Abstract
Nuclear power stands as an immediate and sustainable soluton for satisfying the emerging energy crisis in India. Successful execution of any national ‘nuclear power program’ is keyed to its effective ‘high level nuclear waste’ management strategy. Towards this, India has recently developed sodium-barium-borosilicate glass matrix to immobilize sulfate containing high level waste. Currently, efforts are underway to explore the possibilities of using the same matrix or its modified versions to condition nuclear wastes likely to be generated from ‘closed thorium fuel cycle’. Apart from conventionally used ‘hot wall induction furnace technology’, India has recently acquired expertise in operations of indegineously developed ‘Joule heated ceramic melter’ and ‘Cold crucible induction melter’ for development of suitable inert glass matrices.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
Ensuring energy security to next generation is one of the most important and fundamental responsibilities we need to shoulder these days. Given the diminishing non-renewable energy resources and limited technological progresses achieved in the renewable energy sectors in India, Nuclear Energy appears to be the most promising ‘immediate’ solution to the impending energy crisis. Besides being sustainable and environment friendly (carbon free), the unique feature that makes nuclear energy so special among all other options is that the fuel materials get rejuvenated themselves while being spent. This specific phenomenon of nuclear fuel reduces the dependency of nuclear technology on the availability of fresh fuel materials in long run. Tables 2.1 and 2.2 summarize the various advantageous features of nuclear energy and compare them with the other energy resources being considered in India.
For example, comparison of the energy density values (Table 2.1) suggests that 1 kg of coal can power a 100-W light bulb for nearly 4 days whereas equivalent amount of natural gas can do it for 6 days and uranium in a light water reactor can power it for just more than 140 years. Qualitative estimate of domestic energy resources suggest that with the available coal (~53 billion tons) and hydrocarbon (~12 billion tons), electricity equivalent to ~10,660 GWe years and ~5,833 GWe can be produced respectively. On the other hand, available uranium (~61,000 tons) in thermal and fast reactors and thorium (~225,000 tons) are capable of generating electricity equivalent to ~320 GWe years, ~42,000 GWe years and ~155,000 GWe years respectively. Besides these, hydroelectricity and non-conventional renewable energy sources (wind, biomass etc.) are likely to produce 69 GWe-year/year and 33 GWe-year/year respectively (Anantharaman 2007; Srivastava et al. 2007).
To satisfy the future energy demand due to the population growth and growing aspirations for better quality of life, India has geared up herself to produce more nuclear energy (from present day 2,770 MWe to 63,000 MWe by 2,032) through a three stage nuclear power program, linking closed U fuel cycle (using Pressurized Heavy Water Reactors, PHWRs) with closed Pu fuel cycle (using Liquid Metal Fast Breeder Reactors, LMFBRs) and closed Th fuel cycle (Advanced Thermal Reactors, ATRs; Fig. 2.1). For smooth transition from Pu fuel cycle to Th fuel cycle, Advanced Heavy Water Reactor (AHWR) is being constructed where expertise acquired from successful PHWR operations will be utilized to initiate and gain all-round experiences in the Th fuel cycle. As of today, India has already gained good amount of reactor operational experiences in all the three closed nuclear fuel cycles and steadily marching towards effective integration of the overall program. One very important aspect for successful execution of Nuclaer Power Program is to have a successful plan for deep geological disposal of its high-level nuclear wastes (HLWs). In this direction, India has already mastered different vitrification technologies and very recently has developed novel borosilicate and aluminosilicate glass matrices to condition its radioactive wastes. This chapter aims to give a general review on HLW immobilization experience acquired by Bhabha Atomic Research Centre (BARC), India.
2.2 High Level Nuclear Wastes (HLW)
Generation of energy from nuclear fuels requires execution of certain coordinated activities which are collectively defined as ‘nuclear fuel cycle’. Broadly, nuclear fuel cycle is divided into two parts, namely, front-end and back-end. As shown in the Fig. 2.2, the front-end part includes all those activities related to mining, milling, enrichment, fuel fabrication and energy generation. The back-end part comprises cooling of irradiated fuels (also known as spent fuels) within the water tank (Tarapur: 1,200 ton capacity, Kalpakkam: 800 ton capacity), spent fuel reprocessing (Reprocessing facilities at Trombay, Tarapur and Kalpakkam), nuclear waste immobilization (Waste Immobilization Plants at Trombay, Tarapur and Kalpakkam), interim storage of vitrified waste products (Solid Storage Surveillance Facility, Tarapur and Vitrified Waste Storage Facility, Kalpakkam) and deep geological disposal of conditioned wastes.
Besides this, nuclear fuel cycles are also classified as ‘open-’ or ‘closed-’ ones depending on whether the spent fuels are directly disposed off within deep geological repositories or reprocessed before-hand to extract the valuables. Recently, the closed fuel cycle option has gained more importance as it can fetch multiple benefits including lower environmental stress/risk and more economical nuclear power generation. This is accomplished by recycling of uranium (235U, 233U), and/or plutonium (239Pu) as nuclear fuel components for further energy generation, usage of 137Cs and 90Sr for cancer treatment, usage of 238Pu, 244Cm for Radioactive Thermoelectric Generator, use of 241Am for safety (e.g. smoke detector) and security applications and recovery of expensive platinum group of elements etc. Reprocessing of spent fuels however leads to the generation of HLWs, which generally contain as high as 99 % of radioactivity witnessed in a given fuel cycle.
Chemistry of HLWs is extremely complicated due to multiple factors, namely, the nature and content of fissile radionuclide, type of reactors, pellet-cladding interactions, neutron flux, burn-up, cooling period, spent fuel reprocessing route, partial to total dissolution of cladding materials, corrosion of storage tanks, piping materials, etc. (Ando et al. 2000; Dey and Bansal 2006; Dey and Wattal 2011; Dey et al. 2011; Grover et al. 2006, 2007, 2008, 2010; Kutty et al. 2008a, b; Sengupta et al. 2004). Major inventory of radionuclides present within the HLWs is essentially fission products followed by minor actinides and activation products. A typical light water reactor (LWR) fuel, which is commonly employed for nuclear power generation, consists of 3–5 % fissile 235U and 95 % of fertile 238U. Within the reactor, the fuel is exposed to thermal neutron flux (~0.03 eV) that causes two principal types of nuclear reactions:
-
Fission: 235U + 1no → fission fragments + 2–3 neutrons (1–2 MeV) + energy
-
no capture and β decay: 238U + 1no → 239U → 239Np → 239Pu
Fission fragments are produced in the form of bimodal distribution of elements (the fission yield vs atomic mass) whose atomic masses are approximately half that of the fissioned uranium (Fig. 2.3). Although many hundreds of fission product isotopes are formed in the reactor, most of them have very short half lives and decay within days to weeks of their formation. Neutron capture reactions, followed by β decay, lead to the formation of transuranium elements (Z > 92), of which Pu is abundant. Hence, the concentration of Pu in the fuel increases with time, and isotopes such as 239Pu can then be fissioned, providing up to one-third of the energy generated in a nuclear power plant. The energy of the neutron spectrum can be adjusted so that higher energy (>1 MeV) ‘fast’ neutrons can be used to fission 238U and the minor actinides, such as Np, Cm and Am. The gross elemental inventory present within a typical irradiated nuclear fuel is shown in the Fig. 2.4.
In India, nuclear power plants are distributed across the country which necessitates transportation of ‘water-cooled’ spent fuels to the reprocessing facilities in specially designed ‘Type B’ casks made up of steel and lead. At the reprocessing facility, fuel bundles are dismantled and each of the fuel pins are chopped into small pieces (2–5 cm long) before they are transfered to a basket in the dissolver containing concentrated acidic solutions. Within the dissolver, extraction of valuables is done through a ‘solvent extraction reprocessing route’ called PUREX (plutonium uranium reduction extraction) which involves dissolution of the fuel meat in concentrated nitric acid solution and subsequent extraction of actinides using 30 % tributyl phosphate (TBP) in an inert diluent mixture of paraffins with 12–14 carbon atoms or pure n-dodecane. The PUREX process is totally amenable for automation and remote handling and the experiences acquired over the last 40 years has given the confidence that the technology can be employed to recover useful U and Pu to as high as 99.8–99.9 %. The recent modifications in PUREX process, namely, substitution of ferrous sulphamate with hydrazine stabilized uranous in the partitioning cycle, and evaporation followed by acid reduction using formaldehyde etc., have not only reduced the volume of the waste but have also made the spent fuel cleaner through reduction in material corrosion. With these changes, volume of acidic HLWs could be restricted to 600L/tone of HM processing. At the end of the process, the HLWs are made alkaline by mixing them with NaOH to reduce corrosive nature and stored within underground steel tanks (Dey and Bansal 2006; Dey and Wattal 2011; Dey et al. 2011). Representative composition of HLWs generated at Trombay and Tarapur plant are given in the Table 2.3.
Reprocessing of ThO2 (thoria) based spent fuels is different from those of UO2 or PuO2 or mixed oxide (UO2-PuO2) due to its inert nature. The reprocessing route adopted for spent ThO2 based fuels is known as acid THOREX (thorium extraction) process, which uses TBP in hydrocarbon diluent (usually Shell Sol-T, dodecane or n-paraffin) for the recovery of 233U alone or both 233U and thorium (Dey and Bansal 2006; Dey and Wattal 2011; Dey et al. 2011). Initial experiments with pure ThO2 pellets showed that upto 95 % dissolution of the material could be achieved in 13 M HNO3 + 0.03 M HF + 0.1M Al(NO3)3 mixture within 92 h (Hejzlar et al. 2000; Dey and Bansal 2006; Dey and Wattal 2011; Dey et al. 2011). Presence of MgO upto 1.5wt % in the sintered pellet facilitates the dissolution even faster. For ThO2 – 4 % PuO2 pellets, ~95 % material dissolution could be achieved in ~50 h time. The reasons behind additions of HF and Al(NO3)3 in nitric acid (HNO3) were to promote better dissolution and minimization of free F− corrosion activities respectively. Table 2.4 shows the HLW composition which is likely to be generated upon reprocessing of spent thoria fuel exposed to a burn up of about 10,000 MWd/T.
2.3 Immobilization of HLW’s: The Geological Connection
As described in the previous section, HLWs are very complex solutions cantaining various radionuclides of 30–40 different elements. Since most of these radionuclides are toxic in nature and emit α-particles or β- and/or γ- rays during radioactive decay, they need to be concentrated and contained securely so as to avoid any environmental contamination. The time frame over which a particular HLW needs to be isolated from biosphere depends on its radiochemistry and it is usually until the period its radioactivity gets reduced to that of natural radiation background i.e. uranium ore (Fig. 2.5). Therefore in most of the cases, the isolation period varies from 105 to 106 years. Since this is a quite a long time scale as far as human control and monitoring is concerned, efforts are also being pursued to develop required technology so as to separate long-lived radionuclides from HLWs and transmute them in ‘accelerator based sub-critical system’. If successful, this technology will bring down the isolation period to as low as 103–104 years. However, until such technology is made available, all the efforts towards HLW disposal should aim for an arrangement which will prevent radionuclides to reach biosphere for ~1 million years (IAEA 1992, 2005, 2007; Ojovan and Lee 2005, 2007; Lee et al. 2006).
The solution for this problem came from the geological community which was active during the middle part of the past century (Blasewitz et al. 1973; Roy 1975, 1979; Ringwood 1978, 1985; Ewing 1979; Ringwood et al. 1979; Haaker and Ewing 1981; Hench et al. 1984; Donald et al. 1997), in the form of ‘multiple barrier system (MBS)’ concept (Fig. 2.6). MBS consists of two basic units, namely, ‘engineered barrier system (EBS)’ and ‘natural barrier system (NBS)’.
The EBS is made up of four components, namely, waste form, canister, overpack and buffer. The ‘waste form’ is the inert matrix (amorphous/crystalline/crypto-crystalline) which primarily incorporates the HLW components within its structure. It is then contained within a stainless steel (AISI 304L grade) container called ‘canister’ and two or three such canisters are put inside (one above the other) another stainless steel (AISI 304L grade) container called overpack. Such overpacks are then placed inside underground interim storage facility and finally inside drilled holes dug within suitable host rock such as granite, basalt etc. This actually acts as natural barrier (NBS). The gap in between the overpack and surrounding host rock are to be filled up by backfill (broken rock fragments) and buffer (a composite of clay minerals, graphite and sand) materials. It may be noted here that geological repository need to be constructed at a depth of 500–1,000 m from ground so as to minimize or exclude the possible effects of unforeseen surficial geological processes in future, if any. Such deep burial also makes the repository environment extremely harsh due to simultaneous operations of different fields such as thermal-, hydrological-, biological-, chemical-, radiation- and mechanical fields (Fig. 2.6). Computational estimates show that under such environment, stainless steel containers are not likely to last more than few thousand years, which makes proper selection of nuclear waste form material even more challenging (Crowley 1997; Ewing 1999; OECD 1999; IAEA 2001; Rempe 2008).
To solve this issue, in 1950s Canadian geoscientists came up with the suggestion to synthesize aluminosilicate glass of nephelinite bulk composition to condition HLWs. The idea of trying such composition was essentially derived from natural analogue studies which indicated that radionuclides present within Na+ rich HLW (which is made alkaline by adding NaOH) very commonly occur within nephelinite and can remain within it over millions of years. The reason behind suggesting glass matrix was essentially to take benefit of amorphous structure which can incorporate wide range of ions and molecular clusters within its network. To elaborate this aspect a set of three cartoons are shown in the Fig. 2.7.
The first schematic diagram (Fig. 2.7a) shows the planar view of a quartz crystal where Si4+ cations are linked to O2− anions in all the three dimensions (framework silicate) in such a way that both short-range ordering (SRO) and long-range ordering (LRO) exist within the network. However, in case of SiO2 glass (Fig. 2.7b), where although Si4+ and O2− are found to polymerize in all the three directions, but because of the presence of randomness in the ionic linkages no LRO arrangement is seen in the network (only SRO is present). A comparison between these two structures clearly suggests that SiO2 glass can incorporate more number of ions as it offers wider range of locations (cation-, anion positions, free volumes of different sizes and shapes) for entrapment. The glass structure further opens up when alkali or alkaline earth cations get into the structure (Fig. 2.7c). Incorporation of such cations requires charge compensation and this is satisfied through depolymerization of the network. Such adjustment in the network due to introduction of modifier (alkali/alkaline earth cations) leads to the formation of non-bridging oxygens (NBO; oxygen linked to Si4+ and Na+) from bridging oxygen precursors (BO; oxygen linked to two Si4+ cations) and conversion of Si4+ cations from Q4 type to Q3, Q2 and Q1 types (where Qn signifies n number of BOs linked with one Si4+ cation). This type of network modification, not only makes the glass matrix more suitable for wider range of radionuclide entrapment (Fig. 2.7d) but also reduces its synthesis temperature as can be noted from quartz – albite binary system. However, too much depolymerization of the network often leads to significant reduction in leach resistance of the glass matrix, which is not acceptable as far as waste form application is concerned.
Therefore, while selecting a waste glass matrix composition, one has to carefully balance between acceptable product durability properties (e.g. microstructure, leach resistance, mechanical properties etc.) and processing constraints (e.g. pouring temperature, viscosity, electrical conductivity, corrosiveness of the melt, etc.). A good example of such decision making can be seen if one goes back through the history and looks into the origin of the idea of using borosilicate glass matrices for waste form application. As mentioned earlier, it was initially thought to use nephelinite based glass compositions for HLW immobilization and when it was tried in laboratory scale it was noted that for homogeneous loading of sufficient (so as to make the overall technology economical) amount of ‘simulated HLW’ within the aluminosilicate melts the synthesis temperature had to be as high as 1,400–1,500 °C. Such temperature range was however difficult to attain in the ‘hot wall induction furnaces (metallic melter pot)’ which used to be primarily employed in Waste Immobilization Plants of those yester years. The reason being process pots and suscepters of such vitrification furnaces are made up of Alloy 690 (a nickel based Superalloy; Cr: 27–31 wt %, Fe: 7–11, Al: 0.5 max., Cu: 0.5 max., Si: 0.5 max., Mn: 0.5 max, S: 0.01 max., C0.05 max., Ni: balance) whose melting point is less than 1,400 °C. To solve this problem, the idea of replacing Al2O3 component of the aluminosilicate glass by B2O3 flux was adopted and this has drastically reduced the synthesis temperature to 950–1,000 °C. This finally gave birth to the concept of using borosilicate glass matrices for immobilization of HLWs.
Among the leading petrologists and geochemists of the then era Prof. Krauskopf took the leading role in championing the borosilicate waste glass matrix concept. Although the concept had several adavantageous features, namely, fixation of wide range of elements within the amorphous network, high order leach resistance, and extensive experience on its plant scale production, etc. (Ewing 1979; Hench et al. 1984; Donald et al. 1997), one serious drawback which worried many scientists was its metastable nature and the possibility of uncontrolled nucleation and growth leading to the degradation of the material properties. A contemporary geomaterial scientist Prof. Rustom Roy came up with the suggestion of using crystalline ceramics (in the form of ‘Supercalcine’), analogus to ‘accessory minerals present within magmatic rocks’, for conditioning of HLWs (Roy 1975, 1979; Haaker and Ewing 1981). However, due to poor leach resistance of the material, the suggestion was not much appreciated. Few years later, an experimental petrologist, Prof. Ringwood synthetically prepared rock like multiphase material called ‘SYNROC’ from TiO2 rich bulk compositions (Ringwood 1978, 1985; Ringwood et al. 1979). SYNROC was found to be very promising in terms of its leach resistance and radionuclide incorporation. However, its complicated synthesis process made it a second choice for waste immobilizers. Thus for the last 50 years borosilicate glass remained to be the most preferred choice for the waste immobilizers and it is likely to continue in near future as well especially within the countries including India that excercise closed nuclear fuel cycle.
2.4 Plant Scale Immobilization of HLW
Research and development on HLW immobilization started in India during 1970s (Dey and Bansal 2006; Raj et al. 2006; Dey and Wattal 2011; Dey et al. 2011). Initial efforts were mostly focused towards sodium borosilicate glass system, which by that time was already established as the most feasible option. Experimental and theoretical results on the Na2O-B2O3-SiO2 system suggest that the system can be broadly divided into three domains namely, Zone I: no glass forming zone, Zone II: glass forming zone and Zone III: immiscibility zone (Fig. 2.8). Representative microstructures of these three domains are shown in the Fig. 2.9, which indicate that bulk compositions within Zone I result in the formation of fused mass due to the presence of crystals whereas melts produced within Zone III get rapidly unmixed into silicate and borosilicate fractions. Homogeneous glasses can be obtained from bulk compositions falling within Zone II but significant variations in product durability factors (e.g. leach resistance etc.) and processing constraints (e.g. pouring temperature etc.) were witnessed. Vaswani et al. (1979) studied the Zone II domain and mapped it in terms of equivalent leach resistance, pouring temperatures and microstructural homogeneity (Fig. 2.8).
Na2O – B2O3 – SiO2 liquidus projection diagram (m1: 724 ± 2 °C, m2: 520 ± 10 °C, m3: 830 ± 10 °C) showing the (1) no glass forming-, (2) glass forming- and (3) immiscibility zones (also see Fig. 2.9). Inset 1 shows the leach rate (gm/cm2 day) map of glass forming zone as prepared by Vaswani et al. (1979). Inset 2 shows the temperature dependence of the immiscibility region
Representative microstructures of (a) no glass forming zone, (b) immiscibility zone and (c) glass forming zone of Na2O-B2O3-SiO2 system (also see Fig. 2.8)
Following the IAEA guidelines for acceptable product durability properties and processing constraints, few base glass compositions having homogeneous microstructure, leach resisance of the order of 10−4 gm/cm2-day or better, and pouring temperature ~950 °C or lower, were chosen for conditioning of Indian HLWs. However, during the experimental stage, it was noted that although ~950 °C was good enough for plant scale operation, it was too high to retain Cs- and Ru radionuclides within the sodium borosilicate melts. To reduce the volatility of Cs through forming Cs-titanate complexes, Ti4+ was added into sodium borosilicate melts in the form of rutile (TiO2). This on one hand reduced Cs-volatility but enhanced the phase separation (crystallization and/or immiscibility), either during the molten stage or due to thermal treatment as expected within geological repository condition (due to geothermal gradient and radionuclide decay especially 137Cs and 90Sr). An example of U- and Fe-oxide phase separation within sodium borosilicate melt is shown in the Fig. 2.10.
(a) Cartoon showing the possible way of uniform incorporation of uranium within glass matrix (BO bridging oxygen, NBO non-bridging oxygen, Na+ sodium cation, U4+ uranium cation). Back Scattered Electron images show microstructural differences between (b) homogeneous and (c) heterogeneous waste glass matrices. (d) UMα, (e) FeKα and (f) SiKα X-ray maps show heterogeneous elemental distributions within thermally annealed borosilicate glass matrices
It is quite understandable that uniform distributions of U- and Fe-cations within glass network (as shown by the cartoon, Fig. 2.10a) will lead to the formation of homogeneous glass (Fig. 2.10b). However, clustering of these cations within the amorphous network, either due to abundance or thermal treatment, may lead to phase separation (heterogeneous microstructure) as shown by the back-scattered electron (BSE)- and X-ray images (Fig. 2.10c–f). The concerns regarding phase separations are twofold, namely, deterioration of product durability properties, and difficulties in materials processing. Phase separation within borosilicate glasses often leads to the formation of silica depleted domains which have very different leach resistance, mechanical properties, and thermal behavior relative to the surrounding matrix. Remobilization of Fe2+/Fe3+ from Fe-rich domains by hydrothermal liquid is a well documented phenomenon in nature and so sodium borosilicate glasses with segregated FeO-rich domains are likely to exhibit inferior leach resistance on the whole than otherwise (the scenario may change if Fe occurs as spinel/olivine/pyroxene etc.). U-oxides are known to be leach resistant phases, however preferential partitioning of actinides and other fission products through lattice substitution may make them acting as ‘hotspots’ within borosilicate glass matrix. This not only can lead to devitrification of the surrounding areas but may also initiate crack formation leading to degradation of mechanical properties. Apart from these, phase separation within borosilicate melt affects the viscocity which in turn thwarts transferring them into Steel canisters. For homogeneous loading of HLW components within borosilicate melt and easy pouring of the same within stainless seel canister, it is necessary to maintain its viscosity close to 50 poise, but crystallization often increases the viscocity drastically. Beside this, crystallization/seggregation of phases like spinel, eskolite, platinum group of elements etc., which are denser than the surrounding melt, settle down on the furnace floor, and therefore disturb the vitrification process either by clogging the drain valve or by deviating electrical flow lines.
Similar to the phase crystallization, liquid-liqid immiscibility also causes multiple difficulties in the vitrification of HLW. This aspect is very important in Indian context as some volume of ‘sulfate containing legacy HLW’ was in store at BARC, Trombay (Jahagirdar and Wattal 1998; Kaushik et al. 2006; Mishra et al. 2008; Raj and Kaushik 2009; Yeotikar et al. 2011). Within the sodium borosilicate melt, sulphate rich domain unmixes in similar way as sulphide rich melt segregates from the magma. In the case of HLW loaded borosilicate melt, the sulfate rich domain are essentially composed of water soluble thenardite (Na2SO4) which preferentially partions heat-generating and γ-ray emitting 137Cs radionuclide and occurs in association with Na-aluminate and Na-chromate. These clusters are better known as ‘yellow phase’ and being lighter in density (than borosilicate melt) they float on the top of the melt surface and hamper release of gaseous reaction product from melt volume resulting in its swelling.
Since alkali earth sulfates are known to be more leach resistant than alkali ones, alkali earth borosilicate glass systems were considered for immobilization of sulfate containing HLW. At the initial stage, extensive research works were focused on PbO-B2O3-SiO2 system as homogeneous lead borosilicate glasses are known to form at lower temperatures and posses acceptable leach resistance (Jahagirdar and Wattal 1998). Figure 2.11a shows the development of continuous ‘surface layer’ on leached matrix of lead borosilicate glass pellet which was exposed to static simulated hydrothermal solution (90°C, pH 7) for 400 days. The X-ray line scans taken across the pellet clearly indicates the successive development of pore free leached matrix and surface layer on the pellet (without any discontinuity in between) which are suggestive of good chemical durability of the matrix. Structural analysis of such surface layers developed on leached waste glass matrices indicated the presence of smectite, montmorillonite, etc., which in turn are commonly reported from altered basaltic glasses. However, owing to the poor solubility limit of sulfate, this glass system was discarded and fresh search for newer system was initiated.
It is of common knowledge that sulfate bearing minerals are abundant in sedimentary rocks and rare in magmatic rocks. Barite (BaSO4) is known to be the most abundant sulfate bearing mineral and existence of barite ore deposits over geological time scale indicates its leach resistant character. To understand the rarity of sulfate bearing minerals in magmatic rocks and the characteristic of barite which has most abundant sulfate bearing phase on earth’s crust, insight was sought from ‘valance bond theory’. According to the theory, the mean bond valance for SO4 2− group is 1.5 valence units (vu) which means that, any oxygen atom linked to the S6+ cation receives ~1.5 vu from it, and can accept ~0.50 vu (max.) from any other coordinating cations so as to polymerize the two units. This limits polymerization of sulfate with silicate tetrahedra (1.0 vu) and suggests that the oxygen atom linked to sulfate tetrahedra can bond only with octahedrally or higher coordinated mono-, di-, or trivalent cations e.g. Ba2+. Given cognizance to these observations, it was decided to add varying amounts of BaO to sodium borosilicate glass compositions and monitor the base glass properties in terms of microstructure, pouring temperature and swelling index (Kaushik et al. 2006). Homogeneous base glasses having pouring temperature lower than 950 °C and swelling index less than 2.6 were then loaded with varying amounts of simulated sulfate bearing wastes. The waste glasses so produced were once again subjected to above mentioned assessments. Of these glasses, those capable of incorporating more than 20wt % simulated waste were then ground and the respective powders were subjected to static leach experiments using distilled water at 90 °C for 1 month duration. From these glasses, the one showing highest order of leach resistance and high waste loading was then finally selected for plant scale operation. Details of this glass (SB 44) are given in the Table 2.5 and the representative microstructure is shown in the Fig. 2.12.
2.5 Plant Scale Vitrification Process
Indian experience in plant scale vitrification process is essentially gathered from hot wall induction furnace operation over last two decades. In the simplest form, the furnace has three major components, namely, process pot, susceptor and induction coils, of which, the first two are made up of Alloy 690 (Fig. 2.13a). The process pot has three major parts namely, top dish, cell and bottom dish. The cell is made by welding Alloy 690 sheath and it is joined with top and bottom dishes by welding. During operation, susceptor is heated by multizone induction furnace and it radiates heat to process pot to raise its temperature to the desired limit. Thermowell made up of Alloy 690 is attached to process pot to monitor its temperature. Operating temperatures of process pot depends on the pouring temperature of the waste glass and its process flow chart. To the process pot, nitric acid based HLW and glass additive slurry are charged through different channels. The mixture is then subjected to multistage vitrification process, which includes feeding (100–105 °C), evaporation (105–120 °C), calcination (300–700 °C), fusion – melt formation (700–850 °C) and soaking (950–1,000 °C). After soaking, the homogeneous melt is poured within the stainless steel canister (324 mm outer diameter, 10 mm wall thickness, 770 mm length; each containing ~45 l of HLW loaded glass (~90 kg) incorporating 0.6 million Curies generating ~2 kW of decay heat), cooled in air and a lid is placed on it by welding. Three such canisters are put inside the stainless steel overpack (356 mm outer diameter, 10 mm wall thickness, 2,000 mm length), decontaminated using ultrasonic bath and stored within underground storage. The entire vitrification process is carried out in dedicated cells protected with radiation shielding windows and each equipped with state-of-the-art remote handling systems e.g. servo-manipulators, in-cell crane, grapping tools, product positioning/removing trolleys, closed-circuit television cameras, remote welding machine, etc. An elaborate off-gas cleaning system consisting of condenser, scrubber, chiller, demister and absolute HEPA filter is used to treat the gas before discharge through a 100 m tall stack to the atmosphere. All the vitrification plants have the central data acquisition and control system to monitor and control the critical process parameters during vitrification operation (Raj et al. 2006).
Schematic diagrams showing indigeneously developed vitrification furnaces e.g. (a) hot wall induction furnace, (b) Joule heated ceramic melter (Modified after Banerjee et al. (2011)) and (c) cold crucible furnace (Modified after Sugilal (2008)), being used in India for immobilization of high level nuclear wastes
Besides hot wall induction furnace, India has recently acquired operational experience in Advanced Vitrification System (AVS) which is essentially a Joule heated ceramic melter furnace (Fig. 2.13b). Robust continuous vitrification technology, high throughput and higher processing temperature are the main advantages of this process. In the simplest form, electric current across Alloy 690 electrodes immersed in borosilicate melt is used to generate heat by Joule effect (Q = I2R) which maintains the molten condition and sustains evaporation, calcination, fusion and soaking (Banerjee et al. 2011). In the Indian type ceramic melter, the glass contact refractory is made up of high alumina-zirconia-silica fused cast blocks followed by layers of back-up refractory and insulation materials, all encased in a water cooled stainless steel box mounted on a suitable structural support. For vitrification, both HLW and glass formers are directly introduced into the melting chamber and the slurries are heated to as high as 1,150 °C. The waste-glass melt (Table 2.5) so produced, is drained within the canister. It may be noted here that, longevity of vitrification furnaces is essentially limited by the degradation of metallic components under service conditions (Kain et al. 2005; Sengupta et al. 2006, 2007, 2008, 2009, 2011a; Sengupta 2011).
2.6 Future Scopes
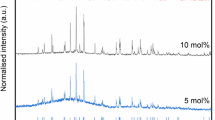
It can be understood from the previous sections that India has already acquired significant experience on immobilization of HLW. With the introduction of newer type of nuclear fuels and more efficient reprocessing technology, the scope for improving the existing knowledgebase has increased tremendously. One example in this regard is, conditioning of HLW derived from Th-fuel cycle. As vitrification of HLW in plant scale is a complex task that requires clearances from different national and international nodal bodies, technologists involved in the process are usually reluctant to change an established flow-sheet. Keeping this in mind, attempts have been made to immobilize Th-bearing simulated HLW within the same base glass composition which is being used for conditioning of sulfate bearing HLW. Preliminary results show that although sodium-barium-borosilicate glass can homogeneously incorporate higher amount of thoria (~5mol %) within its amorphous network with respect to other conventionally used glass matrices, its solubility (of thoria) gets reduced in the presence of other components (Mishra et al. 2007). Hence, engineering the glass structure to incorporate higher amounts of Th-HLW within it (Table 2.6) or identifying a different glass matrix with solubility has become essential (Yalmali et al. 2007).
One of the major technological blockages in improving the glass matrix composition is the limitation of furnace operating temperatures. To address this problem, Cold Crucible Induction Melter (CCIM) is now being developed at BARC which will offer longer melter life, higher temperature availability, higher waste loading, more tolerance towards noble metal precipitation and higher specific capacity (Sugilal 2008; Banerjee et al. 2011). In this process, glass is directly heated within a segmented crucible (manufactured from contiguous segments forming a cylindrical volume, but separated by a thin layer of electrically insulating material) using high frequency electromagnetic induction. To avoid corrosion of metallic components as observed in metallic melter or ceramic melter, each segment of the cold crucible is fed with circulating water system which produces a solidified protective glass layer on the inner side of the melter. With the potential entry of cold crucible induction melters in the near future, it is strongly felt that the old idea of using aluminosilicate glass matrices for HLW immobilization may once again get rejuvenated (Sengupta et al. 2011b).
It can be said that India has already acquired required experience and expertise to immobilize high level nuclear wastes. All these efforts are made with a commitment to a statement of an American Indian – ‘we do not inherit the Earth from our ancestors but we borrow it from our children’ (Ojovan and Lee 2005).
References
Anantharaman K (2007) Role of nuclear power in the Indian Energy Scenario. Gond Geol Mag 9:141–148
Ando Y, Nishihara K, Takano H (2000) Estimation of spent fuel compositions from light water reactors. J Nucl Sci Tech 37:924–933
Banerjee K, Raj K, Wattal PK (2011) Process technology for vitrification of high-level radioactive liquid waste. In: Proceedings of international conference on peaceful uses of atomic energy −2009, vol 2. Nuclear Fuel Cycle, Vigyan Bhavan, 29 Sept–1 Oct 2009, pp 489–495
Blasewitz AG, Richardson GL, McElroy JL, Mendel JE, Schneider KJ (1973) Management of radioactive wastes from fuel reprocessing. In: Organization for economic cooperation and development (ed), pp 615–654
Dey PK, Bansal NK (2006) Spent fuel reprocessing; A vital link in Indian nuclear power program. Nucl Eng Des 236:723–729
Dey PK, Wattal PK (2011) Back-end of fuel cycle. In: Proceedings of international conference on peaceful uses of atomic energy −2009, vol 2. Nuclear Fuel Cycle, Vigyan Bhavan, 29 Sept–1 Oct 2009, pp 343–349
Dey PK, Gupta AK, Achuthan PV, Kaushik CP, Yalmali V (2011) Thorium utilization – back-end challenges. In: Proceedings of international conference on peaceful uses of atomic energy −2009, vol 2. Nuclear Fuel Cycle, Vigyan Bhavan, 29 Sept–1 Oct 2009, pp 350–357
Donald IW, Metcalfe BL, Taylor RNJ (1997) The immobilization of high level radioactive wastes using ceramics and glasses. J Mater Sci 32:851–887
Ewing RC (1979) Natural glasses: analogues for radioactive waste forms. In: McCarthy GJ (ed) Scientific basis for nuclear waste management, vol 1. Plenum, New York, pp 57–68
Grover V, Sengupta P, Bhanumurthy K, Tyagi AK (2006) Electron Probe Microanalysis (EPMA) investigations in the CeO2-ThO2-ZrO2 system. J Nucl Mater 350:169–172
Grover V, Sengupta P, Tyagi AK (2007) Sub-solidus phase relations in CeO2-YSZ and ThO2-YSZ systems: XRD, high-temperature XRD and EPMA studies. Material Sci Eng B138:246–250
Grover V, Banerji A, Sengupta P, Tyagi AK (2008) Raman, XRD and microscopic investigations on CeO2-Lu2O3 and CeO2-Sc2O3 systems: a sub-solidus phase evolution study. J Solid State Chem 181:1930–1935
Grover V, Cavan SV, Sengupta P, Tyagi AK (2010) CeO2-YO1.5-NdO1.5 system: an extensive phase relation study. J Eur Ceramic Soc 30:3137–3143
Haaker RF, Ewing RC (1981) Naturally occurring crystalline phases: analogues for radioactive waste forms, Pacific Northwest Lab. PNL – 3505/UC-70, Richland, Washington
Hejzlar P, Driscoll MJ, Todreas NE (2000)Impact of fuel choices on spent fuel characteristics for once through heavy metal cooled reactors. In: Proceedings of committee meeting (TCM) on “core physics and engineering aspects of emerging nuclear energy systems for energy generation and transmutation” held in Argonne, Illinois, USA, 28 Nov–1 Dec 2000. IAEA, Vienna, IAEA-TECDOC- 1356, pp 168–185
Hench LL, Clark DE, Campbell J (1984) High level waste immobilization forms. Nucl Chem Waste Manag 5:149–173
International Atomic Energy Agency (1992) Design and operation of high level waste vitrification and storage facilities, Technical Reports Series No. 339:50
International Atomic Energy Agency (2001) Monitoring of geological repositories for high level waste, IAEA-TECDOC-1208:25
International Atomic Energy Agency (2005) Status and trends in spent fuel reprocessing, IAEA-TECDOC-1467:101
International Atomic Energy Agency (2007) Spent fuel and high level waste: chemical durability and performance under simulated repository conditions, IAEA-TECDOC-1563:29
Jahagirdar PB, Wattal PK (1998) Vitrification of sulphate bearing high level wastes in borosilicate matrix. Waste Manage 18:265–273
Kain V, Sengupta P, De PK, Banerjee S (2005) Case reviews on the effect of microstructure on the corrosion behavior of austenitic alloys for processing and storage of nuclear waste. Metal Mater Transact 36A:1075–1084
Kaushik CP, Mishra RK, Sengupta P, Kumar A, Das D, Kale GB, Raj K (2006) Barium borosilicate glass- a potential matrix for immobilization of sulfate bearing high level radioactive liquid waste. J Nucl Mater 358:129–138
Kutty TRG, Kulkarni RV, Sengupta P, Khan KB, Bhanumurthy K, Sengupta AK, Panakkal JP, Kumar A, Kamath HS (2008a) Development of CAP Process for fabrication of ThO2-UO2 fuels Part II: characterization and property evaluation. J Nucl Mater 373:309–318
Kutty TRG, Nair MR, Sengupta P, Basak U, Kumar A, Kamath HS (2008b) Characterization of (Th-U)O2 fuel pellets made by impregnation technique. J Nucl Mater 374:9–19
Lee WE, Ojovan MI, Stennett MC (2006) Immobilization of radioactive waste in glasses, glass composite materials and ceramics. Adv App Ceram 105:3–12
Mishra RK, Sengupta P, Kaushik CP, Tyagi AK, Kale GB, Raj K (2007) Studies on immobilization of thorium in barium borosilicate glass. J Nucl Mater 360:143–150
Mishra RK, Sudarsan V, Sengupta P, Vatsa RK, Tyagi AK, Kaushik CP, Das D, Raj K (2008) Role of sulphate in structural modifications of sodium barium borosilicate glasses developed for nuclear waste immobilization. J Am Ceram Soc 91:3903–3907
Ojovan MI, Lee WE (2005) An introduction to nuclear waste immobilization. Elsevier, The Netherlands
Ojovan MI, Lee WE (2007) New developments in glassy nuclear wasteforms. Nova Science Pub Inc, New York
Organisation for Economic Co-operation and Development (1999) Confidence in the long term safety of deep geological repositories. NEA/OECD, Paris
Raj K, Kaushik CP (2009) Glass matrices for vitrification of radioactive waste – an update on R & D efforts, IOP conference series. Mater Sci Eng 2:1–6
Raj K, Prasad KK, Bansal NK (2006) Radioactive waste management practices in India. Nucl Eng Des 236:914–930
Rempe NT (2008) Deep geological repositories. The Geological Society of America, USA
Ringwood AE (1978) Safe disposal of high level nuclear reactor wastes: a new strategy. Australian National University Press, Canberra
Ringwood AE (1985) Disposal of high level nuclear wastes: a geological perspective. Min Mag 49:159–176
Ringwood AE, Kesson SE, Ware NG, Hibberson W, Major A (1979) Immobilization of high level nuclear reactor wastes in SYNROC. Nature 278:219–223
Roy R (1975) Ceramic science of nuclear waste fixation. Am Ceram Soc Bull 54:459
Roy R (1979) Science underlying radioactive waste management: status and needs. In: McCarthy GJ (ed) Scientific basis for nuclear waste management, vol 1. Plenum, New York, pp 1–20
Sengupta P (2011) Interaction study between nuclear waste glass melt and ceramic melter bellow liner materials. J Nucl Mater 411:181–184
Sengupta P, Gawde PS, Bhanumurthy K, Kale GB (2004) Diffusion reaction between Zircaloy 2 and Thoria. J Nucl Mater 325:180–187
Sengupta P, Mittra J, Kale GB (2006) Interaction between borosilicate melt and Inconel. J Nucl Mater 350:66–73
Sengupta P, Kaushik CP, Mishra RK, Kale GB (2007) Microstructural characterization and role of glassy layer developed on Inconel 690 during a nuclear high-level waste vitrification process. J Am Ceram Soc 90:3057–3062
Sengupta P, Soudamini N, Kaushik CP, Jagannath Mishra RK, Kale GB, Raj K, Das D, Sharma BP (2008) Corrosion of alloy 690 process pot by sulfate containing high level radioactive waste at feed stage. J Nucl Mater 374:185–191
Sengupta P, Kaushik CP, Kale GB, Das D, Raj K, Sharma BP (2009) Evaluation of Alloy 690 process pot at the contact with borosilicate melt pool during vitrification of high level nuclear waste. J Nucl Mater 392:379–385
Sengupta P, Rogalla D, Becker HW, Dey GK, Chakraborty S (2011a) Development of graded Ni-YSZ composite coating on Alloy 690 by Pulsed Laser Deposition technique to reduce hazardous metallic nuclear waste inventory. J Hazard Mater 192:208–221
Sengupta P, Fanara S, Chakraborty S (2011b) Preliminary study on calcium aluminosilicate glass as a potential host matrix for radioactive 90Sr – an approach based on natural analogue study. J Hazard Mater 190:229–239
Srivastava A, Chandra S, Grover RB (2007) Energy indicators for sustainable energy and electricity growth in India. Gond Geol Mag 9:251–260
Sugilal G (2008) Experimental analysis of the performance of cold crucible induction glass melter. Appl Therm Eng 28:1952–1961
Vaswani GA, Jahgirdar PB, Rastogi RC, Sundar Rajan NS (1979) Development of suitable radioactive waste product with low formation temperature and improved leach resistance – a practical approach, Internal report BARC-1028. Bhabha Atomic Research Centre, Mumbai
Yalmali VS, Deshingkar DS, Wattal PK, Bharadwaj SR (2007) Preparation and characterization of vitrified glass matrix for high level waste form MOX fuel reprocessing. J Non-Cryst Sol 353:4647–4653
Yeotikar RG, Kaushik CP, Shah JG (2011) Matrices for immobilization of HLW and their characterization. In: Proceedings of international conference on peaceful uses of atomic energy −2009, vol 2. Nuclear Fuel Cycle, Vigyan Bhavan, 29 Sept–1 Oct 2009, pp 475–482
Acknowledgements
Pranesh Sengupta is grateful to the organizers of Alexander von Humboldt Foundation (Germany) Kolleg ‘Earth-Future (2011)’, held at Periyar University, Salem (India) from 7th to 9th September, 2011, for inviting the contribution.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sengupta, P., Kaushik, C.P., Dey, G.K. (2013). Immobilization of High Level Nuclear Wastes: The Indian Scenario. In: Ramkumar, M. (eds) On a Sustainable Future of the Earth's Natural Resources. Springer Earth System Sciences. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32917-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-32917-3_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32916-6
Online ISBN: 978-3-642-32917-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)