Abstract
As one of the major parts of the biosphere, trees will play a significant role in the near future because of an increasing demand for wood as the most important natural raw material. Wood is generated by the vascular cambium and enables water transportation as well as providing mechanical support to the tree. Furthermore, it is the main renewable source for paper, buildings, furniture, boards and fuel. In recent decades intriguing developments in cell, molecular and structural biology have led to an integrated view of wood formation, from its start in the cambium by cell division, via cell expansion and cell wall thickening, to programmed cell death. These complex processes involve the interaction of both exogenous factors, such as photoperiod and temperature, and endogenous regulators, such as phytohormones. In addition, the coordinated expression of the numerous genes implicated in the biosynthesis of the major wood components—cellulose, hemicelluloses and lignin—drives the ordered development of wood. The huge amount of literature in the different fields of wood formation cannot be reviewed here in detail; rather, the aim of this chapter is to give a brief overview of the essential steps leading to mature wood cells, with an emphasis on current progress obtained by modern techniques which have increased our understanding of wood formation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Perennial woody plants dominate many natural land ecosystems. Their major difference to annual herbs is their long life cycle which, in trees, may span several centuries, encompassing germination, seedling, juvenility, maturity, senescence and finally death. To sustain their competitiveness through the course of this long life cycle, trees acquired multiple adaptation strategies which, as a whole, can only rarely be found in annual plant species. These strategies comprise fast and extensive metabolite translocation in response to sudden incidents in the environment, the storage of nutrients in vegetative organs, the responsiveness to seasonal changes in climate and water supply, a long distance communication system via various signals, as well as the competence of producing wood for durable stability and as a transport organ.
Biological production of wood depends on mineral and nutrient supply via the root system, on a balanced storage system and on a prompt and flexible metabolite translocation throughout the growing seasons, whether these are characterised by seasonal climates or by wet and dry periods. Today, wood as a raw material has taken an increasing significance as one of the most important renewable resources for meeting the growing demand for bioenergy, construction materials, wood-pulp for paper production and, of course, as the major onshore carbon sink. With respect to the worldwide on-going discussion on climate change and the need for alternative CO2-neutral energy sources, typical plantation tree species have been the focus of renewed public interest. During the last decades, a few tree species have been established globally as model systems in tree research, such as Populus spec. in seasonal climates (Sterky et al. 2004; Tuskan et al. 2006) and Eucalyptus spec. in warmer and more clement climates (Foucart et al. 2006; Gion et al. 2011; Sexton et al. 2012). Poplar has become the model hardwood species in the northern hemisphere because of its relatively small genome, its ability to be easily propagated and to be genetically transformed (e.g. Tuominen et al. 1995; Hoenicka et al. 2012). Poplar and Eucalyptus are fast-growing trees, and hence, in their respective climate zones, they are also of growing economic importance for large-scale biomass production. In addition, other important tree systems are loblolly pine (e.g. Ralph et al. 1997) and black locust (Magel et al. 1995).
A lot of research on xylogenesis has also been conducted on primary growth systems such as the annual herbaceous species Zinnia elegans (Oda and Fukuda 2012). After isolating mesophyll cells from the leaves, they can be induced through appropriate hormonal treatments to re-differentiate into tracheary elements (Endo et al. 2008). This system can give valuable information on the role of the different substances required for wood development. Although this less-complex Zinnia cell culture system has been able to provide a lot of information on the signals that control plant vascular cell differentiation (Fukuda 2004), it has some shortcomings as a model for the process of wood formation in trees because the products remain as single cells and show no cycle of activity and dormancy (Chaffey 1999). Arabidopsis, however, which is the most advanced model system in plant molecular biology, under appropriate growth conditions, shows substantial secondary thickening in the hypocotyls (Chaffey et al. 2002) and can hence also provide valuable information with respect to wood formation (Zhang et al. 2011), despite its lack of perenniality.
In order to manage wood as an important resource in the future, it is necessary to understand the process of wood formation in trees more deeply. In recent decades considerable progress has been made concerning the cellular, physiological and molecular processes that underlie wood production. This research has revealed that the development of secondary xylem from the cambium is a very complex process which is under the control of numerous genes, as well as a combination of exogenous and endogenous factors involved in the different steps of cell differentiation. Most important are endogenous factors, such as hormones and various factors acting downstream of hormones, including transcription factors (TFs) and receptor kinases (RKs), together with their peptide ligands (Nieminen et al. 2012). This review, therefore, highlights current progress in research into these cellular processes of wood formation.
2 Structure and Function of the Cambium
2.1 Meristematic Features
The cambium is a lateral secondary meristem that derives from the procambium, which in turn originates from the apical meristem (Larson 1994). The cambium develops out of the procambium when parenchyma cells between the vascular bundles start to divide in order to generate a vascular cylinder. As soon as such a cylinder is active, radial files of secondary xylem cells are delivered to the inside and radial phloem files to the outside by so-called periclinal cell divisions. Within each file of both phloem and xylem cells, one initial cell remains in the cambium and gives rise to daughter cells. These daughter cells either become phloem or xylem mother cells. Both types of cells, i.e. initials as well as mother cells, are cytologically almost identical and are called the cambial zone.
The cambial zone has two main functions: cell division and setting out patterns for differentiation. Initials retain the potential to differentiate into either xylem or phloem mother cells. They divide relatively infrequently, however, because of the importance of maintaining an undifferentiated state. Thus, the cambial zone consists of dividing initials, which maintain themselves, together with both xylem and phloem mother cells as their products. By intervening cell division, a mother cell differentiates until it becomes a mature cell. Since, in most species, xylem mother cells divide more than phloem mother cells, much more xylem is produced than phloem. Ratios vary between approximately 1:1 in the tropical hardwood Mimusops elengi (Ghouse and Hashmi 1983) and 15:1 in Thuja occidentalis (Bannan 1955). Most species have xylem to phloem ratios in the range between 4 and 10:1. Recently, progress has been made on the regulation of the xylem vs. phloem ratio in poplar. It has been found that when the transcription factor PtaLBD1 (which is a member of the LATERAL ORGAN BOUNDARIES DOMAIN (LBD) family) is overexpressed, secondary phloem formation increases, suggesting that this transcription factor is involved in the regulation of secondary phloem formation (Yordanov et al. 2010). In addition, the idea that the cambial zone consists of a meristematic region in the centre and two regions of differentiating phloem and xylem mother cells has been confirmed by gene expression data using microarrays from 20 μm thick sections of the cambial zone in aspen (Schrader et al. 2004a). Peak expression from the phloem mother cell region is most often associated with genes representing anticlinal divisions, whereas the xylem mother cell region is rich in cell cycle genes.
In contrast to apical meristems, cells of the cambial zone contain two morphologically distinct cell types: elongated fusiform cells that give rise to axially oriented cells within the xylem and phloem and almost isodiametric ray cells that generate horizontal cell systems such as parenchyma and tracheary elements within the rays. Fusiform initials show higher periclinal divisions than ray initials, which expand in a radial direction to keep step with incremental radial growth. The ratio between ray and fusiform initials depends mainly on the species. With increasing age and cambial dilatation, fusiform initials can differentiate to ray initials in order to keep the species-specific ratio between both constant. Microgenomic analysis has revealed cell type-specific gene expression patterns between ray and fusiform initials of poplar, indicating that photosynthesis genes are overrepresented in ray cambial cells in order to provide a photosynthetic system in rays (Goué et al. 2008). Regarding cell wall-related genes, those involved in pectin metabolism are overrepresented in ray cambial cells while those involved in xyloglucan metabolism are overrepresented in fusiform cambial cells (Goué et al. 2008).
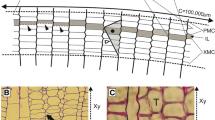
Turning now to the cell position, the cambium can show a storeyed structure. This storeyed form occurs when fusiform cells are arranged in approximately horizontal layers with the cell ends on the same plane when viewed in the tangential section. Wood produced from a storeyed cambium shows a characteristic stratified structure, e.g. as in Aesculus, Diospyros, Swietenia and Dalbergia (Fig. 1a). A storeyed cambium is typical of tree species with short fusiform initials and is both phylogenetically more advanced and more frequent in tropical species than in species from temperate climate zones. In addition to fibres, both rays and vessel elements can show a stratified structure, e.g. as in Dalbergia (Fig. 1a). In contrast, in a non-storeyed cambium, the tips of fusiform cells end at different planes, leading to a wood cell structure with overlapping cell ends, e.g. as in Populus, Fraxinus, Quercus and conifers such as Pinus (Fig. 1b). Furthermore, species with non-storeyed cambia usually have long fusiform initials. The length of the fusiform cells depends on the species and on cambial age. The mean cell length of cambial initials and the final length of mature wood cells increase with tree age. In the case of fibres, their final length is determined by the length of fusiform initials and the degree of intrusive tip growth. In gymnosperm trees the length of fusiform cells ranges from 1,100 μm to 4,000 μm, while in angiosperm trees they vary between 170 μm and 940 μm (Larson 1994). As a consequence, therefore, of the complexity of the two differently oriented subsystems—axial and radial—many different phloem and xylem cell types are produced by the cambial zone within the tree. Tracheids, vessel elements, fibres and axial parenchyma cells emerge from fusiform cambial cells whereas ray parenchyma cells and ray tracheids emerge from cambial ray cells (Fig. 2).
(a) Tangential section of developing wood from Dalbergia riparia showing the stratified structure of all cell types (Courtesy of Dr. A. Olbrich). (b) Tangential section of developing wood from Pinus wallichiana showing an interlocked non-stratified cell structure (Courtesy of V. Haag); f fibre, r ray, rd resin duct, t tracheid, v vessel element. Bar 100 μm
Regarding the regulation of the development of these specific cell types, the ectopic expression of some major transcription factors (VASCULAR-RELATED NAC-DOMAIN6 (VND6) and VND7) has been identified for vessels in Arabidopsis and Populus leaves (Yamaguchi et al. 2008). Microscopy of fusiform cells has shown all the characteristic features for high rates of protein biosynthesis and secretory activity (Catesson 1990; Arend and Fromm 2003). The new wall that needs to be formed during periclinal cell division is very large, and therefore, the rate of cell wall biosynthesis is extremely high in the active cambial zone. High rates of xylem cell formation also correlate with a high number of cambial cells (Gregory 1971; Uggla et al. 1998) because the latter differentiate to wood cells. Hence, both the number of xylem mother cells and the duration of the cell cycle in these cells are important for the rate of wood production. In various conifer species, for example, the shortest average duration of fusiform cambial cells across the cambial zone is in the range of 7–11 days (Mellerowicz et al. 2001). Both the number of mother cells and their division rate may be controlled by independent mechanisms. Since cell wall properties vary among cambial cells and their close derivatives, cell fate seems to be determined at an early stage (Catesson and Roland 1981; Catesson et al. 1994).
During periclinal division of fusiform cells, the phragmoplast and the newly formed cell plate must traverse the large central vacuole to reach the upper and lower ends of the cell. In fusiform cambial cells a cytoplasmic strand, derived from the Golgi vesicles, extends through the vacuole and the developing cell plate. In contrast, cambial ray cells do not possess large vacuoles; during division they show a complete, well-developed, mitotic apparatus which follows the usual mitosis pattern in meristematic plant cells (Fig. 3). The nascent periclinal walls of fusiform cambial cells are of a cellulosic nature with a high content of methylated pectin (Catesson 1989, 1990; Catesson et al. 1994). When the periclinal wall merges with the already existing radial wall, the latter is locally digested until the middle lamella is reached, and it then becomes continuous with the middle lamella of the newly formed tangential cell wall (Catesson and Roland 1981).
Transverse view of cells in the active cambial zone of spruce. Fusiform cells (f) show large vacuoles (v), thin cell walls and conspicuous large nuclei (n). A dividing ray cell (r) has no large vacuoles and exhibits a well-developed mitotic apparatus, as shown here in the state of a telophase with decondensing chromosomes (c) and the developing cell plate (arrows). Bar, 5 μm (After Arend and Fromm 2004)
In addition to periclinal divisions, which lead to an increase in stem diameter, cambial initials show anticlinal divisions perpendicular to the stem surface, when wood production displaces the cambium outwards. In species with storeyed cambia, the newly formed cell wall is oriented radially, and new radial cell files are generated during anticlinal divisions. In species with non-storeyed cambia, anticlinal divisions occur pseudotransversely, i.e. the initials divide by generating a sloping anticlinal wall and by following intrusive tip growth. The orientation of this pseudotransverse division can be leftwards (S) or rightwards (Z) (Zagorska-Marek 1995). Tangential expansion of the cambium refers to both an increase in the number of cells and in their sizes. The majority of anticlinal divisions have been observed to occur within a single layer of cells within the cambial zone of aspen (Schrader et al. 2004a). The rate of anticlinal cell division is also much lower than the rate of periclinal cell division.
For vascular tissue development to occur normally, it has been shown that a peptide signal secreted from the phloem binds to a receptor-like kinase (PHLOEM INTERCALATED WITH XYLEM, PXY) in cambial cells (Fisher and Turner 2007; Hirakawa et al. 2008). Interestingly, xylem and phloem are no longer separated but are intermixed in the loss-of-function pxy mutant. Furthermore, it has been suggested that a Populus class III HD ZIP gene, popREVOLUTA (PRE) plays a fundamental role in regulating the patterning of secondary vascular tissues. In transgenic lines expressing a microRNA-resistant form of PRE abnormal formation of cambia occurs within the stem cortex with phloem developing inwards and xylem outwards (Robischon et al. 2011).
2.2 Seasonal Activity
In temperate latitudes, trees grow synchronously with the seasons and are able to endure periods unfavourable for growth by dormancy. According to Lang (1987), dormancy is the temporary absence of visible growth of any plant structure containing a meristem. Dormancy may also be regarded, however, as a developmental process parallel to active growth and not only a temporary inactive state. Especially in trees of boreal forests with very cold winters, the cycling between activity and dormancy is important for survival. Trees have to accurately synchronise the timing of their active and dormant states with the seasonal changes in order to be able to grow in tough climatic conditions. Cambial dormancy consists of two stages, rest and quiescence (Catesson 1994; Larson 1994, Fig. 4). Rest is controlled by endogenous signals and, in this stage, the cambium cannot divide. Rest can be overcome by giving a chilling treatment to the tree upon which the cambium makes the transition from rest to quiescence and regains responsiveness to auxin. After chilling occurs, therefore, warm temperatures can induce reactivation (Heide 1993). Recently, it has been shown in poplar that chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits in order to trigger a release from dormancy (Rinne et al. 2011). In the ensuing quiescent stage, favourable growth conditions can induce cambial divisions. Generally, the quiescent dormant stage ends during cambial reactivation in spring. In trees from temperate climates, changes in photoperiod and temperature are the dominant environmental signals regulating this seasonal growth-dormancy cycling (Fig. 4).
Transitions in seasonal growth-dormancy cycling in trees from temperate climates. Photoperiod is known to govern growth cessation in late summer/autumn, and in particular, short days are responsible for the cambial transition into the first resting phase (rest). Prolonged exposure to chilling temperatures in early winter initiates the second resting phase (quiescence) and will also release trees from dormancy. After the temperature passes a critical threshold in spring, growth resumes
Within the perennial life cycle of a tree, the cambium functions by renewal of xylem and phloem each year. Distinct differences occur in cambial cytology, however, between its active and dormant states. In several hardwood species, it has been shown that fusiform cambial cells are densely cytoplasmic with many small vacuoles during dormancy while during activity they are highly vacuolated (Robards and Kidway 1969; Sennerby-Forsse 1986; Farrar and Evert 1997; Arend and Fromm 2000). Cambial activity and the width of the cambial zone change during the season. The dormant cambial zone of poplar consists of only 3–4 layers of dense cytoplasmic cells with numerous small vacuoles, lipid droplets as storage material and thick cell walls. No cytoplasmic streaming occurs in these dormant cells. After reactivation, the cambial zone shows 6–7 cell layers per radial file, with thin cell walls and fewer larger vacuoles that appear electron transparent (Arend and Fromm 2000). These differences indicate that the thick walls during dormancy store material that can be metabolised in spring. Cambial cells also increase in radial width during reactivation, causing thinner radial walls. There is strong evidence that the wall thickening during dormancy can be attributed to the conspicuous development of cellulose microfibrils (Catesson 1994), while wall thinning is in part due to incomplete wall lysis (Funada and Catesson 1991). In Aesculus hippocastanum, the thicker wall of the dormant cambial cells is more highly structured than the amorphous cell wall of active cambia, as indicated by the presence of a ‘herring-bone’ lamellate structure (Chaffey et al. 1998). Also, cortical microtubules show a different orientation: they have been found to be axially oriented during dormancy but randomly orientated in active cambial cells, corresponding to the pattern of the microfibrils (Chaffey et al. 1998). In addition, the nature of pectins changes between activity and dormancy. Active cambium contains more hot-water-extractable pectin than dormant cambium (Baier et al. 1994; Ermel et al. 2000). Studies on poplar have indicated that during dormancy, pectin methyl esterase is upregulated while during cambial activity, pectin methylation is increased (Baier et al. 1994; Ermel et al. 2000; Follet-Gueye et al. 2000).
In temperate latitudes active and dormant states of the cambium cause a distinct annual ring structure with a characteristic earlywood/latewood pattern. Earlywood is produced during the first part of the growing season during peak radial growth and is characterised by high vessel or tracheid size and thin cell walls. It coincides with an increase in cambial zone width generated by the number of xylem mother cells. In contrast, latewood is formed during the last part of the growing season. With regard to xylem cell length, it has been found that it increases from a minimum in earlywood to a maximum in latewood (Bissett and Dadswell 1950) both because fusiform cambial cells elongate during the growing season and because during latewood formation intrusive fibre tip growth increases. Finally, xylem cell length decreases sharply at the annual ring boundary. Concerning the chemical composition, significant differences have been found in the distribution of sugar units in hemicelluloses between earlywood and latewood in Norway spruce. Latewood contains clearly more galactoglucomannan than earlywood and conversely less pectins. Lipophilic extractives are also less concentrated in latewood (Bertaud and Holmbom 2004).
The transition from early- to latewood is mainly controlled by environmental conditions. Willow twigs severed from an adult tree in August in mid-Europe are in the state of latewood production. When such cuttings are placed in a nutrient solution under increased temperature, the cambium begins to build earlywood (Fromm 1997). In the cambial region of Pinus densiflora, the transition from earlywood to latewood occurs concurrently with a decrease in the total amount of indole-3-acetic acid (IAA) after it has peaked, suggesting the involvement of IAA in the control of latewood formation (Funada et al. 2001). While the total amount of IAA does not change with latewood initiation in the cambial region of Pinus sylvestris, nonetheless, its radial distribution pattern is altered (Uggla et al. 2001), indicating that IAA probably has a role in defining the altered developmental pattern associated with latewood formation. In Pinus radiata and P. sylvestris, latewood formation seems to be correlated to an increase in the concentration of abscisic acid (ABA) (Jenkins and Shepherd 1974; Wodzicki and Wodzicki 1980).
In contrast to trees growing in temperate latitudes, trees of tropical regions with consistent day length and temperature generally do not exhibit a distinctive tree ring structure, and their cambium is more or less active over the whole year. Seasonality can occur in tropical zones, however, and wood structure also responds to changing weather conditions, i.e. during drought periods narrow wood cells are formed by the cambium, while during a rainy period cells with wide lumina are produced. For example, in Bolivian rainforests climate-growth analysis has indicated that rainfall plays a major role in tree growth (Brienen and Zuidema 2005). Amazonian trees growing in zones with dry periods drop their leaves during drought and build new leaves, as well as wood, shortly after the beginning of the rainy season (Vetter and Botosso 1989; Alves and Angyalossy-Alfonso 2000). Also flooding can cause cambial rest, and hence a ring structure, because root activity is reduced in oxygen-free conditions (Worbes 1985, 1995).
2.3 Cambial Reactivation
Well-coordinated changes in the cellular structure, physiology and metabolism of cambial cells define the transition between active and dormant states. The cytological and structural aspects of cambial reactivation in spring, as well as cambial activity and cessation, have been extensively studied (Catesson 1994; Larson 1994). Recently, it has been shown that tree social status also affects cambial activity. Activity starts earlier, stops later and lasts longer in dominant silver-fir trees than in intermediate and suppressed ones (Rathgeber et al. 2011). In general, in temperate climate zones, the formation of phloem starts before xylem differentiation in many diffuse-porous and coniferous species in spring. In contrast, in ring-porous species, phloem and xylem formation start simultaneously.
Cambial activity is a temporary event, characterised by a special physiological condition of the meristematic cells. A particularly future-oriented field of research in this area lies in the transduction of seasonally conditioned signals (e.g. day length, temperature) controlling cambial activity. Temperature plays a key role during cambial reactivation in spring. It has been shown that localised heating of tree stems can induce cambial reactivation in evergreen conifers (Oribe et al. 2001, 2003) and in hybrid poplar (Begum et al. 2007). In poplar, however, the buds of the trees had not yet burst, indicating that there is no close temporal relationship between bud burst and cambial reactivation. The heat-induced xylem differentiation in hybrid poplar stems was the same as that of xylem cells formed in the normal way, indicating that an increase in the temperature of the stem is one of the most important factors in cambial reactivation (Begum et al. 2007). Furthermore, in order to protect the sensitive cambial cells in spring from sudden reductions in temperature, several cold hardiness-related genes are superinduced during the early stage of reactivation (Druart et al. 2007).
Cambial reactivation in spring starts before any significant photosynthetic activity occurs in the tree. As a result, an alternative source of energy and carbon is needed for cell division in spring. Induction of sucrose synthase and various invertases during the early phase of reactivation in the cambium shows that sucrose is split into hexoses that can be metabolised via glycolysis. During cambial reactivation, high amounts of sucrose are required for cell growth. The uptake of sucrose into cambial cells might be under the control of a PM H+-ATPase, which is demonstrated by immunolocalisation in the cambial and wood formation zone of poplar (Arend et al. 2002). Also, fats are metabolised via beta-oxidation, and the glyoxylate cycle and the level of amino acids are increased due to the degradation of storage proteins in spring (Druart et al. 2007). During cambial reactivation induced by artificial heating of Cryptomeria japonica stems, the levels of starch granules and lipid droplets decreased in the cambium, indicating that these might also be needed as sources of energy for the initiation of cambial cell division and xylem differentiation (Begum et al. 2010). In addition, immunolocalisation demonstrated plasmodesmatal trafficking of storage proteins during cambial reactivation in Populus nigra, indicating that lectin-like reserve proteins, or their degradation products, may be transferred through the plasmodesmata of phloem parenchyma and rays (Fuchs et al. 2010a). In cambial initials, the first cell division coincides with a massive increase in plasmodesmata numbers, in particular at the division wall (Fuchs et al. 2010b). In addition, the onset of rapid xylem production in spring correlates to a marked increase in stem respiration (Lavigne et al. 2004). Surprisingly, in wood rays of poplar, the metabolic pathways related to flower induction are already high in February (Larisch et al. 2012), indicating that reactivation from dormancy had already begun at this time of the year. In contrast, in July, the pathways related to active growth, such as lignin biosynthesis, nitrogen assimilation and defence, were enriched in rays (Larisch et al. 2012).
As early as the 1930s, studies based on bioassays supported the hypothesis that hormones from growing buds in tree stems can induce downward cambial reactivation in spring (Söding 1937; Avery et al. 1937). Subsequently, some of the key signalling molecules related to cambial reactivation, such as plant hormones, have been identified by biochemical and molecular approaches (Sundberg et al. 2000; Moyle et al. 2002; Tanino 2004). In recent years these hormone treatment studies have been complemented by studies using transgenic plants with modified hormonal signalling. An entire chapter of the present volume is dedicated on the role of hormones in regulating xylem development (see chapter “The Role of Hormones in Controlling Vascular Differentiation”).
It is known that auxin plays a major role in wood formation. A radial concentration gradient of auxin has been found within the cambial zones of both Populus and Pinus, with the highest concentrations present in dividing cambial cells (Sundberg et al. 2000). This gradient correlated with the expression pattern of auxin signalling genes (Moyle et al. 2002) and is generated when auxin is synthesised at the shoot apex (Sundberg and Uggla 1997) and transported basipetally within the stem (Little and Savidge 1987; Schrader et al. 2003; Björklund et al. 2007). When auxin transport is inhibited, wood formation is suppressed in Pinus shoots (Sundberg et al. 1994), suggesting that auxin is required for secondary xylem development. Apart from the endogenous hormonal concentration, however, the sensitivity of cambial cells to hormones alters seasonally and plays a significant role in activation (Lachaud 1989). Transgenic poplar trees with decreased auxin responsiveness show fewer cambial cell divisions (Nilsson et al. 2008), and the level of cambial auxin signalling seems to be controlled separately by the rate of auxin transport and by the level of cambial responsiveness to auxin (Baba et al. 2011).
Apart from auxin, gibberellin acts as a mobile shoot-derived signal activating the onset of xylem production in Arabidopsis (Ragni et al. 2011). During cambial reactivation in spring, a transient induction of a gene encoding a gibberellin biosynthesis enzyme can be observed in poplar (Druart et al. 2007), suggesting a role for gibberellin in the activation of cambial growth. Furthermore, in transgenic plants with either enhanced gibberellin signalling (Mauriat and Moritz 2009) or biosynthesis (Eriksson et al. 2000), wood production is increased. Interestingly, increased gibberellin signalling also enhances polar auxin transport in poplar (Björklund et al. 2007) indicating an interrelationship between each of these hormones in the regulation of cambial activity.
Cytokinins are also involved in the regulation of auxin transport (Bishopp et al. 2011). Cytokinin signalling is necessary for cambial functioning in the roots of Arabidopsis (Matsumoto-Kitano et al. 2008) as well as during cambial development in poplar, where cytokinin primary response genes and cytokinin receptors are involved (Nieminen et al. 2008). Finally, it has been demonstrated that ethylene is involved in the formation of tension wood in poplar (Love et al. 2009, see also chapter “Biology and Chemistry of Tension Wood”) as well as in tracheary element differentiation of Zinnia cell cultures (Pesquet and Tuominen 2011). Ethylene signalling has also been found to be connected to jasmonate signalling in Arabidopsis, with elevated jasmonate signalling, causing an increase in secondary growth and stem diameter (Zhu et al. 2011).
Apart from hormones, ions might also play an important role in cambial reactivation. Immediately before the resumption of cell division, a strong temporary increase in calcium concentrations has been observed in the cambium of both beech (Follet-Gueye et al. 1998) and poplar (Arend and Fromm 2000). This increase may be involved in the regulation of cambial reactivation because calcium is known to activate several enzymes such as lipases and amylases which play a role in the hydrolysis of lipids and starch respectively.
2.4 Transition to Dormancy
In temperate latitudes, growth cessation occurs in response to shortening day length and coincides with autumnal senescence, leaf shedding, completion of bud set and cambial cessation. Dormancy consists of many interrelated subprocesses that are active during the different periods. For example, prior to the transition to dormancy, E2F phosphorylation is elevated in the cambium of hybrid aspen (Espinosa-Ruiz et al. 2004). After entering dormancy, increase in drought resistance and acquisition of frost resistance are characteristic subprocesses in buds (Rohde and Boerjan 2001; Ruttink et al. 2007). Also, in the stem, genes related to cold hardiness and defence are overrepresented in winter during dormancy, as shown by whole transcriptome analysis in poplar (Ko et al. 2011).
Since cessation of cambial cell division in aspen in mid-August occurs before the temperature becomes suboptimal (Druart et al. 2007), temperature may not play a critical role in growth cessation. In correlation to the cessation of cell division, transcript levels of cell cycle genes decline in autumn, while those for enzymes involved in phospholipid biosynthesis (necessary for the synthesis of new vacuolar membranes), lipid desaturation, dehydrins and cold-regulated proteins are induced (Druart et al. 2007). The development of cold hardiness coincides with the breakdown of starch in autumn, and the generated carbohydrates serve for metabolites such as sucrose, raffinose and cryoprotectants. Interestingly, poplar FT (a RAF-kinase-inhibitor-like protein) and CONSTANS(CO) (a nuclear zinc-finger protein) have been identified as mediators of short-day signals for growth cessation, since growth does not stop upon exposure to short days when FT1 and CO homologues of poplar (P. trichocarpa) are overexpressed in transgenic aspen (P. tremula × P. tremuloides) (Böhlenius et al. 2006).
At the end of the growing season, when trees enter dormancy, hormones also play a role. The sensitivity of the cambium to auxin, which plays a key role in regulating wood formation, is lost (Little and Bonga 1974). The molecular basis of short-day-induced growth cessation and dormancy in the cambial meristem involves a decrease of auxin responsiveness, although basipetal auxin transport remains active (Baba et al. 2011) and cambial auxin levels remain stable (Uggla et al. 1998; Schrader et al. 2003, 2004b). In addition, the timing of the cessation of cambial cell division caused by short days differs between northern and southern genotypes of hybrid poplar and is coincident with the changes in the pattern of expression of the auxin-regulated genes (Resman et al. 2010). In the cambial region of Eucommia ulmoides Oliv., the expression of ABP1, one of the putative receptors of auxin, was found to be high, low and remarkably scarce in the active, quiescent and resting stages, respectively (Hou et al. 2006). This would suggest a role for ABP1 in mediating auxin-dependent regulation of cambial activity. Results also show that ABP1 expression is improved by IAA but inhibited by ABA (Hou et al. 2006), indicating a possible role for ABA in the cambial activity-dormancy cycle. In addition, expression of the Arabidopsis mutant abi1 gene alters ABA sensitivity, stomatal development, and growth morphology in poplar, indicating that ABA acts as a negative regulator of shoot growth and, furthermore, has a role in shoot branching by inhibiting lateral bud outgrowth (Arend et al. 2009).
2.5 Within-Tree Variations
Cambial age has an important effect on the structure of cambial cells and their derivatives. Wood produced during the early years of cambial growth is called juvenile wood. Juvenile wood is more elastic, thus allowing flexibility, while mature wood is stiffer because it has to carry a greater mechanical load with increasing age. In comparison to mature wood, juvenile wood in poplar is characterised by shorter fusiform cambial cells as well as shorter xylem cells that are derived from these, a lower crystallinity of the fibres, a larger microfibril angle, thinner secondary walls, a higher density of vessels and a lesser amount of latewood (Hejnowicz and Hejnowicz 1958; Kroll et al. 1992). The large S2 microfibril angle in juvenile wood causes increased longitudinal shrinkage as well as decreased transverse shrinkage in sawn lumber during drying. Chemically, juvenile wood shows a lower cellulose content and a higher content of lignins and hemicelluloses. Since earlywood cells predominate, overall wood density decreases in line with lower strength properties (modulus of elasticity and modulus of rupture). Thus, in comparison to mature wood, juvenile wood exhibits marked differences in strength, stability and stiffness and is generally considered to be of inferior quality (Clark et al. 2006; Jordan et al. 2006; Mansfield et al. 2007). In addition, in gymnosperms it tends to contain more compression wood and a higher incidence of spiral grain. Juvenile wood is suitable for the characterisation of the molecular mechanisms controlling MFA and mechanical strength. Thus, transcriptome profiling of juvenile wood with contrasting levels of stiffness from Pinus radiata has identified putative candidate genes involved in microfibril orientation and cell wall mechanics (Li et al. 2011). Since the global proportions of construction timber originating from plantations is growing rapidly, the amount of juvenile wood provided for further processing is also increasing.
Depending on the species, the structure of root and branch wood may also differ from stem wood. The pattern of cell divisions in root cambia differs little from those in stem cambia. In root wood cellular changes are most prominent with increasing distance to the stem. In general, root wood cells have wider lumina and reduced cell wall thickness. Especially in angiosperm trees, the density and size of vessels increase in roots, showing a homogenous distribution throughout the whole growth ring. For instance, in species with ring-porous stem wood, the vessels in the root wood exhibit a diffuse-porous pattern (Fig. 5a, b). In conifers, root tracheids have wider lumina compared to stem tracheids. Also, root tracheids often have bordered pits on radial walls which occur in pairs, whereas in stem wood tracheids, these bordered pits occur only singly in most species. With regard to cell length, tracheids increase in length as their distance from the stem increases and differences between early- and latewood are diminished (Fig. 5c, d). Furthermore, the amount of parenchyma increases in the root wood of both angiosperms and gymnosperms.
(a) Transverse view of stem wood from Quercus robur showing a ring-porous pattern of vessels. (b) Transverse view of root wood from the same species shows a diffuse-porous vessel distribution. (c) Transverse view of stem wood from Picea abies showing a characteristic tree ring with early- and latewood. (d) Transverse view of root wood with diminishing differences between early- and latewood. f fibres, r ray, rd resin duct, t tracheids, v vessel. Bar 200 μm (Courtesy of Dr. A. Olbrich)
Turning now to branches, these have often been a source of cambial studies because sampling is relatively easy. When rates of radial and circumferential branch expansion, age and eccentricity are considered, the patterns and consequences of anticlinal divisions in branch material differ little from those in stem material (Larson 1994). Often, branch wood has an increased density in comparison to stem wood, and generally, xylem elements in branches are smaller than those of stems. Vessels from angiosperm branch wood often have reduced lumina while in gymnosperms the number of resin ducts is greater. Also, changes in chemical composition occur in branch wood, e.g. in spruce branches there are higher concentrations of polyoses, lignin and resin than in the stem.
3 Cell Expansion
Following cell division in the cambial zone, xylem mother cells leave the meristem and wood development progresses through the expansion of differentiating cells. By combining symplastic growth—when neighbouring cells differentiate together—and intrusive growth—when they move past each other—wood cells develop to their final shape. Within the xylem elongation zone, all cells undergo expansion but with individual cell types differing in the extent, type and direction of their enlargement.
In regard to the direction of enlargement, for example, while axial parenchyma cells enlarge primarily radially, vessel elements expand tangentially as well as radially, leading to the lateral displacement of neighbouring cells. In regard to the type of growth, meanwhile, both symplastic growth and intrusive growth are involved in the enlargement of a vessel element. Intrusive growth can relocate neighbouring cells out of their position. Ray cells expand mainly in the radial direction whereas fibres grow radially as well as longitudinally through intrusive tip growth. Tip growth can be very extensive, with the length of the fibres sometimes exceeding by severalfold that of fusiform cambial cells. The dimension of expansion, and eventually cell morphology, is controlled by the anisotropic extension of the primary cell wall (Cosgrove 2005), which depends on both the orientation of cellulose microfibrils and the extent of turgor pressure (Tyerman et al. 2002). The orientation of cellulose microfibrils, which determine the direction of cell expansion, is controlled by cortical microtubules. In differentiating conifer tracheids, cortical microtubules have been shown to be randomly orientated when radial primary walls are formed, but then to change their orientation progressively from longitudinal to transverse as the cells expand (Funada et al. 1997). Since the extent of expansion differs between cell types, controlling mechanisms such as turgor pressure and/or cell wall plasticity must be regulated differentially. Also, the incorporation of hemicelluloses, modification of cellulose and subsequent remodelling of the primary wall play a key role in defining cell morphology.
With regard to turgor regulation, it is well known that potassium is essential for cell expansion during wood formation in trees. In poplar, the K+ concentration in the cambial zone and developing xylem is much higher than in the mature xylem and phloem (Fromm 2010). The K+ concentration is also significantly higher in differentiating vessels in comparison to young fibres (Langer et al. 2002). The pattern of K+ in developing xylem cells also correlates well with the size of the newly formed vessels and fibres. When poplars were grown under non-limiting K+ regimes, K+ content increased within the cambium and also the vessel size clearly increased in contrast to fibre size (Fig. 6, Langer et al. 2002). Furthermore, the developing xylem zone was threefold larger in comparison to plants grown under K+ depletion. When poplars were treated with tetraethylammonium (TEA), a K+ channel blocker, the size of the differentiating vessels was significantly reduced (Fig. 6). In contrast, the size of newly developed fibres was neither influenced by K+ supply nor by TEA treatment. The osmotic function of K+ seems, therefore, to be restricted to vessel and cambial cell enlargement. Moreover, in the poplar cambium, a seasonal variation has been observed, with a high potassium content in spring and summer and a significant reduction in autumn and winter, correlating with the radial width and the osmotic potential of the cambial zone (Wind et al. 2004).
Relative cambium K+ content and potassium-dependent vessel lumen. (a) The rel. K+ content of the cambium increases with root potassium supply from 1 to 11 mM. (b) Plants supplied for 2 weeks with K+ and treated with TEA (5 mM) show reduced vessel size under TEA treatment as well as 1 mM K+. In contrast, fibre lumen does not respond significantly to TEA treatment or different K+ supply. (c) TEA-treated twigs have reduced vessel size compared to untreated twigs (d), Bar 20 μm (According to Langer et al. 2002)
With regard to the molecular analysis of K+-dependent wood formation, so far, ten K+ channels have been identified from the poplar genome (Ache et al. 2010). In close correlation with the increasing cambial K+ concentration in spring is the expression of two ion channels named PTORK (P. tremula outward rectifying K + channel) and PTK2 (P. tremula K + channel 2), which are induced at temperatures >10–15 °C during cambial reactivation. The biophysics of these two poplar K+ channels have been measured by the double-electrode voltage-clamp technique after injection of their gene products’ cRNAs into Xenopus oocytes. The results indicate that depolarization of the membrane elicits an outward rectifying current. PTORK, therefore, is under the control of the membrane potential, as well as external K+ concentration, and releases K+ in a voltage and K-dependent manner. In contrast to PTORK, PTK2 mediates both the uptake and release of K+ in response to changes in membrane potential, calcium and pH (Langer et al. 2002; Fromm and Hedrich 2007). By using immunofluorescence microscopy PTORK labelling can be localised in the plasmalemma of differentiating fibres and vessel-associated cells (VACs) of mature wood rays (Fig. 7, Arend et al. 2005). Since PTORK was absent in vessels, however, it is assumed that this channel might limit the radial expansion of differentiating fibres by mediating K+ efflux. Moreover, the function of PTORK in VACs of mature wood might be to enable the release of K+ into vessels from where it can be remobilised within the shoot.
Immunolocalisation of PTORK in poplar stems. (a) In young fibres the PTORK-specific fluorescence labelling is concentrated along the plasma membrane (arrows). (b) Light micrograph of the same section as shown in (a). (c) A vessel-associated ray cell shows PTORK labelling at the membrane site facing the vessel element. (d) Light micrograph of the same section as shown in (c) Bar 10 μm (According to Arend et al. 2005)
In addition to ion channels, the plasma membrane H+-ATPase can also be detected in developing xylem and cambial cells, as well as in VACs during active growth (Arend et al. 2002, 2004). It is assumed that the PM H+-ATPase generates the proton-motive force necessary for the uptake of K+ and other nutrients into developing wood cells, as well as VACs from the xylem stream.
With regard to the control of cell wall extension, there is convincing evidence, in line with the acid growth hypothesis, that auxin plays a significant role by activating a plasma membrane H+-ATPase (Brett and Waldron 1990). By pumping protons from the cytoplasm into the cell wall, the produced acidification causes a loosening of the cell wall structure, and therefore, a turgor-driven extension becomes possible. Cell wall-loosening enzymes also play an important role in cell wall extension, however. The main load-bearing molecules in the primary wall are cellulose microfibrils coated with xyloglucan and linked by xyloglucan bridges (Mellerowicz et al. 2001). Therefore, wall expansion depends mainly on the ability to break these xyloglucan bridges and cut the hydrogen bondings between xyloglucans and cellulose. Of fundamental importance in these processes are cell wall-loosening enzymes such as xylanase, endoglucanases and xyloglucan endotransglucosylase/hydrolase (XET; Mellerowicz et al. 2001; Cosgrove 2005), the last of which is involved in the incorporation of newly synthesised xyloglucan into the existing macromolecular network. Other enzymes that contribute to cell expansion are xyloglucan-specific glucanases, which hydrolyse xyloglucan (Matsumoto et al. 1997) and endoglucanases, that are involved either in the degradation of noncrystalline cellulose (Ohmiya et al. 2000) or in the biosynthesis of cellulose during growth (del Campillo 1999).
Of special interest in mediating cell wall extension are expansins. These are proteins bound to the cell wall that are able to regulate the rheological properties of the cell wall (McQueen-Mason 1997). They bind at the interface between cellulose microfibrils and matrix polysaccharides, showing no hydrolysing activity but reversibly disrupting non-covalent bonds in a pH-dependent manner (McQueen-Mason and Cosgrove 1995). Expansins are supposed to be involved in the radial expansion and tip growth of fusiform cambial cells and developing xylem cells. Tip growth requires the softening of the pectinous middle lamella of neighbouring cells which would otherwise provide resistance to the intruding growing tip. Also, the formation of Ca2+-bound pectins in the middle lamella of neighbouring cells can limit the growing fibre tip (Catesson et al. 1994). Thus, the amount and composition of pectins might be assigned an important role in expansion. For example, in poplar cell elongation, tip growth is initiated concomitantly with cell division, and the final morphology of fibres is affected by the function of pectin methyl esterase (PME), which acts on the pattern of methyl-esterification of pectic homogalacturonan in the compound middle lamella, and therefore serves to constrain both intrusive and symplastic cell growth (Siedlecka et al. 2008; Pelloux et al. 2007). Wall plasticity, then, could be controlled by PME through its regulation of the status of pectin methylation. Upregulation of Populus PME1 induces de-esterification of homogalacturonan and inhibits fibre elongation. Downregulation of this gene, however, stimulates both fibre elongation and the radial expansion of fibres and vessels (Siedlecka et al. 2008). Since spectra of PME isoforms vary across the developmental gradients of wood-forming tissues (Mellerowicz and Sundberg 2008), the sequential expression of different PMEs could play a significant role in the dynamics of cell expansion.
4 Cell Wall Thickening
4.1 Structure and Composition of the Cell Wall
Both before and during enlargement, young cells have primary cell walls which are composed of 30–50 % pectins, 20–30 % cellulose, 20–25 % hemicelluloses, up to 10 % proteins and a considerable amount of water. Cellulose microfibrils are embedded within a matrix of hemicelluloses, pectins and glycoproteins. Within the hemicelluloses the main component is xyloglucans, which are closely connected with cellulose microfibrils by hydrogen bonds (Hoson 1991) in order to control cell wall expansion. Pectins are mainly composed of polygalacturonic acid and rhamnogalacturonan, which generate a strongly hydrophilic gel surrounding the cellulose-hemicellulose network. After completion of cell elongation, pectins are combined through Ca2+ in order to prevent further cell expansion. In addition, the primary cell wall consists of structural proteins (glycoproteins) which are mainly composed of hydroxyproline-rich proteins (HRGPs), proline-rich proteins (PRPs) and glycine-rich proteins (GRPs). Furthermore, numerous enzymes have been found in the primary wall, such as peroxidases and laccases, which can catalyse lignifications, as well as cellulase and pectinase, which are important during cell wall degradation, e.g. during the formation of perforation plates in differentiating vessels. Phosphatases, invertases, pectinases and pectin methylesterases have also been found in the primary wall.
In terms of chemical composition, distinct differences occur between primary and secondary cell walls. While the main components of primary walls in the developing xylem are pectin, cellulose, hemicelluloses and protein, secondary walls are composed mainly of cellulose, hemicelluloses and lignin. The transition from the primary to the secondary wall is mainly characterised by a decrease in cell wall water content, in association with a decrease in wall porosity caused by a tight accumulation of cellulose microfibrils, as well as by the cross-linking of lignin that displaces water and fills the available interspaces (Fujino and Itoh 1998). The biosynthesis of the secondary cell wall can already start before cell expansion ceases, and this process of formation can be easily identified with polarised light microscopy, since cellulose consists of crystalline zones which cause double refraction of light and numerous cellulose microfibrils are parallel oriented in the secondary wall (Fig. 8).
Secondary wall formation and lignification in wood from Pinus sylvestris grown at a cold location in Kevo (North Finland) where a clear gap occurs between S2 formation and lignification. (a) Light micrograph of phloem and wood formation zone. (b) The same section viewed under polarised light indicating S2 formation. (c) Lignified cell walls shown by lignin autofluorescence. (d) Combined photograph showing (in false colours) S2 formation in red, lignifications in yellow and lignified S2 walls in orange. Note that the sieve cells of the phloem also show secondary wall thickening (b, d), which is characteristic of the secondary phloem of the Pinaceae (Abbe and Crafts 1939). CZ cambial zone, EZ elongation zone, LD lignin deposition, MX mature xylem, PH phloem, RB ring border, rd resin duct, SW secondary wall formation. Bar 100 μm (Courtesy of Dr. A. Olbrich)
During secondary cell wall formation, a reprogramming of wall biosynthesis occurs. Zhong and Ye (2007) identified fibre- and vessel-element-specific master switches that activate transcription factors which induce secondary wall programmes (see also chapter “Transcriptional Regulation of Wood Formation in Tree Species”) that at least partially overlap between fibres and vessel elements and coordinate expression of cell wall-related genes. For instance, in poplar, wood-associated NAC domain transcription factors (Ptr WNDs) are master switches activating a series of downstream transcription factors involved in the regulation of secondary wall biosynthesis during wood formation (Zhong et al. 2011). With regard to genes encoding cell wall-related enzymes, various gene families have been characterised in tree species. These include, for example, genes encoding carbohydrate-active enzymes (CAZymes; Aspeborg et al. 2005), cellulose synthases (CesAs; Djerbi et al. 2005), cellulose synthase-like synthases (CSLs; Suzuki et al. 2006), pectin methyl esterases (PMEs; Pelloux et al. 2007), XTH genes encoding xyloglucan (XG) endotransglucosylases and hydrolases (XETs and XEHs; Baumann et al. 2007; Nishikubo et al. 2007) and expansins (Sampedro et al. 2006).
Mature wood cell walls are composed of multiple layers which differ in microfibril angle and ratios of cellulose to lignin, hemicellulose and pectin. The outermost layer is the compound middle lamella (CML) which consists of the middle lamella and the primary walls, followed by three layers of secondary cell walls (S1, S2, S3). Before the secondary wall is formed, the CML is rich in pectin and xyloglucan. In contrast, after completion of secondary wall formation, the CML has a high lignin concentration, e.g. 68 % in poplar. After definition of cell size and shape, cell wall thickening generally consists of the lamination of S1, S2 and S3 layers with distinct microfibril angles, in a spiral orientation either to the right or to the left (Fig. 9).
Each of these wall layers is composed of parallel-arranged cellulose microfibrils, lignin and hemicelluloses. The amount of these macromolecules within the different cell layers can be influenced by abiotic factors such as mechanical stress. The S1 layer is formed first and has a relatively flat microfibril angle (MFA) with regard to the cell axis (60°–80°). Here the microfibrils are arranged in a spiral orientation. Since it is an intermediate layer between the primary wall and the S2, the S1 is only 0.1–0.4 μm thick (Fig. 9). The S2, meanwhile, is the thickest of the secondary wall layers (1–10 μm) and has the lowest microfibril angle (4°–30°). For instance, in the S2 of normal wood fibres from poplar, the MFA is 4° while it is 13° in wood fibres under tension (Lautner et al. 2012). The MFA of the S2 layer varies both longitudinally and radially within the tree stem. The microfibril angle strongly affects the mechanical properties of the wood cell; with an increasing angle, wood becomes less rigid and its swelling and shrinkage properties change. The S2 layer is most important for mechanical stability and the properties of the wood are affected mainly by the S2. Most wood biomass is also in the S2 layers of fibres. Eventually, the microfibrils reorientate themselves to a transverse helix, and the innermost layer of the secondary wall, the S3, is formed. This is only 0.5–1.0 μm thick and the MFA is flat (60°–90°), similar to the S1.
Wood cells of different types show variations in their secondary wall formation. For example, xylem fibres need more time to differentiate than vessels. In poplar, vessel elements and their contact cells are the first to form a secondary wall (Murakami et al. 1999). The S2 layer is proportionally thinner in vessels when compared to fibres (Harada and Coté 1985); however, both fibres and vessels show a three-layered secondary cell wall. Next to vessels, fibres can also show pectin-like fibrillar cell wall deposits lining the lumen-facing side of the cell wall (Arend et al. 2008). To ensure water transport is the most important function of newly formed wood, and the differentiation of the various cell types to achieve this has to occur in a coordinated manner. Each cell, therefore, has to receive spatial information in order to develop in a concerted action with neighbouring cells. Numerous vessel elements must be joined together end-to-end to form a functional, often several metre long, vessel. Additionally, pit connections between neighbouring cells have to be developed without any skips. In expanding vessels pits and perforations are free of microtubules (Chaffey et al. 1999), and secondary wall deposition does not occur in the area of pits.
4.2 Lignification
The secondary wall is assumed to be composed of intimate lignin cross-linking between cellulose layers (Kerr and Goring 1975). Lignification is an irreversible process, and lignified cell walls can be identified easily by fluorescence microscopy (Fig. 8) and UV microspectrophotometry (see chapter “Topochemical and Electron Microscopic Analyses on the Lignification of Individual Cell Wall Layers During Wood Formation and Secondary Changes”). Lignin gives compression and bending strength to the wood and imparts hydrophobic qualities on the cell wall, required for the transport of water. Water transportation itself occurs mainly in earlywood cells of the sapwood, as shown by computer tomography (Fromm et al. 2001). Thus, lignin strengthens xylem cells and inhibits collaboration through transpiration-induced negative pressures. It also prevents lateral water diffusion through the cell wall and hence facilitates longitudinal water transport. In addition, lignified cell walls are resistant against microbiotic attack (Nicholson and Hammerschmidt 1992). Lignin occurs at a concentration of 16–31 % in the wood and is derived from the three monolignols p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, which give rise to H, G and S units, respectively. These units differ from each other in their degree of methoxylation. Several enzymes required for the biosynthesis of monolignols have been localised in membranes of the ER and Golgi-derived vesicles, indicating that monolignols are secreted in order to be incorporated in the cell wall (Mellerowicz et al. 2001; Kenada et al. 2008). During polymerization of lignin, monolignol radicals caused by dehydrogenation reactions undergo radical coupling in muro (Boerjan et al. 2003). Vessel elements are lignified first in angiosperms, while fibres lignify later and finally even pit membranes are lignified within the heartwood (Fromm et al. 2003). When the S1 layer is completed during wood formation, lignification starts, particularly at cell corners and then in the middle lamella, and progresses towards the cell lumen after the formation of the S2 and S3 layers (Terashima et al. 1993). Across the gradient of the developing xylem, lignin composition shifts from having more p-coumaryl and coniferyl alcohol incorporated within it to later having more sinapyl alcohol (Mellerowicz et al. 2001). The composition and content of lignin vary substantially among different taxa, cell types and wall layers. For example, lignin of angiosperms (hardwood) is composed mainly of coniferyl and sinapyl monolignols, with traces of p-coumaryl alcohol, while in gymnosperms (softwood) it is mainly coniferyl alcohol that occurs. Insights into gene function and biosynthesis of lignin have been given by transgenic work focusing on a reduction of lignin content as well as manipulation of the S-to-G ratio and monolignol incorporation (Chiang 2006; Voelker et al. 2010).
4.3 Cellulose Formation
In addition to lignin, wood consists of 40–50 % cellulose which provides the basis for the tensile strength of the cell wall. The cellulose content, as well as the microfibril angle (MFA), has a significant effect on the properties of wood, particularly the modulus of elasticity (MOE) or stiffness. Cellulose appears in the form of microfibrils (MF) which are ~3–10 nm thick (Somerville 2006). Recently, the structure of the microfibrils of spruce wood cellulose has been studied using a range of spectroscopic methods coupled to small-angle neutron and wide-angle X-ray scattering (Fernandes et al. 2011). Results suggest that microfibrils consist of about 24 chains and that these are possibly twisted, with the level of disorder increasing towards the surfaces (Fernandes et al. 2011). The microfibrillar cellulose is largely crystalline; microfibrils can either be accumulated laterally in two crystalline forms, cellulose Ialpha or cellulose Ibeta, or they can form an amorphous, paracrystalline structure (Jarvis 2003). Within the cell wall, MFs sum up into macrofibrils which are organised in tangential or random sectors (Donaldson 2007). The size of the macrofibrils depends on the lignin and hemicellulose content, but they vary between 15 and 500 nm in thickness and 4 and 7 μm in length within wood cell walls.
By using freeze-fracture preparations, it can be shown that cellulose is synthesised by plasma membrane-bound rosettes: the cellulose synthase complexes (Brett 2000). Each rosette synthesises cellulose from UDP-glucose and consists of six globules, each of which is assumed to contain six CesA proteins (Mutwil et al. 2008). Cellulose synthase genes carry the type A catalytic domain and are therefore called CesA genes (Holland et al. 2000), involved in both primary and secondary wall biosynthesis. CesA genes have cell-specific expression patterns and have been cloned from the wood-producing tissues of trees, including poplar (Sterky et al. 1998; Wu et al. 2000) and pine (Allona et al. 1998). In the Populus genome, 18 CesA genes have been identified (Djerbi et al. 2005; Suzuki et al. 2006). In Arabidopsis mutant, expression analyses have demonstrated that three CesA proteins are different from those involved in primary wall biosynthesis and play key roles in secondary wall biosynthesis (Mutwil et al. 2008). Expression analysis indicates that homologues of these three proteins are also the most abundant CesAs during wood formation in Populus and Eucalyptus (Aspeborg et al. 2005; Djerbi et al. 2004; Prassinos et al. 2005; Bhaudari et al. 2006; Ranik and Myburg 2006). During cellulose synthesis, the moving rosettes release cellulose fibrils into the cell wall. The rosettes are synthesised within the endoplasmic reticulum and transported via Golgi vesicles into the plasma membrane (Haigler and Brown 1986).
Since a parallel correlation exists between cellulose microfibrils and cortical microtubules, a constraint model has been established which describes a rail-like system consisting of microtubules that guide the cellulose synthase complexes in a defined direction (Heath 1974; Baskin 2001). Genetic and pharmacological studies have provided experimental evidence for this model. To visualise CesA, it was fused with a yellow fluorescent protein (YFP) in order to show that the microtubules and microfibrils are pharmacologically disrupted in a way that supports a direct mechanism for the guidance of cellulose deposition by microtubules (Paredez et al. 2006a, b). Furthermore, by using a green fluorescent fusion protein (GFP:CesA), it can be shown that the cortical microtubules also position the delivery of cellulose synthase complexes to the plasma membrane (Paredez et al. 2006a, b). Since microtubules consist of tubulin, some isoforms of the family of α-tubulin and β-tubulin are highly expressed during xylem secondary wall formation in Populus (Oakley et al. 2007). Furthermore, a strong association exists between MFA and α-tubulin in Pinus taeda (Gonzalez-Martinez et al. 2006).
Two models have been proposed regarding the rail system guiding cellulose synthesis through cortical microtubules (Nick 2008). The monorail model envisages that the cellulose-synthesising complexes are moved along microtubules driven by a microtubule-dependent motor. In contrast, the guardrail model envisages that the cellulose-synthesising complexes are moved by the force from the crystallising cellulose (Nick 2008). Since the practical discrimination between these two models is not easy, further investigations are required in order to get more insights into this process.
4.4 Hemicellulose Deposition
Apart from lignin and cellulose, wood is made up of hemicelluloses which account for about 25–35 % of the dry weight of wood and therefore are one of the main wood components. Generally they either occur as heteropolymer-like glucomannan, galactoglucomannan, arabinogalactan and glucuronoxylan or as homopolymer such as galactan, arabinan and ß-1,3-glucan. The biosynthesis of hemicelluloses occurs in the Golgi apparatus in two major steps. Firstly, the formation of the backbone occurs through polysaccharide synthases, and then, secondly, side chain residues catalysed by glycosyltransferases are added (Keegstra and Raikhel 2001). In the secondary walls of dicotyledons 4-O-methylglucuronoxylan is the most important hemicellulose (York and O’Neill 2008). Xylans are composed of 1,4-linked ß-d-xylopyranosyl backbone residues, which are nearly always substituted with mono- or disaccharide side chains. Xylan coats microfibrils in secondary walls and seems not to be distributed uniformly within the wood cell wall. For instance, it is associated with the thick cellulose microfibrils in the S1 and S2 layers but not with the thin microfibrils in the S1 layer of beech fibres, as shown by Awano et al. (2000) using immuno-field emission SEM. During secondary wall formation, the biosynthesis of xylan is specifically upregulated (Gregory et al. 1998), and xylan deposition increases in the secondary walls of tracheary elements in comparison to the primary walls (Ingold et al. 1988). It has been demonstrated that in poplar family members, GT43 is involved in the biosynthesis of xylan backbones and that the poplar GT8D is essential for the biosynthesis of the xylan reducing end sequence (Lee et al. 2011). In general, genes for hemicellulose biosynthesis are highly expressed in developing wood of Populus (Geisler-Lee et al. 2006) and are especially upregulated in the formation of secondary walls (Aspeborg et al. 2005).
5 Programmed Cell Death
Xylem cell death is an important part of the wood formation programme and a prerequisite for the transport of water in vessels and tracheids, commonly known as tracheary elements (TEs). The necessary components for cell death seem to be produced early in xylem differentiation. Inhibitors, as well as storage of hydrolytic enzymes in inactive forms in the vacuole, prevent cell death until vacuolar rupture, which triggers autolytic hydrolysis of the cell contents. There are significant differences between different xylem cell types, however. While vessel elements need only a couple of days to differentiate and die, the lifetime of libriform fibres is estimated to be approximately one month in Populus tremula and Picea abies (Bollhöner et al. 2012). Dead vessels, therefore, are often bordered by still living fibres, as shown in poplar (Fig. 10). Finally, ray parenchyma cells can live several decades before they die (Nakaba et al. 2006). Another main difference occurs between water-transporting TEs and fibres. While TEs die rapidly after vacuolar bursting and hydrolysis of cell contents, fibres die slower and disintegrate cellular contents well before cell death (Bollhöner et al. 2012). In poplar vessels, membrane characteristics change and the cytoplasm becomes very sparse in appearance while the organelles show increasing degradation. Subsequently, the collapse of the vacuole causes an abrupt loss of the protoplast in order to develop a functioning vessel (Arend and Fromm 2003). In poplar fibres, however, death is accompanied by a diminishing tonoplast and the cytoplasm mixes with the vacuolar content. Long before cell death, fibres show DNA breaks, and the cytoplasmic contents start to be hydrolysed gradually before vacuolar rupture (Courtois-Moreau et al. 2009). The slow death programme of fibres occurs in a more controlled fashion and also indicates autophagy as a degradation process where autophagic bodies enclose cytoplasmic contents and move them to the vacuole for degradation. Lastly, fibres show a turgor reduction and the remaining organelles swell, the vacuole bursts and organelles become autolysed.
With regard to morphological changes, studies of Zinnia mesophyll cells, which can be induced to transdifferentiate into TEs in vitro (Fukuda 1996), have given a comprehensive understanding of the cell death of TEs. When Zinnia TEs die, the first indication is the swelling of the vacuole, and a subsequent change in tonoplast permeability (Kuriyama 1999) before a rapid vacuolar collapse appears (Groover et al. 1997). This is considered to be the moment of death. After vacuolar collapse cytoplasmic streaming ceases, hydrolytic enzymes are released from the vacuole, cytoplasmic enzymes are activated by acidification and nuclear DNA is degraded rapidly within 10–20 min (Obara et al. 2001). Organelles such as the endoplasmic reticulum (ER) and Golgi vesicles start swelling, and the cellular contents degrade (Fukuda 1997). Following cellular hydrolysis, enzymes that are resistant to the lytic environment modify the cell walls of dying TEs. Moreover, lignin deposition, which has started prior to cell death, continues after dying (Steward 1966; Pesquet et al. 2010). Similar to TEs also in fibres, bulk lignifications occur after cell death in poplar (Courtois-Moreau et al. 2009).
Various signals are related to xylem cell death and play a key role in the correct timing of cellular autolysis. Thermospermine has a protective role against premature xylem maturation and death. In the Zinnia cell culture, spermidine treatment prolongs tracheary element differentiation, and the size of the differentiating cells increases (Muniz et al. 2008). Ethylene signalling also plays a role in TE maturation. In addition, cell death is transcriptionally regulated as one part of the wood formation process. Recently identified NAC domain transcription factors induce expression of both cell death and secondary wall-related genes (Ohashi-Ito et al. 2010; Zhong et al. 2007, 2010). Another NAC transcription factor seems to inhibit TE cell death (Yamaguchi et al. 2010). Also calcium seems to play an important role in vacuolar rupture. It increases during cell death, and the blocking of Ca2+ inward channels has been shown to suppress vacuolar rupture in Zinnia TEs (Groover and Jones 1999). Furthermore, numerous proteases, lipases and nucleases are stored in the vacuole until release and cause hydrolysis of cellular contents. For example, cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole and mega-autolysis of cellular contents after vacuolar rupture during xylogenesis in Arabidopsis roots (Avci et al. 2008). Also several different genes of vacuolar-processing enzymes (VPEs) are expressed during maturation of fibres in the Populus stem (Moreau et al. 2005; Courtois-Moreau et al. 2009), indicating a possible role in cell death. Furthermore, structurally related proteins called metacaspases seem to play a regulative role in xylem cell death because their genes are expressed at the very last stages of wood formation (Courtois-Moreau et al. 2009). Other important enzymes are nucleases that are responsible for degradation of genomic DNA. In Zinnia cell cultures, three main nucleases have been shown that are involved in differentiating TEs (Ito and Fukuda 2002). The control of the timing of vacuolar rupture is very important but little is known about this process.
6 Conclusions
In the last decades an extensive amount of literature addressing wood formation has been published. Most of the data were obtained on woody models such as Populus, Eucalyptus and Pinus. For a deeper understanding of the molecular mechanisms of cell wall biosynthesis, however, herbaceous plant species such as Arabidopsis thaliana and Zinnia elegans are also valuable systems. Nevertheless, trees are of course the main model system for wood formation partly because of the complicated functioning of the cambium through their seasonal cycle and partly because the different maturation of various wood cell types, as well as heartwood formation, can only be studied in trees. Recent advances in cell and molecular biological techniques have broadened our knowledge of the fascinating process of wood production. An increasing amount of genomic data has become available for trees, and transgenic plants have been designed to either overexpress or silence a gene of interest. By combining various disciplines such as genomics, cell biology, biochemistry, anatomy and electrophysiology, it has become possible to obtain information on gene action in the context of structural cellular components.
References
Abbe LB, Crafts AS (1939) Phloem of white pine and other coniferous species. Bot Gaz 100:695–722
Ache P, Fromm J, Hedrich R (2010) Potassium-dependent wood formation in poplar: seasonal aspects and environmental limitations. Plant Biol 12:259–267
Allona I, Quinn M, Shoop E, Swope K, St. Cyr S, Carlis J, Riedl J, Retzel E, Campbell MM, Sederoff R, Whetten RW (1998) Analysis of xylem formation in pine by cDNA sequencing. Proc Natl Acad Sci USA 95:9693–9698
Alves ES, Angyalossy-Alfonso V (2000) Ecological trends in the wood anatomy of some Brazilian species. 1. Growth rings and vessels. IAWA J 21:3–30
Arend M, Fromm J (2000) Seasonal variation in the K, Ca and P content and distribution of plasma membrane H+-ATPase in the cambium of Populus trichocarpa. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 67–70
Arend M, Fromm J (2003) Ultrastructural changes in cambial cell derivatives during xylem differentiation in poplar. Plant Biol 5:255–264
Arend M, Fromm J (2004) Die Rolle von Kalium und H+-ATPasen bei der Holzbildung. AFZ-DerWald 19:1032–1033
Arend M, Weisenseel MH, Brummer M, Osswald W, Fromm J (2002) Seasonal changes of plasma membrane H+-ATPase and endogenous ion current during cambial growth in poplar plants. Plant Phys 129:1651–1663
Arend M, Monshausen G, Wind C, Weisenseel MH, Fromm J (2004) Effect of potassium deficiency on the plasma membrane H+-ATPase of the wood ray parenchyma in poplar. Plant Cell Environ 27:1288–1296
Arend M, Stinzing A, Wind C, Langer K, Latz A, Ache P, Fromm J, Hedrich R (2005) Polar-localised poplar K+ channel capable of controlling electrical properties of wood-forming cells. Planta 223:140–148
Arend M, Muninger M, Fromm J (2008) Unique occurrence of pectin-like fibrillar cell wall deposits in xylem fibres of poplar. Plant Biol 10:763–770
Arend M, Schnitzler J-P, Ehlting B, Hänsch R, Lange T, Rennenberg H, Himmelbach A, Grill E, Fromm J (2009) Expression of the Arabidopsis mutant abi1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Phys 151:2110–2119
Aspeborg H, Schrader J, Coutinho PM, Stam M, Kallas Å, Nilsson P, Denman S, Amini B, Sterky F, Master E et al (2005) Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiol 137:983–997
Avci U, Earl Petzold H, Ismail IO, Beers EP, Haigler CH (2008) Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J 56:303–315
Avery GS, Burkholder PR, Creighton HB (1937) Production and distribution of growth hormone in shoots of Aesculus and Malus, and its probable role in stimulating cambial activity. Am J Bot 24:51–58
Awano T, Takabe K, Fujita M, Daniel G (2000) Deposition of glucuronoxylans on the secondary cell wall of Japanese beech as observed by immune-scanning electron microscopy. Protoplasma 212:72–79
Baba K, Karlberg A, Schmidt J, Schrader J, Hvidsten TR, Bako L, Bhalerao RP (2011) Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc Nat Acad Sci USA 108:3418–3423
Baier M, Goldberg R, Catesson A-M, Liberman M, Bouchemal N, Michon V, Herve du Penhoat C (1994) Pectin changes in samples containing poplar cambium and inner bark in relation to the seasonal cycle. Planta 193:446–454
Bannan MW (1955) The vascular cambium and radial growth in Thuja occidentalis L. Can J Bot 33:113–138
Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215:150–171
Baumann MJ, Eklof JM, Michel G, Kallas AM, Teeri TT, Czjzek M, Brumer H (2007) Structural evidence for the evolution of xyloglucanase activity from xyloglucan endotransglycosylases: biological implications for cell wall metabolism. Plant Cell 19:1947–1963
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2007) Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Ann Bot 100:439–447
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2010) Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Ann Bot 106:885–895
Bertaud F, Holmbom B (2004) Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci Tech 38:245–256
Bhaudari S, Fujino T, Thammanagowda S, Zhang DY, Xu FY, Joshi CP (2006) Xylem-specific and tension stress-responsive coexpression of KORRIGAN endoglucanase and three secondary wall-associated cellulose synthase genes in aspen trees. Planta 224:828–837
Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, Helariutta K, Mähönen AP, Sakakibara H, Helariutta Y (2011) Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr Biol 21:927–932
Bissett IJW, Dadswell HE (1950) The variation in cell length within one growth ring of certain angiosperms and gymnosperms. Aust For 14:17–29
Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B (2007) Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulate polar auxin transport and has a common transcriptome with auxin. Plant J 52:499–511
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54(1):519–546
Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312:1040–1043
Bollhöner B, Prestele J, Tuominen H (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63(3):1081–1094
Brett CT (2000) Cellulose microfibrils in plants: biosynthesis, deposition, and integration into the cell wall. Int Rev Cytol 199:161–199
Brett C, Waldron K (1990) Physiology and biochemistry of plant cell walls. Unwin Hyman, London
Brienen RJW, Zuidema PA (2005) Relating tree growth to rainfall in Bolivian rain forests: a test for six species using tree ring analysis. Oecologia 146:1–12
Catesson A-M (1989) Specific characters of vessel primary walls during the early stages of wood differentiation. Biol Cell 67:221–226
Catesson A-M (1990) Cambial cytology and biochemistry. In: Iqbal M (ed) The vascular cambium, vol 7, Research studies in botany and related applied fields. Research Studies Press, Taunton, MA, pp 63–112
Catesson A-M (1994) Cambial ultrastructure and biochemistry: changes in relation to vascular tissue differentiation and the seasonal cycle. Int J Plant Sci 155:251–261
Catesson A-M, Roland JC (1981) Sequential changes associated with cell wall formation and fusion in the vascular cambium. IAWA Bull 2:151–162
Catesson A-M, Funada R, Robertbaby D, Quinetszely M, Chuba J, Goldberg R (1994) Biochemical and cytochemical cell-wall changes across the cambial zone. IAWA J 15:91–101
Chaffey N (1999) Cambium: old challenges – new opportunities. Trees 13:138–151
Chaffey NF, Barnett JR, Barlow PW (1998) A cycle of microtubule rearrangement accompanies the seasonal cycle of wall thickening within the vascular cambium of Aesculus hippocastanum L. taproots. New Phytol 139:623–635
Chaffey N, Barnett J, Barlow P (1999) A cytoskeletal basis for wood formation in angiosperm trees: the involvement of cortical microtubules. Planta 208:19–30
Chaffey N, Cholewa E, Regan S, Sundberg B (2002) Secondary xylem development in Arabidopsis: a model for wood formation. Physiol Plant 114:594–600
Chiang VL (2006) Monolignol biosynthesis and genetic engineering of lignin in trees, a review. Environ Chem Lett 4(3):143–146
Clark A, Daniels RF, Jordan L (2006) Juvenile/mature wood transition in loblolly pine as defined by annual ring specific gravity, proportion of latewood, and microfibril angle. Wood Fiber Sci 38:292–299
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850–861
Courtois-Moreau CL, Pesquet E, Sjodin A, Muniz L, Bollhöner B, Kaneda M, Samuels L, Jansson S, Tuominen H (2009) A unique program for cell death in xylem fibers of Populus stem. Plant J 58:260–274
Del Campillo E (1999) Multiple endo-1,4-D-glucanase (cellulase) genes in Arabidopsis. Curr Top Dev Biol 46:39–61
Djerbi S, Aspeborg H, Nilsson P, Sundberg B, Mellerowicz E, Blomqvist K, Teeri TT (2004) Identification and expression analysis of genes encoding putative cellulose synthases (CesA) in the hybrid aspen, Populus tremula (L.) × P. tremuloides (Michx.). Cellulose 11:301–312
Djerbi S, Lindskog M, Arvestad L, Sterky F, Teeri TT (2005) The genome sequence of black cottonwood (Populus trichocarpa) reveals 18 conserved cellulose synthase (CesA) genes. Planta 221:739–746
Donaldson L (2007) Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci Technol 41:443–460
Druart N, Johansson A, Baba K, Schrader J, Sjödin A, Bhalerao RR, Resman L, Trygg J, Moritz T, Bhalerao RP (2007) Environmental and hormonal regulation of the activity-dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. Plant J 50:557–573
Endo S, Pesquet E, Tashiro G, Kuriyama H, Goffner D, Fukuda H, Demura T (2008) Transient transformation and RNA silencing in Zinnia tracheary element differentiating cell cultures. Plant J 53:864–875
Eriksson ME, Israelsson M, Olsson O, Moritz T (2000) Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotechnol 18:784–788
Ermel FF, Follet-Gueye M-L, Cibert C, Vian B, Morvan C, Catesson A-M, Goldberg R (2000) Differential localization of arabinan and galactan side chains of rhamnogalacturonan 1 in cambial derivatives. Planta 210:732–740
Espinosa-Ruiz A, Saxena S, Schmidt J, Mellerowicz E, Miskolczi P, Bako L, Bhalerao R (2004) Differential stage-specific regulation of cyclin-dependent kinases during cambial dormancy in hybrid aspen. Plant J 38:603–615
Farrar JJ, Evert RF (1997) Seasonal changes in the ultrastructure of the vascular cambium of Robinia pseudoacacia. Trees 11:191–202
Fernandes AN, Thomas LH, Altaner CM, Callow P, Forsyth VT, Apperley DC, Kennedy CJ, Jarvis MC (2011) Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci USA 108:E1195–E1203
Fisher K, Turner S (2007) PXY, a receptor–like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol 17:1061–1066
Follet-Gueye ML, Verdus MC, Demarty M, Thellier M, Ripoll C (1998) Cambium pre-activation in beech correlates with a strong temporary increase of calcium in cambium and phloem but not in xylem cells. Cell Calcium 24(3):205–211
Follet-Gueye ML, Ermel FF, Vian B, Catesson A-M, Goldberg R (2000) Pectin remodeling during cambial derivative differentiation. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 289–294
Foucart C, Paux E, Ladouce N, San-Clemente H, Grima-Pettenati J, Sivadon P (2006) Transcript profiling of a xylem vs phloem cDNA subtractive library identifies new genes expressed during xylogenesis in Eucalyptus. New Phytol 170:739–752
Fromm J (1997) Hormonal physiology of wood growth in willow (Salix viminalis L.): effects of spermine and abscisic acid. Wood Sci Technol 31:119–130
Fromm J (2010) Wood formation of trees in relation to potassium and calcium nutrition. Tree Phys 30:1140–1147
Fromm J, Hedrich R (2007) The role of potassium in wood formation of poplar. In: Sattelmacher B, Horst WJ (eds) The Apoplast of higher plants: compartment of storage, transport and reactions. Springer, New York, pp 137–149
Fromm J, Sautter I, Matthies D, Kremer J, Schumacher P, Ganter C (2001) Xylem water content and wood density in spruce and oak trees detected by high-resolution computed tomography. Plant Phys 127:416–425
Fromm J, Rockel B, Lautner S, Windeisen E, Wanner G (2003) Lignin distribution in wood cell walls determined by TEM and backscattered SEM techniques. J Struct Biol 143:77–84
Fuchs M, Ehlers K, Will T, van Bel AJE (2010a) Immunolocalization indicates plasmodesmal trafficking of storage proteins during cambial reactivation in Populus nigra. Ann Bot 106:385–394
Fuchs M, van Bel AJE, Ehlers K (2010b) Season-associated modifications in symplasmic organization of the cambium in Populus nigra. Ann Bot 105:375–387
Fujino T, Itoh T (1998) Changes in the three dimensional architecture of the cell wall during lignifications of xylem cells in Eucalyptus tereticornis. Holzforschung 52:111–116
Fukuda H (1996) Xylogenesis: initiation, progression and cell death. Annu Rev Plant Physiol Plant Mol Biol 47:299–325
Fukuda H (1997) Tracheary element differentiation. Plant Cell 9:1147–1156
Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev 5:379–391
Funada R, Catesson A-M (1991) Partial cell wall lysis and the resumption of meristematic activity in Fraxinus excelsior cambium. IAWA Bull ns 12:439–444
Funada R, Abe H, Furusawa O, Imaizumi H, Fukuzawa K, Ohtani J (1997) The orientation and localization of cortical microtubules in differentiating conifer tracheids during cell expansion. Plant Cell Physiol 38(2):210–212
Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M (2001) Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora Sieb. Et Zucc. stems in relation to earlywood-latewood transition and cessation of tracheid production. Holzforschung 55:128–134
Geisler-Lee J, Geisler M, Coutinho PM, Segerman B, Nishikubo N, Takahashi J, Aspeborg H, Djerbi S, Master E, Andersson-Gunnerås S et al (2006) Poplar carbohydrate-active enzymes (CAZymes). Gene identification and expression analyses. Plant Physiol 140:946–962
Ghouse AKM, Hashmi S (1983) Periodicity of cambium and of the formation of xylem and phloem in Mimusops elengi L., an evergreen member of tropical India. Flora 173:479–487
Gion JM, Carouché A, Deweer S, Bedon F, Pichavant F, Charpentier JP, Baillères H, Rozenberg P, Carocha V, Ognouabi N et al (2011) Comprehensive genetic dissection of wood properties in a widely-grown tropical tree: Eucalyptus. BMC Genomics 12:301
González-Martínez SC, Wheeler NC, Ersoz E, Nelson CD, Neale DB (2006) Association genetics in Pinus taeda L. I. Wood property traits. Genetics 175:399–409
Goué N, Lesage-Descauses M-C, Mellerowicz EJ, Magel E, Label P, Sundberg B (2008) Microgenomic analysis reveals cell type-specific gene expression patterns between ray and fusiform initials within the cambial meristem of Populus. New Phytol 180:45–56
Gregory RA (1971) Cambial activity in Alaskan white spruce. Am J Bot 58:160–171
Gregory ACE, O’Connell AP, Bolwell GP (1998) Xylans. Biotechnol Genet Eng Rev 15:439–455
Groover A, Jones AM (1999) Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol 119:375–384
Groover A, DeWitt N, Heidel A, Jones A (1997) Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma 196:197–211
Haigler CH, Brown RM Jr (1986) Transport of rosettes from the Golgi apparatus to the plasma membrane in isolated mesophyll cells of Zinnia elegans during differentiation to tracheary elements in suspension culture. Protoplasma 134:111–120
Harada H, Coté WA Jr (1985) Structure of wood. In: Biosynthesis and biodegradation of wood components. Academic, Orlando, FL, pp 1–44
Heath IB (1974) A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J Theor Biol 48:445–449
Heide O (1993) Daylength and thermal time responses of bud burst during dormancy release in some northern deciduous trees. Physiol Plant 88:531–540
Hejnowicz A, Hejnowicz Z (1958) Variation of length of vessel members and fibres in the trunk of Populus tremula L. Acta Soc Bot Pol 27:131–159
Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H (2008) Non-cell autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci USA 105:15208–15213
Hoenicka H, Lautner S, Klingberg A, Koch G, El-Sherif F, Lehnhardt D, Zhang B, Burgert I, Odermatt J, Melzer S, Fromm J, Fladung M (2012) Influence of over-expression of the FLOWERING PROMOTING FACTOR 1 gene (FPF1) from Arabidopsis on wood formation in hybrid poplar (Populus tremula L. × P. tremuloides Michx.). Planta 235:359–373
Holland N, Holland D, Helentjaris T, Dhugga KS, Xoconostle-Cazares B, Delmer DP (2000) A comparative analysis of the plant cellulose synthase (CesA) gene family. Plant Phys 123:1313–1323
Hoson T (1991) Structure and function of plant cell walls: immunological approaches. Int Rev Cytol 130:233–268
Hou H-W, Zhou Y-T, Mwange K-N, Li W-F, He X-Q, Cui K-M (2006) ABP1 expression regulated by IAA and ABA is associated with the cambium periodicity in Eucommia ulmoides Oliv. J Exp Bot 57(14):3857–3867
Ingold E, Sugiyama M, Komamine A (1988) Secondary cell wall formation: changes in cell wall constituents during the differentiation of isolated mesophyll cells of Zinnia elegans to tracheary elements. Plant Cell Physiol 29:295–303
Ito J, Fukuda H (2002) ZEN1 is a key enzyme in the degradation of nuclear DNA during programmed cell death of tracheary elements. Plant Cell 14:3201–3211
Jarvis M (2003) Chemistry: cellulose stacks up. Nature 426:611–612
Jenkins PA, Shepherd KR (1974) Seasonal changes in levels of indoleacetic acid and abscisic acid in stem tissues of Pinus radiata. N Z J For Sci 4:511–519
Jordan L, He R, Hall DB, Clark A, Daniels RF (2006) Variation in loblolly pine cross-sectional microfibril angle with tree height and physiographic region. Wood Fiber Sci 38:390–398
Keegstra K, Raikhel N (2001) Plant glycosyltransferases. Curr Opin Plant Biol 4:219–224
Kenada M, Rensing KH, Wong JCT, Banno B, Mansfield SD, Samuels AL (2008) Tracking monolignols during wood development in lodgepole pine. Plant Phys 147:1750–1760
Kerr AJ, Goring DAI (1975) The ultrastructural arrangement of the wood cell wall. Cellulose Chem Technol 9:563–573
Ko JH, Prassinos C, Keathley D, Han KH (2011) Novel aspects of transcriptional regulation in the winter survival and maintenance mechanism of poplar. Tree Phys 31:208–225
Kroll RE, Ritter DC, Gertjejansen RO, Au KC (1992) Anatomical and physical properties of balsam poplar (Populus balsamifera L.) in Minnesota. Wood Fiber Sci 24:13–24
Kuriyama H (1999) Loss of tonoplast integrity programmed in tracheary element differentiation. Plant Physiol 121:763–774
Lachaud S (1989) Participation of auxin and abscisic acid in the regulation of seasonal variations in cambial activity and xylogenesis. Trees 3:125–137
Lang GA (1987) Dormancy: a new universal terminology. Hort Sci 22:817–820
Langer K, Ache P, Geiger D, Stinzing A, Arend M, Wind C, Regan S, Fromm J, Hedrich R (2002) Poplar potassium transporters capable of controlling K+ homeostasis and K+-dependent xylogenesis. Plant J 32:997–1009
Larisch C, Dittrich M, Wildhagen H, Lautner S, Fromm J, Polle A, Hedrich R, Rennenberg H, Müller T, Ache P (2012) Poplar wood rays are involved in seasonal remodeling of tree physiology. Plant Physiol 160(3):1515–1529
Larson PR (1994) The cambium: development and structure. Springer, Berlin
Lautner S, Zollfrank C, Fromm J (2012) Microfibril angle distribution of poplar tension wood. IAWA J 33(4):431–439
Lavigne MB, Little CHA, Riding RT (2004) Changes in stem respiration rate during cambial reactivation can be used to refine estimates of growth and maintenance respiration. New Phytol 162:81–93
Lee C, Teng Q, Zhong R, Ye ZH (2011) Molecular dissection of xylan biosynthesis during wood formation in poplar. Mol Plant 4:730–747
Li X, Wu HX, Southerton SG (2011) Transcriptome profiling of Pinus radiata juvenile wood with contrasting stiffness identifies putative candidate genes involved in microfibril orientation and cell wall mechanics. BMC Genomics 12:480–496
Little CHA, Bonga JM (1974) Rest in the cambium of Abies balsamea. Can J Bot 52:1723–1730
Little CHA, Savidge RA (1987) The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul 6:137–169
Love J, Björklund S, Vahala J, Hertzberg M, Kangasjärvi J, Sundberg B (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci USA 106:5984–5989
Magel EA, Monties B, Drouet A, Jay-Allemand C, Ziegler H (1995) Heartwood formation: biosynthesis of heartwood extractives and “secondary” lignifications. In: Sandermann H Jr, Bonnet-Masimbert M (eds) Eurosilva – contribution to forest tree physiology. INRA, Versailles, pp 35–56
Mansfield SD, Iliadis L, Avramidis S (2007) Neural network prediction of bending strength and stiffness in western hemlock (Tsuga heterophylla Raf.). Holzforschung 61:707–716
Matsumoto T, Sakai F, Hayashi T (1997) A xyloglucan-specific endo-1,4-ß-glucanase isolated from auxin-treated pea stems. Plant Phys 114:661–667
Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105:20027–20031
Mauriat M, Moritz T (2009) Analyses of GA20ox-and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J 58:989–1003
McQueen-Mason S (1997) Plant cell walls and the control of growth. Biochem Soc Trans 25:204–214
McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls (analysis of wall hydrolysis, stress relaxation, and binding). Plant Phys 107(1):87–100
Mellerowicz EJ, Sundberg B (2008) Wood cell walls: biosynthesis, developmental dynamics and their implications for wood properties. Curr Opin Plant Biol 11:293–300
Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W (2001) Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol 47:239–274
Moreau C, Aksenov N, Lorenzo M, Segerman B, Funk C, Nilsson P, Jansson S, Tuominen H (2005) A genomic approach to investigate developmental cell death in woody tissues of Populus trees. Genome Biol 6:R34
Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. Plant J 31(6):675–685
Muñiz L, Minguet EG, Singh SK, Pesquet E, Vera-Sirera F, Moreau-Courtois CL, Carbonell J, Blázquez MA, Tuominen H (2008) ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development 135:2573–2582
Murakami Y, Funada R, Sano Y, Ohtani J (1999) The differentiation of contact cells and isolation cells in the xylem ray parenchyma of Populus maximowiczii. Ann Bot 84:429–435
Mutwil M, Debold S, Persson S (2008) Cellulose synthesis: a complex. Curr Opin Plant Biol 11:252–257
Nakaba S, Sano Y, Kubo T, Funada R (2006) The positional distribution of cell death of ray parenchyma in a conifer, Abies sachalinensis. Plant Cell Rep 25:1143–1148
Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
Nick P (2008) Control of cell axis. In: Nick P (ed) Plant microtubules – development and flexibility. Springer, Berlin, pp 3–46
Nieminen K, Immanen J, Laxell M, Kauppinen L, Tarkowski P, Dolezal K, Tähtiharju S, Elo A, Decourteix M, Ljung K et al (2008) Cytokinin signaling regulates cambial development in poplar. Proc Natl Acad Sci USA 105:20032–20037
Nieminen K, Robischon M, Immanen J, Helariutta Y (2012) Towards optimizing wood development in bioenergy trees. New Phytol 194:46–53
Nilsson J, Karlberg A, Antti H, Lopes-Vernaza M, Mellerowicz E, Perrot-Rechenmann C, Sandberg G, Bhalerao RP (2008) Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20:843–855
Nishikubo N, Awano T, Banasiak A, Bourquin V, Ibatullin F, Funada R, Brumer H, Teeri TT, Hayashi T, Sundberg B, Mellerowicz EJ (2007) Xyloglucan endotransglycosylase (XET) functions in gelatinous layers of tension wood fibers in poplar – a glimpse into the mechanism of the balancing act of trees. Plant Cell Physiol 48:843–855
Oakley RV, Wang YS, Ramakrishna W, Harding SA, Tsai CJ (2007) Differential expansion and expression of α- and β-tubulin gene families in Populus. Plant Physiol 145:961–973
Obara K, Kuriyama H, Fukuda H (2001) Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiol 125:615–626
Oda H, Fukuda H (2012) Secondary cell wall patterning during xylem differentiation. Curr Opin Plant Biol 15:38–44
Ohashi-Ito K, Oda Y, Fukuda H (2010) Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell 22:3461–3473
Ohmiya Y, Samejima M, Shiroishi M, Amano Y, Kanda T, Sakai F, Hayashi T (2000) Evidence that endo-1,4-ß-glucanases act on cellulose in suspension-cultured poplar cells. Plant J 24:147–158
Oribe Y, Funada R, Shibagaki M, Kubo T (2001) Cambial reactivation in the partially heated stem in an evergreen conifer Abies sachalinensis. Planta 212:684–691
Oribe Y, Funada R, Kubo T (2003) Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees 17:185–192
Paredez A, Wright A, Ehrhardt DW (2006a) Microtubule cortical array organization and plant cell morphogenesis. Curr Opin Plant Biol 9:571–578
Paredez AR, Somerville CR, Ehrhardt DW (2006b) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312:1491–1495
Pelloux J, Rustérucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase (PME) structure and function. Trends Plant Sci 12:267–277
Pesquet E, Tuominen H (2011) Ethylene stimulates tracheary element differentiation in Zinnia elegans cell cultures. New Phytol 190:138–149
Pesquet E, Korolev AV, Calder G, Lloyd CW (2010) The microtubule-associated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Curr Biol 20:744–749
Prassinos C, Ko JH, Han KH (2005) Transcriptome profiling of vertical stem segments provides insights into the genetic regulation of secondary growth in hybrid aspen trees. Plant Cell Physiol 46:1213–1225
Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS (2011) Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell 23:1322–1336
Ralph J, MacKay JJ, Hatfield RD, O’Malley DM, Whetten RW, Sederoff RR (1997) Abnormal lignin in a loblolly pine. Science 277:235–239
Ranik M, Myburg AA (2006) Six new cellulose synthase genes from Eucalyptus are associated with primary and secondary cell wall biosynthesis. Tree Physiol 26:545–556
Rathgeber CBK, Rossi S, Bontemps JD (2011) Cambial activity related to tree size in a mature silver-fir plantation. Ann Bot 108:429–438
Resman L, Howe G, Jonsen D, Englund M, Druart N, Schrader J, Antti H, Skinner J, Sjodin A, Chen T, Bhalerao RP (2010) Components acting downstream of short day perception regulate differential cessation of cambial activity and associated responses in early and late clones of hybrid poplar. Plant Phys 154:1294–1303
Rinne PLH, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjarvi J, van der Schoot C (2011) Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23:130–146
Robards AW, Kidway P (1969) A comparative study of the ultrastructure of resting and active cambium of Salix fragilis L. Planta 84:239–249
Robischon M, Du J, Miura E, Groover A (2011) The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiol 155:1214–1225
Rohde A, Boerjan W (2001) Insights into bud development and dormancy in poplar. In: Huttunen S, Heikkilä H, Bucher J, Sundberg B, Jarvis P, Matyssek R (eds) Trends in European tree physiology research. Kluwer, The Netherlands, pp 33–52
Ruttink T, Arend M, Morreel K, Strome V, Rombauts S, Fromm J, Bhalerao R, Boerjan W, Rohde A (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19:2370–2390
Sampedro J, Carey RE, Cosgrove DJ (2006) Genome histories clarify evolution of the expansin superfamily: new insights from the poplar genome and pine ESTs. J Plant Res 119:11–21
Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP (2003) Polar auxin transport in the wood forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA 100:10096–10101
Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, Bhalerao RP (2004a) Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J 40:173–187
Schrader J, Nilsson J, Mellerowicz E, Berglund A, Nilsson P, Hertzberg M, Sandberg G (2004b) A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 16:2278–2292
Sennerby-Forsse L (1986) Seasonal variation in the ultrastructure of the cambium in young stems of willow (Salix viminalis) in relation to phenology. Plant Physiol 67:529–537
Sexton TR, Henry RJ, Harwood CE, Thomas DS, McManus LJ, Raymond C, Henson M, Shepherd M (2012) Pectin methylesterase genes influence solid wood properties of Eucalyptus pilularis. Plant Physiol 158:531–541
Siedlecka A, Wiklund S, Péronne MA, Micheli F, Lésniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicsz EJ (2008) Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol 146:554–565
Söding H (1937) Wuchsstoff und Kambiumtätigkeit der Bäume. Jahrb Wiss Bot 84:639–670
Somerville C (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22:53–78
Sterky F, Regan S, Karlsson J, Hertzberg M, Rohde A, Holmberg A, Amini B, Bhalerao R, Larsson M, Villarroel R, Van Montagu M, Sandberg G, Olsson O, Teeri TT, Boerjan W, Gustafsson P, Uhlén M, Sundberg B, Lundeberg J (1998) Gene discovery in the wood-forming tissues of poplar: analysis of 5692 expressed sequence tags. Proc Natl Acad Sci USA 95:13330–13335
Sterky F, Bhalerao RR, Unneberg P, Segerman B, Nilsson P, Brunner AM, Campaa LC, Lindvall JJ, Tandre K, Strauss SH, Sundberg B, Gustafsson P, Uhlen M, Bhalerao RP, Nilsson O, Sandberg G, Karlsson J, Lundeberg J, Jansson S (2004) A Populus EST resource for plant functional genomics. Proc Natl Acad Sci USA 101:13951–13956
Steward CM (1966) Excretion and heartwood formation in living trees. Science 153:1068–1074
Sundberg B, Uggla C (1997) Origin and dynamics of indoleacetic acid under polar transport in Pinus sylvestris. Physiol Plant 104:22–29
Sundberg B, Tuominen H, Little C (1994) Effects of the indole-3-acetic acid (IAA) transport inhibitors N-1-naphthylphthalamic acid and morphactin on endogenous IAA dynamics in relation to compression wood formation in 1-year-old Pinus sylvestris (L) shoots. Plant Physiol 106:469–476
Sundberg B, Uggla C, Tuominen H (2000) Cambial growth and auxin gradients. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 169–188
Suzuki S, Li LG, Sun Y, Chiang VL (2006) The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol 142:1233–1245
Tanino K (2004) Hormones and endodormancy induction in woody plants. J Crop Improv 10:157–199
Terashima N, Fukushima K, He LF, Takabe K (1993) Comprehensive model of the lignified plant cell wall. In: Jung HG (ed) Forage cell wall structure and digestibility. ASA-CSSA-SSSA, Madison, WI, pp 247–270
Tuominen H, Sitbon F, Jacobsson C, Sandberg G, Olsson O, Sundberg B (1995) Altered growth and wood characteristics in transgenic hybrid aspen expressing Agrobacterium tumefaciens T-DNA indoleacetic-acid-biosynthetic genes. Plant Physiol 109:1179–1189
Tuskan GA et al (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604
Tyerman SD, Niemietz CM et al (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25(2):173–194
Uggla C, Mellerowicz EJ, Sundberg B (1998) Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol 117:113–121
Uggla C, Magel E, Moritz T, Sundberg B (2001) Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Phys 125(4):2029–2039
Vetter RE, Botosso PC (1989) Remarks on age and growth rate determination of Amazonian trees. IAWA Bull ns 10:133–145
Voelker SL, Lachenbruch B, Meinzer FC, Jourdes M, Ki C, Patten AM, Davin LB, Lewis NG, Tuskan GA, Gunter L, Decker SR, Selig MJ, Sykes R, Himmel ME, Kitin P, Shevchenko O, Strauss SH (2010) Antisense down-regulation of 4CL expression alters lignification, tree growth and saccharification potential of field-grown poplar. Plant Physiol 154:874–886
Wind C, Arend M, Fromm J (2004) Potassium-dependent cambial growth in poplar. Plant Biol 6:30–37
Wodzicki TJ, Wodzicki AB (1980) Seasonal abscisic acid accumulation in stem and cambial region of Pinus sylvestris and its contribution to the hypothesis of a late-wood control system in conifers. Physiol Plant 48:443–447
Worbes M (1985) Structural and other adaptations to long-term flooding by trees in Central Amazonia. Amazoniana 9:459–484
Worbes M (1995) How to measure growth dynamics in tropical trees – a review. IAWA J 16:337–351
Wu L, Joshi CP, Chiang VL (2000) A xylem-specific cellulose synthase gene from aspen (Populus tremuloides) is responsive to mechanical stress. Plant J 22:495–502
Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55:652–664
Yamaguchi M, Ohtani M, Mitsuda N, Kubo M, Ohme-Takagi M, Fukuda H, Demura T (2010) VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 22:1249–1263
Yordanov YS, Regan S, Busov V (2010) Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell 22:3662–3677
York SW, O’Neill MA (2008) Biochemical control of xylan biosynthesis – which end is up? Curr Opin Plant Biol 11:258–265
Zagórska-Marek B (1995) Morphogenetic waves in cambium and figured wood formation. In: Iqbal M (Hrgs) Encyclopedia of plant anatomy, Band 9, Teil 4, the cambial derivatives. Gebrüder Borntraeger, Berlin, pp 69–69
Zhang J, Elo A, Helariutta Y (2011) Arabidopsis as a model for wood formation. Curr Opin Biotechnol 22:293–299
Zhong R, Ye Z-H (2007) Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10:564–572
Zhong R, Richardson EA, Ye ZH (2007) Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta 225:1603–1611
Zhong R, Lee C, Ye Z-H (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3:1087–1103
Zhong R, McCarthy RL, Lee C, Ye ZH (2011) Dissection of the transcriptional program regulating secondary walls biosynthesis during wood formation in Poplar. Plant Physiol 157:1452–1468
Zhu Z, An F, Feng Y, Li P, Xue L, Mu A, Jiang Z, Kim JM, To TK, Li W et al (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108:12539–12544
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Fromm, J. (2013). Xylem Development in Trees: From Cambial Divisions to Mature Wood Cells. In: Fromm, J. (eds) Cellular Aspects of Wood Formation. Plant Cell Monographs, vol 20. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36491-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-642-36491-4_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36490-7
Online ISBN: 978-3-642-36491-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)