Abstract
Treatment of chondral and osteochondral defects in the knee is actually extremely demanding for orthopedic surgeons, and no “gold standard” has still been determined. Tissue engineering has introduced the possibility to repair or replace damaged tissue by implanting several types of synthetic “scaffolds.” Among these, at the beginning of the last decade, has come in use a new, nonbiological, synthetic cartilage implant made of polyvinyl alcohol-hydrogel (PVA-H). The use of polyvinyl alcohol-hydrogel mini cylindrical prosthesis of 1–1.5 cm diameter has been thought to exactly cover the damaged area in order to allow the patient to walk immediately after surgery, having interposed between the damaged area and the opposing cartilage surface a sound damper bearing realized in the laboratory that consists of a hydrogel with the closest characteristics and similar as possible to those of normal hyaline articular cartilage. Short- and long-term studies have confirmed that the use of polyvinyl alcohol-hydrogel prostheses offers pain relief and maintenance of good knee joint function, confirmed by the statistically and clinically significant progressive improvement of patient’s baseline and postoperative scores.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Introduction
The resistant and elastic articular hyaline cartilage covers the ends of the bones which form a joint and makes these bones smooth, soft, and blunt and able to move freely relative to one another to the extent permitted by constraints represented by muscles and ligaments. Hyaline cartilage is a unique tissue and is divided into several layers, consisting for the most part of fluid, and a rich network of proteins, among which is especially collagen, and of relatively few cells, so-called chondrocytes, is responsible for the production of matrix proteins. The composition of articular cartilage varies among the different zones and also varies within the same joint and between different joints, even if the components are always the same.
Cartilage acts as a shock absorber. This ability is derived from the abundance of the liquid in its context, which is able to compress itself significantly, thus performing two functions: loading endurance and stress protection of chondrocytes and matrix proteins.
Cartilage should be imagined as a fabric in continuous formation and degradation. In reality, what goes towards a continuous production and destruction are the molecules that constitute the extracellular matrix. These processes, although opposite, are continuous and always balanced: as many molecules are formed, others die. Unfortunately, aging and trauma have particularly important damaging effects. In fact, cartilage, unlike other tissues of the body, does not have the ability to effectively heal after traumatic injury, excessive wear, or age-related degeneration, and thus a lesion, initially maybe small, tends to extend gradually, damaging much of the affected joint, thus exposing the bone surfaces that it was designed to protect. The latter, at this point, come into contact, and friction against each other causes pain and limitation of joint range of motion. Articular cartilage damage may progress to osteoarthritis over time due to enzymatic degradation and mechanical wear. Cartilage injuries that reach the subchondral bone elicit a reparative response through the formation of a hematoma and a fibrin clot, but the endeavor of the inflammatory repair through the arrival of mesenchymal stem cells from the bone marrow and fibroblast brings forth fibrocartilage repair tissue which has been demonstrated to be unable to fully withstand to mechanical degeneration, and the chondral defect may progress to osteoarthritis.
Apart from its anatomical constitution, a joint will be able to work correctly until its internal environment remains normal, and this is especially true for the weight-bearing joints, like the knee. It is well known that a correct knee joint function depends on excellent lubrication and uniform distribution of loads in different compartments. Chondral and osteochondral lesions of the knee cause an important derangement of the function. In 1743, William Hunter stated that “from Hippocrates to the present age, it is universally allowed that ulcerated cartilage is a troublesome thing and that once destroyed it is not repaired.” For this reason, the treatment of these defects has become, especially in recent years, one of the most investigated areas of interest in the orthopedic practice, and multiple types of treatments have been proposed in order to obtain optimal healing of an articular hyaline cartilage injury.
There are numerous surgical techniques available depending on the severity of the injury, its extent, its location, the age of the patient, and their expectations. Treatment options can be divided in those who try to regenerate hyaline damaged cartilage and those who look only to repair the lesion. The restorative surgical techniques lead to the reconstruction of architecture of the articular cartilage, restoring normal function and eliminating all disabling symptoms. Unfortunately, in literature, no surgical restorative technique has demonstrated guaranteed good long-term results associated to hyaline cartilage regrowth. In contrast, the goal of the reparative surgical techniques is to reconstruct in the best manner the chondral defect, relieving patient’s symptoms even without anatomical restoration of the smooth profile of the articular surfaces (“glass-like”). Among these techniques, the most used are the bone marrow-stimulating procedures such as the subchondral drilling introduced by Pridie and microfractures introduced by Steadman et al. (2003). In the first technique, the trabecular bone is drilled, allowing bone marrow mesenchymal stem cells and fibroblasts to invade the lesion along with the blood to form a clot. The repair tissue is mainly fibrous tissue and fibrocartilage. The latter is the most popular and frequently used: during the microfracture technique, the cartilage tidemark is abraded and small holes are created perpendicular to the subchondral bone plate to a depth of 3–4 mm using an awl, with the holes placed approximately 4–5 mm apart to allow bleeding into the defect. Also the microfractures usually result in a fibrous-fibrohyaline unstructured repair tissue. This tissue lacks the biomechanical and viscoelastic features of hyaline cartilage. The potential short-term improvement in symptoms is usually followed by repair tissue failure and potentially by gradual deterioration to osteoarthritis and return of symptoms. The effectiveness of these treatments is today often debated, and they are commonly associated with a concomitant high tibial osteotomy. Osteochondral autografts, where one cylinder of bone and healthy cartilage from a non-loaded area of the same joint is picked and immediately transplanted to the injured part, or osteochondral allografts, where fresh or prolonged refrigerated osteochondral allografts are press fit applied to cover the defect, are the restorative procedures. In the case of the popular mosaicplasty introduced by Hangody et al. (2001), during which several autologous osteochondral cylinders are harvested from less weight-bearing areas of the femoral condyles and transferred into the defect area and implanted in a mosaic pattern, fibrocartilage has been found to grow between the different osteochondral plugs (Beyerlein 2003; Bosch et al. 2003). Autologous chondrocyte implantation, introduced in 1994 by Lars Peterson and Mats Brittberg (Brittberg et al. 1994) through a pilot study of 23 patients with 39 months of follow-up, seems the most promising technique among the biologic regenerative techniques. This type of treatment involves two separate surgical procedures. In the first step, during a knee arthroscopy executed under general or spinal anesthesia and tourniquet-controlled bloodless field, associated meniscal injuries are treated and the characteristics in size, depth, and location of the chondral defect are evaluated and recorded, and a small amount of healthy cartilage tissue (usually small pieces of cartilage, 300–500 mg in total) is taken from a less weight-bearing area, usually the medial or lateral rim of the trochlea, for culture and subsequent implantation. After cell culturing, a second surgery is performed to implant the cells into the cartilage defect area. This method, since it involves the reimplantation of the patient’s own cultured chondrocytes, is nowadays diffusely used and is the object of keen interest and evaluation everywhere (Peterson et al. 2000; Browne 2000), even if it leads to the formation of a tissue similar to hyaline cartilage, but with the biomechanical resistance, elasticity, and duration characteristics typical of fibrocartilage. But not all patients are ideal candidates for such treatment, being indicated in young patients with traumatic defects and contraindicated in older patients with degenerative damage of the cartilaginous articular contour. First generation ACI has been performed with the use of a periosteum flap sutured at the corners of the rapider defect in order to allow the cells to spread and expand. Due to the technical difficulties and to the development of new materials, several membranes have been used over time as scaffolds to support the initial repair tissue and provide a bed for the seeding and culturing of cells.
Despite the various treatment options available, symptomatic articular cartilage defects continue to represent a therapeutic challenge for knee surgeons, due also to their very frequent arthroscopic incidence, reported up to 63 % by Curl et al. (1997), 61 % in the 1,000 consecutive arthroscopies studied by Hjelle et al. (2002), 66 % in the 999 consecutive arthroscopies studied by Aroen et al. (2004), and 67 % of the 25,124 patients reviewed by Widuchowski et al. (2007). Among these patients, between 5 % and 20 % present with degree III–IV lesions needing surgical treatment.
Principles and Historical Background
In order to relieve patient’s symptoms, among other possible treatment options, since 2002 has come in use a new, nonbiological method of treating chondral and osteochondral defects using synthetic cartilage implants made of polyvinyl alcohol-hydrogel (PVA-H) (SaluCartilage, Salumedica now Cartiva, Carticept Medical) (Fig. 1).
Polyvinyl alcohol has broad medical device applications, where extensive biocompatibility testing has demonstrated the suitability of cross-linked matrices, typically as hydrogels (PVA-Hs), for critical patient contact uses including permanent implant and blood contact applications. Such devices have been in use for more than 20 years. The resulting devices exploit the unique combination of strength, swellability, lubricity, and flexibility of the hydrogel materials. Various forms of PVA hydrogels have been investigated as an artificial cartilage replacement since the early 1970s due to its rubber elastic physical properties (Nishinari et al. 1983; Hyon et al. 1989; Kobayashi et al. 2003, 2005). More recent improvements in the hydrogel formation process, high polymer content cryogels (hydrogels formed by a sequential freeze-thaw process) have been manufactured to have tensile strengths in the range of 1–17 MPa, comparable to that of human normal articular cartilage (Noguchi et al. 1991). In the following years, this new PVA cryogel material has undergone extensive preclinical tests, animal implant studies, and a European trial study in order to demonstrate its safety and reliability.
Once obtained, this new PVA-H material has undergone animal studies, preclinical tests, and a European trial study in order to demonstrate its reliability and biocompatibility before its definitive clinical use.
The nonclinical safety of PVA hydrogel implants has been studied in a variety of animal models. As an artificial articular cartilage, replacement by implanting the PVA hydrogel plug into white rabbits for up to 52 weeks showed that PVA hydrogel caused minimal inflammatory reaction to the surrounding tissue and synovial membrane (Kobayashi et al. 2003). Intra-articular and intramuscular implant in these rabbits by press-fit insertion of 4 mm cylinders of PVA-H or UHMWPE in the trochlea found that, relative to the polyethylene group, PVA-H caused less postoperative inflammation (only in the initial stages), no cartilaginous degeneration, and no synovitis. Oka et al. (2000) and Kobayashi et al. (2005) reported biocompatibility of PVA hydrogels as artificial meniscus after 1 and 2 years of implantation in the bilateral knees of mature rabbits. These authors reported that during the study, they did not observe no wear, no dislocation, nor breakage of the PVA hydrogels even after 2 years application in vivo. In fact, PVA gels, with 80–90 % water content by weight, were implanted subcutaneously or intramuscularly into rabbits and no adverse effects were noticed in the surrounding tissue: these facts have led the authors to definitely confirm the good biocompatibility of the material (Tamura et al. 1986).
To improve bone integration with the implant, a hybrid implant of titanium fiber-mesh/PVA condylar plug was developed and studied by Oka et al. (2000) and Chang et al. (1997). The prototype devices, as well as implants of alumina and titanium, were implanted into the femoral condyles of 24 dogs. The composite osteochondral device caused only minimal damage in the tibial articular cartilage and menisci, while alumina and titanium after 8 and 24 weeks caused ulceration and cartilage loss and exposition of subchondral bone. Measurements in thickness and fluid pressure of the gap formed between a glass plate and PVA-H and PE specimens under loading. Oka et al. (2000) found that PVA-H had a thicker fluid film, lower peak stress, and a longer duration of sustained stress than PE, suggesting a greater damping effect.
The above nonclinical evaluations were conducted in order to evaluate mechanical and functional behavior of the PVA hydrogel material and demonstrate the biocompatibility of the material. The PVA-H has been subjected to compression, shear, cyclic sliding wear, as well as testing to demonstrate its biphasic response to loading and has shown biomechanical characteristics remarkably similar to native cartilage. Extensive biocompatibility testing and long-term implants further substantiate material safety with respect to an absence of any toxic, carcinogenic, allergic, or immunogenic reactions. Consequently, PVA-H matches the characteristics of hyaline cartilage better than other synthetic material introduced in the market to date.
Once the biocompatibility and the safety of the material were ascertained, a European Clinical Trial Study has been conducted. In this study, 104 patients were operated by 35 surgeons and showed, at the 3 months follow-up, 24 points 2000 IKDC score improvement that was statistically and clinically significant. In 2002, the product was CE marked.
Materials and Methods
From December 2002 to February 2007, surgical implantation of polyvinyl alcohol-hydrogel synthetic prosthesis in the treatment of focal grades III and IV chondral and osteochondral defects of the knee was performed on 25 patients and a total of 26 knees among one institution and by one surgeon. The material is a transparent synthetic polymer with 308,000 Da molecular weight and 40 % of water content, in cylindrical shape of various diameters (6 mm, 8 mm, 10 mm, and 15 mm).
Patient selection was as per the literature indications for ACI, OATS, mosaicplasty, and osteochondral allograft transplantation. The inclusion criteria were degree III and IV chondral or osteochondral symptomatic defects of the knee, focal unicompartmental defects with 15–20 mm maximum extent, patient’s age limited from fourth to seventh decade, absence of severe angular deformities or articular instabilities, absence of other compartment pathologies, good bone stock quality and quantity, and previous MRI and arthroscopic confirmation of defect’s site, size, and degree owing to the Outerbridge classification (Outerbridge 1961). Exclusion criteria included infection, pregnancy, generalized chondromalacia, tumor focal defects, uncorrected ligament instability, previous joint replacement, and varus or valgus deformity. Of the 25 patients (one operated bilaterally), it has been possible to conduct follow-ups on 18 patients. Another patient answered a telephone questionnaire, but was not able to be back for clinical and imaging follow-up. These patients, 11 men and 7 women, whose mean age was 54.4 years (range 40–70 years), presented in 17 cases a medial femoral condyle osteochondral lesion and in three cases a lateral femoral condyle lesion. The defects were located in the right knee in five cases and in the left knee in 14 cases. Sixteen implants (80 %) were 10 mm and 4 (20 %) were 15 mm in diameter. One patient has had two implants and one patient has been treated bilaterally. All patients underwent a complete physical examination after we obtained a thorough history of their symptoms. All the patients complained of knee joint pain, during several months before surgery, without any history of recent trauma. On physical examination, all the patients were found to have medial (17 cases) or lateral (3 cases) joint line tenderness, restriction of deep flexion, and inability to squat. At their last follow-up, all patients underwent a complete physical examination and MRI imaging of the operated knee. The patients gave their informed consent prior to their inclusion in the study. The research was conducted according to the principles of the Declaration of Helsinki.
Clinical and Radiological Assessment
Physical general status and knee joint function were recorded and analyzed preoperatively and at latest follow-up based on the SF 36 and the 2000 International Knee Documentation Committee (IKDC) assessment developed by the International Cartilage Research Society. All patients’ results were graded according to the IKDC score. Plain film and magnetic resonance imaging were taken preoperatively and at follow-ups. It is important to emphasize that we always confirmed the indication to the synthetic cartilage prosthesis implantation by an arthroscopic examination of the knee joint in order to evaluate the real size and degree of the weight-bearing area defect of the femoral condyles. MRI was performed following surgery to assess placement of the implant at the time of follow-up and to retrieve eventual adverse reactions. Two experienced musculoskeletal radiologists, who were unaware of the clinical history, reviewed the final MRI studies.
