Abstract
More than two decades ago, Kenneth Nealson and Charles Myers published a seminal manuscript, describing an organism that can couple growth to the respiratory reduction of manganese oxide, an extracellular electron acceptor. This was the starting point of research aiming to elucidate mechanisms of extracellular respiration in a γ-proteobacterium named Shewanella oneidensis. This research is manifested in a nearly confusing multitude of publications that are sometimes even contradictory. It is the aim of this chapter to give a thorough overview of our knowledge about the biochemistry of metal respiration in S. oneidensis. This chapter starts off with a technological survey describing the molecular toolbox we have in our hands to genetically modify S. oneidensis. Thereafter, the path of electrons from the cytoplasmic membrane to the cell surface is followed, and thereby potential proteins for this electron transport and the transfer onto terminal metallic electron acceptors are brought to the reader’s attention. Moreover, the potential role of further proteins is analyzed that are not necessarily involved in the electron transport chain to ferric iron or manganese oxides per se but still seem to provide a selective advantage for the organism. Throughout the text it will become clear that the list of open questions concerning S. oneidensis physiology is still, even after decades of research and although it is the best studied dissimilatory metal reducer, extensive, and that there is room for more fascinating questions that can be addressed using the system S. oneidensis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Shewanella oneidensis: A Novel Model Organism
S.oneidensis is a gram-negative γ-proteobacterium originally isolated from Oneida Lake in Upstate New York, USA, due to its capability of reducing manganese (IV) oxides (Myers and Nealson 1988; Venkateswaran et al. 1999). In some older publications it is referred to as Shewanella putrefaciens (all changes in names are reviewed in (Gralnick et al. 2006)). S. oneidensis is an obligate respirer and can use a variety of compounds as electron acceptor for anaerobic respiration such as ferric iron, manganese dioxide, and uranium (VI) as well as nitrate, nitrite, sulfur, thiosulfate, fumarate, sulfite, dimethylsulfoxide, and trimethylamine-N-oxide (Burns and DiChristina 2009; Cruz-Garcia et al. 2007; Gralnick et al. 2006; Myers and Nealson 1988; Schwalb et al. 2002, 2003; Shirodkar et al. 2011). S.oneidensis can further grow aerobically with oxygen as terminal electron acceptor. The organism is believed to live in stratified environments at the oxic/anoxic interface where a multitude of electron acceptors could be available (Venkateswaran et al. 1999). S.oneidensis uses fermentation end products (lactate, formate, and H2) and N-acetylglucosamine (the chitin monomer) as carbon and electron sources (Scott and Nealson 1994; Yang et al. 2006).
The interest in S. oneidensis and organisms belonging to the genus Shewanella has greatly increased during the last two decades. A good surrogate for this growing interest is the number of publications dealing with Shewanella, which increased from almost zero in the period from 1988 to 1990 to more than 1,500 between 2009 and the mid of 2011 (Fig. 1).
Currently, this “boom” for metal reducing bacteria like S. oneidensis is to a large extent caused by potential applications in biotechnology, mainly remediation of soils and aquifers contaminated with radionuclides, and the utilization of these bacteria as biocatalysts in microbial fuel cells for electricity production. Both aspects will be reviewed in later chapters of this book (see “Bioremediation via Microbial Metal Reduction” and “Dissimilatory Metal Reducers Producing Electricity: Microbial Fuel Cells”). The benefits of using Shewanella species to study these processes led to genome sequencing projects for 23 Shewanella species so far, mostly funded by the department of energy (DOE) of the USA.
The available genome sequences as well as several proteomic and transcriptomic studies of S. oneidensis lead to a good understanding of systems biology of the organism under several conditions. In 2005, a Shewanella knowledgebase was established to organize available data and integrate experimental as well as in silico approaches to describe the “system” Shewanella (Karpinets et al. 2010). The physiology of S. oneidensis is certainly the reason why researcher compiled an enormous amount of data so far, but researchers used especially this dissimilatory iron reducer since it is fairly easy to handle in the laboratory. The next short paragraph will give a quick survey about different ways to grow and manipulate S. oneidensis.
Shewanella oneidensis MR-1 in the laboratory. The cultivation and manipulation of S. oneidensis MR-1 under laboratory conditions is very easy compared to other metal reducing organisms. It can be cultivated aerobically in LB medium and on agar plates where it grows at a temperature optimum of 30 °C and a doubling time of approximately 40 min. Genetic systems for S. oneidensis are well established. Scientist can take advantage of several types of plasmids and antibiotic selection markers. Table 1 gives an overview about plasmids that are routinely used for Shewanella research and typical concentrations of antibiotics that have to be added for plasmid maintenance.
The most common technique for DNA transfer in Shewanella is conjugation with an Escherichia coli donor strain. The usual donor strains are E. coli S17-1 (Thormann et al. 2005) and E. coli WM3064 (Saltikov and Newman 2003). The latter one, E. coli WM3064 is a diaminopimelic acid (DAP) auxotrophic strain that has to be grown in LB medium supplemented with 0.3 mM DAP. This strain allows for a rapid selection of transconjugants since plating on LB agar without DAP can eliminate the donor strain.
Besides conjugation, standard protocols for electroporation according to (Sambrook et al. 1989) using ice-cold water can be utilized. Transformation efficiency is low compared to E. coli but can be increased by prolonged phenotypic expression (up to 12 h) on SOC medium (Bücking et al. 2010). Myers et al. reported the use of a sorbitol treatment of the cells prior to electroporation (Myers and Myers 1997c).
Many different suicide plasmids have been used to perform directed mutagenesis based on R6K ori and SacB counterselection (Myers and Myers 2001). In our studies, a yeast cloning-based vector system has turned out to be particularly useful (Bücking et al. 2010; Shanks et al. 2006). Additionally, a Cre lox gene excision system has been successfully adapted to Shewanella (Learman et al. 2009). For random mutagenesis several groups have applied transposon tools based on Tn5, Tn10, and mariner transposons (Beliaev and Saffarini 1998; Bouhenni et al. 2005; Newman and Kolter 2000). Site-directed integration of DNA can also be accomplished using a TN7 platform (Thormann et al. 2004).
In summary, genetic work with at least S. oneidensis MR-1 but potentially also with other members of the genus is almost as easy and fast as working with E. coli. This certainly encouraged several groups to work with S. oneidensis and accelerated the process of identifying how this organism can grow with ferric iron and other terminal extracellular electron acceptors.
2 Components of the Electron Transport Chain to Ferric Iron and Manganese in S. oneidensis
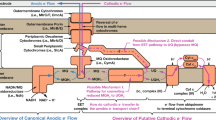
S. oneidensis evolved an extended respiratory chain to the cell surface. This allows the organism to transport the electrons to sparsely soluble electron acceptors like ferric or manganese (oxyhydr)oxides (Fig. 2).
Generally, c-type cytochromes seem to play an important role in the respiratory electron transport chains to the cell surface. The sheer number of 41 c-type cytochrome encoding genes (9 corresponding proteins putatively localized in the cytoplasmic membrane, 5 in the outer membrane, 27 in the periplasm) in the genome of S. oneidensis is a first indication for this. As a comparison, E. coli has only seven genes encoding c-type cytochromes (yhjA, nrfA, nrfB, napB, napC, torC, torY) (Blattner et al. 1997; Meyer et al. 2004; Romine et al. 2008). Cells of Shewanella cultivated under anoxic conditions appear dark red in color due to the expression of a multitude of heme containing proteins. These proteins are crucial for dissimilatory metal reduction since mutants in the cytochrome maturation machinery of S. oneidensis (encoded by the ccm-genes) are unable to respire on extracellular electron acceptors like ferric iron or manganese oxides (Bouhenni et al. 2005; Carpentier et al. 2005).
The number of c-type cytochromes seems to be a selective advantage for the organism but leads to a number of complications when researchers try to outline a path of electron transfer. Multiple c-type cytochromes are coexpressed under anoxic conditions. Transcriptome studies showed that in a large number of cases the availability of oxygen rather than the available anaerobic terminal electron acceptor seems to be the trigger for c-type cytochrome expression (Beliaev et al. 2005). Hence, here seems to be a fundamental difference to E. coli physiology. E. coli regulates expression of respiratory enzymes according to the potential free energy that is available from the reduction (Gunsalus 1992; Unden and Bongaerts 1997). In Shewanella, multiple respiratory pathways seem to be coexpressed under iron reducing conditions. It is therefore very well conceivable that electron transfer pathways in Shewanella are much more intertwined than in E. coli. We recently proposed such a kind of interconnection between different respiratory pathways regarding the function of the soluble fumarate reductase FccA in iron reduction (see below) (Schuetz et al. 2009). The complication for researchers arises from the fact, that c-type cytochromes do not seem to catalyze specific electron transfer reactions in most cases (Bretschger et al. 2007; Gao et al. 2010; Myers and Myers 2003b). Consequently, single mutants in S. oneidensis cytochromes frequently do not show a clear phenotype, since multiple cytochromes are coexpressed (Myers and Myers 2003b). Bretschger et al. published a study that underlines the redundancy driven complexity of the electron transport chains in S. oneidensis (Bretschger et al. 2007). The reduction potential of 34 different cytochrome mutants toward poorly soluble iron and manganese was compared. Only deletion of one cytochrome (MtrA, metal reducing protein A) resulted in the inability to reduce iron- or manganese-oxides, while several mutants showed only minor defects. It will become evident in further sections that this cytochrome MtrA has a dual function, which could very well be the reason for this defined phenotype.
The following considerations regarding the electron transport chain to Fe(III) or Mn(IV) will be separated in three parts following the electron flow from the cytoplasmic membrane through the periplasm and to the outer membrane in S. oneidensis.
2.1 Electron Transfer from the Cytoplasmic Membrane to the Periplasm
CymA (SO_4591). The standard reduction potential of the Fe3+(aq)/Fe2+(aq) couple (at pH 0) is 770 mV, and therefore almost as high as the oxygen/water couple with 820 mV. However, and as outlined by Prof. Majzlan in “Minerals and Aqueous Species of Iron and Manganese As Reactants and Products of Microbial Metal Respiration”, this high redox potential is relevant only for acidophilic iron reduction. At neutral pH, the redox potential of ferric (oxyhydr)oxides varies between –177 and +24 mV (Majzlan “Minerals and Aqueous Species of Iron and Manganese As Reactants and Products of Microbial Metal Respiration”). Due to this low redox potential ubiquinone (E°′ = + 66 mV) is not suitable as electron carrier in the cytoplasmic membrane and instead menaquinone (E°′ = –74 mV) is necessary. Consequently, mutants in the menaquinone synthesis pathway are unable to grow with ferric iron as terminal electron acceptor (Saffarini et al. 2002). It is well established that electrons are transported from the menaquinol pool to the periplasm by the catalytic activity of a tetraheme c-type cytochrome protein called cytoplasmic membrane protein A (CymA). As a member of the NapC/NirT protein family, the putative role of CymA is to direct electron flow from the menaquinol pool to several oxidoreductases located in the periplasm (Fig. 3; (Myers and Myers 1997b; Schwalb et al. 2003)). Interestingly, menaquinol was recently shown to be not only a substrate for CymA but also a cofactor for the enzyme (McMillan et al. 2012).
Deletion of the cymA gene from the chromosome leads to a mutant that is unable to use not only ferric iron as terminal electron acceptor, but also nitrate, nitrite, fumarate, and DMSO (Gao et al. 2009; Myers and Myers 1997b; Schwalb et al. 2003). A cymA deletion mutant is furthermore affected in its ability to use manganese oxides as electron acceptors (Bretschger et al. 2007). In the arsenate respiring strain Shewanella sp. strain ANA-3, CymA is furthermore electron donor for the periplasmic arsenate reductase (Murphy and Saltikov 2007).
Interestingly, the apparent midpoint potential of CymA (revealed using protein film voltammetry) is with –200 mV below the potential of the menaquinol/menaquinone couple. It was therefore hypothesized that a predominantly reduced menaquinole pool is necessary to enable electron transfer (Firer-Sherwood et al. 2008; Hartshorne et al. 2007). Of note, in terms of bioenergetics, the energy conservation associated with respiration of ferric iron is most likely associated with electron input into the menaquinone pool. CymA and all further proteins that build the electron transport chain to ferric iron serve therefore most probably only by means of menaquinone recycling and are not involved in proton pumping. Therefore, minor differences in the redox potentials of the cytochromes involved in electron transport to ferric iron might be sufficient since they exclusively serve as a wire that connects the menaquinole pool to ferric iron. Interestingly, CymA expression is necessary and sufficient to convert E. coli into a dissimilatory iron reducer if nitrilotriacetic acid (NTA) chelated ferric iron is added as electron acceptor. We could show in further experiments that at least in E. coli ferric NTA can pass the outer membrane and that CymA is the terminal reductase in this strain. Although a conductive connection to the surface of E. coli was not established, these experiments allowed concluding that iron reduction in gram-negative bacteria seems to be primarily limited by the lack of access of the terminal electron acceptor to a ferric reductase and that ferric iron reduction is not necessarily a process that demands a specific ferric iron reductase. Instead, c-type cytochromes in general can reduce a wide variety of ferric iron species and other organic and inorganic electron acceptors as well (Gescher et al. 2008).
2.2 Periplasmic Electron Transfer Reactions
Isolated periplasmic fractions from S. oneidensis cells have a bright to dark red color, which is due to the multitude of c-type cytochromes that are localized to the periplasm. This multitude can be easily separated and visualized using SDS polyacrylamide gels and subsequent heme activity staining (Fig. 4). Several research groups used heme containing protein bands excised from SDS gels or whole protein pools of the cell to conduct mass spectrometry (MS) in order to reveal the proteins that correlate to the detected heme containing protein bands. An overview of the results is presented in Table 2. While information about the function of most of these proteins is sparse, at least some evidence for the function of nine of the detected periplasmic c-type cytochromes is available. Hence, we will now direct our attention to the potential physiological function of these proteins.
Heme stain of a periplasmic protein fraction derived from S. oneidensis cells grown under ferric iron reducing conditions (Schuetz et al. 2009). Copyright © American Society for Microbiology
FccA (SO_0970). FccA is the respiratory fumarate reductase of S. oneidensis. Its structure was solved by Leys et al. (1999). This enzyme can be distinguished from other respiratory fumarate reductases by two characteristics. First, it is a soluble, monomeric periplasmic protein while most other fumarate reductases consist of more than one subunit and are bound to the cytoplasmic membrane (Thauer et al. 1977). Second, FccA contains an N-terminal tetraheme domain which is not present in other fumarate reductases (Morris et al. 1994). The flavin domain contains a noncovalently bound FAD close to the active site (Pessanha et al. 2009). Surprisingly, FccA is the most prominent cytochrome in the periplasm of cells that were grown under ferric iron reducing conditions (Fig. 4). Due to the metabolic burden that is accompanied by the massive production of an enzyme having four heme and one FAD cofactor, it seems likely that FccA might have a function even under ferric iron reducing conditions. In vitro and in vivo experiments revealed that FccA can be directly reduced by the membrane bound cytochrome CymA (Schuetz et al. 2009; Schwalb et al. 2002). An fccA deletion mutant in S. oneidensis shows an interesting phenotype when analyzed carefully (Myers and Myers 1997a; Schuetz et al. 2009): the initial ferric iron reduction rate is faster when compared to the wild type, but the increase in colony forming units is not as high as in wild type cells (Fig. 5a; (Schuetz et al. 2009)). This small difference in growth rate becomes more prominent in competitive growth experiments (Fig. 5b, unpublished results). Since, the slower rate of ferric iron reduction in the wild type is not coupled to a decrease in cell growth or fitness, it was proposed that FccA could act as a transient electron storage protein or capacitor that is filled with electrons via reaction kinetics that are faster than electron transfer to an extracellular electron acceptor. This electron storage protein could allow for an initial growth rate higher than that of the ΔfccA mutant. For a certain time, this electron uptake could ensure that the respiratory chain is not stalled due to the limiting terminal electron transfer reactions and the subsequently reduced electron transfer proteins. This strategy would also allow Shewanella cells to use a carbon and electron source even if no terminal electron acceptor is present, since the transient electron storage would act as an intermediate electron acceptor. A recent publication supports this idea since it reports on electron storage within Shewanella biofilms measured with an electrode of a microbial fuel cell (Uria et al. 2011).
a Growth and iron reduction curves of S. oneidensis wild type and ΔfccA mutant strain (Schuetz et al. 2009). The growth medium contained 10 mM hydrous ferric oxide and 5 mM lactate. Error bars indicate standard deviations. Copyright © American Society for Microbiology. b Competitive growth experiments with S. oneidensis wild type and ΔfccA. S.oneidensis wild type and ΔfccA cells were mixed in a 1:1 ratio and subsequently added to medium containing 50 mM ferric citrate and 25 mM lactate. An aliquot of the sample was spread on LB agar and the relative amount of wild type and ΔfccA cells was determined using PCR. Subsequently, 1 % of the culture was transferred to fresh medium when 35 mM ferric citrate were reduced and the relative amount of cells was determined. This procedure was repeated twice. All experiments were conducted in independent triplicates (unpublished data)
A similar hypothesis, but concerning periplasmic c-type cytochromes in general, was raised by Esteve-Nunez et al. (2008) for Geobacter metallireducens and by Rodrigues et al. (2006) for Desulfovibrio vulgaris.
If FccA is a transient electron storage protein, then it has to be connected to the catabolic electron flow to ferric iron. Evidence for a function in the electron transfer chain to ferric iron is derived from an in vitro experiment with membrane fractions from S. oneidensis cells. It was recently demonstrated that the outer membrane of S. oneidensis contains a protein complex that consists of the periplasmic cytochrome MtrA, the β-barrel protein MtrB, and the outer membrane decaheme cytochrome MtrC (also called OmcB in older studies). This complex will be discussed in more detail later. For now it is relevant to know that the complex can catalyze electron transfer across a membrane and that the decaheme cytochrome MtrC can directly reduce ferrihydrite. When purified and dithionite reduced FccA is added in the presence of ferrihydrite to catalytic amounts of Shewanella membranes (containing this complex) an oxidation of FccA coupled to ferric iron reduction can be detected ((Schuetz et al. 2009); Fig. 6). This redox process is most probably due to the MtrABC complex since the addition of membranes derived from a ΔmtrA strain did not lead to FccA driven ferrihydrite reduction (Schuetz et al. 2009).
Taken together, FccA could likely fulfill a dual function: first as a fumarate reductase and second as an electron storage protein in the periplasm that is directly connected to CymA at the cytoplasmic membrane and MtrA (in form of the MtrABC complex) at the outer membrane.
MtrA (SO_1777). MtrA is a 32 kDa monomeric decaheme c-type cytochrome. It was one of the first proteins identified to be necessary for iron reduction (Beliaev and Saffarini 1998). This central function for metal reduction was confirmed by numerous groups (Bretschger et al. 2007; Hartshorne et al. 2009; Schicklberger et al. 2010) while in one publication no growth deficiency of an ΔmtrA strain was detected (Gao et al. 2010). It is not clear why the mutant used by Gao et al. behaved that differently, but in our eyes the authors might have selected for a suppressor mutant that could overcome the impact of the mtrA deletion.
The cellular localization of MtrA is also under debate: until now, three publications have examined the localization of MtrA under ferric iron reducing conditions. Pitts et al. ( 2003 and Schuetz et al. (2009) detected MtrA (via SDS-PAGE and heme staining or MS analysis) associated to membranes and in a soluble state in the periplasm. Ross and coworkers (2007) detected MtrA only in the membrane fraction. These contradicting results might be due to the different methods that were used for the isolation of periplasmic fractions. Pitts et al. and Schuetz et al. used polymyxin B to insert holes in the outer membrane, while Ross et al. used an osmotic shock method accompanied with the destabilization of the outer membrane using EDTA.
Further evidence for a function in periplasmic electron transfer is derived from connection of in vitro and heterologous expression experiments. In vitro, MtrA can be directly reduced by CymA, which is bound to the cytoplasmic membrane. The rate of electron transfer is slightly higher when compared to FccA reduction. It is furthermore known that MtrA similar to CymA is a reductase acting on chelated ferric iron forms (Pitts et al. 2003). E. coli cells that express MtrA and CymA together respire Fe(III)-NTA faster than E. coli cells that express CymA alone (Schuetz et al. 2009). It was therefore hypothesized that the higher reduction rate in CymA and MtrA expressing cells is due to expression of a second ferric NTA reduction site that is directly connected to CymA (Fig. 7).
Fe(III)-NTA reduction of the E. coli strain expressing CymA and MtrA. a Scheme of the electron transfer reactions in the periplasm of the constructed E. coli strain. Heme cofactors are indicated by a diamond. b Ferric iron reduction by the constructed strain expressing CymA and MtrA (▲), and the strain expressing CymA only (△) (Schuetz et al. 2009). 10 mM ferric NTA were added to mineral media as the sole electron acceptor, while glycerol (50 mM) was used as carbon and electron source. Both strains were induced with 0.43 mM anhydrotetracycline. Copyright © American Society for Microbiology
It is so far unknown whether this periplasmic MtrA can serve as electron donor for MtrA that is localized to the outer membrane within the MtrABC complex. Nevertheless, it was shown that purified reduced MtrA could serve as electron donor for FccA catalyzed fumarate reduction (Fig. 8). Therefore, MtrA and FccA can exchange electrons and the electron flow might solely be directed by the availability of the electron acceptor.
FccA mediated electron transfer from MtrA to fumarate a Scheme of the tested electron transfer reaction. Heme cofactors are indicated by a diamond. b Redox status of initially reduced MtrA in the presence of fumarate and FccA (Schuetz et al. 2009). 1.2 μmol MtrA were reduced with dithionite and incubated for 10 min with fumarate. Addition of 120 pmol FccA (indicated with an arrow) caused a rapid reoxidation of MtrA as visualized using the absorption maximum of reduced c-type cytochromes at 552 nm. Copyright © American Society for Microbiology
MtrD (SO_1782). MtrD is a decaheme c-type cytochrome, which is very similar to MtrA. Only one MS study identified peptides from this protein so far (Zhang et al. 2011). The mtrD gene is transcribed in an operon with mtrD and mtrF, which are believed to be involved in metal detoxification (Gao et al. 2010; McLean et al. 2008). The role of MtrD in iron reduction is controversial: a ΔmtrD strain had a strong negative effect in a competition study by Gao et al. while a similar mutant had an insignificant effect in a study by Bretschger et al. (2007); (Gao et al. 2010).
CcpA (SO_2178). CcpA has strong similarities to proteins of the c-type cytochrome peroxidase (CCP) family, which can be found in yeast and bacteria. In bacteria CCPs are diheme proteins with two c-type heme groups (Cabiscol et al. 2000). They seem to protect the bacterial cell from oxidative damage caused by hydrogen peroxide or the peroxide-based formation of hydroxyl radicals via the Fenton reaction (Atack and Kelly 2007; Pauleta et al. 2004). Exposure of bacterial cells to excess oxygen increases the amount of hydrogen peroxide but paradoxically bacterial CCPs are often up-regulated under microoxic or anoxic conditions (Atack and Kelly 2007).
All bacterial CCP proteins investigated so far have a conserved tertiary structure. They are two domain proteins, with a single heme group per domain covalently bound to the cysteines of the general binding motif of c-type cytochromes, CXXCH. As expected, CcpA of S. oneidensis has this typical three-dimensional structure and in vitro tests revealed the typical catalytic activity (Schütz et al. 2011); Fig. 9a). Furthermore, a deletion of the ccpA gene from the S. oneidensis chromosome resulted in a decreased capability to remove hydrogen peroxide from the medium and a decreased growth rate when cells were grown under microoxic conditions with ferric iron as terminal electron acceptor ((Schütz et al. 2011); Fig. 9b).
a Three-dimensional structure of CcpA from S. oneidensis. The structure of CcpAHis was solved by X-ray crystallography to a resolution of 1.8 Å. b Growth of S. oneidensis wild type and ΔccpA mutant. Cells were grown in independent triplicates under microoxic conditions in medium containing 50 μM oxygen, 50 mM ferric citrate and 15 mM lactate. Growth was determined via the quantification of colony forming units on LB-agar plates. The inset shows a control experiment performed in media that was further reduced with 1.5 mM titanium citrate (Schütz et al. 2011). Copyright © American Society for Microbiology
Electron transfer from CymA to CcpA was detectable only in the presence of the small monoheme c-type cytochrome ScyA (see below). This is interesting, since electron transfer between c-type cytochromes was so far regarded as being rather unspecific. The interplay between ScyA and CcpA provides proof that this does not necessarily have to be the case. Although CcpA is not directly connected to CymA, hydrogen peroxide could theoretically function as terminal electron acceptor under anoxic conditions with an electron transport chain that potentially consists of CymA, ScyA, and CcpA (Fig. 10). But more importantly, the anaerobic respiratory chain can provide electrons for the detoxification of reactive oxygen species. It might be beneficial to fuel peroxidases using anaerobic electron transport chains since this will guarantee a fast response to hydrogen peroxide evolution in case of oxygen inflow into so far anoxic growth environments.
ScyA (SO_0264). It was outlined above that ScyA (SO_0264) is a small monoheme cytochrome (8.3 kDa) that is necessary for the reduction of the diheme peroxidase CcpA. ScyA can be directly reduced by CymA (Schütz et al. 2011). Interestingly, we and others were unable to construct a markerless scyA deletion mutant. Hence, we had to use a conditional mutant to study the ΔscyA phenotype. It is so far not known why this scyA locus might be necessary for the cell.
NrfA (SO_3980). NrfA is a 50 kDa pentaheme protein that is catalyzing nitrite reduction in S. oneidensis. NrfA is upregulated under nitrate reducing conditions (Beliaev et al. 2002). Transcriptional and mutational analyses suggest that CymA (instead of NrfH in other γ-protebacteria) is the electron-donating enzyme to NrfA (Gao et al. 2009) (Fig. 11). A biochemical analysis of probable further electron donating and accepting enzymes for NrfA was so far not conducted. A nrfA mutant reduced similar amounts of ferric iron compared to the wild type within 24 h (Bretschger et al. 2007).
IfcA-1 (SO_1421). IfcA is a tetraheme c-type cytochrome that was only described in S. frigidimarina so far (Dobbin et al. 1999). In this strain, it was specifically induced under iron reducing conditions. The purified protein has fumarate reductase activity in vitro but seems not to be operating under fumarate reducing conditions (Dobbin et al. 1999).
STC (also called CctA) (SO_2727). STC is a small tetraheme c-type cytochrome. It has been extensively characterized structurally and electrochemically (Fonseca et al. 2009; Leys et al. 2002; Paquete et al. 2010; Qian et al. 2011a). The exact role in the physiology of Shewanella remains unclear. Coursolle and Gralnick (2010) propose a role in periplasmic electron transfer as rather unspecific electron shuttling protein, eventually even between different respiratory pathways.
Otr (SO_4144). Otr is an octaheme c-type cytochrome that has S4O62−, NO2, and NH2OH reduction activities in vitro (Atkinson et al. 2007; Mowat et al. 2004). Still, the physiological role remains to be explored.
Other periplasmic cytochromes. As it was listed above, a number of further periplasmic cytochromes were detected using mass spectrometry (Table 2). So far, these cytochromes were not studied in more detail and are currently not connected to a physiological function in S. oneidensis. Bretschger et al. (2007) tested mutants in these cytochromes for a putative defect in ferric iron reduction but the amount of ferric iron reduced after 24 h was in most cases even above the level reached by the wild type. In a recent study, Gao et al. (2010) were using mainly competitive growth experiments to assess the fitness of several mutants. There study revealed that most of the constructed mutants in c-type cytochromes were negatively affected in fitness as compared to the wild type (Gao et al. 2010). Still, it is so far not possible to assign specific functions to these proteins and the ecological reason for their expression has to be studied in the future. Nevertheless, one hypothesis regarding their function could be that they expand the transient electron storage capacity and might link electron transfer to further potential electron acceptors, and hence widen the potential electron transfer network in the periplasm of S. oneidensis, as will be discussed in the following paragraphs.
A highly dynamic network formed by periplasmic c-type cytochromes could promote a rapid dispatch of transiently stored electrons to various electron acceptors. For instance, when cells are in contact with extracellular Fe(III) (oxyhydr)oxides, electrons derived from CymA are transferred via MtrA to MtrC, which is oxidized by the Fe(III) (oxyhydr)oxide or extracellular electron shuttles. In the absence of such a poorly soluble electron acceptor, oxidation of MtrC, and consequently of MtrA, is blocked, and electrons are transferred from CymA either directly or indirectly via MtrA to the transiently electron-storing FccA. FccA can be rapidly reoxidized by MtrA when MtrA is oxidized by outer membrane MtrC once cells are contacting Fe(III) (oxyhydr)oxides again. In the presence of fumarate, a direct oxidation of FccA by fumarate would bypass the outer membrane electron transfer.
Depending on mineral identity, ferric iron can have the lowest redox potential of all usable respiratory electron acceptors of S. oneidensis. Therefore, the midpoint redox potentials of proteins involved in the electron transport chain to ferric iron should theoretically also allow electron transfer to other periplasmic or surface exposed terminal reductases of S. oneidensis. Hence, the respiratory chain to ferric iron could be furthermore connected to electron transport chains to electron acceptors such as nitrate, nitrite, DMSO, or manganese oxides (Fig. 12). Of note, one of the other periplasmic cytochromes that are not as highly expressed as FccA or MtrA is the nitrite reductase NrfA. Furthermore, we detected (using MS-analysis) the terminal nitrate reductase NapA and the terminal DMSO reductase DmsA and B in cells grown with ferric iron as terminal electron acceptor. In addition, MtrC, the potential final reductase of the electron transport chain to ferric iron, has also manganese oxide reducing activity. Hence, if all these pathways are connected like and together with MtrA and FccA, then the availability of nitrate, nitrite, manganese oxide, or DMSO could furthermore result in a dispatch of electrons derived from reduced MtrA and FccA.
Potential components of a periplasmic and outer membrane electron transfer network. Indicated are terminal reductases, that were detected using mass spectrometry and that might be involved in the network. Already elucidated electron transfer connections are indicated by an arrow. Redox potentials of the reactions are given E0` in mV (Thauer et al. 1977) (Majzlan “Minerals and Aqueous Species of Iron and Manganese As Reactants and Products of Microbial Metal Respiration”). The possible function of other c-type cytochromes (c-Cyt) is indicated
The existence of a periplasmic electron transfer network could also explain the partial or general lack of phenotypes observed in mutants defective in a number of periplasmic c-type cytochromes. It is furthermore in line with data recorded by Firer-Sherwood and coworkers (2008). In their study, the authors subjected the multiheme cytochromes CymA, STC, MtrA, OmcA, and MtrC of S. oneidensis to protein film voltammetry and determined not only apparent midpoint potentials but also windows of redox potentials that allow for electron uptake and abstraction. The widths of the individual redox potential windows have a large overlapping region, which potentially allows for rapid interprotein electron transfer (Table. 3; (Firer-Sherwood et al. 2008)).
The electron transfer network and the ecological niche of S. oneidensis. In terms of respiratory energy generation, S. oneidensis does not seem to be a specialist but rather a generalist. The strain reduces a wide variety of possible electron acceptors but oxidizes carbon sources under anoxic conditions only to the level of acetate. The energy yield that can be gained via the respiratory chains is not fully exploited completely in some cases. For instance, the electron transport chain to nitrate goes via CymA, and hence possible further coupling sites that could be used (given the redox potential of the electron acceptor) are not exploited (Gao et al. 2009). Furthermore, regulation of the expression of terminal oxidoreductases is rather relaxed and a multitude of these enzymes is detectable in the periplasm (Beliaev et al. 2005). Moreover, it was pointed out that substrate level rather than oxidative phosphorylation is the primary energy source of S. oneidensis under anoxic conditions (Hunt et al. 2010). Therefore, it seems as if the ecological strategy of S. oneidensis is a rapid consumption of available carbon sources with the least number of possible reactions. This has to be connected with a rapid dispatch of electrons on available electron acceptors, which could very well be achieved by the realization of an electron transfer network in the periplasm.
2.3 Electron Transfer Reactions at the Outer Membrane
2.3.1 The Electron Conduit Through the Outer Membrane
The hydrophobic thickness of the outer membrane of gram-negative bacteria is approximately 25 Å (Lomize et al. 2006). Therefore, tunneling-based electron transfer through the membrane is not possible even if cytochromes are associated with the periplasmic and the extracellular site of the membrane. The electron conduit over the outer membrane of S. oneidensis is composed of three proteins that build a membrane-spanning complex. The β-barrel protein MtrB (SO_1776) is connected to the decaheme cytochrome MtrA (SO_1777) on the periplasmic site and the outer membrane cytochrome MtrC (SO_1778) at the outer surface. It was demonstrated in vitro that this complex has the capacity to transport electrons over a liposomal membrane (Hartshorne et al. 2009). Hence, the in vivo function of this complex is most likely outer membrane spanning electron transfer (Fig. 12).
Interestingly, a mutant in mtrB is unable to use ferric iron (ferric citrate or hydrous ferric oxide) or manganese oxide as electron acceptor (Beliaev and Saffarini 1998; Bretschger et al. 2007). Thereby, MtrB is besides MtrA the only protein that is definitely necessary for these respiratory processes (Beliaev and Saffarini 1998; Shyu et al. 2002). The impact of the mutation can certainly be explained by the integral function of MtrB for the MtrABC complex. But earlier publications nicely displayed that MtrB might influence ferric iron reduction also on further levels. Strikingly, deletion of mtrB causes mislocalization of outer membrane cytochromes into the cytoplasmic membrane or the periplasm (Myers and Myers 2002a). Hence, it seems possible that MtrB has an influence on the transport of these proteins through the periplasm and furthermore the assembly into the outer membrane.
Building the MtrAB complex. Surprisingly, it was found that MtrB is not detectable in a ΔmtrA mutant (Hartshorne et al. 2009; Schicklberger et al. 2010). Since the genes for MtrA and MtrB most likely form an operon, it was first sought for an effect of the mtrA deletion on mtrB transcription or RNA stability. Still, quantitative PCR excluded this possibility. Instead, MtrA seems to affect the periplasmic stability of MtrB, because a mutant in the periplasmic protease DegP (SO_3942) did not require MtrA for MtrB stability (Schicklberger et al. 2010). DegP is well known for its major role in the prevention of a toxic accumulation of unfolded outer membrane proteins in the periplasm in a variety of species including E. coli (Bos et al. 2007). The so far established pathway of β-barrel protein transport to the outer membrane necessitates activity of the periplasmic chaperones Skp and SurA that bind nascent β-barrel proteins and guide them through the periplasm (Bos et al. 2007; Sklar et al. 2007; Spiess et al. 1999). It was proposed that MtrA (besides its function in electron transport) is also a periplasmic chaperone or scaffold, which is specific for MtrB. However, the detailed mechanism of MtrABC complex formation and of the exact role of MtrA in the stability of MtrB still has to be shown experimentally.
Excursus—Distribution of MtrAB-like modules. Interestingly, modules similar to MtrAB are present in bacteria belonging to the α, β, γ, and δ group of the proteobacteria (Table 3). With the exception of S. denitrificans, 18 sequenced Shewanella strains contain up to nine copies of an MtrAB like module. S. denitrificans cannot grow with ferric iron as terminal electron acceptor which might explain the lack of a module similar to MtrAB. In S. oneidensis, it was shown that MtrAB like modules are involved in dissimilatory metal and DMSO reduction (Gralnick et al. 2006). The module involved in dissimilatory iron, manganese, and uranium reduction is in 3’direction flanked by the gene for the outer membrane cytochrome MtrC, whereas the module that is necessary for DMSO reduction is adjacent to the two 5’ encoded DMSO reductase genes (dmsA, dmsB; Fig. 13). A number of Shewanella strains contain another MtrAB like module that is in 5’direction flanked by an outer membrane cytochrome (MtrF) that is highly similar to MtrC (mtrDEF cluster; Fig. 13; (Bücking et al. 2010).
Gene clusters similar to the mtrCAB cluster were found in a number of pathogenic Vibrio strains and in the known ferric iron reducer Rhodoferax ferrireducens. While the cluster might be important for metal oxide respiration in R. ferrireducens, in Vibrio it might be a way for iron acquisition, and hence a pathogenicity factor. Interestingly, it was discovered that proteins with a high similarity to MtrAB are necessary for the back reaction of iron reduction meaning the oxidation of Fe(II): In Rhodopseudomonas palustris TIE-1 these proteins are necessary for phototrophic Fe(II) oxidation (pioABC; Fig. 13; (Jiao and Newman 2007) and in Sideroxydans lithotrophicus ES-1 the module seems to be important for chemotrophic Fe(II) oxidation (mtoA; (Liu et al. 2012)). Modules similar to MtrAB were also detected in a number of other bacteria. Currently, we do not know what the function of these modules might be.
In summary, MtrAB like modules are widely distributed and seem to comprise a strategy for electron transfer over the outer membrane. Table 4 shows the distribution and putative functions of the modules in different classes of proteobacteria (representative examples of sequenced organisms are shown). While the outer membrane cytochrome MtrC is necessary for ferric iron reduction in S. oneidensis, other surface exposed modules seem furthermore connectable to complexes similar to MtrAB and might determine the terminal electron acceptor or the electron donor of the respiratory pathway.
2.3.2 Outer Membrane Cytochromes
Many studies on the role of outer membrane cytochromes (OMC) have been published to date. Surprisingly, it is still a matter of ongoing research to assign specific functions to the individual proteins. S. oneidensis contains the genetic information for five putative outer membrane cytochromes (Meyer et al. 2004). All of these are exposed to the cell surface when expressed in S. oneidensis from a plasmid-encoded copy of the gene (Bücking et al. 2010; Richter et al. 2010). The deduced amino acid sequences of OmcA, MtrC, MtrF, and SO_1659 indicate 10 putative heme attachment sites, while SO_2931 is expected to be a diheme c-type cytochrome Fig. 14.
The different modes of terminal extracellular electron transfer in S. oneidensis. Schematic overview of a indirect electron transfer to Fe(III) using an endogenous electron shuttle c indirect electron transfer to Fe(III) using an exogenous electron shuttle e direct electron transfer to Fe(III) g cell using pili coated with final reductases for Fe(III) reduction. b d f and h represent an example for the more general overview shown in a, c, e, and g, respectively
OmcA and MtrC. The function of OmcA and MtrC has been studied best so far. They were shown to be lipoproteins exposed to the outer surface of the cell (Myers and Myers 2003a, 2004a). Both proteins form a high affinity complex in the outer membrane (Shi et al. 2006). Surprisingly, Reardon et al. (2010) outlined that the overall distribution of these cytochromes on the cell surface is not similar, which seems contradictory to the detection of a high affinity complex. When S. oneidensis cells were grown with ferrihydrite as electron acceptor, MtrC was shown to localize in close association to iron precipitates on the cell surface. In contrary, OmcA was distributed more diffusely on the cell exterior and was also detected outside of the cell in the layer of extracellular polymeric substances. Both proteins are capable of transferring electrons to various soluble and insoluble ferric iron species including ferrihydrite (Reardon et al. 2010). Furthermore, both proteins seem to contain hematite binding motifs as described by Lower et al. (2008) using phage display. Nevertheless, single gene deletion mutants in omcA or mtrC have differing phenotypes. In general, mtrC mutants show stronger growth deficiencies under dissimilatory metal reducing conditions (Myers and Myers 2001, 2002a). This might in part be due to the formation of the MtrABC complex that was mentioned before. Myers et al. showed that within 24 h an mtrC deletion mutant had only 10–15 % of the reducing activity toward manganese oxide, ferric citrate, or amorphous ferric oxide compared to the wild type (Myers and Myers 2002a). However, prolonged incubation seems to result in the evolution of suppressor mutations that compensate partly for the loss of outer membrane cytochromes. In the study of Myers et al., the amount of ferrous iron in mutant and wild type samples was almost equal after 4 days (Myers and Myers 2002a). In a recent study from our lab, we could show that a strain deficient in all outer membrane cytochromes (later on referred to as ΔOMC), was able to regain the ability to reduce ferric iron due to the occurrence of a suppressor mutation (Bücking et al. 2012).
An omcA single deletion mutant was shown to possess lower MnO2 reduction rates, whereas activity toward soluble electron acceptors and iron (oxyhydr)oxides remained unaffected or, in the case of Fe(III), mildly affected (Myers and Myers 2001) (Coursolle and Gralnick 2010). Expression of OmcA in the above mentioned ΔOMC strain did not complement the mutant for ferric iron reduction or growth in a microbial fuel cell but was surprisingly sufficient to allow for manganese oxide reduction, although on a lower level compared to the wild type or the MtrC expressing ΔOMC strain (Bücking et al. 2010).
MtrF. Deletion of the mtrF gene alone did not lead to decreased ferric citrate, ferrihydrite, MnO2, or U(VI) reduction rates (Marshall et al. 2006; Myers and Myers 2002a). Surprisingly, even a pronounced stimulating effect of the mutation on ferrihydrite reduction has been reported (Bretschger et al. 2007). It seems that MtrF is not expressed under the tested iron reducing conditions, since experiments conducted with a ΔOMC strain expressing MtrF from a plasmid encoded copy revealed that the activity of MtrF is highly similar to MtrC. Consequently, MtrF expression can rescue the ΔOMC strain when ferric iron, manganese oxide or an anode is provided as terminal electron acceptor (Bücking et al. 2010). Hence, it can be further hypothesized that the similarity between MtrC and MtrF allows for the formation of a MtrABF complex into the outer membrane of S. oneidensis cells.
Recently, the crystal structure of MtrF was solved. Using this structural data, the authors aimed at elucidating the actual electron transfer mechanism from MtrF to a metal surface. MtrF shows a surface exposure of heme 5 and 10 of 250 and 300 Å2, respectively (Clarke et al. 2011). Based on a previous report, the authors suggested that those terminal hemes have sufficient surface exposure such that they could be involved in direct contact with the iron surface (Clarke et al. 2011). This direct electron transfer would require a distance of less than 15 Å between the catalytic heme group and the poorly soluble metal oxide (Kerisit and Rosso 2007). Interestingly, the structure of MtrF shows besides the surface exposed heme containing domain, two domains that hold heme cofactors that are not surface exposed but solvent accessible. These domains furthermore hold β-barrel structures that are similar to flavin mononucleotide (FMN) binding domains. Still, FMN was not found in this region even after soaking the crystals in an FMN solution. Clark et al. suggest that these domains could interact with soluble electron shuttles, potentially FMN (see below and chapter 4).
Of note, MtrF is upregulated under oxic conditions in a specific medium that led to an autoaggregation of S. oneidensis cells. Therefore, McLean and coworkers (2008) hypothesized that MtrF might have a function in the aerobic reduction-based detoxification of metals like uranium or technetium that become sparsely soluble upon reduction. A hypothesis which points in the same direction was formulated by Gao et al. (2010) regarding chromium stress.
SO_2931 and SO_1659. Physiological expression of SO_2931 and SO_1659 has so far never been reported (Elias et al. 2006; Kolker et al. 2005). In line with these results, deletion of SO_2931 did neither hamper current production in a microbial fuel cell nor reduction of ferric iron (Bretschger et al. 2007). Expression of these cytochromes in the ΔOMC strain did not result in a phenotypic alteration, although they were surface exposed (Bücking et al. 2010; Richter et al. 2010). Therefore, it is currently unknown if these proteins are involved in other reactions or are nonfunctional.
Additional necessary and supporting factors. DiChristina et al. (2002) showed that the type II secretion system (T2SS) of S. oneidensis is necessary for dissimilatory iron reduction. Mutants in important parts of the T2SS are defective in the secretion of OmcA and MtrC (Shi et al. 2008), in DMSO reduction (Gralnick et al. 2006) and in the production of conductive nanowires (see below (Gorby et al. 2006)). It seems most likely that the T2SS is necessary for the translocation of the terminal reductases across the outer membrane to the surface of the bacterial cell. Support for this hypothesis comes from a study by Shi et al. (2008) Here, the authors observed a proteinase K resistance of MtrC and OmcA in cells with a nonfunctional version of the T2SS, while a functional T2SS lead to outer membrane cytochrome degradation. Apparently, outer membrane cytochromes are only surface localized in the presence of a T2SS and are localized to the periplasmic site of the membrane in the absence of this export machinery.
Interestingly, Qian et al. provided evidence for the possible function of a protein similar to TonB-dependent receptor proteins in dissimilatory iron reduction in S. oneidensis. The authors could show that this protein, SO_2907, is capable of binding soluble Fe(III) forms and that a deletion mutant is affected in reduction of ferric citrate. It was therefore speculated that SO_2907 might be involved in iron transport to the periplasm which would represent another physiological strategy to bring ferric iron in contact with potential reductases (Qian et al. 2011b). Still, mutants in outer membrane cytochromes show strong phenotypes in ferric citrate reduction which underlines the fact that the cell surface is the major site for ferric iron reduction (Bücking et al. 2010). Nevertheless, it will be interesting to see in further experiments which role this potential ferric iron transport route might have in comparison to the established electron transport to ferric iron via outer membrane cytochromes.
3 Extending the Extended Respiratory Chain
For a long time, electron transfer to poorly soluble metal oxides was believed to be realized via direct electron transfer reactions catalyzed by cytochrome proteins on the cell surface. This hypothesis was challenged by Lies et al. (2005) using an innovative experiment. The authors synthesized ferric iron containing porous glass beads. This glass material prohibited direct contact of the ferric iron to the bacterial cell. Nevertheless, S. oneidensis was capable of reducing this trapped iron. This was evidence for a way of distributing electrons to poorly soluble electron acceptors that can be micrometers away from a bacterial cell. Since then, several ideas have been proposed and several mechanisms have been elucidated how indirect electron transfer reactions could be realized by the cell itself or using exogenous electron shuttling components.
Endogenous electron shuttles. Endogenous electron shuttles could interact between a metal oxide surface and a bacterial cell and thereby enabling reduction at a distance. If this shuttling compound could be reused several times by the organism that secretes it, then the energy invested in the synthesis of that compound could be almost negligible.
A first hypothesis for an endogenous electron shuttle of S. oneidensis was raised by Diane Newman. She speculated about a possible function of quinones as endogenous electron shuttles (Newman and Kolter 2000), because she could show that menaquinone synthesis mutants, unable to respire on extracellular electron acceptors, could be rescued using menaquinone-related substances that were detected in the supernatant of wild type cells. Later, it became evident; that the secreted substances allowed the mutants to overcome the gap in the menaquinone synthesis pathway and that they were not involved in electron shuttling (Myers and Myers 2004b).
In 2008, two groups published reports on the role of flavins as electron shuttles. They were detected in the growth medium of S. oneidensis cells and in microbial fuel cells (Marsili et al. 2008; von Canstein et al. 2008). Interestingly, it was demonstrated that their presence strongly enhanced ferric iron and anode reducing activity of S. oneidensis cells (Marsili et al. 2008; Ross et al. 2009). Prof. Gralnick will discuss their role in metal reduction in more detail in “On the Role of Endogenous Electron Shuttles in Extracellular Electron Transfer” of this book. Furthermore, Prof. Kappler will introduce the reader to exogenous electron shuttles like humic substances that are ubiquitously distributed in a variety of habitats (see “Humic Substances and Extracellular Electron Transfer”).
Nanowires. Pilus like cellular appendages that are conductive are termed nanowires. These nanowires can be several microns long, and hence enable a dissimilatory metal reducer to reach a distantly localized electron acceptor. They were first described in Geobacter (Reguera et al. 2005) and later also discovered in S. oneidensis (El-Naggar et al. 2010; Gorby et al. 2006). While they are necessary for the reduction of ferric oxides by G. sulfurreducens, S. oneidensis produces them under conditions of electron acceptor limitation. S. oneidensis nanowires seem to be decorated with outer membrane cytochromes that are necessary for electron transport along the pilus. Consequently, nonconductive but morphologically similar pili are produced in the absence of MtrC and OmcA. Therefore, it seems as if electrons are transported from outer membrane cytochrome to outer membrane cytochrome along the pilus which necessitates a distance of less than 14 Å between individual proteins (Gorby et al. 2006). Curiously, it is so far unknown what the structural component of these pili in S. oneidensis is. Moreover, conductivity was so far only shown for dried samples. Hence, a number of further experiments have to be conducted to find out the physiological function that these appendages might have for S. oneidensis.
Membrane vesicles. A number of publications report the production of outer membrane vesicles by gram-negative bacteria. Their production seems to be a stress response and they seem to be important tools for nutrient acquisition, biofilm development, as well as pathogenesis (Kulp and Kuehn 2010). Yuri Gorby reported that Shewanella strain CN32 produces such vesicles and that they can reduce ferric iron in the presence of hydrogen (Gorby et al. 2008). Hydrogen apparently reduces the outer membrane cytochromes localized to the membrane vesicles. Furthermore it seems likely, that these vesicles can be reduced by Shewanella itself and can then act as complex endogenous electron shuttles. Still, so far we are not able to assess the influence that these vesicles might have in environmental iron reduction.
Iron chelators. Iron assimilation depends for a number of strains on the production of siderophors that are able to chelate sparsely soluble ferric iron and to convert it thereby into organic ligand complexed, and thus dissolved Fe(III) (Braun and Hantke 2011). One major group of siderophors consists of derivatives of hydroxamic acid. S. oneidensis has the genetic information for hydroxamate biosynthesis. Furthermore, S. oneidensis produces soluble Fe(III) when grown under anoxic conditions either with ferrihydrite or with ferrihydrite plus fumarate as terminal electron acceptor (Jones et al. 2010). Surprisingly, hydroxamate biosynthesis is not involved in the solubilization of ferrihydrite under the tested conditions. Instead, a different yet uncharacterized organic chelator is produced as was shown by Jones et al. (2010). The authors could find a number of mutants that are apparently not able to produce this soluble iron, as detected using a novel microelectrode screening assay. Unfortunately, it is so far not known how this organic chelator is produced by S. oneidensis and what chemical structure it has. Still, the detected mutants that are not able to produce dissolved organic-Fe(III) complexes are furthermore unable to reduce ferric citrate or ferrihydrite.
4 Concluding Remarks
S. oneidensis shows an astonishing versatility in terms of electron acceptors that can be used under anoxic conditions. The versatility seems to be mostly due to a variety of c-type cytochromes that enable electron transport to terminal electron acceptors. There is growing evidence for a second function of c-type cytochromes as electron storage proteins. The presence of these capacitors seems to go along with a fitness benefit for the organism. Hence, the often-asked question why Shewanella produces numerous c-type cytochromes under anoxic conditions might have this function as an answer. Not the expression of certain singular electron transport pathways but the simultaneous expression of a multitude of pathways might result in a concentration of cytochromes that is high enough to enable this capacitor function. If we further follow this hypothesis, it seems also reasonable to assume that it is of a higher benefit to split the capacitor function into several partly independent pathways that can communicate with each other. Thereby, a rapid oxidation of all cytochromes is guaranteed in response to the availability of a variety of electron acceptors. Still, further experiments are necessary to elucidate the redox-behavior of the c-type cytochrome pool. Furthermore, competition experiments between Shewanella as a versatile organisms and more specialized organisms like Geobacter would be helpful to understand if the network leads to a growth advantage for Shewanella species. Moreover, it seems necessary to dissect the ecological niche of Shewanella in more detail in the environment to test whether the proposed advantages of versatility and capacitor function of c-type cytochromes apply in the environment as well as under laboratory conditions. Last but not least, a further focus of future research should be to elucidate which role electron shuttles play in environmental metal reduction.
References
Atack JM, Kelly DJ (2007) Structure, mechanism and physiological roles of bacterial cytochrome c peroxidases. Adv Microb Physiol 52:73–106
Atkinson SJ, Mowat CG, Reid GA, Chapman SK (2007) An octaheme c-type cytochrome from Shewanella oneidensis can reduce nitrite and hydroxylamine. FEBS Lett 581:3805–3808
Beliaev AS, Saffarini DA (1998) Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J Bacteriol 180:6292–6297
Beliaev AS, Thompson DK, Khare T, Lim H, Brandt CC, Li G, Murray AE, Heidelberg JF, Giometti CS, Yates J 3rd, Nealson KH, Tiedje JM, Zhoui J (2002) Gene and protein expression profiles of Shewanella oneidensis during anaerobic growth with different electron acceptors. OMICS 6:39–60
Beliaev AS, Klingeman DM, Klappenbach JA, Wu L, Romine MF, Tiedje JM, Nealson KH, Fredrickson JK, Zhou J (2005) Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. J Bacteriol 187:7138–7145
Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462
Bos MP, Robert V, Tommassen J (2007) Biogenesis of the gram-negative bacterial outer membrane. Annu Rev Microbiol 61:191–214
Bouhenni R, Gehrke A, Saffarini D (2005) Identification of genes involved in cytochrome c biogenesis in Shewanella oneidensis, using a modified mariner transposon. Appl Environ Microbiol 71:4935–4937
Bouhenni RA, Vora GJ, Biffinger JC, Shirodkar S, Brockman K, Ray R, Wu P, Johnson BJ, Biddle EM, Marshall MJ, Fitzgerald LA, Little BJ, Fredrickson JK, Beliaev AS, Ringeisen BR, Saffarini DA (2010) The role of Shewanella oneidensis MR-1 outer surface structures in extracellular electron transfer. Electroanal 22:856–864
Braun V, Hantke K (2011) Recent insights into iron import by bacteria. Curr Opin Chem Biol 15:328–334
Bretschger O, Obraztsova A, Sturm CA, Chang IS, Gorby YA, Reed SB, Culley DE, Reardon CL, Barua S, Romine MF, Zhou J, Beliaev AS, Bouhenni R, Saffarini D, Mansfeld F, Kim BH, Fredrickson JK, Nealson KH (2007) Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol 73:7003–7012
Brown RN, Romine MF, Schepmoes AA, Smith RD, Lipton MS (2010) Mapping the subcellular proteome of Shewanella oneidensis MR-1 using sarkosyl-based fractionation and LC-MS/MS protein identification. J Proteome Res 9:4454–4463
Bücking C, Popp F, Kerzenmacher S, Gescher J (2010) Involvement and specificity of Shewanella oneidensis outer membrane cytochromes in the reduction of soluble and solid-phase terminal electron acceptors. FEMS Microbiol Lett 306:144–151
Bücking C, Piepenbrock A, Kappler A, Gescher J (2012) Outer membrane cytochrome independent reduction of extracellular electron acceptors in Shewanella oneidensis. Microbiology (Epub ahead of print)
Burns JL, DiChristina TJ (2009) Anaerobic respiration of elemental sulfur and thiosulfate by Shewanella oneidensis MR-1 requires psrA, a homolog of the phsA gene of Salmonella enterica serovar typhimurium LT2. Appl Environ Microbiol 75:5209–5217
Cabiscol E, Piulats E, Echave P, Herrero E, Ros J (2000) Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J Biol Chem 275:27393–27398
Carpentier W, De Smet L, Van Beeumen J, Brige A (2005) Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol 187:3293–3301
Clarke TA, Edwards MJ, Gates AJ, Hall A, White GF, Bradley J, Reardon CL, Shi L, Beliaev AS, Marshall MJ, Wang Z, Watmough NJ, Fredrickson JK, Zachara JM, Butt JN, Richardson DJ (2011) Structure of a bacterial cell surface decaheme electron conduit. Proc Natl Acad Sci U S A 108:9384–9389
Coursolle D, Gralnick JA (2010) Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol Microbiol
Cruz-Garcia C, Murray AE, Klappenbach JA, Stewart V, Tiedje JM (2007) Respiratory nitrate ammonification by Shewanella oneidensis MR-1. J Bacteriol 189:656–662
DiChristina TJ, Moore CM, Haller CA (2002) Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J Bacteriol 184:142–151
Dobbin PS, Butt JN, Powell AK, Reid GA, Richardson DJ (1999) Characterization of a flavocytochrome that is induced during the anaerobic respiration of Fe3+ by Shewanella frigidimarina NCIMB400. Biochem J 342(Pt 2):439–448
El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA (2010) Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci U S A 107:18127–18131
Elias DA, Monroe ME, Marshall MJ, Romine MF, Belieav AS, Fredrickson JK, Anderson GA, Smith RD, Lipton MS (2005) Global detection and characterization of hypothetical proteins in Shewanella oneidensis MR-1 using LC-MS based proteomics. Proteomics 5:3120–3130
Elias DA, Monroe ME, Smith RD, Fredrickson JK, Lipton MS (2006) Confirmation of the expression of a large set of conserved hypothetical proteins in Shewanella oneidensis MR-1. J Microbiol Methods 66:223–233
Elias DA, Yang F, Mottaz HM, Beliaev AS, Lipton MS (2007) Enrichment of functional redox reactive proteins and identification by mass spectrometry results in several terminal Fe(III)-reducing candidate proteins in Shewanella oneidensis MR-1. J Microbiol Methods 68:367–375
Esteve-Nunez A, Sosnik J, Visconti P, Lovley DR (2008) Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ Microbiol 10:497–505
Firer-Sherwood M, Pulcu GS, Elliott SJ (2008) Electrochemical interrogations of the Mtr cytochromes from Shewanella: opening a potential window. J Biol Inorg Chem 13:849–854
Fonseca BM, Saraiva IH, Paquete CM, Soares CM, Pacheco I, Salgueiro CA, Louro RO (2009) The tetraheme cytochrome from Shewanella oneidensis MR-1 shows thermodynamic bias for functional specificity of the hemes. J Biol Inorg Chem 14:375–385
Gao H, Yang ZK, Barua S, Reed SB, Romine MF, Nealson KH, Fredrickson JK, Tiedje JM, Zhou J (2009) Reduction of nitrate in Shewanella oneidensis depends on atypical NAP and NRF systems with NapB as a preferred electron transport protein from CymA to NapA. ISME J 3:966–976
Gao H, Barua S, Liang Y, Wu L, Dong Y, Reed S, Chen J, Culley D, Kennedy D, Yang Y, He Z, Nealson KH, Fredrickson JK, Tiedje JM, Romine M, Zhou J (2010) Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration. Microbiol Biotechnol 3:455–466
Gescher JS, Cordova CD, Spormann AM (2008) Dissimilatory iron reduction in Escherichia coli: identification of CymA of Shewanella oneidensis and NapC of E. coli as ferric reductases. Mol Microbiol 68:706–719
Gorby Y, McLean J, Korenevsky A, Rosso K, El-Naggar MY, Beveridge TJ (2008) Redox-reactive membrane vesicles produced by Shewanella. Geobiology 6:232–241
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci U S A 103:11358–11363
Gralnick JA, Vali H, Lies DP, Newman DK (2006) Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1. Proc Natl Acad Sci U S A 103:4669–4674
Gunsalus RP (1992) Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol 174:7069–7074
Hartshorne RS, Jepson BN, Clarke TA, Field SJ, Fredrickson J, Zachara J, Shi L, Butt JN, Richardson DJ (2007) Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J Biol Inorg Chem 12:1083–1094
Hartshorne RS, Reardon CL, Ross D, Nuester J, Clarke TA, Gates AJ, Mills PC, Fredrickson JK, Zachara JM, Shi L, Beliaev AS, Marshall MJ, Tien M, Brantley S, Butt JN, Richardson DJ (2009) Characterization of an electron conduit between bacteria and the extracellular environment. Proc Natl Acad Sci U S A 106:22169–22174
Hunt KA, Flynn JM, Naranjo B, Shikhare ID, Gralnick JA (2010) Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J Bacteriol 192:3345–3351
Jiao Y, Newman DK (2007) The pio operon is essential for phototrophic Fe(II) oxidation in Rhodopseudomonas palustris TIE-1. J Bacteriol 189:1765–1773
Jones ME, Fennessey CM, DiChristina TJ, Taillefert M (2010) Shewanella oneidensis MR-1 mutants selected for their inability to produce soluble organic-Fe(III) complexes are unable to respire Fe(III) as anaerobic electron acceptor. Environ Microbiol 12:938–950
Karpinets TV, Romine MF, Schmoyer DD, Kora GH, Syed MH, Leuze MR, Serres MH, Park BH, Samatova NF, Uberbacher EC (2010) Shewanella knowledgebase: integration of the experimental data and computational predictions suggests a biological role for transcription of intergenic regions. Database (Oxford) 2010. baq012
Kerisit S, Rosso KM (2007) Kinetic Monte Carlo model of charge transport in hematite (alpha-Fe(2)O(3)). J Chem Phys 127:124706
Kolker E, Picone AF, Galperin MY, Romine MF, Higdon R, Makarova KS, Kolker N, Anderson GA, Qiu X, Auberry KJ, Babnigg G, Beliaev AS, Edlefsen P, Elias DA, Gorby YA, Holzman T, Klappenbach JA, Konstantinidis KT, Land ML, Lipton MS, McCue LA, Monroe M, Pasa-Tolic L, Pinchuk G, Purvine S, Serres MH, Tsapin S, Zakrajsek BA, Zhu W, Zhou J, Larimer FW, Lawrence CE, Riley M, Collart FR, Yates JR 3rd, Smith RD, Giometti CS, Nealson KH, Fredrickson JK, Tiedje JM (2005) Global profiling of Shewanella oneidensis MR-1: expression of hypothetical genes and improved functional annotations. Proc Natl Acad Sci U S A 102:2099–2104
Kulp A, Kuehn MJ (2010) Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64:163–184
Learman DR, Yi H, Brown SD, Martin SL, Geesey GG, Stevens AM, Hochella MF Jr (2009) Involvement of Shewanella oneidensis MR-1 LuxS in biofilm development and sulfur metabolism. Appl Environ Microbiol 75:1301–1307
Leys D, Tsapin AS, Nealson KH, Meyer TE, Cusanovich MA, Van Beeumen JJ (1999) Structure and mechanism of the flavocytochrome c fumarate reductase of Shewanella putrefaciens MR-1. Nat Struct Biol 6:1113–1117
Leys D, Meyer TE, Tsapin AS, Nealson KH, Cusanovich MA, Van Beeumen JJ (2002) Crystal structures at atomic resolution reveal the novel concept of “electron-harvesting” as a role for the small tetraheme cytochrome c. J Biol Chem 277:35703–35711
Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK (2005) Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol 71:4414–4426
Liu J, Wang Z, Belchik SM, Edwards MJ, Liu C, Kennedy DW, Merkley ED, Lipton MS, Butt JN, Richardson DJ, Zachara JM, Fredrickson JK, Rosso KM, Shi L (2012) Identification and characterization of MtoA: a decaheme c-type cytochrome of the neutrophilic Fe(II)-oxidizing bacterium Sideroxydans lithotrophicus ES-1. Front Microbiol 3:37
Lomize AL, Pogozheva ID, Lomize MA, Mosberg HI (2006) Positioning of proteins in membranes: a computational approach. Protein Sci 15:1318–1333
Lower BH, Lins RD, Oestreicher Z, Straatsma TP, Hochella MF Jr, Shi L, Lower SK (2008) In vitro evolution of a peptide with a hematite binding motif that may constitute a natural metal-oxide binding archetype. Environ Sci Technol 42:3821–3827
Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z, Boyanov MI, Lai B, Kemner KM, McLean JS, Reed SB, Culley DE, Bailey VL, Simonson CJ, Saffarini DA, Romine MF, Zachara JM, Fredrickson JK (2006) c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol 4:e268
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973
McLean JS, Pinchuk GE, Geydebrekht OV, Bilskis CL, Zakrajsek BA, Hill EA, Saffarini DA, Romine MF, Gorby YA, Fredrickson JK, Beliaev AS (2008) Oxygen-dependent autoaggregation in Shewanella oneidensis MR-1. Environ Microbiol 10:1861–1876
McMillan DG, Marritt SJ, Butt JN, Jeuken LJ (2012) Menaquinone-7 is specific cofactor in tetraheme quinol dehydrogenase CymA. J Biol Chem 287:14215–14225
Meyer TE, Tsapin AI, Vandenberghe I, de Smet L, Frishman D, Nealson KH, Cusanovich MA, van Beeumen JJ (2004) Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. OMICS 8:57–77
Morris CJ, Black AC, Pealing SL, Manson FD, Chapman SK, Reid GA, Gibson DM, Ward FB (1994) Purification and properties of a novel cytochrome: flavocytochrome c from Shewanella putrefaciens. Biochem J 302(Pt 2):587–593
Mowat CG, Rothery E, Miles CS, McIver L, Doherty MK, Drewette K, Taylor P, Walkinshaw MD, Chapman SK, Reid GA (2004) Octaheme tetrathionate reductase is a respiratory enzyme with novel heme ligation. Nat Struct Mol Biol 11:1023–1024
Murphy JN, Saltikov CW (2007) The cymA gene, encoding a tetraheme c-type cytochrome, is required for arsenate respiration in Shewanella species. J Bacteriol 189:2283–2290
Myers CR, Nealson KH (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron-acceptor. Science 240:1319–1321
Myers CR, Myers JM (1997a) Isolation and characterization of a transposon mutant of Shewanella putrefaciens MR-1 deficient in fumarate reductase. Lett Appl Microbiol 25:162–168
Myers CR, Myers JM (1997b) Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J Bacteriol 179:1143–1152
Myers CR, Myers JM (1997c) Replication of plasmids with the p15A origin in Shewanella putrefaciens MR-1. Lett Appl Microbiol 24:221–225
Myers CR, Myers JM (2002a) MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl Environ Microbiol 68:5585–5594
Myers CR, Myers JM (2003a) Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett Appl Microbiol 37:254–258
Myers CR, Myers JM (2004a) The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett Appl Microbiol 39:466–470
Myers CR, Myers JM (2004b) Shewanella oneidensis MR-1 restores menaquinone synthesis to a menaquinone-negative mutant. Appl Environ Microbiol 70:5415–5425
Myers JM, Myers CR (2001) Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl Environ Microbiol 67:260–269
Myers JM, Myers CR (2002b) Genetic complementation of an outer membrane cytochrome omcB mutant of Shewanella putrefaciens MR-1 requires omcB plus downstream DNA. Appl Environ Microbiol 68:2781–2793
Myers JM, Myers CR (2003b) Overlapping role of the outer membrane cytochromes of Shewanella oneidensis MR-1 in the reduction of manganese(IV) oxide. Lett Appl Microbiol 37:21–25
Newman DK, Kolter R (2000) A role for excreted quinones in extracellular electron transfer. Nature 405:94–97
Paquete CM, Saraiva IH, Calcada E, Louro RO (2010) Molecular basis for directional electron transfer. J Biol Chem 285:10370–10375
Pauleta SR, Cooper A, Nutley M, Errington N, Harding S, Guerlesquin F, Goodhew CF, Moura I, Moura JJG, Pettigrew GW (2004) A copper protein and a cytochrome bind at the same site on bacterial cytochrome c peroxidase. Biochemistry 43:14566–14576
Pessanha M, Rothery EL, Miles CS, Reid GA, Chapman SK, Louro RO, Turner DL, Salgueiro CA, Xavier AV (2009) Tuning of functional heme reduction potentials in Shewanella fumarate reductases. Biochim Biophys Acta 1787:113–120
Pitts KE, Dobbin PS, Reyes-Ramirez F, Thomson AJ, Richardson DJ, Seward HE (2003) Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates. J Biol Chem 278:27758–27765
Qian Y, Paquete CM, Louro RO, Ross DE, Labelle E, Bond DR, Tien M (2011a) Mapping the iron binding site(s) on the small tetraheme cytochrome of Shewanella oneidensis MR-1. Biochemistry 50:6217–6224
Qian Y, Shi L, Tien M (2011b) SO2907, A putative TonB-dependent receptor, is involved in dissimilatory iron reduction by Shewanella oneidensis MR-1. J Biol Chem 286:33973–33980
Reardon CL, Dohnalkova AC, Nachimuthu P, Kennedy DW, Saffarini DA, Arey BW, Shi L, Wang Z, Moore D, McLean JS, Moyles D, Marshall MJ, Zachara JM, Fredrickson JK, Beliaev AS (2010) Role of outer-membrane cytochromes MtrC and OmcA in the biomineralization of ferrihydrite by Shewanella oneidensis MR-1. Geobiology 8:56–68
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Richter K, Bücking C, Schicklberger M, Gescher J (2010) A simple and fast method to analyze the orientation of c-type cytochromes in the outer membrane of Gram-negative bacteria. J Microbiol Methods 82:184–186
Rodrigues ML, Oliveira TF, Pereira IA, Archer M (2006) X-ray structure of the membrane-bound cytochrome c quinol dehydrogenase NrfH reveals novel haem coordination. EMBO J 25:5951–5960
Romine MF, Carlson TS, Norbeck AD, McCue LA, Lipton MS (2008) Identification of mobile elements and pseudogenes in the Shewanella oneidensis MR-1 genome. Appl Environ Microbiol 74:3257–3265
Ross DE, Ruebush SS, Brantley SL, Hartshorne RS, Clarke TA, Richardson DJ, Tien M (2007) Characterization of protein–protein interactions involved in iron reduction by Shewanella oneidensis MR-1. Appl Environ Microbiol 73:5797–5808
Ross DE, Brantley SL, Tien M (2009) Kinetic characterization of terminal reductases OmcA and MtrC involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl Environ Microbiol 75:5218–5226
Saffarini DA, Blumerman SL, Mansoorabadi KJ (2002) Role of menaquinones in Fe(III) reduction by membrane fractions of Shewanella putrefaciens. J Bacteriol 184:846–848
Saltikov CW, Newman DK (2003) Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci U S A 100:10983–10988
Sambrook J, Fritsch EF, Maniatis T, Russell DW (1989) Molecular cloning. Cold Spring Harbor Laboratory Press, New York
Schicklberger M, Bücking C, Schuetz B, Heide H, Gescher J (2010) Involvement of the Shewanella oneidensis decaheme cytochrome MtrA in the periplasmic stability of the beta-barrel protein MtrB. Appl Environ Microbiol 77:1520–1523
Schuetz B, Schicklberger M, Kuermann J, Spormann AM, Gescher J (2009) Periplasmic electron transfer via the c-type cytochromes MtrA and FccA of Shewanella oneidensis MR-1. Appl Environ Microbiol 75:7789–7796. doi:10.1128/AEM.01834-09
Schütz B, Seidel J, Sturm G, Einsle O, Gescher J (2011) Investigation of the electron transport chain to and the catalytic activity of the diheme cytochrome c peroxidase CcpA of Shewanella oneidensis. Appl Environ Microbiol 77:6172–6180. doi:10.1128/AEM.00606-11
Schwalb C, Chapman SK, Reid GA (2002) The membrane-bound tetrahaem c-type cytochrome CymA interacts directly with the soluble fumarate reductase in Shewanella. Biochem Soc Trans 30:658–662
Schwalb C, Chapman SK, Reid GA (2003) The tetraheme cytochrome CymA is required for anaerobic respiration with dimethyl sulfoxide and nitrite in Shewanella oneidensis. Biochemistry 42:9491–9497
Scott JH, Nealson KH (1994) A biochemical study of the intermediary carbon metabolism of Shewanella putrefaciens. J Bacteriol 176:3408–3411
Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O’Toole GA (2006) Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol 72:5027–5036
Shi L, Chen B, Wang Z, Elias DA, Mayer MU, Gorby YA, Ni S, Lower BH, Kennedy DW, Wunschel DS, Mottaz HM, Marshall MJ, Hill EA, Beliaev AS, Zachara JM, Fredrickson JK, Squier TC (2006) Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J Bacteriol 188:4705–4714
Shi L, Deng S, Marshall MJ, Wang Z, Kennedy DW, Dohnalkova AC, Mottaz HM, Hill EA, Gorby YA, Beliaev AS, Richardson DJ, Zachara JM, Fredrickson JK (2008) Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J Bacteriol 190:5512–5516
Shirodkar S, Reed S, Romine M, Saffarini D (2011) The octahaem SirA catalyses dissimilatory sulfite reduction in Shewanella oneidensis MR-1. Environ Microbiol 13:108–115
Shyu JB, Lies DP, Newman DK (2002) Protective role of tolC in efflux of the electron shuttle anthraquinone-2,6-disulfonate. J Bacteriol 184:1806–1810
Sklar JG, Wu T, Kahne D, Silhavy TJ (2007) Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev 21:2473–2484
Spiess C, Beil A, Ehrmann M (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339–347
Sturm G, Gescher J About the periplasmic electron transfer network in S. oneidensis (In preparation)
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Thormann KM, Saville RM, Shukla S, Pelletier DA, Spormann AM (2004) Initial phases of biofilm formation in Shewanella oneidensis MR-1. J Bacteriol 186:8096–8104
Thormann KM, Saville RM, Shukla S, Spormann AM (2005) Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J Bacteriol 187:1014–1021
Unden G, Bongaerts J (1997) Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320:217–234
Uria N, Munoz Berbel X, Sanchez O, Munoz FX, Mas J (2011) Transient storage of electrical charge in biofilms of Shewanella oneidensis MR-1 growing in a microbial fuel cell. Environ Sci Technol 45(23):10250–10256
Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson KH (1999) Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 49 Pt 2:705–724
von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Yang C, Rodionov DA, Li X, Laikova ON, Gelfand MS, Zagnitko OP, Romine MF, Obraztsova AY, Nealson KH, Osterman AL (2006) Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J Biol Chem 281:29872–29885
Zhang H, Yang F, Qian WJ, Brown RN, Wang Y, Merkley EE, Park JH, Monroe ME, Purvine SO, Moore RJ, Shi L, Fredrickson JK, Pasa-Tolic L, Smith RD, Lipton MS (2011) Identification of c-type heme-containing peptides using nonactivated immobilized metal affinity chromatography resin enrichment and higher-energy collisional dissociation. Anal Chem 83:7260–7268
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bücking, C., Schicklberger, M., Gescher, J. (2013). The Biochemistry of Dissimilatory Ferric Iron and Manganese Reduction in Shewanella oneidensis . In: Gescher, J., Kappler, A. (eds) Microbial Metal Respiration. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32867-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-32867-1_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32866-4
Online ISBN: 978-3-642-32867-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)