Abstract
Nonfunctioning pituitary tumours account for one-third of all pituitary neoplasms, with an incidence of 7–9 new cases/106 every year. They are slow-growing benign monoclonal adenomas that do not cause any hormone hypersecretion syndromes. Symptoms arise from local compression of normal pituitary tissue and surrounding structures. The aim of treatment is the preservation of residual pituitary function and alleviation of local compressive mass effects. The available published series show that nonfunctioning adenomas constitute the underlying diagnosis in 45 % of patients with pituitary apoplexy. Apoplexy occurs more often in macroadenomas, men and with antithrombotic use. Headache, ophthalmoplegia, reduced visual acuity and visual filed defects are common presenting features. ACTH, TSH and gonadotropin deficiencies are frequently present and need to be assessed biochemically in patients with suspected pituitary apoplexy. A low serum prolactin at presentation has been linked with the severity of hypopituitarism postsurgical decompression. Patients with nonfunctioning pituitary adenomas who present with apoplexy have reduced 5-year rates of tumour regrowth (11 %) compared to non-apoplectic surgically treated nonirradiated nonfunctioning pituitary adenomas (18–34 % in different series). In those patients operated for pituitary apoplexy, tumour recurrence seems to be more common in patients with residual post-operative tumour.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Nonfunctioning Pituitary Adenomas
1.1 Definition, Incidence and Prevalence
Nonfunctioning pituitary adenomas (NFPAs) are slow-growing, benign, monoclonal adenomas characterized by the absence of clinical and biochemical evidence of pituitary hormonal overproduction. Immunohistochemistry shows that the majority of clinically nonfunctioning adenomas consist of cells staining positive for pituitary hormones (40–65 % for gonadotropins or their subunits, 10 % for ACTH and less for other hormones), whereas in 20–40 % the adenoma cells are immunohistochemically negative (Asa et al. 1986; Croue et al. 1992; Zhao et al. 2000; Dekkers et al. 2006). They account for one-third of all pituitary neoplasms, with an incidence of 7–9 new cases/106 every year. In the general population, the estimated prevalence of pituitary adenomas is 9.3 % with a range of 1.5–26.7 % (Hall et al. 1994; Molitch 2008; Orija et al. 2012). The prevalence of pituitary macroadenomas (diameter >1 cm) is lower and estimated to be only approximately 0.11–0.3 % (Orija et al. 2012). Pituitary microadenomas (diameter <1 cm) are more often functioning, compared with macroadenomas that tend to be nonfunctioning in 80 % of the patients (McComb et al. 1983; Sanno et al. 2003; Fernández-Balsells et al. 2011).
1.2 Clinical Signs and Symptoms
The clinical signs and symptoms of clinically nonfunctioning macroadenomas are determined by mass effects of the tumour. Presenting complaints include headache, visual field defects with or without decreased visual acuity and effects of hypopituitarism. Other presenting symptoms are apoplexy, cranial nerve deficits and optic nerve atrophy (Molitch 2008; Orija et al. 2012). Headache is present in 40–60 % of all patients and is caused by increased intracranial pressure and stretch of the dura mater (Ironside 2003; Wichers-Rother et al. 2004; Dekkers et al. 2006). Visual field defects result from compression of the optic chiasm (Nielsen et al. 2006). Typically macroadenomas cause a bitemporal visual field defect. Asymmetry of the visual field defects may be present between both eyes, depending on the growth pattern of the tumour. In the majority of patients presenting with nonfunctioning macroadenomas, pituitary insufficiency is present to some degree (Dekkers et al. 2006). As the diagnosis of clinically nonfunctioning pituitary adenomas is made by exclusion of hormone overproduction, the evaluation of the medical history and the physical examination should include a search for signs and symptoms of hormonally active pituitary adenomas such as acromegaly, Cushing’s disease, hypogonadism and hyperprolactinaemia.
1.3 Laboratory Findings
Hypopituitarism in patients with macroadenomas results from compression of the pituitary stalk, compression of functioning pituitary tissue or hypothalamic involvement of the pituitary tumour. In terms of pituitary hormone deficiencies, GH deficiency is most commonly present (85 %), followed by LH and FSH deficiency (75 %), with ACTH and TSH deficiencies occurring less commonly (around 35 %) (Nomikos et al. 2004; Wichers-Rother et al. 2004; Dekkers et al. 2006). In addition to pituitary hormone deficiencies, nonfunctioning macroadenomas can be accompanied by hyperprolactinaemia. The secretion and release of prolactin are inhibited by hypothalamic release of dopamine. When the pituitary stalk is compressed by the presence of a tumour, dopamine delivery to the pituitary is disrupted resulting in hyperprolactinaemia. In general, prolactin levels less than 100 ug/l (or 2,000 mIU/l) are compatible with pituitary stalk compression. Levels above 100 ug/l are almost never encountered in clinically nonfunctioning macroadenomas (Karavitaki et al. 2006; Orija et al. 2012). Macroprolactinomas are typically associated with prolactin levels greater than 250 ug/l (1 ug/l is equivalent to 21.2 mIU/l) (Melmed et al. 2011). The high intrasellar pressure that results from the presence of an adenoma in the rigid sella turcica has been linked to the development of hyperprolactinaemia in pituitary adenomas and the residual pituitary function (Arafah et al. 2000). In contrast, during pituitary apoplexy the sudden development of extremely high intrasellar pressure results in ischaemic necrosis of the gland. This is associated with a low serum prolactin during the apoplectic event and development of pituitary hormone deficiencies postsurgical decompression (Zayour et al. 2004). In the assessment of hyperprolactinaemia, the ‘hook effect’ that may result in only mild to moderate elevation of serum prolactin in the context of a macroprolactinoma must be considered. This artefact can be resolved by diluting the serum sample (1:100) and re-assaying for prolactin measurement (Melmed et al. 2011).
1.4 Imaging
On magnetic resonance imaging (MRI) T1-weighted images, adenomas usually appear hypointense (darker) or isointense relative to normal pituitary tissue. After contrast administration, the adenoma usually remains hypointense, while normal pituitary tissue enhances intensely as pituitary adenomas are less vascular compared with the normal gland (Elster 1993; Chaudhary and Bano 2011; Orija et al. 2012). On delayed scanning a reversal of the image contrast may be observed. A variety of advanced MR techniques have been evolved to help evaluate specific cases. Dynamic contrast MRI is the most reliable tool for the evaluation of pituitary adenomas (Chaudhary and Bano 2011). Computerized tomography (CT) is better than MRI at evaluating bony changes, such as changes of the sella turcica, and calcifications. On both unenhanced and enhanced CT imaging, pituitary adenomas usually appear less attenuated compared to the normal pituitary (Orija et al. 2012).
1.5 Progression
Progression from a NFP microadenoma to macroadenoma is a rare event. The proportion of patients with growth of the macroadenoma ranges between 7 and 51 %, and this probably increases during longer duration of follow-up. Eleven and twenty-nine percent patients may show spontaneous regression of tumour volume occurred during long-term follow-up (Dekkers et al. 2006). Clinically silent tumour ischaemia has been suggested as the mechanism linked to tumour regression. This is also likely be the mechanism leading to symptomatic pituitary apoplexy in NFPAs. During a 5-year follow-up study of incidentally found macroadenomas, apoplexy developed in about 9.5 % of cases (Arita et al. 2006).
NFPA recurrence rates are reported to be 6–46 % after transsphenoidal surgery, whereas after post-operative radiotherapy recurrence rates of 0–36 % are reported (Dekkers et al. 2008). At follow-up the incidence of new pituitary insufficiency in patients with NFPAs is not higher than in patients with prolactinoma or acromegaly. Patients with NFPAs had a lower remission percentage compared with those patients with functioning adenomas (Roelfsema et al. 2012).
1.6 Treatment
The main aims for treatment of patients with clinically nonfunctioning macroadenomas are the preservation or restoration of visual function and adequate long-term tumour control. Transsphenoidal surgery is the treatment of choice in patients with visual field defects because this is the only treatment modality leading to immediate decompression of the optic nerve. Surgery improves visual function in approximately 80 % of all patients (Dekkers et al. 2006). Visual recovery can be demonstrated within the first days after surgery (Jakobsson et al. 2002), although improvement of visual function can continue even until 1 year after surgical treatment (Dekkers et al. 2007). Recovery from headaches is likely to occur after surgery for macroadenomas (Wichers-Rother et al. 2004).
If treated conservatively, regular assessments of pituitary endocrine functions and repeat MRI are recommended because remaining pituitary function can be compromised by growth of the macroadenoma. Thereafter, radiological assessment by MRI is recommended with yearly intervals, which may be extended to two yearly intervals in the absence of progression of the macroadenoma. The interval for visual field assessment depends upon the distance between the pituitary adenoma and the optic chiasm (Dekkers et al. 2008).
Post-operatively, visual function if compromised usually improves. However, often pituitary function does not improve after removal of the adenoma and occasionally it may deteriorate (Comtois et al. 1991; Webb et al. 1999; Nomikos et al. 2004; Wichers-Rother et al. 2004). Radiotherapy is usually not given routinely post-operatively in patients without a tumour remnant because the chance of recurrence in these patients is small (Dekkers et al. 2006; Reddy et al. 2011). When radiotherapy is not routinely offered post-operatively, there does not seem to be a plateau in the incidence of adenoma regrowth, and these patients need to be followed long term with pituitary imaging in order to ensure that no recurrence is missed (Reddy et al. 2011). Conventional external beam radiotherapy is associated with the development of hypopituitarism, secondary brain tumours and optic nerve atrophy (Brada et al. 1993; Tsang et al. 1994; Castinetti et al. 2010; Castro et al. 2010). Gamma knife stereotactic radiosurgery has been used in the post-operative treatment of NFPA resulting in low tumour recurrence rates and high tumour control rates (Castinetti et al. 2010), with improved outcome compared to conventional radiotherapy (Wilson et al. 2012).
Patients with NFPAs need to be referred to an endocrinology team, experienced in managing patients with pituitary tumours. Pituitary hormone replacement needs to be initiated and monitored as appropriate. A multidisciplinary approach between endocrinologists and neurosurgeons (and radiotherapy physicians when indicated) is necessary for patients to decide on the appropriateness of surgical intervention.
2 Pituitary Apoplexy in Pituitary Adenomas
2.1 Incidence of Pituitary Apoplexy in Nonfunctioning Pituitary Adenomas
Pituitary apoplexy is defined as a clinical syndrome characterized by sudden onset headache, vomiting, ophthalmoplegia, visual disturbance and altered consciousness due to acute haemorrhage usually into a pre-existing pituitary adenoma (Rajasekaran et al. 2011). The reported prevalence in published series varies between 0.6 and 10 %, with a mean of 2 % of all surgically resected adenomas (Nawar et al. 2008). Recent epidemiological data from Oxford showed that 7.9 % of pituitary adenomas presented with pituitary apoplexy (Fernandez et al. 2010). Classical pituitary apoplexy has to be differentiated from subclinical apoplexy that goes clinically undetected and is discovered on pituitary imaging or surgical specimens.
Forty-five percent of all pituitary tumours that develop apoplexy are nonfunctioning (Nawar et al. 2008). There is a higher incidence of apoplexy in nonfunctioning tumours compared with other pituitary tumours (Randeva et al. 1999; Sibal et al. 2004; Möller-Goede et al. 2011). In a UK population epidemiological study, 6.2 cases of apoplexy/100,000 population were found and all occurred in nonfunctioning adenomas (Fernandez et al. 2010).
Due to the rarity of apoplectic events, the only available evidence in pituitary apoplexy is from retrospective cohort comparison studies and single centre audits. This limits the systematic assessment of interventions in the management of patients with pituitary apoplexy (Rajasekaran et al. 2011).
2.2 Mechanisms for Development of Apoplexy in Pituitary Adenomas
The blood supply to the anterior pituitary lobe is provided by portal vessels through the infundibulum, where the perfusion pressure is known to be low. Pituitary adenomas seem to be susceptible to increments of intrasellar pressure caused by a tumour therefore creating a relative ischaemic state in the adenoma tissue (Bjerre et al. 1982). It has also been suggested that intrinsic vascular changes in pituitary adenomas may contribute to their susceptibility to infarction and haemorrhage (Cardoso and Peterson 1984).
2.3 Risk Factors for Development of Apoplexy in Nonfunctioning Pituitary Adenomas
Risk factors for the development of pituitary apoplexy are present in 4–50 % (mean 26 %) of patients presenting with apoplexy (Nawar et al. 2008). The risk factors studied more systematically include male gender, shown to predispose in some, but not all studies (Möller-Goede et al. 2011), use of anticoagulants (Möller-Goede et al. 2011) and the presence of a macroadenoma as opposed to a pituitary microadenoma (Murad-Kejbou and Eggenberger 2009). A variety of other precipitating factors have been linked to the occurrence of pituitary apoplexy (Table 4.1) (Nawar et al. 2008; Murad-Kejbou and Eggenberger 2009).
2.4 Clinical, Laboratory and Imaging Assessment in Nonfunctioning Pituitary Adenoma Apoplexy
Pituitary apoplexy constitutes a medical emergency, and all patients presenting with symptoms suggestive of pituitary apoplexy need to be assessed urgently, regarding their pituitary hormone reserve (random cortisol, thyroid function tests, LH, FSH, prolactin, testosterone in men/oestradiol in women, IGF-1, GH), serum electrolytes, renal and liver function, clotting and full blood count (Rajasekaran et al. 2011). Haemodynamic and visual function assessment (visual acuity, visual fields, oculomotor nerves) are mandatory.
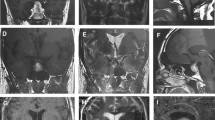
MRI is the radiological investigation of choice and has been found to confirm the diagnosis of pituitary apoplexy in over 90 % of the patients. The appearance on different MRI sequences can be used to date the haemorrhage. In the first 1–2 days post apoplexy, intra-parenchymal haemorrhage is hyperintense on T1-weighted images and hypointense on T2-weighted images (Tosaka et al. 2007; Semple et al. 2008). On days 3–15, haemorrhage appears bright on both T1- and T2-weighted images. After day 15, a fluid level within the haemorrhage may be visualized (Semple et al. 2008). CT scan which is the modality commonly available in the acute setting is diagnostic in a lower proportion of patients, although it can identify macroadenomas (Davis et al. 1985; Semple et al. 2008). In Fig. 4.1, CT and MR imaging from our centre’s archive for a patient who presented with classical apoplexy and was found to have a NFPA is shown.
A 53-year-old man presented to our department with a 2-day history of headache, nausea, vomiting and blurred vision. (a) A haemorrhagic pituitary mass was shown on urgent CT scanning. (b) A pituitary macroadenoma with signs of recent haemorrhage was confirmed on pituitary MRI (T1 sequence with no contrast, white block arrow points to the haemorrhagic area with an intense signal compared to the pituitary adenoma). (c) Two months after the acute event, there was a substantial reduction in the size of the macroadenoma (T1 sequence with no contrast)
2.5 Management of Pituitary Apoplexy
In patients who are haemodynamically unstable, hydrocortisone should be administered after blood sampling for pituitary hormones. Hydrocortisone 100–200 mg as an intravenous bolus is appropriate followed either by 2–4 mg/h by continuous intravenous infusion or by 50–100 mg six hourly by intramuscular injection. Ideally patients should be managed in a Neurosurgical High Dependency Unit, with access to an experienced neurosurgical team and input from endocrinology and ophthalmology. The decision for conservative or surgical management should be taken jointly by neurosurgeons, endocrinologists, ophthalmologists and neuroradiologists in a multidisciplinary manner (Rajasekaran et al. 2011).
