Abstract
This subchapter provides a comprehensive analysis of the facial morphology and growth in a large consecutive sample of Danish children born with nonsyndromic cleft lip and palate based on three-projection infant cephalometry. The following subgroups of clefts are dealt with isolated cleft lip (CL), isolated cleft palate (CP), Robin sequence (RS), unilateral complete cleft lip and palate (UCCLP), and bilateral complete cleft lip and palate (BCCLP). Infants with cleft of the secondary palate, with or without cleft of the primary palate, share a number of characteristic morphological traits when compared to the norm: decreased posterior length of the maxilla, maxillary retrognathia, decreased posterior height of the maxilla, increased width of the maxilla and the nasal cavity, decreased length of the mandible, mandibular retrognathia, and reduced size of the pharyngeal airway. The amount of facial growth in infants with cleft of the secondary palate is close to the norm, but the direction of growth is, in general, more vertical than normal. We have observed that surgery to the lip and anterior part of the hard palate at 2 months of age in UCCLP children influences the development of the maxillary complex, as observed at 22 months of age, in a number of beneficial ways: the premaxilla is no longer relatively protruding, and it is less asymmetric; the nasal septum deviates less toward the noncleft side; the width of the nasal cavity and the posterior part of the maxilla becomes relatively more normal; and the transverse position of the lateral maxillary segment on the noncleft side is closer to normal. However, the posterior height of the maxilla is still reduced to the same degree, the mandible is still short to the same degree, and bimaxillary retrognathia is still present. We are suggesting that subjects with cleft of the secondary palate have a special “intrinsic” facial type, primarily characterized by bimaxillary retrognathia and increased maxillary width, and we are speculating that this facial type could be a “liability factor,” increasing the probability of CP and CLP.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Congenital clefts of the lip and/or palate can arise in isolation or together with other malformations (syndromes) (Gorlin et al. 2001). This chapter deals solely with “nonsyndromic” clefts. Both individuals with unoperated and operated clefts have a face which differs from those of unaffected individuals. Since the introduction of roentgencephalometry more than 70 years ago (Broadbent 1931), hundreds of cephalometric studies, including both unoperated and operated cleft individuals, have suggested that some deviations are directly caused by the primary anomaly, while others are caused by the surgical interventions and the following dysplastic and compensatory growth of the facial bones (e.g., Graber 1949, 1954; Slaughter and Brodie 1949; Ortiz-Monasterio et al. 1959, 1966; Dahl 1970; Pruzansky 1971; Bishara and Olin 1972; Friede and Pruzansky 1972a, b; Bishara 1973; Friede and Johanson 1974; Bishara et al. 1976, 1985, 1986; Friede and Morgan 1976; Friede 1977, 1978, 1998; Friede et al. 1986; Smahel et al. 1987; Ehmann 1989; Mars and Houston 1990; da Silva Filho et al. 1992b, 1998; Capelozza et al. 1993, 1996; Tomanova and Müllerova 1994; Berkowitz 1995; Dahl and Kreiborg 1995; Semb and Shaw 1996; Sandham and Foong 1997; Friede and Enemark 2001). However, the relative importance of the intrinsic factors, the iatrogenic factors, and the functional or adaptive factors for the facial development is still unclear. There are probably several reasons for this. Firstly, comprehensive knowledge of craniofacial morphogenesis in cleft newborns or infants before surgery, based on large, consecutive, well-controlled samples, is very scarce. This situation is not surprising since, in developed countries, the cleft of the lip is surgically treated within the first couple of months after birth. Thus, the possible period of examining the unoperated state is short, and several methodological problems are involved. Secondly, the cephalometric analyses are most often limited to the lateral projection using simplistic cephalometric analyses, typically based on 15–20 reference points and almost invariably measuring maxillary prognathism as the S-N-A angle or similar measurements to the premaxilla, and the use of infant cephalometry has been very limited. These authors are of the opinion that incomplete knowledge about the intrinsic factors related to the cleft anomaly has automatically lead to excessive emphasis on the importance of iatrogenic and adaptive factors in facial development of cleft children.
2 The Danish Experience
In the middle of the 1970s, we decided to take advantage of the very favorable sampling conditions in Denmark in an effort to contribute to the question of the characteristics of facial growth and development in children born with clefts (Jensen et al. 1988). In Denmark, for more than 65 years, all newborns with facial clefts have been recorded at the Institutes for Speech Disorders in Copenhagen and Århus. Repeated follow-up examinations have shown that the registration of clefts in Denmark is highly reliable and nearly complete. The population is homogeneous and stable, and only very few children are lost to follow-up. Furthermore, all primary cleft surgery is performed in one hospital by one surgeon.
Inspired by Pruzansky and Lis (1958), we constructed a three-projection infant cephalometer, which can obtain truly orthogonal lateral, frontal, and axial cephalograms (Kreiborg et al. 1977). A comprehensive cephalometric analysis system was developed including all craniofacial regions (calvaria, cranial base, orbits, maxilla, mandible, airway, cervical spine, and soft tissue profile) (Kreiborg 1981; Heller et al. 1995; Hermann et al. 2001a), and the method was validated (Hermann et al. 2001a). Furthermore, new methods of visualization of differences in craniofacial morphology and growth between different groups were developed using mean plots (Kreiborg 1981; Hermann et al. 2001a), color-coded vector plots (Hermann et al. 2001a), and color-coded surfaces on a 3D CT-model (Darvann et al. 1999).
During the 6 years from 1976 to 1981, there were 359,027 live births in Denmark. A total of 678 newborns of Northern European ancestry with cleft lip, cleft palate, or both were registered in the period. Twenty-four infants died before 22 months of age, and for practical reasons, material uptake had to be omitted in some patients with isolated cleft palate. Only nonsyndromic clefts were included in the study, but 602 of the 678 children (about 90 %) were examined by us (Jensen et al. 1988) and nearly all at both 2 months of age (before any surgical or orthopedic treatment) and at 22 months of age (before closure of the posterior palate in the children with clefts of the secondary palate). All children were treated by the same surgeon (Dr. Poul Fogh-Andersen), and in the children with cleft of the primary palate, the cleft lip was, in all cases, closed using a Tennison procedure. One-third of the children had isolated cleft lip (CL), about 40 % had combined cleft lip and palate (CLP), and about 27 % had isolated cleft palate (CP). The clefts were subclassified according to the method of Jensen et al. (1988).
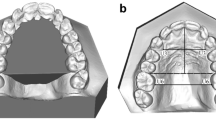
In the 602 children included in the study, cephalograms were obtained in the lateral, frontal, and axial projections by three experienced orthodontists (Dr. Birgit Leth Jensen, Dr. Erik Dahl, and Dr. Sven Kreiborg). In addition, impressions were made of the maxilla, and anthropometric registrations (body height, body length, and head circumference) were carried out. The results of the cephalometric analyses have been presented in a number of publications (Dahl et al. 1982, 1989; Kreiborg et al. 1985; Kreiborg and Cohen 1996; Darvann et al. 2001; Hermann et al. 1999a, b, 2000, 2001a, b, 2002, 2003a, b, 2004; Kreiborg and Hermann 2002). So far, we have analyzed infant craniofacial morphology and early craniofacial growth in detail in three dimensions in the following groups: unilateral incomplete cleft lip (UICL), isolated cleft palate (ICP), Robin sequence (RS), unilateral complete cleft lip and palate (UCCLP) (Fig. 9.1a), and bilateral complete cleft lip and palate (BCCLP) (Fig. 9.1b). In the following, we shall summarize our findings, with emphasis on the unoperated infant to shed light on the intrinsic factors related to the cleft condition (see Fig. 9.2 and Table 9.1), and compare them to data in the literature on unoperated adolescents and adults with clefts.
(a–d) Mean plots in three projections (lateral, frontal, and axial) of the four different cleft groups superimposed on the control group with UICL. The lateral mean plots are aligned on the n-s line and registered at s. The frontal mean plots are aligned on the latero-orbital line and registered at the center point of that line. The axial mean plots are aligned on a line between the two tuber points and registered at the center point of that line. Superimposition of the mean plots for the 2-month-old (a) ICP and UICL groups, (b) RS and UICL groups, (c) UCCLP and UICL groups, and (d) BCCLP and UICL groups
2.1 Cleft Lip (CL)
Isolated CL involves only structures of the embryonic primary palate. The craniofacial morphology in CL subjects has been shown to be fairly normal except for the small region of the cleft including the premaxilla and the incisors. In unoperated bilateral complete CL, the premaxilla may, however, protrude markedly. In unilateral complete CL, the protrusion is less pronounced but asymmetric. In subjects with unoperated unilateral incomplete cleft lip (UICL), the protrusion of the premaxilla is negligible (Hermann et al. 1999a). The interorbital distance in CL subjects seems to be slightly increased compared to the norm (Cohen 1997). The basal part of the maxilla has a normal prognathism in relation to the anterior cranial base, and the mandible is of normal size, shape, and inclination (Dahl 1970; Hermann et al. 1999a). Following lip surgery, the premaxilla is molded into a normal position, and maxillary prognathism measured to point A or ss (subspinale) is normal (Dahl 1970; Han et al. 1995; Hermann et al. 1999a, b, 2000). In conclusion, subjects with UICL have a very close to normal craniofacial morphology from infancy to adult age, and consequently, we have used our group of infants with UICL as a control group in the study of deviations in craniofacial morphology and growth of infants and young children with ICP, RS, UCCLP, and BCCLP since no actual normative cephalometric data for Danish infants and young children are available.
2.2 Cleft Palate (CP)
Isolated cleft palate (ICP) involves only structures of the embryonic secondary palate. In Fig. 9.2a, the mean facial diagrams of the ICP group are superimposed on the mean facial diagram of a group of age-matched infants with UICL (control group). The major deviations in the ICP group were: reduced length and posterior height of the maxilla, maxillary retrognathia, increased width of the maxilla and the nasal cavity, and reduced length of the mandible with mandibular retrognathia. Thus, the ICP group revealed bimaxillary retrognathia. The sagittal jaw relationship was, however, normal. In addition, in the ICP group, the upper airway dimensions were reduced. Bimaxillary retrognathia and a short mandible were previously documented in unoperated older children and adults with ICP (Dahl 1970; Bishara 1972).
2.3 Robin Sequence (RS)
Robin sequence (RS) is defined as a triad of symptoms: isolated cleft palate, micrognathia, and glossoptosis (Gorlin et al. 2001). RS may be part of several syndromes, e.g., Treacher-Collins syndrome (Kreiborg and Cohen 1996; Cohen 1997). In this chapter, only nonsyndromic cases of RS will be discussed. We consider this group as a subgroup of the ICP group (Hermann et al. 2003a). In Fig. 9.2b, the mean facial diagram of the RS group at 2 months of age is superimposed on the mean facial diagram of the control group. The major deviations in the RS group were decreased length and posterior height of the maxilla, maxillary retrognathia, increased width of the maxilla and nasal cavity, and very short mandible with marked mandibular retrognathia. Thus, the RS group revealed bimaxillary retrognathia; the retrognathia was, however, most marked for the mandible, and the sagittal jaw relation was increased. In addition, the RS group had a significantly smaller cranial base angle (n-s-ba) resulting in a smaller depth of the bony nasopharynx than the controls, and the upper airway dimensions were markedly reduced. The degree of maxillary retrognathia was similar in the RS and the ICP group. However, the mandibular retrognathia in the RS group was even more marked than in the ICP subjects. It would seem that RS subjects probably represent the extreme part of the ICP population in terms of mandibular retrognathia and upper airway constriction. As mentioned above, we consider the RS group as a special subgroup of the ICP group. Accordingly, we believe the bimaxillary retrognathia to be intrinsically associated with the cleft of the secondary palate.
2.4 Cleft Lip and Palate (CLP)
Combined clefts of the lip, alveolus, and palate involve structures of both the embryonic primary palate and secondary palate. In Fig. 9.2c, the mean craniofacial morphology in 2-month-old unoperated infants with unilateral complete cleft lip and palate (UCCLP) was compared to the control group (Hermann et al. 1999a). The major deviations in the UCCLP group were decreased posterior length and height of the maxilla; retrognathia of the basal part of the maxilla with relative protrusion of the premaxilla; the width of the maxilla and nasal cavity was markedly increased and the premaxilla deviated to the noncleft side; and the mandible was short and retrognathic. Thus, the UCCLP group revealed bimaxillary retrognathia combined with a relative protrusion of the premaxilla, which deviated to the noncleft side. In addition, in the UCCLP group, the upper airway dimensions were reduced.
Increased width of the midface and nasal cavity was previously reported in unoperated UCCLP infants (Han et al. 1995) and in unoperated adults with UCCLP (Motohashi et al. 1994). Relative protrusion and asymmetry of the premaxilla have also been reported in unoperated UCCLP children, adolescents, and adults (Ortiz-Monasterio et al. 1959, 1966; Bishara et al. 1976, 1985, 1986; Capelozza et al. 1993). The relative protrusion and deviation are probably due to overgrowth in the premaxillary-vomerine complex (Pruzansky 1971; Friede and Morgan 1976; Friede 1978) and due to the lack of structural integrity of the maxilla on one side. This relative protrusion of the premaxilla explains why we found the measurements s-n-ans (S-N-ANS) and s-n-ss (S-N-A) in the infant UCCLP group to be comparable to the values in the control group, despite the fact that the UCCLP group showed significant maxillary retrognathia measured to the basal part of the maxilla.
Dahl et al. (1982) and Hermann et al. (2003a, b) analyzed facial morphology in 2-month-old infants with unoperated bilateral complete cleft lip and palate from our sample. Fig. 9.2d illustrates the mean facial diagram of the BCCLP group superimposed on the mean facial diagram of the control group. The most obvious features in the BCCLP group were protrusion of the premaxilla both in relation to the anterior cranial base and in relation to the basal part of the maxilla; the length of the basal part of the maxilla and posterior maxillary height were decreased; retrognathia of the basal part of the maxilla; markedly increased width of the maxilla and nasal cavity; a short and retrognathic mandible. Thus, the BCCLP group revealed bimaxillary retrognathia with a truly protruding premaxilla. In other words, the protruding premaxilla was situated in a totally retrognathic face with a fairly normal sagittal jaw relationship. In addition, the upper airway dimensions were reduced.
The extreme protrusion of the premaxilla is probably the result of marked overgrowth in the premaxillary-vomerine complex secondary to total lack of structural integrity in the region.
For comparison, Mars and Houston (1990) and da Silva Filho et al. (1998) described groups of adult unoperated patients with BCCLP and found extreme protrusion of the premaxilla and a very convex profile measured as the ANB angle. No measurements were performed to describe the position of the body of the maxilla. Da Silva-Filho et al. (1992a, 1998) also found the mandible to be short and retrognathic and discussed whether this finding was related to the primary anomaly or if it was caused by secondary functional adaptations.
The retrognathia of the basal part of the maxilla and the short and retrognathic mandible found in our sample are, in our opinion, variations intrinsically associated with the cleft of the secondary palate as discussed above.
3 Discussion and Conclusions
The Danish study of craniofacial morphology in untreated cleft infants is the hitherto most comprehensive and well-controlled since it covers a whole population, which is homogeneous and in which central registration of clefts has been carried out for more than 65 years, a registration which has been shown to be highly reliable and nearly complete. Furthermore, all cleft infants are surgically treated at one hospital by one surgeon using the same techniques. All infants were examined with state-of-the-art three-projection cephalometry using the hitherto most comprehensive cephalometric analysis covering all craniofacial regions, and the methods were validated. The study included more than 600 children, and even after breakdown into subgroups, the sample sizes were adequate for statistical testing (except maybe for the RS group). Based on these facts, the findings related to the infant craniofacial morphology at 2 months of age, prior to any surgical or orthopedic treatment, must be considered to represent the “true” malformation, primarily caused by intrinsic factors.
In Table 9.1, the most important findings in the primary anomaly in the Danish infants with RS, ICP, BCCLP, and UCCLP are given, revealing a rather clear pattern. The findings support the suggestion of Dahl (1970) and others that facial clefts should be classified based on the embryonic facial development, i.e., into clefts involving the primary palate only (CL), clefts involving the secondary palate only (CP), and clefts involving structures of both the primary and the secondary palate (CLP). The postnatal facial morphology in these groups differs greatly. Infants with cleft of the secondary palate, with or without cleft of the primary palate, shared a number of characteristic morphological traits when compared to the norm: decreased posterior length of the maxilla, maxillary retrognathia, decreased posterior height of the maxilla, increased width of the maxilla and the nasal cavity, decreased length of the mandible, mandibular retrognathia, and reduced size of the pharyngeal airway. As seen from Table 9.1 and Fig. 9.3, the mandibular involvement was most pronounced in the RS group followed by the ICP and BCCLP groups and, finally, the UCCLP group. A similar pattern was observed for the reduced size of the pharyngeal airway.
As for the maxilla, the increased width of the maxilla and the nasal cavity was most pronounced in the groups with clefts of both the secondary and the primary palate, i.e., BCCLP and UCCLP. None of these groups showed decreased total length of the maxilla or retrognathia of the maxilla when measured to the premaxilla; the reason for this being a true and relative protrusion of the premaxilla, respectively.
In conclusion, a short and retrognathic mandible was a constant finding in infants with cleft of the secondary palate. The reduction in size of the pharyngeal airway in infants with cleft of the secondary palate was clearly related to the short and retrognathic mandible, being most severe in the RS group, which had the added effect of a reduction in the cranial base angle. But, in principle, all four groups had restricted upper airways as part of the primary anomaly. The increased width of the maxilla and nasal cavity was most pronounced in the groups which also had cleft of the primary palate (UCCLP and BCCLP). The UCCLP group was also characterized by relative protrusion of the premaxilla which was positioned asymmetrically, deviating to the noncleft side, whereas in the BCCLP group, the premaxilla showed true protrusion both in relation to the basal part of the maxilla (the lateral segments) and to the anterior cranial base. On average, the premaxilla was found to be positioned in the midline in this group, although most of the individual cases showed some degree of asymmetry. The protrusion of the premaxilla is suggested to be secondary to the primary anomaly of clefting, allowing for overgrowth in the premaxillary-vomerine complex, due to partial or total lack of anatomical integrity in the region.
It has been the aim of this chapter to summarize our findings about the intrinsic variations in facial morphology associated with the different types of cleft malformations to form a basis for valid estimations of the amount of surgical iatrogenesis, especially to the maxillary development, introduced by different surgical procedures and regimes, including the timing of treatment. In Fig. 9.4, the growth changes of the craniofacial skeleton from 2 to 22 months of age in the UCCLP group have been compared to the UICL group (control group) using color-coded surfaces on a 3D CT-model. In both groups, the cleft lip was surgically closed just after the examination at 2 months of age using a Tennison procedure. In the UCCLP group, the anterior part of the palate was closed with a vomer flap at the same time. The method of producing the illustrations will be given below.
(a–d) 3D visualization of locations of growth differences. Locations where UCCLP growth differs significantly (p < 0.01) from UICL growth (2–22 months of age) are colored red (UCCLP < UICL) or blue (UCCLP > UICL). The surface reconstruction shown is of a noncleft subject of comparable age and is used solely for illustration. Cleft side is on patient’s left in the figures. Locations of differences in the (a) magnitude of growth, (b) sagittal, (c) vertical, and (d) transverse growth components are shown
3D landmark locations corresponding to the skeletal landmarks used in the three-projection cephalometric analysis as well as for creating the color-coded surfaces in Fig. 9.4
3.1 Intuitive Visualization of the Location of Growth Differences
Cephalometric measurements in three projections provided growth vectors at each of the 279 (230 skeletal and 49 soft tissue) anatomical landmarks. The growth vectors, computed as the vector difference between corresponding landmark locations at the ages 2 and 22 months, respectively, after alignment to a common coordinate system (Hermann et al. 2000), have been used to form average growth patterns previously shown in Hermann et al. (1999a, b) (UICL, UCCLP) and Hermann et al. (2004) (UICL, BCCLP). Results of comparisons of growth between the UCCLP and the BCCLP groups, respectively, and the control group (UICL) have been shown as color-coded average growth patterns in Hermann et al. (1999a, b) (UCCLP vs. UICL) and Hermann et al. (2004) (BCCLP vs. UICL). These color-coded growth diagrams disclosed the locations of significantly different growth (1, 5, and 10 % levels) in the study group when compared to a reference group, and the diagrams were shown separately for each of the 3 projections (lateral, frontal, and axial), as well as for the growth magnitude and the two growth directions (x and y in each of the projections, respectively). In order to facilitate the effective comprehension of these diagrams, the locations of significant difference are color-coded onto the surface of a skull reconstructed from a CT scan of a single (noncleft) infant. As an example, Fig. 9.4 shows such color-coded surfaces for the comparison of the UCCLP with the UICL (control group). The color-coded surfaces were created by landmarking the 3D CT scan of the single noncleft infant at locations corresponding to the 230 skeletal cephalometric landmarks and color coding the surface in the vicinity of each landmark by a color corresponding to the significance of the growth difference. The landmark locations are shown in Fig. 9.5. A color table was chosen such that colors signify Student’s t-test p values smaller than 0.01. Blue colors correspond to locations where the study group exhibits larger growth than the control group, while the opposite is the case at locations colored red. Regions without any significant differences between the two groups remained gray. In the UICL and UCCLP groups, the frontal and axial projection data were mirrored in order to have all clefts on the left side. Accordingly, the cleft is on the patient’s left side in Fig. 9.4. The spatial extent of colored surface area in the vicinity of a landmark was governed by the distance to its closest landmark, and a maximum extent (spherically from landmark position) was chosen as 40 mm. Color-coded skulls are shown for differences in growth magnitude, as well as for each of the three growth directions (sagittal, vertical, and transverse). The colors for sagittal growth differences were computed from the x-component of the growth vectors in the lateral cephalometric projection and the y-component of the growth vectors in the axial projection. The colors for vertical growth differences were computed from the y-component of the growth vectors in the lateral projection and the y-component of the growth vectors in the frontal projection. The colors for transverse growth differences were computed from the x-component of the growth vectors in the frontal projection and the x-component of the growth vectors in the axial projection. The method of color coding has previously been described and applied for visualization of the growth differences between UCCLP and UICL in Darvann et al. (1999).
Secondary to surgical closure of the lip at 2 months of age in the UCCLP group, we found that the premaxilla was molded into place, demasking the intrinsic maxillary retrognathia and leading to a normal sagittal jaw relationship at 22 months of age. Maxillary growth was, besides the premaxillary molding, characterized by smaller vertical growth on the cleft side and reduced transverse development, which could probably be related to the effects of surgery. The amount of mandibular growth was similar in the two groups. However, the direction of growth was slightly more vertical in the UCCLP group. This growth pattern was probably related to the intrinsic pattern of mandibular development. Otherwise, craniofacial growth seemed to be very similar in the two groups.
We found that surgery to the lip and anterior part of the hard palate at 2 months of age in UCCLP subjects seemed to influence the development of the maxillary complex, as observed at 22 months of age, in a number of beneficial ways: the premaxilla was no longer relatively protruding, and it was less asymmetric; the nasal septum deviated less toward the noncleft side; the width of the nasal cavity and the posterior part of the maxilla became relatively more normal; and the transverse position of the lateral maxillary segment on the noncleft side was closer to normal. The posterior height of the maxilla was, however, still reduced to the same degree; the mandible was still short and retrognathic to the same degree; and bimaxillary retrognathia was still present. The only iatrogenic effect observed was that the lateral maxillary segment on the cleft side had become displaced toward the midsagittal plane anteriorly, resulting in a much too narrow dental arch at the level of the deciduous canine (Hermann et al. 2000). It is noteworthy that several studies of older, unoperated UCCLP children and adults find the maxillary prognathism to be within normal limits or even increased when compared to normative data (Ortiz-Monasterio et al. 1959, 1966; Mars and Houston 1990; Capalozzo et al. 1996). All these studies, however, only measure maxillary prognathism to the A-point or to the point ANS, both located in the relatively protruding premaxilla. Ortiz-Monasterio et al. (1959) concluded based on their findings in unoperated adults with UCCLP that: “The embryonic factor responsible for the facial cleft does not interfere with maxillary growth. This evidence leads us to believe that growth defects of the middle third of the face so frequently seen are caused by early or repeated and aggressive surgery.” We disagree somewhat with this conclusion. Based on our studies of infants with UCCLP, it would seem that maxillary retrognathia in this group is part of the intrinsic variations associated with the cleft malformation of the secondary palate. In the unoperated infant and the unoperated adult, the maxillary retrognathia is, however, partly masked by relative protrusion of the premaxilla, secondary to overgrowth in the premaxillary-vomerine suture. Surgical closure of the lip at 2 months of age molds the premaxilla back into place, demasking the maxillary retrognathia. Thus in the 22-month-old lip-operated UCCLP group, it is our opinion that the bimaxillary retrognathia illustrates the facial type characteristic of the group rather than an iatrogenic effect of cleft surgery (Hermann et al. 1999b, 2000). Thus, we do not consider the maxillary retrognathia observed at 22 months of age as the result of surgical iatrogenesis; rather, we believe it represents a normalization of the “intrinsic facial type” characteristic of subjects with UCCLP; and at 22 months of age, the face is still harmonious with a normal sagittal jaw relationship. We have, at this point in time, not reexamined the sample at older ages and can, therefore, not comment on facial growth and signs.
In conclusion, we are not arguing that cleft surgery does not lead to disturbed maxillary development during the growth period. But we are suggesting that subjects with cleft of the secondary palate have a special “intrinsic” facial type, primarily characterized by bimaxillary retrognathia and increased maxillary width. We are speculating that this facial type could be a “liability factor” increasing the probability of CP or CLP (Hermann et al. 1999a, b). Finally, we suggest that when outcome of cleft surgery in CLP subjects is evaluated at adolescence or adulthood, comparisons should not be made to normal standards, but rather to the adolescent and adult morphology seen in CP subjects.
References
Berkowitz S (1995) Cleft lip and palate. Perspectives in management. Singular Publishing Group, San Diego, pp 13–40
Bishara SE (1972) Cephalometric evaluation of facial growth in operated and non-operated individuals with isolated clefts of the palate. Cleft Palate J 10:239–246
Bishara SE (1973) Cephalometric evaluation of facial growth in operated and non-operated individuals with isolated clefts of the palate. Cleft Palate J 10:239–246
Bishara SE, Olin WH (1972) Surgical repositioning of the premaxilla in complete bilateral cleft lip and palate. Angle Orthod 42:139–147
Bishara SE, Krause CJ, Olin WH, Weston D, Ness JV, Felling C (1976) Facial and dental relationships of individuals with unoperated clefts of the lip and/or palate. Cleft Palate J 13:238–252
Bishara SE, Arrendondo RSM, Vales HP, Jakobsen JR (1985) Dentofacial relationships in persons with unoperated clefts: comparison between three cleft types. Am J Orthod 87:481–507
Bishara SE, Jakobsen JR, Krause JC, Soza-Martinex R (1986) Cephalometric comparisons of individuals from India and Mexico with unoperated cleft lip and palate. Cleft Palate J 23:116–125
Broadbent H (1931) A new x-ray technique and its application to orthodontia. Angle Orthod 1:45–66
Capelozza Filho L Jr, Normando ADC, da Silva Filho OG Jr (1996) Isolated influences of lip and palate surgery on facial growth: comparison of operated and unoperated male adults with UCLP. Cleft Palate Craniofac J 33:51–56
Capelozza L Jr, Taniguchi SM, Da Silva Filho OG Jr (1993) Craniofacial morphology of adult unoperated complete unilateral cleft lip and palate patients. Cleft Palate Craniofac J 30:376–381
Cohen MM Jr (1997) The child with multiple birth defects. Oxford University Press, New York, pp 168–171
da Silva Filho OG, Jr NADC, Capelozza L Jr (1992a) Mandibular morphology and spatial position in patients with clefts: intrinsic or iatrogenic? Cleft Palate Craniofac J 29:369–375
da Silva Filho OG, Jr RAL, Abdo RCC (1992b) Influence of surgery on maxillary growth in cleft lip and/or palate patients. J Craniomaxillofac Surg 20:111–118
da Silva Filho OG, Carvalho Lauris RC, Capelozza Filho L, Semb G (1998) Craniofacial morphology in adult patients with unoperated complete bilateral cleft lip and palate. Cleft Palate Craniofac J 35:111–119
Dahl E (1970) Craniofacial morphology in congenital clefts of the lip and palate. Acta Odontol Scand 28(Suppl 57):1–167
Dahl E, Kreiborg S (1995) Craniofacial malformations. In: Thilander B, Rönning O (eds) Introduction to orthodontics, 2nd edn. Stockholm, Gothia, pp 239–254
Dahl E, Kreiborg S, Jensen BL, Fogh-Andersen P (1982) Comparison of craniofacial morphology in infants with incomplete cleft lip and infants with isolated cleft palate. Cleft Palate J 19:258–266
Dahl E, Kreiborg S, Jensen BL (1989) Roentgencephalometric studies of infants with untreated cleft lip and palate. In: Kriens O (ed) What is a cleft lip and palate? A multi-disciplinary update. Georg Thieme Verlag, Stuttgart, pp 113–115
Darvann TA, Hermann NV, Marsh JL, Kreiborg S (1999) Color-coded 3D models in roentgencephalometry. In: Kalender W (ed) Abstract book, computer assisted surgery and rapid prototyping in medicine. CAS’99, Erlangen, p 34
Darvann TA, Hermann NV, Huebener DV, Nissen RJ, Kane AA, Schlesinger JK, Dalsgaard F, Marsh JL, Kreiborg S (2001) The CT-scan method of 3D form description of the maxillary arch. Validation and an application. Transactions 9th international congress on cleft palate and related craniofacial anomalies, Göteborg, pp 223–233
Ehmann G (1989) Cephalometric findings in normal and unoperated CLAP Fulbe-tribe adults of northern Cameroon. In: Kriens O (ed) What is a cleft lip and palate? A multi-disciplinary update. Georg Thieme Verlag, Stuttgart, pp 121–122
Friede H (1977) Studies on facial morphology and growth in bilateral cleft lip and palate. Thesis, University of Göteborg, Göteborg
Friede H (1978) The vomero-premaxillary suture – a neglected growth site in mid-facial development of unilateral cleft lip and palate patients. Cleft Palate J 15:98–404
Friede H (1998) Growth sites and growth mechanisms at risk in cleft lip and palate. Acta Odontol Scand 56:346–351
Friede H, Enemark H (2001) Long-term evidence for favorable midfacial growth after delayed hard palate repair in UCLP patients. Cleft Palate Craniofac J 38:323–329
Friede H, Johanson B (1974) A follow-up study of cleft children treated with primary bone grafting. Scand J Plast Reconstr Surg 8:88–103
Friede H, Morgan P (1976) Growth of the vomero-premaxillary suture in children with bilateral cleft lip and palate. Scand J Plast Reconstr Surg 10:45–55
Friede H, Pruzansky S (1972a) Longitudinal study of growth in bilateral cleft lip and palate, from infancy to adolescence. Plast Reconstr Surg 49:392–403
Friede H, Pruzansky S (1972b) Changes in profile in complete bilateral cleft lip and palate from infancy to adolescence. Trans Eur Orthod Soc:147–157
Friede H, Figueroa AA, Naegele ML, Gould HJ, Kay CN, Aduss H (1986) Craniofacial growth data for cleft lip patients from infancy to 6 years of age: potential applications. Am J Orthod 90:388–409
Gorlin RJ, Cohen MM Jr, Hennekam RCM (2001) Syndromes of the head and neck, 4th edn. Oxford University Press, New York
Graber TM (1949) A cephalometric analysis of the developmental pattern and facial morphology in cleft palate. Angle Orthod 19:91–100
Graber TM (1954) The congenital cleft palate deformity. J Am Dent Assoc 48:375–395
Han B-J, Suzuki A, Tashiro H (1995) Longitudinal study of craniofacial growth in subjects with cleft lip and palate: from cheiloplasty to 8 years of age. Cleft Palate Craniofac J 32:156–166
Heller A, Kreiborg S, Dahl E, Jensen BL (1995) X-ray: cephalometric analysis system for lateral, frontal, and axial projections. The 5th European craniofacial congress, Copenhagen; 61:33. Abstract
Hermann NV, Jensen BL, Dahl E, Bolund S, Kreiborg S (1999a) A comparison of the craniofacial morphology in 2 months old unoperated infants with unilateral complete cleft lip and palate, and unilateral incomplete cleft lip. J Craniofac Genet Dev Biol 19:80–93
Hermann NV, Jensen BL, Dahl E, Bolund S, Darvann TA, Kreiborg S (1999b) Craniofacial growth in subjects with unilateral complete cleft lip and palate, and unilateral incomplete cleft lip, from 2 to 22 months of age. J Craniofac Genet Dev Biol 19:135–147
Hermann NV, Jensen BL, Dahl E, Bolund S, Kreiborg S (2000) Craniofacial comparisons in 22-month-old lip-operated children with unilateral complete cleft lip and palate and unilateral incomplete cleft lip. Cleft Palate Craniofac J 37:303–317
Hermann NV, Jensen BL, Dahl E, Darvann TA, Kreiborg S (2001a) A method for three-projection infant cephalometry. Cleft Palate Craniofac J 38:299–316
Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E (2001b) Mandibular retrognathia in infants with cleft of the secondary palate. Transactions 9th international congress on cleft palate and related craniofacial anomalies, Göteborg, pp 151–154
Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E, Bolund S (2002) Early craniofacial morphology and growth in children with unoperated isolated cleft palate. Cleft Palate Craniofac J 39:604–622
Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E, Bolund S (2003a) Early craniofacial morphology and growth in children with nonsyndromic Robin Sequence. Cleft Palate Craniofac J 40:131–143
Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E, Bolund S (2003b) Craniofacial morphology and growth comparisons in children with Robin Sequence, isolated cleft palate, and unilateral complete cleft lip and palate. Cleft Palate Craniofac J 40:373–396
Hermann NV, Darvann TA, Jensen BL, Dahl E, Bolund S, Kreiborg S (2004) Early craniofacial morphology and growth in children with bilateral complete cleft lip and palate. Cleft Palate Craniofac J 41:424–438
Jensen BL, Kreiborg S, Dahl E, Fogh-Andersen P (1988) Cleft lip and palate in Denmark 1976–1981. Epidemiology, variability, and early somatic development. Cleft Palate J 25:1–12
Kreiborg S (1981) Crouzon syndrome. A clinical and roentgencephalometric study. Scand J Plast Reconstr Surg 18:1–198
Kreiborg S, Cohen MM Jr (1996) Syndrome delineation and growth in orofacial clefting and craniosynostosis. In: Turvey TA, Vig KWL, Fonseca RJ (eds) Facial clefts and craniosynostosis. Principles and management. WB Saunders, Philadelphia, pp 57–75
Kreiborg S, Hermann NV (2002) Craniofacial morphology and growth in infants and young children with cleft lip and palate. In: Wyszynski D (ed) Cleft lip and palate. From origin to treatment. Oxford University Press, New York, pp 87–97
Kreiborg S, Dahl E, Prydsø U (1977) A unit for infant roentgencephalometry. Dentomaxillofac Radiol 6:29–33
Kreiborg S, Jensen BL, Dahl E, Fogh-Andersen P (1985) Pierre Robin syndrome. Early facial development. Paper presented at 5th international congress on cleft palate and related craniofacial anomalies, Monte Carlo, Abstract
Mars M, Houston WJB (1990) A preliminary study of facial growth and morphology in unoperated male unilateral cleft lip and palate subjects over 13 years of age. Cleft Palate J 27:7–10
Motohashi N, Kuroda T, Capelozza Filho L Jr, de Souza Freitas JA (1994) P-A cephalometric analysis of nonoperated adult cleft lip and palate. Cleft Palate Craniofac J 31:193–200
Ortiz-Monasterio F, Rebeil AS, Valderrama M, Cruz R (1959) Cephalometric measurements on adult patients with nonoperated cleft palates. Plast Reconstr Surg 24:53–61
Ortiz-Monasterio F, Serrano A, Barrera G, Rodriguez-Hoffman VE (1966) A study of untreated adult cleft palate patients. Plast Reconstr Surg 38:36–41
Pruzansky S (1971) The growth of the premaxillary-vomerine complex in complete bilateral cleft lip and palate. Tandlaegebladet 75:1157–1169
Pruzansky S, Lis EF (1958) Cephalometric roentgenography of infants: sedation, instrumentation and research. Am J Orthod 44:159–186
Sandham A, Foong K (1997) The effect of cleft deformity, surgical repair and altered function in unilateral cleft lip and palate. Transactions of the 8th international congress on cleft palate and related craniofacial anomalies, Singapore, pp 673–678
Semb G, Shaw WC (1996) Facial growth in orofacial clefting disorders. In: Vig KWL, Fonseca RJ, Turvey TA (eds) Facial clefts and craniosynostosis. Principles and management. WB Saunders, Philadelphia, pp 28–56
Slaughter WB, Brodie AG (1949) Facial clefts and their surgical management in view of recent research. Plast Reconstr Surg 4:203–224
Smahel Z, Brousilova M, Müllerova Z (1987) Craniofacial morphology in isolated cleft palate prior to palatoplasty. Cleft Palate J 24:200–208
Tomanova M, Müllerova Z (1994) Effects of primary bone grafting on facial development in patients with unilateral complete cleft lip and palate. Acta Chir Plast 36:38–41
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kreiborg, S., Hermann, N.V., Darvann, T.A. (2013). Characteristics of Facial Morphology and Growth in Infants with Clefts. In: Berkowitz, S. (eds) Cleft Lip and Palate. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30770-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-30770-6_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30769-0
Online ISBN: 978-3-642-30770-6
eBook Packages: MedicineMedicine (R0)