Abstract
This chapter describes the use of plasmonic substrates for the laser desorption/ionization in mass spectrometry. Interesting phenomenon such as polarization and wavelength dependence has been observed for the laserdesorption/ionization of molecules from gold-coated porous silicon, goldnanorod arrays, and nanoparticles, and these results suggest the presence of near-field effects on the desorption/ionization process.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Mass spectrometry is an indispensable tool for biochemistry and life science, and it offers the analytical information such as sample composition which is not available from other analytical techniques. The basic principle of mass spectrometry is to generate ions from the inorganic or organic compound and to separate these ions according to their mass-to-charge ratio (m/z). To mass analyze the sample, analyte molecules need to be electrically charged. Thus, ion source is one of the key parts in a mass spectrometer. The analyte may be ionized by a variety of methods, for example, electron ionization [1], field ionization [2], field desorption [3], chemical ionization [4], electrospray ionization [5], and laser-based desorption/ionization. At present, large biomolecules such as proteins and peptides are analyzed exclusively using electrospray ionization (ESI, developed by John Fenn in 1989) and matrix-assisted laser desorption/ionization (MALDI, developed by Koichi Tanaka and Franz Hillenkam [6, 7]).
When the nanostructured surface of metal of high conductivity, for example, gold, is irradiated with laser of certain wavelength at appropriate polarization, collective electron motion, known as localized surface plasmon-polariton oscillation, will be excited. The localized surface plasmon-polariton resonance leads to enhanced photon absorption and huge concentration of optical near-fields at a small volume, which contribute to the enhancement in the surface-enhanced spectroscopy. Although intensive research on the plasmonic electronics and plasmon biosensing is in progress [8, 9], there is little work on the exploitation of the plasmon effect in the desorption/ionization of biomolecule for mass spectrometry. In this chapter, we describe the visible laser desorption/ionization of biomolecules using nanostructured plasmonic substrates.
Three types of nanostructured substrates are described here: gold-coated porous silicon, gold nanorods, and gold nanoparticles. The porous silicon made by electro-chemical etching was coated with gold using argon ion sputtering. The gold nano-rod arrays were fabricated by electrodepositing the gold into the porous alumina template and the subsequent partial removal of the alumina template. A frequency-doubled Nd:YAG laser was used to irradiate gold nanostructured substrate, and the desorbed molecular ions were mass analyzed by a time-of-flight mass spectrometer. The present technique offers a potential analytical method for the low molecular weight analytes which are rather difficult to handle in the conventional matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. With the presence of Au nanoparticles, the UV-MALDI matrix was also found to be photo-ionized by the 532 nm laser even though the photon energy is insufficient for free molecules.
Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry
For laser mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is a very effective and soft method in obtaining mass spectra for synthetic and biological samples, such as peptides and proteins with less molecular fragmentation [6, 7]. Depending on the matrices, laser wavelengths of ultraviolet (UV) and infrared have been employed. The established UV-MALDI method usually employs a nitrogen laser (337 nm), or a frequency-tripled Nd:YAG laser (355 nm) for desorption/ionization, while the Er:YAG lasers (2.94 μm) and CO2 (10.6 μm) are used in the IR-MALDI.
In MALDI, biomolecular analytes are mixed with photo-absorbing chemical matrix of suitable functional groups to assist the energy transfer. Thus, the matrix molecules must possess suitable chromophores to absorb the laser photons efficiently. Besides having high optical absorption coefficients, matrix molecules must possess suitable chemical properties for efficient ionization. All of the matrices that act as protonating agents for analytes contain OH and/or NH2 functional groups. Thus, protons are believed to be transferred from these groups during the ionization process.
Nicotinic acid (NA) was historically the first UV-MALDI matrix for successful detection of peptides and proteins. Ever since, many other better matrices, for example, 2,5-dihydroxybenzoic acid (DHB) and α-cyano-4-hydroxycinnamic acid (CHCA), had also been found. As for IR-MALDI, the laser wavelength of ∼ 3 μm effectively excites the O–H and N–H stretch vibration of the molecules, while the laser wavelength of ∼ 10 μm causes the excitation of C–O stretch and O–H bending vibration [10, 11]. Molecular structures of typical MALDI matrices are shown in Fig. 27.1.
Commonly used MALDI matrices. 2,5-Dihydroxybenzoic acid (DBD), α-cyano-4-hydroxycinnamic acid (CHCA), and sinapinic acid are for UV-MALDI. Succinic acid and glycerol are for IR-MALDI. Glycerol is also used as liquid matrix incorporation with UV-absorbing material (e.g., metallic nano powder, graphite) for UV-MALDI
Except for the light-absorbing analytes, direct photoionization of macromolecules rarely takes place, and the peptides and protein ions observed in laser desorption/ionization are mostly protonated (molecular ions generated by proton attachment, e.g., [M + H]+). For the analytes such as underivatized carbohydrates, due to their poor proton affinity, the molecules are difficult to be protonated and instead are mostly ionized by alkali metal attachment, for example, [M + Na]+ and [M + K]+ [12]. Typical ion species produced by LDI/MALDI are listed in Table 27.1.
The typical laser fluences in UV-MALDI are in the range of 10–100 mJ cm− 1, which correspond to 106–107 W cm− 2 for a pulsed laser of 10 ns pulse width. For IR-MALDI, the required fluence is somewhat 10 times higher than that of UV-MALDI. At the laser threshold, a sharp onset of desorption/ionization takes place. The threshold laser fluence depends on the type of matrix as well as the matrix-to-analyte mixing ratio. The mixing ratio of 5,000 is usually used in MALDI. Although the detailed desorption/ionization mechanism of MALDI had not been thoroughly understood, it is generally believed that the radical ions of the matrix molecules produced by either two-photon ionization or the molecular exciton pooling played crucial roles in ionizing the desorbed analytes via gas-phase interaction [13, 14].
Common UV-MALDI matrices have a wider absorption band than that of IR-MALDI, and the desorption/ionization performance is less affected by photon energies over a certain range of UV wavelength [15, 16]. However, restricted by their optical absorption coefficients, the UV and IR-MALDI matrices do not work with visible or near-IR lasers.
Early investigations on visible-MALDI (VIS-MALDI) were conducted by Tang et al. using rhodamine dyes as matrices [17]. Cornett et al. introduced a binary matrix consisted of rhodamine 6G which was dissolved in a liquid matrix (3-nitro-benzylalcohol) for VIS-MALDI with 532 nm frequency-doubled Nd:YAG laser [18]. Since the liquid matrix is transparent to the visible laser, the optical absorption was due to the dye, and the method is similar to the two-phase matrix method of Tanaka et al. in which the laser energy was absorbed by metal nanoparticles suspended in the liquid glycerol. Other VIS-MALDI studies were performed using Neutral Red [19], 2-amino-3-nitrophenol [20], and rhodamine 575 [21].
Despite their high optical absorption at the visible wavelength, the ionization efficiency with these VIS-MALDI matrices is not as efficient as that with UV-MALDI matrices. Suitable chemical matrix for visible laser has not been found thus far, and the existing UV-MALDI matrices are not accessible by the laser wavelength ranging from 400 nm to ∼ 2.7 μm [22].
Laser Desorption/Ionization with Inorganic Matrix and Nanostructure
Although MALDI is highly sensitive for large biomolecules ( > 700 Da), the detection for analytes of low molecular weight is rather difficult due to the matrix interferences. Thus, in the low mass range, direct laser desorption/ionization (LDI) on surface modified substrates or the use of inorganic matrices becomes alternatives to chemical MALDI. The use of nanoparticles as efficient UV-absorbing matrices was first introduced by Tanaka et al. [6], of which 30 nm cobalt powders were suspended in glycerol solution. A variety of nanomaterials, for example, titanium nitride [22], zinc oxide and titanium oxide [23], and gold nanoparticles [24], had been proposed as inorganic matrices.
