Abstract
Schizophrenia has long been associated with an imbalance in dopamine (DA) neurotransmission, and brain imaging has played an important role in advancing our knowledge and providing evidence for the dopaminergic abnormalities. This chapter reviews the evidence for DA dysfunction in different brain regions in schizophrenia, in particular striatal, extrastriatal, and prefrontal regions, with emphasis on recently published findings. As opposed to the traditional view that most striatal dopaminergic excess, associated with the positive symptoms of schizophrenia, involves the dopaminergic mesolimbic pathway, recent evidence points to the nigrostriatal pathway as the area of highest dysregulation. Furthermore, evidence from translational research suggests that dopaminergic excess may be present in the prodromal phase, and may by itself, as suggested by the phenotype observed in transgenic mice with developmental overexpression of dorso-striatal D2 receptors, be an early pathogenic condition, leading to irreversible cortical dysfunction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The psychotomimetic effects of stimulant drugs, in combination with the observed antipsychotic effects of D2-blocking drugs, gave rise to the initial dopamine (DA) hypothesis of schizophrenia (Carlsson 1977, 1978; van Rossum 1966). Refined and modified in the intervening years, this theory to date remains central to the pathophysiology of schizophrenia (Howes and Kapur 2009; Laruelle et al. 2003; Lieberman et al. 1997).
Based on the observation that DA-enhancing drugs such as amphetamine show psychotomimetic properties while the effectiveness of classic antipsychotic medication, blocking DA D2 receptors, had been shown to directly correlate with its affinity for the D2 receptor (Creese et al. 1976), the dopamine hypothesis initially assumed a general dopaminergic hyperfunction (Carlsson 1977, 1978). However, subsequent findings, including reduced cerebral blood flow in the prefrontal cortex (PFC) of patients with schizophrenia and the observation that negative symptoms such as flattened affect, anhedonia, and compromised cognitive function did not respond to classic antipsychotic treatment targeting D2 receptors, were incompatible with a generally overactive DA system. Accordingly, the dopamine hypothesis was reformulated as an imbalance in DA neurotransmission in different regions of the brain, characterized in particular by hyperactivity in the subcortical DA pathway and hypoactivity in the cortical DA system (Davis et al. 1991; Knable and Weinberger 1997; Weinberger 1987). It was also suggested that DA hyperactivity in subcortical brain regions, in particular striatal areas, accounted primarily for the positive symptoms of schizophrenia, while the negative symptoms were mostly associated with DA hypoactivity in cortical areas, particularly PFC (Abi-Dargham 2004).
In this chapter, we present the history as well as the latest research findings relating to dopamine dysfunction in schizophrenia. The two main new findings that will be emphasized here are the following: Unlike the traditional thinking that most dopaminergic excess causing positive symptoms involves the dopaminergic mesolimbic pathway, recent evidence points to the nigrostriatal pathway, projecting to the anterior caudate, part of the associative striatum, as the area of highest dysregulation. Furthermore, as opposed to the thinking that dopamine is a final common pathway, recent evidence from studies in mice with a developmental overexpression of D2 receptors (Kellendonk et al. 2006) and in patients at high risk for developing schizophrenia (Howes et al. 2009) suggests that dopamine dysregulation may be an early pathogenic mechanism that may lead to further dysregulation of brain function. We will review the evidence and outline future research needed to understand the molecular mechanisms and develop better therapeutic interventions.
2 Evidence for Dopamine (DA) Dysfunction in Schizophrenia
Direct empirical evidence for DA alterations in schizophrenia was initially elusive. Postmortem studies were difficult to interpret due to the possible confounding by prior antipsychotic exposure and the first imaging studies using Positron Emission Tomography (PET) and Single Photon Emission Tomography (SPECT) produced inconsistent findings. Advances in PET and SPECT imaging techniques, such as the development of new, high-affinity radiotracers, made it possible to study DA neurotransmission in several regions of the brain, including striatal, extrastriatal, and prefrontal cortical regions with more anatomical detail than previously possible.
2.1 Striatal DA Alterations
Using PET and SPECT imaging techniques, different paradigms have been employed in order to study aspects of presynaptic and postsynaptic striatal DA alterations in schizophrenia. These approaches include markers of DA synthesis, release and reuptake, as well as DA receptor availability and differences in receptor affinity states (Erritzoe et al. 2003; Laruelle 1998; Seeman et al. 2006).
2.1.1 Presynaptic DAergic Parameters
2.1.1.1 Dopamine Synthesis
DA is synthesized by hydroxylation of the precursor tyrosine to l-DOPA, which then is decarboxylated to DA by aromatic acid decarboxylase (AADC). Radioactive analogues of l-DOPA, such as [11C]l-DOPA and [18F]DOPA, have been used to estimate enzymatic activity of AADC, as an indicator of DA synthesis capacity (Brown et al. 1999; Cumming and Gjedde 1998; Garnett et al. 1978, 1983). However, this is overly simplified and other factors may affect the overall signal measured with [11C]l-DOPA and [18F]DOPA, including delivery of [11C]l-DOPA and [18F]DOPA to the brain, crossing the plasma membrane, storage in presynaptic vesicles as a result of vesicular monoamine transporter (VMAT) activity, release of [11C]l-DOPA and [18F]DOPA, and degradation, all may play a role in addition to the activity of DOPA decarboxylase. Better knowledge of these mechanisms is needed to better understand the exact molecular processes underlying [11C]l-DOPA and [18F]DOPA uptake and their disturbance in schizophrenia.
Nevertheless, a number of studies have used radiolabeled l-DOPA, such as [11C]l-DOPA or [18F]DOPA, in schizophrenia. The first study, by Reith et al. (1994), revealed significantly elevated [18F]DOPA uptake in drug-naïve patients compared to healthy controls. While the majority of the following studies using the same technique replicated this finding (Hietala et al. 1995, 1999; Howes et al. 2009; Lindstrom et al. 1999; McGowan et al. 2004; Meyer-Lindenberg et al. 2002; Nozaki et al. 2009), two studies failed to find markers of elevated DA synthesis capacity: One study reported a small but not significant increase in [18F]DOPA uptake (Dao-Castellana et al. 1997), while another found decreased levels of [18F]DOPA uptake (Elkashef et al. 2000). This discrepancy might be explained by differences in study population: Studies reporting positive findings were all conducted in acutely psychotic, mostly drug-naïve, patients (with the exception of McGowan and colleagues), while the two studies reporting negative or inverse findings were done in chronic, previously medicated, patients. Recently, DA synthesis capacity was investigated in twins discordant for schizophrenia. No elevation in striatal DA synthesis was observed in the unaffected twin, nor in the low-symptomatic medicated twin with chronic schizophrenia when compared to controls, suggesting that increased DA synthesis is not a genetic marker for schizophrenia (Shotbolt et al. 2011). Most recently, Howes et al. (2009) showed increases in [18F]DOPA uptake in associative striatum in patients prodromal for schizophrenia, predicting conversion (Howes et al. 2011b) and progressing further on follow-up after 2 years to involve the sensorimotor striatal subregion (Howes et al. 2011a).
2.1.1.2 Dopamine Release
The next step in DA transmission is the release of DA from the presynaptic terminal. An estimation of DA release from striatal DA neuron terminals can be obtained by measuring changes in D2 binding potential after pharmacologically induced DA release with SPECT and PET imaging techniques (Kegeles et al. 1999; Laruelle 2000; Laruelle et al. 1995). One of the first studies to investigate amphetamine-induced DA release in schizophrenia was conducted by Laruelle et al. (1996), using SPECT and the radiotracer [123I]IBZM, an antagonist at D2/3 receptors. Changes in D2/3 binding potential as an estimation of amphetamine-induced DA release were measured in 15 medication-free patients with schizophrenia and 15 healthy control participants at the level of the striatum as a whole. The decrease in binding potential induced by amphetamine was significantly greater in patients with schizophrenia compared to controls. Furthermore, the amphetamine-induced decrease in D2/3 binding potential was associated with the transient increase in positive symptoms induced by amphetamine in the schizophrenia group (Laruelle et al. 1996). The results of a subsequent PET study using [11C]raclopride as radiotracer were in line with these observations (Breier et al. 1997): The schizophrenia group showed greater amphetamine-induced decreases in [11C]raclopride binding compared to controls, indicative of greater amphetamine-induced release of endogenous DA. Furthermore, the study did not find differences in amphetamine effects on [11C]raclopride binding between medication-free (N = 5) and medication-naïve (N = 6) patients with schizophrenia, indicating that the effect occurs independent of earlier treatment with antipsychotics. Abi-Dargham et al. (1998) have since then replicated these findings. Furthermore, it was shown that changes in amphetamine-induced dopamine release in schizophrenia might be associated with illness phase, as amphetamine-induced transient worsening of psychotic symptoms correlated with changes in [11C]raclopride binding in patients with schizophrenia and no difference in [11C]raclopride binding was seen in patients in remission compared to controls (Laruelle et al. 1999).
The amphetamine paradigm shows stimulated release, as amphetamine increases dopamine levels by reversing DA transport. Another aspect of DA transmission is uncovered with depletion paradigms: The administration of alpha-methyl-para-tyrosine (αMPT), a reversible competitive inhibitor of tyrosine hydroxylase, causes acute depletion of endogenous DA and can be used as a method to assess the degree of baseline (nonstimulated) intrasynaptic DA concentration indirectly measured through D2/3 occupancy (Laruelle et al. 1997), most likely reflecting basal release as a result of neuronal firing. Abi-Dargham et al. (2000) used this technique in combination with SPECT and [123I]IBZM in 18 untreated patients with schizophrenia and 18 matched control participants before and after αMPT-induced DA depletion. Exposure to αMPT led to increased D2 receptor availability in both patients with schizophrenia and healthy controls; however, the effect was larger in schizophrenia patients, indicative of higher baseline DA activity in this group. The magnitude of the effect did not differ between medication-naïve patients with schizophrenia and those previously treated with antipsychotics (Abi-Dargham et al. 2000). Similar results were recently obtained by Kegeles et al. (2010a), who employed the same depletion paradigm in combination with PET and [11C]raclopride. Kegeles and colleagues furthermore showed that the largest effect of acute DA depletion on D2 receptor binding was observed in the associative striatum and not in limbic striatum as was long hypothesized. Recently, it was shown that amphetamine-stimulated DA release and baseline DA activity are related in patients with schizophrenia but not in controls (Abi-Dargham et al. 2009). Pilot data from Canada furthermore suggest that DA release in patients with schizophrenia and individuals at high risk compared to healthy controls is also increased in response to psychosocial stress (Mizrahi 2010). Altogether, studies have consistently demonstrated increased amphetamine-stimulated DA release in schizophrenia. Depletion studies further suggest that schizophrenia is characterized by increased baseline DA activity.
2.1.1.3 Dopamine Reuptake
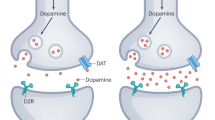
DA transmission in the striatum is regulated by DA transporters (DAT), which are located on the presynaptic membrane of DA terminals and rapidly remove DA from the synaptic cleft (Goto et al. 2007). Several studies have investigated DAT density in schizophrenia, using PET or SPECT, to obtain an index of density of DA terminals and striatal innervation (Hsiao et al. 2003; Laakso et al. 2000, 2001; Laruelle et al. 2000; Lavalaye et al. 2001; Mateos et al. 2005, 2007; Schmitt et al. 2005, 2006, 2008; Yang et al. 2004; Yoder et al. 2004). The majority of studies did not observe differences in DAT availability between antipsychotic-naïve patients with schizophrenia and controls (Hsiao et al. 2003; Laakso et al. 2000; Laruelle et al. 2000; Lavalaye et al. 2001; Schmitt et al. 2005, 2006; Yoder et al. 2004). Two studies reported reduced DAT binding in patients with schizophrenia compared to controls (Laakso et al. 2001; Mateos et al. 2005). However, since the patients were not medication-naïve, the researchers concluded that the observed reduction in DAT binding was secondary to prior treatment with antipsychotics. In order to clarify this issue, Mateos et al. (2007) repeated the experiment in a cohort of antipsychotic-naïve patients with schizophrenia and also found lower DAT density (Mateos et al. 2007). Still, this remains the only study, which reported decreased DAT binding in schizophrenia. So, taken together the evidence suggests that schizophrenia is not associated with changes in DAT density.
Recently, two dual-isotope SPECT studies used [99mTc]TRODAT and [123I]IBZM to simultaneously measure DAT and D2 receptor availability in drug-naïve patients with schizophrenia and controls (Schmitt et al. 2008; Yang et al. 2004). In line with most of the previous findings, no overall differences in DAT or D2 receptor availability were observed. However, in patients but not controls, DAT density correlated positively with D2 receptor availability (Yang et al. 2004), which was most pronounced in patients presenting with predominantly positive symptoms (Schmitt et al. 2008).
2.1.2 Postsynaptic DAergic Parameters
2.1.2.1 DA Receptor Availability
The most extensively studied receptor in schizophrenia is the D2 receptor, which is abundantly present in the human striatum. Numerous studies have measured the density of striatal D2 receptors in schizophrenia, both in vivo and postmortem, and three meta-analyses have reviewed the strength and consistency of the reported findings (Kestler et al. 2001; Laruelle 1998; Zakzanis and Hansen 1998). We should note here that most of these studies refer to both D2 and D3 receptors, as the ligands are not specific to one subtype. Unless otherwise specified, when we mention the D2 receptor in this review, we also refer to both subtypes. The first of these meta-analyses was conducted by Zakzanis and Hansen (1998) and included 17 studies of which 7 were postmortem. The results from 511 patients with schizophrenia and 534 controls suggested that, although D2 receptor availability was elevated in approximately 70 % of the patients, increased receptor density failed to discriminate patients from controls and is therefore unlikely to represent a general marker of schizophrenia (Zakzanis and Hansen 1998). In line with this are the findings of the second meta-analysis, which included 15 in vivo studies on D2 receptor density in schizophrenia, and concluded that there was small but significant elevation of receptor availability along with greater variability in schizophrenia patients compared to controls (Laruelle 1998). The more recent, comprehensive meta-analysis by Kestler et al. (2001) included 20 postmortem studies and 17 in vivo studies on D2 receptor availability in schizophrenia and took into account differences in methodology such as in vivo versus postmortem measures as well as the possible influence of sample characteristics such as age, gender, or medication status. The authors concluded that the data were compatible with the idea of a subgroup of patients with schizophrenia being characterized by elevated D2 density (Kestler et al. 2001). The results of a recent study using the high-affinity D2/3 ligand [18F]fallypride were also in line with this, showing selective alterations in D2 density in schizophrenia patients (5 drug naïve, 16 drug free) (Kegeles et al. 2010b). Meta-analysis of D2 receptor studies in schizophrenia has shown a modest increase in D2 receptor density; however, the findings are likely to be confounded by prior treatment with antipsychotics. Chronic administration of antipsychotic medication has been shown to upregulate D2 receptor density both in preclinical animal models and humans (Burt et al. 1977; Kashihara et al. 1986; Silvestri et al. 2000). A recent PET study in cats suggested that the magnitude of D2 receptor upregulation depends on the percentage occupancy of D2 receptor and the temporal pattern of the antipsychotic administration. A high dose (80 % occupancy of D2 receptor) of haloperidol administered continuously by osmotic mini-pumps over 4 weeks produced a robust upregulation of D2 receptor, still detectable 2 weeks after withdrawal. On the other hand, a lower dose (60 % occupancy D2 receptor) or s.c administration once a day (transient 80 % occupancy for few hours) of haloperidol did not change D2 receptor availability (Ginovart et al. 2009). For patients with schizophrenia, antipsychotics are prescribed both as oral and as depot medication and require occupancy between 60 and 80 % for therapeutic effect, beyond which the risk of side effects increases notably (Farde et al. 1992; Fitzgerald et al. 2000). The majority of studies of D2 receptor availability in schizophrenia used mixed drug-free and drug-naïve patient groups; thus it is likely that increased D2 receptor density may be, at least in part, related to prior exposure to antipsychotic rather than to the disease process per se.
Several lines of research indicate that, in addition to the D2 receptor, the D3 receptor might play an important role in the pathophysiology of schizophrenia (Griffon et al. 1995; Gurevich et al. 1997; Sokoloff et al. 2006). However, until recently it was not feasible to selectively target D3 receptors with neurochemical imaging to obtain a direct measure of D3 in schizophrenia, since the available radiotracers exhibited similar affinities for the D2 and D3 receptors and therefore could not distinguish between them. Using the D2/3 agonist radiotracer [11C]PHNO, which has higher affinity for D3 than for D2, a recent study did not reveal differences in receptor levels between patients with schizophrenia compared to controls (Graff-Guerrero et al. 2009), although a more selective tracer would be needed to replicate this initial finding.
Several postmortem studies have used ligand subtraction methods to measure the distribution of striatal D4 receptors in schizophrenia. However, these studies produced inconsistent results, some reporting increased availability of D4 (Marzella et al. 1997; Murray et al. 1995; Seeman et al. 1993; Sumiyoshi et al. 1995), while others did not find differences in D4 receptors between schizophrenia patients and controls (Lahti et al. 1996, 1998; Reynolds and Mason 1994). More selective tracers are needed to better characterize these receptors.
Most (postmortem) studies did not find altered levels of D1 receptors in the striatum (Cross et al. 1981; Joyce et al. 1988; Knable et al. 1994; Pimoule et al. 1985; Reynolds and Czudek 1988; Seeman et al. 1987); one reported a slight decrease in D1 density (Hess et al. 1987). The results of two in vivo studies of striatal D1 density in schizophrenia are in line with the majority of postmortem findings and do not suggest alterations in striatal D1 receptor levels in schizophrenia (Abi-Dargham et al. 2002; Okubo et al. 1997).
Taken together, it seems that a subgroup of schizophrenia patients is characterized by increased density of D2-like (i.e., D2/3) receptors, independent of age, gender, and prior antipsychotic exposure. The density of striatal D1 receptors on the other hand seems to be unaltered. No conclusive data are available for D4.
2.1.2.2 Balance in D2 Receptor Affinity States
Being the primary target of antipsychotic medication, the D2 receptor plays a major role in schizophrenia and psychosis (Kapur and Mamo 2003; Kapur and Remington 2001), although, as discussed earlier, its involvement in the pathophysiology of psychotic disorders remains unclear (Kestler et al. 2001; Laruelle 1998; Zakzanis and Hansen 1998). The D2 receptor has been shown to exist in two different affinity states: a high-affinity, active state (D high2 ) and a low-affinity, inert state (D low2 ) (Sibley et al. 1982; Willeit et al. 2006). Preclinical work has shown that radiolabeled DA agonists such as (+)-PHNO, which selectively binds to D2/3 receptors in the high-affinity state, in combination with PET can be used to study the distribution of D high2 both in vitro (Nobrega and Seeman 1994) and in vivo (Galineau et al. 2006; Ginovart et al. 2006). The distribution of D high2 using PET and [11C](+)-PHNO was recently demonstrated also in human volunteers (Graff-Guerrero et al. 2008; Willeit et al. 2006).
For schizophrenia, it has been suggested that the observed supersensitivity to DA-enhancing drugs such as amphetamine is the result of an imbalance in D2 affinity states, in particular elevated availability of D high2 receptors (Seeman 2010; Seeman et al. 2005). However, evidence for this conclusions stems primarily from animal models of schizophrenia (for a review see Seeman 2010). Recently, the distribution of D high2 was for the first time studied in patients with schizophrenia, and contrary to what could be expected from preclinical work, no elevation in D high2 was observed in medication-free patients with schizophrenia compared to controls (Graff-Guerrero et al. 2009). Although the authors acknowledge that differences in D high2 between schizophrenia patients and controls could have been masked by endogenous DA, to date, there is no evidence for D high2 dysregulation in schizophrenia.
2.2 Extrastriatal DA Alterations
Several studies have now used high-affinity radiotracers such as [18F]fallypride, [11C]FLB 457, and [123I]epidepride to measure the distribution of D2-type receptors in low-density brain regions such as the thalamus, anterior cingulate cortex, temporal cortex, or substantia nigra in unmedicated and medication-naïve patients with schizophrenia. Some studies found decreased D2 receptor availability in the thalamus (Buchsbaum et al. 2006; Kessler et al. 2009; Talvik et al. 2003, 2006; Yasuno et al. 2004), anterior cingulate cortex (Buchsbaum et al. 2006; Suhara et al. 2002), temporal cortex (Buchsbaum et al. 2006; Tuppurainen et al. 2003), and midbrain (Tuppurainen et al. 2006), while some found no differences in the thalamus (Tuppurainen et al. 2006), anterior cingulate, and temporal cortex (Glenthoj et al. 2006; Kessler et al. 2009; Talvik et al. 2003), and one study found an increase in D2 receptor availability in schizophrenia in the substantia nigra (Kessler et al. 2009). A large recent study did not confirm any of the above reported extrastriatal D2 receptor alterations in schizophrenia (Kegeles et al. 2010b).
Another recent study has used the radiotracer [11C]PE21 in combination with PET to visualize thalamic DAT in patients with schizophrenia, who were either medication-naïve or off medication for at least 6 months (Arakawa et al. 2009). In contrast to striatal brain regions, where DAT seems to be unaffected in schizophrenia, this study reported increased DAT binding in the thalamus of patients with schizophrenia compared to controls. Another study measured extrastriatal DA synthesis capacity using PET and [11C]l-DOPA and found no differences between medication-naïve patients with schizophrenia and controls with regard to DA synthesis capacity in the thalamus or anterior cingulate and temporal cortex (Nozaki et al. 2009), although the ability of this tracer to measure extrastriatal dopamine synthesis is questionable (Cropley et al. 2008).
2.3 Prefrontal Cortical DA Alterations
While D2 receptors are abundantly present in striatal regions of the brain, the predominant DA receptors in prefrontal cortical regions are of the D1 type (Hall et al. 1994). The distribution of D1 receptors in schizophrenia has been studied using the PET radiotracers [11C]NNC 112 or [11C]SCH 23390. However, the few studies that have been conducted produced conflicting results. While Okubo et al. (1997), using [11C]SCH 23390, found a decrease in receptor binding in patients with schizophrenia compared to controls, the study by Karlsson et al. (2002), using the same radiotracer, did not reveal any differences between groups (Karlsson et al. 2002; Okubo et al. 1997). However, patients in this latter study were all drug-naïve, while the former study also included drug-free patients. The more recent study by Hirvonen et al. (2006) reported decreased [11C]SCH 23390 binding in frontotemporal brain regions of previously medicated patients with schizophrenia compared to their unaffected co-twins and healthy comparison twins and higher doses of antipsychotics were associated with greater decreases in D1 receptor binding. Interestingly, unaffected monozygotic co-twins displayed increased receptor binding compared to healthy comparison twins, and unaffected dizygotic co-twins showed intermediate levels. Two other studies used the radiotracer [11C]NNC 112: One reported increased D1 receptor binding in the schizophrenia group, which correlated with deficits in working memory performance (Abi-Dargham et al. 2002), and the second study found increases limited to the drug-naïve patients but not the previously treated ones (Abi-Dargham et al. in press). Studies of D1 receptor availability in schizophrenia are summarized in Table 1.
While it is possible that the discrepancy in findings is due to the influence of antipsychotic medication, it might also be that differences in radioligand properties contributed to the diverging results, as shown by Guo et al. (2003): Using a DA depletion paradigm the researchers could demonstrate that the in vivo binding of the two radiotracers [11C]SCH 23390 and [11C]NNC 112 was affected differentially by changes in endogenous DA, indicating that the increased D1 receptor availability observed in the studies by Abi-Dargham et al. (2002) using [11C]NNC 112 might reflect an upregulation of D1 receptors, secondary to chronically low DA levels. This interpretation would also be consistent with the observed correlation between increased D1 receptor binding and deficits in working memory. However, since both radioligands also bind to the serotonergic 5-HT2A receptor (Ekelund et al. 2007; Slifstein et al. 2007), selective tracers are needed to pursue these investigations of the role of cortical D1 receptors in schizophrenia.
2.4 Dopamine in At-Risk and Prodromal States
The studies described earlier have revealed several aspects of DA dysfunction in patients with established schizophrenia. In order to extend these findings and shed light on the timing of DA dysfunction in schizophrenia, researchers have started recently to examine different aspects of DA transmission in individuals with prodromal signs of schizophrenia and individuals at genetic or psychometric risk for psychosis.
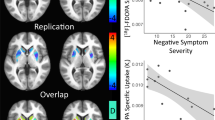
Three studies have investigated striatal DA synthesis capacity with PET and [18F]DOPA. While Huttunen et al. (2008) observed increased [18F]DOPA uptake in the caudate-putamen in low-symptomatic first-degree relatives of patients with schizophrenia compared to controls, the study by Shotbolt et al. (2011) did not reveal any changes in radiotracer uptake in unaffected and completely asymptomatic co-twins of schizophrenia patients compared to controls. Howes et al. (2009) found increased [18F]-DOPA uptake in individuals with prodromal signs of schizophrenia compared to controls. Interestingly, this effect was most pronounced in the associative striatum and correlated positively with severity of prodromal symptoms. Moreover, in a subsequent study the authors could indicate that the increase in DA synthesis capacity observed in individuals in the prodromal phase of the illness progressed over time only in those individuals who later developed schizophrenia, but not in those who did not develop the disease (Howes et al. 2011a). However, in contrast to the findings in prodromal individuals, where the effect was most pronounced in associative striatum, the progression in DA synthesis alteration in those who developed the disease was only seen in sensorimotor striatum (Howes et al. 2011a).
Three other studies have examined stimulated DA release in individuals psychometrically at risk for schizophrenia (i.e., individuals with schizoptypal traits). Soliman et al. (2007) employed a psychosocial stress paradigm in combination with PET and [11C]raclopride to study stress-induced DA release in psychometric schizotypes compared to controls. No changes in DA release in response to stress were observed in normal controls, nor in the “positive” schizotypes (i.e., individuals with perceptual aberrations). Only the “negative” schizotypes (i.e., individuals with physical anhedonia) showed an increase in DA release in response to stress compared to baseline (Soliman et al. 2007). Abi-Dargham et al. (2004) as well as Woodward et al. (2010) studied amphetamine-induced DA release in individuals with schizotypal personality disorder (SPD) and individuals with schizotypal traits, respectively. In the SPD subjects of the first study, amphetamine caused a larger decrease in [123I]IBZM binding compared to controls (Abi-Dargham et al. 2004). Similarly, amphetamine-induced DA release measured indirectly through D2 occupancy with PET and [18F]fallypride correlated positively with schizotypal traits in the second study (Woodward et al. 2010). In accordance with previous findings showing that DA dysregulation might be most pronounced in associative striatum as opposed to limbic or sensorimotor regions (Howes et al. 2009; Kegeles et al. 2010a), the correlation between stimulated DA release and schizotypal traits was strongest in associative striatum.
Finally, one study has investigated striatal D2 receptor availability in six monozygotic and five dizygotic unaffected co-twins of patients with schizophrenia and compared them to control twins without a family history of psychosis. Elevated caudate D2 receptor availability was observed only in the monozygotic co-twins of schizophrenia patients, compared to dizygotic co-twins and controls. No changes, however, were revealed in the dizygotic co-twins compared to controls (Hirvonen et al. 2005).
In summary, studies suggest an increase in DA in schizotypal states and in relation to schizotypal and prodromal symptoms, linking DA dysfunction to the expression of the schizophrenia phenotype. Combined with the findings of Shotbolt et al. (2011) who did not observe any changes in DA synthesis capacity in unaffected co-twins of patients with schizophrenia, this finding suggests that DA dysfunction does not relate to a general genetic vulnerability in the absence of behavioral manifestations but rather represents a biological marker for the very early expression of schizophrenia symptomatology.
2.5 Summary
The most consistently found dopaminergic alteration in schizophrenia is elevated presynaptic DA function in striatal brain regions (see Figs. 1 and 2 for an illustration), in particular associative striatum, as demonstrated by imaging studies showing (1) increased l-DOPA uptake as an index of increased DA synthesis capacity, (2) elevated amphetamine-induced DA release, and (3) elevated D2 receptor occupancy by DA as revealed by the acute DA depletion. DAT seems to be unaffected in schizophrenia, and increased D2 receptor availability was only found in a subgroup of patients. Although preclinical work suggests an imbalance in D2 receptor affinity states being associated with psychosis, the only study in patients with schizophrenia did not find elevated D high2 . The evidence concerning DA alterations in extrastriatal and prefrontal cortical brain areas in schizophrenia seems less consistent. While some studies found elevated levels of D2 receptors in areas such as the thalamus, anterior cingulate, and temporal cortex or the midbrain and substantia nigra, others did not. The results of the few studies on D1 receptor availability in prefrontal cortex in schizophrenia are also conflicting. Findings concerning the distribution of D3 and D4 remain understudied. To date, only one study used PET and the radiotracer [11C]PHNO to investigate D3 receptor in a small sample of patients with schizophrenia compared to controls and found no differences (Graff-Guerrero et al. 2009). Thus, while a lot of effort has been put into the investigation of striatal DA alterations in schizophrenia, research has been less successful with regard to extrastriatal and prefrontal cortical regions. More research is needed to resolve the inconsistencies in findings research has provided so far.
New data bringing new evidence: The DA dysfunction in schizophrenia seems to be most pronounced in AST as opposed to VST as previously assumed. Adapted with permission from Simpson et al. (2010). SMST (sensorimotor striatum), AST (associative striatum), VST (ventral striatum), VTA (ventral tegmental area), SNc (substantia nigra pars compacta), SNr (substantia nigra pars reticulate)
3 Functional and Clinical Implications
As defined in the current, fourth version of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association 2000), schizophrenia is a highly heterogeneous disease, presenting with positive, negative, and cognitive symptoms. Positive symptoms include hallucinations and delusions, while negative symptoms refer to flattened affect, anhedonia, and loss of motivation. Cognitive disturbances are mostly seen in domains such as working memory, executive function, and aspects of social cognition. DA dysregulation plays a role within each of these dimensions. While positive symptoms seem to most directly relate to excessive striatal DA transmission, negative and cognitive symptoms have been associated with decreased DA function in PFC. The latter assumption was primarily based on the known and crucial involvement of the PFC in cognitive and emotional processes (Arnsten 2007, 2011; Goldman-Rakic and Selemon 1997). Still, after decades of research into DA and schizophrenia, it remains elusive how DA dysregulation actually translates into the complex and multifactorial symptoms that characterize the clinical picture of schizophrenia.
3.1 Relating DA Dysfunction to Positive Symptoms
Several of the imaging studies discussed in this chapter have reported associations between alterations in striatal DA function and symptomatology in patients with schizophrenia. Regarding DA synthesis, Hietala et al. (1999) found a negative correlation between striatal F-DOPA uptake and depressive symptoms and a positive correlation with paranoid symptoms, although this was significant at trend level only. Howes et al. (2009) reported a positive correlation between severity of positive prodromal symptoms as well as neuropsychological impairment and increased DA synthesis capacity in associative striatum. However, this was not true for depressive symptoms. With regard to DA release, Laruelle et al. (1996) found that amphetamine-induced decrease in D2 binding potential was associated with positive symptoms. In a subsequent study the authors could furthermore establish a relation between amphetamine-stimulated DA release and illness phase, as amphetamine-stimulated DA release was only increased in patients presenting with acute schizophrenia but not in patients in remission (Laruelle et al. 1999). Recently, Woodward et al. (2010) revealed a positive correlation between stimulated DA release and schizotypal personality traits in healthy individuals.
Assuming that striatal hyperdopaminergia plays a role in the emergence and experience of positive psychotic symptoms, the question arises as to how dopaminergic alterations in striatal brain regions ultimately give rise to the experience of hallucinations and delusions. Altered salience attribution has been suggested as a possible mechanism (Kapur et al. 2005). Burst firing of dopamine neurons in the ventral tegmental area markedly increases dopamine release in the striatum (Floresco et al. 2003) and is believed to mediate the perception of salience or reward associated with stimuli (Berridge and Robinson 1998; Schultz 1998; Stuber et al. 2008). The phasic bursts of dopamine release, which are highly dependent on glutamatergic excitatory afferents, have been shown to be regulated by constant low-frequency tonic firing of dopamine neurons (Goto et al. 2007). Tonic dopamine tone in turn is under control of GABAergic inhibition. Increased levels of tonic dopamine firing may result in decreased amplitude of phasic dopamine burst firing, thus dampening responsivity of this system. Decreased tonic dopamine levels, on the other hand, may result in a heightened responsivity of the phasic dopamine component (Bilder et al. 2004; Goto et al. 2007). Kapur has suggested that, in schizophrenia, dopamine dysregulation results in a psychological state of aberrant salience, in which mundane events and ideas may be attributed with undue significance (Kapur et al. 2005). Thus, a hyperdopaminergic state in striatal brain regions, which most likely reflects dysregulation of the phasic component of DA release (Grace 1991, 1995), is believed to create a condition in which logically unconnected ideas and associations are weaved together and elaborated upon, eventually leading to the emergence of a delusional system. The process of salience attribution has been related to associative and reinforcement learning, in which what is called “reward prediction error” plays a key role (Smith et al. 2006). It is hypothesized that previous reward outcomes are used to form a reward prediction, which is then compared to the actual current reward. The difference between reward prediction and actual outcome is referred to as the reward prediction error (Smith et al. 2006) and has been shown to be mediated, in animals as well as in humans, by dopamine activity in ventral midbrain and striatum (Abler et al. 2006; Bayer and Glimcher 2005; D’Ardenne et al. 2008; Pessiglione et al. 2006). Compared to healthy controls, patients with psychosis seem to exhibit aberrant reward prediction and reward-related learning, both at the behavioral and the neural level (Jensen et al. 2008; Juckel et al. 2006). However, the relationship between dopamine dysfunction in these brain regions, alterations in reward processing, and symptomatology in schizophrenia has not directly been studied and remains speculative.
3.2 Relating DA Dysfunction to Negative and Cognitive Symptoms
As intact dopaminergic neurotransmission is critical for PFC functioning and cognition (Arnsten 2007; Goldman-Rakic 1999), the negative and cognitive symptoms of schizophrenia have been particularly associated with cortical hypodopaminergia (Abi-Dargham et al. 2002; Goldman-Rakic et al. 2004; Lynch 1992), although direct evidence for this association in schizophrenia is missing. The nature of dopamine dysfunction in the cortex remains unclear, although one study showing decreased tyrosine hydroxylase immunolabeling suggests decreased innervation (Akil et al. 2000). Recently, the group of Simpson et al. (2010) suggested a role for the striatum in the etiology of negative and cognitive symptoms of schizophrenia. Based on their preclinical work in D2 overexpressing mice, the researchers demonstrated that striatal DA alterations in form of overexpression of D2 receptors lead to changes in DA turnover and prefrontal D1 receptor stimulation (Kellendonk et al. 2006). Behaviorally, this alteration was accompanied by deficits in working memory (Kellendonk et al. 2006) and operant performance, expressed in both reduced motivation and deficits in timing of the rewards (Drew et al. 2007). It was moreover shown that the deficits in cognitive performance were secondary to the motivational deficit directly resulting from the D2 overexpression (Ward et al. 2009), and remained even after the D2 receptor overexpression had been reversed (Drew et al. 2007). In line with these preclinical findings, studies in individuals with prodromal symptoms of schizophrenia have revealed an association between increased striatal DA synthesis capacity and altered activation in prefrontal cortical brain regions during cognitive engagement (Fusar-Poli et al. 2010, 2011). These studies highlight the concept of dopamine dysregulation occurring early on in the disease process and having pathogenic effects on the rest of the circuitry. It is likely that dopamine dysregulation early on may influence the development and function of other systems, as well as reciprocally, dopamine dysregulation itself may be a consequence of dysregulation in glutamatergic and GABAergic systems. We will discuss the interdependence of these alterations below.
3.3 Neural Circuitry; the Interdependence of Dopaminergic, Glutamatergic, and GABAergic Processes
We have outlined the dopaminergic alterations in schizophrenia in this chapter. There is also clear evidence for GABAergic abnormalities (Lewis and Gonzalez-Burgos 2000) and glutamatergic abnormalities. The hypothesis of a glutamatergic NMDA hypofunction initially derived from the observation that NMDA antagonists induce all three classes of symptoms (negative, cognitive, and positive) observed in schizophrenia and has received additional support from genetic, postmortem, and preclinical studies; for a review, see Moghaddam (2003). These alterations in GABA, dopamine, and glutamate can be interdependent. With imaging, we have observed that acute or chronic NMDA dysfunction can lead to striatal dopamine dysregulation (Kegeles et al. 2002) or to an increase in cortical D1 receptors (Narendran et al. 2005), respectively. It is also believed that alterations in GABAergic interneurons in hippocampus (Zhang and Reynolds 2002) can lead to excess glutamate drive which in turn may lead to a dysregulation of midbrain dopamine activity. Preclinical studies have shown that the hippocampus is involved in the regulation of striatal dopamine by affecting firing patterns of midbrain dopamine cells (Grace 2012; Lodge and Grace 2008) and thus suggested that disinhibition due to NMDA receptor hypofunction on GABAergic interneurons in hippocampus may contribute to the hyperdopaminergic state in striatum (Lisman 2012). Recent work by Schobel and colleagues showed a significant increase in cerebral blood volume (CBV) in the hippocampus CA1 field in the prodromal stage predicting conversion to psychosis. CBV alterations were similar, although smaller in magnitude to those observed in patients with schizophrenia by Schobel et al. (2009), Harrison (2004), and Meyer-Lindenberg et al. (2005). Notably, the CA1 field is densely packed with pyramidal neurons and serves as the primary output area to the hippocampal-VTA loop. In the cortex, hypofunction of NMDA receptors on fast-spiking GABAergic interneurons leads to disinhibition of cortical excitatory neurons and impairment of synchronized oscillatory activity; for a review, see Lisman et al. (2008), which may relate to the cognitive deficits in schizophrenia as well as at least some of the negative symptoms that are less responsive to antipsychotics. In summary, the dopaminergic alterations have to be considered within the overall context of disordered circuitry and transmission in schizophrenia affecting multiple systems. Presynaptic dopamine alterations may be secondary to abnormal regulation by these systems. However, this suggestion does not necessarily mean that dopamine alterations cannot be in turn pathogenic, as observed in the D2 overexpressing mouse model (Kellendonk et al. 2006). More work is needed to understand the sequence of alterations within patients’ brains, but so far it is clear that both the hippocampal excess activity and the subcortical dopaminergic overactivity occur early on, could be related, and precede full onset of the disease.
4 Conclusions
This chapter reviewed evidence for DA dysfunction in schizophrenia. Most of this evidence stems from imaging studies applying PET and SPECT techniques. Due to advances in these imaging techniques it has become possible to study neurochemical alterations in the DA system in several regions of the brain. Accordingly, recent studies were able to examine DA neurotransmission in the different substructures of the striatum and revealed that, in contrast to the prevailing idea of DA hyperactivity in the mesolimbic DA pathway, it is rather hyperactivity in associative striatum that is implicated in schizophrenia pathology, as illustrated in Fig. 2. It further seems that the dysfunction is predominantly presynaptic but also postsynaptic, characterized by increased DA synthesis capacity and increased phasic release to pharmacological and possibly also psychosocial challenges (see Fig. 1). Furthermore, this dysfunction occurs early in the disease and may represent an early pathogenic process leading to further dysregulation.
Despite recent advances in the study of DA dysregulation in schizophrenia, the etiology of DA imbalance remains unknown. It has been assumed that striatal DA hyperactivity results from decreased activity in PFC, due to its functional role in inhibiting subcortical DA transmission (Deutch 1992; Meyer-Lindenberg et al. 2002). Conversely, preclinical work has recently shown that striatal DA abnormalities result in altered PFC DA activity (Kellendonk et al. 2006). The striatum is a complex integrative structure, receiving among others input from the hippocampus, an area of pathology in schizophrenia (Harrison 2004; Meyer-Lindenberg et al. 2005), and in animal models changes in hippocampal activity lead to dysregulation of DA neuronal activity (Lodge and Grace 2008). Future research should combine imaging with translational models of the disease to understand the cellular mechanisms involved in the striatal and cortical dopamine dysfunction in schizophrenia.
References
Abi-Dargham A (2004) Do we still believe in the dopamine hypothesis? New data bring new evidence. The International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 7(Suppl 1):S1–5
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155:761–767
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M (2000) Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci 97:8104–8109
Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719
Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, O’Flynn K, Koenigsberg HW, Van Heertum R, Cooper T, Laruelle M, Siever LJ (2004) Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry 55:1001–1006
Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M (2009) Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry 65:1091–1093
Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, Narendran R, Hwang DR, Laruelle M, Slifstein M. J Psychopharmacol. 2012 Jun;26(6):794-805. Epub 2011 Jul 18
Abler B, Walter H, Erk S, Kammerer H, Spitzer M (2006) Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage 31:790–795
Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA (2000) Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry 47:361–370
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. Author, Washington, DC
Arakawa R, Ichimiya T, Ito H, Takano A, Okumura M, Takahashi H, Takano H, Yasuno F, Kato M, Okubo Y, Suhara T (2009) Increase in thalamic binding of [(11)C]PE2I in patients with schizophrenia: a positron emission tomography study of dopamine transporter. J Psychiatr Res 43:1219–1223
Arnsten AF (2007) Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex 17(Suppl 1):i6–15
Arnsten AF (2011) Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci 29(3):215–23
Bayer HM, Glimcher PW (2005) Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47:129–141
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Bilder RM, Volavka J, Lachman HM, Grace AA (2004) The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29:1943–1961
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 94:2569–2574
Brown WD, Taylor MD, Roberts AD, Oakes TR, Schueller MJ, Holden JE, Malischke LM, DeJesus OT, Nickles RJ (1999) FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology 53:1212–1218
Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J, Kemether E, Oakes TR, Mukherjee J (2006) D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophr Res 85:232–244
Burt DR, Creese I, Snyder SH (1977) Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science 196:326–328
Carlsson A (1977) Does dopamine play a role in schizophrenia? Psychol Med 7:583–597
Carlsson A (1978) Antipsychotic drugs, neurotransmitters, and schizophrenia. Am J Psychiatry 135:165–173
Creese I, Burt DR, Snyder SH, Creese I, Burt DR, Snyder SH (1976) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192:481–483
Cropley VL, Fujita M, Bara-Jimenez W, Brown AK, Zhang XY, Sangare J, Herscovitch P, Pike VW, Hallett M, Nathan PJ, Innis RB (2008) Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18F]FDOPA. Psychiatry Res 163:171–182
Cross AJ, Crow TJ, Owen F (1981) 3H-Flupenthixol binding in post-mortem brains of schizophrenics: evidence for a selective increase in dopamine D2 receptors. Psychopharmacology 74:122–124
Cumming P, Gjedde A (1998) Compartmental analysis of dopa decarboxylation in living brain from dynamic positron emission tomograms. Synapse 29:37–61
D’Ardenne K, McClure SM, Nystrom LE, Cohen JD (2008) BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 319:1264–1267
Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Levy D, Remy P, Crouzel C, Artiges E, Feline A, Syrota A, Martinot JL (1997) Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res 23:167–174
Davis KL, Kahn RS, Ko G, Davidson M (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486
Deutch AY (1992) The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm 36:61–89
Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD (2007) Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci 27:7731–7739
Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN, Hwang Y, Hwang DR, Abi-Dargham A, Laruelle M (2007) In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol 9:117–125
Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ (2000) 6-(18)F-DOPA PET study in patients with schizophrenia. Positron emission tomography. Psychiatry Res 100:1–11
Erritzoe D, Talbot P, Frankle WG, Abi-Dargham A (2003) Positron emission tomography and single photon emission CT molecular imaging in schizophrenia. Neuroimaging Clin North Am 13:817–832
Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544
Fitzgerald PB, Kapur S, Remington G, Roy P, Zipursky RB (2000) Predicting haloperidol occupancy of central dopamine D2 receptors from plasma levels. Psychopharmacology (Berl) 149:1–5
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973
Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Grasby PM, McGuire PK (2010) Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry 67:683–691
Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Montgomery AJ, Grasby PM, McGuire P (2011) Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry 16:67–75
Galineau L, Wilson AA, Garcia A, Houle S, Kapur S, Ginovart N (2006) In vivo characterization of the pharmacokinetics and pharmacological properties of [11C]-(+)-PHNO in rats using an intracerebral beta-sensitive system. Synapse 60:172–183
Garnett ES, Firnau G, Chan PK, Sood S, Belbeck LW (1978) [18F]fluoro-dopa, an analogue of DOPA, and its use in direct external measurements of storage, degradation, and turnover of intracerebral dopamine. Proc Natl Acad Sci USA 75:464–467
Garnett ES, Firnau G, Nahmias C (1983) Dopamine visualized in the basal ganglia of living man. Nature 305:137–138
Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA (2006) Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97:1089–1103
Ginovart N, Wilson AA, Hussey D, Houle S, Kapur S (2009) D2-receptor upregulation is dependent upon temporal course of D2-occupancy: a longitudinal [11C]-raclopride PET study in cats. Neuropsychopharmacology 34:662–671
Glenthoj BY, Mackeprang T, Svarer C, Rasmussen H, Pinborg LH, Friberg L, Baare W, Hemmingsen R, Videbaek C (2006) Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry 60:621–629
Goldman-Rakic PS (1999) The “psychic” neuron of the cerebral cortex. Ann N Y Acad Sci 868:13–26
Goldman-Rakic PS, Selemon LD (1997) Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 23:437–458
Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV (2004) Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology 174:3–16
Goto Y, Otani S, Grace AA (2007) The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53:583–587
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Grace AA (1995) The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend 37:111–129
Grace AA (2012) Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 62:1342–1348
Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S (2008) Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp 29:400–410
Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S (2009) The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology 34:1078–1086
Griffon N, Sokoloff P, Diaz J, Levesque D, Sautel F, Schwartz JC, Simon P, Costentin J, Garrido F, Mann A et al (1995) The dopamine D3 receptor and schizophrenia: pharmacological, anatomical and genetic approaches. Eur Neuropsychopharmacol 5(Suppl):3–9
Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A (2003) Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology 28:1703–1711
Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN (1997) Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch Gen Psychiatry 54:225–232
Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L (1994) Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 11:245–256
Harrison PJ (2004) The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 174:151–162
Hess EJ, Bracha HS, Kleinman JE, Creese I (1987) Dopamine receptor subtype imbalance in schizophrenia. Life Sci 40:1487–1497
Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Kirvela O, Ruotsalainen U et al (1995) Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet 346:1130–1131
Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Eronen E, Ruotsalainen U, Salokangas RK (1999) Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res 35:41–50
Hirvonen J, van Erp TG, Huttunen J, Aalto S, Nagren K, Huttunen M, Lonnqvist J, Kaprio J, Hietala J, Cannon TD (2005) Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry 62:371–378
Hirvonen J, van Erp TG, Huttunen J, Aalto S, Nagren K, Huttunen M, Lonnqvist J, Kaprio J, Cannon TD, Hietala J (2006) Brain dopamine d1 receptors in twins discordant for schizophrenia. Am J Psychiatry 163:1747–1753
Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 35:549–562
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM (2009) Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 66:13–20
Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P (2011a) Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry 16:885–886
Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P (2011c) Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 168(12):1311–17
Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC (2003) Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophr Res 65:39–46
Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S, Solin O, Ilonen T, Korkeila J, Ristkari T, McGlashan T, Salokangas RK, Hietala J (2008) Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry 63:114–117
Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S (2008) The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology 33:473–479
Joyce JN, Lexow N, Bird E, Winokur A (1988) Organization of dopamine D1 and D2 receptors in human striatum: receptor autoradiographic studies in Huntington’s disease and schizophrenia. Synapse 2:546–557
Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A (2006) Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29:409–416
Kapur S, Mamo D (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27:1081–1090
Kapur S, Remington G (2001) Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry 50:873–883
Kapur S, Mizrahi R, Li M (2005) From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res 79:59–68
Karlsson P, Farde L, Halldin C, Sedvall G (2002) PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry 159:761–767
Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S (1986) Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biol Psychiatry 21:650–656
Kegeles LS, Zea-Ponce Y, Abi-Dargham A, Rodenhiser J, Wang T, Weiss R, Van Heertum RL, Mann JJ, Laruelle M (1999) Stability of [123I]IBZM SPECT measurement of amphetamine-induced striatal dopamine release in humans. Synapse 31:302–308
Kegeles LS, Martinez D, Kochan LD, Hwang DR, Huang Y, Mawlawi O, Suckow RF, Van Heertum RL, Laruelle M (2002) NMDA antagonist effects on striatal dopamine release: Positron emission tomography studies in humans. Synapse 43:19–29
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M (2010a) Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67:231–239
Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T, Harkavy-Friedman JM, Gil R, Laruelle M, Abi-Dargham A (2010b) Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry 68:634–641
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER (2006) Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49:603–615
Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, Dawant B, Zald D, Meltzer HY (2009) Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry 65:1024–1031
Kestler LP, Walker E, Vega EM (2001) Dopamine receptors in the brains of schizophrenia patients: a meta-analysis of the findings. Behav Pharmacol 12:355–371
Knable MB, Weinberger DR (1997) Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol (Oxford, England) 11:123–131
Knable MB, Hyde TM, Herman MM, Carter JM, Bigelow L, Kleinman JE (1994) Quantitative autoradiography of dopamine-D1 receptors, D2 receptors, and dopamine uptake sites in postmortem striatal specimens from schizophrenic patients. Biol Psychiatry 36:827–835
Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Rakkolainen V, Syvalahti E, Hietala J (2000) Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry 157:269–271
Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Syvalahti E, Hietala J (2001) Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res 52:115–120
Lahti RA, Roberts RC, Conley RR, Cochrane EV, Mutin A, Tamminga CA (1996) D2-type dopamine receptors in postmortem human brain sections from normal and schizophrenic subjects. Neuroreport 7:1945–1948
Lahti RA, Roberts RC, Cochrane EV, Primus RJ, Gallager DW, Conley RR, Tamminga CA (1998) Direct determination of dopamine D4 receptors in normal and schizophrenic postmortem brain tissue: a [3H]NGD-94-1 study. Mol Psychiatry 3:528–533
Laruelle M (1998) Imaging dopamine transmission in schizophrenia: a review and meta-analysis. Q J Nucl Med 42:211–221
Laruelle M (2000) Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20:423–451
Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF et al (1995) SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med 36:1182–1190
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 93:9235–9240
Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB (1997) Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17:162–174
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R (1999) Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 46:56–72
Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D’Souza DC, Krystal J, Seibyl J, Baldwin R, Innis R (2000) Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry 47:371–379
Laruelle M, Kegeles LS, Abi-Dargham A (2003) Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci 1003:138–158
Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken JB, Gersons BP, van Royen EA (2001) Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res 47:59–67
Lewis DA, Gonzalez-Burgos G (2000) Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Res Bull 52:309–317
Lieberman JA, Sheitman BB, Kinon BJ (1997) Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 17:205–229
Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, Langstrom B (1999) Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry 46:681–688
Lisman J (2012) Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012 Jun;22(3):537–544
Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA (2008) Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31:234–242
Lodge DJ, Grace AA (2008) Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res 14:97–104
Lynch MR (1992) Schizophrenia and the D1 receptor: focus on negative symptoms. Prog Neuropsychopharmacol Biol Psychiatry 16:797–832
Marzella PL, Hill C, Keks N, Singh B, Copolov D (1997) The binding of both [3H]nemonapride and [3H]raclopride is increased in schizophrenia. Biol Psychiatry 42:648–654
Mateos JJ, Lomena F, Parellada E, Font M, Fernandez E, Pavia J, Prats A, Pons F, Bernardo M (2005) Decreased striatal dopamine transporter binding assessed with [123I] FP-CIT in first-episode schizophrenic patients with and without short-term antipsychotic-induced parkinsonism. Psychopharmacology 181:401–406
Mateos JJ, Lomena F, Parellada E, Mireia F, Fernandez-Egea E, Pavia J, Prats A, Pons F, Bernardo M (2007) Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology 191:805–811
McGowan S, Lawrence AD, Sales T, Quested D, Grasby P (2004) Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch Gen Psychiatry 61:134–142
Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF (2002) Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 5:267–271
Meyer-Lindenberg A, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF (2005) Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386
Mizrahi R (2010) Advances in PET analyses of stress and dopamine. Neuropsychopharmacology 35:348–349
Moghaddam B (2003) Bringing order to the glutamate chaos in schizophrenia. Neuron 40:881–884
Murray AM, Hyde TM, Knable MB, Herman MM, Bigelow LB, Carter JM, Weinberger DR, Kleinman JE (1995) Distribution of putative D4 dopamine receptors in postmortem striatum from patients with schizophrenia. J Neurosci 15:2186–2191
Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, Kegeles LS, Talbot PS, Huang Y, Hwang DR, Khenissi L, Cooper TB, Laruelle M, Abi-Dargham A (2005) Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 162:2352–2359
Nobrega JN, Seeman P (1994) Dopamine D2 receptors mapped in rat brain with [3H](+)PHNO. Synapse 17:167–172
Nozaki S, Kato M, Takano H, Ito H, Takahashi H, Arakawa R, Okumura M, Fujimura Y, Matsumoto R, Ota M, Takano A, Otsuka A, Yasuno F, Okubo Y, Kashima H, Suhara T (2009) Regional dopamine synthesis in patients with schizophrenia using L-[beta-11C]DOPA PET. Schizophr Res 108:78–84
Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M (1997) Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 385:634–636
Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006) Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442:1042–1045
Pimoule C, Schoemaker H, Reynolds GP, Langer SZ (1985) [3H]SCH 23390 labeled D1 dopamine receptors are unchanged in schizophrenia and Parkinson’s disease. Eur J Pharmacol 114:235–237
Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve SE, Etienne P, Evans AC, Lal S, Shevell M, Savard G, Wong DF, Chouinard G, Gjedde A (1994) Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci U S A 91:11651–11654
Reynolds GP, Czudek C (1988) Status of the dopaminergic system in post-mortem brain in schizophrenia. Psychopharmacol Bull 24:345–347
Reynolds GP, Mason SL (1994) Are striatal dopamine D4 receptors increased in schizophrenia? J Neurochem 63:1576–1577
Schmitt GJ, Meisenzahl EM, Frodl T, La Fougere C, Hahn K, Möller HJ, Dresel S (2005) The striatal dopamine transporter in first-episode, drug-naive schizophrenic patients: evaluation by the new SPECT-ligand[99mTc]TRODAT-1. J Psychopharmacol 19:488–493
Schmitt GJ, Frodl T, Dresel S, la Fougere C, Bottlender R, Koutsouleris N, Hahn K, Möller HJ, Meisenzahl EM (2006) Striatal dopamine transporter availability is associated with the productive psychotic state in first episode, drug-naive schizophrenic patients. Eur Arch Psychiatry Clin Neurosci 256:115–121
Schmitt GJ, la Fougere C, Dresel S, Frodl T, Hahn K, Möller HJ, Meisenzahl EM (2008) Dual-isotope SPECT imaging of striatal dopamine: first episode, drug naive schizophrenic patients. Schizophr Res 101:133–141
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA (2009) Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66:938–946
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Seeman P (2010) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2 receptors. CNS Neurosci Ther 9(7):777–89
Seeman P, Bzowej NH, Guan HC, Bergeron C, Reynolds GP, Bird ED, Riederer P, Jellinger K, Tourtellotte WW (1987) Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neuropsychopharmacology 1:5–15
Seeman P, Guan HC, Van Tol HH (1993) Dopamine D4 receptors elevated in schizophrenia. Nature 365:441–445
Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’Dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T (2005) Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A 102:3513–3518
Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T (2006) Psychosis pathways converge via D2high dopamine receptors. Synapse 60:319–346
Shotbolt P, Stokes PR, Owens SF, Toulopoulou T, Picchioni MM, Bose SK, Murray RM, Howes OD (2011) Striatal dopamine synthesis capacity in twins discordant for schizophrenia. Psychol Med 41:1–8
Sibley DR, De Lean A, Creese I (1982) Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. J Biol Chem 257:6351–6361
Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, Kapur S, Zipursky RB, Wilson AA, Christensen BK, Seeman P (2000) Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 152:174–180
Simpson EH, Kellendonk C, Kandel E (2010) A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65:585–596
Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu X, Laruelle M, Abi-Dargham A (2007) [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT(2A) receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab 27:1733–1741
Smith A, Li M, Becker S, Kapur S (2006) Dopamine, prediction error and associative learning: a model-based account. Network (Bristol, England) 17:61–84
Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C (2006) The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5:25–43
Soliman A, O’Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A (2007) Stress-induced dopamine release in humans at risk of psychosis: a [(11)C]Raclopride PET Study. Neuropsychopharmacology 33:2033–2041
Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A (2008) Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321:1690–1692
Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L (2002) Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry 59:25–30
Sumiyoshi T, Stockmeier CA, Overholser JC, Thompson PA, Meltzer HY (1995) Dopamine D4 receptors and effects of guanine nucleotides on [3H]raclopride binding in postmortem caudate nucleus of subjects with schizophrenia or major depression. Brain Res 681:109–116
Talvik M, Nordstrom AL, Olsson H, Halldin C, Farde L (2003) Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. The International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 6:361–370
Talvik M, Nordstrom AL, Okubo Y, Olsson H, Borg J, Halldin C, Farde L (2006) Dopamine D2 receptor binding in drug-naive patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res 148:165–173
Tuppurainen H, Kuikka J, Viinamaki H, Husso-Saastamoinen M, Bergstrom K, Tiihonen J (2003) Extrastriatal dopamine D 2/3 receptor density and distribution in drug-naive schizophrenic patients. Mol Psychiatry 8:453–455
Tuppurainen H, Kuikka JT, Laakso MP, Viinamaki H, Husso M, Tiihonen J (2006) Midbrain dopamine D2/3 receptor binding in schizophrenia. Eur Arch Psychiatry Clin Neurosci 256:382–387
van Rossum JM (1966) The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther 160:492–494
Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, Kandel ER, Balsam PD (2009) Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci 123:720–730
Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44:660–669
Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA (2006) High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59:389–394
Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, Doop M, Kessler RM, Zald DH (2010) Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry 168(4):418–26
Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH (2004) Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: a dual-isotope SPECT study. Am J Psychiatry 161:1496–1498
Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T, Takano A, Nakayama K, Halldin C, Farde L (2004) Low dopamine D(2) receptor binding in subregions of the thalamus in schizophrenia. Am J Psychiatry 161:1016–1022
Yoder KK, Hutchins GD, Morris ED, Brashear A, Wang C, Shekhar A (2004) Dopamine transporter density in schizophrenic subjects with and without tardive dyskinesia. Schizophr Res 71:371–375
Zakzanis KK, Hansen KT (1998) Dopamine D2 densities and the schizophrenic brain. Schizophr Res 32:201–206
Zhang ZJ, Reynolds GP (2002) A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 55:1–10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kuepper, R., Skinbjerg, M., Abi-Dargham, A. (2012). The Dopamine Dysfunction in Schizophrenia Revisited: New Insights into Topography and Course. In: Gross, G., Geyer, M. (eds) Current Antipsychotics. Handbook of Experimental Pharmacology, vol 212. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-25761-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-642-25761-2_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-25760-5
Online ISBN: 978-3-642-25761-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)