Abstract
Auxin transport is a central process in plant growth and development and as a result is highly regulated. The amount and direction of auxin transport is defined by a set of auxin influx and efflux carriers with precise localization that lead to long-distance polar auxin transport. These auxin transport proteins are regulated by transcriptional and posttranslational mechanisms and through protein-targeting machinery that directs them to the appropriate plasma membrane location. A variety of signals initiate regulatory changes in the abundance, activity, or localization of these proteins, with plant hormones, light, and other environmental signaling implicated in this process. Recent evidence indicates that changing levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) may also fine-tune the activity or synthesis of these proteins. This insight has been obtained by using mutants or treatments that alter the levels of ROS or RNS and demonstration of changing auxin transport and abundance of transport proteins. The molecular mechanisms by which ROS and RNS lead to changes in auxin transport are not yet clear but likely include changes in protein synthesis and abundance. This chapter briefly introduces the key proteins and antioxidant molecules that control the levels of ROS and RNS and focuses on the evidence linking these changes to altered auxin transport.
María Fernández-Marcos and Luis Sanz authors contributed equally to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Reactive Oxygen Species
- Nitric Oxide
- Auxin Transport
- Reactive Nitrogen Species
- Adventitious Root Formation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Important roles of reactive oxygen species (ROS) and reactive nitrogen species (RNS) have recently been described in many plant developmental processes (Swanson and Gilroy 2010; Mittler et al. 2011) including guard cell physiology, flower development, root hair elongation, and most relevantly cellular differentiation in the root apex and modulation of auxin transport via action on PIN auxin efflux proteins (Bashandy et al. 2010; Tsukagoshi et al. 2010). ROS and RNS make excellent signaling molecules as their toxicity requires they be kept at low levels in cells, which allows subtle changes in their synthesis to lead to large magnitude differences in their levels, like other important signaling molecules, such as calcium and protons. As a result, there is a precise balance between synthesis and scavenging of these molecules that allows their level to be carefully modulated.
ROS include H2O2, O2 •−, and 1O2, which are by-products of aerobic cellular metabolism. Their accumulation is determined by the balance between production and detoxification by antioxidants. In both plants and animals, ROS can be generated through respiratory burst/NADPH oxidases (Suzuki et al. 2011; Marino et al. 2012), while ROS levels are reduced through antioxidant protein networks including thioredoxins, glutathione/glutaredoxins, and peroxidases (Mittler et al. 2011) or by chemical antioxidants, such as flavonoids or ascorbate (Hernandez et al. 2009; Pollastri and Tattini 2011). Plants with mutations in the genes encoding proteins that synthesize ROS or antioxidants have linked ROS to plant development (Mittler et al. 2011).
The most abundant RNS in plants is nitric oxide (NO), which is a gaseous free radical displaying a broad spectrum of regulatory functions involved in physiological processes (Delledonne 2005). In animals, nitric oxide synthase (NOS) defines NO levels, but no obvious plant NOS homolog has yet been identified. Some studies have shown the importance of a NOS-like pathway in mediating NO responses in plants, while other studies suggest that the enzyme nitrate reductase (NR) is more important (Besson-Bard et al. 2008a, b). The diverse enzymatic and nonenzymatic reactions in plant NO synthesis are illustrated in Fig. 1. NO levels and bioactivity are also controlled by scavengers, which include class 1 hemoglobin (Hb1) (Fig. 1; Dordas et al. 2004; Perazzolli et al. 2004; Igamberdiev and Hill 2004) in a reaction that is dependent on the presence of both NAD(P)H and AHb1 (Igamberdiev et al. 2005). Strong hypoxic induction of the AHb1 gene (At2g16060) occurs in Arabidopsis under conditions that also induce enzymes of nitrogen metabolism (Klok et al. 2002), suggesting a mechanism for a rapid and transient elevation of NO levels, followed by scavenging to prevent cellular damage.
Formation of reactive nitrogen species (RNS) with emphasis on the diverse enzymatic or nonenzymatic reactions potentially involved in NO synthesis in plants. AtNOA Arabidopsis thaliana nitric oxide associated, RNOH substituted derivatives of hydroxylamine. NO nitric oxide, NO 3 − nitrate, NO 2 − nitrite, N 2 O 3 nitrogen trioxide, NO 2 • nitrogen dioxide radical, O 2 •− superoxide, ONOO − peroxynitrite. Adapted from Moreau et al. (2010)
Both RNS and ROS directly modulate the activity of proteins through posttranslational modification (PTM). PTMs mediated by RNS, such as cysteine S-nitrosylation or tyrosine nitration (Moreau et al. 2010; Arc et al. 2011), can result in an alteration of diverse protein functions. Similarly, ROS leads to oxidation of specific cysteine residues, which control protein activity. The best described ROS-dependent regulation of a mammalian protein is tyrosine phosphatase 1B (PTP1B), where the activity of the protein has been shown to be regulated by a specific single cysteine oxidation which in turn regulates the insulin signaling pathway (Tonks 2006). Recent data suggest that protein nitration and S-nitrosylation could be more than a biological marker of nitrosative stress and could participate in protein turnover or signal transduction in plants (Corpas et al. 2007; Ischiropoulos 2009; Stamler et al. 1992; Jaffrey and Snyder 2001). The specificity of these modifications indicates that they may act as regulatory switches in signal transduction pathways (Hess et al. 2005), analogous to protein phosphorylation (Spickett et al. 2006). Recent evidence, using mutants or chemical treatments that raise or lower ROS and RNS, suggests that auxin transport proteins (or proteins that control their synthesis or targeting) may be targets of ROS and/or RNS regulation by oxidation, nitration, or S-nitrosylation.
2 ROS Regulation of Auxin Transport
ROS have been reported to modulate polar auxin transport by influencing auxin efflux and influx-dependent transport. Recent genetic analysis indicates that polar auxin transport is impaired in plants with altered ROS accumulation. Plants with defects in genes encoding thioredoxin reductase (ntra and ntrb) and an enzyme of glutathione biosynthesis (cad2) exhibit altered ROS homeostasis, due to the absence of these antioxidant proteins (Bashandy et al. 2010). The ntra ntrb cad2 triple mutant has impaired auxin transport and developmental phenotypes consistent with altered auxin transport including PIN-shaped inflorescences and reduced lateral root formation (Bashandy et al. 2010). In addition, atgrxs17 and ntra ntrb cad2 plants show auxin-related morphological phenotypes and reduced expression of the auxin-responsive reporter, DR5 pro :GUS indicating that they have deficiencies related to auxin action or auxin accumulation (Bashandy et al. 2010; Cheng et al. 2011). Consistent with this latter possibility, root growth defects of this triple mutant are rescued by auxin treatment, suggesting a defect in transport, rather than signaling (Bashandy et al. 2010). Decreased transcript levels for auxin efflux and influx carriers in response to elevated ROS suggest that ROS may modulate auxin polar transport at the level of synthesis of transport proteins (Blomster et al. 2011; Tognetti et al. 2012). Intriguingly, the auxin transport inhibitor TIBA and O3 treatment (used as a tool to produce an apoplastic ROS burst) exhibited similar transcriptional effects on auxin-responsive genes (Blomster et al. 2011). Similarities between the responses to oxidative stress and auxin transport inhibitors suggest ROS may act on plant morphology by inhibiting auxin transport either at the level of synthesis or turnover of auxin transport proteins.
Elevated ROS may also alter auxin transport by affecting the stability of auxin efflux carriers. The fluorescence of GFP fusions to PIN1, PIN2, PIN3, and PIN7 are decreased in the root tips of primary roots, but not adventitious roots, treated with BSO (Koprivova et al. 2010; Bashandy et al. 2010). One set of authors suggest that since BSO did not abolish transcription of PIN1, and the effect of BSO was complemented by dithiothreitol, the authors conclude that as yet an uncharacteristic post-transcriptional redox mechanism regulates the accumulation of PIN proteins, and thus auxin transport, in the root tips (Koprivova et al. 2010). Taken together, these data suggest the intriguing result that BSO treatment decreases PIN protein abundance with both transcriptional and post-translational role implicated.
Mutations in genes encoding proteins that are involved in the synthesis of chemical antioxidants, such as flavonols, suggest an additional link between ROS and auxin transport (Pollastri and Tattini 2011). Flavonols have been shown to regulate auxin transport and dependent physiological processes, including root elongation, gravitropism, and branching (Brown et al. 2001; Buer and Muday 2004; Peer et al. 2004; Buer and Djordjevic 2009; Lewis et al. 2011). Auxin transport is elevated in inflorescences, hypocotyls, and roots of plants with the tt4-2 mutation, which make no flavonoids (Murphy et al. 2000; Brown et al. 2001). A comparison of the root gravitropic responses of wild-type and several tt4 alleles identified a delay in root gravitropism when flavonoid synthesis is abolished, which is reversed by chemical complementation by naringenin (Buer and Muday 2004; Buer et al. 2006; Lewis et al. 2011). Flavonoids promote gravitropism presumably by regulating auxin movement in the root tip that modulates differential growth (Buer and Muday 2004). Finally, factors that regulate flavonoid biosynthesis also affect auxin transport, such as light levels (Jensen et al. 1998; Rashotte et al. 2003), wounding and pathogen attacks (Mathesius et al. 1998; Berleth and Sachs 2001), ethylene levels (Lewis et al. 2011), and gravity stimulation (Buer and Muday 2004; Buer et al. 2006).
What has not yet been demonstrated is whether the role of flavonols is to alter ROS in the root and thereby regulate auxin transport through ROS signaling pathways or through more direct mechanisms (Pollastri and Tattini 2011). The levels of ROS species are elevated in plants with defects in flavonol synthesis, consistent with flavonols acting as antioxidants in vivo (Lewis and Muday, unpublished observation), but other mechanisms of flavonol regulation of auxin transport have been described. Quercetin has been shown to block auxin transport when ABCB proteins are expressed in heterologous systems (Fig. 2; Geisler et al. 2005; Bouchard et al. 2006). In addition, ABCB4 was shown to be epistatic to TT4 by double mutant analysis, indicating that flavonols act through ABCB4 to control basipetal auxin transport and gravitropism (Lewis et al. 2007). The inhibition of auxin transport includes disruptions of a complex between an ABCB protein and an immunophilin protein that is needed for maximal auxin transport (Bailly et al. 2008). Whether this protein complex is sensitive to oxidation state in the cell has not yet been reported. An additional intriguing possibility is that ROS and/or RNS control the activity of flavonols by converting them to a semiquinone state, which may have different inhibitory properties. In this scenario, the oxidized flavonol could then be reduced, restoring its capacity to inhibit protein complex formation. Resolving the role of flavonols in regulation of auxin transport via modulation of ROS levels awaits further experimentation.
3 RNS Regulation of Auxin Transport
Auxin transport has a central role in auxin-regulated growth processes. Despite the effort to understand the mechanism of NO regulation of polar auxin transport, our knowledge is still limited. Hu et al. (2005) showed that gravistimulation of soybean primary roots induces asymmetric accumulation of NO, and this NO generation is stimulated by auxin since NPA treatments inhibit NO accumulation and gravitropic bending, suggesting that lateral auxin transport is essential for asymmetric NO generation.
Interestingly, high levels of endogenous NO in the cue1/nox1 background produce a drastic reduction in auxin movement from the root shoot junction to the root tip (acropetal or rootward auxin transport), through use of [3H]IAA radiotracer assays as described previously (Fig. 2; Lewis and Muday 2009; Fernández-Marcos et al. 2011). Additionally, high levels of endogenous or applied NO reduce the fluorescence of a PIN1:GFP fusion which participates in rootward auxin transport, without altering significantly PIN1 transcript levels (Fernández-Marcos et al. 2011). In contrast, the fluorescence of a GFP-fusion reporter for PIN2, which mediates basipetal or shootward IAA transport, was not altered significantly suggesting a specific effect of NO on rootward auxin transport in primary roots mediated by changes in PIN1 protein levels (Fernández-Marcos et al. 2011). Likewise, acropetal auxin transport is enhanced in mutants with lower levels of NO such as atnoa1, supporting the hypothesis that altered NO levels cause altered auxin transport capacity (Fernández-Marcos et al. 2011; unpublished data).
In a recent report, Bai et al. (2012) propose that treatment with 3-O-C10-HL (N-acyl-homoserine lactones, AHLs) promotes auxin-dependent adventitious root formation, possibly through H2O2- and NO-dependent cGMP signaling in mung bean (Vigna radiata) seedlings. This treatment is able to stimulate the generation of H2O2, NO, and the synthesis of cGMP to activate adventitious root formation. Treatment with 3-O-C10-HL enhances hypocotyl auxin basipetal transport and this effect can be reversed by scavenging H2O2 or NO, suggesting that these molecules act within a single pathway to promote hypocotyl basipetal auxin transport and adventitious root formation.
4 Other ROS and RNS Connections to Auxin
ROS and RNS can also affect the dynamics of the actin cytoskeleton and may alter actin-dependent targeting of auxin transport proteins. At the level of subcellular dynamics and polar targeting, there is increasing evidence that auxin regulates polar auxin transport by inhibiting PIN endocytosis (Dhonukshe et al. 2008; Lin et al. 2012; Nagawa et al. 2012), which is actin dependent (Geldner et al. 2001). Whether ROS have an effect on auxin distribution as a result of their regulation of the cytoskeleton, vesicle trafficking, and membrane dynamics remains to be elucidated. However, it is already known that NO affects the functioning of the actin cytoskeleton. In response to NO levels, actin cables change their orientation from longitudinal to oblique and cellular cross-wall domains become actin depleted/depolymerized (Kasprowicz et al. 2009). Additionally, actin-dependent vesicle trafficking is also affected. This was demonstrated through the analysis of recycled wall material transported to newly formed cell plates (Kasprowicz et al. 2009). Thus, the dynamic actin cytoskeleton could be considered as a downstream effector of NO signaling in planta (Kasprowicz et al. 2009).
4.1 Auxin Promotes ROS Accumulation
Auxin induces changes in redox status leading to a more oxidizing cellular environment (Takahama 1996; Joo et al. 2001; Jiang and Feldman 2003; Li et al. 2009; Wang et al. 2010; De Tullio et al. 2010). This change in redox status is mainly due to the generation of several ROS, such as hydrogen peroxide (H2O2) (Brightman et al. 1988; Joo et al. 2001) and superoxide ions (O2 •−) (Schopfer 2001). These ROS may be generated by oxidation of IAA (Kawano 2003) or, indirectly, as a consequence of auxin affecting the activities or synthesis of redox-associated systems (Takahama 1996; Kisu et al. 1997; Jiang and Feldman 2003; Pignocchi et al. 2003).
Redox processes are important for regulating root growth. This regulation may act through mechanisms dependent (Duan et al. 2010) or independent (Tsukagoshi et al. 2010) on the auxin signaling pathway. ROS accumulation in the quiescent center (QC) is an interesting example of how auxin induces changes in redox status. The redox status of the QC, where auxin is strongly accumulated, is different from that in adjacent rapidly dividing cells. The QC has a more oxidizing environment (Kerk and Feldman 1995; Sanchez-Fernandez et al. 1997; Kerk et al. 2000; Jiang and Feldman 2003; Liso et al. 2004) and a large group of transcripts associated with regulating redox status are localized to this tissue (Jiang et al. 2010).
Auxin also promotes ROS accumulation during gravitropic bending (Joo et al. 2001). Gravistimulation elicits a transient increase in intracellular ROS. The action of asymmetrically applied H2O2 in causing root curvature does not depend upon auxin redistribution, suggesting that ROS play a role as a downstream component in the auxin response pathway. Increased ROS concentrations may in turn trigger nitric oxide (NO) generation by nitrate reductase (NR) (Wang et al. 2010) and NO synthase (NOS)-like enzymes (Neill et al. 2008; Li et al. 2009). This probably occurs through the rapid phosphorylation of MAP kinase 6 (MAPK6) (Kovtun et al. 2000; Wang et al. 2010) and/or the action of the protein kinase OX1 and involves Ca2+ (Rentel et al. 2004). Removal of NO with an NO scavenger or inhibition of NO synthesis via NO synthase inhibitors or an inhibitor of nitrate reductase reduces gravitropic bending, indicating that NO synthesis is an important component of the gravitropic response (Hu et al. 2005).
Additional experiments have revealed possible mechanisms of auxin-induced ROS synthesis. The activation of PtdIns 3-kinase and NADPH oxidase is required for auxin-induced production of ROS, regulating plant cell expansion through the activation of Ca2+ channels (Joo et al. 2001; Foreman et al. 2003). Recent results suggest that NADPH oxidase may also be regulated by the Feronia (FER) receptor-like kinase (Duan et al. 2010) and RAC/ROP GTPases (Tao et al. 2002; Xu et al. 2010). Specifically, FERONIA acts as a surface regulator of the RAC/ROP signaling pathway which in turn regulates NADPH oxidase-dependent ROS production (Wu et al. 2011) (Fig. 3).
4.2 ROS Represses Auxin-Inducible Promoters
Several lines of evidence suggest that ROS may modulate auxin sensitivity by repressing auxin-inducible gene expression (Navarro et al. 2006; Wang et al. 2007; Ludwikow and Sadowski 2008; Bashandy et al. 2010; Iglesias et al. 2010; Blomster et al. 2011; Cheng et al. 2011). Indeed, auxin-resistant mutants axr1 and axr3 are less sensitive to ROS than wild-type plants (Koprivova et al. 2010). This process seems to involve changes in MAPK activity. Specifically, ROS can activate an Arabidopsis MAPK, ANP1, which initiates a phosphorylation cascade involving two stress MAPKs, AtMPK3 and AtMPK6 (Kovtun et al. 2000). The activated MAPK cascade plays a dual role in regulation of gene expression activating stress-response genes that protect plants from diverse environmental stresses and repressing auxin-inducible promoters (Kovtun et al. 2000). Thus, the ANP-mediated MAPK cascade represents a molecular link between oxidative stress and the plant growth hormone auxin (Kovtun et al. 2000). In this scenario, NO may also collaborate with ROS to repress auxin-inducible promoters. Increased NO accumulation in cue1/nox1 mutant, where endogenous NO levels are enhanced, depletes auxin-dependent reporter expression in the apical auxin maximum (Fernández-Marcos et al. 2011).
4.3 RNS Regulation of Auxin Signaling
The synergistic effects of auxin and NO have been well characterized in the regulation of a variety of physiological processes of plants. One of the best described NO functions in plants is their involvement in the auxin-regulated signaling cascades determining root growth and morphology. During the last decade it has been reported that NO is involved in the promotion of adventitious roots (Pagnussat et al. 2002), in primary root growth and lateral root formation (Correa-Aragunde et al. 2004; Fernández-Marcos et al. 2011), in root hair development (Lombardo et al. 2006), and in gravitropic responses (Hu et al. 2005).
The role of NO in root development and the cross talk with hormones such as auxin is an emerging area of study. We found that high levels of NO, released by NO donors or using NO overaccumulating mutants (cue1/nox1), produced a decrease in the primary root length by reducing root meristem size and cell division rates (Fernández-Marcos et al. 2011). As auxin gradients are important factors in the regulation of these processes, the spatial pattern of the auxin response reporter DR5 pro :GUS/GFP after NO treatment and in the cue1/nox1 background was analyzed, showing an alteration in the root apical auxin maximum. NO also reduces elongation of root cells (Fernández-Marcos et al. 2012). It has been reported that attenuation of auxin transport and signaling delayed gibberellin (GA)-induced RGA (a DELLA protein) degradation (Fu and Harberd 2003), and as a consequence the NO-inhibition of elongation in the elongation-differentiation zone (EDZ) could be due to the promotion of DELLA activity and, consequently, PIN1 degradation in the presence of high levels of NO (Fernández-Marcos et al. 2012).
Consistent with robust changes in signaling and transcription, numerous NO-regulated genes have been identified. These genes are involved in different functional and biological processes (Huang et al. 2002; Polverari et al. 2003; Parani et al. 2004; Palmieri et al. 2008). However, the direct molecular targets of NO remain poorly documented in plants. Only a few intracellular S-nitrosylated proteins have been identified in plants (Astier et al. 2011; Lindermayr et al. 2005; Tanou et al. 2009). A recent and promising example is the NO-mediated modulation of auxin signaling through posttranslational modification of the TIR1 auxin receptor. S-nitrosylation of TIR1 promotes its interaction with Aux/IAA repressors, thereby facilitating their degradation (Fig. 2; Terrile et al. 2012).
5 Conclusions
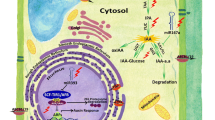
In summary, ROS/RNS are important regulators of auxin-dependent growth and development through their effects on several distinct aspects of auxin biology. Recent reports indicate that auxin transport is perturbed in mutants affected in ROS/RNS homeostasis and/or under treatments to produce a burst of ROS/RNS. These results collectively suggest the existence of a regulatory loop between ROS and auxin transport with profound implications for a broad array of signaling processes (Fig. 4).
The schematic diagram shows how ROS and NO impact auxin signaling and response by affecting its biosynthesis and distribution. (3) Joo et al. (2001); (5) Kawano (2003); (1) Hu et al. (2005); (4) Wang et al. (2010); (7) Astier et al. (2011); (6) Blomster et al. (2011); (8) Cheng et al. (2011); (2) Fernández-Marcos et al. (2011); (9) Terrile et al. (2012)
References
Arc E, Galland M, Cueff G, Godin B, Lounifi I, Job D, Rajjou L (2011) Reboot the system thanks to protein post-translational modifications and proteome diversity: how quiescent seeds restart their metabolism to prepare seedling establishment. Proteomics 11:1606–1618
Astier J, Besson-Bard A, Wawer I, Parent C, Rasul S, Jeandroz S, Dat J, Wendehenne D (2011) Nitric oxide signalling in plants: cross-talk with Ca2+ protein kinases and reactive oxygen species. In: Foyer CH, Zhang H (eds) Nitrogen metabolism in plants in the post-genomic era. Annual plant review, vol 42. Wiley, Oxford
Bai X, Todd CD, Desikan R, Yang Y, Hu X (2012) N-3-oxo-decanoyl-l-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol 158:725–736
Bailly A, Sovero V, Vincenzetti V, Santelia D, Bartnik D, Koenig BW, Mancuso S, Martinoia E, Geisler M (2008) Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J Biol Chem 283:21817–21826
Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010) Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22:376–391
Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4:57–62
Besson-Bard A, Courtois C, Gauthier A, Dahan J, Dobrowolska G, Jeandroz S, Pugin A, Wendehenne D (2008a) Nitric oxide in plants: production and cross-talk with Ca2+ signalling. Mol Plant 1:218–228
Besson-Bard A, Pugin A, Wendehenne D (2008b) New insights into nitric oxide signalling in plants. Annu Rev Plant Biol 59:21–39
Blomster T, Salojarvi J, Sipari N, Brosche M, Ahlfors R, Keinanen M, Overmyer K, Kangasjarvi J (2011) Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol 157:1866–1883
Bouchard R, Bailly A, Blakeslee JJ, Oehring SC, Vincenzetti V, Lee OR, Paponov I, Palme K, Mancuso S, Murphy AS, Schulz B, Geisler M (2006) Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J Biol Chem 281:30603–30612
Brightman A, Barr R, Crane F, Morre D (1988) Auxin-stimulated NADH oxidase purified from plasma membrane of soybean. Plant Physiol 86:1264–1269
Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126:524–535
Buer CS, Djordjevic MA (2009) Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J Exp Bot 60:751–763
Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16:1191–1205
Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140:1384–1396
Cheng NH, Liu JZ, Liu X, Wu Q, Thompson SM, Lin J, Chang J, Whitham SA, Park S, Cohen JD, Hirschi KD (2011) Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem 286:20398–20406
Corpas F, Barroso J, Carreras A, Valderrama R (2007) Nitrosative stress in plants: a new approach to understand the role of NO in abiotic stress. In: Lamattina L, Polacco JC (eds) Nitric oxide in plant growth, development and stress physiology. Plant cell monographs. Springer, Heidelberg, pp 187–205
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
De Tullio MC, Jiang K, Feldman LJ (2010) Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiol Biochem 48:328–336
Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8:390–396
Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrasek J, Seifertova D, Tejos R, Meisel LA, Zazimalova E, Gadella TW Jr, Stierhof YD, Ueda T, Oiwa K, Akhmanova A, Brock R, Spang A, Friml J (2008) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 105:4489–4494
Dordas C, Hasinoff BB, Rivoal J, Hill RD (2004) Class 1 haemoglobins, nitrate and NO levels in hypoxic maize cell suspension cultures. Planta 219:66–72
Duan Q, Kita D, Li C, Cheung AY, Wu HM (2010) FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107:17821–17826
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci USA 108:18506–18511
Fernández-Marcos M, Sanz L, Lorenzo O (2012) Nitric oxide: an emerging regulator of cell elongation during primary root growth. Plant Signal Behav 7:196–200
Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743
Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, Ejendal KF, Smith AP, Baroux C, Grossniklaus U, Muller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44:179–194
Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Hernandez I, Alegre L, Van Breusegem F, Munne-Bosch S (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14:125–132
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Huang X, Kiefer E, von Rad U, Ernst D, Foissner I, Durner J (2002) Nitric oxide burst and nitric oxide-dependent gene induction in plants. Plant Physiol Biochem 40:625–631
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Igamberdiev AU, Baron K, Manac'h-Little N, Stoimenova M, Hill RD (2005) The haemoglobin/nitric oxide cycle: involvement in flooding stress and effects on hormone signalling. Ann Bot 96:557–564
Iglesias MJ, Terrile MC, Bartoli CG, D'Ippolito S, Casalongue CA (2010) Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol 74:215–222
Ischiropoulos H (2009) Protein tyrosine nitration - an update. Arch Biochem Biophys 484:117–121
Jaffrey SR, Snyder SH (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001(86):pl1
Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116:455–462
Jiang K, Feldman LJ (2003) Root meristem establishment and maintenance: the role of auxin. J Plant Growth Regul 21:432–440
Jiang K, Zhu T, Diao Z, Huang H, Feldman LJ (2010) The maize root stem cell niche: a partnership between two sister cell populations. Planta 231:411–424
Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126:1055–1060
Kasprowicz A, Szuba A, Volkmann D, Baluska F, Wojtaszek P (2009) Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. J Exp Bot 60:1605–1617
Kawano T (2003) Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 21:829–837
Kerk N, Feldman LJ (1995) A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development 121:2825–2833
Kerk NM, Jiang K, Feldman LJ (2000) Auxin metabolism in the root apical meristem. Plant Physiol 122:925–932
Kisu Y, Harada Y, Goto M, Esaka M (1997) Cloning of the pumpkin ascorbate oxidase gene and analysis of a cis-acting region involved in induction by auxin. Plant Cell Physiol 38:631–637
Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14:2481–2494
Koprivova A, Mugford ST, Kopriva S (2010) Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep 29:1157–1167
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97:2940–2945
Lewis DR, Muday GK (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protocols 4:437–451
Lewis DR, Miller ND, Splitt BL, Wu GS, Spalding EP (2007) Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-Like ABC transporter genes. Plant Cell 19:1838–1850
Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK (2011) Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156:144–164
Li JH, Liu YQ, Lü P, Lin HF, Bai Y, Wang XC, Chen YL (2009) A signalling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiol 150:114–124
Lin D, Nagawa S, Chen J, Cao L, Chen X, Xu T, Li H, Dhonukshe P, Yamamuro C, Friml J, Scheres B, Fu Y, Yang Z (2012) A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr Biol 22:1319–1325
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Liso R, De Tullio MC, Ciraci S, Balestrini R, Larocca N, Bruno L, Chiappetta A, Bitonti MB, Bonfante P, Arrigoni O (2004) Localization of ascorbic acid, ascorbic acid oxidase, and glutathione in roots of Cucurbita maxima L. J Exp Bot 55:2589–2597
Lombardo C, Graziano C, Polacco J, Lamattina L (2006) Nitric oxide is a positive regulator of root hair development. Plant Signal Behav 1:28–33
Ludwikow A, Sadowski J (2008) Gene networks in plant ozone stress response and tolerance. J Integr Plant Biol 50:1256–1267
Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17:9–15
Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14:23–34
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Moreau M, Lindermayr C, Durner J, Klessig DF (2010) NO synthesis and signaling in plants – where do we stand? Physiol Plant 138:372–383
Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211:315–324
Nagawa S, Xu T, Lin D, Dhonukshe P, Zhang X, Friml J, Scheres B, Fu Y, Yang Z (2012) ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol 10:e1001299
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59:165–176
Pagnussat G, Simontachi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Palmieri MC, Sell S, Huang X, Scherf M, Werner T, Durner J, Lindermayr C (2008) Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: a bioinformatics approach. J Exp Bot 59:177–186
Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman DW, Goldman SL (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J 2:359–366
Pasternak T, Potters G, Caubergs R, Jansen MA (2005) Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J Exp Bot 56:1991–2001
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16:1898–1911
Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M (2004) Arabidopsis non-symbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 16:2785–2794
Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH (2003) The function of ascorbate oxidase in tobacco. Plant Physiol 132:1631–1641
Pollastri S, Tattini M (2011) Flavonols: old compounds for old roles. Ann Bot 108:1225–1233
Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M (2003) Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol Plant Microbe Interact 16:1094–1105
Rashotte AM, Poupart J, Waddell CS, Muday GK (2003) Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133:761–772
Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, Knight MR (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427:858–861
Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inze D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA 94:2745–2750
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28:679–688
Spickett CM, Pitt AR, Morrice N, Kolch W (2006) Proteomic analysis of phosphorylation, oxidation and nitrosylation in signal transduction. Biochim Biophys Acta 1764:1823–1841
Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J (1992) S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA 89:444–448
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Swanson S, Gilroy S (2010) ROS in plant development. Physiol Plant 138:384–392
Takahama U (1996) Effects of fusicoccin and indole-3-acetic acid on the levels of ascorbic acid and dehydroascorbic acid in the apoplast during elongation of epicotyl segments of Vigna angularis. Physiol Plant 98:731–736
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G, Molassiotis A, Job D (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60:795–804
Tao LZ, Cheung AY, Wu HM (2002) Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14:2745–2760
Terrile MC, París R, Calderón-Villalobos LI, Iglesias MJ, Lamattina L, Estelle M, Casalongué CA (2012) Nitric oxide influences auxin signalling through S-nitrosylation of the Arabidopsis transport inhibitor response1 auxin receptor. Plant J 70:492–500
Tognetti VB, Muhlenbock P, Van Breusegem F (2012) Stress homeostasis - the redox and auxin perspective. Plant Cell Environ 35:321–333
Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7:833–846
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616
Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17:1784–1790
Wang P, Du Y, Li Y, Ren D, Song CP (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22:2981–2998
Wu HM, Hazak O, Cheung AY, Yalovsky S (2011) RAC/ROP GTPases and auxin signaling. Plant Cell 23:1208–1218
Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143:99–110
Acknowledgment
Research in the Lorenzo laboratory is financed by grants BIO2011-26940, CSD2007-00057 (TRANSPLANTA) from the Ministerio de Educación y Ciencia (Spain) and SA048A10-2 from Junta de Castilla y León. L.S. is supported by a Marie Curie European Reintegration Grant (FP7-PEOPLE-ERG-2008). We acknowledge grants from the National Science Foundation Arabidopsis 2010 program (IOB-0820717) and United States Department of Agriculture and Food Research Initiative (2009-65116-20436) to GKM.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Fernández-Marcos, M., Sanz, L., Lewis, D.R., Muday, G.K., Lorenzo, O. (2013). Control of Auxin Transport by Reactive Oxygen and Nitrogen Species. In: Chen, R., Baluška, F. (eds) Polar Auxin Transport. Signaling and Communication in Plants, vol 17. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-35299-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-35299-7_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-35298-0
Online ISBN: 978-3-642-35299-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)