Operative Technique
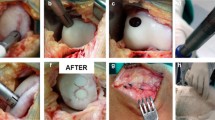
The knee joints have been initially examined arthroscopically under peripheric anesthesia in order to verify the location, the size, and the degree of the chondral defect and to treat all the associated lesions. The Cartiva implants have been inserted using the specific instrumentation. The first 5 cases were implanted after an initial arthroscopic knee joint evaluation through a minimally invasive mini-open procedure, but the subsequent 20 implants were implanted arthroscopically (Fig. 2). For the arthroscopic procedure, a specific instrumentation has been developed by the author after several cadaveric workshops. The Cartiva implants used for this indication were 10 mm or 15 mm in diameter and 10 mm in depth. The procedure is very similar to that used for osteochondral autograft or allograft transplantation: A sizer is carefully positioned in the center of the defect and a guide pin is drilled into the center of the defect. Then, after having established the real size of the defect, the drill of the correct diameter size (10 or 15 mm) is used to create a drill hole 10 mm in depth, which is then cleaned and with extreme attention compacted in order to create a perfect hole with a perfectly clean base and margins able to accept the synthetic implant that is introduced in a press-fit manner taking care to seat it exactly flush to the surrounding normal chondral margin. We mostly have applied just one implant, but in three cases two were implanted. After implantation, the mini-arthrotomy incision is closed with a few stitches, leaving the arthroscopic accesses open, without applying any drainage. Our postoperative protocol is based on same-day free weight bearing as tolerated, with or without antebrachial walking aids, with knee soft bandage, monitoring patients for adverse events. We never recommended the use of CPM machines, since patients are usually able to regain complete knee flexion-extension in the first few postoperative days.
Statistical Analysis
Continuous variables, e.g., age, were summarized by descriptive statistics (N, mean, standard deviation, standard error, median, minimum, and maximum), and categorical variables, e.g., location of implant, were summarized by counts and percentages. A paired student’s t-test was used to test for a significant difference between the baseline and follow-up IKDC scores. Statistical tests with a corresponding p-value less than 0.05 were considered significant. No adjustment for multiplicity was needed nor performed (Tables 1 and 2).
Results
All 25 patients treated with synthetic cartilage implants made of polyvinyl alcohol-hydrogel (PVA-H) had a grade III or IV chondral or osteochondral defect no larger than 20 mm in diameter as confirmed by magnetic resonance imaging and arthroscopy. The implantation of the synthetic cartilage prostheses has been carried out in all patients without any intraoperative complication (Fig. 3). All patients received a single implant per knee with the exception of one patient who received two. One patient received bilateral treatment.
The mean follow-up for this series is 68.4 months with a range of 38–96 months. The mean (SE) preoperative IKDC score across all 19 knees receiving an implant was 32.525 (2.0387), ranging from a low of 19.54 to a high of 47.12 points. At last follow-up, the majority of patients showed significant improvement over preoperative values of IKDC score, with an average (SE) increase of 43.27 (5.2543) points (p < 0.001). Individual change from preoperative scores ranged from a slight loss of 4.60 to a gain of 71.27 points. Nearly half (47.4 %) of the patients had an improvement of more than 50 points in their IKDC score (Fig. 4).
Implant characteristics and IKDC outcomes through the final assessment in patients with knee chondral defects treated with PVA-H implants. a Percentage of knees by location and size of implant. Eighteen patients were enrolled with one patient experiencing bilateral defects, for a total of 19 knees receiving treatment. One patient received two 10 mm implants in the same knee. b Percentage of knees by the duration of time between initial preoperative and final assessments. c Mean (±95 % confidence interval) IKDC scores at the initial preoperative and final assessments and the change from preoperative IKDC score. *P < 0.050 for change from preoperative in IKDC by Pearson’s paired t-test. d Percentage of knees by change from preoperative IKDC score. A positive change score reflects an improvement in IKDC score from pre-op to the final assessment
On patient assessment according to the IKDC questionnaire, at last follow-up, 85 % of patients indicated improvement after the procedure and were satisfied with the results of the procedure. Follow-up MRI images revealed normal healing process, without signs of osteolysis or wear. No synovial joint reaction has been observed, but a 64-year-old female, treated at the very beginning of our learning curve, after persistent medial pain on weight bearing solved after 2 months, had implant dislocation at 1 year and required knee replacement. As said before, all patients have shown improvement of knee function and knee scores, in many cases over 50 points of IKDC, except 3. Of these, the first maintained a rather good quality of life for over 5 years with approximately the same level of functionality she enjoys now, after implant removal and knee replacement during 2008. The second case was a 43-year-old female with a posttraumatic chondral defect in a valgus knee. The patient experienced post-op pain: the implant was removed at another institution 6 months post-op and was converted to OATS. The third case was a 49-year-old male with a severe arthritic pre-op knee and may not have been ideal candidate for Cartiva, but was too young for a total knee replacement at the time of operation and, despite a severe knee worsening during the last year that will need in the short future a knee replacement, has, at over 6 years follow-up, an IKDC score of 33.33 from a pre-op of 37.93.
The results with pre-op and final follow-up evaluation scores are analytically reported in the Table 3.
Discussion
As recent chapters have demonstrated, chondral lesions can be encountered in up to 67 % of knee arthroscopies, and being 20 % of these are III–IV degree lesions (Curl et al. 1997). Treatment of this pathology is therefore a critical issue. Several treatment options have been introduced over the years and have shown satisfactory results in the short-/medium-term follow-ups. The tissue-based treatments still need more relevant clinical studies in order to confirm their success, since there is a significant shortcoming in the quality of repaired tissue. Routine surgical techniques to repair cartilage often result in significant fibrocartilage tissue formation with the biomechanical resistance, elasticity, and duration characteristics typical of fibrocartilage. Apart from the quality of the repair tissue, not all patients are the ideal candidates for such tissue-based treatments, being these mostly indicated only in the young patients, under 50 years of age, with traumatic defects and are not indicated in older patients with degenerative damage of the cartilaginous articular surfaces.
Tissue engineering has introduced the possibility to repair or replace damaged tissue by implanting several types of synthetic “scaffolds” that are produced in the laboratory. non-absorbable. The use of polyvinyl alcohol-hydrogel mini cylindrical prosthesis of 1–1.5 cm. diameter has been thought to exactly cover the damaged area in order to allow the patient to walk immediately after surgery, having been interposed between the area damaged and the opposing cartilage surface.
This technique has a number of advantages compared to others: it needs only one short surgical procedure for implantation, does not damage the articular cartilage at the donor site, and is followed by immediate weight bearing and short rehabilitation program, enabling the patient to shortly get back to his/her daily living or sport activities.
The use of polyvinyl alcohol-hydrogel prostheses for pain relief and maintenance of good knee joint function is supported by the clinical evidence presented in this and other studies (Browne 2000; Bosch et al. 2003). In these papers, the results reported are though at short term (6 and 4 months, respectively). Bosch et al. (2003) has stated that SF-36 shows at 6 months “clear improvement in quality of life. Patients are again leading active life (cycling, walking, jogging, snowboarding),” but has also concluded that “the first positive observations should be followed by further studies over longer periods.” This is the case of our study in which the mid-/long-term efficacy of these devices is confirmed by the statistically and clinically significant progressive improvement of patient’s IKDC baseline and post-op scores.
Conclusion
In conclusion, synthetic cartilage grafts can represent a valid treatment in the presence of painful deep chondral knee defects. But in order to obtain good-excellent results and to prevent complications, it is our belief that surgeons must respect the knee joint and keep strict inclusion criteria:
-
III and IV degree chondral or osteochondral symptomatic defects
-
Focal unicompartmental defects with 15–20 mm maximum extent
-
Patient’s age limited from fourth to seventh decade
-
Absence of angular deformities or articular instabilities
-
Arthroscopic confirmation and grading of the defect
The surgical procedure is not difficult, but it is important that the orthopedic surgeon respects the correct indications and puts all his/her efforts in obtaining the correct alignment and seating of the implant just flush to the normal healthy chondral margin. This is in our opinion the most important step to be fulfilled in order to obtain good and excellent results. This also means that the implantation of a synthetic cartilage device is a procedure that has to be offered to the patient as an intermediate procedure, a bridge solution, which will provide an immediate analgesic and functional improvement, enabling the patients to get back to their previous activities. We are always updating our follow-ups in order to be able to confirm the good results and the procedure’s benefits also in the long-term period and are also working to obtain various implant sizes that can more easily adapt to the articular damage encountered.
References
Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L (2004) Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 32(1):211–215
Beyerlein J, Imhoff AB (2003) Salucartilage–a new synthetic replacement for the arthroscopic treatment of focal osteonecrosis. Arthroscopy 16:34–39
Bosch U, Meller R, Troger H-J, Zeichen J (2003) Salucartilage–a synthetic cartilage replacement for the minimally invasive treatment of osteochondral defects. Arthroscopie 16:40–43
Brittberg M, Peterson L et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895
Browne JE, Branch TP (2000) Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg 8:180–189
Chang Y-S, Oka M, Gu H-O, Kobayashi M, Toguchida J, Nakamura T, Hayami T (1997) Histologic comparison of tibial articular surfaces against rigid materials and artificial articular cartilage. J Biomed Mater Res 37:51–59
Curl W, Krome J, Gordon ES, Rushing J, Smith BP, Poehling G (1997) Cartilage injuries: a review of 31,516 knee arthroscopies. Arthrosopy 13:456–460
Hangody L, Feczko P, Bartha P, Bodo G, Kish G (2001) Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res 3:262–267
Hjelle K, Solheim E, Strand T, Muri R, Brittberg M (2002) Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18(7):730–734
Hyon S-H, Cha W-I, Ikada Y (1989) Preparation of transparent poly (vinyl alcohol) hydrogel. Polym Bull 22:119–122
Kobayashi M, Toguchida J, Oka M (2003) Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 24:639–647
Kobayashi M, Chang YS, Oka M (2005) A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 26:3243–3248
Nishinari K, Watase M, Ogino K, Nambu M (1983) Simple extension of poly (vinyl alcohol) gels. Polym Commun 24:345–347
Noguchi T, Yamamuro T, Oka M, Kumar P, Kotoura Y, Hyon S, Ikada Y (1991) Poly (vinyl alcohol) hydrogel as an artificial articular cartilage: evaluation of biocompatibility. J Appl Biomater 2:101–107
Oka M, Ushio K, Kumar P, Ikeuchi K, Hyon SH, Nakamura T, Fujita H (2000) Development of artificial articular cartilage. Proc Inst Mech Eng 214:59–68
Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Joint Surg Br 43:752–757
Peterson L, Minasc T, Brittberg M et al (2000) Two to nine year outcome after autologous chondrocyte implantation for chondral defects of the knee. Clin Orthop Relat Res 374:212–234
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG (2003) Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 19(5):477–484
Tamura K, Ike O, Hitomi S, Isobe J, Shimizu Y, Nambu M (1986) A new hydrogel and its medical application. ASAIO Trans 32:605–608
Widuchowski W, Widuchowski J, Trzaska T (2007) Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14(3):177–182
Further Reading
Bray JC, Merrill EW (1973) Poly(vinyl alcohol) hydrogels for synthetic articular cartilage material. J Biomed Mater Res 7:431–443
Buckwalter JA, Mankin J (1998) Articular cartilage repair and transplantation. Arthritis Rheum 41:1331–1342
Stammen JA, Williams S, Ku DN, Guldberg RE (2001) Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 22:799–806
Watase M, Nishinari K (1985) Large deformation of hydrogels of poly (vinyl alcohol), agarose and kappa-carrageenan. Makromol Chem 186:1081–1086
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Sciarretta, F.V. (2015). Surgical Treatment of Osteochondral Defects in the Knee with Polyvinyl Alcohol-Hydrogel Implants. In: Doral, M.N., Karlsson, J. (eds) Sports Injuries. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36569-0_151
Download citation
DOI: https://doi.org/10.1007/978-3-642-36569-0_151
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36568-3
Online ISBN: 978-3-642-36569-0
eBook Packages: MedicineReference Module Medicine