Abstract
RNA interference (RNAi) is an evolutionarily conserved, sequence-specific gene-inactivation system that plays an essential role in many biological processes, such as genome defense against mobile DNA elements or regulation of factors involved in plant and animal development. In higher plants and invertebrates, it also functions as a powerful antiviral mechanism. To overcome antiviral RNAi, viruses have evolved suppressor proteins which counteract host RNAi-based antiviral processes and target one or more key points in the RNAi machinery. Here, we review recent progress in our understanding of the mechanism and function of antiviral RNAi in plants and on the viral responses through the expression of silencing suppressor proteins. As a counter-attack RNAi may also regulate innate immunity in plants and contribute to a novel layer of defense against pathogen attack. We also discuss emerging evidence that viruses use RNAi to manipulate host gene expression to modify the cellular environment for the benefit of invading viruses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Viruses are obligate intracellular pathogens that manipulate and exploit the molecular mechanisms of the host to survive in a hostile cellular environment. The presence of viruses and their propagation induces diverse mechanisms in the host for combating viral infection at both the single-cell and the whole-organism levels. Viruses are obligate intracellular pathogens infecting almost all living organisms. During the replication of viruses at a given point of their life cycle, they reach the stage of ssRNA or dsRNA, which can trigger host defense responses. These mechanisms range from RNA interference (RNAi) a mechanism mainly found in plants and lower eukaryotes, to the sophisticated interferon-regulated gene response of higher animals (Ding 2010; Csorba et al. 2009; Ding and Voinnet 2007).
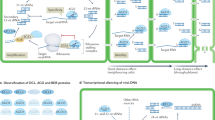
RNAi plays an essential role in many plant biological processes, including developmental timing and patterning, transposon control, DNA methylation, and chromatin modification as well as antiviral defense. Although it operates through multiple pathways, RNAi relies on a set of core processes triggered by double-stranded (ds) or self-complementary foldback RNAs that are processed into 21–24-nt-short small-interfering (si)RNA or micro (mi)RNA duplexes by the RNase III-type DICER enzymes (Bartel 2004; Bernstein et al. 2001; Baulcombe 2004). These miRNAs and siRNAs guide a multiprotein effector complex, the RNA-induced silencing complex (RISC) (Hammond et al. 2000; Tomari and Zamore 2005), of which Argonaute protein (AGO) is the slicer component showing similarity to RNase H (Song et al. 2004; Liu et al. 2004; Tomari and Zamore 2005). RISC is the executioner of RNAi, inhibiting target RNA expression. The specific recognition of target sequences is guided by the si/miRNAs through a base-pairing mechanism, whereas the slicing of target RNA is carried out by the AGO proteins at the post-transcriptional level (Fig. 1) (Bartel 2004; Almeida and Allshire 2005; Brodersen et al. 2008; Eamens et al. 2008).
Endogenous small RNA pathways in plants. a Plant miRNA genes (MIRs) are transcribed into primary miRNA transcripts by RNA polymerase II (Pol II) and the transcript contains a cap and polyA tail. This primary transcript forms a hairpin structure that is cleaved by DCL1 to produce the miRNA/miRNA* duplex. The duplex is methylated by HEN1. The mature miRNA duplex is bound by AGO 1, and the miRNA* strand is eliminated. Plant miRNAs have near-perfect complementarity to their target sites and they bind to these sites to guide the cleavage of target mRNAs, although there are examples where the translation of the mRNA is suppressed without cleavage. The cap-binding complex (CBP) promotes miRNA biogenesis but is not essential. b transacting siRNAs belong to a special class of endogenous siRNAs. Ta-siRNAs require components of both the miRNA and siRNA pathways for their biogenesis. They are produced from long non-coding transcripts made by Pol II that are converted into dsRNA by an RNA-dependent RNA polymerase 6 (RDR6) and SGS3. This long dsRNA is then cleaved in a phased dicing reaction by DCL4 to generate 21-nt ta-siRNAs which are methylated by HEN 1. The phase of the dicing reaction is determined by the initial miRNA cleavage site. Ta-siRNAs target mRNAs in a similar way as miRNAs. c The vast majority of endogenous siRNAs in plants form a complex population of more than 100,000 different siRNAs transcribed from thousands of loci. The biogenesis of the heterochromatic siRNAs requires RNA polymerase IV (PolIV), which is unique to the plant kingdom and homologues to DNA-dependent RNA polymerase II. Pol IV produces a transcript that is converted to a dsRNAs precursor by RDR2 and subsequently cleaved by DCL3 to produce 24-nt ra-siRNAs that are methylated by HEN 1 and are bound by AGO 4 or AGO 6. AGO-bound ra-siRNAs can induce heterochromatin formation or cleavage of Pol V (another plant-specific RNA polymerase)-produced transcripts, which serves as an amplification loop to produce more ra-siRNAs. CLASSY 1 (CLSY1) is a putative chromatin-remodeling protein that acts together with Pol IV
The model plant Arabidopsis thaliana encodes four DICER paralogues with specialized functions (Baulcombe 2004). Dicer-like (DCL)-1 mainly contributes to the production of miRNAs from non-coding, imperfect stem-loop precursor RNAs (Voinnet 2009), whereas populations of 21, 22 and 24-nt-long short-interfering (si)RNAs are synthesized from long, perfectly or near-perfectly base-paired dsRNAs through the action of DCL4, DCL2, and DCL3, respectively (Brodersen and Voinnet 2006; Vazquez 2006; Chapman and Carrington 2007).Upon processing, small RNAs (sRNA) are incorporated into RISC containing one of the ten AGO proteins that are involved in RNAi in Arabidopsis, thereby controlling the expression of genes involved in various pathways.
Besides its essential roles in development, RNAi constitutes the primary natural plant immune system against viruses. Several lines of evidence underline the antiviral role of RNAi; first, viral siRNAs are invariably detected during viral infection, which is a hallmark of the activation of antiviral RNAi (Hamilton and Baulcombe 1999; Szittya et al. 2002). Second, most if not all plant viruses have evolved virulence factors called viral suppressors of RNAi (VSRs) to overcome the RNAi-based host defense. (Silhavy et al. 2002; Ding and Voinnet 2007; Burgyan and Havelda 2011). Furthermore, plants infected by viruses defective in VSR expression rapidly recover from virus-induced diseases and symptoms (Ratcliff et al. 1997, 1999; Silhavy et al. 2002). Third, plants carrying mutations in components of the RNAi pathway are more susceptible to some, but not all, virus infections (Ding and Voinnet 2007).
2 RNAi-Based Antiviral Immunity
Viruses are potentially both initiators and targets of antiviral plant response (Ratcliff et al. 1997), and it has been also demonstrated that these responses are RNA-mediated and that viral siRNAs (vsiRNAs), derived from viral RNA templates, represent the specificity determinant of this antiviral response. The main steps in the formation of an effective antiviral RNAi are (1) activation of RNAi in the cell by the incoming viral RNA, where structured or double-stranded RNAs are recognized by plant Dicer-like (DCL) enzymes, producing vsiRNAs; (2) the protection of vsiRNAs by 2′-O methylation by HEN1; (3) antiviral RISC assembly, in which vsiRNAs are recruited by AGO proteins; (4) targeting cognate viral RNAs by vsiRNA-guided RISC. Alternatively, vsiRNAs or aberrant viral RNAs can enter the plant RNA-dependent RNA polymerase (RDR)-mediated amplification cycle to enhance the antiviral silencing response (Fig. 2).
Antiviral RNAi in plant and its suppression by virus-encoded silencing suppressors. RNAi is initiated by the recognition of viral dsRNAs or partially double-stranded hairpin RNAs, which are processed to viral siRNAs (vsiRNAs) by dsRNA-specific RNases called Dicer-like proteins (DCL2/3/4). In the next step, HSP90-activated AGO1/2/7 is loaded with vsiRNA, thereby forming large RISC, which has likely incorporated other proteins such as the GW motif-containing AGO interactors. Afterward, the vsiRNA-loaded RISC targets viral RNAs by slicing or translational arrest. Secondary vsiRNAs are produced in an amplification loop through the actions of RNA-dependent RNA polymerases (RDRs) and cofactors (SGS3 and SDE5). As indicated, viral silencing suppressors can disrupt these pathways at multiple points preventing the assembly of different effectors or inhibiting their actions
2.1 Activation of RNAi and Production of vsiRNAs
The majority of known plant viruses have RNA genomes and replicate via double-stranded RNA replication intermediates, and it is widely assumed that these dsRNA molecules are the main trigger of antiviral RNAi. However, it turned out that induction of the antiviral response is much more complex. The probability that viral RNAs are present in a naked form in the plant cell is very small. The majority of viral RNAs are in an encapsidated form or in other protein complexes for replication or movement. Moreover, viral replication usually takes place inside specialized replication compartments (Burgyan et al. 1996), and the viral dsRNA replication intermediates can immediately be unwound by viral or host RNA helicases (Ahlquist 2002). It is likely that highly structured single-stranded viral RNAs with stem-loop structures are also recognized by the silencing machinery and directly diced by plant DCLs into vsiRNAs (Fig. 2). The sequencing and experimental data of vsiRNAs strongly support this model since the resulting vsiRNA molecules are imperfect duplexes (Molnar et al. 2005) have a non-random distribution along the viral genome, and they map asymmetrically to the positive strand of the viral RNA (Molnar et al. 2005; Ho et al. 2006; Donaire et al. 2009; Qi et al. 2009; Pantaleo et al. 2010; Szittya et al. 2010). Similarly, in the case of Cauliflower mosaic virus (CaMV), the 35S polycistronic transcript of this dsDNA virus contains a highly structured 600-nt leader, which is the major vsiRNA source (Moissiard and Voinnet 2006). However, this massive production of leader-derived vsiRNAs does not restrict viral accumulation. They may serve as a decoy diverting the silencing machinery from viral transcripts (Blevins et al. 2011). In viroid-infected plants, the strandedness of viroid-specific siRNAs is also asymmetrical and they are preferentially derived from the highly structured plus sense viroid RNA sequence (Itaya et al. 2007), although recent deep sequencing data have shown a more symmetrical origin of viroid siRNAs (Navarro et al. 2009, 2012). Furthermore, in plants infected by the Potyvirus Turnip mosaic virus (TuMV), which has a positive ssRNA genome that expresses a polyprotein, the sequenced vsiRNAs showed similar amounts of (+) and (−) strand vsiRNAs (Ho et al. 2006). This result may suggest that the TuMV-derived vsiRNAs are processed mostly from dsRNA. It is likely that the strandedness and the composition of vsiRNAs are virus specific and strongly depend on the replication strategy, compartmentalization, and other different characteristics of the given virus.
