Abstract
Dictyostelium has become an important model system to study the molecular details of the signalling pathways controlling gradient sensing and cell polarisation that control localised activation of the actin–myosin cytoskeleton responsible for evolutionary highly conserved mechanisms of chemotactic cell movement up chemoattractant gradients. 3′-5′ cyclic AMP is the chemoattractant that controls the chemotactic cell movements that result in aggregation of up to several hundred thousand cells, slug formation, migration and fruiting body formation. The coordination of these complex cell movements require long-range cAMP mediated cell–cell signalling based on periodic initiation of cAMP signals in the aggregation centre and slug tip and relay by surrounding cells, resulting in highly dynamic patterns of cAMP wave propagation. Model calculations have shown that the dynamic feedbacks between autocatalytic cell–cell cAMP signalling and cAMP-mediated collective chemotactic cell movement result in emergent properties that readily explain multicellular morphogenesis. cAMP signalling not only controls cell movement but also acts as a key morphogen to control cell differentiation, which in turn affects cell type specific cell–cell signalling and cell movement, adding an additional layer of feedback. To fully understand the multicellular morphogenesis of this organism at the level of cell behaviours, it will be needed to integrate the detailed cell type proportioning mechanisms in models describing cell–cell signalling and movement. Dictyostelium is likely to be the first eukaryotic organism where it will be possible to quantitatively understand how multicellular development and morphogenesis arise as emergent properties from a few relatively simple collective cell behaviours.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aggregation Centre
- Prespore Cell
- Prestalk Cell
- Guanine Nucleotide Exchange Factor Activate
- Myosin Thick Filament
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
One of the central aims of the study of development is to understand how distinct cellular behaviours e.g. division, differentiation, apoptosis and shape changes and movement are coordinated in space and in time to result in emergent properties at the tissue and organism level. The coordination of these cellular behaviours requires extensive signalling between cells of different types and cells and their environment. Signalling is mostly mediated by chemical and mechanical signals, although other forms of communication through electrical fields and light are also known. In slime moulds, signalling between cells and the environment plays an essential role in processes such as the location of food through sensing of metabolites and quorum sensing, during which process the cells determine their own density relative to the available level of food supplies (Kessin 2001; Gole et al. 2011). The latter process controls the decision to either keep on looking for nutrients or to start the starvation induced multicellular developmental cycle to make gametes or spores. Food is located through chemotaxis towards metabolites such as folic acid secreted by bacteria. During the vegetative phase of the life cycle, cells secrete chemorepellent signals that results in dispersal of cells, increasing their probability of finding new food reserves (Kakebeeke et al. 1979). These repellents have been poorly characterised and little is known about the signal transduction pathways. Once the cells start to develop, they signal each other and this results in the aggregation of hundred to several hundred of thousands of cells which depending on the prevailing environmental conditions can either result in the entry in the sexual cycle which includes the formation of macrocysts through cell fusion followed by meiosis and hatching as described elsewhere in this volume (O’Day 1979; Lewis and O’Day 1985) or in the entry of the asexual developmental cycle which results in the formation of fruiting bodies. In this chapter, we will describe cell–cell signalling mechanisms that the cells use to coordinate their movement behaviour during development, discuss briefly how they contribute and interact with signals controlling differentiation and we will highlight how the interactions between cell–cell signalling movement and differentiation control the emergent morphogenesis at the organismal level (Weijer 2004). We will also discuss key intra-cellular signalling mechanisms and pathways that coordinate the molecular mechanism underlying cell–cell signalling and chemotactic cell movement.
2 Cell Movement and Signalling to the Cytoskeleton
Cell movement is characterised by a series of complex behaviours. It is generally thought that cell movement involves cycles of pseudopod or lamellipod extension at the front end of a migrating cell, coupled with retraction in the rear end of the cell (Fig. 1a). During these processes new cell-substrate contacts are made in the front and broken in the back. These behaviours are primarily brought about by dynamic changes in the actin–myosin cytoskeleton and interacting components such as transmembrane adhesion molecules (Ridley et al. 2003). Extension is believed to be driven by localised polymerisation of actin filaments resulting in a pushing force to the membrane. Once the membrane is extended new contacts with the substrate need to be made and this involves the formation of transient focal adhesions. In the back of the cells, actin filaments depolymerise and contacts with the substrate need to be broken. This requires force and this involves the action of myosin II mini filaments which are involved in contracting the actin filament network, which could also play a role in stimulating actin filament depolymerisation. Since the membrane is not able to stretch much, it appears likely that continuous membrane synthesis needs to be coordinated with actin polymerisation and pseudopod or filopod extension, while depolymerisation may be coupled to localised membrane resorption (Bretscher and AguadoVelasco 1998). Indeed fast migrating cells such as Dictyostelium have been shown to turn over their membranes every 10 min and mutants defective in membrane secretion are defective in locomotion (Bretscher and AguadoVelasco 1998; Zanchi et al. 2010). There is likely an important role for different classes of unconventional myosins that link the cytoskeleton to the membrane, in both tension sensing and movement of components of the cytoskeletal machinery along the membrane (Chen et al. 2012). Deletion of several of these myosins especially of class I myosins have been shown to result in severe movement defects (Fukui et al. 1989; Wessels et al. 1991; Dai et al. 1999; Falk et al. 2003). At the same time that the cell is extending, the cellular contents of the cell needs to be displaced and this involves likely transport of material along both the actin and microtubule network involving different classes of motor proteins. The cytoskeletal machinery appears always to be running as shown by the fact that cells extend and retract pseudopods essentially continuously. Non-stimulated cells extend pseudopods in random locations around the cell, resulting in a very low persistence of directional migration resulting in what is known as a random walk (Soll et al. 2003; Sasaki et al. 2007).
a, b Cells moving in response to cAMP gradient. Pseudopods extend in the direction of the gradient extend. Pseudopods extending away chemoattractant source retract. Extension is driven by actin polymerisation (green). Retraction is driven by assembly of Myosin II filaments (blue). Direction of extension and retraction are indicated by small black arrows. The circular arrow in the extending pseudopod indicates a strong local autocatalytic feedback mechanism operating between signal detection by cAMP receptors and actin polymerisation at levels of highest external signal. The blocking arrows indicate long-range inhibition resulting in retraction of pseudopods pointing away from the chemoattractant source possibly resulting from a competition for limited resources. c Simplified signal transduction scheme from receptor to chemotaxis. cAMP binds to the cAMP receptor. This activates a heterotrimeric G protein, which in turn activates Ras. Downstream of Ras there are at least four parallel signal transduction pathways involving PI3 kinase signalling, TorC2 signalling, signalling through PlA2 and through cAMP to the actin–myosin cytoskeleton. There may also be Ras-independent signalling pathways, possibly through the Dock180/Elmo family of Rac Gef’s
3 Cellular Behaviours During Chemotaxis
There is evidence that cells can modulate the rate of cytoskeletal dynamics in response to extracellular factors and can move faster or slower depending on the factor and its local concentration (Rifkin and Goldberg 2006; Song et al. 2006). This process is known as chemokinesis. Chemokinesis will result in cells avoiding areas of these factors if they stimulate movement and in accumulation of cells if they inhibit movement. In case where movement stimulating factors are secreted in an autocrine manner, this will result in cell dispersal. Through these mechanisms, cells of different types can also influence each others behaviours and they may well play a role during the development of Dictyostelium. However, another more efficient mode of control of cell motility is well established during the development of Dictyostelium and this is chemotaxis. During chemotaxis in Dictyostelium, cells measure a gradient of a chemoattractant and then use this information to bias the extension or persistence of protrusions up or down the direction of the gradient (Wang et al. 2011; Cai et al. 2012). Although chemotaxis can result from a temporal measurement of the concentration at different locations, the evidence suggests that in Dictyostelium the cells measure a spatial gradient over the length of the cell, although due to the adaptation in the chemosensory systems the temporal component also plays a role in chemotaxis in vivo during aggregation as will be discussed in more detail later (Fisher et al. 1989; Iijima et al. 2002).
Starving Dictyostelium cells have been shown to be able to respond accurately to steep but also to very shallow gradients of the chemoattractant 3′-5′cyclic-AMP (cAMP) (Andrew and Insall 2007; Fuller et al. 2010). One view of the underlying mechanism of chemotaxis is that in the presence of an external gradient of a chemoattractant such as cAMP, the cells persistently extend successive pseudopods in the direction of rising cAMP concentration, while at the same time by some global inhibition mechanism suppressing the extension of lateral pseudopods (Van Haastert and Devreotes 2004). In this view, there is a direct coupling between the concentration of the chemoattractant and the local rate of actin polymerisation driving the formation of a new pseudopod. A competing view is that cells extend pseudopods a constant cell specific internal rate of around three pseudopods per minute and that this process is not dependent on the chemoattractant concentration (Andrew and Insall 2007). Furthermore, the direction of pseudopod extension is essentially random, although it has been suggested that pseudopods and new pseudopods form preferentially close to the site where the preceding pseudopod was initiated. This process results in a cell having several pseudopods at any given time and chemotaxis results from the fact that the pseudopod in a higher local cAMP concentration persists, while a pseudopod in a region of lower cAMP concentration is retracted. This process will result in a gradual movement of the cell up a chemoattractant gradient (Andrew and Insall 2007; King and Insall 2009). In this model, cells move in the right direction essentially by an error correction process, continuously updating their directionality in response to the signal. The latter has been shown to work remarkably well in detailed model computations (Neilson et al. 2011) making the assumption that there is an internal competition for available resources (Fig. 1a, b). These models include local activation and local and global inhibition working on different time scales.
4 Signalling During Chemotaxis
Much current research is directed towards understanding the molecular mechanism by which cells detect cAMP gradients, polarise their cytoskeleton and move in response to cAMP gradients (Fig. 1c) (Chen et al. 2007; Franca-Koh et al. 2007; Insall and Andrew 2007). Cells can respond very reliably to very shallow gradient of cAMP, where it is estimated that the difference in occupied cAMP receptors between the front and the back is just few percent, while the total number of cAMP receptors occupied is very low (Ueda et al. 2001). Therefore, it is assumed that an internal amplification mechanism exists that amplifies the weak external signal gradient into a large internal signal gradient that results in a reliable polarisation of the actin–myosin cytoskeleton and directional movement up the cAMP gradient (Fig. 1a, b). It is currently though that there exists a gradient sensing mechanism, the chemical compass, which is followed by an internal amplification mechanism that controls the polarisation of the cytoskeleton, which may well involve the cytoskeleton itself (Bourne and Weiner 2002; Franca-Koh and Devreotes 2004). This view is based on the observation that the initial signalling events, receptor activation and G protein activation occur in the presence of inhibitors of actin polymerisation, but that for full-blown activation of downstream pathways such as Ras and phosphatidyl-inositide 3 kinase (PI3 kinase) activation among others, actin polymerisation is required (Devreotes and Janetopoulos 2003). To explain the differences in cytoskeletal organisation especially actin polymerisation between the front and the back of the cell it has been suggested that the compass is based on a local excitation global inhibition (LEGI) model (Ma et al. 2004). In this model, a local stimulus results in the local production of an excitation variable (receptor occupancy, G protein activation) of limited range as well as a long-range inhibitory signal. The difference between excitation and inhibition is assumed to be proportional to an effector output such as actin polymerisation, thus resulting in a front-to-back gradient of activation (Iglesias and Devreotes 2008). Another property of this system is that the excitation normally increases faster in time than the inhibitor; the LEGI mechanism can provide perfect adaptation, but it does not necessarily provide any amplification (Iglesias 2012; Wang et al. 2012). Since the response of individual cells to a standard stimulus has been shown to be essentially bimodal, i.e. some cells respond where others do not. This has been taken to imply that the LEGI module is followed by an amplification module with cell specific variable thresholds. This results in individual cells in a population responding at different concentrations.
Much work has gone into identifying the components of the compass, the mechanism of short-range activation and long-range inhibition as well as the source of signal amplification. It has become clear that there is no amplification, for instance through ligand-induced clustering and redistribution at the level of the receptors. The receptors remain homogenously distributed in the membrane in the presence of a chemotactic gradient (Ueda et al. 2001). The receptors have been shown to diffuse in the membrane, where they activate G heterotrimeric proteins, but there is also no evidence for differential distribution of G proteins in the membrane in response to a chemoattractant gradient (Jin et al. 2000). Furthermore, there is no evidence for amplification nor for adaptation at the level of G protein activation, as measured by dissociation of Gα and Gβγ subunits using a fluorescence resonance energy transfer (FRET)-based approach (Janetopoulos et al. 2001). The next step in the cascade is activation of the Ras oncogenes RasG and RasC (Kae et al. 2004; Sasaki et al. 2007; Sasaki and Firtel 2009). There may be some amplification of the signal at this stage. The activation of Ras is thought to result from the balance by activation of a RasGef (Guanine Nucleotide Exchange Factor activating Ras) and inactivation by a RasGap (GTPase Activating Protein) (Takeda et al. 2012). This has been suggested to act as an incoherent feed-forward mechanism. Activated Ras is then involved in the activation of several downstream pathways such as the activation of the TORC2 complex and PI3 kinase which in turn result in the activation of PkB (Charest et al. 2010). There is a strong amplification at the level of the activation of PI3 kinase, which involves translocation of PI3 kinase to the membrane in a Ras-dependent manner and a dissociation of PTEN from the membrane (Iijima and Devreotes 2002). The difference between these competing reactions, synthesis and degradation results in an amplification of the response, which may be further amplified by a feedback of actin polymerisation of PI3 kinase localisation. Activated PkB in combination with PkBR1 result in the phosphorylation of several targets and may play a role in the activation of actin polymerisation and inhibition of myosin thick filament assembly in the front (Chung and Firtel 1999; Chung et al. 2001; Kamimura et al. 2008). Surprisingly, it has been shown that knockout of all PI3 kinase signalling (five PI3 kinases and Pten) results in cells that can still do chemotaxis rather well (Hoeller and Kay 2007; Kay et al. 2008). This has led to investigation of further parallel pathways, involving other phospholipases such as Phospholipase A2 as well provide further evidence of a role for cGMP signalling in cell polarisation and chemotaxis (Chen et al. 2007; Veltman et al. 2008; Kamimura and Devreotes 2010) (Fig. 3). Furthermore, there is recently increasing evidence that parallel signalling pathways to the cytoskeleton may involve members of unconventional Gefs of the Dock180/Elmo families (Para et al. 2009; Pakes et al. 2012; Yan et al. 2012). It remains to be discovered how the activation of these components are coupled to the cAMP receptors, and whether the responses are G protein dependent.
Activation of Rac has been shown to destabilise the Wave/Scar complex which in turn results in the formation of the Arp2/3 complex and nucleation of new actin filaments from existing filaments (Bear et al. 1998; Blagg and Insall 2004; Ibarra et al. 2005). These new filaments are capped by capping proteins and cross-linked to each other and membranes by a host of proteins (Eddy et al. 1997). Localised actin polymerisation in the leading edge then results in the generation of protrusive forces as described above.
Actin polymerisation in a broad front results in the formation of lamellipodia, however, cells are known to form large numbers of filopodia, fine protrusive structures. Filopodia may be involved in gradient sensing or in force generation and assist in movement, since deletion of components that results in ablation of filopodia often also result in defects of cell migration. It appears that actin polymerisation in filopodia is dependent on that local activation of formins, rather than being dependent on the activation of the Arp2/3 complex and these are shown to have interaction with wave (Schirenbeck et al. 2005a, b). Unconventional myosins, especially myosin VII, play a key role in bundling and possibly transport of components along the actin bundles in the filopodia as well as in the control of cell substrate adhesion via interactions with talin (Maniak 2001; Faix and Rottner 2006; Galdeen et al. 2007).
Cells move by extending pseudopods at their leading edge, a process driven by localised actin polymerisation which requires the action of members of the myosin I family and inhibition of the formation of myosin II thick filaments through phosphorylation of the tail of the myosin heavy chain on several threonine residues (Yumura et al. 2005; Bosgraaf and van Haastert 2006; Goldberg et al. 2006). To move, cells need to pull up their back ends and suppress the extension of lateral pseudopods. This involves the formation and contraction of actin–myosin II thick filament networks, actin depolymerisation and is dependent on internal cAMP levels (Falk et al. 2003; Zhang et al. 2003). To move forward, the cells must gain traction from the substrate on which they are moving. This requires the formation of multiple transient (10–20 s) cell-substrate contact sites that are actin rich and have been shown to transduce traction forces to the substrate (Bretschneider et al. 2004; Uchida and Yumura 2004). It appears that cells may undergo alternating phases of actin driven extension at the front and myosin II driven contraction at the back (Iwadate and Yumura 2008). Much work is directed towards the investigation of the molecular mechanisms resulting in signal detection and cell polarisation, this has been extensively reviewed elsewhere recently and will not be covered here in detail (Willard and Devreotes 2006; Janetopoulos and Firtel 2008). In addition to this, more recent methods have been developed to investigate the traction and motive forces produced by migrating cells and to use these methods to characterise the molecular mechanisms that translate cell polarisation into directed movement (Del Alamo et al. 2007; Meili et al. 2010; Bastounis et al. 2011). This is a complex area of research that will undoubtedly gain in importance in the future, since it allows a functional quantitative characterisation of how signalling is translated into motive forces underlying directed cell movement.
5 Cell–Cell Signalling Controlling Cell Movement During Multicellular Development
Since Dictyostelium development takes place in the absence of food, under starvation conditions only limited cell divisions occur during multicellular development. Morphogenesis therefore primarily results from the movement of individual differentiating cells into a relatively complex structure, the proportions of which are essentially independent of how many cells exactly aggregate. Key questions are, which signals guide the movement behaviour of thousands of cells during development, which signals control differentiation and how do cell–cell signalling, movement and differentiation interact to form a fruiting body?
6 Aggregation
Starvation induces changes in the gene expression programme that results in the cells acquiring the ability to respond to cAMP gradients by chemotaxis through mechanisms described above, but importantly they also acquire the ability to produce and secrete and degrade cAMP (Saran et al. 2002; Iranfar et al. 2003). Aggregation is caused by periodic cAMP synthesis and secretion by cells in the aggregation centre. The cells initially become chemically excitable and will produce cAMP when stimulated with cAMP. Binding of cAMP to the serpentine transmembrane cAMP receptor results in stimulation of signal transduction cascade that leads to the activation of an adenylylcyclase (ACA), that within tens of seconds produces cAMP part of which is secreted to the outside (Fig. 2). The secreted cAMP binds to the receptor and thus is part of an autocatalytic feedback loop resulting in a rapid increase of cAMP production. However, stimulation of the receptor also activates an adaptation process that with a small time delay results in the inhibition of ACA activation and a cessation of cAMP production. Since cAMP diffuses away into the extracellular medium and is also degraded by secreted cAMP phosphodiesterases, this results in a drop in cAMP levels, which in turn results in de-adaptation of the cells (Fig. 2). Both excitation and adaptation depend on the receptor-dependent activation of a heterotrimeric G protein, resulting in activation of Ras, which in turn activates PI3 kinase, which phosphorylates phosphatidylinositide(4,5)phosphate (PIP2) to phosphatidyl-(3,4,5)-phosphate (PIP3). PIP3 then activates many downstream pathways leading to chemotaxis and ACA activation (Mahadeo and Parent 2006). The activation of ACA is critically dependent on the PIP3-dependent binding of cytosolic regulator of adenylyl cyclase (CRAC) to the membrane where it is activated which is required for adenylyl cyclase activation and chemotaxis (Comer et al. 2005).
cAMP signalling activates cAMP relay and chemotaxis. Extracellular cAMP binds to a transmembrane serpentine cAMP receptor and activates two distinct signal transduction pathways. One pathway leading to organisation of the actin–myosin cytoskeleton and chemotaxis. The second pathway results in activation of the aggregation stage adenylylcyclase (ACA). Activation of ACA results in cAMP production part of which is secreted and binds to the receptor to form a positive feedback loop where a little extracellular cAMP results in the production of more. Binding of cAMP to the receptor also activates an adaptation process that inhibits both ACA activation and the chemotactic signal transduction pathway. Once adaptation reaches a full response, the production of cAMP ceases and cAMP secretion stops. Since cAMP is continuously degraded by an extracellular cAMP phosphodiesterase, extracellular cAMP levels fall which allow the cells to de-adapt and start a new cAMP response
There are no special cells that form aggregation centres and they form in a stochastic manner. Aggregation centres from cells will produce and secrete cAMP continuously at a low but increasing level. Most of this secreted cAMP will be degraded by the secreted cAMP phosphodiesterase. However, since gene expression is heterogeneous, some cells will produce and secrete a little more cAMP than others. Due to this heterogeneity in gene expression and stochastic distribution of cells, by chance there will be an area where some cells can just produce enough cAMP to start the amplification of the signal through positive feedback (Gregor et al. 2010; Kamino et al. 2011). This locally produced cAMP diffuses to neighbouring cells, which now detect an above threshold signal to which they can respond and therefore amplify the signal and pass it on to their neighbours, resulting in the formation of travelling waves of cAMP. Since the cAMP waves direct the chemotactic movement of the cells to their source, this will result in an increased local cell density in the region that initiated the signal, making it more likely that the cells in this region will fire again once they are de-adapted. Through these feedbacks, the group of cells that started signalling will establish itself as an aggregation centre. cAMP receptor mutants with lower affinity for cAMP show altered patterns of wave propagation but also in extreme cases result in mutants that can still propagate waves, but these wave fragments do not set up aggregation centres. This is caused by the fact that the time it takes between two oscillations is too long and the cells will disperse again by random movement resulting in the generation of waves in other random locations (Dormann et al. 2001b). Several centres will arise in random locations and compete to attract cells. Faster oscillating centres will encroach on slower oscillating centres and finally can wipe them out. Successful centres will typically send out a series of around 20 cAMP waves that will attract up to several hundred thousands of other cells to the initial aggregation centre to form a mound.
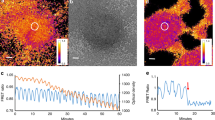
Detection and amplification of this signal by surrounding cells coupled with desensitisation of the cAMP producing cells results in the propagation of waves of cAMP away from the aggregation centre (Fig. 3). Cells detect the rising phase of the wave and move in the direction of increasing cAMP concentration, once the waves passes; the cells are adapted and are insensitive to the falling phase of the wave and therefore do not turn around and chase the wave once it has passed. These cAMP waves therefore guide the cells towards the aggregation centre, where they accumulate into a three-dimensional aggregate, the mound (Dormann and Weijer 2001, 2003). During the synchronised chemotactic movement phase cells elongate, while during the falling phase of the waves, the cells are amoeboid in shape. The large-scale spatiotemporal patterns of cells behaviour can be visualised as changes in light scattering since moving elongated cells scatter more light (Fig. 3a). These wave can thus successfully be used to visualise and measure the spatiotemporal dynamics of wave propagation at all stages of development (Fig. 3b–d). Recently, this method has been used to perform a high throughput analysis of cAMP signalling mutants (Sawai et al. 2007). Initially, the cells move towards the aggregation centre as individuals, but after 10–20 waves have passed they form bifurcating aggregation streams, in which the cells make head to tail contacts via a calcium-independent adhesion molecule, contact site A and side to side contacts via a calcium-dependent cadherins (Wong et al. 2002; Harris et al. 2003). Stream formation is dependent on the localisation of ACA in the rear of the aggregating cells, resulting in polarised cAMP secretion from the back of the cells (Kriebel et al. 2003). cAMP wave propagation can be observed at the individual cell level by following the localised translocation of PIP3 at the leading edge of the cell (Dormann et al. 2002, 2004). The number of cells in aggregation streams appears to be controlled by the local concentration of a secreted extracellular high molecular weight protein complex, counting factor, which through modulation of movement and adhesion may control the numbers of cells that stably migrate in an aggregation stream (Jang and Gomer 2008).
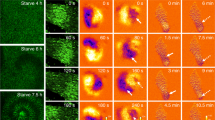
Optical density waves reflect cAMP waves at different stages of development. a Aggregation centres initiate cAMP waves. These propagate from the centre outward (black arrow). Cells detect the rising phase of the cAMP wave and move in the direction of higher cAMP concentrations (bottom figures and arrows, colour of cells and arrows indicates degree of light scattering). During their chemotactic movement, the cells elongate and this changes their light scattering properties. When during the rising phase of the wave, many cells move in synchrony, this results in increased light scattering waves reflecting the rising phase of the cAMP signal. b Spiral optical density waves during the early aggregation phase, when the cells are still in a monolayer on agar. c Optical density waves in a streaming aggregate. In the body of the aggregate, multi-armed spiral waves rotate counter clockwise throwing off individual wave-fronts that propagate down the streams to the periphery of the aggregate white arrow indicates the direction of wave propagation, the black arrow indicates the direction of cell movement. d A slug migrating to the right, showing two dark optical density waves that travel from right to left (white arrow), cells move to the right following the tip (black arrow)
7 Mound and Slug Formation
After the cells have aggregated they form a hemi-spherical structure, the mound. Mounds are characterised by rotating waves of cAMP that direct the counter rotational periodic movement of the cells. Cells start to differentiate into prespore and prestalk cells during aggregation, based on physiological biases like nutritional state and cell cycle position at the time of starvation already present in the population before aggregation (Weeks and Weijer 1994; Araki et al. 1997). As a result, there is little correlation between the time of arrival in the mound and differentiation fate. Therefore, initially the prestalk and prespore cell types display a salt and pepper distribution in the mound (Fig. 5a). A subpopulation of prestalk cells sort out to form the tip and the slug tip guides the movement of all other cells thus acting as an organiser (Weijer 2004). External cAMP has been shown to be able to affect cell sorting of neutral red labelled prestalk cells at the mound stage (Matsukuma and Durston 1979; Sternfeld and David 1981). More recently, use of a temperature-sensitive ACA mutant has shown that ACA activity is required in vivo for cells to be able to sort to the tip (Patel et al. 2000). The tips action as an organiser can be mimicked by the periodic injection of cAMP pulses of the right frequency and duration (Matsukuma and Durston 1979; Dormann and Weijer 2001), suggesting that the tip is a source of periodic cAMP waves, in agreement with the fact that prestalk cells express ACA and the extracellular cAMP phosphodiesterase pdeA (Verkerke-van Wijk et al. 2001; Weening et al. 2003). More recently, it has become clear that prestalk cells likely generate more force in response to a chemotactic signal than prespore cells. This can be the result of the increased myosin expression and assembly by cells in the prestalk zone (Eliott et al. 1991, 1993). More recently, it has been shown that it likely also involves differential regulation of the actin cytoskeleton especially through filamin (Blagg et al. 2011). Furthermore, it is possible that differential expression of adhesion molecules such as DdCad the Dictyostelium cadherin analogue may contribute to cell sorting (Wong et al. 2002; Sriskanthadevan et al. 2011).
It is not yet known which signals control tip cell fate (see below), but it is becoming clear that to proceed from the aggregate to the mound stage cell–cell adhesion and or contact start to play an important role. Mutants defective in the putative single pass transmembrane contact molecules lagC, lagD cannot proceed beyond the aggregation stage and are defective in tip formation (Kibler et al. 2003). There is evidence that Dictyostelium may possess several integrin-like adhesion molecules (Cornillon et al. 2008) and it has been known that mutants in talin, paxillin and a lim domain protein which are thought to couple adhesion molecules to the actin cytoskeleton are all defective in cell sorting (Chien et al. 2000; Tsujioka et al. 2004; Bukharova et al. 2005). Sorting of prestalk cells towards the tip requires the invasive movement of prestalk cells through a tightly packed mass of other (Tasaka and Takeuchi 1979; Weijer 2004; Kay and Thompson 2009). Myosin II is absolutely required for progression beyond the mound stage and it is known that prestalk cells express higher levels of myosin II (Eliott et al. 1991; Springer et al. 1994). For prestalk cells to sort, they need to assemble more myosin thick filaments than prespore cells (Singer and Dormann Weijer 2013). There is also evidence that cell type specific changes in the organisation of the actin cytoskeleton may affect cell sorting, since mutants affecting the cell type specific regulation of filamin, a major actin cross-linking protein and RapGapA which controls Rap1 activity involved in control of adhesion both affect cell sorting out of prestalk cells (Parkinson et al. 2009; Blagg et al. 2011). Together all these observations suggest that tip formation is the result of cellular properties specific to tip forming cells. Tip cells express high levels of ACA resulting in increased signalling ability, therefore allowing them to set up a new independent signalling centre that can outcompete the aggregation centre organising the mound. They also assemble more myosin II thick filaments, critical to their ability to produce more force in response to a cAMP signal (Clow et al. 2000). This latter property, allows them to push other cells aside and re-aggregate within the mound to form the tip.
In slugs, optical density waves can be seen to propagate from the middle of the prestalk zone to the back, reflecting the periodic movement of the cells forward (Fig. 3). These optical waves are strictly dependent on the tip. Cells in the tip often rotate perpendicular to the direction of slug migration, especially when it is lifted from the substrate. In the back of the slug, the cells move periodically forward and all cells move on average with slug speed. It has been shown that the assumptions cAMP wave propagation and chemotaxis in response to these waves is in principle sufficient to explain morphogenesis from single cell via aggregation, stream and mound formation to cell sorting and slug formation. The interactions between cell signalling and cell movement can be described by relatively simple mathematical models in a robust way and it would appear that these process are sufficient to explain Dictyostelium morphogenesis (Fig. 4) (Vasiev and Weijer 2003; Umeda and Inouye 2004). However, the situation is almost certainly more complex since strains lacking the aggregation stage ACA can still form slugs, when they overexpress the catalytic subunit of protein kinase A, suggesting either that there either exists an ACA-independent mechanism to produce periodic cAMP signals, for instance involving cAMP generation by other adenylylcyclases ACB and or ACG and the recently discovered cAMP stimulated cAMP phosphodiesterase (Meima et al. 2003) or that there exists altogether different mechanisms that can control cell movement such as contact following (Umeda and Inouye 2002). The latter mechanism does, however, not explain which signals direct the movement of the cells in the tip. The hypothesis that prestalk cells generate much of the force for migration in the slug has been supported by traction force measurements produced by measuring the local deformation of elastic gels by migrating slugs (Rieu et al. 2005). These measurements show that the posterior region of the slug generates much of the motive force during migration. Measurements of forces in slugger mutants have further suggested that the anterior-like cells provide the motive force for the prespore zone of the slug (Rieu et al. 2009). These results all suggest that cell-type specific differences in cell mechanics differences are critical for cell sorting and slug migration.
Model calculation of wave propagation and cell movement from aggregation to slug migration using a hydrodynamic model. The top row depicts the aggregation up to the mound stage. The first image starts with the randomly distributed cells (yellow) which are organised by a spiral wave of cAMP (red). They form aggregation streams and finally a hemispherical mound (Vasiev et al. 1997). The middle row shows cell sorting and the formation of a slug. The mound consists of two cells types: 20 % yellow prestalk cells and 80 % blue prespore cells. They are initially randomly mixed. The cAMP waves (purple) organise the movement of the cells. In the model, the assumption is that the prestalk cells are more excitable and develop more movement force in response to a cAMP wave. As a result of this, they move towards the centre of the mound and up to form the tip. The separation of the cells feeds back on the signal propagation resulting in the formation of a twisted scroll wave. This leads to an intercalation of the cells and an upward extension of the slug (Vasiev and Weijer 1999). The bottom row shows that a slug organised by a scroll wave can move (Vasiev and Weijer 2003)
8 Differentiation
It is well established that Dictyostelium slugs can form from as few as a few hundred cells and can contain up to several million cells, while the proportions between spore and stalk cells remain relatively constant (Rafols et al. 2001; Maruo et al. 2004). A major goal is to understand the relationship between cell movement and the signals that control differentiation. These signals must be able to maintain the correct proportioning of the prespore and prestalk celltypes in an environment of extensive cell movement and changes in shape of the slug. In the slug, the different cell types are arranged in a simple axial pattern, pstA cells in the tip, a band of pstO cells that form the upper and part of the lower cup of the fruiting body, prespore cells, precursors for spores, which are intermingled anterior-like cells and rearguard cells precursor to the lower cup and basal disk in the back of the slug (Fig. 5a) (Williams 2006; Yamada et al. 2010). It seems evident that this requires adaptive signalling dynamics, but the signals and the details of their regulation are not yet understood in detail (Fig. 5b). cAMP pulses control the expression of aggregation stage genes necessary for cAMP relay and cell–cell contact and cAMP is necessary for prespore gene expression in later development (Saran et al. 2002; Iranfar et al. 2003). Prespore cells in turn produce DIF (differentiation inducing factor, a small chlorinated hexaphenone), which controls the differentiation of pstO cells (Kay and Thompson 2001; Maeda et al. 2003; Thompson et al. 2004; Saito et al. 2006). DIF spreads by simple diffusion from the prespore zone in adjacent regions where it controls the differentiation of prestalk O cells and possibly rearguard cells (Fukuzawa et al. 2003). Cells in the pstA zone express ACA and studies investigating the cyclic AMP-dependent nuclear translocation of the transcription factor statA have shown that cAMP levels are high in the tip, while cAMP is lower elsewhere in the slug (Dormann et al. 2001a; Verkerke-van Wijk et al. 2001), compatible with the idea that all cells in the tip relay the cAMP signal and only the anterior-like cells in the rest of the slug. The signals and signalling pathways that control the expression of functionally important tip enriched molecules such as ACA and myosin II presumably involve signalling through the Stat pathway (Wang and Williams 2010; Araki and Williams 2012), but the exact details remain to be established. It also remains to be seen how accurate cell type proportioning is achieved in slugs despite their vastly different sizes and dynamic changes in shape.
Cell types and cell type proportioning in Dictyostelium. a During aggregation cells start to differentiate into prestalk (dark grey) and prespore cells (light grey). Since cells arrive in the mound in random order they from a salt and pepper distribution of cell types. After a variable time of movement, the prestalk cells re-aggregate to form the tip and the initial prestalk zone. The tip guides the movement of all the other cells and the structure elongates to from a slug that topples over and migrates away. In the slugs, there are at least four cell types. The tip is made up of PstA (dark grey) cells, followed by a cohort of PstO (light grey) cells, which together form the prestalk zone. The prestalk zone is followed by the prespore zone in which mainly prespore cells are intermingled with anterior like cells, cells of prestalk character that do not sort but express ACA and relay the cAMP signal. In the back of the slug, the rearguard cells are found. b Prespore and prestalk cells differentiate from vegetative stage cells. The early differentiation in aggregation stage cells requires cAMP pulses. The cells then differentiate into prestalk and prespore cells. Prestalk cells initiate cAMP waves and extracellular cAMP is needed for prespore gene expression. Prespore cells make DIF which is necessary for PstO cell differentiation. Prestalk cells secrete DIF-ase that inactivates DIF. Prestalk cells differentiate into stalk cells and prespore cells differentiate into spores. Stalk differentiation is inhibited by NH3. An open question is how cell-type proportioning works quantitatively which is the subject of further modelling studies
The switch from migrating slugs to culmination appears to be controlled by a fall in ammonia concentration. The identification of a number of ammonia transporters some of which are expressed in the very tip and when deleted show a slugger phenotype supports the importance of ammonia as a morphogen (Kirsten et al. 2005, 2008; Singleton et al. 2006). Ammonia signals most likely through the histidine kinase DhkC to the response regulator domain of the internal cAMP phosphodiesterase RegA, which is a major determinant in the control of intracellular cAMP levels (Singleton et al. 1998; Saran et al. 2002). High ammonia is expected to result in activation of regA and low internal cAMP levels. A drop in ammonia is expected to result in a rise of intracellular cAMP and stalk cell differentiation.
In conclusion, Dictyostelium is besides being a system of choice to investigate the molecular mechanisms underlying cell polarity and chemotaxis also as an excellent model system to investigate the basic cell–cell signalling mechanisms that underlie multicellular tissue formation and morphogenesis.
References
Andrew N, Insall RH (2007) Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol 9(2):193–200
Araki T, Williams JG (2012) Perturbations of the actin cytoskeleton activate a Dictyostelium STAT signalling pathway. Eur J Cell Biol 91(5):420–425
Araki T, Abe T, Williams JG, Maeda Y (1997) Symmetry breaking in Dictyostelium morphogenesis: evidence that a combination of cell cycle stage and positional information dictates cell fate. Dev Biol 192(2):645–648
Bastounis E, Meili R, Alonso-Latorre B, del Alamo JC, Lasheras JC, Firtel RA (2011) The SCAR/WAVE complex is necessary for proper regulation of traction stresses during amoeboid motility. Mol Biol Cell 22(21):3995–4003
Bear JE, Rawls JF, Saxe CL (1998) SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J Cell Biol 142(5):1325–1335
Blagg SL, Insall RH (2004) Control of SCAR activity in Dictyostelium discoideum. Biochem Soc Trans 32(Pt 6):1113–1114
Blagg SL, Battom SE, Annesley SJ, Keller T, Parkinson K, Wu JM, Fisher PR, Thompson CR (2011) Cell type-specific filamin complex regulation by a novel class of HECT ubiquitin ligase is required for normal cell motility and patterning. Development 138(8):1583–1593
Bosgraaf L, van Haastert PJ (2006) The regulation of myosin II in Dictyostelium. Eur J Cell Biol 85:969–979
Bourne HR, Weiner O (2002) A chemical compass. Nature 419(6902):21
Bretscher MS, AguadoVelasco C (1998) Membrane traffic during cell locomotion. Curr Opin Cell Biol 10(4):537–541
Bretschneider T, Diez S, Anderson K, Heuser J, Clarke M, Muller-Taubenberger A, Kohler J, Gerisch G (2004) Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr Biol 14(1):1–10
Bukharova T, Weijer G, Bosgraaf L, Dormann D, van Haastert PJ, Weijer CJ (2005) Paxillin is required for cell-substrate adhesion, cell sorting and slug migration during Dictyostelium development. J Cell Sci 118(Pt 18):4295–4310
Cai H, Huang CH, Devreotes PN, Iijima M (2012) Analysis of chemotaxis in Dictyostelium. Methods Mol Biol 757:451–468
Charest PG, Shen Z, Lakoduk A, Sasaki AT, Briggs SP, Firtel RA (2010) A Ras signaling complex controls the RasC-TORC2 pathway and directed cell migration. Dev Cell 18(5):737–749
Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, Iglesias PA, Devreotes PN (2007) PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell 12(4):603–614
Chen CL, Wang Y, Sesaki H, Iijima M (2012) Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis. Sci Signal 5(209):ra10
Chien S, Chung CY, Sukumaran S, Osborne N, Lee S, Ellsworth C, McNally JG, Firtel RA (2000) The Dictyostelium LIM domain-containing protein LIM2 is essential for proper chemotaxis and morphogenesis. Mol Biol Cell 11(4):1275–1291
Chung CY, Firtel RA (1999) PAKa is required for cytoskeletal regulation in Dictyostelium cells during chemotaxis and regulated via Akt/PKB pathway. Mol Biol Cell 10:1521
Chung CY, Potikyan G, Firtel RA (2001) Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol Cell 7(5):937–947
Clow PA, Chen T, Chisholm RL, McNally JG (2000) Three-dimensional in vivo analysis of Dictyostelium mounds reveals directional sorting of prestalk cells and defines a role for the myosin II regulatory light chain in prestalk cell sorting and tip protrusion. Development 127:2715–2728.
Comer FI, Lippincott CK, Masbad JJ, Parent CA (2005) The PI3K-mediated activation of CRAC independently regulates adenylyl cyclase activation and chemotaxis. Curr Biol 15(2):134–139
Cornillon S, Froquet R, Cosson P (2008) Regulation of cellular adhesion in Dictyostelium: involvement of Sib proteins. Eukaryot Cell 7:1600–1605
Dai JW, TingBeall HP, Hochmuth RM, Sheetz MP, Titus MA (1999) Myosin I contributes to the generation of resting cortical tension. Biophys J 77(2):1168–1176
Del Alamo JC, Meili R, Alonso-Latorre B, Rodriguez-Rodriguez J, Aliseda A, Firtel RA, Lasheras JC (2007) Spatio-temporal analysis of eukaryotic cell motility by improved force cytometry. Proc Natl Acad Sci USA 104(33):13343–13348
Devreotes P, Janetopoulos C (2003) Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem 278(23):20445–20448
Dormann D, Weijer CJ (2001) Propagating chemoattractant waves coordinate periodic cell movement in Dictyostelium slugs. Development 128(22):4535–4543
Dormann D, Weijer CJ (2003) Chemotactic cell movement during development. Curr Opin Genet Dev 13(4):358–364
Dormann D, Abe T, Weijer CJ, Williams J (2001a) Inducible nuclear translocation of a STAT protein in Dictyostelium prespore cells: implications for morphogenesis and cell-type regulation. Development 128(7):1081–1088
Dormann D, Kim JY, Devreotes PN, Weijer CJ (2001b) cAMP receptor affinity controls wave dynamics, geometry and morphogenesis in Dictyostelium. J Cell Sci 114(Pt 13):2513–2523
Dormann D, Weijer G, Parent CA, Devreotes PN, Weijer CJ (2002) Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr Biol 12(14):1178–1188
Dormann D, Weijer G, Dowler S, Weijer CJ (2004) In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci 117(Pt 26):6497–6509
Eddy RJ, Han J, Condeelis JS (1997) Capping protein terminates but does not initiate chemoattractant-induced actin assembly in Dictyostelium. J Cell Biol 139(5):1243–1253
Eliott S, Vardy PH, Williams KL (1991) The distribution of myosin-II in Dictyostelium discoideum slug cells. J Cell Biol 115:1267–1274
Eliott S, Joss GH, Spudich A, Williams KL (1993) Patterns in Dictyostelium discoideum—the role of myosin-II in the transition from the unicellular to the multicellular phase. J Cell Sci 104:457–466
Faix J, Rottner K (2006) The making of filopodia. Curr Opin Cell Biol 18(1):18–25
Falk DL, Wessels D, Jenkins L, Pham T, Kuhl S, Titus MA, Soll DR (2003) Shared, unique and redundant functions of three members of the class I myosins (MyoA, MyoB and MyoF) in motility and chemotaxis in Dictyostelium. J Cell Sci 116(Pt 19):3985–3999
Fisher PR, Merkl R, Gerisch G (1989) Quantitative analysis of cell motility and chemotaxis in Dictyostelium discoideum by using an image processing system and a novel chemotaxis chamber providing stationary chemical gradients. J Cell Biol 108:973–984
Franca-Koh J, Devreotes PN (2004) Moving forward: mechanisms of chemoattractant gradient sensing. Physiology (Bethesda) 19:300–308
Franca-Koh J, Kamimura Y, Devreotes PN (2007) Leading-edge research: PtdIns(3,4,5)P3 and directed migration. Nat Cell Biol 9(1):15–17
Fukui Y, Lynch TJ, Brzeska H, Korn ED (1989) Myosin I is located at the leading edges of locomoting Dictyostelium amoebae. Nature 341:328–331
Fukuzawa M, Abe T, Williams JG (2003) The Dictyostelium prestalk cell inducer DIF regulates nuclear accumulation of a STAT protein by controlling its rate of export from the nucleus. Development 130(4):797–804
Fuller D, Chen W, Adler M, Groisman A, Levine H, Rappel WJ, Loomis WF (2010) External and internal constraints on eukaryotic chemotaxis. Proc Natl Acad Sci USA 107(21):9656–9659
Galdeen SA, Stephens S, Thomas DD, Titus MA (2007) Talin influences the dynamics of the myosin VII-membrane interaction. Mol Biol Cell 18(10):4074–4084
Goldberg JM, Wolpin ES, Bosgraaf L, Clarkson BK, Van Haastert PJ, Smith JL (2006) Myosin light chain kinase A is activated by cGMP-dependent and cGMP-independent pathways. FEBS Lett 580(8):2059–2064
Gole L, Riviere C, Hayakawa Y, Rieu JP (2011) A quorum-sensing factor in vegetative Dictyostelium discoideum cells revealed by quantitative migration analysis. PLoS ONE 6(11):e26901
Gregor T, Fujimoto K, Masaki N, Sawai S (2010) The onset of collective behavior in social amoebae. Science 328(5981):1021–1025
Harris TJ, Ravandi A, Awrey DE, Siu CH (2003) Cytoskeleton interactions involved in the assembly and function of glycoprotein-80 adhesion complexes in dictyostelium. J Biol Chem 278(4):2614–2623
Hoeller O, Kay RR (2007) Chemotaxis in the absence of PIP3 gradients. Curr Biol 17(9):813–817
Ibarra N, Pollitt A, Insall RH (2005) Regulation of actin assembly by SCAR/WAVE proteins. Biochem Soc Trans 33(Pt 6):1243–1246
Iglesias PA (2012) Chemoattractant signaling in dictyostelium: adaptation and amplification. Sci Signal 5(213):pe8
Iglesias PA, Devreotes PN (2008) Navigating through models of chemotaxis. Curr Opin Cell Biol 20(1):35–40
Iijima M, Devreotes P (2002) Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109(5):599–610
Iijima M, Huang YE, Devreotes P (2002) Temporal and spatial regulation of chemotaxis. Dev Cell 3(4):469–478
Insall R, Andrew N (2007) Chemotaxis in Dictyostelium: how to walk straight using parallel pathways. Curr Opin Microbiol 10(6):578–581
Iranfar N, Fuller D, Loomis WF (2003) Genome-wide expression analyses of gene regulation during early development of Dictyostelium discoideum. Eukaryot Cell 2(4):664–670
Iwadate Y, Yumura S (2008) Actin-based propulsive forces and myosin-II-based contractile forces in migrating Dictyostelium cells. J Cell Sci 121(Pt 8):1314–1324
Janetopoulos C, Firtel RA (2008) Directional sensing during chemotaxis. FEBS Lett 582:2075–2085
Janetopoulos C, Jin T, Devreotes P (2001) Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291(5512):2408–2411
Jang W, Gomer RH (2008) Combining experiments and modelling to understand size regulation in Dictyostelium discoideum. J R Soc Interface 5:S49–S58
Jin T, Zhang N, Long Y, Parent CA, Devreotes PN (2000) Localization of the G protein beta gamma complex in living cells during chemotaxis. Science 287(5455):1034–1036
Kae H, Lim CJ, Spiegelman GB, Weeks G (2004) Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep 5(6):602–606
Kakebeeke PIJ, De Wit RJW, Kohtz SD, Konijn TM (1979) Negative chemotaxis in Dictyostelium and Polysphondylium. Exp Cell Res 124:429–433
Kamimura Y, Devreotes PN (2010) Phosphoinositide-dependent protein kinase (PDK) activity regulates phosphatidylinositol 3,4,5-trisphosphate-dependent and -independent protein kinase B activation and chemotaxis. J Biol Chem 285(11):7938–7946
Kamimura Y, Xiong Y, Iglesias PA, Hoeller O, Bolourani P, Devreotes PN (2008) PIP3-independent activation of TorC2 and PKB at the cell’s leading edge mediates chemotaxis. Curr Biol 18(14):1034–1043
Kamino K, Fujimoto K, Sawai S (2011) Collective oscillations in developing cells: insights from simple systems. Dev Growth Differ 53(4):503–517
Kay RR, Thompson CR (2001) Cross-induction of cell types in Dictyostelium: evidence that DIF-1 is made by prespore cells. Development 128(24):4959–4966
Kay RR, Thompson CR (2009) Forming patterns in development without morphogen gradients: scattered differentiation and sorting out. Cold Spring Harb Perspect biol 1(6):a001503
Kay RR, Langridge P, Traynor D, Hoeller O (2008) Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol 9(6):455–463
Kessin R (2001) Dictyostelium. Cambridge University Press, Cambridge
Kibler K, Svetz J, Nguyen TL, Shaw C, Shaulsky G (2003) A cell-adhesion pathway regulates intercellular communication during Dictyostelium development. Dev Biol 264(2):506–521
King JS, Insall RH (2009) Chemotaxis: finding the way forward with Dictyostelium. Trends Cell Biol 19(10):523–530
Kirsten JH, Xiong Y, Dunbar AJ, Rai M, Singleton CK (2005) Ammonium transporter C of Dictyostelium discoideum is required for correct prestalk gene expression and for regulating the choice between slug migration and culmination. Dev Biol 287(1):146–156
Kirsten JH, Xiong Y, Davis CT, Singleton CK (2008) Subcellular localization of ammonium transporters in Dictyostelium discoideum. BMC Cell Biol 9:71
Kriebel PW, Barr VA, Parent CA (2003) Adenylyl cyclase localization regulates streaming during chemotaxis. Cell 112(4):549–560
Lewis KE, O’Day DH (1985) The regulation of sexual development in Dictyostelium discoideum: cannibalistic behaviour of the giant cell. Can J Microbiol 31:423–428
Ma L, Janetopoulos C, Yang L, Devreotes PN, Iglesias PA (2004) Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in dictyostelium cells. Biophys J 87(6):3764–3774
Maeda M, Sakamoto H, Iranfar N, Fuller D, Maruo T, Ogihara S, Morio T, Urushihara H, Tanaka Y, Loomis WF (2003) Changing patterns of gene expression in dictyostelium prestalk cell subtypes recognized by in situ hybridization with genes from microarray analyses. Eukaryot Cell 2(3):627–637
Mahadeo DC, Parent CA (2006) Signal relay during the life cycle of Dictyostelium. Curr Top Dev Biol 73:115–140
Maniak M (2001) Cell adhesion: ushering in a new understanding of myosin VII. Curr Biol 11(8):R315–R317
Maruo T, Sakamoto H, Iranfar N, Fuller D, Morio T, Urushihara H, Tanaka Y, Maeda M, Loomis WF (2004) Control of cell type proportioning in Dictyostelium discoideum by differentiation-inducing factor as determined by in situ hybridization. Eukaryot Cell 3(5):1241–1248
Matsukuma S, Durston AJ (1979) Chemotactic cell sorting in Dictyostelium discoideum. J Embryol Exp Morphol 50:243–251
Meili R, Alonso-Latorre B, del Alamo JC, Firtel RA, Lasheras JC (2010) Myosin II is essential for the spatiotemporal organization of traction forces during cell motility. Mol Biol Cell 21(3):405–417
Meima ME, Weening KE, Schaap P (2003) Characterization of a cAMP-stimulated cAMP phosphodiesterase in Dictyostelium discoideum. J Biol Chem 278(16):14356–14362
Neilson MP, Veltman DM, van Haastert PJ, Webb SD, Mackenzie JA, Insall RH (2011) Chemotaxis: a feedback-based computational model robustly predicts multiple aspects of real cell behaviour. PLoS Biol 9(5):e1000618
O’Day DH (1979) Aggregation during sexual development in Dictyostelium discoideum. Can J Microbiol 25:1416–1426
Pakes NK, Veltman DM, Rivero F, Nasir J, Insall R, Williams RS (2012) ZizB, a novel RacGEF regulates development, cell motility and cytokinesis in Dictyostelium. J Cell Sci 125:2457–2465
Para A, Krischke M, Merlot S, Shen Z, Oberholzer M, Lee S, Briggs S, Firtel RA (2009) Dictyostelium Dock180-related RacGEFs regulate the actin cytoskeleton during cell motility. Mol Biol Cell 20(2):699–707
Parkinson K, Bolourani P, Traynor D, Aldren NL, Kay RR, Weeks G, Thompson CR (2009) Regulation of Rap1 activity is required for differential adhesion, cell-type patterning and morphogenesis in Dictyostelium. J Cell Sci 122(Pt 3):335–344
Patel H, Guo KD, Parent C, Gross J, Devreotes PN, Weijer CJ (2000) A temperature-sensitive adenylyl cyclase mutant of Dictyostelium. EMBO J 19(10):2247–2256
Rafols I, Amagai A, Maeda Y, MacWilliams HK, Sawada Y (2001) Cell type proportioning in Dictyostelium slugs: lack of regulation within a 2.5-fold tolerance range. Differentiation 67(4–5):107–116
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302(5651):1704–1709
Rieu JP, Barentin C, Maeda Y, Sawada Y (2005) Direct mechanical force measurements during the migration of Dictyostelium slugs using flexible substrata. Biophys J 89(5):3563–3576
Rieu JP, Saito T, Delanoe-Ayari H, Sawada Y, Kay RR (2009) Migration of Dictyostelium slugs: anterior-like cells may provide the motive force for the prespore zone. Cell Motil Cytoskelet 66(12):1073–1086
Rifkin JL, Goldberg RR (2006) Effects of chemoattractant pteridines upon speed of D. discoideum vegetative amoebae. Cell Motil Cytoskelet 63(1):1–5
Saito T, Taylor GW, Yang JC, Neuhaus D, Stetsenko D, Kato A, Kay RR (2006) Identification of new differentiation inducing factors from Dictyostelium discoideum. Biochim Biophys Acta 1760(5):754–761
Saran S, Meima ME, Alvarez-Curto E, Weening KE, Rozen DE, Schaap P (2002) cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection. J Muscle Res Cell Motil 23(7–8):793–802
Sasaki AT, Firtel RA (2009) Spatiotemporal regulation of Ras-GTPases during chemotaxis. Methods Mol Biol 571:333–348
Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, Meili R, Devreotes PN, Firtel RA (2007) G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol 178(2):185–191
Sawai S, Guan XJ, Kuspa A, Cox EC (2007) High-throughput analysis of spatio-temporal dynamics in Dictyostelium. Genome Biol 8(7):R144
Schirenbeck A, Arasada R, Bretschneider T, Schleicher M, Faix J (2005a) Formins and VASPs may co-operate in the formation of filopodia. Biochem Soc Trans 33(Pt 6):1256–1259
Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J (2005b) The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol 7(6):619–625
Singleton CK, Zinda MJ, Mykytka B, Yang P (1998) The histidine kinase dhkC regulates the choice between migrating slugs and terminal differentiation in Dictyostelium discoideum. Dev Biol 203(2):345–357
Singleton CK, Kirsten JH, Dinsmore CJ (2006) Function of ammonium transporter A in the initiation of culmination of development in Dictyostelium discoideum. Eukaryot Cell 5(7):991–996
Soll DR, Wessels D, Heid PJ, Voss E (2003) Computer-assisted reconstruction and motion analysis of the three-dimensional cell. Sci World J 3:827–841
Song L, Nadkarni SM, Bodeker HU, Beta C, Bae A, Franck C, Rappel WJ, Loomis WF, Bodenschatz E (2006) Dictyostelium discoideum chemotaxis: threshold for directed motion. Eur J Cell Biol 85:981–989
Springer ML, Patterson B, Spudich JA (1994) Stage-specific requirement for myosin II during Dictyostelium development. Development 120:2651–2660
Sriskanthadevan S, Zhu Y, Manoharan K, Yang C, Siu CH (2011) The cell adhesion molecule DdCAD-1 regulates morphogenesis through differential spatiotemporal expression in Dictyostelium discoideum. Development 138(12):2487–2497
Sternfeld J, David CN (1981) Cell sorting during pattern formation in Dictyostelium. Differentiation 20:10–21
Takeda K, Shao D, Adler M, Charest PG, Loomis WF, Levine H, Groisman A, Rappel WJ, Firtel RA (2012) Incoherent feedforward control governs adaptation of activated ras in a eukaryotic chemotaxis pathway. Sci Signal 5(205):ra2
Tasaka M, Takeuchi I (1979) Sorting out behaviour of disaggregated cells in the absence of morphogenesis in Dictyostelium discoideum. J Embryol Exp Morphol 49:89–102
Thompson CR, Fu Q, Buhay C, Kay RR, Shaulsky G (2004) A bZIP/bRLZ transcription factor required for DIF signaling in Dictyostelium. Development 131(3):513–523
Tsujioka M, Yoshida K, Inouye K (2004) Talin B is required for force transmission in morphogenesis of Dictyostelium. EMBO J 23(11):2216–2225
Uchida KS, Yumura S (2004) Dynamics of novel feet of Dictyostelium cells during migration. J Cell Sci 117(Pt 8):1443–1455
Ueda M, Sako Y, Tanaka T, Devreotes P, Yanagida T (2001) Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science 294(5543):864–867
Umeda T, Inouye K (2002) Possible role of contact following in the generation of coherent motion of Dictyostelium cells. J Theor Biol 219(3):301–308
Umeda T, Inouye K (2004) Cell sorting by differential cell motility: a model for pattern formation in Dictyostelium. J Theor Biol 226(2):215–224
Van Haastert PJ, Devreotes PN (2004) Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol 5(8):626–634
Vasiev B, Weijer CJ (1999) Modeling chemotactic cell sorting during Dictyostelium discoideum mound formation. Biophys J 76(2):595–605
Vasiev B, Weijer CJ (2003) Modelling of Dictyostelium discoideum slug migration. J Theor Biol 223(3):347–359
Vasiev B, Siegert F, Weijer CJ (1997) A hydrodynamic model for Dictyostelium discoideum mound formation. J Theor Biol 184(4):441
Veltman DM, Keizer-Gunnik I, Van Haastert PJ (2008) Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J Cell Biol 180(4):747–753
Verkerke-van Wijk I, Fukuzawa M, Devreotes PN, Schaap P (2001) Adenylyl cyclase A expression is tip-specific in Dictyostelium slugs and directs StatA nuclear translocation and CudA gene expression. Dev Biol 234(1):151–160
Wang HY, Williams JG (2010) Synergy between two transcription factors directs gene expression in Dictyostelium tip-organiser cells. Int J Dev Biol 54(8–9):1301–1307
Wang Y, Chen CL, Iijima M (2011) Signaling mechanisms for chemotaxis. Dev Growth Differ 53(4):495–502
Wang CJ, Bergmann A, Lin B, Kim K, Levchenko A (2012) Diverse sensitivity thresholds in dynamic signaling responses by social amoebae. Sci Signal 5(213):ra17
Weeks G, Weijer CJ (1994) The Dictyostelium cell cycle and its relationship to differentiation. FEMS Microbiol Lett 124(2):123–130
Weening KE, Wijk IV, Thompson CR, Kessin RH, Podgorski GJ, Schaap P (2003) Contrasting activities of the aggregative and late PDSA promoters in Dictyostelium development. Dev Biol 255(2):373–382
Weijer CJ (2004) Dictyostelium morphogenesis. Curr Opin Genet Dev 14(4):392–398
Wessels D, Murray J, Jung G, Hammer JA III, Soll DR (1991) Myosin IB null mutants of Dictyostelium exhibit abnormalities in motility. Cell Motil Cytoskelet 20:301–315
Willard SS, Devreotes PN (2006) Signaling pathways mediating chemotaxis in the social amoeba Dictyostelium discoideum. Eur J Cell Biol 85(9–10):897–904
Williams JG (2006) Transcriptional regulation of Dictyostelium pattern formation. EMBO Rep 7(7):694–698
Wong E, Yang C, Wang J, Fuller D, Loomis WF, Siu CH (2002) Disruption of the gene encoding the cell adhesion molecule DdCAD-1 leads to aberrant cell sorting and cell-type proportioning during Dictyostelium development. Development 129(16):3839–3850
Yamada Y, Kay RR, Bloomfield G, Ross S, Ivens A, Williams JG (2010) A new Dictyostelium prestalk cell sub-type. Dev Biol 339(2):390–397
Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L, Veenstra TD, Parent CA, Jin T (2012) A Gbetagamma effector ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev Cell 22(1):92–103
Yumura S, Yoshida M, Betapudi V, Licate LS, Iwadate Y, Nagasaki A, Uyeda TQ, Egelhoff TT (2005) Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium. Mol Biol Cell 16(9):4256–4266
Zanchi R, Howard G, Bretscher MS, Kay RR (2010) The exocytic gene secA is required for Dictyostelium cell motility and osmoregulation. J Cell Sci 123(Pt 19):3226–3234
Zhang H, Heid PJ, Wessels D, Daniels KJ, Pham T, Loomis WF, Soll DR (2003) Constitutively active protein kinase A disrupts motility and chemotaxis in Dictyostelium discoideum. Eukaryot Cell 2(1):62–75
Acknowledgments
This work was supported by the BBSRC and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Weijer, C.J. (2013). Signalling During Dictyostelium Development. In: Romeralo, M., Baldauf, S., Escalante, R. (eds) Dictyostelids. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38487-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-38487-5_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38486-8
Online ISBN: 978-3-642-38487-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)