In one study, surgery within the first 8 days of presentation was associated with improved visual acuity and visual field defects, compared with surgical management at later stages (Randeva et al. 1999). Surgery by an experienced neurosurgeon in pituitary disease is recommended as opposed to a non-specialist on-call neurosurgical team. Deterioration of the patients visual or neurological function warrants further assessment for surgical intervention (Rajasekaran et al. 2011).
2.6 Recurrence of Apoplectic and Non-apoplectic Nonfunctioning Pituitary Adenoma
Recurrence of NFPA following apoplexy is reported as 10.8 % (4/37 – a mean follow-up of 5.5 years) (Chen et al. 2010), 11.1 % (all patients had incomplete tumour removal following surgery and mean follow-up 6.6 years) (Pal et al. 2011) and 12.4 % in 185 patients with subclinical pituitary adenoma apoplexy (37.3 % of these patients had a nonfunctioning adenoma – mean follow-up 7.4 years) (Zhang et al. 2009).
Non-apoplectic NFPAs not treated with radiotherapy seem to relapse more often compared with apoplectic nonfunctioning adenomas. Recurrence rates that have been reported include 32 % at a mean of 5.4 years (Turner et al. 1999), 34.8 % at a mean time of 6.1 years in a series of 155 patients with relapse in 20.4 % of cases 20 years or longer after the initial operation (Reddy et al. 2011) and 33.5 % at median follow-up of 4.1 years with overall recurrence rates of 24.4 and 51.5 % at 5 and 10 years, respectively (O’Sullivan et al. 2009). The relapse rate at 5 years was 53 % in those with a residual post-operative extrasellar tumour compared with 20 % with an intrasellar tumour (Reddy et al. 2011). The time to regrowth was shorter for those with an extrasellar remnant post-operatively, 3.3 ± 2.17 years for those with a cavernous sinus remnant and 5.3 ± 3.1 years for those without cavernous involvement. There were only two recurrences observed in those who had no residual tumour on the post-operative scans, one at 5.3 years and the other at 25.8 years (Reddy et al. 2011). Whether a conservative versus a surgical approach in nonfunctioning adenomas pituitary apoplexy alters the rate of recurrence is not known.
Nonfunctioning pituitary adenomas with silent ACTH staining do not seem to relapse more often compared with null-cell adenomas (Bradley et al. 2003; Cho et al. 2010; Reddy et al. 2011), although not all cohort studies have shown that (Cooper et al. 2010). However, when they do relapse their course tends to be more aggressive (Bradley et al. 2003; Cho et al. 2010).
3 Ten Practical Tips for Nonfunctioning Pituitary Adenomas and Pituitary Apoplexy
-
1.
Patients with pituitary tumours need to be referred to an endocrinology team experienced in managing patients with pituitary pathology.
-
2.
Pituitary hormone levels need to be assessed and replacement needs to be initiated and monitored as appropriate.
-
3.
Hyperprolactinaemia occurs in NFPAs and prolactinomas; however, prolactin values above 100 ug/l (or 2,000 mIU/l) almost never occur in clinically nonfunctioning macroadenomas.
-
4.
The decision for conservative or surgical management should be taken jointly by neurosurgeons, endocrinologists, ophthalmologists and neuroradiologists in a multidisciplinary manner.
-
5.
Post-operatively, patients with NFPAs need to be assessed and managed as inpatients for pituitary hormone deficiencies. We routinely commence oral hydrocortisone replacement post pituitary surgery pending 9 am serum cortisol assessment.
-
6.
Patients who have been diagnosed with pituitary tumour should be given clear information regarding the signs and symptoms of pituitary apoplexy and the precipitating factors (Rajasekaran et al. 2011).
-
7.
In patients with suspected pituitary apoplexy who are haemodynamically unstable, intravenous hydrocortisone should be administered after drawing blood samples for baseline endocrine function tests.
-
8.
Patients with pituitary apoplexy should be managed in a Neurosurgical High Dependency Unit with access to an experienced neurosurgical team and input from endocrinology and ophthalmology specialists.
-
9.
Patients with pituitary apoplexy and severe neuro-ophthalmic signs such as severely reduced visual acuity, severe and persistent or deteriorating visual field defects or deteriorating level of consciousness should be considered for surgical management (Rajasekaran et al. 2011).
-
10.
Non-apoplectic NFPAs not treated with radiotherapy seem to relapse more often compared with apoplectic nonfunctioning adenomas. Patients need to have long-term follow-up with clinical assessment and imaging.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- CT:
-
Computerized tomography
- FSH:
-
Follicle-stimulating hormone
- GH:
-
Growth hormone
- IGF-1:
-
Insulin-like growth factor 1
- LH:
-
Luteinizing hormone
- MRI:
-
Magnetic resonance imaging
- NFPAs:
-
Nonfunctioning pituitary adenomas
- PRL:
-
Prolactin
- TSH:
-
Thyroid-stimulating hormone
References
Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab. 2000;85:1789–93.
Arita K, Tominaga A, Sugiyama K, Eguchi K, Iida K, Sumida M, Migita K, Kurisu K. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg. 2006;104:884–91.
Asa SL, Gerrie BM, Singer W, Horvath E, Kovacs K, Smyth HS. Gonadotropin secretion in vitro by human pituitary null cell adenomas and oncocytomas. J Clin Endocrinol Metab. 1986;62:1011–9.
Bjerre P, Gyldensted C, Riishede J, Lindholm J. The empty sella and pituitary adenomas. A theory on the causal relationship. Acta Neurol Scand. 1982;66:82–92.
Brada M, Rajan B, Traish D, Ashley S, Holmes-Sellors PJ, Nussey S, Uttley D. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38:571–8.
Bradley KJ, Wass JA, Turner HE. Non-functioning pituitary adenomas with positive immunoreactivity for ACTH behave more aggressively than ACTH immunonegative tumours but do not recur more frequently. Clin Endocrinol (Oxf). 2003;58:59–64.
Cardoso ER, Peterson EW. Pituitary apoplexy: a review. Neurosurgery. 1984;14:363–73.
Castinetti F, Regis J, Dufour H, Brue T. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat Rev Endocrinol. 2010;6:214–23.
Castro DG, Cecilio SA, Canteras MM. Radiosurgery for pituitary adenomas: evaluation of its efficacy and safety. Radiat Oncol. 2010;5:109.
Chaudhary V, Bano S. Imaging of the pituitary: recent advances. Indian J Endocrinol Metab. 2011;15 Suppl 3:S216–23.
Chen L, White WL, Spetzler RF, Xu B. A prospective study of nonfunctioning pituitary adenomas: presentation, management, and clinical outcome. J Neurooncol. 2010;102:129–38.
Cho HY, Cho SW, Kim SW, Shin CS, Park KS, Kim SY. Silent corticotroph adenomas have unique recurrence characteristics compared with other nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 2010;72:648–53.
Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer. 1991;68:860–6.
Cooper O, Ben-Shlomo A, Bonert V, Bannykh S, Mirocha J, Melmed S. Silent corticogonadotroph adenomas: clinical and cellular characteristics and long-term outcomes. Horm Cancer. 2010;1:80–92.
Croue A, Beldent V, Rousselet MC, Guy G, Rohmer V, Bigorgne JC, Saint-Andre JP. Contribution of immunohistochemistry, electron microscopy, and cell culture to the characterization of nonfunctioning pituitary adenomas: a study of 40 cases. Hum Pathol. 1992;23:1332–9.
Davis PC, Hoffman Jr JC, Tindall GT, Braun IF. CT-surgical correlation in pituitary adenomas: evaluation in 113 patients. AJNR Am J Neuroradiol. 1985;6:711–6.
Dekkers OM, de Keizer RJ, Roelfsema F, Vd Klaauw AA, Honkoop PJ, van Dulken H, Smit JW, Romijn JA, Pereira AM. Progressive improvement of impaired visual acuity during the first year after transsphenoidal surgery for non-functioning pituitary macroadenoma. Pituitary. 2007;10:61–5.
Dekkers OM, Pereira AM, Roelfsema F, Voormolen JH, Neelis KJ, Schroijen MA, Smit JW, Romijn JA. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2006;91:1796–801.
Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2008;93:3717–26.
Elster AD. Modern imaging of the pituitary. Radiology. 1993;187:1–14.
Fernández-Balsells MM, Murad MH, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, Lampropulos JF, Natividad I, Perestelo-Pérez L, Ponce de León-Lovatón PG, Erwin PJ, Carey J, Montori VM. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2011;96:905–12.
Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010;72:377–82.
Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994;120:817–20.
Ironside JW. Best Practice No 172: pituitary gland pathology. J Clin Pathol. 2003;56:561–8.
Jakobsson KE, Petruson B, Lindblom B. Dynamics of visual improvement following chiasmal decompression. Quantitative pre- and postoperative observations. Acta Ophthalmol Scand. 2002;80:512–6.
Karavitaki N, Thanabalasingham G, Shore HC, Trifanescu R, Ansorge O, Meston N, Turner HE, Wass JA. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf). 2006;65:524–9.
Koga T, Miyao M, Sato M, Hirota K, Kakuyama M, Tanabe H, Fukuda K. Pituitary apoplexy during general anesthesia in beach chair position for shoulder joint arthroplasty. J Anesth. 2010;24:476–8.
Liberale G, Bruninx G, Vanderkelen B, Dubois E, Vandueren E, Verhelst G. Pituitary apoplexy after aortic abdominal aneurysm surgery: a case report. Acta Chir Belg. 2006;106:77–80.
Maïza JC, Bennet A, Thorn-Kany M, Lagarrigue J, Caron P. Pituitary apoplexy and idiopathic thrombocytopenic purpura: a new case and review of the literature. Pituitary. 2004;7:189–92.
McComb DJ, Ryan N, Horvath E, Kovacs K. Subclinical adenomas of the human pituitary. New light on old problems. Arch Pathol Lab Med. 1983;107:488–91.
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA, Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88.
Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37:151–71.
Möller-Goede DL, Brändle M, Landau K, Bernays RL, Schmid C. Pituitary apoplexy: re-evaluation of risk factors for bleeding into pituitary adenomas and impact on outcome. Eur J Endocrinol. 2011;164:37–43.
Murad-Kejbou S, Eggenberger E. Pituitary apoplexy: evaluation, management, and prognosis. Curr Opin Ophthalmol. 2009;20:456–61.
Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008;23:75–90.
Nielsen EH, Lindholm J, Bjerre P, Christiansen JS, Hagen C, Juul S, Jørgensen J, Kruse A, Laurberg P. Frequent occurrence of pituitary apoplexy in patients with non-functioning pituitary adenoma. Clin Endocrinol (Oxf). 2006;64:319–22.
Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas – a study on 721 patients. Acta Neurochir (Wien). 2004;146:27–35.
O’Sullivan EP, Woods C, Glynn N, Behan LA, Crowley R, O’Kelly P, Smith D, Thompson CJ, Agha A. The natural history of surgically treated but radiotherapy-naive nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 2009;71:709–14.
Orija IB, Weil RJ, Hamrahian AH. Pituitary incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26:47–68.
Pal A, Capatina C, Tenreiro AP, Guardiola PD, Byrne JV, Cudlip S, Karavitaki N, Wass JA. Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clin Endocrinol (Oxf). 2011;75:501–4.
Rajasekaran S, Vanderpump M, Baldeweg S, Drake W, Reddy N, Lanyon M, Markey A, Plant G, Powell M, Sinha S, Wass J. UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (Oxf). 2011;74:9–20.
Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf). 1999;51:181–8.
Reddy R, Cudlip S, Byrne JV, Karavitaki N, Wass JA. Can we ever stop imaging in surgically treated and radiotherapy-naive patients with non-functioning pituitary adenoma? Eur J Endocrinol. 2011;165:739–44.
Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012;15:71–83.
Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol. 2003;149:123–7.
Semple PL, Jane JA, Lopes MB, Laws ER. Pituitary apoplexy: correlation between magnetic resonance imaging and histopathological results. J Neurosurg. 2008;108:909–15.
Sibal L, Ball SG, Connolly V, James RA, Kane P, Kelly WF, Kendall-Taylor P, Mathias D, Perros P, Quinton R, Vaidya B. Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases. Pituitary. 2004;7:157–63.
Tosaka M, Sato N, Hirato J, Fujimaki H, Yamaguchi R, Kohga H, Hashimoto K, Yamada M, Mori M, Saito N, Yoshimoto Y. Assessment of hemorrhage in pituitary macroadenoma by T2-weighted gradient-echo MR imaging. AJNR Am J Neuroradiol. 2007;28:2023–9.
Tsang RW, Brierley JD, Panzarella T, Gospodarowicz MK, Sutcliffe SB, Simpson WJ. Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30:557–65.
Turner HE, Stratton IM, Byrne JV, Adams CB, Wass JA. Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation – a follow-up study. Clin Endocrinol (Oxf). 1999;51:281–4.
Webb SM, Rigla M, Wägner A, Oliver B, Bartumeus F. Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab. 1999;84:3696–700.
Wichers-Rother M, Hoven S, Kristof RA, Bliesener N, Stoffel-Wagner B. Non-functioning pituitary adenomas: endocrinological and clinical outcome after transsphenoidal and transcranial surgery. Exp Clin Endocrinol Diabetes. 2004;112:323–7.
Wilson PJ, De-Loyde KJ, Williams JR, Smee RI. A single centre’s experience of stereotactic radiosurgery and radiotherapy for non-functioning pituitary adenomas with the Linear Accelerator (Linac). J Clin Neurosci. 2012;19:370–4.
Yahagi N, Nishikawa A, Matsui S, Komoda Y, Sai Y, Amakata Y. Pituitary apoplexy following cholecystectomy. Anaesthesia. 1992;47:234–6.
Zayour DH, Selman WR, Arafah BM. Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: relation to pituitary function. J Clin Endocrinol Metab. 2004;89:5649–54.
Zhang F, Chen J, Lu Y, Ding X. Manifestation, management and outcome of subclinical pituitary adenoma apoplexy. J Clin Neurosci. 2009;16:1273–5.
Zhao D, Tomono Y, Tsuboi K, Nose T. Immunohistochemical and ultrastructural study of clinically nonfunctioning pituitary adenomas. Neurol Med Chir (Tokyo). 2000;40:453–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Theodoraki, A., Vanderpump, M.P.J. (2014). Nonfunctioning Pituitary Tumour Apoplexy. In: Turgut, M., Mahapatra, A., Powell, M., Muthukumar, N. (eds) Pituitary Apoplexy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38508-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-38508-7_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38507-0
Online ISBN: 978-3-642-38508-7
eBook Packages: MedicineMedicine (R0)