In the case of circular ssDNA geminiviruses, early reports suggested that vsiRNAs are likely derived from dsRNAs formed by overlapping sense-antisense transcripts (Blevins et al. 2006; Ding and Voinnet 2007; Akbergenov et al. 2006). Indeed, it has been shown very recently that the majority of viral siRNAs accumulating during geminiviral infection are plant RDR-independent primary siRNAs, and dsRNA precursors of these siRNAs are likely generated by bidirectional readthrough transcription of circular viral DNA by RNA polymerase II. In contrast to transgenic mRNA, geminiviral mRNAs appear to be poor templates for RDR-dependent production of secondary siRNAs (Aregger et al. 2012). These findings demonstrate that the generation of vsiRNAs is virus specific, indicating that plant DCLs are adapted to different viral replication and expression strategies and are able to recognize different RNA structures formed during virus life cycles.
The Arabidopsis thaliana genome encodes four DCLs for sRNA processing: DCL1 to DCL4. Specific DCLs have major functions in specific silencing pathways but functional redundancy exists between members: DCL1 contributes to miRNA production and has no or little role in the antiviral response. DCL2, DCL3, and DCL4 are able to recognize viral structures and, respectively, generate vsiRNAs of 22, 24, and 21 nt in length (Deleris et al. 2006; Blevins et al. 2006; Aregger et al. 2012).
Biogenesis of vsiRNAs needs the coordinated and hierarchical action of DCL enzymes, and RNA virus infection is mainly affected by DCL4 and to a lesser extent by DCL2. Inactivation of DCL4 reveals the surrogate antiviral role of DCL2. Inactivation of both DCL2 and DCL4 was necessary and sufficient to restore systemic infection of a suppressor-deficient virus, indicating the crucial role of DCL4 and DCL2 in the antiviral response (Deleris et al. 2006; Bouche et al. 2006; Llave 2010). This finding is further supported by the observation that in an immune precipitation experiment, AGO1 was found loaded with 21- and 22-nt vsiRNAs in Nicotiana benthamiana plants infected with p19 mutant tombusvirus (Burgyan et al., unpublished result). Interestingly in the absence of DCL4, the resultant 22-nucleotide viral siRNAs alone did not guide efficient silencing in a CMV–Arabidopsis (virus–host) combination (Wang et al. 2011).
Upon DNA virus infection, the production of 24-nt vsiRNAs by DCL3 is also sufficient for virus-induced gene silencing (Blevins et al. 2006). DCL3-dependent 24-nt-long vsiRNAs have also been detected in Tobacco rattle virus (TRV) and CMV-infected wild-type (wt) plants or Turnip crinkle virus (TCV)-infected dcl4/dcl2 double-mutant Arabidopsis plants (Deleris et al. 2006; Qu et al. 2008; Zhang et al. 2012).
The participation of DCL1 in the antiviral silencing induced by RNA viruses is very limited since DCL1-dependent vsiRNAs are hardly detected in the dcl2/dcl3/dcl4 triple-mutant plants (Deleris et al. 2006; Bouche et al. 2006; Blevins et al. 2006). However, DCL1 promotes DCL3- and DCL4-derived vsiRNAs accumulation upon dsDNA (CaMV) or ssDNA (geminivirus) infection. Very likely, DCL1 excises the stem-loop structures of CaMV 35S leader transcripts, which are very similar to pri- or pre-miRNAs, and renders them more accessible to other DCLs (Moissiard and Voinnet 2006). Recent findings suggest that this leader region is transcribed into long sense- and antisense-RNAs, which serve as a template for a massive quantity of 21-, 22-, and 24-nt viral siRNAs (Blevins et al. 2011). An opposite effect of DCL1 was observed in plants infected with TCV: the disruption of DCL1 function led to higher expression of DCL4 and DCL3 and enhanced antiviral response, suggesting that these proteins are under DCL1-negative control (Qu et al. 2008).
In several virus–plant combinations, in addition to core dicing and slicing components of RNAi, RNAi-mediated viral immunity requires host RDRs to amplify the antiviral plant response and produce viral secondary siRNAs (Fig. 2). Arabidopsis encodes six identified RDRs (Wassenegger and Krczal 2006), and they play a role in the amplification of the silencing response by production of secondary vsiRNAs (Voinnet 2005; Diaz-Pendon et al. 2007; Llave 2010). The amplification and the high level of secondary vsiRNA in many virus infections depend on the combined activity of the host-encoded RDRs such as RDR1, RDR2, and RDR6, suggesting that aberrant viral single-stranded RNAs lacking quality control marks might be converted by RDR enzymes to dsRNAs, which could serve as a substrate for secondary vsiRNA production (Donaire et al. 2008; Diaz-Pendon et al. 2007; Bao et al. 2009; Garcia-Ruiz et al. 2010; Qu 2010). These siRNAs, upon incorporation into RISC, execute effector steps of RNAi and also direct further amplification rounds by releasing the cleaved target RNAs, thus providing additional templates for RDR enzymes (Voinnet 2005; Vaucheret 2006).
It has also been suggested that the RDR-dependent secondary vsiRNAs can drive a more effective antiviral response (Vaistij and Jones 2009; Wang et al. 2010). However, this is not a general phenomenon for all plant viruses since other studies have shown that loss-of-function mutations in RDR6 have no detectable impact on the production of vsiRNAs and virus accumulation in Arabidopsis plants infected with TRV, TCV, and cr-TMV (Dalmay et al. 2000, 2001; Blevins et al. 2006; Deleris et al. 2006; Szittya et al. 2010).
These findings indicate that although there are very conserved steps in the silencing-based antiviral response, plants are able to respond specifically to different viruses, demonstrating the versatility of this antiviral surveillance mechanism.
2.2 Assembly of Antiviral RNA-Induced Silencing Complexes
The Arabidopsis genome contains ten AGO proteins, AGO1 to AGO10, and they are the catalytic components of the effector complexes (RISC) of RNAi. AGO proteins interact with small RNAs to inhibit gene expression by target cleavage or translational arrest in all RNAi-related pathways known so far (Fig. 1). Endogenous or exogenous small RNAs are loaded into distinct AGO-containing effector complexes to guide them to their RNA target molecules (Ding and Voinnet 2007; Vaucheret 2008; Hutvagner and Simard 2008). In plants, the loading of siRNAs into a particular AGO complex is preferentially, but not exclusively, dictated by their 5′ terminal nucleotides (Mi et al. 2008; Takeda et al. 2008). It has been shown previously that both AGO1 and AGO7 function to ensure the efficient clearance of viral RNAs and that AGO7 appears to work as a surrogate slicer in the absence of AGO1 (Qu et al. 2008). Moreover, it is likely that AGO1 is capable of targeting viral RNAs with more compact structures, whereas AGO7 favors less structured RNA targets (Qu et al. 2008). Our knowledge is still limited about plant si/miRNA RISC assembly, although a recently developed in vitro system has provided further insights into plant RISC development and RNA targeting mediated by this effector (Iki et al. 2010, 2011). Accumulating evidence has shown that AGO2 is also involved in RNAi-based antiviral responses (Harvey et al. 2011; Scholthof et al. 2011; Wang et al. 2011; Zhang et al. 2011, 2012; Jaubert et al. 2011). Moreover, complementation assays in ago2-1 plants with different AGO2 forms demonstrated the requirement for AGO2 catalytic residues for antiviral activity, emphasizing its role in antiviral defense in plants (Carbonell et al. 2012). Indeed a recent report has shown a direct evidence for AGO2 slicer activity (Schuck et al. 2013).
2.3 Viral RNA Targeting by Antiviral RISC
The DCL-mediated processing of viral dsRNA regions into vsiRNA in theory could be enough for viral RNA degradation. However, dcl2/dcl3, dcl2/dcl4, and dcl3/dcl4 mutant plants infected with TRV had approximately equivalent levels of vsiRNAs but only dcl2/dcl4 plants showed strong viral symptoms and high virus titer (Deleris et al. 2006). Strikingly, high levels of viral siRNAs in the absence of AGO1, AGO2, or both were insufficient to confer resistance, suggesting that AGO1 and AGO2 are essential for the antiviral activities of viral siRNAs downstream of DCL-mediated siRNA biogenesis (Wang et al. 2011).
AGO1 was suggested to be involved in antiviral silencing, as hypomorphic ago1 mutants are hypersensitive to CMV infection (Morel et al. 2002). Pull-down experiments revealed that AGO1 recruits vsiRNAs and the AGO1-vsiRNA complex is a major player in antiviral defense (Zhang et al. 2006). In addition, it has been reported that both AGO2 and AGO5 can bind CMV-derived vsiRNAs, selecting for short RNAs having 5′ A and C nucleotides, respectively (Takeda et al. 2008; Mi et al. 2008).
More direct evidence for the existence of antiviral RISC comes from studies with the positive-strand RNA Cymbidium ringspot virus (CymRSV). Two vsiRNA-containing silencing complexes, which co-fractionated with miRNA-containing complexes, were detected in infected plants: the smaller one at approximately the AGO1-siRNA size (150 kDa), the so-called minimal RISC, and a high molecular weight (670 kDa) multiprotein complex (Pantaleo et al. 2007) probably analogous to animal RISC (Pham et al. 2004). A similar complex was isolated in separate experiments involving another tombusvirus. This complex contained vsiRNAs and exhibited in vitro nuclease activity, which preferentially targeted homologous viral sequences (Omarov et al. 2007). Strikingly, viral RNA was targeted in a non-random fashion in hot spots by the antiviral RISC in Cym19stop suppressor mutant virus-infected plants (Pantaleo et al. 2007). Those regions of viral RNA that show hot spots for vsiRNA generation probably form strong secondary structures, which are selectively recognized by DCLs. However, these hot spots are poor targets for RISC-mediated cleavage, since RNA sequences possessing strong secondary structures are not accessible to RISC (Szittya et al. 2002; Ameres et al. 2007; Pantaleo et al. 2007). The accessibility of the viral RNA is probably also influenced by encapsidation, formation of replication complexes containing host and viral proteins and compartmentalization of virus replication. It has been shown recently that there is asymmetry in the strandedness of virus-derived siRNAs, showing that the majority of viral siRNAs have plus-stranded viral sequences (Molnar et al. 2005; Ho et al. 2006; Donaire et al. 2009; Qi et al. 2009; Szittya et al. 2010). This finding suggests that viral siRNA-guided RISC should target more frequently the viral strand having negative polarity than the plus-stranded viral RNA. Indeed, in previous experiments, strand-specific sensors were used for sensing antiviral RISC-mediated cleavages, and the sensor RNAs carrying (−) strand sequences were better targets than the (+) strand sensors (Pantaleo et al. 2007; Szittya et al. 2010). It is worth noting that the level of negative-strand viral RNA is a rate-limiting factor for viral replication; thus preferential targeting of the negative viral strand could make the antiviral silencing response very efficient and very attractive for plant defense. The analysis of 5’ RNA cleavage products of sensor RNAs and viral RNAs reveals the presence of non-templated U residues at the cleavage site (Pantaleo et al. 2007), this is the signature of RISC action (Shen and Goodman 2004), confirming the presence of RISC-mediated slicing.
According to the current model of virus-induced RNAi (Fig. 2), a large amount of vsiRNA originates from partially base-paired regions of plus-stranded viral RNAs (Molnar et al. 2005; Ding and Voinnet 2007; Szittya et al. 2010). Thus, plus-stranded vsiRNAs could also potentially target plus-stranded viral RNA through translational arrest. Indeed, recent findings suggest that translational arrest could also be a widespread way to inhibit gene expression by plant miRNAs and siRNAs (Brodersen et al. 2008; Lanet et al. 2009). Moreover, a novel role of AGO4 has been suggested for specific translational control of viral RNA (Bhattacharjee et al. 2009). AGO7 was also shown to function as a surrogate slicer in the absence of AGO1 in the clearance of viral RNA of TCV and favors less structured RNA targets (Qu et al. 2008). More recently, Scholthof and co-workers reported that a N. benthamiana AGO (NbAGO) with similarity to Arabidopsis AGO2 is involved in antiviral defense against TBSV. The activity of this NbAGO2 was shown to be directly associated with anti-TBSV RNAi, suggesting that NbAGO2 might primarily function in antiviral defense (Scholthof et al. 2011).
3 Strategies of Antiviral RNAi Suppression
The fact that most viruses have evolved viral suppressors of RNAi (VSRs) underlines the antiviral nature of RNAi and reveals a pathogen counter-defensive strategy leading to the active suppression of host surveillance (Voinnet et al. 1999; Silhavy and Burgyan 2004; Ding and Voinnet 2007; Burgyan and Havelda 2011). Indeed, plant viruses often cause severe symptoms and damage, demonstrating an efficient counter-defensive viral strategy. The first VSRs were identified more than a decade ago (Anandalakshmi et al. 1998; Brigneti et al. 1998; Kasschau and Carrington 1998), which was followed by the discovery of several additional VSRs (Silhavy and Burgyan 2004; Burgyan and Havelda 2011). In the past, more than 50 individual VSRs have been identified from almost all plant virus genera, underlining the need of their expression for successful virus infection (Ding and Voinnet 2007; Diaz-Pendon and Ding 2008; Csorba et al. 2009). Available data suggest that virtually all plant viruses encode at least one suppressor, but in many cases viruses encode more than one (e.g., carmo-, clostero-, crini- and begomoviruses) (Csorba et al. 2009). VSRs are surprisingly diverse within and across kingdoms, with no obvious sequence homology, and are therefore considered to be the outcome of recent evolutionary processes. The various VSRs are able to target all steps of the RNAi pathway, such as viral RNA recognition, dicing, RISC assembly, RNA targeting, and amplification (Fig. 2). Since the first discovery of VSRs, a lot of effort has gone into understanding the molecular basis of RNAi suppression. However, our knowledge of the molecular mechanisms of action of VSRs is still restricted to only a few cases, in large part because the majority of VSRs have essential viral functions besides being RNAi suppressors. This multifunctional nature of VSRs often causes serious difficulties in the exploration of their mechanisms, because the inactivation of VSRs often leads to a loss of viability of the given virus.
3.1 Viral Suppressors Interacting with RNAi-Related RNAs
The inhibition of viral RNA recognition and the subsequent dicing by plant DICER effectors is not a frequent strategy of known VSRs. Two viral proteins have been identified which inhibit the processing of dsRNA to siRNAs in agroinfiltration assays: P14 of Pothos latent aureusvirus (PoLV) and P38 of Turnip crinkle virus (TCV). In addition, P38 and P14 have been shown to bind dsRNA in a size-independent way (Merai et al. 2006, 2005). However, the dsRNA-binding properties of P38 have been questioned using transgenic plants (Schott et al. 2012). Instead, p38 was shown to inhibit antiviral RNAi through AGO1 binding and interference with the AGO1-dependent homeostatic network, which leads to the inhibition of Arabidopsis DCLs (Azevedo et al. 2010). Genetic evidence was also reported, that P38 specifically inhibits Dicer-like 4 (DCL4) activity, which has been shown to be the primary antiviral DICER in the Arabidopsis model plant (Deleris et al. 2006). In addition, the P6 VSR of CaMV (Love et al. 2007) has been shown to interfere with vsiRNA processing. P6 was previously described as a viral translational transactivator protein essential for virus biology. Importantly, P6 has two importin-alpha-dependent nuclear localization signals, which are mandatory for CaMV infectivity. A recent discovery showed that one of the nuclear functions of P6 is to suppress RNAi by interacting with double-stranded RNA-binding protein 4 (DRB4), which is required for the function of DCL4 (Haas et al. 2008).
The most common suppression strategy, evolved by several viral genera, is duplex siRNA sequestration (Lakatos et al. 2006; Merai et al. 2006; Csorba et al. 2007, 2009; Ding and Voinnet 2007; Wu et al. 2010), which prevents assembly of the RISC effector complex. These siRNA-binding VSRs are unrelated proteins, although they share analogous biochemical properties, suggesting their independent evolution in different viruses. The P19 protein of tombusviruses, probably the best-known VSR so far, prevents RNAi by siRNA sequestration through binding duplex siRNAs with high affinity (Silhavy et al. 2002). Crystallographic studies have shown that P19 forms a tail-to-tail homodimer, which acts like a molecular caliper, measuring the length of siRNA duplexes and binding them in a sequence-independent manner, selecting for the 19-bp-long dsRNA region of the typical siRNA duplex (Vargason et al. 2003; Ye et al. 2003). Thus, the P19 VSR evolved to bind and inactivate vsiRNAs, which are the most conserved elements of the RNAi pathway. Indeed, the P19 VSR was also able to inhibit siRNA-directed RNA cleavage and to suppress the assembly of the RISC in vitro (Lakatos et al. 2006; Schuck et al. 2013) or in vivo systems (Dunoyer et al. 2004). In addition, recent findings also demonstrate that P19 inhibits the spread of the siRNA duplex identified as the signal of systemic RNAi (Dunoyer et al. 2010). In a very recent report, Schott and co-workers strongly criticized the reliability of in vitro siRNA-binding assays, which are generally used to identify the molecular mechanism of RNAi inhibition. Using transgenic Arabidopsis lines expressing four distinct VSRs (the P38, the P15 protein of Peanut clump virus, the Hc-Pro of TuMV, and the tombusviral P19), they concluded that the siRNA/miRNA sequestration strategy of P19 is an exception instead of representing a common suppression strategy of VSRs. In contrast, previous studies using virus-infected plants have generated direct in vivo evidence, using pull-down approaches, that P19 s of CymRSV and Carnation Italian ringspot virus (CIRV), Hc-Pro of Tobacco etch virus (TEV), and P21 of Beet yellows virus efficiently bind vsiRNAs in virus-infected plants (Lakatos et al. 2004, 2006; Lozsa et al. 2008). Importantly, virus-expressed Hc-Pro also bound plant miRNA duplexes (Lakatos et al. 2006). However, P19 expressed by CIRV efficiently bound vsiRNAs but failed to bind miRNA duplexes (Lozsa et al. 2008). These findings indicate that transgenically expressed VSRs may not really reflect their normal biological function. It has also been reported that transgenically expressed P19 and HC-Pro VSRs prevent the essential siRNA and miRNA 2′-O methylation steps (Yu et al. 2006; Ebhardt et al. 2005), which appears to protect small RNAs from oligouridinilation and subsequent degradation (Li et al. 2005). Thus, it seems that the inhibitory effect of VSRs may require the temporal and spatial co-expression of the suppressor and the endogenous siRNAs and miRNAs (Lozsa et al. 2008).
The 2b protein of Cucumovirus Tomato aspermy virus (TAV) and B2 of the insect-infecting Flock House virus also show siRNA-binding activity; however, studies have shown that the structures of silencing suppressor proteins and their mode of binding siRNAs do not share any similarity (Chen et al. 2008; Chao et al. 2005).
In sum, plants fail to confine the virus infection and spread, since vsiRNAs are sequestered by siRNA-binding VSRs before they can be incorporated into the RNAi based–antiviral effector complexes.
The P14 protein of PoLV binds long and short dsRNAs (including duplex siRNAs) in a size-independent way (Merai et al. 2005, 2006). P14 may interact with viral dsRNAs, inhibiting the RNA-silencing machinery on two levels: (1) by siRNA sequestration (Merai et al. 2006), and (2) by interfering with DCL4-mediated vsiRNA processing. The inhibition of DCL4 by P38 has been confirmed experimentally (Deleris et al. 2006). In contrast to dsRNA-binding VSRs, the AC4 suppressor of African cassava mosaic virus binds single-stranded small RNAs bound by AGOs and prevents holo-RISC assembly. AC4 also inhibits miRNA-mediated negative regulation of endogenous genes (Chellappan et al. 2005). Rice stripe virus NS3 and Grapevine virus A P10 proteins are also able to sequester ss-siRNA molecules (Xiong et al. 2009; Zhou et al. 2006) implying, in part at least, a similar strategy to AC4.
A very similar outcome is achieved by adopting a completely different strategy in the case of the Crinivirus Sweet potato chlorotic stunt virus (SPCSV). An SPCSV-encoded RNaseIII endonuclease cleaves the 21-, 22- and 24-vsiRNAs into 14-bp products, which are inactive in the RNAi pathways (Cuellar et al. 2009).
The V2 protein from Tomato yellow leaf curl virus has been shown to be an efficient suppressor of RNAi (Zrachya et al. 2007; Glick et al. 2008), and V2 was proposed to interact with the tomato protein SGS3 in infected plant cells (Glick et al. 2008). However, in vitro studies on V2 show that it outcompetes SGS3 protein for binding a dsRNA with 5′ ssRNA overhangs, whereas a V2 mutant lacking the suppressor function in vivo cannot efficiently overcome SGS3 binding (Fukunaga and Doudna 2009). These findings not only predict a new type of RNA-binding VSR but also may reveal a new RNA intermediate, which is essential for SDS3-/RDR6-dependent siRNA formation in the plant (Kumakura et al. 2009).
3.2 Viral Suppressors Interacting with RNAi-Related Host Proteins
The inhibiton of antiviral RNAi also happens through direct or indirect interactions between VSRs and the protein components of RISC. The 2b protein of CMV, one of the first described VSRs (Brigneti et al. 1998), prevents the spread of the long-range silencing signal, thus facilitating systemic virus infection (Guo and Ding 2002). Consistently, the 2b protein of Fny-CMV has been found to physically interact with the PAZ and part of the PIWI-domain of AGO1, inhibiting the slicing activity of AGO1 (Zhang et al. 2006). The Fny-CMV 2b protein was found to preferentially co-localize with the AGO1 protein in the cell’s nucleus and also in cytoplasmic foci (Mayers et al. 2000). Fny-CMV 2b protein expression phenocopies the ago1–27 mutant phenotype (Zhang et al. 2006). The VSR mutant CMV-Δ2b can be rescued by the dcl2/dcl4 host double mutant, which is impaired in vsiRNA production, suggesting that 2b is dispensable for infection and spread in a host defective in small RNA-directed immunity (Diaz-Pendon et al. 2007). The 2b protein is also known to bind siRNAs and long dsRNA (Goto et al. 2007) and to inhibit the production of viral secondary siRNAs (Diaz-Pendon et al. 2007). Thus, cucumovirus 2b proteins have a dual mode of silencing inhibition, either by sequestering siRNAs or by interacting with AGO1 and preventing RISC assembly.
The P0 protein of the phloem-limited poleroviruses also targets the AGO1 protein, the core component of RISC for degradation (Pazhouhandeh et al. 2006; Bortolamiol et al. 2007; Baumberger et al. 2007). The P0 protein is indispensable for viral infection since null mutations of P0 in Beet western yellows virus (BWYV) and Potato leafroll virus strongly diminish or completely abolish virus accumulation (Mayo and Ziegler-Graff 1996). In contrast to the RNA-binding VSRs, P0 has no RNA-binding activity (Zhang et al. 2006; Csorba et al. 2010). Instead, it interacts with the SCF family of E3-ligase S-phase kinase-related protein1 (SKP1) components, orthologous to Arabidopsis ASK1 and ASK2, by means of its minimal F-box motif and promotes AGO1 degradation (Pazhouhandeh et al. 2006; Baumberger et al. 2007; Bortolamiol et al. 2007). The mutation of the F-box motif of P0 leads to the loss of silencing suppressor activity, suggesting the involvement of the proteasome pathway in AGO1 degradation. However, P0-mediated AGO1 degradation is insensitive to proteasome inhibitors, which is inconsistent with the idea that AGO1 is targeted by P0 for ubiquitination and proteasome-dependent degradation. Furthermore, P0 does not appear to interact directly with AGO1 (Baumberger et al. 2007; Bortolamiol et al. 2007; Csorba et al. 2010). Indeed, it has been found that P0 cannot interfere with the slicer activity of pre-programmed siRNA-/miRNA-containing AGO1, but it prevents the de novo formation of siRNA-/miRNA-loaded AGO1 (Csorba et al. 2010). The transgenic expression of P0 in Arabidopsis leads to severe developmental abnormalities, similar to those induced by mutants affecting the miRNA pathways. This effect of P0 is accompanied by AGO1 protein decay in planta and enhanced levels of several miRNA-target transcripts (Bortolamiol et al. 2007).
Two recent studies illustrated a new strategy for VSR binding to AGO whereby the viral proteins mimic the cellular glycine/tryptophan (GW) repetitive motif (Azevedo et al. 2010; Giner et al. 2010). The GW linear peptide motif has been identified in different silencing-related proteins, and it functions as an “Ago hook” to interact with AGO proteins (Till and Ladurner 2007; Karlowski et al. 2010). The AGO-interacting GW motif has been identified in the largest subunit, NRPD1b, of RNA polymerase V in plants (El-Shami et al. 2007), in human GW182 protein (Behm-Ansmant et al. 2006; Eulalio et al. 2008; Liu et al. 2005), and in the Tas3 homologue of the GW182 RITS complex component in Schizosaccharomyces pombe (Buhler et al. 2006; Verdel et al. 2004). In addition, the RdDM effector KTF1 contains several GW motifs and was identified as an AGO4-binding element (Bies-Etheve et al. 2009; He et al. 2009). It was shown that the P38 VSR protein contains two GW repeats and interacts with A. thaliana AGO1 but not AGO4 (Azevedo et al. 2010). Direct AGO1 binding to the individual N-terminal and C-terminal GW motifs of P38 in vitro was also detected, and the interaction was abolished by changing GW to GA. Point mutations in the P38 GW residues are sufficient to abolish TCV virulence, which is restored in the A. thaliana ago1 hypomorphic mutant, uncovering both physical and genetic interactions between the two proteins. It has been suggested that P38 compromises small RNA loading into AGO1 by interacting with non-loaded AGO1 (Azevedo et al. 2010). It was also shown that AGO1 inactivation by P38 profoundly impacts the cellular availability of the four Arabidopsis DCLs, uncovering an AGO1-dependent, homeostatic network that functionally connects these factors (Azevedo et al. 2010). Recently, it was shown that P38 also interacts with Arabidopsis AGO2; however, this interaction also depends on a region of P38, which is outside of the GW motif (Zhang et al. 2012). The P1 VSR of Sweet potato mild mottle ipomovirus (SPMMV) also uses a GW Ago hook to inhibit siRNA-/miRNA-loaded RISC activity (Giner et al. 2010). It has been shown that the interaction between P1 and small RNA-loaded AGO1 is specific and direct. The suppression activity mapped to the N-terminal part of P1, containing three GW motifs, and site-directed mutagenesis proved that these motifs are essential for both binding and suppression of AGO1 functions. An attractive model is therefore suggested for the mechanism of silencing suppression mediated by P1, that is, it can mimic essential AGO1 interactors and outcompete them for RISC binding. Indeed, these AGO1 interactors have recently been identified in Arabidopsis (Garcia et al. 2012). Alternatively, P1 might compromise base-pairing between the small RNA-loaded AGO1 and the target RNA (Giner et al. 2010).
The action of P38 and P1 VSRs indicates a new mechanism for the suppression of RNAi by mimicking the plant GW proteins that hook up to AGOs, and it might represent a widespread strategy by pathogens to counteract RNAi-based host defenses.
3.3 Other RNAi Suppressor Strategies
Recent work has revealed a new strategy for antiviral silencing suppression through the specific induction of the plant miRNA miR-168, which has an important function in AGO1 homeostasis (Vaucheret et al. 2006; Varallyay 2010). It has been observed that CymRSV-infection enhances the expression of AGO1 mRNA; however, this was not followed by increased AGO1 protein levels. It was also shown that the P19 protein specifically increased the expression of miR-168, which controls the AGO1 mRNA level. By contrast, p19 suppressor mutant virus infection did not result in miR-168 induction and enhanced accumulation of the AGO1 protein, suggesting that the P19 VSR inhibits the translation of AGO1 mRNA by enhancing the endogenous miR-168 level to alleviate the antiviral function of the AGO1 protein (Varallyay et al. 2010). However, the mechanism behind the specific induction of miR-168 and the inhibition of AGO1 translation is not fully understood. Importantly, it has also been demonstrated that in virus-infected plants, P19 is not able to efficiently bind miRNA duplexes, including miR-168 (Lozsa et al. 2008) suggesting that the specific induction of miR-168 by P19 is not mediated through miR-168 binding. The down-regulation of AGO1 protein may have some collateral effects similar to the recently described AGO1 quenching by P38, which impacts the cellular availability of the four Arabidopsis DCLs, compromising the AGO1-dependent homeostatic network (Azevedo et al. 2010). An insufficient level of AGO1 protein in virus-infected plants can lead to the mis-regulation of miRNA targets, resulting in disordered host cell gene expression, which could facilitate the development of viral symptoms.
Host factors involved in both RNAi and viral replication have been proposed to play roles in RNAi suppression during infection by Red clover necrotic mosaic virus (RCNMV). The putative host factor involved in both processes could be the DCL1 protein, because miRNA biogenesis is inhibited by virus replication and dcl1 mutant plants show reduced susceptibility to RCNMV infection (Takeda et al. 2005). In the suggested scenario, DCL1 and its homologues are recruited by the viral replication complex and are therefore depleted from the silencing pathways.
4 Effects of Viral Suppressors on Endogenous RNAi Pathways and Induced Viral Symptoms
The antiviral RNAi pathway and the components of the endogenous RNAi pathways overlap therefore VSRs may also have the capacity to interfere with many aspects of these endogenous pathways. This hypothesis has been supported by the observation that many VSRs are pathogenicity determinants and responsible for virus-induced symptoms (Voinnet 2005). Furthermore, in many cases, transgenic plants expressing VSRs constitutively show developmental abnormalities that mimic the phenotype of miRNA deficient hypomorphic dcl1 mutant plants (Chapman et al. 2004; Dunoyer et al. 2004; Kasschau et al. 2003; Mallory et al. 2002; Jay et al. 2011).
4.1 Viral Suppressors Interaction with Endogenous Small (si-, mi- and hcRNAs) RNAs
siRNA-binding VSRs (e.g., P19, Hc-Pro, P122, and P21) can interact with endogenous siRNA and miRNA biogenesis and can result in the overexpression of siRNA-regulated genes. Indeed, Arabidopsis plants stably expressing the P19 protein altered the function of miRNAs and induced developmental defects by preventing the activity of the mature miRNA strand through the sequestering miRNA/miRNA* duplexes (Dunoyer et al. 2004). Furthermore, transgenic Arabidopsis lines expressing P38, P15, Hc-Pro, and P19 were also found to be compromised for the loading of siRNAs into AGO1. However, in the case of P38, the inhibition of siRNA loading was mainly the consequence of its direct binding to AGO1. Surprisingly, only P19 was found to efficiently prevent both siRNA and miRNA loading into AGO1. Interestingly, all VSR-transgenic plants efficiently inhibited miRNA-mediated target regulation (Schott et al. 2012) independently of miRNA sequestration. The use of these transgenic systems provides a tool to study the components of the RNAi pathways. However, they do not necessarily reproduce faithfully the molecular effects of viral infections on the RNAi pathways. It is important to note that during infections, the expression of VSRs is restricted to virus-infected tissues and compartments and is also limited by time.
Other VSRs target different stages of the RNAi pathway. The V2 VSR of TYLCV interacts directly with the tomato SGS3 protein. Since SGS3 is also required for the endogenous tasiRNA pathway, the potential inactivation of SGS3 by V2 also may affect the development of TYLCV disease symptoms. Accordingly, both TYLCV-infected tomatoes and tomato plants carrying a mutation within the sgs3 locus exhibit an aberrant leaf phenotype (Glick et al. 2008). Some of the VSRs can inhibit HEN1-mediated methylation (Lozsa et al. 2008). All endogenous classes of plant small RNAs are 2’-O methylated by HEN1, which protects them from degradation (Yu et al. 2005; Park et al. 2005; Li et al. 2005). In agreement with this, transgenic Arabidopsis plants expressing different VSR proteins, including HC-Pro, P21, and P19, inhibited HEN1-mediated methylation (Yu et al. 2006). However, in natural virus infection, it was found that the HEN1-dependent methylation of viral siRNAs was variable, depending on the virus species. It was shown that P19 had only a slight effect on viral siRNA 3′ modification; however, Hc-Pro significantly inhibited the 3′modification of vsiRNAs and miRNAs as well (Lozsa et al. 2008). These results further emphasize the intrinsic difference between the use of VSR-expressing transgenic plants and natural infections.
VSRs can also suppress RNAi-based antiviral immunity through protein–protein interaction. Several VSRs were shown to interact with AGO1, including P38 (Azevedo et al. 2010), P1 of SPMMV (Giner et al. 2010) and the 2b protein of the CMV Fny strain (Zhang et al. 2006). The poleroviral P0 and P0(PE) of the Enamovirus Pea enation mosaic virus-1 VSR proteins were suggested to act as an F-Box protein targeting AGO proteins for degradation, thereby preventing RISC assembly (Baumberger et al. 2007; Bortolamiol et al. 2007; Csorba et al. 2010; Derrien et al. 2012; Fusaro et al. 2012). Inhibiting AGO1 function during virus infection can influence the regulation of miRNA-regulated endogenous genes. Accordingly, 2b, P0, and P1 are pathogenicity determinants (Pfeffer et al. 2002; Voinnet et al. 1999; Brigneti et al. 1998), and AGO1 suppression by P38 profoundly modifies a homeostatic interaction network linking the four Arabidopsis DCLs. This is thought to result from reduced levels of miR162-containing AGO1, which enhances DCL1 accumulation which in turn decreases DCL4 and DCL3 levels through an unidentified mechanism (Azevedo et al. 2010).
In Arabidopsis, the AGO1 level is maintained by an autoregulatory feedback loop by miR-168 (Vaucheret et al. 2006). The P19 VSR of CymRSV specifically increases the expression of miR-168 to alleviate plant antiviral function. Therefore, P19 can also inhibit the translation of AGO1 mRNA by enhancing endogenous miR-168 levels (Varallyay et al. 2010).
In plants, siRNAs can also target homologous genomic DNA sequences for cytosine methylation, a process known as RNA-directed DNA methylation (RdDM). This mechanism transcriptionally silences many endogenous loci, including transposons and DNA repeats, and can be utilized as a defense against DNA viruses (Matzke et al. 2009). As a consequence, several viruses encode proteins that can suppress RdDM including the P6 protein of CaMV (Haas et al. 2008) and the C2 protein (also known as AL2 and AC2) of geminiviruses (Wang et al. 2005; Voinnet et al. 1999; van Wezel et al. 2002; Buchmann et al. 2009; Raja et al. 2008; Yang et al. 2012). It was shown that geminivirus AL2 and L2 (AL2 related) proteins interact with and inactivate an adenosine kinase, which is required for efficient production of S-adenosyl methionine, an essential methyltransferase cofactor. This inhibition protects viral DNA from methylation (Raja et al. 2008). Indeed, transgenic expression of AL2 or L2 causes a global reduction in cytosine methylation and therefore geminivirus proteins can reverse RdDM by non-specifically inhibiting cellular transmethylation reactions (Buchmann et al. 2009).
4.2 The Effect of Viral siRNAs on Host Transcripts
During virus infection, DCL4 and DCL2 act in a hierarchical manner to produce 21- and 22-nt-long viral siRNAs (Deleris et al. 2006; Fusaro et al. 2006). These vsiRNAs can be incorporated into AGO complexes and can inhibit the expression of viral RNAs (Pantaleo et al. 2007; Omarov et al. 2007; Llave 2010). Furthermore, it was also demonstrated by several groups that recombinant viruses carrying endogenous sequences can guide AGO-containing effector complexes to endogenous mRNA transcripts (Becker and Lange 2010). These observations led to the hypothesis that vsiRNAs can also specifically regulate host gene expression if host genes share sequence complementarity with vsiRNA. Accordingly, bioinformatic analysis of small RNA deep–sequencing data from a crucifer-infecting strain of Tobacco mosaic virus (TMV-Cg)-infected Arabidopsis showed that a large set of host genes could be potentially targeted by TMV-Cg-derived vsiRNAs. A small subset of the predicted host targets were selected for experimental validation and two of them, which encode a cleavage and polyadenylation specificity factor (CPSF30, At1g30460) and an unknown protein similar to translocon-associated protein alpha (TRAP α, At2g16595), respectively, yielded a positive result upon cleavage validation by 5′-RACE assays (Qi et al. 2009). In another study, it was reported that vsiRNAs derived from the CaMV 35S leader sequence bear near-perfect sequence complementarity to Arabidopsis transcripts, and evidence was provided that at least one of the vsiRNAs specifically down-regulated the Arabidopsis At1g76950 mRNA (Moissiard and Voinnet 2006). Moreover, it was found in two independent studies that vsiRNAs from the Y-satellite of CMV specifically cleave the mRNA of Nicotiana tabacum magnesium protoporphyrin cheletase subunit I (CHLI, the key gene involved in chlorophyll synthesis), thus inducing the typical yellow symptoms in tobacco. It was demonstrated using 5’-RACE, GFP sensor experiment, and transgenic plants that vsiRNA-directed cleavage of the CHLI host gene is solely responsible for the Y-satellite-induced symptoms (Shimura et al. 2011; Smith et al. 2011). Similarly, viroid-derived siRNAs resulting from the host defensive response, via RNAi, can target plant cell mRNAs and trigger a signal cascade leading to symptoms such as severe leaf albinism (Navarro et al. 2012). Moreover, a recent study analyzing the data sets by integrating the vsiRNAs and degradom libraries from Vitis vinifera identified a pool of vsiRNAs, which are responsible for the cleavage of host mRNAs. Among them, several encode putative proteins involved in ribosome biogenesis and in biotic and abiotic stresses. The bioinformatic analysis of these data also showed that a large fraction of vsiRNAs did not induce host mRNA cleavage, suggesting that relatively few vsiRNAs are involved in the inhibition of host mRNAs (Miozzi et al. 2013).
vsiRNAs can also target homologous genomic DNA sequences through RdDM to interfere with gene expression at a transcriptional level. A PVX vector carrying the promoter sequence of the GFP transgene triggered transcriptional gene silencing through the sequence-specific epigenetic modification of DNA and chromatin (Jones et al. 1999, 2001). Furthermore, CaMV infection of a transgenic oilseed rape line containing an herbicide tolerance gene regulated by the 35S promoter, altered the expression of the herbicide tolerance gene such that plants became susceptible to the herbicide. Susceptibility to the herbicide was most likely the result of transcriptional gene silencing of the transgene (Al-Kaff et al. 1998).
These observations suggest that viruses might also be able to harness the RNAi-based antiviral plant response for the benefit of the virus and that vsiRNAs which create a cellular environment more suitable for viral proliferation (i.e., by targeting host defense factors) may be positively selected (Ruiz-Ferrer and Voinnet 2009).
5 RNAi may Regulate Innate Immunity in Plants
In plants, RNAi is considered as the primary antiviral defense system (Ding and Voinnet 2007). However, plants also respond to pathogen infection using innate immunity, which is also a conserved and evolutionary ancient mechanism (Ding and Voinnet 2007; Jones and Dangl 2006; Chisholm et al. 2006). To recognize invading pathogens, plant genomes contain two major classes of innate immune receptors. The primary immune response comprises the pattern recognition receptors (PRRs) that recognize conserved pathogen-associated molecular patterns (PAMPs) by the diverse transmembrane pattern recognition receptors (PRRs) and activate PAMP-triggered immunity (PTI). However, to counteract PTI and establish infection in susceptible host, pathogens can suppress PTI, usually through secreted proteins called effectors. To recognize effector proteins, plant genomes have also evolved large numbers of resistance (R) genes with nucleotide-binding (NB) and leucine-rich repeat (LRR) domains (Meyers et al. 2005), which function as a second line of defense that mediate intercellular recognition of specific pathogen effectors and initiate effector-triggered immunity (ETI). ETI is considered to be an accelerated and amplified version of the PTI response. The ETI signaling cascade often leads to disease resistance, usually through a hypersensitive cell death response (HR) at the infection site (Jones and Dangl 2006). There is no evidence for a viral PAMP so far. However, several viral proteins including VSRs (e.g., P126 of TMV, P38) can function as effector proteins that trigger R gene-dependent hypersensitive responses in specific hosts (Zvereva and Pooggin 2012). Although R-genes provide protection from diverse pathogens, their large numbers and cell death-triggering activity are potential threats to plant fitness (Tian et al. 2003). Despite the enormous agricultural importance of R-genes, the mechanisms regulating their expression are not well understood. Recently, small RNAs emerged as important gene expression regulators of various cellular processes. In plants, based on their biogenesis, we distinguish two main classes of endogeneous small RNAs: miRNAs and siRNAs. Despite differences in their biogenesis, one group of siRNAs also regulates protein-coding genes very similarly to miRNAs and these are called ta-siRNAs or secondary siRNAs. The formation of ta-siRNAs from a non-coding RNA (TAS) precursor is dependent on a miRNA trigger (Fig. 1). Recent studies have revealed the presence of a regulatory cascade that affects a large portion of NB-LRR resistance genes. The initiator of the cascade is a member of the miR-482 superfamily, and this targets the coding sequence for the P-loop motif in the mRNA sequences of NB-LRR genes. This targeting causes mRNA cleavage and initiates the production of RDR-6-dependent secondary siRNAs that also have the potential to regulate homologous NB-LRR resistance genes (Shivaprasad et al. 2012; Li et al. 2012; Zhai et al. 2011). The miR-482-mediated silencing of NB-LRR transcripts is lost in virus- and bacteria-infected leaves, very likely due to the action of pathogen-encoded proteins. Based on these data, it was proposed that miRNAs and secondary siRNAs regulate the pathogen-inducible expression of NB-LRR innate immune receptor proteins and the RNAi pathway could also function to achieve inducible expression of NB-LRR genes (Shivaprasad et al. 2012).
6 The Effect of Environmental Signals on RNAi-Based Defense Against Viral Pathogens
Plant–virus interactions are also strongly influenced by environmental factors, especially by temperature. Outbreaks of virus diseases are frequently associated with low temperature, while at high temperature, viral symptoms are often attenuated (heat masking) and the virus load of the infected plants is low (Johnson 1922; Hull 2002). Several studies showed that rapid spread of virus diseases and the development of severe symptoms is often associated with low temperatures (Hine et al. 1970; Gerik et al. 1990). Furthermore, thermotherapy is used to free vegetative material from infecting viruses (Manganaris et al. 2003). RNAi is the primary antiviral defense system in plants, and it was reported that RNAi-mediated plant defense is temperature dependent. At low ambient temperature (15 °C), the virus-triggered RNAi pathway is inhibited and the level of vsiRNAs is dramatically reduced, thus leading to enhanced virus susceptibility. In contrast, the antiviral RNAi pathway is activated and the amount of vsiRNAs gradually increases with rising temperature (Szittya et al. 2003). Moreover, it was reported that ssDNA geminivirus-induced RNAi is also altered by temperature, similar to RNA viruses. Cassava geminivirus-induced RNAi was increased by rising temperature, with the appearance of less symptomatic newly developed leaves, and vsiRNAs accumulated to higher levels. However, there was a difference in the vsiRNA accumulation level among the non-recovery-type and recovery-type geminiviruses at high ambient temperature (30 C). Recovery-type viruses accumulated moderately, while non-recovery-type viruses accumulated very high level of vsiRNAs during infection at high temperature (Chellappan et al. 2005). To determine the underlining mechanism of the enhanced RNAi-mediated antiviral defense at higher temperature, a TCV-Arabidopsis model system was used in a recent study. Arabidopsis Col-0 plants, together with plants with defects in RNAi pathway components, were infected with TCV and kept at 18–26 °C after inoculation. Contradicting previous reports (Qu et al. 2005; Scholthof et al. 2011), TCV actually produced higher levels of viral RNAs at 26° C than at 18 °C, suggesting that TCV RNA replication is stimulated by elevated growth temperature. However, as was expected, host RNAi activity was likewise stimulated at higher temperature. Plants containing loss-of-function mutations within DCL2, AGO2, and HEN1 died on TCV infection at higher temperature. This suggests the existence of an RNAi pathway comprised of at least DCL2, AGO2, and HEN1 that functions to partially attenuate the stress caused by TCV infections at higher temperature (Zhang et al. 2012).
7 Concluding Remarks and Future Direction
Plant viruses have provided a large contribution to our understanding of RNAi pathways. The high diversity of VSRs interfering with different steps of the plant RNAi pathway provides an invaluable molecular tool to dissect endogenous RNAi pathways, although in many cases, we are far from fully understanding the mechanisms behind this suppression. Indeed, the widely used transient expression assays or VSR-expressing transgenic plants may lead to an oversimplified view of their function. On the one hand, the use of these systems as molecular tools yields novel insights and broadens our understanding of the molecular details of the plant’s RNAi pathways. As a result, during the last decade, we have learned a lot about RNAi-based antiviral immunity in plants. However, this is very likely just the beginning of a long journey. We still do not understand how viral RNAs and replication intermediates are recognized by the plant mRNA quality control and surveillance machinery. Since many viruses replicate in a well-defined compartment, it is also not clear when and where this recognition happens. We do not know whether VSRs have endogenous interactors that are essential for silencing suppression. Further understanding of the contribution of vsiRNAs to disease induction is needed, and we also need to investigate their role in possible epigenetic modifications. Since DCL4-produced tasiRNAs were shown to function non-cell autonomously (Chitwood et al. 2009), it also needs to be determined whether DCL4-produced vsiRNAs can move from cell to cell and their possible influence on gene expression in neighboring cells need to be understood.
References
Ahlquist P (2002) RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 296:1270–1273
Akbergenov R, Si-Ammour A, Blevins T, Amin I, Kutter C, Vanderschuren H, Zhang P, Gruissem W, Meins F Jr, Hohn T, Pooggin MM (2006) Molecular characterization of geminivirus-derived small RNAs in different plant species. Nucleic Acids Res 34:462–471
Al-Kaff NS, Covey SN, Kreike MM, Page AM, Pinder R, Dale PJ (1998) Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science 279:2113–2115
Almeida R, Allshire RC (2005) RNA silencing and genome regulation. Trends Cell Biol 15:251–258
Ameres SL, Martinez J, Schroeder R (2007) Molecular basis for target RNA recognition and cleavage by human RISC. Cell 130:101–112
Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB (1998) A viral suppressor of gene silencing in plants. Proc Natl Acad Sci U S A 95:13079–13084
Aregger M, Borah BK, Seguin J, Rajeswaran R, Gubaeva EG, Zvereva AS, Windels D, Vazquez F, Blevins T, Farinelli L, Pooggin MM (2012) Primary and secondary siRNAs in geminivirus-induced gene silencing. PLoS Pathog 8:e1002941
Azevedo J, Garcia D, Pontier D, Ohnesorge S, Yu A, Garcia S, Braun L, Bergdoll M, Hakimi MA, Lagrange T, Voinnet O (2010) Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev 24:904–915
Bao H, Guo H, Wang J, Zhou R, Lu X, Shi S (2009) MapView: visualization of short reads alignment on a desktop computer. Bioinformatics 25:1554–1555
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363
Baumberger N, Tsai CH, Lie M, Havecker E, Baulcombe DC (2007) The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr Biol 17:1609–1614
Becker A, Lange M (2010) VIGS–genomics goes functional. Trends Plant Sci 15:1–4
Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E (2006) mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 20:1885–1898
Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366
Bhattacharjee S, Zamora A, Azhar MT, Sacco MA, Lambert LH, Moffett P (2009) Virus resistance induced by NB-LRR proteins involves Argonaute4-dependent translational control. Plant J 58:940–951
Bies-Etheve N, Pontier D, Lahmy S, Picart C, Vega D, Cooke R, Lagrange T (2009) RNA-directed DNA methylation requires an AGO4-interacting member of the SPT5 elongation factor family. EMBO Rep 10:649–654
Blevins T, Rajeswaran R, Shivaprasad PV, Beknazariants D, Si-Ammour A, Park HS, Vazquez F, Robertson D, Meins F Jr, Hohn T, Pooggin MM (2006) Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res 34:6233–6246
Blevins T, Rajeswaran R, Aregger M, Borah BK, Schepetilnikov M, Baerlocher L, Farinelli L, Meins F Jr, Hohn T, Pooggin MM (2011) Massive production of small RNAs from a non-coding region of Cauliflower mosaic virus in plant defense and viral counter-defense. Nucleic Acids Res 39:5003–5014
Bortolamiol D, Pazhouhandeh M, Marrocco K, Genschik P, Ziegler-Graff V (2007) The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr Biol 17:1615–1621
Bouche N, Lauressergues D, Gasciolli V, Vaucheret H (2006) An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J 25:3347–3356
Brigneti G, Voinnet O, Li WX, Ji LH, Ding SW, Baulcombe DC (1998) Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J 17:6739–6746
Brodersen P, Voinnet O (2006) The diversity of RNA silencing pathways in plants. Trends Genet 22:268–280
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320:1185–1190
Buchmann RC, Asad S, Wolf JN, Mohannath G, Bisaro DM (2009) Geminivirus AL2 and L2 proteins suppress transcriptional gene silencing and cause genome-wide reductions in cytosine methylation. J Virol 83:5005–5013
Buhler M, Verdel A, Moazed D (2006) Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell 125:873–886
Burgyan J, Havelda Z (2011) Viral suppressors of RNA silencing. Trends Plant Sci 16:265–272
Burgyan J, Rubino L, Russo M (1996) The 5′-terminal region of a tombusvirus genome determines the origin of multivesicular bodies. J Gen Virol 77:1967–1974
Carbonell A, Fahlgren N, Garcia-Ruiz H, Gilbert KB, Montgomery TA, Nguyen T, Cuperus JT, Carrington JC (2012) Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 24:3613–3629
Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR (2005) Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat Struct Mol Biol 12:952–957
Chapman EJ, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet 8:884–896
Chapman EJ, Prokhnevsky AI, Gopinath K, Dolja VV, Carrington JC (2004) Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev 18:1179–1186
Chellappan P, Vanitharani R, Fauquet CM (2005) MicroRNA-binding viral protein interferes with Arabidopsis development. Proc Natl Acad Sci U S A 102:10381–10386
Chen HY, Yang J, Lin C, Yuan YA (2008) Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b. EMBO Rep 9:754–760
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814
Chitwood DH, Nogueira FT, Howell MD, Montgomery TA, Carrington JC, Timmermans MC (2009) Pattern formation via small RNA mobility. Genes Dev 23:549–554
Csorba T, Bovi A, Dalmay T, Burgyan J (2007) The p122 subunit of Tobacco Mosaic Virus replicase is a potent silencing suppressor and compromises both small interfering RNA- and microRNA-mediated pathways. J Virol 81:11768–11780
Csorba T, Pantaleo V, Burgyan J (2009) RNA silencing: an antiviral mechanism. Adv Virus Res 75:35–71
Csorba T, Lozsa R, Hutvagner G, Burgyan J (2010) Polerovirus protein P0 prevents the assembly of small RNA-containing RISC complexes and leads to degradation of ARGONAUTE1. Plant J 62:463–472
Cuellar WJ, Kreuze JF, Rajamaki ML, Cruzado KR, Untiveros M, Valkonen JP (2009) Elimination of antiviral defense by viral RNase III. Proc Natl Acad Sci U S A 106:10354–10358
Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101:543–553
Dalmay T, Horsefield R, Braunstein TH, Baulcombe DC (2001) SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J 20:2069–2078
Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O (2006) Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313:68–71
Derrien B, Baumberger N, Schepetilnikov M, Viotti C, De Cillia J, Ziegler-Graff V, Isono E, Schumacher K, Genschik P (2012) Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc Natl Acad Sci U S A 109:15942–15946
Diaz-Pendon JA, Ding SW (2008) Direct and indirect roles of viral suppressors of RNA silencing in pathogenesis. Annu Rev Phytopathol 46:303–326
Diaz-Pendon JA, Li F, Li WX, Ding SW (2007) Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell 19:2053–2063
Ding SW (2010) RNA-based antiviral immunity. Nat Rev Immunol 10:632–644
Ding SW, Voinnet O (2007) Antiviral immunity directed by small RNAs. Cell 130:413–426
Donaire L, Barajas D, Martinez-Garcia B, Martinez-Priego L, Pagan I, Llave C (2008) Structural and genetic requirements for the biogenesis of tobacco rattle virus-derived small interfering RNAs. J Virol 82:5167–5177
Donaire L, Wang Y, Gonzalez-Ibeas D, Mayer KF, Aranda MA, Llave C (2009) Deep-sequencing of plant viral small RNAs reveals effective and widespread targeting of viral genomes. Virology 392:203–214
Dunoyer P, Lecellier CH, Parizotto EA, Himber C, Voinnet O (2004) Probing the microRNA and small interfering RNA pathways with virus-encoded suppressors of RNA silencing. Plant Cell 16:1235–1250
Dunoyer P, Schott G, Himber C, Meyer D, Takeda A, Carrington JC, Voinnet O (2010) Small RNA duplexes function as mobile silencing signals between plant cells. Science 328:912–916
Eamens A, Vaistij FE, Jones L (2008) NRPD1a and NRPD1b are required to maintain post-transcriptional RNA silencing and RNA-directed DNA methylation in Arabidopsis. Plant J 55:596–606
Ebhardt HA, Thi EP, Wang MB, Unrau PJ (2005) Extensive 3′ modification of plant small RNAs is modulated by helper component-proteinase expression. Proc Natl Acad Sci U S A 102:13398–13403
El-Shami M, Pontier D, Lahmy S, Braun L, Picart C, Vega D, Hakimi MA, Jacobsen SE, Cooke R, Lagrange T (2007) Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes Dev 21:2539–2544
Eulalio A, Huntzinger E, Izaurralde E (2008) GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol 15:346–353
Fukunaga R, Doudna JA (2009) dsRNA with 5′ overhangs contributes to endogenous and antiviral RNA silencing pathways in plants. EMBO J 28:545–555
Fusaro AF, Matthew L, Smith NA, Curtin SJ, Dedic-Hagan J, Ellacott GA, Watson JM, Wang MB, Brosnan C, Carroll BJ, Waterhouse PM (2006) RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep 7:1168–1175
Fusaro AF, Correa RL, Nakasugi K, Jackson C, Kawchuk L, Vaslin MF, Waterhouse PM (2012) The Enamovirus P0 protein is a silencing suppressor which inhibits local and systemic RNA silencing through AGO1 degradation. Virology 426:178–187
Garcia D, Garcia S, Pontier D, Marchais A, Renou JP, Lagrange T, Voinnet O (2012) Ago hook and RNA helicase motifs underpin dual roles for SDE3 in antiviral defense and silencing of nonconserved intergenic regions. Mol Cell 48:109–120
Garcia-Ruiz H, Takeda A, Chapman EJ, Sullivan CM, Fahlgren N, Brempelis KJ, Carrington JC (2010) Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip Mosaic Virus infection. Plant Cell 22:481–496
Gerik JS, Duffus JE, Perry R, Stenger DC, Van Maren AF (1990) Etiology of tomato plant decline in the California desert. Phytopathology 80:1352–1356
Giner A, Lakatos L, Garcia-Chapa M, Lopez-Moya JJ, Burgyan J (2010) Viral protein inhibits RISC activity by argonaute binding through conserved WG/GW motifs. PLoS Pathog 6:e1000996
Glick E, Zrachya A, Levy Y, Mett A, Gidoni D, Belausov E, Citovsky V, Gafni Y (2008) Interaction with host SGS3 is required for suppression of RNA silencing by tomato yellow leaf curl virus V2 protein. Proc Natl Acad Sci U S A 105:157–161
Goto K, Kobori T, Kosaka Y, Natsuaki T, Masuta C (2007) Characterization of silencing suppressor 2b of cucumber mosaic virus based on examination of its small RNA-binding abilities. Plant Cell Physiol 48:1050–1060
Guo HS, Ding SW (2002) A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J 21:398–407
Haas G, Azevedo J, Moissiard G, Geldreich A, Himber C, Bureau M, Fukuhara T, Keller M, Voinnet O (2008) Nuclear import of CaMV P6 is required for infection and suppression of the RNA silencing factor DRB4. EMBO J 27:2102–2112
Hamilton AJ, Baulcombe DC (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286:950–952
Hammond SM, Bernstein E, Beach D, Hannon GJ (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293–296
Harvey JJ, Lewsey MG, Patel K, Westwood J, Heimstadt S, Carr JP, Baulcombe DC (2011) An antiviral defense role of AGO2 in plants. PLoS ONE 6:e14639
He XJ, Hsu YF, Zhu S, Wierzbicki AT, Pontes O, Pikaard CS, Liu HL, Wang CS, Jin H, Zhu JK (2009) An effector of RNA-directed DNA methylation in arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell 137:498–508
Hine RB, Osborne WE, Dennis RE (1970) Elevation and temperature effects on severity of maize dwarf mosaic virus in sorghum in Arizona. Plant Disease Reporter 54:1064–1068
Ho T, Pallett D, Rusholme R, Dalmay T, Wang H (2006) A simplified method for cloning of short interfering RNAs from Brassica juncea infected with Turnip mosaic potyvirus and Turnip crinkle carmovirus. J Virol Methods 136:217–223
Hull R (2002) Matthews’ Plant Virology, 4th edn. Academic Press, San Diego
Hutvagner G, Simard MJ (2008) Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9:22–32
Iki T, Yoshikawa M, Nishikiori M, Jaudal MC, Matsumoto-Yokoyama E, Mitsuhara I, Meshi T, Ishikawa M (2010) In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol Cell 39:282–291
Iki T, Yoshikawa M, Meshi T, Ishikawa M (2011) Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J 31:267–278
Itaya A, Zhong X, Bundschuh R, Qi Y, Wang Y, Takeda R, Harris AR, Molina C, Nelson RS, Ding B (2007) A structured viroid RNA serves as a substrate for dicer-like cleavage to produce biologically active small RNAs but is resistant to RNA-induced silencing complex-mediated degradation. J Virol 81:2980–2994
Jaubert M, Bhattacharjee S, Mello AF, Perry KL, Moffett P (2011) ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol 156:1556–1564
Jay F, Wang Y, Yu A, Taconnat L, Pelletier S, Colot V, Renou JP, Voinnet O (2011) Misregulation of AUXIN RESPONSE FACTOR 8 underlies the developmental abnormalities caused by three distinct viral silencing suppressors in Arabidopsis. PLoS Pathog 7:e1002035
Johnson J (1922) The relation of air temperature to the mosaic disease of potatoes and other plants. Phytopathology 12:438–440
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jones L, Hamilton AJ, Voinnet O, Thomas CL, Maule AJ, Baulcombe DC (1999) RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 11:2291–2301
Jones L, Ratcliff F, Baulcombe DC (2001) RNA-directed transcriptional gene silencing in plants can be inherited independently of the RNA trigger and requires Met1 for maintenance. Curr Biol 11:747–757
Karlowski WM, Zielezinski A, Carrere J, Pontier D, Lagrange T, Cooke R (2010) Genome-wide computational identification of WG/GW Argonaute-binding proteins in Arabidopsis. Nucleic Acids Res 38:4231–4245
Kasschau KD, Carrington JC (1998) A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95:461–470
Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, Krizan KA, Carrington JC (2003) P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev Cell 4:205–217
Kumakura N, Takeda A, Fujioka Y, Motose H, Takano R, Watanabe Y (2009) SGS3 and RDR6 interact and colocalize in cytoplasmic SGS3/RDR6-bodies. FEBS Lett 583:1261–1266
Lakatos L, Szittya G, Silhavy D, Burgyan J (2004) Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J 23:876–884 Epub 2004 Feb 2019
Lakatos L, Csorba T, Pantaleo V, Chapman EJ, Carrington JC, Liu YP, Dolja VV, Calvino LF, Lopez-Moya JJ, Burgyan J (2006) Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J 25:2768–2780
Lanet E, Delannoy E, Sormani R, Floris M, Brodersen P, Crete P, Voinnet O, Robaglia C (2009) Biochemical Evidence for Translational Repression by Arabidopsis MicroRNAs. Plant Cell 21:1762–1768
Li J, Yang Z, Yu B, Liu J, Chen X (2005) Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol 15:1501–1507
Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B (2012) MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci U S A 109:1790–1795
Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305:1437–1441
Liu J, Rivas FV, Wohlschlegel J, Yates JR 3rd, Parker R, Hannon GJ (2005) A role for the P-body component GW182 in microRNA function. Nat Cell Biol 7:1261–1266
Llave C (2010) Virus-derived small interfering RNAs at the core of plant-virus interactions. Trends Plant Sci 15:701–707
Love AJ, Laird J, Holt J, Hamilton AJ, Sadanandom A, Milner JJ (2007) Cauliflower mosaic virus protein P6 is a suppressor of RNA silencing. J Gen Virol 88:3439–3444
Lozsa R, Csorba T, Lakatos L, Burgyan J (2008) Inhibition of 3′ modification of small RNAs in virus-infected plants require spatial and temporal co-expression of small RNAs and viral silencing-suppressor proteins. Nucleic Acids Res 36:4099–4107
Mallory AC, Reinhart BJ, Bartel D, Vance VB, Bowman LH (2002) A viral suppressor of RNA silencing differentially regulates the accumulation of short interfering RNAs and micro-RNAs in tobacco. Proc Natl Acad Sci U S A 99:15228–15233
Manganaris GA, Economou AS, Boubourakas IN, Katis NI (2003) Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22:195–200
Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ (2009) RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21:367–376
Mayers CN, Palukaitis P, Carr JP (2000) Subcellular distribution analysis of the cucumber mosaic virus 2b protein. J Gen Virol 81:219–226
Mayo MA, Ziegler-Graff V (1996) Molecular biology of luteoviruses. Adv Virus Res 46:413–460
Merai Z, Kerenyi Z, Molnar A, Barta E, Valoczi A, Bisztray G, Havelda Z, Burgyan J, Silhavy D (2005) Aureusvirus P14 is an efficient RNA silencing suppressor that binds double-stranded RNAs without size specificity. J Virol 79:7217–7226
Merai Z, Kerenyi Z, Kertesz S, Magna M, Lakatos L, Silhavy D (2006) Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J Virol 80:5747–5756
Meyers BC, Kaushik S, Nandety RS (2005) Evolving disease resistance genes. Curr Opin Plant Biol 8:129–134
Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, Chen S, Hannon GJ, Qi Y (2008) Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133:116–127
Miozzi L, Gambino G, Burgyan J, Pantaleo V (2013) Genome-wide identification of viral and host transcripts targeted by viral siRNAs in Vitis vinifera. Mol Plant Pathol 14:30–43
Moissiard G, Voinnet O (2006) RNA silencing of host transcripts by cauliflower mosaic virus requires coordinated action of the four Arabidopsis Dicer-like proteins. Proc Natl Acad Sci U S A 103:19593–19598
Molnar A, Csorba T, Lakatos L, Varallyay E, Lacomme C, Burgyan J (2005) Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J Virol 79:7812–7818
Morel JB, Godon C, Mourrain P, Beclin C, Boutet S, Feuerbach F, Proux F, Vaucheret H (2002) Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell 14:629–639
Navarro B, Pantaleo V, Gisel A, Moxon S, Dalmay T, Bisztray G, Di Serio F, Burgyan J (2009) Deep sequencing of viroid-derived small RNAs from grapevine provides new insights on the role of RNA silencing in plant-viroid interaction. PLoS ONE 4:e7686
Navarro B, Gisel A, Rodio ME, Delgado S, Flores R, Di Serio F (2012) Small RNAs containing the pathogenic determinant of a chloroplast-replicating viroid guide the degradation of a host mRNA as predicted by RNA silencing. Plant J 70:991–1003
Omarov RT, Ciomperlik JJ, Scholthof HB (2007) RNAi-associated ssRNA-specific ribonucleases in Tombusvirus P19 mutant-infected plants and evidence for a discrete siRNA-containing effector complex. Proc Natl Acad Sci U S A 104:1714–1719
Pantaleo V, Szittya G, Burgyan J (2007) Molecular bases of viral RNA targeting by viral small interfering RNA-programmed RISC. J Virol 81:3797–3806
Pantaleo V, Saldarelli P, Miozzi L, Giampetruzzi A, Gisel A, Moxon S, Dalmay T, Bisztray G, Burgyan J (2010) Deep sequencing analysis of viral short RNAs from an infected Pinot Noir grapevine. Virology 408:49–56
Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS (2005) Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A 102:3691–3696
Pazhouhandeh M, Dieterle M, Marrocco K, Lechner E, Berry B, Brault V, Hemmer O, Kretsch T, Richards KE, Genschik P, Ziegler-Graff V (2006) F-box-like domain in the polerovirus protein P0 is required for silencing suppressor function. Proc Natl Acad Sci U S A 103:1994–1999
Pfeffer S, Dunoyer P, Heim F, Richards KE, Jonard G, Ziegler-Graff V (2002) P0 of beet Western yellows virus is a suppressor of posttranscriptional gene silencing. J Virol 76:6815–6824
Pham JW, Pellino JL, Lee YS, Carthew RW, Sontheimer EJ (2004) A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell 117:83–94
Qi X, Bao FS, Xie Z (2009) Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE 4:e4971
Qu F (2010) Antiviral role of plant-encoded RNA-dependent RNA polymerases revisited with deep sequencing of small interfering RNAs of virus origin. Mol Plant Microbe Interact 23:1248–1252
Qu F, Ye X, Hou G, Sato S, Clemente TE, Morris TJ (2005) RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J Virol 79:15209–15217
Qu F, Ye X, Morris TJ (2008) Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci U S A 105:14732–14737
Raja P, Sanville BC, Buchmann RC, Bisaro DM (2008) Viral genome methylation as an epigenetic defense against geminiviruses. J Virol 82:8997–9007
Ratcliff F, Harrison BD, Baulcombe DC (1997) A similarity between viral defense and gene silencing in plants. Science 276:1558–1560
Ratcliff FG, MacFarlane SA, Baulcombe DC (1999) Gene silencing without DNA. rna-mediated cross-protection between viruses. Plant Cell 11:1207–1216
Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60:485–510
Scholthof HB, Alvarado VY, Vega-Arreguin JC, Ciomperlik J, Odokonyero D, Brosseau C, Jaubert M, Zamora A, Moffett P (2011) Identification of an ARGONAUTE for antiviral RNA silencing in Nicotiana benthamiana. Plant Physiol 156:1548–1555
Schott G, Mari-Ordonez A, Himber C, Alioua A, Voinnet O, Dunoyer P (2012) Differential effects of viral silencing suppressors on siRNA and miRNA loading support the existence of two distinct cellular pools of ARGONAUTE1. EMBO J 31:2553–2565
Schuck J, Gursinsky T, Pantaleo V, Burgyán J, Behrens SE (2013) AGO/RISC-mediated antiviral RNA silencing in a plant in vitro system. Nucleic Acids Res doi:10.1093/nar/gkt193
Shen B, Goodman HM (2004) Uridine addition after microRNA-directed cleavage. Science 306:997
Shimura H, Pantaleo V, Ishihara T, Myojo N, Inaba J, Sueda K, Burgyan J, Masuta C (2011) A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog 7:e1002021
Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC (2012) A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24:859–874
Silhavy D, Burgyan J (2004) Effects and side-effects of viral RNA silencing suppressors on short RNAs. Trends Plant Sci 9:76–83
Silhavy D, Molnar A, Lucioli A, Szittya G, Hornyik C, Tavazza M, Burgyan J (2002) A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J 21:3070–3080
Smith NA, Eamens AL, Wang MB (2011) Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog 7:e1002022
Song JJ, Smith SK, Hannon GJ, Joshua-Tor L (2004) Crystal structure of Argonaute and its implications for RISC slicer activity. Science 305:1434–1437
Szittya G, Molnar A, Silhavy D, Hornyik C, Burgyan J (2002) Short defective interfering RNAs of tombusviruses are not targeted but trigger post-transcriptional gene silencing against their helper virus. Plant Cell 14:359–372
Szittya G, Silhavy D, Molnar A, Havelda Z, Lovas A, Lakatos L, Banfalvi Z, Burgyan J (2003) Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J 22:633–640
Szittya G, Moxon S, Pantaleo V, Toth G, Rusholme Pilcher RL, Moulton V, Burgyan J, Dalmay T (2010) Structural and functional analysis of viral siRNAs. PLoS Pathog 6:e1000838
Takeda A, Tsukuda M, Mizumoto H, Okamoto K, Kaido M, Mise K, Okuno T (2005) A plant RNA virus suppresses RNA silencing through viral RNA replication. EMBO J 24:3147–3157
Takeda A, Iwasaki S, Watanabe T, Utsumi M, Watanabe Y (2008) The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol 49:493–500
Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J (2003) Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423:74–77
Till S, Ladurner AG (2007) RNA Pol IV plays catch with Argonaute 4. Cell 131:643–645
Tomari Y, Zamore PD (2005) Perspective: machines for RNAi. Genes Dev 19:517–529
Vaistij FE, Jones L (2009) Compromised virus-induced gene silencing in RDR6-deficient plants. Plant Physiol 149:1399–1407
van Wezel WR, Dong X, Liu H, Tien P, Stanley J, Hong Y (2002) Mutation of three cysteine residues in Tomato yellow leaf curl virus-China C2 protein causes dysfunction in pathogenesis and posttranscriptional gene-silencing suppression. Mol Plant Microbe Interact 15:203–208
Varallyay E, Valoczi A, Agyi A, Burgyan J, Havelda Z (2010) Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. EMBO J 29:3507–3519
Vargason J, Szittya G, Burgyan J, Hall TM (2003) Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115:799–811
Vaucheret H (2006) Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 20:759–771
Vaucheret H (2008) Plant Argonautes. Trends Plant Sci 13:350–358
Vaucheret H, Mallory AC, Bartel DP (2006) AGO1 homeostasis entails coexpression of MIR168 and AGO1 and preferential stabilization of miR168 by AGO1. Mol Cell 22:129–136
Vazquez F (2006) Arabidopsis endogenous small RNAs: highways and byways. Trends Plant Sci 11:460–468
Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D (2004) RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303:672–676
Voinnet O (2005) Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet 6:206–220
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136:669–687
Voinnet O, Pinto YM, Baulcombe DC (1999) Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci U S A 96:14147–14152
Wang H, Buckley KJ, Yang X, Buchmann RC, Bisaro DM (2005) Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J Virol 79:7410–7418
Wang XB, Wu Q, Ito T, Cillo F, Li WX, Chen X, Yu JL, Ding SW (2010) RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proc Natl Acad Sci U S A 107:484–489
Wang XB, Jovel J, Udomporn P, Wang Y, Wu Q, Li WX, Gasciolli V, Vaucheret H, Ding SW (2011) The 21-Nucleotide, but Not 22-Nucleotide, Viral Secondary Small Interfering RNAs Direct Potent Antiviral Defense by Two Cooperative Argonautes in Arabidopsis thaliana. Plant Cell 23:1625–1638
Wassenegger M, Krczal G (2006) Nomenclature and functions of RNA-directed RNA polymerases. Trends Plant Sci 11:142–151
Wu Q, Wang X, Ding SW (2010) Viral suppressors of RNA-based viral immunity: host targets. Cell Host Microbe 8:12–15
Xiong R, Wu J, Zhou Y, Zhou X (2009) Characterization and subcellular localization of an RNA silencing suppressor encoded by Rice stripe tenuivirus. Virology 387:29–40
Yang LP, Fang YY, An CP, Dong L, Zhang ZH, Chen H, Xie Q, Guo HS (2013) C2-mediated decrease in DNA methylation, accumulation of siRNAs, and increase in expression for genes involved in defense pathways in plants infected with beet severe curly top virus. Plant J 73:910–917
Ye K, Malinina L, Patel DJ (2003) Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 426:874–878
Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307:932–935
Yu B, Chapman EJ, Yang Z, Carrington JC, Chen X (2006) Transgenically expressed viral RNA silencing suppressors interfere with microRNA methylation in Arabidopsis. FEBS Lett 580:3117–3120
Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, Li Y, Gonzalez AJ, Yan Z, Kitto SL, Grusak MA, Jackson SA, Stacey G, Cook DR, Green PJ, Sherrier DJ, Meyers BC (2011) MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev 25:2540–2553
Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH (2006) Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20:3255–3268
Zhang X, Zhao H, Gao S, Wang WC, Katiyar-Agarwal S, Huang HD, Raikhel N, Jin H (2011) Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(*)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol Cell 42:356–366
Zhang X, Zhang X, Singh J, Li D, Qu F (2012) Temperature-dependent survival of Turnip crinkle virus-infected arabidopsis plants relies on an RNA silencing-based defense that requires dcl2, AGO2, and HEN1. J Virol 86:6847–6854
Zhou Z, Dell’Orco M, Saldarelli P, Turturo C, Minafra A, Martelli GP (2006) Identification of an RNA-silencing suppressor in the genome of Grapevine virus A. J Gen Virol 87:2387–2395
Zrachya A, Glick E, Levy Y, Arazi T, Citovsky V, Gafni Y (2007) Suppressor of RNA silencing encoded by Tomato yellow leaf curl virus-Israel. Virology 358:159–165
Zvereva AS, Pooggin MM (2012) Silencing and innate immunity in plant defense against viral and non-viral pathogens. Viruses 4:2578–2597
Acknowledgments
We apologize to those colleagues whose work we were unable to cite because of space restrictions. GS and JB are funded by grants from OTKA, K-101793 and NK-105850, respectively.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Szittya, G., Burgyán, J. (2013). RNA Interference-Mediated Intrinsic Antiviral Immunity in Plants. In: Cullen, B. (eds) Intrinsic Immunity. Current Topics in Microbiology and Immunology, vol 371. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37765-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-37765-5_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37764-8
Online ISBN: 978-3-642-37765-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)