces of silicate layers. Such the inhomogeneous dispersions due to the hydrogen bond and the electrostatic force could be mitigated by silanating the OMMT.
3.2 Retention of Transparency with Varying Contents
Our PMMA/OMMT nanocomposites have favorable macroscopic transparency as shown in Fig. 15.6. Although the translucency increases in shades of brown in accordance with the weight fraction of OMMT, the appearance might be adequate for their use as esthetic orthodontic wires.
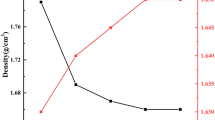
The total light transmittance (Tt), parallel light transmittance (Tp), and the haze are unique quantification (Fig. 15.7). Although they all do not directly indicate the degree of transparency and the relative rate of Tp decreases with increasing amount of OMMT, it is considered to be reasonable that the relatively small deterioration that accompanies the increase of OMMT content renders it almost macroscopically equal transparency to that of the original PMMA.
3.3 Strengthening of PMMA by Intercalated Platelets
The flexural modulus of the PMMA/OMMT nanocomposites increased successfully and almost linearly, relative to that of pristine PMMA, with increasing amount of OMMT content (Fig. 15.8). The improvement in reinforcement may possibly be due to shear deformation and stress transfer to the silicate layers that have exceptionally high tensile modulus. Both an anisotropic morphology of the crystals and an improved wettability by organic modification with PMMA contribute to this phenomenon. Therefore, it is considered that by raising the swelling power of the OMMT or by silanating them, the mechanical properties of the nanocomposites will improve substantially. This would be accompanied by an increase volume of intercalated polymer chains or an increase in the number of exfoliated silicate layers. Further studies will target direct evidence of this.
4 Future Prospects
Introduction of an entirely new concept in development of esthetic orthodontic wires is just beginning. However, from our initial experimental data, PMMA/OMMT nanocomposites show considerable promise as the esthetic wire in the near future. Moreover, PMMA is a preferable biomaterial also in terms of safety such that it does not easily be degraded, and undesirable potential effects of benzene rings are inhibited by the use of it.
Esthetic transparent FRP wires, as Eliades had remarked, will be commercially available as a combination of GFRP structure wires and super-fiber reinforced PMMA/OMMT nanocomposite structure ones during the next several years if the industry finds that introducing them to the market will be profitable.
5 Conclusions
For a technological advancement and an application in polymer nanocomposites of layered silicates, poly(methyl methacrylate)/organically modified montmorillonite (PMMA/OMMT) nanocomposites were prepared using a solution intercalation method. It was found that the polarity, viscosity, and hydrophile-lipophile properties affect the dispersion of the silicate platelets, intercalation of the PMMA molecules into the silicate galleries, and partial exfoliation of the OMMT. It is also noteworthy that the nanocomposites appear to remain stable even though they have been processed by compression molding at temperature 503.15 K. In addition to the good mechanical properties and the optical properties, human body-friendly and environmentally friendly properties of both layered silicates and PMMA are encouraging for their application to esthetic transparent orthodontic wires and many other uses for dental practice. A novelty and an application potentiality of our challenge are beneficial for evolving dentistry.
References
Eliades T (2007) Am J Orthod Dentofacial Orthop 131:253
Russell JS (2005) J Orthod 32:146
Watari F, Kobayashi M, Yamagata S, Nagayama K, Imai T, Nakamura S (1996) Biomechanics 9:469
Imai T, Watari F, Yamagata S, Kobayashi M, Nagayama K, Toyoizumi H, Nakamura S (1998) Biomaterials 19:2195
Watari F, Yamagata S, Imai T, Kobayashi M, Nakamura S (1998) J Mater Sci 33:5661
Imai T, Yamagata S, Watari F, Kobayashi M, Nagayama K, Toyoizumi H, Uga M, Nakamura S (1999) Dent Mater J 18:167
Imai T, Watari F, Yamagata S, Kobayashi M, Nagayama K, Nakamura S (1999) Am J Orthod Dentofacial Orthop 116:533
Dowling AH, Stamboulis A, Fleming GJP (2006) J Dent 34:802
Dowling AH, Fleming GJP (2007) J Dent 35:309
Atai M, Solhi L, Nodehi A, Mirabedini SM, Kasraei S, Akbari K, Babanzadeh S (2009) Dent Mater 25:339
Fornes TD, Paul DR (2003) Polymer 44:4993
Zhang K, Park BJ, Fang FF, Choi HJ (2009) Molecules 14:2095
Zeng C, Lee LJ (2001) Macromolecules 34:4098
Huang X, Brittain WJ (2001) Macromolecules 34:3255
Meneghetti P, Qutubuddin S (2004) Langmuir 20:3424
Fan X, Xia C, Advincula RC (2005) Langmuir 21:2537
Liu L, Song L, Hu Y, Chen H (2006) Polym Compos 27:660
Marras SI, Kladi KP, Tsivintzelis I, Zuburtikudis I, Panayiotou C (2008) Acta Biomater 4:756
Yamagata S, Akasaka T, Uo M, Ushijima N, Nodasaka Y, Iida J, Watari F (2009) Nano Biomed 1:151
Yamagata S, Hamba Y, Akasaka T, Uo M, Iida J, Watari F (2011) Nano Biomed 3:217
Okamoto M, Morita S, Taguchi H, Kim YH, Kotaka T, Tateyama H (2000) Polymer 41:3887
Meneghetti P, Qutubuddin S (2005) J Colloid Interface Sci 288:387
Hossain MD, Kim WS, Hwang HS, Lim KT (2009) J Colloid Interface Sci 336:443
Messersmith PB, Giannelis EP (1994) Chem Mater 6:1719
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Yamagata, S., Iida, J., Watari, F. (2014). FRP Esthetic Orthodontic Wire and Development of Matrix Strengthening with Poly(methyl methacrylate)/Montmorillonite Nanocomposite. In: Pandey, J., Reddy, K., Mohanty, A., Misra, M. (eds) Handbook of Polymernanocomposites. Processing, Performance and Application. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38649-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-642-38649-7_19
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38648-0
Online ISBN: 978-3-642-38649-7
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)