Direct LDI on various substrates, for example, graphite [25], silicon, titania sol-gel [26], and metal-coated porous alumina [27], has also been studied. In particular, porous silicon, which has high absorption in the ultraviolet region, has received considerable attention due to its reported high sensitivity [28, 29]. Such method is named as desorption/ionization on silicon (DIOS). Other silicon-based substrates include silicon nanowires [30], column/void silicon network [31], nano-grooves [27], and nano-cavities [32]. Matrix-less IR laser desorption/ionization was also reported on flat silicon surface [33].
Time-of-Flight Mass Spectrometry
Molecular ions can be analyzed by a number of different instruments, for example, time-of-flight mass spectrometer (TOF-MS), magnetic sector mass spectrometer, quadrupole ion trap mass spectrometer (QIT-MS), and Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR-MS) [10, 11]. In particular, the TOF-MS is used almost exclusively for ions produced by laser desorption/ionization. The ions generated upon the irradiation of pulsed laser are separated during their flight along the field-free path and arrive at the detector at different time depending on their m ∕ z. The flight time is given as
where e is the electron charge, L is the flight tube length, and U is the accelerating voltage. Equation 27.1 can be converted to m ∕ z = 2eU(t ∕ L)2 = At 2, where A is the calibration constant which is determined by least squares fitting of this equation to a series of known m ∕ z peak and their measured arrival time. To account for the other effects, say, electronic delay, a second calibration constant, B is usually introduced, and the calibration equation becomes [10]
For TOF-MS, the initial ion velocity produced from the laser plume and the initial position of the generated ions can contribute to the measurement errors and reduce the resolution of the instrument. To certain extent, the initial velocities’ effect can be reduced by using the delayed extraction, in which the accelerating voltage is applied only after certain time interval (typically tens to hundreds ns) after the laser pulse [10]. The initial velocity/energy distribution can also be compensated by using an ion reflector or reflectron. The TOF mass spectrometers used in our experiment are equipped with both reflectron and delayed ion extraction.
Surface Plasmon-Polariton
Surface plasmon-polariton is the collective oscillation of electron density on the metal surface [34]. At surface plasmon-polariton resonance, all the free electrons within the conduction band oscillate in phase and lead to a huge concentration of optical near-field at a small volume (Fig. 27.2). The localized electronic oscillation which is coupled with the optical near-field is usually referred as localized plasmon-polariton.
For the nanoparticles of noble metal, for example, silver and gold, the surface plasmon-polariton resonance takes place at the visible light, and their optical properties can be described by the Mie-extinction, σ ext , and
where σ abs , and σ sca are the absorption and scattering cross section, respectively. Figure 27.3 shows the calculated normalized extinction cross section of the nanoparticles for various noble metals. The particles diameter is assumed to be 40 nm, and the Mie-extinction [35, 36] is solved numerically. For gold, silver, and copper, the nanoparticles have unique absorption bands in the visible region (see Fig. 27.3), and they can be easily identified by the color of their scattered light. The intense red color of the aqueous dispersion of the colloidal gold nanoparticles is the manifestation of the localized surface plasmon-polariton resonance, which peaks at ∼ 520 nm.
For the nanoparticles which are much smaller than the exciting optical wavelength (r ≪ λ, where r is the radius of the nanoparticle and λ is the optical wavelength), the extinction cross section is primarily due to the dipole oscillation, and the Mie theory reduces to dipole approximation [37, 38]:
where \(V = \frac{4} {3}\pi {r}^{3}\), ω is the angular frequency of the exciting light, c is the speed of light, \(\epsilon (\omega ) = \epsilon _{1}(\omega ) + i\epsilon _{2}(\omega )\) is the dielectric function of the nanoparticles, and ε m is dielectric function of the surrounding medium. The resonance condition for surface plasmon-polariton is fulfilled when
if ε2(ω) is small or weakly dependent on ω [37].
The surface plasmon-induced electromagnetic field enhancement on the metallic nanoparticles had been known to be accountable for the surface-enhanced Raman spectroscopy (SERS) [39], and nonlinear optical response such as second harmonic generation [40], and optical frequency mixing [41]. Besides electromagnetic enhancement, the metal-absorbates’ electronic coupling had also been known to have contributed to the chemical enhancement for SERS [42, 43]. Upon absorption on the metal surface, the interaction between the absorbate molecules and the electron gas on the metal surface results in the broadening and shifting in energy of the free molecular states [42]. Thus, even though the states’ transition of the free molecule may be too energetic to be excited by, say, a visible laser, a near resonance could be found for the laser once the molecule is adsorbed on the metal surface.
The exploitation of localized surface plasmon-polariton resonance using ordered nanoparticle arrays and aggregates includes optical near-field lithography [44] and plasmonic waveguides [45]. Functionalized or conjugated gold nanoparticles, which have high binding affinity to specific analytes, are used for biosensing [46] and DNA detection [47]. Selective laser photothermal therapy using nanoparticles had also been proposed for cancer treatment [48].
Plasmon-Induced Desorption
Desorption of Metallic Ions Because the surface plasmon resonance is strongly damped, the local heating which is due to the joule losses on the metal surface could take place. For the gold nanoparticles which have small heat capacity, the heat transfer was estimated to be in the picosecond time scale, and the high lattice temperature can be reached rapidly [38]. However, due to the strong electron oscillation, the plasmon-induced nonthermal desorption had been reported for several metals. By irradiating the roughened silver surface with visible or near-ultraviolet laser, two prominent peaks were observed in the kinetic-energy distribution of Ag+ ions produced from the surface [49].
In another experiment where the surface plasmon was coupled using the attenuated total reflection method, similar results were also obtained for the metal atoms desorbed from the Au, Al, and Ag films [50]. While the lower energy peak was generally referred as thermal peak, they attributed the peak of the higher kinetic energy to the nonthermal electronic process. Nonthermal visible laser desorption of alkali atoms was also reported for sodium particles and sodium film [51, 52]. Theoretical model that involves energy coupling of surface plasmon via ion collision had also been put forward to support the plasmon hypothesis [53].
Desorption of Absorbates On the metal surface, the adsorbed molecules produce physical or chemical bonding via, at least by part, interaction with the electron gas on the metal substrate. At plasmon resonance, due to the collective motion of electron gas, the strong optical near-field enhancement and associated strong modulation of electronic energy levels take place (Fig. 27.4).
This plasmonic process near the metallic surface produces the highly excited plasmonic sideband states due to the nonlinear optical effect. Regardless of the nature of the bonding between molecules and metallic substrate, the plasmonic sideband formation results in the optical near-field excitation of the bonding, and some of the highly excited bonding state can exert stochastic transition into the dissociation states of the absorbed molecules (see Fig. 27.4).
Fabrication of Gold Nanostructure Substrates
Although the gold nanoparticles, for example, gold colloids, have a strong optical extinction at ∼ 520 nm due to the surface plasmon resonance, the colloids aggregate when mixed with certain analytes, and the plasmon frequency redshifts drastically to a longer wavelength. This poses a problem to a non-tunable laser for excitation. When used as the matrix, the chemical contents in the colloids’ buffer solution can also contribute to the background noise. Instead of gold colloids, recently, we demonstrated the use of gold nanostructure for nonorganic matrix-based laser desorption/ionization [54, 55].
Two different substrates were tested in our experiments: gold-coated porous silicon and gold nanorod arrays. The porous silicon with random structure was used as the nanostructured template and was coated with gold using argon ion sputtering. Depending on the type of the silicon, the nanostructure of the porous silicon can be tailored by the etching condition [56, 57].
The vertically aligned gold nanorod arrays, which had more regular surface morphology, were fabricated by electrodeposition of gold into the nanopores of the porous alumina template [58, 59]. The diameter of the gold nanorods follows the pores of the alumina template, and the aspect ratio can be controlled through the deposition time. The porous alumina template with ordered nanopore arrays can be easily fabricated using anodic oxidation. The pore diameter can be tuned from ∼ 10 to 100 nm depending on the electrolyte and the anodization voltage [60, 61]. Compared to the colloids’ aggregates and the electrochemically roughened surface, which are of random structure, the surface morphology of the gold nanorod arrays can be better controlled and fabricated reproducibly.
Fabrication of Gold-Coated Porous Silicon
Owing to their photoluminescence properties, the porous silicon has attracted considerable research interest since their first discovery by Canham [56]. Porous silicon can be fabricated easily using electrochemical etching. Depending on the type of silicon, etching parameters such as etching current, time, etchant concentration, and illumination are reported to affect the pore size and the porosity of the etched silicon [57].
Owing to their high UV absorption, porous silicon has also been used as substrate in direct UV-LDI (DIOS). Encouraged by the success of DIOS, the morphology of the porous silicon with random structure was used as the fabrication template and was coated with gold using argon ion sputtering. However, it is noted that in our experiment, the operating laser wavelength was different from that of DIOS.
Anodic Etching with Hydrofluoric Acid The porous silicon in our experiment was made by anodic etching of 0.02 Ω cm n-type silicon (Nilaco, Japan) using aqueous solution of ∼ 23 wt.% hydrofluoric acid (HF). The etching was conducted at ∼ 5 mA/cm2 for 2 min under white light LED illumination. The etching was performed in a Teflon etching cell with platinum as the counter electrode. A super bright LED produced approximately 5 mW/cm2 front illumination to the etching surface. Schematics in Fig. 27.5 show the electrochemical etching of the silicon using a Teflon etching cell. With illumination, macropores with diameter of 50–100 nm and pore depth of 100–200 nm were formed (microporous: dia. < 2 nm, mesoporous: dia. = 2–50 nm, macroporous: dia. > 50 nm).
Post-etching When the freshly etched porous silicon was coated with gold using argon ion sputtering coater, the pores appeared to be fully covered by the gold and lost its nanostructural identity. Such substrate produced no observable ion signal for most of the analytes for 532 nm laser irradiation. To increase the pore size, the freshly etched porous silicon was further treated with piranha (H2SO4/H2O2 = 1/3) for 4 min, followed by 10 % HF etching. The Piranha treatment oxidized the porous silicon lightly and formed a thin layer of silicon oxide. After stripped by 10 % HF, the pores were enlarged to 100–200 nm.
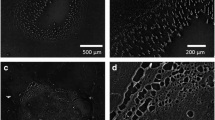
Metallization of Porous Silicon The porous silicon was metalized with gold using argon ion sputtering coater with thickness control. The scanning electron microscopy inspection showed that the coating was not a continuous layer, and gold formed particles on the porous silicon structure with its size about the thickness of the coating. The porous silicon with and without gold coating is shown in Fig. 27.6. The cross-sectional view of the gold-coated porous silicon is depicted in Fig. 27.7. The depths of the irregular pore range are in the range of few hundreds of nm.
The coated surface had also been analyzed using Auger electron spectroscopy to confirm the complete coverage of gold on the porous silicon structure. The specular reflectivity of the gold-coated porous silicon is shown in Fig. 27.8. The measurement was made at normal incidence. The macroporous silicon is an efficient light trap, and it has high optical extinction extended to the visible region. After coated with ∼ 10 nm gold, its reflectivity has increased significantly in the red and near-infrared region. However, the visible region of ∼ 500 nm remained very much unchanged due to the surface plasmon-polariton resonance.
Throughout the experiment, the porous silicon was coated with 10 ∼ 15 nm thick gold. The porous silicon of other different etching conditions had also been examined for their performance in desorption/ionization. Decreasing the etching current and the strength of light illumination reduced the pores’ density, size, and depth, and the ion signals became weaker. Increasing the pore depth by longer etching time and current did not improve the ion yields.
Gold Nanorod Arrays
In order to have better understanding of the desorption/ionization from the metallic nanostructure, it is desirable to have substrates with more regular and better defined surface morphology. Lithography method offers the best control over the nanostructure size, shape, and spacing, but the techniques are expensive and with limited effective area. In comparison, template method is inexpensive and can be used to pattern a large area of surface.
Nanoporous anodic alumina has been a favorable template material or mask for fabricating nanoparticle arrays [62, 63]. With suitable electrolytes and appropriate anodization condition, a high density of ordered pores can be formed easily. The pore diameter can be tuned from ∼ 10 to > 100 nm by varying the anodization condition [60–62, 64]. Usually sulfuric acid is used for fabricating the alumina of pore size 10 ∼ 20 nm, whereas oxalic acid and phosphoric acid are used for bigger pore size. The pore size generally increases with the anodization voltage; however, the self-ordering takes place only under limited voltage condition.
Porous alumina membranes were first used by Martin et al. in the synthesis of gold nanorods [65, 66]. The Au was electrochemically deposited within the pores, and subsequently, the Au nanorods were released and re-dispersed into organic solvent, followed by polymer stabilization. Because the excessive use of organic chemicals and polymers (which are essential to stabilize the nanoparticles) would likely contribute to the background noise in the mass spectrum, a modified approach was adopted in this work.
Fabrication of Nanorod Arrays In our experiment, the embedded Au nanorods were partially released, and the nanorods were held by the template, preventing the aggregation of particle without using stabilizing agent. A schematic describing the fabrication processes is depicted in Fig. 27.9.
Fabrication processes of the gold nanorods substrate. (a) Porous alumina (Al2O3) template fabricated by anodic oxidation using aqueous solution of ∼ 20 wt.% sulfuric acid. (b) Pulsed electrodeposition of gold within the pores of the porous alumina. (c) Partial removal of the alumina template using aqueous solution of 8 % v ∕ v phosphoric acid. (d) The scanning electron micrograph of the porous alumina template embedded with gold nanorods. (e) The nanorods emerged after partial removal of the alumina template using aqueous solution of phosphoric acid
Aluminum sheet or the aluminum film coated on the glass or silicon substrate could be used as the starting material. Sulfuric acid ( ∼ 20 wt.%) was used as the electrolyte for the anodic oxidation of aluminum. Platinum counter electrode was used in the anodic oxidation as well as in the electrodeposition of gold. The aluminum was oxidized at the anodization voltage of ∼ 12 V for 5–10 min to form porous alumina. The pore diameter was in the range of ∼ 15 nm. As shown in Fig. 27.9a, a thin barrier layer was also formed at the bottom of the pores. Although it was possible to remove the barrier layer using etching method, at ∼ 12 V anodization voltage, the barrier was thin enough that the gold could be electrodeposited directly within the pores at moderate voltage. The aqueous solution of 40 mM chlorauric acid (HAuCl4) was used as the working electrolyte. The pulsed electrodeposition was conducted at ∼ 12 V with the duty cycle of 1/10 and pulse repetition rate of 1 s− 1. After several minutes, the deposited surface became ruby red in color, and the grown nanorods were embedded inside the porous alumina as illustrated in Fig. 27.9b.
It is noted that, the quality of the alumina template also depends on its initial surface roughness, and multiple anodizing steps are usually used to produce high-quality alumina template [63]. Occasionally, we oxidize the aluminum at higher voltage (e.g., ∼ 20 V) for a few minutes and gradually reduced anodization voltage to ∼ 12 V. Higher anodization voltage had been known to produce more ordered and bigger nanopores [60, 61, 64]. Beginning the anodization with slightly higher voltage had been found to have more homogeneous deposition of gold onto the template.
Post-etching of the Alumina Template To trap the analyte molecules on the gold surface, the embedded nanorods were partially exposed to the surface (Fig. 27.9c) by chemical etching of the alumina template. The etching was done by using aqueous solution of ∼ 8 % v/v phosphoric acid.
Figure 27.9d, e show the SEM micrographs of the porous alumina embedded with gold nanorods and the appearance of gold nanorods after partial removal of the alumina template, respectively. The diameter of the gold nanorods was ∼ 15 nm, and the average lengths could be fabricated in the range of ∼ 50 to ∼ 200 nm depending on the deposition condition. The SEM image of the fabricated gold nanorods viewed at 45∘ tilt angle is shown in Fig. 27.10.
All the rods were oriented at the same direction with their major (long) axis perpendicular to the surface. Thus, the oscillation direction of the localized plasmon resonance could be selectively excited by the TM- or TE-polarized light. The optical electric field was perpendicular to the substrate surface (i.e., along the major axis of the gold nanorods) when it was TM-polarized and vice versa when it was TE polarized. Unless otherwise stated, the standard substrate used in this study consisted of gold nanorods with length of ∼ 100 nm and diameter of ∼ 15 nm.
Reflectivity of Gold Nanorods Figure 27.11 shows the normalized reflectivity of the gold nanorod substrate measured at normal incidence (light source not polarized). Compared to the porous silicon, the substrate consisted of vertically aligned gold nanorod arrays possesses a more regular surface morphology. Owing to its ordered structure, the reflectivity of the gold nanorod substrate shows a distinct optical absorption at ∼ 520 nm, which coincides spectrally with the surface plasmon resonance of spherical nanoparticles. These visible absorption bands can be excited efficiently by a frequency-doubled Nd:YAG laser at 532 nm.
Figure 27.11b shows the specular reflectivity of the gold nanorod substrate measured using the 532 nm laser with different optical polarization. The length of the nanorods was ∼ 100 nm, and the measurement was taken at 60∘ incidence angle, which was close to our LDI experimental condition. Normalized reflectivity of flat aluminum and gold film is included for comparison. The reflected optical intensity was normalized to that of TE-polarization. Unlike the flat metal in which the reflectivity is minimum for TM-polarized light [67], the ∼ 100 nm gold nanorods have a higher optical absorption for the 532 nm laser at TE polarization due to the transverse plasmon resonance [38, 59].
Experimental Details
Time-of-Flight Mass Spectrometer
The laser desorption/ionization experiment was performed with a 2.5 m time-of-flight mass spectrometer (JEOL 2500) with delayed ion extraction. The instrument can be operated in linear or reflectron mode. A simplified schematic showing the mass spectrometer in reflectron mode is shown in Fig. 27.12. The acceleration voltage for ions was 20 kV. The vacuum pressures in the ion source and the detector were 7. 5 ×10− 5 and 5 ×10− 7 torr, respectively. The primary laser source for the desorption/ionization experiment was a frequency-doubled Nd:YAG laser which was operated at 532 nm wavelength and pulse width of 4 ns. The gold nanostructured substrate was attached to a modified target plate and was irradiated by the laser at 60∘ to the surface normal. Pictures showing the time-of-flight spectrometer and the target plated are depicted in Figs. 27.13 and 27.14, respectively.
The output of the laser was originally at TE-polarization, and a calcite polarizer was added to increase the polarization extinction ratio. The laser polarization on the target was adjusted using a half-wave plate. The laser spot size on the target substrate was about ∼ 200 μm in diameter. Unless otherwise stated, the mass spectra were acquired at optimized laser fluence which was estimated to be in the range of few 10 to ∼ 100 mJ/cm2. For comparison, the frequency-tripled Nd:YAG laser (355 nm wavelength, no further polarized) was also used to investigate the wavelength dependence. Throughout the experiment, the substrate was scanned, and 40–60 laser shots were used to acquire the mass spectra.
Sample Preparation
All chemicals and analytes were obtained commercially and used without further purification. Bovine insulin was prepared in the aqueous solution of 1 % trifluoroacetic acid (TFA). Lys–Lys, Lys–Lys–Lys–Lys–Lys, bradykinin, and melittin were dissolved in water. Lactose was prepared in the aqueous solution of sodium chloride ( ∼ 10 ppm) to promote cationization. The citric buffer was prepared by mixing the aqueous solution of citric acid 10 mM with the diammonium citrate (10 mM) at the ratio of 1/2. Working stocks containing the analyte were prepared in the concentration of 1 ∼ 10 pmol/μl. About 0.2–1 μl of the working stock was pipetted onto the gold nanorod substrate, and the droplet was gently dried using warm air blower. When the droplet was dried, the gold nanorod substrate loaded with the analytes was transferred into the vacuum chamber of the time-of-flight mass spectrometer.
Laser Desorption/Ionization (LDI) with Gold Nanostructure
LDI with Gold-Coated Porous Silicon
Figure 27.15a shows the mass spectrum of 5 pmol bradykinin (1,060 Da) obtained by irradiating the 532 nm visible laser on the analytes deposited on the gold-coated porous silicon. Besides the protonated ions, [M + H]+, the alkali metal ion adducts, [M + Na]+ and [M + K]+, are also observed in the mass spectrum.
On the bare porous silicon (no gold coating), no molecular ion signal was observed at the same or higher laser fluence (Fig. 27.15b). This clearly shows that the gold nanostructure rather than the porous silicon template contributes to the desorption/ionization of the analytes. In an attempt to desorb/ionize the bradykinin from the flat gold surface ( ∼ 50 nm Au film coated on the flat silicon surface) using the 532 nm laser, only very weak signal was obtained at high laser power (Fig. 27.15c). This indicates that the gold nanostructure was essential in assisting the desorption/ionization of analytes.
LDI with Gold Nanorods
Polarization Dependence Due to the random structure of the porous silicon substrate, the optical polarization of the incidence light was not well defined. The optical polarization effect was studied using the gold nanorods. The mass spectra of angiotensin I (1,296 Da) obtained using the gold nanorod substrate with different laser polarization are shown in Fig. 27.16.
Mass spectra of angiotensin I (1,296 Da) obtained from the gold nanorods excited by the laser of different optical polarization. (a) and (b): the optimum optical polarization for short nanorods (length ∼ 50 nm) was TM. (c) and (d): the optimum polarization was reversed to TE for longer nanorods (length ∼ 100 nm)
The laser polarization incidence on the substrate was adjusted using a half-wave plate, without significant change in the incidence laser fluence. The same laser fluence was applied to obtain mass spectra of different laser polarization.
For the shorter nanorods with length < 50 nm, the molecular ion signal became maximum when the laser was TM-polarized and minimum when TE polarized (Fig. 27.16a, b). These short nanorods behave optically as particles on the flat surface, and the dependence of optical absorption on the laser polarization is similar to that of gold thin film. Such optical property could also be influenced by the dielectric alumina template.
As the length of the gold nanorods increases, (e.g., ∼ 100 nm), the optimum polarization at the excitation wavelength of 532 nm was reversed to TE (Fig. 27.16c, d). The reverse of the optimum polarization was due to the excitation of transverse surface plasmon resonance of the gold nanorods. In sum, the obtained mass spectra which are sensitive to the laser polarization agree reasonably with their optical properties. In the following experiments, the gold nanorods were of ∼ 100 nm in length, and the optical polarization was optimized at TE. As for the gold-coated porous silicon, significant polarization dependence was not observed, but the polarization was arbitrarily maintained at TM.
Post-etching of Gold Nanorods Substrate The chemical etching of the alumina template was also a key process in obtaining good mass spectrum of the deposited analyte. The desorption/ionization efficiency was found to be quite dependent on the etching time. Figure 27.17 shows the mass spectra of 5 pmol melittin (2,847 Da) acquired from the gold nanorods prepared with different etching times. In Fig. 27.17, T o denotes the time at which the chemical etching of the alumina template has just reached the gold nanorods. The SEM images of the gold nanorod substrate taken after the LDI experiment are shown in Fig. 27.18. The analyte ion signals increased as the gold nanorods started to emerge from the template. The optimum condition was achieved at about T o + 13 min when ∼ 100 nm nanorods were almost completely released from the template. Further etching of the alumina template caused the nanorods to topple (see Fig. 27.18c), and the ion signal started to diminish. Excessive etching detached some of the nanorods from the alumina template (Fig. 27.18d), and it became difficult to observe ion signals.
The mass spectra of 5 pmol melittin (2,847 Da) acquired from the gold nanorods of different etching time. T o denotes the time at which the chemical etching of the alumina template had just reached the gold nanorods, and no ion was detected before T o . The nanorods started to protrude from the template after T o , and for this substrate, ion signal reached maximum at T o + 13 min. Nanorods started to topple after T o + 19 min, and the ion signal decreased. After T o + 30 min, some nanorods were detached from the substrate, and the melittin ions were difficult to be detected
Wavelength Dependence The frequency-doubled (532 nm) and frequency-tripled (355 nm) output from the Nd:YAG laser were used to study the laser wavelength dependence. The mass spectra of 5 pmol pentalysine (Lys–Lys–Lys–Lys–Lys, 659 Da) acquired from the gold-coated porous silicon and gold nanorods are shown in Fig. 27.19. The visible LDI using 532 nm laser is depicted in Fig. 27.19a, c, and the UV-LDI is shown in Fig. 27.19b, d. The UV laser fluence irradiated on the substrate was estimated to be slightly higher than the visible laser, but accurate comparison was difficult. Instead, the background ions, for example, Au+ ions (Fig. 27.19c, d), were used as the indirect references for comparison. For both substrates, the 532 nm laser gave better ion yields with less background noise (Fig. 27.19a, c). Although ions were also observed with the 355 nm laser, the desorption/ionization was not as efficient, and the background ions appeared to be stronger, probably due to the higher laser threshold fluence. The background ions might be due to the contaminants absorbed from the air or vacuum chamber.
Desorption/Ionization of Low-Molecular-Weight Analytes Because ions could be produced without the addition of chemical matrix, the present method may find application in the analysis of low-molecular-weight analytes, which are rather difficult to handle in the conventional MALDI. Figure 27.20a, b show, respectively, the mass spectra of 5 pmol lactose (342 Da) acquired from the gold nanorods and gold-coated porous silicon by using 532 nm laser. Both substrates produced similar mass spectra. Although gold atoms were consumed during the laser desorption/ionization, no obvious damage was observed on the substrate at the moderate laser fluence, which was the threshold for most of the peptides and small carbohydrates. The gold cluster ions, \(\mathrm{Au}_{n}^{+}\), were easily identified and were used as the references for mass calibration.
The mass spectra for ∼ 6 pmol Lys–Lys (274 Da) acquired from the gold nanorods and stainless steel target plate by using 532 nm are shown in Fig. 27.21. At high laser fluence, Lys–Lys ion signals were also observable from the stainless steel (Fig. 27.21b), but the most intense and homogeneous ion signals were produced from the gold nanorods (Fig. 27.21a). Besides Au+, some background ions ( < 300 m/z) were also present in the low mass region, which were probably due to contaminants and hydrocarbons which were absorbed by the nanorods from the air or vacuum.
Addition of Proton Source Probably due to the lack of acid or proton source on the gold nanostructured substrate, the protonation of peptide and protein was not as efficient as the MALDI method. Besides glycerol (which is the usually used liquid matrix), it had been reported recently that the citric buffer (mixture of citric acid and ammonium citrate) could also work efficiently as the proton donor [26].
The visible laser desorption/ionization of 650 fmol bradykinin and 2 pmol bovine insulin from the gold nanorods with the addition of citric buffer during the sample preparation is shown in Fig. 27.22a, b, respectively. The molar ratio of analyte to citric acid was ∼ 1/350. The citric buffer significantly improved the analytes’ ion yields and the resolution of the mass spectra. The ions contributed by the citric buffer were also observed in the mass spectra. The peaks at m/z 215 and 231 (in Fig. 27.22a) correspond to the sodium and potassium ion adducts of the citric acid ([M + Na]+ and [M + K]+). The yet determined background peak at m/z 183 was probably due to the contaminant absorbed on the gold nanorods.
For the two-phase matrix, the nano-powders have to be suspended in the liquid glycerol to prevent agglomeration, and the ion signals deteriorate after complete evaporation of glycerol in the vacuum [22]. In contrast, the nanostructures allow the use of various solid proton sources. Besides citric acid, several compounds with suitable hydroxyl groups, for example, tartaric acid, xylitol, and mannitol, were also found to be useful in analytes ionization.
Surface-Enhanced Raman Spectroscopy (SERS) from Gold Nanorods In a separate experiment, we demonstrated that the gold nanorods used in our experiments were also SERS-active. The Raman experiment was conducted using Jasco NRS-2100 micro-Raman monochromator spectrometer. The analyte, rhodamine 6G, was dissolved in water, and a continuous-wave 488 nm argon laser was used for excitation. The operating laser power was about 0.35 mW, and the exposure time was 10 s. The Raman spectroscopy of 5 pmol rhodamine 6G (R6G) deposited on the gold nanorods is shown in Fig. 27.23a. For comparison, the signal obtained from the aluminum substrate under the same experimental condition is shown in Fig. 27.23b, and the respective mass spectra acquired by using 532 nm laser are shown in Fig. 27.23c, d.
Raman signals of 5 pmol rhodamine 6G (471 Da) obtained from (a) the gold nanorods and (b) aluminum substrate. The excitation wavelength was 488 nm. The prominent peaks are at 1,365, 1,510, 1,575, and 16,550 cm− 1. (c) and (d) are the respective mass spectra acquired by using 532 nm laser. The R6G ions appeared as (M–Cl)+
Gold Nanoparticle-Assisted Excitation of UV-Absorbing MALDI Matrixby Visible Laser
In this section, we demonstrate the excitation of UV-absorbing MALDI matrix and the desorption/ionization of the doped biomolecular analytes by using a frequency-doubled Nd:YAG laser (532 nm), assisted by gold nanoparticles [68].
In UV-MALDI, the photoionization routes to produce radical ions include direct two-photon ionization [13] and exciton pooling of the excited matrix molecules [14]. The ionization potential (IP) for DHB is 8 eV, and the energy for first excited state is 3.466 eV [69]. Because the IP is still higher for the two-photon energy from the typical UV laser (e.g., nitrogen laser), the photo/thermal hypothesis was proposed [70], of which the energy deficit is made up by the thermal energy. For the exciton pooling, it is readily accessible by one UV photon, and two or more excited matrix molecules pool their energies to form radical ions or higher excited states of which the transfer of proton to the target analytes takes place.
In our experiment, Au thin film ( ∼ 10 nm) was first coated on the matrix surface, and the Au nanoparticles were prepared and deposited directly on matrix surface by ablating the Au film in the vacuum. Coating the bio-sample with Au thin film is common in the secondary ion mass spectrometry (SIMS) [71], and recently, metallic coating has also been reported to improve the UV-MALDI imaging [72]. However, in our experiment, the bulk Au film on the laser spot will be ablated, and the remaining Au nanoparticles were used to excite the matrix molecule using visible laser, of which the photon energy is insufficient for the free molecules.
Laser Ablation of Au Film The experiment began by ablating the Au film which was pre-coated on the MALDI matrix to form nanoparticles (Fig. 27.24). The laser fluence was adjusted to the ablation threshold, and the Au cluster ions were monitored directly from the mass spectrum. As the Au thin film was of ∼ 10 nm, the laser threshold fluence for ablation was much lower compared to that of bulk Au. The laser spot size on the target was about ∼ 0.2 mm in diameter, and the operating laser fluence was estimated to be 50 ∼ 100 mJ/cm2.
After few laser shots, the Au ions were significantly reduced, and the mass spectra were acquired directly or with slight adjustment of the laser fluence. Unless otherwise stated, the mass spectra for matrix and analytes were usually acquired after 5 ∼ 10 initial laser shots and from the accumulation of 30–100 single-shot mass spectra on the same laser spot. Each mass spectrum in the following section represents the ions produced from a fixed position, that is, from the same crater/hole formed by the laser ablation.
Figure 27.25a shows the scanning electron microscope (SEM) image of the Au thin film ablated by the Nd:YAG laser after several laser shots. The target was irradiated at ∼ 60∘ incident angle, and the ablated crater was of an elliptical shape with a major axis of ∼ 200 μm. A close-up inspection on the ablated region showed the formation of gold nanoparticles with size ranging from < 10 to 50 nm (Fig. 27.25b).
Several processes could be conceivable for the formation of the gold nanoparticles on the sample surface, for example, the melting of the Au film, and the redeposition of the ablated Au clusters back onto the surface. Some nanoparticles could also be embedded into the matrix after ablation due to migration. Considerable heating was also expected in the initial ablation laser shots.
Radical Matrix Ions Produced by Visible Laser The typical first laser shot mass spectrum is shown in Fig. 27.26a. The first laser shot ablated most of the Au atoms, and the mass spectrum was dominated by intense Au cluster ions. Because the Au film was deposited on the matrix surface, the laser ablation Au film was less likely to assist the desorption of the underneath matrix. Only very weak or no matrix ion signal was observed at first few laser shots. Figure 27.26b shows the Au nanoparticles assisted visible-MALDI mass spectrum of the super-DHB (2,5-dihydroxybenzoic acid [DHB, 154 Da] and 2-hydroxy-5-methoxybenzoic acid [HMB, 168 Da]).
The desorbed ions consist of DHB+ ∙, [DHB+H]+, [DHB+Na]+, [DHB–OH]+, HMB+ ∙, and [HMB+H]+, which are the typical matrix-related ions observed in UV-MALDI. Gold ions Au+ also appeared in the mass spectrum but with relatively lower abundance. Irradiating the matrix without Au coating produces no observable matrix ions (see Fig. 27.26c).
The mass spectra of analytes obtained by the visible laser-excited MALDI matrix are shown in Figs. 27.27 and 27.28. Figure 27.27a–d show the single-shot mass spectra that correspond to the successive laser shots. The mass spectra are dominated by the Au cluster ions during first few laser shots, and no analyte ion is observed (Fig. 27.27a, b). The m/z for [M+H]+ is denoted as * in the mass spectra. The analyte and the matrix-related ions became apparent after the 5th laser shot and lasted for more than 50 laser shots from a single spot. Since the initial ablation laser shots did not produce any analyte ions except the huge abundance of Au clusters, “these initial ions” will be excluded during the analysis.
Materials and Film Thickness Dependence Figure 27.28a shows the mass spectrum of the bradykinin (1,060 Da) acquired by accumulation of 26 single-shot spectra from the same laser spot. Protonated bradykinin ion, [M+H]+, was observed as the base peak with some contribution from metal ion adducts ([M+Na]+ and [M+Au]+). [DHB+Au]+ was, however, not observed.
Comparison was also made with the platinum and silver nanoparticles prepared with the same laser ablation method. Visible-MALDI mass spectra of bradykinin assisted by platinum and silver nanoparticles are shown, respectively, in Fig. 27.28b, c. Although analyte ion signal was also observed from platinum nanoparticles, the ion intensity was not as strong as that of Au nanoparticles at the same laser fluence. In the case of silver, the mass spectrum was dominated by the silver ion adducts of the matrix, [DHB+Ag]+, and the protonation of analyte was largely suppressed. The abundance of [M+Ag]+ ion appeared to be higher than [M+H]+.
The matrix-assisted visible laser desorption/ionization of angiotensin I from the Au coatings of different initial thickness is shown in Fig. 27.29. The initial Au film thickness was found to affect the optimization of laser fluence for the film ablation and the generation of analyte ions. The optimum initial thickness was in the range of 5 ∼ 10 nm (see Fig. 27.29a, b), and for thicker coating ( 25 nm), higher laser fluence was needed to produce the equivalent ablation effect as well as for the desorption/ionization of analytes. As shown in Fig. 27.29c, the ion intensity was also reduced probably due to larger average size of the nanoparticles formed from the thicker Au film.
Thermometer Molecule and Wavelength Dependence The thermal property of the Au-assisted visible laser MALDI was examined using 4-chlorobenzyl pyridinium chloride (4CBP) as the thermometer molecule. This thermometer molecule was synthesized according to the literature [73]. The pyridine containing benzyl chloride was heated at 60 ∘C for 5 ∼ 6 h, and the white solid salts were collected after the pyridine was evaporated. The pyridinium salts were used without further purification.
The survival yield of the thermometer molecule, S, reflects the internal energy gained from the neighboring matrix molecules either in the solid phase or gas phase [74] and is given as
where I(M+) and I(F+) are the abundances of molecular ion, M+, and the fragment ion, F+ of 4CBP, respectively. If comparison, the measurement was conducted with three different laser wavelengths (355, 532, and 1,064 nm), and the thermometer molecule was mixed with the matrix (super-DHB) at a molar ratio of ∼ 500. For the case of visible (532 nm) and IR (1,064 nm) lasers, the mixture was coated with ∼ 10 nm Au, and after 5–10 laser ablation shots, the laser fluence was slightly reduced to acquire the mass spectra. For UV-MALDI, the same sample (with Au coating) was irradiated with the UV laser (355 nm) at optimum laser fluence, without laser ablation.
The mass spectra and the survival yield of 4CBP acquired using UV, visible, and IR lasers are shown in Fig. 27.30a–c, respectively. The highest survival yield for 4CBP was obtained with UV-MALDI as the UV laser excited the matrix electronically (Fig. 27.30a, survival yield ∼ 0.9). For the Au nanoparticle-assisted MALDI using 532 nm visible laser (Fig. 27.30b), the survival yield of 4CBP exceeded 0.8 at the typical threshold laser fluence but is lower than that of the UV irradiation, indicating some heating effect produced by the gold nanoparticles. In Fig. 27.30c, the mass spectrum was obtained with the IR laser of 1,064 nm wavelength which is away from the surface plasmon resonance and is less energetic to induce sufficient electronic excitation. Although the matrix radical ions were also observed, it was of much lower abundance and of poor shot-to-shot repeatability. The low survival yield ( ∼ 0.65) of 4CBP also indicates considerable heating when the IR laser was used. In sum, the results suggest that the excitation of matrix molecules with the presence of gold nanoparticles is rather wavelength dependent, and the heating effect produced by the nanoparticles could not solely contribute to the observed ions.
SERS with Au Nanoparticles Formed by Laser Ablation In a separate experiment, we demonstrated that the Au nanoparticles prepared by the laser ablation method were also SERS-active by performing the Raman spectroscopy on rhodamine 6G. About 0.5 μL of rhodamine 6G ( ∼ 1 mM) was deposited on the glass substrate, and after being dried, the sample was coated with 10 nm Au using sputtering deposition. The Au-coated rhodamine 6G was irradiated by one ablation laser shot with 532 nm laser in vacuum. The Raman spectroscopy was conducted using micro-Raman monochromator spectrometer (NRS-2100, Jasco, Japan), and the excitation source was a continuous-wave 488 nm argon laser. The operating laser power was about 3 mW, and the exposure time was 10 s.
The Raman spectrum of Au-coated rhodamine 6G after laser ablation is shown in Fig. 27.31a. The prominent peaks are at 1,195, 1,365, 1,539, and 1,651 cm− 1. Raman spectra obtained from those without laser ablation and without Au coating are shown, respectively, in Fig. 27.31b, c. Although SERS signal was also observed from the un-ablated Au coating (Fig. 27.31b), as the Au film may form nanostructure on the surface rather than a homogeneous layer, the intensity is much weaker compared to that in Fig. 27.31a where Au nanoparticles were formed by the laser ablation.
Ionization Mechanism Regarding the ionization mechanism for DHB, it is generally believed that in the case of UV excitation (e.g., using nitrogen laser or frequency-tripled Nd:YAG laser), the main processes involve multiphotons photo-ionization, and/or the exciton pooling, in which two or more excited matrix molecules pool their energies to form radical ions [14]. For DHB, the ionization potential (IP) is ∼ 8 eV, and the energy for first excited state is 3.466 eV [69]. For the visible laser (532 nm wavelength, 2.33 eV) employed in our experiment, at least three or more photons are needed for multiphoton ionization and two photons for excitation. Although the presence of Au nanoparticles increases the thermal heating of the matrix molecules, which could lead to the phase evaporation and molecular desorption, it remains questionable if the thermal process is sufficient for the excitation and ionization of the matrix molecules.
As the gold nanoparticles were also SERS-active, the chemical and electromagnetic field enhancements, which are the two prominent mechanisms for SERS, may also play considerable roles in the excitation of the UV-absorbing DHB matrix by the visible laser. For example, as the gold nanoparticles couple the photon to surface plasmon efficiently, the induced electromagnetic field enhancement could promote the nonlinear optical processes for the multiple photon excitation and photoionization of the matrix molecules. The chemical enhancement, of which the optical absorption band was broadened or red-shifted due to the interaction between absorbate molecules and the conduction electrons on the metal surface, also allows the excitation of matrix molecules by the visible laser. Besides nanoparticles, the inhomogeneous Au film remnants, which were in the form of nanostructure left on the ablated crater, could also contribute to similar assistant effects.
Discussion and Future Prospect
For the silicon based-substrate, the macroporous structure or grooves of ∼ 100 nm (but not smaller) are required to produce good ion signal [27, 29]. Since the nanorods, which is ∼ 15 nm in diameter, work equally well with the gold-coated porous silicon, the macroporous feature is not essentially required in the case of gold. Due to the inert nature of gold, the nanorod substrate can be kept in the atmospheric environment and reused after proper cleaning, without significant deterioration in performance.
Regarding the desorption/ionization mechanism, because the gold porous silicon and nanorods essentially resemble the finely divided particles, it may function as the two-phase matrix (inorganic particles in liquid suspension), in which the rapid heating of the substrate and the resultant peak surface temperature leads to the desorption/ionization of the residual solvent molecules that may subsequently assist the formation of analyte ions [75, 76]. The observed alkali ion adducts in some of the spectra also suggest that the ions were formed by the gas-phase cationization which are likely thermal driven.
The sensitivity of the LDI was also found to be enhanced with the addition of proton source. Besides citric acid and glycerol (which have been frequently used in MALDI), we found that certain sugar alcohol (e.g., xylitol and mannitol) and tartaric acid are also useful as proton donors. In the case where proton source was added, they were excited by the nanostructure as their optical absorption is almost negligible in the near-UV and visible region, and the ionization of analytes took place probably in the gas phase. Regarding the desorption/ionization mechanism, it is usually assumed that the heating is the only process induced by the nanoparticles or nanostructure upon photon absorption. However, photo heating and the peak temperature might not be the exclusive mechanism for ion desorption, and localized effect, such as enhanced electric field had also been proposed [76]. As seen from the laser wavelength and polarization dependence, the optimum condition of the desorption/ionization was achieved when the surface plasmon resonance is excited. Certain degree of optical-field enhancement is also expected at the plasmon resonance as we had obtained the surface-enhanced Raman spectrum of rhodamine 6G from the gold nanorods. However, it remains uncertain whether this is merely due to the plasmon-enhanced optical absorption.
In the visible-MALDI experiment, we showed that with the presence of Au nanoparticles, the UV-MALDI matrix could also be photoionized by the 532 nm laser even though the photon energy is insufficient for free molecules. Since the 532 nm laser (2.33 eV) was employed in our experiment, at least three or more photons are needed for direct photo/thermal multiphoton process and two photons for excitation. Although the presence of Au nanoparticles increases the thermal heating of the matrix molecules, which could lead to the phase evaporation and molecular desorption, it remains questionable if the thermal process is sufficient for the excitation and ionization of the matrix molecules. Thus, in addition to heating effect, a combination of several processes could contribute to the observed ions. As gold nanoparticles couple the photon to surface plasmon efficiently, the induced electromagnetic field enhancement, which promotes nonlinear optical processes, introduces the possibility of two or multiple photon excitation of the matrix molecules. The chemical enhancement in the SERS, of which the optical absorption band was broadened or red-shifted due to the interaction between absorbates and conduction electrons on the metal surface, also allows the excitation of matrix molecules by the visible laser.
Before ending this chapter, here are some updates on the works done by other researchers in this interesting field. By tuning the laser wavelength to close to the surface plasmon peak, the strong optical absorption has allowed Spencer and co-worker to detect as little as 50 attomole of peptide from one single aerosol that contains ∼ 2,000 gold nanoparticles of 5 nm diameter [77]. Also with gold nanoparticles as laser absorbing matrix, Shibamoto et al. have detected ultra trace amount of sample molecules, which is less than several hundred zeptomoles with laser desorption/ionization [78]. Using gold nanorods, laser desorption/ionization has also been performed with near-infrared laser (wavelength = 1,064 nm) that excites the longitudinal surface plasmon resonance (LSPR) modeof the gold nanorods [79, 80].
Finally, it is noted that the optical near-field enhancement is only active in the vicinity of the nanostructure and is characterized by the penetration depth corresponding to the size of the nanoparticles [81]; thus, a metallic tip sharpened to sub-wavelength radius may be used as a scanning nano-probe to desorb the biomolecules with nanometer resolution. The Au nanoparticles may also find application in selective ionization, of which only the specific sides of the bio-sample that are bound to the particles are ionized by the laser.
References
F.H. Field, J.L. Franklin, Electron Impact Phenomena and the Properties of Gaseous Ions, 1st edn. (Academic, New York, 1957)
R. Gomer, M.G. Inghram, J. Am. Chem. Soc. 77, 500, (1955)
H.D. Beckey, Int. J. Mass Spectrom. 2, 500, (1969)
M.S.B. Munson, F.H. Field, J. Am. Chem. Soc. 88, 2621 (1966)
J.B. Fenn, M. Mann, C.K. Meng, S.F. Wong, C.M. Whitehouse, Science 246, 64 (1989)
K. Tanaka, H. Waki, Y. Ido, S. Akita, Y. Yoshida, T. Yoshida, T. Matsuo, Rapid Commun. Mass Spectrom. 2, 151 (1988)
M. Karas, F. Hillenkamp, Anal. Chem. 60, 2299 (1988)
E. Hutter, J.H. Fendler, Adv. Mater. 16, 1685 (2004)
P. Englebienne, A.V. Hoonacker, M. Verhas, Spectroscopy 17, 255 (2003)
F. Hillenkamp, J. Peter-Katalinić, MALDI MS (Wiley-VCH, Weinheim, 2007)
J.H. Gross, Mass Spectrometry (Springer, Berlin, 2004)
T.J.P. Naven, D.J. Harvey, Rapid Commun. Mass Spectrom. 10, 829 (1996)
H. Ehring, M. Karas, F. Hillenkamp, Org. Mass Spectrom. 27, 472 (1992)
R. Zenobi, R. Knochenmuss, Mass Spectrom. Rev. 17, 337 (1998)
X. Chen, James A. Carroll, R.C. Beavis, J. Am. Chem. Soc. 9, 885 (1998)
K. Dreisewerd, Chem. Rev. 103, 395 (2003)
K. Tang, S.L. Allman, R.B. Jones, C.H. Chen, Org. Mass Spectrom. 27, 1389 (1992)
D.S. Cornett, M.A. Duncan, I.J. Amster, Anal. Chem. 65, 2608 (1993)
C.J. Smith, S.Y. Chang, E.S. Yeung, J. Mass Spectrom. 30, 1765 (1995)
L.C. Chen, D. Asakawa, H. Hori, K. Hiraoka, Rapid Commun. Mass Spectrom. 21, 4129 (2007)
C. Yang, X. Hu, A.V. Loboda, R.H. Lipson, J. Am. Soc. Mass Spectrom. 21, 294 (2010)
M. Schürenberg, K. Dreisewerd, F. Hillenkamp, Anal. Chem. 71, 221 (1999)
T. Kinumi, T, Saisu, M. Takayama, H. Niwa, J. Mass Spectrom. 35, 417 (2000)
J.A. McLean, K.A. Stumpo, D.H. Russell, J. Am. Chem. Soc. 127, 5304 (2005)
J. Sunner, E. Dratz, Y.C. Chen, Anal. Chem. 67, 4335 (1995)
C.-T. Chen, Y.-C. Chen, Rapid Commun. Mass Spectrom. 18, 1956 (2004)
S. Okuno, R. Arakawa, K. Okamoto, Y. Matsui, S. Seki, T. Kozawa, S. Tagawa, Y. Wada, Anal. Chem. 77, 5364 (2005)
J. Wei, J.M. Buriak, G. Siuzdak, Nature 399, 243 (1999)
Z. Shen, J.J. Thomas, C. Averbuj, K.M. Broo, M. Engelhard, J.E. Crowell, M.G. Finn, G. Siuzdak, Anal. Chem. 73, 612 (2001)
E.P. Go, J.V. Apon, G. Luo, A. Saghatelian, R.H. Daniels, V. Sahi, R. Dubrow, B.F. Cravatt, A. Vertes, G. Siuzdak, Anal. Chem. 77, 1641 (2005)
J.D. Cuiffi, D.J. Hayes, S.J. Fonash, K.N. Brown, A.D. Jones, Anal. Chem. 73, 1292 (2001)
N.H. Finkel, B.G. Prevo, O.D. Velev, L. He, Anal. Chem. 77, 1088 (2005)
S.H. Bhattacharya, T.J. Raiford, K.K. Murray, Anal. Chem. 74, 2228 (2002)
R.H. Ritchie, Surf. Sci. 34, 1 (1973)
H.C. van de Hulst, Light Scattering by Small Particles (Dover, New York, 1981)
H. Du, Appl. Opt. 43, 1951, (2004)
U. Kreibig, M. Vollmer, Optical Properties of Metal Clusters (Springer, Berlin, 1995)
S. Link, M.A. El-Sayed, Int. Rev. Phys. Chem. 19, 409 (2000)
M. Moskovits, Rev. Mod. Phys. 57, 783 (1985)
R. Antoine, M. Pellarin, B. Palpant, M. Broyer, B. Prével, P. Galletto, P.F. Brevet, H.H. Girault, J. Appl. Phys. 84, 4532 (1998)
M. Danckwerts, L. Novotny, Phys. Rev. Lett. 98, 026104 (2007)
K. Arya, R. Zeyher, Phys. Rev. B 24, 1852 (1981)
A. Campion, J.E. Ivanecky III, C.M. Child, M. Foster, J. Am. Chem. Soc. 117, 11807 (1995)
P.G. Kik, S.A. Maier, H.A. Atwater, Mater. Res. Soc. Symp. Proc. 705, Y3.6 (2001)
S.A. Maier, P.G. Kik, H.A. Atwater, S. Meltzer, E. Harel, B.E. Koel, A.A.G. Requicha, Nat. Mater. 2, 229 (2003)
D.J. Maxwell, J.R. Taylor, S. Nie, J. Am. Chem. Soc. 124, 9606 (2002)
Y.C. Cao, R. Jin, C.A. Mirkin, Science 297, 1536 (2002)
I. El-Sayed, X. Huang, M. El-Sayed, Cancer Lett. 239, 129 (2006)
M.J. Shea, R.N. Compton, Phys. Rev. B 47, 9967 (1993)
I. Lee, T.A. Callcott, E.T. Arakawa, Phys. Rev. B 47, 6661 (1993)
W. Hoheisel, K. Jungmann, M. Vollmer, R. Weidenauer, F. Träger, Phy. Rev. Lett. 60, 1649 (1988)
J. Brewer, H.G. Rubahn, Chem. Phys. 303, 1 (2004)
R.H. Ritchie, J.R. Manson, P.M. Echenique, Phys. Rev. B 49, 2963 (1994)
L.C. Chen, T. Ueda, M. Sagisaka, H. Hori, K. Hiraoka, J. Phys. Chem. C 111, 2409 (2007)
L.C. Chen, J. Yonehama, T. Ueda, H. Hori, K. Hiraoka, J. Mass Spectrom. 42, 346 (2007)
L.T. Canham, Appl. Phys. Lett. 57, 1046 (1990)
O. Bisi, S. Ossicini, L. Pavesi, Surf. Sci. Rep. 38, 1 (2000)
M.S. Sander, L.-S. Tan, Adv. Funct. Mater. 13, 393 (2003)
J. Pérez-Juste, I. Pastoriza-Santos, L.M. Liz-Marzán, P. Mulvaney, Coord. Chem. Rev. 249, 1870 (2005)
H. Masuda, K. Yada, A. Osaka, Jpn. J. Appl. Phys. 37, L1340 (1998)
O. Jessensky, F. Müller, U. Gösele, Appl. Phys. Lett. 72, 1173 (1998)
H. Masuda, K. Fukuda, Science 268, 1466 (1995)
H. Masuda, M. Satoh, Jpn. J. Appl. Phys. 35, L126 (1996)
S. Ono, N. Masuko, Surf. Coat. Technol. 169, 139 (2003)
C.A. Foss Jr., G.L. Hornyak, J.A. Stockert, C.R. Martin, J. Phys. Chem. 96, 7497 (1992)
C.R. Martin, Science 266, 1961 (1994)
M. Born, E. Wolf, Principles of Optics, 2nd edn. (Pergamon, Oxford, 1964)
L.C. Chen, K. Mori, H. Hori, K. Hiraoka, Int. J. Mass Spectrom. 279, 41 (2009)
V. Karbach, R. Knochenmuss, Rapid Commun. Mass Spectrom. 12, 968 (1998)
D.A. Allwood, P.E. Dyer, R.W. Dreyfus, Rapid Commun. Mass Spectrom. 11, 499 (1997)
A. Delcorte, N. Medard, P. Bertrand, Anal. Chem. 74, 4955 (2002)
G. McCombie, R. Knochenmuss, J. Am. Soc. Mass. Spectrom. 17, 737 (2006)
J. Naban-Maillet, D. Lesage, A. Bossée, Y. Gimbert, J. Sztáray, K. Vékey, J.-C. Tabet, J. Mass. Spectrom. 40, 1 (2005)
G. Luo, I. Marginean, A. Vertes, Anal. Chem. 74, 6185 (2002)
S. Zumbuhl, R. Knochenmuss, S. Wulfert, F. Dubois, M.J. Dale, R. Zenobi, Anal. Chem. 70, 707 (1998)
S. Alimpiev, S. Nikoforov, V. Karavanskii, T. Minton, J. Sunner, J. Chem. Phys. 115, 1891 (2001)
M.T. Spencer, H. Furutani, S.J. Oldenburg, T.K. Darlington, K.A. Prather, J. Phys. Chem. C 112, 4083 (2008)
K. Shibamoto, K. Sakata, K. Nagoshi, T. Korenaga, J. Phys. Chem. C 113, 17774 (2009)
E.T. Castellana, R.C. Gamez, M.E. Gómez, D.H. Russell, Langmuir 26, 6066 (2010)
F. Gámez, P. Hurtado, P.M. Castillo, C. Caro, A.R. Hortal, P. Zaderenko, B. Martínez-Haya, Plasmonics 5, 125 (2010)
M. Ohtsu, H. Hori, Near-Field Nano-Optics (Kluwer/Plenum Publishers, New York, 1999)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Chen, L.C., Hori, H., Hiraoka, K. (2013). Laser Desorption and Ionization Mass Spectrometry Using PlasmonicSubstrates. In: Ohtsu, M. (eds) Handbook of Nano-Optics and Nanophotonics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-31066-9_28
Download citation
DOI: https://doi.org/10.1007/978-3-642-31066-9_28
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-31065-2
Online ISBN: 978-3-642-31066-9
eBook Packages: Physics and AstronomyReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics