Abstract
An important role of oxygen free radicals and scavengers has been suggested in the development of tissue damage in various organs such as the heart or the gastrointestinal (GI) tract. The production of oxygen free radicals is closely associated with hypoxemia. Although a lot of details of their generation and action are known, there are many questions still to be answered, including whether the correlation between the hypoxemia and the production of oxygen free radicals are causative factors in the development of tissue damage or if these changes in the levels of oxygen free radicals are associated with other biochemical factors. If the role of oxygen free radicals is of importance in tissue damage, then it is important to better understand regulatory mechanisms.
Many researchers have suggested that the main causative factor of gastric mucosal tissue damage is the generation of oxygen free radicals. Accepting that argument, there are various endogenous (catalase, peroxidase, glutathione peroxidase, superoxide dismutase) and exogenous (like ascorbic acid, vitamins A, E, and retinoids) defensive mechanisms (scavengers) present in tissues that ought to reduce the actions of oxygen free radicals or to prevent tissue damage.
The answers to these questions have remained open up till now; the roles of certain pathways became clear and scientifically proven. We should be aware that there are many simultaneous mechanisms present during the development of tissue injury or various diseases. Researchers are able to examine only one (or few) step(s) of mechanisms and conclude certain step of action overemphasized.
During the last three decades, we examined the potential role of oxygen free radicals and scavengers in animal experiments to collect evidences for the development of human GI disorders. We suggested that oxygen free radicals are really causative factors in the development of various GI disorders; however, we had suggested that besides oxygen free radicals many other mechanisms might also play crucial role in tissue injury.
The aims of this chapter are to give further details on the role of oxygen free radicals and antioxidants in earlier suggested and proved mechanisms of tissue injury (in connection to biochemical tissue parameters, neural and hormonal influences).
Our observations were carried out in vitro on isolated cells (stable cultured cell lines or freshly isolated gastric mucosal cells), in vivo in animal experiments, in human healthy subjects, and in patients with different GI mucosal disorders.
We deeply analyzed the effects of scavenger retinoids in experimental models, in human healthy subjects, and in patients with different GI disorders. We found that:
-
1.
The damaging and protective mechanisms of various compounds can be defined on isolated cellular system: (a) Ethanol causes direct damage on freshly isolated cells and on the cells of various stable cell lines (non-secreting mouse myeloma, human hepatoma). (b) The release of free radicals can be shown by ESR-spin trapping, during the development of ethanol-induced direct cellular damage. (c) Indomethacin (IND) did not produce direct cellular damage, although it aggravates the ethanol-induced direct cellular injury. (d) Human H. pylori alone and in combination with IND had no direct cellular mucosal damaging effect and did not aggravate the effect of ethanol. (e) Histamine and pentagastrin have cytoprotective effects in vivo; however, no direct cytoprotective effects could be demonstrated at cellular level. (f) The direct cytoprotective effects of scavengers can be detected at cellular level, which does not depend on the chemical structures.
-
2.
The retinoids prevent the chemical-induced (ethanol, HCl, sodium salicylate, IND) gastric mucosal damage without the alternation of gastric acid secretion.
-
3.
The actions of scavengers represent special type of gastric cytoprotection.
-
4.
The cell protecting action could be detected on isolated cells; however, no direct correlation exists between cellular actions and the results of animal experiments.
-
5.
The gastric cytoprotective effects of retinoids do not depend on the (a) presence of vitamin A activity, (b) the number of unsaturated double bonds, (c) presence of a characteristic structure and their terminal structure, nor (d) on the alteration of vascular permeability. The mucosal gastroprotective action of retinoids depends on (a) intact vagal nerve, (b) intact adrenals, and (c) dose-dependent inhibition of ATP-ADP transformation, which results a dose-dependent increase of ATP-cAMP transformation.
-
6.
The cytoprotective effects of retinoids differ from those of prostaglandins.
-
7.
The cAMP is an intracellular signal for the development of GI mucosal damage and prevention.
-
8.
The presence of GI mucosal protection can be proven in human healthy subjects and in patients with different disorders.
These results clearly proved that:
-
1.
The GI cytoprotection produced by scavengers (dominantly retinoids) exists in various experimental experiments, in human healthy subjects, and in patients with different GI disorders.
-
2.
There are significant correlations between the scavenging properties of different compounds and intact vagal nerve function.
-
3.
There are significant correlations between mucosal protective effects of scavengers and intact adrenals.
-
4.
There are significant correlations between GI mucosal protective effects and GI mucosal biochemistry: (a) dose-dependent ATP level reduction and (b) dose-dependent increase of ATP-cAMP transformation and sulfhydryl groups in GI tissues.
-
5.
The scavengers play essential roles in GI mucosal protection against drug-induced GI mucosal damage.
-
6.
The actions of oxygen free radical and scavengers – in comparison with other mechanism – can be evaluated only by time-sequence analysis of results involved in the development of GI mucosal damage and prevention.
-
7.
The oxygen free radicals and scavengers are involved in the development of experimental mucosal damage and prevention.
-
8.
The oxygen free radicals and scavengers are involved in cells, tissues, and organ mucosal damage and protection against drug-induced GI mucosal damage (human peptic ulcer disease, inflammations like gastritis, precancerous dysplasia, in situ cancer, or further developed states (in terms of causative factors and as well as of prevention); however, alterations in tissue biochemical parameters and neural and hormonal influences indicate very close correlations to the changes in oxygen free species.
It has been generally concluded that:
-
1.
Many mechanisms are running parallel to each other during the development of tissue (or cell) injury and protection (including oxygen free radicals, tissue energy status, vascular permeability), which can be modified by neural and hormonal and pharmacological influences.
-
2.
The final development of tissue injury and protection produced by different noxious agents differs at the levels of cells, in vivo animal experiments, and in humans (healthy subjects, GI patients).
-
3.
The interpretation of obtained results significantly depends on the applied level of observation.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Adrenals
- Animal experiments
- Cellular energy systems
- Human healthy subjects
- Isolated cells
- Neural and hormonal actions
- Oxygen free radicals
- Patients with different gastrointestinal disorders
- Scavengers
- Sulfhydryls
- Vagal nerve
Introduction
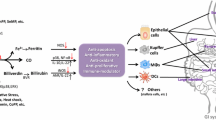
The induction of oxygen free radicals by damaging agents represents a very important event in the development of various diseases. The introduction of oxygen free radicals and scavenging agents has been suggested by researchers to obtain a new general prospective in the explanation for the development of diseases similarly to the stress theory given by Hans Selye in 1936 (Selye 1936). The cellular mitochondrion has a key role in the generation of oxygen free radicals (the schematic role of mitochondrion in cell death is presented in Fig. 76.1), which is a very complex and not fully evaluated processes. The underlying mechanisms involved appear at subcellular, cellular, tissue, organ, and body levels (Tapodi et al. 2005).
Schematic presentation of pathways leading to cell death (Tapodi et al. 2005)
The oxygen free radicals can be induced by different chemicals, viruses, bacterial or physical factors, as well as immunological events. The release of oxygen free radical can be detected by Spin method; however, this method is not commonly used in everyday medical research, especially in everyday medical practice. Consequently, we have to obtain other easy, usable methods to measure oxygen free radical levels in cells, animal experiments, and human observations.
Easily detectable enzymes (catalase, peroxidase, glutathione peroxidase, superoxide dismutase) and some endogenous components (vitamins C, A, E) are present in the body to reduce damage. The induction of these enzymes follows the release of oxygen free radicals. Vitamins having scavenging properties are able to reduce the actions of oxygen free radicals and might be useful in decreasing the development of oxygen free radical-induced tissue damage. Levels of oxygen species and scavengers are also easy to measure directly during experimental conditions. These observations can also be performed in human beings or in patients with certain conditions.
The crucial point of human research is that we are able to perform chemical observations from blood samples (which in many cases does not represent the damaged target organ in the measurement of oxygen free radicals during the test), and importantly, we can investigate the protective properties of scavenging agents and other drugs applied in treatment of diseases. The tissue samples are achievable during surgical intervention by the removal or biopsy of the target organ. The timing is also a crucial factor in the measurement of free radicals due to their short lifetime. Generally, the measurements of the serum level of various scavengers (vitamins or other compounds) can be accepted for further analysis and used for not only research purposes but also for preventative screening programs. Clinical research should be tempered by the many controversial findings in this area of research findings.
The mechanistic effects of oxygen free radicals and scavengers (drugs) can be evaluated at levels of cells, in animal experiments, in human healthy subjects, and in patients with different disorders. We summarize some of these issues that arise when studying gastrointestinal (GI) disorders.
Basic Methods of the Study
First General Approaches
Free radicals have been implicated in the development of GI tissue damage caused by physical agents (e.g., mechanic irritation, trauma, ionizing radiation), chemicals (such as carbon tetrachloride, paraquat, alcohol, HCl, concentrated NaCl, and NaOH), or ischemia/reperfusion (Mózsik et al. 1987).
Many structurally unrelated chemicals damage the gastric mucosa of experimental animals and humans. The most widely used chemicals in animals are 0.6 M HCl, 0.2 M NaOH, absolute alcohol (96 % v/v), hypertonic NaCl, and drugs like acidified aspirin, indomethacin (IND), reserpine, or epinephrine. Gastric erosions and ulcers also develop during stress induced by water immersion of experimental animal.
The list of agents that prevent acute gastric ulcers is diverse. Namely, the lesions can be prevented not only by classical antisecretory drugs such as anticholinergic, gastrin antagonist, histamine receptor antagonists, and proton pump inhibitors but also by non-antisecretory or “cytoprotective” doses of prostaglandins (PG), SH agents, somatostatin, vitamin A, other retinoids, body protective compound (BPC), and membrane-stabilizing component (Intal®) and by many other chemically unrelated drugs or scavengers.
In our experimental models, we used damaging agents like absolute (96 % v/v) ethanol, 0.6 M HCl, 0.2 M NaOH, 25 % v/v NaCl, acidified aspirin (100 mg suspended in 1 % methylcellulose prepared with 0.2 M HCl), and IND (20 mg/kg) given intraperitoneally (i.p.).
As protective compounds, the following nonenzymatic antioxidants were administered intragastrically (i.g.), i.p., or subcutaneously (s.c.) 30 min before the administration of damaging agents: dimethyl sulfoxide (DMSO), butylated hydroxytoluene (BHT), propyl gallate, ascorbic acid, sodium benzoate, mannitol, and N,N′-diphenyl-p-phenylenediamine (DPPD). Vitamin A, β-carotene, PGI2, PGFα2, atropine, or cimetidine were injected s.c. at the time of administration of IND (the antisecretory drugs were given in cytoprotective doses).
Enzymes scavenging free radicals were also applied i.v. or i.p. at 30 before ethanol or acidified aspirin treatment: superoxide dismutase (SOD) (3,200 μg/mg protein), catalase (20,000 μg/mg protein), and peroxidase (46 purpurogallin μ/mg solid).
Early Biochemical Studies
Rat gastric mucosa was obtained by scraping. The SOD activity was measured by the method of Misra and Fridovich (Misra and Fridovich 1972). The enzyme activity was expressed as IU/mg mucosal protein.
Lipid peroxidation was measured by four parameters in rat gastric mucosal homogenate, obtained from rats sacrificed at different time after administration of necrotizing agents as described by Lunec and Dormandy (1979). Conjugated dienes (absorbance at 242 nm) and products absorbing at 270 nm were measured in the chloroform phase against a solvent phase with spectrofluorometry (excitation 320 nm, emission 390 nm). Fluorescent lipid peroxidation products were measured in the same phase with spectrofluorometry (excitation 320 nm, emission 390 nm). Malondialdehyde (MDA) was quantified by the thiobarbituric acid method (Yu and Sinnhuber 1967).
The following results were obtained:
-
1.
Lipid peroxidation after acidified aspirin determined by fluorescence emission at 390 nm roughly doubled in 1, 3, and 6 min after administration of necrotizing agent, while it remained unchanged after ethanol. Conjugated dienes measured by the changes in absorbance at 242 nm of separated chloroform phases slightly decreased after ethanol administration, while it remained at baseline after aspirin. Products determined by absorbance at 270 nm were apparent at 1 min after acidified aspirin and diminished at 1 and 3 min following ethanol (Recknagel and Ghoshal 1966). Levels of thiobarbituric acid-reactive compounds (of which MDA was the major fraction) did not change any time measured in the gastric mucosa following ethanol or aspirin application. The concentration of MDA in the gastric mucosa of rats given IND significantly increased, and its values were unchanged after administration of various doses of atropine, cimetidine, as well as the combination of atropine and cimetidine.
-
2.
The nonenzymatic antioxidants versus acute gastric mucosal damage.
Antioxidants were given to test their ability in reduction mucosal damage caused by either ethanol or aspirin. In the ethanol model, propyl gallate at 0.1, 1, or 10 mg/kg dose was effective in reducing the extent of mucosal lesions to 61 %, 63 % or 15 % of control, respectively. DMSO, BTH, ascorbinic acid, mannitol, sodium benzoate, and DPPD were effective in the dose range with three orders of magnitude. In the aspirin model, all four drugs decreased gastric mucosal damage at least in one dose. The reduction of gastric damage by antioxidants ranged from 40 % of controls for propyl gallate to 18 % for BHT.
-
3.
Enzymatic antioxidants versus acute gastric mucosal damage.
SOD, catalase, and peroxidase given s.c. or i.p. in ethanol and aspirin models were ineffective. Administering the enzymes i.v. (after ligating the renal blood vessels to prolong their circulatory half-life) SOD (1,500 U/100 g) was still ineffective in reducing mucosal damage induced by either ethanol or aspirin. Catalase (100,000 U/100 g) decreased gastric lesions induced by either ethanol or aspirin by 59 %. The combination of SOD was ineffective in ethanol-induced damage but effective in acidified aspirin-induced gastric mucosal injury.
-
4.
Vitamin A and β-carotene significantly decreased gastric mucosal damage; nevertheless, the change in gastric mucosal SOD activity was not consistent. The SOD activity in the gastric mucosa increased to 147 % after ethanol administration, while it decreased to 1 % in 0.6 M HCl, 0.2 M NaOH, and 25 % NaCl models after 1 h. When these animals were treated (prior the administration of ethanol) with vitamin A, β-carotene, prostaglandin-I2 (PGI2), atropine, or cimetidine, then SOD activity turned to 44–81 %, except of β-carotene – that value remained at 3 % of control in ethanol model. Surprisingly, the SOD activity significantly increased after administration of vitamin A, β-carotene, PGI2, atropine, and cimetidine in HCl model.
-
5.
Similar changes of oxygen free radical content could be obtained independently from the chemical structure of applied necrotizing agent (ethanol, NaOH, concentrated NaCl, HCl) (Mózsik et al. 1987).
These earlier published results clearly indicated that some association exists between the development and prevention of acute gastric mucosal damage produced by different chemical agents and the amount of measurable oxygen free radicals (Mózsik et al. 1987).
We concluded from these observations the following:
-
1.
Probably the obtained results in lipid peroxidation do not depend (or only at least partially) on the chemical structure of given necrotizing agents.
-
2.
We received opposing information of the very early and very late time period of experimental observations suggesting the need of time sequence observations.
-
3.
We need to identify further parameters along with evaluation of oxygen free radical system.
-
4.
The results obtained with nonenzymatic and enzymatic antioxidants and classical drug experiments showing that these experimental conditions were able to produce similar changes in the oxygen free radicals in association to produce and to prevent the acute gastric mucosal damage in rats.
Following these observations, we carried out a scientifically planned systematic approach to understand the changes in oxygen free radicals and cellular and organ damage. Consequently, observations were carried out on isolated cells (cell lines and freshly isolated gastric mucosal cells), in different animal experiments, in human healthy subjects, and in patients with different GI disorders. We analyzed the effect of scavenger (especially the retinoids) treatments in further observations. We aimed to understand the main points (roles) of oxygen free radicals and scavengers from isolated cells, via animal experiments, human healthy subjects, and patients with different GI disorders. The time period of these observations ranged from a very short time to several years.
As clinicians, we wanted to introduce these results to medical practice for prevention of different GI diseases (like acute inflammation, chronic inflammation, premalignant states, malignant diseases of various organs of GI tract, drug-induced side effects, and nutrition).
Experimental Models
In Vitro Studies on Isolated Cells
These observations were performed on primary cell cultures and cell lines and divided into short-term (Bódis et al. 1996, 1997a, b, c, 1998; Szabó et al. 1996a) and long-term studies (Szabó et al. 1996b, 1997a, b, 2000).
Short-Term Studies
In the short-term toxicity studies, freshly isolated cells (Nagy et al. 1994; Szabó et al. 1996a, b), a non-secreting mouse myeloma cell line (Sp2/0-Ag14, CRL, obtained from American Type Culture Collection), and a human hepatocellular carcinoma line (HEP G2 cell line, HB 8065) were used. Cells were incubated with Helicobacter pylori (H. pylori) suspension (10+6 to 10+8 bacteria/mL) for 30 min alone and also in combined with IND. These observations were carried out to test the acute cellular toxicity.
Preincubation with prostacyclin (PGI2Na) (10−6–10−4 M for 60 min), histamine (10−8–10−6 M for 60 min), pentagastrin (10−8–10−4 M) with and without prostacyclin, or BPC (10−8–10−4 M) was used to test the acute cytoprotective effects produced by these compounds.
Long-Term Studies
Freshly isolated rat gastric mucosal cells and the above-mentioned myeloma cell lines (Sp2/0-Ag14 and HEP G2) were incubated for 5 min, 1 h, 4 h, and 24 h. Ethanol (1, 5, 15, and 20 % v/v) and ethanol (applied in the same concentration) with pyrazole (10−5 M) were used during co-incubation as cytotoxicity studies.
DMSO (10−3 M), glutathione (GSH, 10−3 M), d-mannitol (10−3 M), dimethyl-1-pyrroline N-oxide (DMPO, 10−3 M), and β-carotene (10−6 M) were applied for the evaluation of their cytoprotective effects.
The cell viability was studied on the cellular levels of plasma membrane, mitochondria, and nucleus by trypan blue exclusion method (Baur et al. 1975), measurement of the linkage of lactate-dehydrogenase (Bergmeyer and Bern 1974) or succinic-dehydrogenase activities in culture media (Mosmann 1983), and ethidium bromide-DNA fluorescence assay (Dey and Majumder 1989) to determine nucleic acid stability. Determination of free radicals was carried out by electron spin resonance (ESR).
These observations resulted as:
-
1.
Ethanol directly damaged the cells of different stable cultures and from freshly isolated gastric mucosa (ED50 value is 15 % v/v).
-
2.
IND by itself did not produce any direct cellular damage; however, it aggravates the ethanol-induced direct cellular injury.
-
3.
H. pylori alone and in combination with IND had no direct cellular damaging effect, and it did not enhance injury caused by other compounds.
-
4.
Histamine and pentagastrin had direct cytoprotective effects in vivo; however, these compounds did not have direct cellular preventing effects. There was a significant discrepancy between the results obtained in vitro and in vivo circumstances (Bódis et al. 1997a).
The results obtained in cell cultures and in freshly isolated gastric mucosal cells clearly indicate that these results represent only a small part of oxygen free radical production and cellular (tissue, organ, and body) protection (regarding potential therapeutic possibilities). These results surely cannot be directly applied for the global understanding of the essential points in oxygen free radical production but might give some knowledge for gastric mucosal damage prevention in living organs.
In Vivo Studies
Animal Experiments
Clinical science has to return to animal models in case certain medical and therapeutic questions cannot be answered. As more information gathered on oxygen free radicals and scavengers from international research, we thought that we understand the changes of oxygen free radical-scavenger system in association with other certain measurable parameters.
Measurements were carried out directly on tissues of target organ (and not from serum) with the aim to recognize the role of oxygen free radical in damage of different tissues and to show the possible protective effects of scavengers by the suppression of oxygen free radicals in different organs.
We focused on the analysis of the potential mechanisms and regulation of scavengers’ action. We tried to understand the correlation between scavenger action and mucosal biochemistry (including the changes of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), cyclic adenosine monophosphate (cAMP), and lactate levels). Biochemical examinations were carried out simultaneously from the same tissue samples. Other theoretically important biochemical parameters were calculated from the results of measured values, like adenylate pool and “energy charge.” The value of energy charge equals to 1, when all adenosine compounds are in phosphorylated form, and equals to zero, when all adenosine compounds are in dephosphorylated form. The results of these experiments were able to give answer for the causative role of oxygen free radicals and of scavengers’ preventive properties. We found close correlation between the actions of scavenger and GI cytoprotection (Robert et al. 1979; Mózsik and Jávor 1991; Mózsik 2005, 2006; Mózsik et al. 1976, 1981a, b, 1992, 2010, 2011).
We also wanted to receive some answer for the potential hormonal and neural regulation of these mechanisms. The following animal experimental models were applied: (1) pylorus-ligated rats (Shay et al. 1945); (2) gastric mucosal damage produced by intragastric administration of 0.6 M, 0.2 M NaOH, 96 % v/v ethanol, and 25 % NaCl in 1-h experiments; (3) IND (20 mg/kg s.c. given)-induced gastric mucosal damage in 4-h experiments; (4) sodium salicylate (200 mg/kg i.g. given)-induced gastric mucosal damage in 4-h experiments; (5) surgical and chemical (atropine treatment) vagotomy prior the experiments (1-, 4-, 24-h experiments); and (6) surgical adrenalectomy prior the experiments (1-h experiments) alone and surgical adrenalectomy combined with glucocorticoid and mineralocorticoid supplementation given immediately after surgical intervention.
The antioxidant/oxidant reactions were determined by measurements of tissue activity of catalase, peroxidase, glutathione peroxidase, and SOD and by glutathione, thiobarbiturate, and oxygen free radical levels.
The following important results were obtained:
-
1.
The chemical agents (0.6 M HCl, 0.2 M NaOH, 96 % v/v ethanol, concentrated NaCl) resulted same gastric mucosal macroscopic and microscopic view in 1-h experiments in rats. Furthermore, the total of 50 % of gastric mucosal damage over 1 h can be obtained at the first 5 min after i.g. administration of necrotizing agents (Mózsik et al. 1983; Mózsik and Jávor 1988).
-
2.
Small doses of atropine (0.1 mg/kg), cimetidine (5 mg/kg), pentagastrin, histamine, and sodium cromoglycate produce gastric mucosal protection without the presence of any gastric acid inhibitory effect (“true cytoprotection”). These antisecretory compounds also inhibit the chemical-induced mucosal damage when applied in doses having gastric acid inhibitory effects (Jávor et al. 1983). The “gastric cytoprotection” was defined in rats by André Robert in 1979; however, our clinical observations proved earlier that the duodenal ulcer in patients healed without any inhibition of gastric acid secretion in 1965 (Mózsik et al. 1965, 1967) and the healing rates by atropine, cimetidine, and carbenoxolone were equal and superior to that of placebo in randomized, prospective, and multiclinical studies (Tárnok et al. 1979; Mózsik et al. 2010, 2011).
-
3.
The retinoids are classical scavenger compounds (consisting of about 600 plant spices over the world), which are widely used in the human nutrition. We studied the effects of retinoids in the gastric acid secretion and gastric mucosal damage produced by different chemical agents as. 0.6 M HCl, 0.2 M NaOH, 96 % v/v ethanol and concentrated NaCl (given i.g.), aspirin, acidified aspirin, IND, and epinephrine (Jávor et al. 1983; Mózsik 2005). Surprisingly, we registered that these compounds are not able to inhibit the gastric acid secretion in 4-h pylorus-ligated rats; however, these compounds inhibit the i.g. given chemical agent-induced gastric mucosal damage (Jávor et al. 1983). Thereafter we systematically studied the antisecretory properties and gastric mucosal protective effects of vitamin A, β- and α-carotene, β-cryptoxanthin, zeaxanthin, lutein, capsorubin, capsanthin, capsanthol, and lycopene in animal experiments. The presence of vitamin A activity, terminal structure, and number of unsaturated double bonds were analyzed. These results indicated that vitamin A, β-carotene, β-cryptoxanthin, zeaxanthin, and lutein inhibited the gastric mucosal damage without presence of gastric acid inhibitory effect, while capsorubin, capsanthin, capsanthol, and lycopene had not mucosal protective action. The results of these observations indicated clearly that no correlation exists (a) between the cytoprotective effects of retinoids and vitamin A activity, (b) between their cytoprotective effects and their terminal chemical structures, and (c) between cytoprotection and number of unsaturated double bonds (Jávor et al. 1983; Mózsik 2005) (Table 76.1):
-
(a)
Gastric cytoprotection does not depend on vitamin A activity. For example, the vitamin A activity of β-carotene (β, β-carotene) was completely demolished by minor modifications such as the introduction of a hydroxyl group at C-3(3′) and substitution of E-end group with β-end group. Although zeaxanthin (3,3′-dihydroxy-β, β-dihydroxy-β-carotene) is not precursor of vitamin A, it does not exert a cytoprotective effect similar to that of vitamin A precursors β-carotene (β, β-carotene and β-cryptoxanthin (3-hydoxy-β, β-carotene)).
-
(b)
Gastric cytoprotective property cannot be related exclusively to a conjugated polyene chain present in carotenoids, since acyclic lycopene (γ,γ-carotene) with an undecene chromophore and cyclic capsorubin (3,3′-dihydroxy-κ,κ-carotene-6,6′-dione) with a nonene-dione chromophore are inactive. Furthermore, cytoprotective zeaxanthin and lutein have different chromophore systems.
-
(c)
Gastric cytoprotection by asymmetrical C40 carotenoids cannot be explained by a splitting of the molecules at the central double bond, which would result in two C20 units with or without cytoprotective properties. According to such conversion, one might expect that capsanthin (3,3′-dihydroxy-β,K- carotene- 6′–one) would be cytoprotective, as its molecule can be built up from an active (½ zeaxanthin) and an inactive (½ capsorubin) C20 units. The lack of gastric cytoprotection by capsanthin argues against such a conversion. It should be noted that capsanthol, the reduction product of capsanthin, is also inactive.
-
(a)
-
4.
The gastric mucosal protection produced by atropine, cimetidine, and retinoids in 1-h experiments is the similar time course and by biochemical backgrounds (Jávor et al. 1983; Abdel Salam et al. 1997).
Characterization of Gastric Mucosal (Cyto)Protective Effects of Retinoids
-
1.
β-carotene dose-dependently inhibits the ethanol-induced gastric mucosal damage (in 1-h experiments); however, this mucosal preventive effect disappears after bilateral surgical vagotomy (Vincze et al. 1997; Sütő et al. 1989; Mózsik et al. 1991, 1995). It is important that the chemical vagotomy cannot decrease the β-carotene-induced gastric cytoprotective effect.
-
2.
The tissue level of PGE2 and PGF1α (as terminal product of prostacyclin I2) significantly decreased after 0, 1, and 10 mg/kg β-carotene application in 1-h experimental model following bilateral surgical vagotomy; meanwhile, the levels of these cellular components are remained unchanged during β-carotene-induced gastric cytoprotection.
-
3.
In animals after surgical vagotomy, the presence of β-carotene can be detected in the gastric mucosa. No elevation was obtained in the liver content of vitamin A and β-carotene, and gastric mucosal protection cannot be detected (Király et al. 1992). These observations clearly indicate that the intact vagal nerve is basically necessary for the development of gastric mucosal protection.
-
4.
When the surgical vagotomy was carried out, we were not able to demonstrate any gastric cytoprotection by β-carotene. However, when the animal received dexamethasone (given in doses of 0.2, 0.4, 0.6 mg/kg s.c.) for 1 week time following the surgical vagotomy, gastric cytoprotection by β-carotene could be demonstrated. The absence of mineralocorticoid (triamcinolone) did not modify the β-carotene-induced gastric cytoprotection. When the animals were treated with triamcinolone (given in doses of 1–10–100 mg/kg s.c.) or dexamethasone without vagotomy, those did not modify the gastric cytoprotective effect of retinoids (Sütő et al. 1989).
-
5.
The extent of changed permeability of the gastric mucosal was measured by the exudation of Evans blue at 1 h in ethanol-treated animals without and with β-carotene administration. The permeability increased gradually (from the 5 to 60 min after ethanol administration); however, the extent of gastric mucosal vascular permeability remained unchanged. Meanwhile, the gastric cytoprotection by β-carotene could be detected (Mózsik 2005), indicating that the retinoid-induced gastric cytoprotection is an independent process from the actual permeability of gastric mucosa. These results together with result mentioned under 4 and 5 clearly prove that the intact adrenals basically necessary for the development of gastric mucosal damage.
-
6.
The gastric mucosal preventive effects of retinoids (especially β-carotene) and PGI2 were compared in ethanol and HCl models of rats in relation to relapse time for the development of gastric mucosal prevention after the usage of necrotizing agents. By molecular pharmacological studies, we identified the ED50 values for β-carotene and PGI2 on the gastric mucosal damage. These values are 10 mg/kg orally given for β-carotene and 5 μg/kg s.c. given for PGI2 in rat experiments.
We used an acid-dependent (1.0 ml from 0.6 M HCl i.g.) and a nonacid-dependent (1.0 ml from 96 % v/v ethanol i.g.) experimental model (Gasztonyi et al. 1996). The animals were sacrificed at 0, 5, 15, 30, and 60 min after the administration of necrotizing agents. The protective agents were given at 30 min before the onset of experiments. The number and severity of gastric mucosal lesions were measured and calculated.
It was found that both these components (β-carotene and PGI2) prevented the different necrotizing agents-induced gastric mucosal damage; however, their effects differed in time. The PGI2 inhibited the mucosal damage during the first period (from 0 to 15 min); meanwhile, the β-carotene acted between 15- and 60-min time period (late phase). Of course, we used different doses of β-carotene (1 and 10 mg/kg. i.g.) and of PGI2 (5 and 50 μg/kg s.c.). The obtained results indicated a dose-dependent action (Mózsik 2005, 2006).
-
7.
Systematic observations were carried out in the experimental models (see f), as we wanted to obtain simultaneously other experimental data on the actions of a free radical (β-carotene) and a natural gastric mucosal protective agent (PGI2). We measured the tissue activities of catalase, glutathione peroxidase, and SOD in the ethanol model (using 1 and 10 mg/kg β-carotene, 5 and 50 μg/kg PGI2) according to the internationally accepted methods (Mózsik 2005, 2006).
In the other parts of these observations, various essential components were determined from the gastric mucosa, such as ATP, ADP, AMP, cAMP, lactate, adenylate pool (calculated as sum of ATP + ADP + AMP), energy charge (obtained as ATP + 0.5 ADP/ADP + ADP + AMP, according to Atkinson’s formula), and the ratio of ATP/ADP (Mózsik et al. 1989a; Mózsik 2006). The Atkinson formula can give information on the extent of phosphorylation and dephosphorylation in cells. As we mentioned earlier, the value obtained from Atkinson’s formula is theoretically “1,” when all adenosine compounds are in phosphorylated form, and its value is “0,” when all adenosine compounds are in dephosphorylated form (Atkinson 1971a, b).
The measurements of above parameters in gastric mucosa represented a special step of these types of observations. There were a big number of researchers in this field, who emphasized the presence of tissue hypoxia triggering oxygen free radical generation. The actual tissue level of ATP represents equilibrium between the ATP breakdown and resynthesis in the tissue level (not in the serum). These processes are regulated by several hormones, mediators, drugs, and pathological conditions. However, it is very important to note that the high (near to 1.0 according to Atkinson’s formula) level of tissue ATP can be obtained only in well oxygenized condition, whereas hypoxia can be demonstrated by the increase of tissue level of lactate. Our observations (using very simple experimental models) something else happened suggesting another phenomenon in the generation of oxygen free radicals (Mózsik et al. 1989a, b). When necrotizing agents produce gastric mucosal damage in association with the generation of oxygen free radicals, naturally the tissue ATP would decrease. In our observations, the ATP level turned back to the normal level, suggesting that the presence of hypoxia surely can be surely excluded.
The changes of catalase, glutathione peroxidase, SOD activity, and MDA concentration did not change after simple administration of gastric mucosal damage protective agents (at 30 min) (except of glutathione peroxidase, which showed an increase) in ethanol and HCl models. Administration of β-carotene (given in 1 and 10 mg/kg doses orally) or PGI2 (given in 5 and 50 μg/kg s.c.) enhanced the originally seen changes in catalase, glutathione peroxidase, SOD activity, and MDA concentration. It is also interesting that these changes of above parameter were detected at 1 min after administration of gastric necrotizing agents (Mózsik 2005). A very important result was obtained from the classical biochemical examinations too. The tissue values of ATP decreased in both experimental models after giving the necrotizing agents in the first 15 min and thereafter turned back to the original level. These observations exclude the presence of hypoxemia in the gastric mucosa, when a lot of oxygen free radical reactions started. Furthermore, the decreased level of “energy change” also turned back to the original level in the gastric mucosa of both β-carotene- and PGI2-treated groups of animals, which also gave another proof to exclude the presence of tissue hypoxia during generation of oxygen free radicals (Mózsik 2005). The changes in tissue levels of adenosine phosphates and in the further calculated parameters (exception of “energy charge”) appeared in a dose-dependent manner.
These results proved that the productions of oxygen free radicals are involved in development of tissue damage, whereas the actions of antioxidants are involved in tissue protection. However, many other changes in the tissue biochemical parameters (metabolisms of adenosine compounds) and neural and hormonal controls (intact vagal nerve, intact adrenal) in gastric mucosal permeability are also involved in the mechanisms of damaging and protective effects. On the other hand, the damaging and preventive actions appear together with many other cellular, functional, biochemical, and vascular events (Mózsik et al. 2003a, b; Mózsik et al. 2005, 2007, 2011).
-
8.
In the last series of animal observations, the details of β-carotene-induced gastric mucosal protection were evaluated. The ED50 value was determined for IND in experiments using IND in 20 mg/kg s.c. dose (Karádi and Mózsik 2000). The gastric mucosal damage appeared during the forthcoming 4-h period (the extent of mucosal damage increased up 4 h).
The number and severity of mucosal lesions and tissue level of cAMP and PGE2 were measured at the 0, 1, 2, 3, and 4 h after administration of IND (Mózsik et al. 1998). The tissue level of PGE2 and cAMP significantly decreased in the gastric mucosa at the beginning; meanwhile, the gastric mucosal lesions appeared dominantly from 2 h. The PGE2 and cAMP turned back gradually to their original tissue level, when the gastric mucosal damage reached the peak value. In detailed analysis, it was observed that there is a very significant correlation between the decrease of gastric mucosal cAMP (but not in case of PGE2) and extent of gastric mucosal damage in 4-h IND-treated animals. The prevention of IND-induced gastric mucosal damage by β-carotene is a dose-dependent process, and β-carotene is able to elevate the tissue level of cAMP dose-dependently (at 1 h after IND administration).
The results of these observations indicated that (a) the development of gastric mucosal damage by IND is a cAMP-dependent process and (b) the gastric protecting effect of β-carotene is also cAMP dependent.
Clinical Observations
Observations in Healthy Human Subjects
Randomized, prospective, and multiclinical studies were carried out in healthy human subjects (n = 12 in each group) to test the cytoprotective effects of 0.125 mg/kg i.m. cimetidine (12.5 mg/kg i.m.) and vitamin A (100,000 IU i.m) on gastric acid output (basal acid output, BAO; maximal acid output, MAO) (Morón et al. 1984; Mózsik et al. 1986). These observations showed no inhibition by these compounds on gastric acid secretory responses.
Many drugs and chemicals cause damage of the GI mucosa in healthy subjects and in patients with different disorders. The nonsteroidal anti-inflammatory drug (NSAID) intake may lead to the development of GI mucosal damage. We need to emphasize that these drugs are widely used in the treatment of different cardiovascular and connective tissue diseases. Furthermore, the applications of these drugs in these disorders are based on evidence-based medicine; however, these drugs can be contraindicated in several disorders (which is also evidence based).
There are no questions that NSAIDs produce GI mucosal damage by several mechanisms. To test the effects of scavenger (vitamin A) and other drugs (applied in non-antisecretory doses) (see above) on the drug-induced gastric mucosal damage, IND (which is a nonselective cyclooxygenase inhibitor) was applied in 3 × 25 mg/day dose, which seldom causes gastric mucosal damage. Detected microbleeding (and other examinations) reflected the extent of mucosal damage (Morón et al. 1984; Nagy et al. 1984). These studies were carried out in prospective, randomized, and multiclinical studies by the cytoprotective doses of atropine, cimetidine, and vitamin A. The gastric basal microbleeding is about 2 ml/day without application of any drug. The gastrointestinal mucosal bleeding could increase up to 8–9 ml/day after IND administration and could be decreased by atropine, cimetidine (given in non-antisecretory doses), and vitamin A.
It was interesting to note that the effect of vitamin A was the same in the prevention of IND-induced gastric mucosal damage to those produced by atropine and cimetidine. In these series of observations, we could demonstrate the gastric mucosal protective effect of vitamin A (as antioxidant agents).
The prevention of drug-induced gastric mucosal damage by scavengers is clinically important event, which is closely associated to GI cytoprotection (Mózsik et al. 2010, 2011).
Clinical Observation in Patients with Different Gastrointestinal Disorders: Healing Effects of Vitamin A in Gastric Ulcer Disease in Patients
Observations were carried out in patients with chronic gastric ulcer. Ulcer healing rates by atropine, cimetidine, carbenoxolone, and vitamin A were studied in randomized, prospective, and multiclinical study. In patients who underwent gastroscopy, the presence of gastric ulcer and ulcer sizes were determined at the 0, 2, and 4 weeks of treatment (Mózsik et al. 1986; Patty et al. 1982, 1983). All of above treatments were effective in these patients with chronic gastric ulcers. In few patients, the ulcer did not heal completely at the end of 4 weeks. The pharmacodynamic actions of these drugs can be evaluated by the follow of the ulcer sizes found during further endoscopic examinations of unhealed patients.
We demonstrated that the healing rate by vitamin A was significantly higher (the produced by other drugs) at 2 weeks after the onset of clinical observations (Mózsik 2005, 2006; Mózsik et al. 2007).
Observations in Patients with Various Acute and Chronic Gastrointestinal Disorders
The serum levels of vitamin A, β-carotene, β-cryptoxanthin, zeaxanthin, lutein, capsorubin, capsanthol, and capsanthin were systematically measured by HPLC method in the patients with different GI disorders: chronic H. pylori positive and negative gastritis; hepatitis C infection; alcoholic hepatitis; ileitis terminalis; ulcerative colitis; alcoholic cirrhosis; colonic adenoma with mild, moderate, and severe dysplasia; and esophageal, gastric, pancreatic, hepatocellular, and colorectal cancers and in situ adenocarcinoma of colon polyps (Table 76.2) (Rumi et al. 1999, 2000, 2001a, b). These patients represent the acute, chronic, or permanent diseases. Forty-seven healthy human subjects were used as control group. It is important to emphasize that the body mass index, serum cholesterol, serum albumin, total serum protein, and hemoglobin (Hgb) indicated normal values in the control group and in patients’ groups.
Observations in Patients with Different Gastrointestinal Tumors
Observations were carried out in 107 patients with esophageal (n = 8), gastric (n = 1), liver (n = 15), pancreatic (n = 10), colorectal (n = 53), and in situ colon (n = 9) cancer, including 70 males (50 ± 12 years) and females (9 ± 10 years). Fifty-seven healthy persons (in matched group) were used as control (healthy), and 600 healthy blood donors were used for the control of Leiden mutation. A total of 764 patients with GI cancer and healthy subjects were included into this part of our observations.
Increased blood coagulation has been suggested to have a role in the development of chronic inflammatory diseases and cancer. The Leiden mutation is “relatively frequent” genetic markers for thrombophilia (Mózsik et al. 2001). The Leiden mutation was detected by PCR technique in the patients mentioned above along with serum measurements of retinoids by HPLC (Mózsik et al. 2002, 2005a).
Our results showed a decrease in vitamin A and zeaxanthin (in some times in β- and α-carotene) levels; meanwhile, the prevalence of Leiden mutation increased in patients’ sera (21 % in esophageal, 23 % in gastric, 25 % in liver, 21 % in pancreatic, 21 % in colon, and 55 % in in situ colon cancer). The main results of correlations between the Leiden mutation and vitamin A and between the vitamin A and zeaxanthin are summarized in Fig. 76.2.
Prevalence of Leiden mutation in patients with different gastrointestinal tumors, expressed in percent values (a). Correlation between the prevalence of Leiden mutation versus serum level of vitamin A in patients with different GI tumors (b). Correlation between the prevalence of Leiden mutation versus serum level of zeaxanthin in patients with different GI tumor (c). n indicates the number (in parenthesis) of examined patients
It was interesting to note that the preventive effects of retinoids showed the same pattern by chemical structures in different acute and chronic diseases including the precancerous states and cancers of different origin as those were obtained in animal experiments (Table 76.3) (Mózsik et al. 2003a, b).
Clinically interesting and important is that the decrease in retinoid levels in patients with different diseases did not affect the tissue levels of β-carotene, which remained dominantly unchanged (meanwhile the vitamin A level decrease in all groups). Then, it can be suggested that some regulatory mismatch exists in these groups of patients with cancer, since the β-carotene is a provitamin for vitamin A, so for some reason the transformation from β-carotene to vitamin A was not apparent. The measurements of retinoids represent a way to approach the nutritional factors; meanwhile, the identification of Leiden mutation indicates a small part from the genetic factors.
Discussion
The roles of neural, hormonal systems and immunological mechanisms are emphasized in the homeostatic regulation of living organs. The possible correlations between these systems seem to be very complex and mostly unknown processes. The advantages in these possible roles of neural, hormonal systems and immunological mechanisms offer a uniform explanation for very different physiological mechanisms. On the other hand, the theory of stress also offered a uniform explanation for the development of various diseases. In that case, the form of stress is nonspecific; however, the tissue reactions by the organs are specific.
The theory of oxygen free radicals and antioxidants (scavengers) aimed to give also a uniform explanation for the development of tissue damages in different organs and their prevention. The causative factors for the development of oxygen free radicals and their prevention are not clearly defined. Independently, the key role of tissue hypoxemia has been permanently emphasized. Many chemical agents, drugs, physical influences, and immunological actions are able to release various elements of oxygen free radicals. These states of damages in different organs indicate a similarity to stress theory (viz., the causative factors are different). However, it is very surprising that the reactions in different organs represent main biochemical parameters (changes in catalase, glutathione peroxidase, SOD, increase of MDA and other products); the appearance of microscopic and macroscopic changes of organs is common. The reactions of different organs are not organ specific; these mechanisms significantly differ from those involved in the stress theory by Selye.
Our presented results indicate clearly:
-
1.
Different chemical agents (such HCl, NaOH, concentrated NaCl, concentrated ethanol) produce the same pathological, biochemical parameters and changes in the tissue energy metabolism and oxygen free radicals, and furthermore, these changes simultaneously appear after the application of the necrotizing agents.
-
2.
The presence of tissue hypoxia can be biochemically excluded during these processes (by the direct measurements of tissue level of ATP and lactate). It is true that the tissue level of ATP decreases after administration of necrotizing agents; however, its value turned back to the normal level during the experiments, which prove directly the unimpaired function of oxidative phosphorylation.
-
3.
The changes of oxygen free radicals in organs are associated with many other changes in the tissue metabolisms (tissue levels of ATP, ADP, AMP, “energy change,” vascular permeability, catalase, glutathione peroxidase, SOD, MDA, PGs, and sulfhydryls) with normal hormonal and neural regulation. In other words, the changes in oxygen free radicals represent only a part of changes in the biochemical parameters involved in tissue injury. Furthermore, we can obtain very close and significant correlations between the closely accepted oxygen free radicals and other biochemical parameters (Mózsik 2005, 2006).
-
4.
The release of oxygen free radicals (and/or other tissue mediators) initiates tissue damage probably generally in the body; however, the gastric mucosal damage by oxygen free radicals can be apparent in both innervated and surgically denervated gastric tissue. Surprisingly, the scavenger-induced gastric mucosal protection will develop in gastric tissue with intact vagal innervation. These results indicate that the potential key role of oxygen free radicals differs in the whole body and the different organs (this organ was in our case the stomach). It was interesting to see these differences after surgical vagotomy. The surgical vagotomy decreased significantly the tissue level of PGE2 and PGFα1 (which is the terminal product of PGI2); meanwhile, the levels of these components remained unchanged in animals treated with necrotizing agents. These results prove in animals that the scavenger-induced gastric cytoprotection is an independent phenomenon from the tissue PG system. Similar observations were obtained by β-carotene in rats treated with IND; however, the development of IND-induced gastric mucosal damage was found to be a cAMP-dependent process.
-
5.
The GI cytoprotection is a glucocorticoid-dependent process, since after surgical adrenalectomy no cytoprotection can be obtained by retinoids, and becomes apparent after glucocorticoid supplementation. The absence of mineralocorticoid has no modifying effect on the scavenger-induced GI mucosal protection.
-
6.
The chemical structures, vitamin A activity, and number of unsaturated bonds in the chemical structure were systematically studied in animal observations. We could observe that the retinoid-induced gastric cytoprotection does not depend on the (a) chemical structure, (b) presence of vitamin A activity, and (c) the number of unsaturated bonds present. Similar results were obtained in patients with different disorders, when the serum levels of retinoids were measured. Surprisingly, the serum level of vitamin A showed a significant correlation to severity (Crohn Disease Activity Index, CDAI) in Crohn’s diseases (Mózsik et al. 2003a).
-
7.
The harmful and beneficial effects of the oxygen free radicals and scavengers can be detected easily on the level of cells in stable and freshly isolated cells, in animal experiments, in human healthy subjects, and in patients with different disorders, representing the very wide scale of tissue mucosal damage by oxygen free radicals. We are aware of harmful effects caused by drugs, chemicals, physical agents, viruses, and bacteria involving the release of oxygen free radicals and their preventions with antioxidants (scavengers) obtained on isolated cells and in animal experiments might not be fully applied for the understanding of their actions in healthy human subjects and in patients.
-
8.
The time-sequence analysis obtained in different experimental models – with and without application of antioxidants (scavengers), involving the liberation of oxygen free radicals during physicochemical events and application of different chemical compounds resulting mucosal damage – can offer the understanding of mechanism involved in the oxygen free radicals and of antioxidants (Mózsik et al. 1989a,b).
-
9.
The damaging and protective effects of oxygen free radicals and scavengers can be proved on isolated cells (freshly isolated cells, stable cell culture line), in different animal experiments, in human healthy subjects, and in patients with different GI disorders. Consequences of such approaches on the oxygen free radicals and scavenger involve wide scale physiological mechanisms.
-
10.
Finally, we can state that the productions of oxygen free radical and their modification by antioxidant scavengers have a key role in the development of tissue injury and prevention; however, a lot of other changes are present during these processes. Further investigations are needed to name the most important element of above changes.
Abbreviations
- ADP:
-
Adenosine diphosphate
- AMP:
-
Adenosine monophosphate
- ATP:
-
Adenosine triphosphate
- BAO:
-
Basal acid output
- BHT:
-
Butylated hydroxytoluene
- BPC:
-
Body protective compound
- cAMP:
-
Cyclic adenosine monophosphate
- CDAI:
-
Crohn disease activity index
- DMSO:
-
Dimethyl sulfoxide
- DPPD:
-
N,N'-diphenyl-p-phenylenediamine
- ESR:
-
Electron spin resonance
- GI:
-
Gastrointestinal
- GSH:
-
Glutathione
- H. pylori :
-
Helicobacter pylori
- Hgb:
-
Hemoglobin
- i.g.:
-
Intragastrically
- i.p.:
-
Intraperitoneally
- i.v.:
-
Intravenously
- IND:
-
Indomethacin
- MAO:
-
Maximal acid output
- MDA:
-
Malondialdehyde
- PG:
-
Prostaglandin
- PGE2 :
-
Prostaglandine-E2
- PGF1α :
-
Prostaglandine-F1α
- PGI2 :
-
Prostaglandine-I2
- s.c.:
-
Subcutaneously
- SOD:
-
Superoxide dismutase
References
Abdel Salam OM, Szolcsányi J, Mózsik G (1997) The indomethacin-induced gastric mucosal damage in rats. Effect of gastric acid, acid inhibition, capsaicin-type agents and prostacyclin. J Physiol Paris 91:7–19
Atkinson DE (1971a) Regulation of enzyme function. Annu Rev Microbiol 23:47–68
Atkinson DE (1971b) Adenine nucleotides as stochiometric coupling agents agenst in metabolism and as regulatory modifiers:the adenylate energy charhe. In: Vogel HJ (ed) Metabolic ragulations, vol 5. Academic, New York, pp 1–20
Baur H, Kasperek S, Pfaff E (1975) Criteria of viability of isolated liver cells. Z Physiol Chem 356:827–838
Bergmeyer HU, Bern TE (1974) Colorimetric assay with L(+)lactate, NAD, phenazone, methosulphate and INT. In: Bergmeyer HU (ed) Methods in enzymatic analysis. Akademic Press, New York, pp 579–582
Bódis B, Karádi O, Abdel-Salam OME, Vörös S, Nagy L, Mózsik Gy (1996) Direct cellular effect of Helicobacter pylori alone and with cotreatments of ethanol and indomethacin in mixed isolated rat gastric mucosa cells. Dig Dis Sci 41:430
Bódis B, Karádi O, Abdel-Salam OME, Faludi R, Nagy L, Mózsik Gy (1997a) Organoprotection and cytoprotection of histamine differs in rats. Inflammopharmacology 5:29–41
Bódis B, Karádi O, Nagy L, Dohocky C, Kolega M, Mózsik Gy (1997b) Direct cellular effects of some mediators, hormones and growth factor-like agents on denervated (isolated) rat gastric mucosal cells. J Physiol Paris. 91:183–187
Bódis B, Karádi O, Németh P, Dohocky C, Kolega, Mózsik Gy (1997c) Evidence for direct cellular protective effect of PL-10 substances (synthetized parts of body protecting compound, BPC) and their specificity to gastric mucosal cells. Life Sci 61:243–248
Bódis B, Németh P, Mózsik Gy (1998) Organoprotection and Cytoprotection in the Stomach. Akadémia Kiadó, Budapest
Dey CS, Majumder GC (1989) A simple quantitative method of estimation of cell intactness based on ethidium bromide fluorescence. Biochem Int 17:367–374
Gasztonyi B, Király Á, Sütő G, Vincze Á, Karádi O, Mózsik Gy (1996) A comparative study on the adenine nucleotide metabolism of the acid-dependent and non-acid-dependent acute mucosal injury in the rats. Inflammopharmacology 4:351–360
Jávor T, Bata M, Lovász L, Morón F, Nagy L, Patty I, Szabolcs J, Tárnok F, Tóth G, Mózsik G (1983) Gastric cytoprotective effects of vitamin A and other carotenoids. Int J Tissue React 5:289–296
Karádi O, Mózsik Gy (2000) Surgical and chemical vagotomy on the gastrointestinal mucosal defense. Akadémiai Kiadó, Budapest, pp 1–81
Király A, Sütö G, Vincze A, Tóth G, Matus Z, Mózsik Gy (1992) Correlation between the cytoprotective effect of beta-carotene and its gastric mucosal level in indomethacin (IND) treated rats with or without acute surgical vagotomy. Acta Physiol Hung 80:213–218
Lunec J, Dormandy TL (1979) Fluorescent lipid-peroxidation products in synovial fluid. Clin Sci (Lond) 56:53–59
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Morón F, Mózsik G, Nagy L, Patty I, Tárnok F, Jávor T (1984) Evidence for the existence of gastric cytoprotective effect of atropine and cimetidine in humans. Acta Physiol Hung 64:367–372
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mózsik Gy (2005) Neural,hormonal and pharmacological regulation of retinoid-induced gastrointestinal mucosal damage. Recent Res Dev Life 3:131–207
Mózsik Gy (2006) Molecular pharmacology and biochemistry of gastroduodenal mucosal damage and protection. In: Mózsik Gy (ed) Discoveries in Gastroenterology (1960–2005). Akadémiai Kiadó, Budapest, pp 139–224
Mózsik Gy, Dömötör A, Rumi Gy, Szekeres Gy (2007) Gastrointestinal cytoprotection: from basic science to clinical perspectives. Inflammopharmacology 15:1–12
Mózsik Gy, Szabo IL, Czimmer J (2011) Approaches to gastrointestinal cytoprotection from isolated cells, via animal experiments to healthy human and patients subjects with different gastrointestinal dosorders. Curr Pharm Des 17:1556–1572
Mózsik G, Jávor T (1988) A biochemical and pharmacological approach to the genesis of ulcer disease. A model study of ethanol-induced injury to gastric mucosa in rats. Dig Dis Sci 33:92–105
Mózsik Gy, Jávor T (1991) Therapy of ulcers with sulfhydryl and nonsulfhydryl antioxidants. In: Swabb EA, Szabó S (eds) Ulcer disease. Investigation and basis for therapy. Marcel Dekker, New York/Basel/Hong Kong, pp 321–341
Mózsik Gy, Jávor T, Dobi S (1965) Clinical pharmacological analysis of long term parasympatholytic treatment. In: Magyar I (ed) Acta tertii conventus medicinae internae hungarici. Gastroenterologia. Akadémiai Kiadó, Budapest, pp 709–715
Mózsik Gy, Jávor T, Dobi S, Petrássy K, Szabó A (1967) Development of “pharmacological denervation phenomenon” in patients with atropine treatment. Eur J Pharmacol 1:391–395
Mózsik Gy, Vizi F, Beró T, Kutas J (1976) Biochemical evaluation of mucosa tissues around the gastric, duodenal, and jejunal ulcer in patients. In: Mózsik Gy, Jávor T (eds) Progress of peptic ulcer. Akadémiai Kiadó, Budapest, pp 265–285
Mózsik Gy, Figler M, Nagy L, Patty I, Tárnok F (1981) Gastric and small intestinal energy metabolism in mucosal damage. In: Mózsik Gy, Hänninen O, Jávor T (eds) Advances in physiological sciences. Gastrointestinal defense mechanisms, vol 29. Pergamon Press/Akadémiai Kiadó, Oxford/Budapest, pp 213–277
Mózsik Gy, Nagy L, Tárnok F (1981) Feed-back mechanism systems between the ATP – adenylate cycléase – cAMP and ATP – N + -K + -dependent ATPase – ADP in the rat and human gastric fundic mucosa in relation to gastric secretion. In: Gáti T, Szollár L, Ungváry Gy (eds) Advances in physiological sciences. Nutrition, digestion, metabolism, vol 012. Pergamon Press/Akadémiai Kiadó, Oxford/Budapest, pp 157–173
Mózsik Gy, Morón F, Figler M, Jávor T, Nagy L, Patty I, Tárnok F (1983) Interrelationships between membrane-bound ATP-dependent energy systems, gastric mucosal damage produced by NaOH, hypertonic NaCl, HCl, and alcohol, and prostacyclin-induced gastric cytoprotection in rats. Prostaglandins Leukot Med 12:423–436
Mózsik Gy, Morón F, Nagy L, Ruzsa CS, Tárnok F, Jávor T (1986) Evidence of the gastric cytoprotective effects of vitamin A, atropine and cimetidine on the development of gastric mucosal damage produced by administration of indomethacin in healthy subjects. Int J Tissue React 8:85–90
Mózsik Gy, Pihan G, Szabó S, Jávor T, Czeglédi B, Tigyi A, Tárnok F, Zsoldos T (1987) Free radicals, nonsulhydryl antioxidants, drugs, and vitamins i acute gastric mucosal injury and protection. In: Szabó S, Mózsik Gy (eds) New pharmacology of ulcer disease. Experimental and new therapeutic approaches. Elsevier Science, New York, pp 197–207
Mózsik Gy, Figler M, Garamszegi M, Jávor T, Nagy L, Sütő G, Vincze Á, Zsoldos T (1989a) Mechanism of gastric mucosal cytoprotection. I. Time-sequence analysis of gastric mucosal membrane-bound ATP-dependent energy systems, oxygen free radicals and macroscopically appearance of gastric cytoprotection by PGI2 and β-carotene in HCL- model of rats. In: Hayashi E, Niki M, Kondo M, Yoshikawa T (eds) Medical, biochemical, and chemical aspects of free radicals. Elsevier Science, Amsterdam, pp 1421–1425
Mózsik Gy, Figler M, Garamszegi M, Jávor T, Nagy L, Sütő G, Vincze Á, Zsoldos T (1989b) Mechanism of gastric mucosal cytoprotection. II. Time-sequence analysis of gastric mucosal membrane-bound ATP-dependent energy systems, oxygen free radicals and appearance of gastric mucosal damage. In: Hayashi E, Niki M, Kondo M, Yoshikawa T (eds) Medical, biochemical, and chemical aspects of free radicals. Elsevier Science, Amsterdam, pp 1427–1431
Mózsik Gy, Király Á, Garamszegi M, Jávor T, Nagy L, Sütő G (1991) Failure of prostacyclin,β-carotene, atropine and cimetidine to produce gastric cyto- and general mucosal protection in surgically vagotomized rats. Life Sci 49:1383–1389
Mózsik Gy, Király Á, Sütő G, Vincze Á (1992) ATP breakdown and resynthesis in the development of gastrointestinal mucosal damage and its prevention in animals and human (An overview of 25 years ulcer research study). Acta Physiol Hung 80:39–80
Mózsik G, Bódis B, Garamszegi M, Karádi O, Király Á, Nagy L, Sütő G, Tóth G, Vincze Á (1995) Role of vagal nerve in the development of gastric mucosal injury and its prevention by atropine, cimetidine, β-carotene and prostacyclin in rats. In: Szabó S, Tache Y, Glavin G (eds) Neuroendocrinology of gastrointestinal ulceration. Plenum Press, New York, pp 175–190
Mózsik G, Bódis B, Karádi O, Király A, Nagy L, Rumi G, Süto G, Szabó I, Vincze A (1998) Cellular mechanisms of beta-carotene-induced gastric cytoprotection in indomethacin-treated rats. Inflammopharmacology 6:27–40
Mózsik Gy, Nagy Z, Nagy A, Rumi G, Karádi O, Czimmer J, Matus Z, Tóth G, Pár A (2001) Leiden mutation (as genetic) and environmental (retinoids) sequences in the acute and chronic inflammatory and premalignant colon disease in human gastrointestinal tract. J Physiol Paris 95:489–494
Mózsik G, Figler M, Gasztonyi B, Karádi O, Losonzy H, Nagy A, Nagy Z, Pár A, Rumi G Jr, Sütó G, Vincze A (2002) Prevalence of Leiden mutation in various gastrointestinal disorders. Orv Hetil 143:447–450
Mózsik Gy, Nagy Á, Nagy Zs, Pár A (2003a) A comparative study to prevalence of activated protein C in different gastrointestinal and thrombophylic patients vs. healthy subjects in Hungary. Gastroenterology 122 (Suppl) A430–A431
Mózsik Gy, Rumi Gy, Figler M, Nagy Zs, Gasztonyi B, Bódis B, Debreceni A, Matus Z (2003b) Correlations between the serum level of retinoids in patients with Crohn disease on dependence its activity. Gastroenterology 122 (Suppl):A430
Mózsik G, Rumi G, Dömötör A, Figler M, Gasztonyi B, Papp E, Pár A, Pár G, Belágyi J, Matus Z, Melegh B (2005a) Involvement of serum retinoids and Leiden mutation in patients with esophageal, gastric, liver, pancreatic, and colorectal cancers in Hungary. World J Gastroenterol 28:7646–7650
Mózsik G, Szolcsányi J, Rácz I (2005b) Gastroprotection induced by capsaicin in healthy human subjects. World J Gastroenterol 11:5180–5184
Mózsik Gy, Past T, Dömötör A, Kuzma M, Perjési P (2010) Production of orally applicable new drug or drug combinations from natural origin capsaicinoids for human medical therapy. Curr Pharm Des 16:1197–1208
Nagy L, Mózsik G, Feledi E, Ruzsa C, Vezekényi Z, Jávor T (1984) Gastric microbleeding measurements during one day treatment with indomethacin and indomethacin plus sodium salicylate (1:10) in patients. Acta Physiol Hung 64:373–377
Nagy L, Szabó S, Morales RE, Plebani M, Jenkins JM (1994) Identification of subcellular targets and sensitive tests of ethanol-induced damage in isolated rat gastric mucosal cells. Gastroenterology 107:907–914
Patty I, Benedek S, Deák G, Jávor T, Kenéz P, Nagy L, Simon L, Tárnok F, Mózsik G (1982) Controlled trial of vitamin A therapy in gastric ulcer. Lancet 2:876
Patty I, Benedek S, Deák G, Jávor T, Kenéz P, Morón F, Nagy L, Simon L, Tárnok F, Mózsik G (1983) Cytoprotective effect of vitamin A and its clinical importance in the treatment of patients with chronic gastric ulcer. Int J Tissue React 5:301–307
Recknagel RO, Ghoshal AK (1966) Quantitative estimation of peroxidative degeneration of rat liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning. Exp Mol Pathol 5:413–426
Robert A, Nemazis JE, Lancaster C, Hanchar A (1979) Cytoprotection by prostaglandins in rats Prevention of gastric produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 77:433–443
Rumi G, Szabó I, Vincze A, Matus Z, Tóth G, Rumi G, Mózsik Gy (1999) Decrease in serum levels of vitamin A and zeaxanthin in patients with colorectal polyp. Eur J Gastroenterol Hepatol 11:305–308
Rumi G, Szabó I, Vincze A, Matus Z, Tóth G, Mózsik GY (2000) Decrease of serum carotenoids in Crohn’s disease. J Physiol Paris 94:159–161
Rumi G, Matus Z, Tóth G, Pár A, Nagy Z, Vincze A, Rumi G, Mózsik Gy (2001a) Changes of serum carotenoids in patients with esophageal, gastric, hepatocellular, pancreatic and colorectal cancer. J Physiol Paris 95:239–242
Rumi G, Pár A, Matus Z, Rumi Gy, Mózsik Gy (2001b) The defensive effects of retinoids in the gastrointestinal tract (animal experiments and human observations). Akadémiai Kiadó, Budapest, pp 1–79
Selye HA (1936) Syndrome produced by diverse nocuous agents. Nature 138:32
Shay H, Komarov SA, Fels SS, Meranze D, Gurenstein M, Siplet H (1945) A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5:46–61
Sütő G, Garamszegi M, Jávor T, Vincze A, Mózsik G (1989) Similarities and differences in the cytoprotection induced by PGI2 and beta-carotene in experimental ulcer. Acta Physiol Hung 73:155–158
Szabó I, Bódis B, Nagy L, Mózsik Gy (1996a) Methods for the examination of the direct injury and protective actions on the isolated gastric mucosal cells. Hung Med Wkly J 137:2687–2689
Szabó I, Bódis B, Nagy L, Mózsik Gy (1996b) Comparative studies of isolated gastric mucosa mixed cells and hepatoma and myeloma cell lines with ethanol indomethacin, and their combination. Inflammopharmacology 5:83–91
Szabó I, Bódis B, Nagy L, Mózsik Gy (1997a) Comparative studies on isolated mucosal mixed cells and hepatoma, myeloma cell lines with ethanol, indomethacin and their combinations. Inflammopharmacology 5: 83–89
Szabó I, Bódis B, Németh P, Mózsik Gy (1997b) Usage of scavengers on stable cultured (SP2/0-AG14 and HEP G2) cell lines as an approach to reveal the mechanisms of cytoprotection, In: Mózsik Gy, Nagy L, Király Á (eds) Twenty five years of peptic ulcer research in Hungary. From basic science to clinical practice 1975–1995. Akadémia Kiadó, Budapest, pp 275–285
Szabó I, Rumi Gy, Bódis B, Németh P, Mózsik Gy (2000) Gastrin and pentagastrin enhance the tumour proliferation of human stable cultured gastric adenocarcinoma cells. J Physiol Paris 94:71–74
Tapodi A, Debreceni B, Hanto K, Bognar Z, Wittmann I, Gallyas F, Varbiro G, Sumegi B (2005) Pivotal role of Akt activation in mitochondrial protection and cell survival by poly (ADP- ribose)polymerase-1 inhibition in oxidative stress. J Biol Chem 280:35767–35775
Tárnok F, Jávor T, Mózsik Gy, Nagy L, Patty I, Rumi Gy, Solt I (1979) A prospective multiclinical study comparing the effects of placebo, carbenoxolone, atropine, cimetidine in patients with duodenal ulcer. Drugs Exp Clin Res 5:157–166
Vincze Á, Király Á, Sütő G, Karádi O, Mózsik G (1997) Role of neurohumoral and local mucosal factors in b-carotene induced gastroprotection in the rats. In: Mózsik Gy, Nagy L, Király Á (eds) Twenty five years of peptic ulcer research in Hungary. From basic sciences to clinical practice. Akadémiai Kiadó, Budapest, pp 265–273
Yu TC, Sinnhuber RO (1967) An improved 2-thiobarbituric acid (TBA) procedure for the measurement of autoxidation in fish oils. J Am Oil Chem Soc 44:256–258
Acknowledgment
This study was supported by the National Office for Research and Technology, “Baros Gábor” Program (REG-DD-09-2-2009-0087, CAPSATAB).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Szabo, I.L., Czimmer, J., Mózsik, G. (2014). Translational Research on the Roles of Free Radicals and Antioxidants in Gastrointestinal Disorders. In: Laher, I. (eds) Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30018-9_138
Download citation
DOI: https://doi.org/10.1007/978-3-642-30018-9_138
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30017-2
Online ISBN: 978-3-642-30018-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences