Abstract
Segniliparaceae, a family within the order Actinomycetales and suborder Corynebacterineae, comprises the sole genus Segniliparus with two species. The two type strains have been isolated from human sputum and are described to be opportunistic pathogens. They are aerobic, mesophilic, and chemoorganotroph. The most distinctive characteristic is the presence of ultralong C60–C100 mycolic acids. These are non-oxygenated α-mycolates with high levels of cis unsaturation, a feature solely present on Segniliparus species Hong (PLoS One 7:e39017, 2012). The type strain of Segniliparus rotundus is CDC 1076T (=ATCC BAA-972T = CIP 108378T = DSM 44985T) and of Segniliparus rugosus CDC 945T (=ATCC BAA-974T = CIP 108380T = DSM 45245T). Of each species, further strains have been isolated, mainly from the human habitat.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Taxonomy, Historical and Current
Short Description of the Family
Segniliparaceae Butler et al. 2005
Segniliparaceae (Seg’ni.li.par.a’ce.ae. N.L. masc. n. Segniliparus type genus of the family; -aceae ending to denote a family; N.L. fem. pl. n. Segniliparaceae, the Segniliparus family).
Members of the family are non-spore forming and nonmotile. The genus Segniliparus is the only genus in the family with the two species Segniliparus rotundus and S. rugosus (Butler et al. 2005). The family belongs to the suborder Corynebacterineae, order Actinomycetales, subclass Actinobacteridae, class Actinobacteria, and phylum Actinobacteria (Garrity and Holt 2001; Ludwig et al. 2012; Stackebrandt et al. 1997; Zhi et al. 2009). The cells are rod-shaped without any branching. They are aerobic, strongly acid-fast, and produce multiple chemical functional groups of high-molecular-mass, nonpolar, mycolic acids.
Molecular Analyses
The DNA–DNA association value between the type strains of Segniliparus rotundus and S. rugosus was <28 % using the hydroxyapatite method with an optimum reassociation temperature of 70 °C (Butler et al. 2005). A complete genome sequence has been obtained for Segniliparus rotundus (Sikorski et al. 2010) and a high-quality draft genome sequence for S. rugosus has been published by Earl et al (2011), revealing genome sizes of 3.16 and 3.64 megabases and a DNA coding region of 92.3 % and 86.4 %, respectively. The number of genes associated with the general COG functional categories is similar in both strains; however, there is a larger proportion of genes not in COGs in S. rugosus (46.1 %) compared to S. rotundus (41.3 %) (Earl et al. 2011; Sikorski et al. 2010).
Phylogenetic Structure of the Family andits Genus
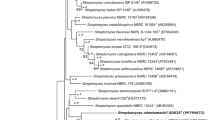
The 16S rRNA gene sequences of the two type strains of the genus Segniliparus differ by only 1.1 % (Sikorski et al. 2010) (Fig. 40.1 ). The next closest relatives of the genus Segniliparus, as based on 16S rRNA gene sequences, are the members of the genus Rhodococcus, family of Nocardiaceae, which share 93.3–94.8 % 16S rRNA genes sequence similarity with strain Segniliparus rotundus CDC 1076T (Ludwig et al. 2012; Sikorski et al. 2010). A BLAST survey against the nucleotide database identified only very few entries at a similarity above 93 %, e.g., as obtained from wastewater (Del Casale et al. 2011), suggesting a rather limited ecological distribution of the genus Segniliparus.
Phylogenetic reconstruction of the family Segniliparaceae based on 16S rRNA and created using the neighbor-joining algorithm with the Jukes-Cantor correction. The sequence datasets and alignments were used according to the All-Species Living Tree Project (LTP) database (Yarza et al. 2010; http://www.arb-silva.de/projects/living-tree). The tree topology was stabilized with the use of a representative set of nearly 750 high-quality type strain sequences proportionally distributed among the different bacterial and archaeal phyla. In addition, a 40 % maximum frequency filter was applied in order to remove hypervariable positions and potentially misplaced bases from the alignment. Scale bar indicates estimated sequence divergence
Phenotypic Analyses
Segniliparus Butler et al. 2005
Segniliparus (Seg.ni.li.pa’rus. L. adj. segnis slow; Gr. adj. liparos fat/fatty; N.L. masc. n. Segniliparus the slow fatty one, the one with slow fats, to indicate the possession of slowly reacting fatty acids, i.e., late-eluting mycolic acids detected with HPLC). The genus comprises two species, Segniliparus rotundus and S. rugosus.
The main features of the sole genus in the family Segniliparaceae are listed in Table 40.1 . Segniliparus species form nonmotile rod-shaped cells with 0.4–0.9 μm width and 1.0–4.5 μm length. The cells are aerobic and acid–alcohol fast. Spores or aerial mycelium have not been observed, though the cells are occasionally V-shaped but not with true branching (Butler et al. 2005). The cells grow in 3–4 days on days on Löwenstein–Jensen (LJ), Middlebrook 7H10, and Middlebrook 7H11 agar at an optimal temperature of 33 °C and yield nonpigmented, non-photochromogenic colonies that do not produce a diagnostic odor. The growth on heart infusion (HI) agar is poor. The cells show arylsulfatase activity but are negative for niacin production. A semiquantitative catalase test produces bubbles of >45 mm (Butler et al. 2005). A definite range of salinity tolerance is not known, but growth tolerance with sodium chloride on LJ and American Trudeau Society (ATS) media in 14 days is described to be positive (Butler et al. 2005). Urea is hydrolyzed but acetamide, adenine, casein, citrate, esculin, hypoxanthine, tyrosine, and xanthine are not. d-glucose, maltose, and trehalose are used as carbon sources and produce acid, whereas adonitol, l-arabinose, cellobiose, dulcitol, i-erythritol, galactose, i-myoinositol, lactose, mannose, melibiose, raffinose, l-rhamnose, salicin, and sodium citrate are not (Butler et al. 2005). Utilization of d-fructose, glycerol, d-mannitol, d-sorbitol, and sucrose is variable. The API CORYNE test kit numerical profile is 2040 000 and revealed that the two species are positive for β-glucosidase and pyrazinamidase activities but negative for alkaline phosphatase, β-galactosidase, β-glucuronidase, α-glucosidase, N-acetyl-β-glucosaminidase, and pyrrolidonyl arylamidase activity at 33 °C. The antimicrobial susceptibility patterns have been determined from several strains of both species using serial twofold broth microdilution assays and are listed in detail in Table 40.2 . The species have the same fatty acid composition, with prominent fatty acids of C10 : 0, C14 : 0, C16 : 0, and tuberculostearic acid (Butler et al. 2005). The quinones are mainly MK-8(H4) and MK-8(H2) with some MK-8(H6) and traces of MK9(H2) (Sikorski et al. 2010). The species do not produce a Rhodococcus equi-specific ChoE virulence factor (Butler et al. 2005). The gas–liquid chromatography thermal cleavage product of the mycolic acids is a C24:0 acid–methyl ester. The high-performance liquid chromatography mycolic acid pattern consists of three late-emerging groups of peaks with the final peak co-eluting with the high-molecular-mass internal standard. Thin-layer chromatography demonstrates three nonpolar α+ (C84-C100) -, α (C73-C83) -, and α’ (C60-C66) – mycolic acid chemical functional groups. These ultralong non-oxygenated α-mycolates with high levels of cis unsaturation are a special and sole phenotypic characteristic of Segniliparus species. Overall 65 homologous mycolic acids have been observed in Segniliparus. The overall length of the mycolic acids, which is among the longest lipids known in cell biology, is distinctly atypical of rapid growing mycolata (Hong et al. 2012; Lanéelle et al. 2013).
Isolation, Enrichment, and Maintenance Procedures
The type strain of S. rotundus was isolated from human sputum in a public health laboratory in Tennessee, USA, in 2005 or before (Butler et al. 2005). The type strain of S. rugosus was isolated from human sputum in a public health laboratory in Alabama, USA, in 1998 (Earl et al. 2011). Information of case histories on the type strains is not available. Several other strains have been isolated from humans, for some of them also case histories have been published (Butler et al. 2007; Hansen et al. 2009; Koh et al. 2011).
Strains of the genus Segniliparus do not require special procedures for maintenance and long-term storage. Can be stored frozen at –24 °C in appropriate medium or water containing 43 % glycerol and in liquid nitrogen in appropriate medium or water containing 5 % dimethylsulfoxide without loss of viability. Long-term preservation is by lyophilization with 20 % skin milk.
Ecology
Habitat
The type strains of the genus Segniliparus have been isolated from human sputum in 2005 or before (Butler et al. 2005; Earl et al. 2011). Further strains have been isolated from patients with cystic fibrosis (S. rugosus, most probably USA, but also in Australia) and bronchiectasis (S. rotundus, South Korea), from sputum, bronchus, or nasal samples (Butler et al. 2007; Hansen et al. 2009; Koh et al. 2011). The presence of S. rugosus in Ixodes ricinus ticks was identified by denaturating gradient gel electrophoresis (DGGE) of 16S rRNA gene amplicons and subsequent sequencing of the DGGE band. The ticks were collected in Sunnmøre, Norway, in May/June/September 2010 both as host-seeking ticks and feeding ticks picked from cats and dogs (Tveten and Sjåstad 2011). This suggests that transmission of S. rugosus between mammalian hosts can take place via ticks (Tveten and Sjåstad 2011). Further isolates of S. rugosus have been obtained from a subadult female California sea lion (Zalophus californianus) stranded on the beach of San Onofre, California, USA, in April 2010 (Evans 2011). Though in environmental databases hardly any 16S sequences related to Segniliparus are present, this finding addresses the question of whether S. rugosus could be free-living in the oceans or part of the flora of any number of ocean-dwelling vertebrates or invertebrates (Evans 2011; Sikorski et al. 2010).
Pathogenicity, Clinical Relevance
Although both S. rugosus and S. rotundus are officially classified to belong to risk group 1 (TRBA 2010), occasionally members of the species are suspected to behave as opportunistic pathogens in immunocompromised humans. This appears to be specifically true for humans suffering from cystic fibrosis (CF) (Butler et al. 2007; Hansen et al. 2009) and lung diseases such as tuberculosis and bronchiectasis (Koh et al. 2011). Hence, it is supposed that Segniliparus species can cause pneumonia in patients with bronchiectasis (Koh et al. 2011). Clinically, the CF cases exhibited a marked and rapid decline in lung function and radiologic studies over a short period of time which was not characteristic of CF or infections usually associated with this disease (Butler et al. 2007). However, the public health significance of Segniliparus species is still unclear. Also other mammalians such as sea lions may be affected by Segniliparus species (Evans 2011). Potentially, members of Segniliparus may be transmitted via ticks (Tveten and Sjåstad 2011), but may also originate from an environmental source (Butler et al. 2007).
References
Classification of bacteria and archaea in risk groups. www.baua.de. TRBA 466. 2010.
Butler WR, Floyd MM, Brown JM, Toney SR, Daneshvar MI, Cooksey RC et al (2005) Novel mycolic acid-containing bacteria in the family Segniliparaceae fam. nov., including the genus Segniliparus gen. nov., with descriptions of Segniliparus rotundus sp. nov. and Segniliparus rugosus sp. nov. Int J Syst Evol Microbiol 55:1615–1624
Butler WR, Sheils CA, Brown-Elliott BA, Charles N, Colin AA, Gant MJ et al (2007) First isolations of Segniliparus rugosus from patients with cystic fibrosis. J Clin Microbiol 45:3449–3452
Del Casale A, Flanagan PV, Larkin MJ, Allen CCR, Kulakov LA (2011) Analysis of transduction in wastewater bacterial populations by targeting the phage-derived 16S rRNA gene sequences. FEMS Microbiol Ecol 76:100–108
Earl AM, Desjardins CA, Fitzgerald MG, Arachchi HM, Zeng Q, Mehta T et al (2011) High quality draft genome sequence of Segniliparus rugosus CDC 945T = (ATCC BAA-974T). Stand Genomic Sci 5:389–397
Evans RH (2011) Segniliparus rugosus—associated bronchiolitis in California sea lion. Emerg Infect Dis 17:311–312
Garrity GM, Holt JG (2001) The road map to the manual. In: Garrity GM, Boone DR, Castenholz RW (eds) Bergey’s manual of systematic bacteriology. Springer, New York, pp 119–169
Hansen T, Van Kerckhof J, Jelfs P, Wainwright C, Ryan P, Coulter C (2009) Segniliparus rugosus infection, Australia. Emerg Infect Dis 15:611–613
Hong S, Cheng T-Y, Layre E, Sweet L, Young DC, Posey JE et al (2012) Ultralong C100 mycolic acids support the assignment of Segniliparus as a new bacterial genus. PLoS One 7:e39017
Koh W-J, Choi G-E, Lee S-H, Park YK, Lee NY, Shin SJ (2011) First case of Segniliparus rotundus pneumonia in a patient with bronchiectasis. J Clin Microbiol 49:3403–3405
Lanéelle M-A, Eynard N, Spina L, Lemassu A, Laval F, Huc E et al (2013) Structural elucidation and genomic scrutiny of the C60–C100 mycolic acids of Segniliparus rotundus. Microbiology 159:191–203
Ludwig W, Euzéby J, Schumann P, Busse HJ, Trujillo ME, Kämpfer P, Whitman WB (2012) Road map of the phylum Actinobacteria. In: Goodfellow M, Kämpfer P, Busse H-J, Trujillo ME, Suzuki Ki, Ludwig W, Whitman WB (eds) Bergey’s manual of systematic bacteriology, vol 5, 2nd edn. Springer, New York, pp 1–28
Sikorski J, Lapidus A, Copeland A, Misra M, Rio TGD, Nolan M et al (2010) Complete genome sequence of Segniliparus rotundus type strain (CDC 1076T). Stand Genomic Sci 2:203–211
Stackebrandt E, Rainey FA, Ward-Rainey NL (1997) Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int J Syst Bacteriol 47:479–491
Tveten A-K, Sjåstad KK (2011) Identification of bacteria infecting Ixodes ricinus ticks by 16S rDNA amplification and denaturing gradient gel electrophoresis. Vector Borne Zoonotic Dis 11:1329–1334
Yarza P, Ludwig W, Euzéby J, Amann R, Schleifer K-H, Glöckner FO, Rosselló-Móra R (2010) Update of the all-species living tree project based on 16S and 23S rRNA sequence analyses. Syst Appl Microbiol 33:291–299
Zhi X-Y, Li W-J, Stackebrandt E (2009) An update of the structure and 16S rRNA gene sequence-based definition of higher ranks of the class Actinobacteria, with the proposal of two new suborders and four new families and emended descriptions of the existing higher taxa. Int J Syst Evol Microbiol 59:589–608
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Sikorski, J. (2014). The Family Segniliparaceae . In: Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F. (eds) The Prokaryotes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30138-4_279
Download citation
DOI: https://doi.org/10.1007/978-3-642-30138-4_279
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30137-7
Online ISBN: 978-3-642-30138-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences