Abstract
The task of the Genetic Toxicology is to use recognized test methods in order to identify the genotoxic or mutagenic potential of pharmaceuticals and chemicals. The genotoxic or mutagenic potential is what we call the potential health risk of a substance with respect to its ability to cause cell mutations. Substances with this potential are known as mutagens and genotoxicity studies, also called mutagenicity studies, help identify such substances.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Ingo Stammberger, Andreas Czich and Knut Braun
The task of the Genetic Toxicology is to use recognized test methods in order to identify the genotoxic or mutagenic potential of pharmaceuticals and chemicals. The genotoxic or mutagenic potential is what we call the potential health risk of a substance with respect to its ability to cause cell mutations. Substances with this potential are known as mutagens and genotoxicity studies, also called mutagenicity studies, help identify such substances.
Early warnings that a possible new drug product may have a genotoxic effect are of immense importance for drug development. If a potential new drug is proven to have a genotoxic potential, this generally leads to termination of development of that drug, thereby preventing human exposure to drugs with mutagenic effects (except cytostatic drugs).
In addition, in the last decade, the testing and identification of impurities was recognized as important step in the toxicological risk assessment of the drug substance. The identification of a genotoxic potential and the determination of acceptable limits for genotoxic impurities in active substances is a difficult issue and now a days addressed in several publications and Guidelines. The data set usually available for genotoxic impurities is quite variable and is the main factor that dictates the process used for the assessment of acceptable limits. A generally applicable approach was defined by implementing the Threshold of Toxicological Concern (TTC). A TTC value of 1.5 μg/day intake of a genotoxic impurity is considered to be associated with an acceptable risk (excess cancer risk of <1 in 100,000 over a lifetime) for most pharmaceuticals (Kroes et al. 2004). From this threshold value, a permitted level in the active substance can be calculated based on the expected daily dose and the duration of treatment (EMEA 2006). However, higher limits may be justified under certain conditions, e.g., based on the therapeutic area.
Numerous test methods are available in order to assess the genotoxic potential of drug substances. There are essentially three main categories of genotoxic damage that can be caused by a substance:
-
1.
Gene mutations
-
2.
Chromosome aberrations and genome mutations
-
3.
DNA damage and DNA repair
For the assessment of potential genotoxic impurities, a combination of in silico analysis (see Chap. 41 “In-Silico ADME Modeling”) and the detection of a direct DNA damaging potential of the compound is considered to be sufficient. For the detection of the direct DNA damaging potential, the bacterial reverse mutation test (Ames Test) is recommended by the Guidelines as stand-alone assay.
Gene mutations include base pair substitutions and frameshift mutations, where base pair substitutions arise from the substitution of one or several base pairs in the DNA, and frameshift mutations arise from an insertion or deletion involving a number of base pairs that is not a multiple of three and consequently disrupts the triplet reading frame, usually leading to the creation of a premature termination (stop) codon and resulting in a truncated protein product.
Chromosomal aberrations include both numerical and structural aberrations. Numerical aberrations are changes in the number of chromosomes of the normal number characteristic of the animals utilized (aneugenicity). Structural aberrations are classified into two types, chromosome or chromatid aberrations (clastogenicity). Chromosomal mutations and related events are the cause of many human genetic diseases and there is evidence that chromosomal mutations and related events are involved in cancer development.
DNA damage can happen in several ways. For example, energy production in cells can produce toxic molecules, such as the so-called free radicals. They can react with the bases in the DNA and modify them, thus preventing the genetic code from being used properly. Those DNA damages then need to be repaired. All cells have evolved a system of recognizing DNA errors, fixing them and in special cases repairing them. This DNA repair mechanism can be faulty, which in itself results in a genotoxic effect.
There are specific assays available to detect each of these types of genotoxic damage, and a battery of tests is often used to determine if a substance causes any or all of these three types of damage. An overview of which assays can be used to detect a given type of genotoxic effect is shown in Table 52.1.
These assays are generally performed by applying a test substance to well characterized cell systems (e.g., bacterial or mammalian cell cultures) and evaluating changes in the growth and characteristics of the cells. This may involve assessing the speed to form colonies and the size of the colonies as well as using markers to label chromosome structure or to monitor DNA synthesis.
Usually most bacteria and cell lines do not possess the full capability for metabolizing pro-mutagens and pro-cancerogens. To overcome this deficiency substances are tested in the presence of an exogenous metabolic activation system (S9-mix) as well as in its absence. Adding the S9-mix to the cell culture allows for metabolism of the test substance by enzymes not present in the bacterial cells. The S9-mix is a postmitochondrial supernatant fraction from liver supplemented with a NADP-generating system. It is generally made from rat liver induced with polychlorinated biphenyls, however, livers from hamsters, monkeys, or other species may be used depending on the anticipated metabolism of the substance being tested. An S9-mix has been successfully used in eukaryotic in vitro systems for the metabolic activation of various compounds.
In the last decade, a lot of effort was done to revise the ICH (International Congress on Harmonization) guidelines for genotoxicity testing of pharmaceuticals. Importantly, there are a number of changes to the previous testing strategy that reflects a desire to reduce the false positive rate in the in vitro test battery. For instance, the top dose was lowered for noncytotoxic drugs in the mammalian cell assays. In addition, one testing option eliminates the need to conduct the in vitro mammalian cell assays (and just conduct Ames and an in vivo assay with two endpoints included). New assays and endpoints were introduced (Micronucleus Assay in vitro for regulatory purposes, Comet Assay in vivo) and a few assays might be used as follow-up assays very soon (PigA Assay).
The test guidelines for new pharmaceuticals recommend that for follow-up testing to evaluate positive results in genotoxicity test in vitro, in vivo assays should be conducted in two tissues. In practice this is usually achieved by performing an erythrocyte chromosome-damage test in bone marrow and a genotoxicity assay in liver, because some genotoxic carcinogens are known to be positive in the liver but not in the bone marrow assays. In current regulatory guidance the rodent Comet assay is considered as a useful follow-up test in case of positive results in in vitro genotoxicity assays. The Comet Assay is recognized as a useful tool for the evaluation of genotoxicity in organs/cell types that cannot easily be evaluated with other standard tests, e.g., in skin and stomach.
In addition the implementation of Genetic Toxicology endpoints into repeat dose toxicity studies was evaluated and recommended by the scientific community, to reduce the numbers of animals within toxicological studies. Those endpoint were mainly the Micronucleus Assay and the Comet Assay.
References and Further Reading
Dearfield KL, Thybaud V, Cimino MC, Custer L, Czich A, Harvey JS, Hester S, Kim JH, Kirkland D, Levy DD, Lorge E, Moore MM, Ouédraogo-Arras G, Schuler M, Suter W, Sweder K, Tarlo K, van Benthem J, van Goethem F, Witt KL (2011) Follow-up actions from positive results of in vitro genetic toxicity testing. Environ Mol Mutagen 52(3): 177–204. doi:10.1002/em.20617. Epub 2010 Oct 20. Review. PubMed PMID: 20963811
EMEA (2006) Guideline on the limits of genotoxic impurities. Committee For Medicinal Products for Human Use, The European Medicines Agency, London, 2006, CPMP/SWP/5199/02, EMEA/CHMP/QWP/251344/2006
ICH S2 (2011) International conference on harmonization. Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use S2(R1) 9 Nov 2011. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step2/S2_R1__Guideline.pdf. Accessed 3 Sep 2011
Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG et al. (2004) Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol 42: 65–83
UKCOM (2000) Committee on mutagenicity of chemicals in food, consumer products and the environment. Guidance on a strategy for testing of chemicals for mutagenicity. Department of Health, UK. Environmental and molecular mutagenesis. doi:10.1002/em192. Available at http://www.advisorybodies.doh.gov.uk/com/guidance. pdf. Accessed 03 Sept 2011
WHO (2007) WHO/IPCS (World Health Organisation/ International Programme on Chemical Safety). Draft Guidance on Mutagenicity Testing for Chemical Risk Assessment. Available at http://www.who.int/ipcs/methods/harmonization/areas/mutagenicity_testing. Accessed on 03 Sep–Oct 2011
Bacterial Reverse Mutation Test
PURPOSE/RATIONALE
The bacterial reverse mutation test (Ames Test) investigates the ability of chemicals and drugs to induce reverse (back) mutations in bacteria, which involves base pair substitutions additions and/or deletions (frameshift mutations) of one or a few DNA base pairs. The bacterial strains used in the test system have mutations in genes coding for enzymes required for the biosynthesis of the amino acids histidine (Salmonella typhimurium) and tryptophan (Escherichia coli). If a test substance causes a mutation that restores function to the enzyme gene, then the bacteria will be able to grow and produce colonies. An extensive data base exists based on the use of this test, and it has been found that compounds producing a positive result in the bacterial reverse mutation test have a high potency to induce cancer in animal studies.
PROCEDURE
The commonly used strains for S. typhimurium are TA100 and TA1535 (base pair substitution), TA98 and TA1537 (frame shift mutations) and TA102 for cross-link mutations. The preferred strain of E. coli to detect base pair substitution mutations is WP2.
Bacteria of an overnight nutrient broth culture are mixed with the test compound or solvent (as a negative control), S9-mix or buffer, and molten top agar and poured into a petri dish containing a layer of minimal agar. After incubation for approximately 48 h at approx. 37°C in the dark, colonies (representing the number of revertants) are counted by hand or an automatic colony counter. The method can be modified by including a preincubation step or by using a higher amount of S9-mix in the test system. Preincubation would involve incubating the test compound, S9-mix or buffer, and bacteria for a short period before pouring this mixture onto plates of minimal agar. According to the current ICH S2R Guideline, one experiment is sufficient to determine the mutagenic potential. The assay can be conducted as plate or preincubation assay.
Rational for the Dose Selection
The highest dose level in each experiment should correspond:
-
either to 5,000 μg/plate
-
or to a dose level corresponding to the highest soluble concentration in the solvent
-
or to a dose level producing a precipitation in the plates or to a dose level which induces bacteria toxicity, whatever the solubility
EVALUATION
To evaluate the result of a test compound, the number of revertant colonies has to be determined for each concentration used in the test system as well as for the negative (solvent or untreated) and positive control. Bacterial toxicity is expressed as a thinning of the bacterial lawn or in a reduction in the number of colonies compared to the negative control. Bacterial toxicity and precipitation should be taken into consideration when assessing the mutagenicity of a substance. The following criteria should be met to consider a bacterial reverse mutation test as valid.
-
The number of revertant colonies on both negative (solvent or untreated) control plates should be in a historical control range described in literature or determined in the laboratory.
-
The positive control should induce a significant increase in the number of revertant colonies.
-
At least five dose levels for the test article are analyzable (i.e., number of revertant colonies can be determined), for each strain in each experimental condition.
-
The highest dose level fulfills the rational for the highest dose level selection
A test compound is classified as inducing point mutations if it causes at least two of the following criteria:
-
It produces at least a two- to threefold increase in the mean number of revertants per plate depending on the strain used of at least one of the tester strains over the mean number of revertants per plate of the appropriate vehicle control at complete bacterial background lawn
-
It induces a dose-related increase in the mean number of revertants per plate of at least one of the tester strains over the mean number of revertants per plate of the appropriate vehicle control in at least two to three concentrations of the test compound at complete bacterial background lawn
-
The increase number of revertant colonies is reproducible
-
The number of revertant colonies exceeds the upper value of the range of the historical negative control data
Moreover, biological significance needs to be discussed, taking into consideration the criteria mentioned above.
If the test substance does not achieve either of the above criteria, it is not considered as showing evidence of mutagenic activity in this system.
CRITICAL ASSESSMENT OF THE METHOD
The advantages of the bacterial reverse mutation test are the ease with which it can be performed, the short amount of time required and the low cost. The test should be regarded as the first test in the GLP testing strategy for the detection of a genotoxic potential of a test compound. The test is as well required as part of the nonclinical testing strategy before the start of Phase I in the clinical development.
MODIFICATIONS OF THE METHOD
Mini-Ames and Ames II
The bacterial reverse mutation test does not detect all compounds with the potential to induce point mutations. For some chemical series, modifications of the test system are necessary. For example, the potential for azo compounds to induce point mutations can only be detected by using an S9-mix prepared by hamster liver. To get an indication early in the development of a test compound for its potential to induce point mutations, modified test systems of the standard bacterial reverse mutation test are performed as a screening test. These are the so-called Mini-Ames test, performed on smaller agar dishes, or the Ames IITM test, performed with microtiter plates. The test design of the screening method should be as much as possible related to the standard method, e.g., same top dose, to reach highest comparability between both methods. Both screening tests have the same principle as the standard test, and use S. typhimurium strains. The advantages of both are that they need much less compound and have a higher throughput in the number of tests per week.
The Bacterial Reverse Mutation Test in the Light of Genotoxic Impurity (GTI) Testing
Genotoxic impurities need to be investigated as well for their potential to induce point mutations. Currently, the need for testing is triggered by in silico alerts. If a compound shows an in silico alert, testing in the Ames Assay is required. If the Ames Assay is positive, the compound is classified as a Genotoxic impurity (GTI) In this case the so-called staged TTC (Threshold of Toxicological Concern) will trigger the further handling of the GTI and no further testing is required as described in Müller et al. (2006) and McGovern and Jacobson-Kram (2006). A new ICH Guidance dealing with Genotoxic impurities is under evaluation (ICH M7).
References and Further Reading
Alvares A-P, Bickers D-R, Kappas A (1973) Polychlorinated biphenyls: a new type of inducer of cytochrome P 448 in the liver. Proc Natl Acad Sci USA 70: 1321–1325
Ames B-N, Durston W-W, Yamasaki E, Lee F-D (1973) Carcinogens are mutagens. A simple test system combining liver homogenate for activation and bacteria for detection. Proc Natl Acad Sci USA 70: 2281–2285
Ames B-N, McCann J, Yamasaki E (1975) Methods for detecting carcinogens and mutagens with the Salmonella/mammalian microsome mutagenicity test. Mutat Res 31: 347–364
EC Directive 2000/32/EC, L 136, Annex 4D, B.13/B.14
Flückiger-Isler S, Baumeister M, Braun K, Gervais V, Hasler-Nguyen N, Reimann R, Van Gompel J, Wunderlich H-G, Engelhardt G (2004) Assessment of the performance of the Ames IITM assay: a collaborative study with 19 coded compounds. Mutat Res 558: 181–197
Kamber M, Flückiger-Isler S, Engelhardt G, Jaeckh R, Zeiger E (2009) Comparison of the Ames II and traditional Ames test responses with respect to mutagenicity, strain specificities, need for metabolism and correlation with rodent carcinogenicity. Mutagenesis 24: 359–366
Levin D-E, Hollstein M, Christman M-F, Schwiers E-A, Ames B-N (1982) A new Salmonella tester strain with A.T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci USA 79: 7445–7449. Genetics
Maron D-M, Ames B-N (1983) Revised methods for the Salmonella mutagenicity test. Mutat Res 113 173–215
McCann J, Springarn N-E, Kobory J, Ames B-N (1975) Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci USA 72: 979–983
McGovern T, Jacobson-Kram D (2006) Regulation of genotoxic and carcinogenic impurities in drug substances and products. Trends Anal Chem 100(1): 681–685
Mortelmans K, Zeiger E (2000) The Ames Salmonella/microsome mutagenicity assay. Mutat Res 455: 29–60
Müller L, Mauthe RJ, Riley CM, Andino MM, Antonis DD, Beels C, DeGeorge J, De Knaep AG, Ellison D, Fagerland JA, Frank R, Fritschel B, Galloway S, Harpur E, Humfrey CD, Jacks AS, Jagota N, Mackinnon J, Mohan G, Ness DK, O’Donovan MR, Smith MD, Vudathala G, Yotti L (2006) A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regul Toxicol Pharmacol 44(3):198–211. Epub 2006 Jan 18. PubMed PMID: 16412543
OECD Guideline For Testing Of Chemicals, 471. Bacterial Reverse Mutation Test, adopted 21 July 1997
U.S. EPA: OPPTS 870.5100 (1998) Health effects test guidelines bacterial reverse mutation test
L5178Y tk+/– Mouse Lymphoma Test (MLA)
PURPOSE/RATIONALE
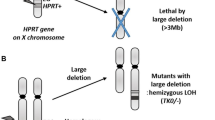
The MLA is used for the detection of point mutations, structural aberrations, and aneugenicity. The principle of the assay is that cells deficient in thymidine kinase (TK) due to the tk+/– or tk–/– mutation are resistant to the cytotoxic effects of the pyrimidine analogue trifluoro-thymidine (TFT). TK proficient cells are sensitive to TFT. This influences the cellular metabolism and leads finally to an inhibition of further cell division. Thus mutant cells are able to proliferate in the presence of TFT, whereas normal cells, which contain TK, are not. The major advantage of the assay is its ability to detect a broad range of mutagenic events represented by optimal detection of both large and small colonies.
The assay was described by Clive and coworkers (Clive et al. 1972) as a mutational assay system using the TK locus in mouse lymphoma cells. In the following years, he and his coworkers undertook a large-scale of investigation of the potential and optimal conduct of the assay. This included the use of the above-mentioned TFT to select tk mutants, a comparison of the hypoxanthine guanine phosphoribosyl transferase (hprt) and tk loci, an analysis of the best expression time for tk mutant selection and a description of distinct “large” and “small” colony tk mutants (Moore et al. 1985).
Originally, the assay was performed mainly in soft agar plates. In 1986, a protocol variation was successfully applied using cloning cells in liquid medium in 96-well microtiter plates instead of soft agar in Petri dishes (Cole et al. 1986). As a result, a better discrimination between small and large colonies was possible. Using a variety of chemicals, Doerr and Moore conducted an extensive evaluation of the correlation between gross chromosome aberration induction in mouse lymphoma cells and small colony tk mutant induction (Moore and Doerr 1990). From these studies it was clear that chemicals that induce small colony tk mutants also induce gross aberrations in the mouse lymphoma cells. Such mutants could result from alterations that: (a) affect expression of the tk and linked loci (chromosome 11 events); (b) affect the tk and other single essential loci in the genome, i.e., multiple point mutations; (c) affect the tk locus and have a chromosome event (other than chromosome 11) that results in slow growth.
In 1998, it was demonstrated that L5178Y cells contain two mutant p53 alleles (Clark et al. 1998). It is possible that the dysfunctional p53 protein in L5178Y cells may account for the sensitivity of these cells to mutagens and for the assay’s capability to detect the chromosomal rearrangements and mitotic recombination often seen in the later stages of cancer development.
A number of recommendations and comments were made on the conduct of the MLA (Sofuni et al. 1997), including heterogeneity and the use of single or duplicate cultures, cytotoxicity parameters, relative survival and relative total growth, strategies for dose range finding, and the statistical analysis of MLA data. From the International Working Group of Genotoxicity Testing a very detailed description of the protocol, the validity criteria and the evaluation was published in 2003, 2006, and 2007 (Moore et al. 2003, 2006, 2007). A detailed discussion on the selection of the top dose can be found in Moore et al., 2011.
PROCEDURE
The genotoxic potential of a compound in the MLA is generally evaluated with and without metabolic activation by S9 liver homogenate obtained from rats pretreated with Aroclor or Phenobarbital/ßNaphtoflavone. The addition of the S9-mix allows for metabolism of the test compound by enzymes not present in mouse lymphoma cells. The cells were exposed to the compound for 3–4 h or 24 h without a metabolic activation system and for 3 h with metabolic activation. Cells were then grown for a 48-h phenotypic expression period (two cultures per concentration). The phenotypic expression period is the time required for the mutant (TK deficient) phenotype to be expressed, i.e., loss of preexisting TK and depletion of the preexisting TMP pool. Cells were then cloned and incubated for 10–13 days with the selection agent (TFT) while their cloning efficiencies were checked in nonselective medium.
The ICH4 committee concluded in 1995 that there is the capability of detecting point mutations and most substances that induce chromosome aberrations (including aneugens). Therefore the chromosome aberration assays and the Mouse Lymphoma assay are currently considered interchangeable, if the Protocol recommendations of the ICH S2 documents are fulfilled. That means: (a) in the case of a negative result following 3–4 h (treatment with and without S9), a continuous treatment of 24 h without activation was considered advisable; (b) a requirement for the use of the microwell cloning protocol rather than agar cloning to better discriminate small and large colonies; and (c) the use of an appropriate positive control inducing a higher proportion of small colonies. However, further validity criteria were described in the OECD Guideline 476 and the ICH2b Guideline. One major point for the interpretation of the data is the fulfillment of the toxicity criteria (approximately 20%). In addition, in 2007, the Global evaluation Factor (GEF) was introduced, to judge if a compound is positive. This factor is based on the Induced Mutant Frequency (IMF), which is defined as the increase above the spontaneous background mutant frequency. For the microwell technique, the GEF has been defined to be equal to 126 × 10−6 (Moore et al. 2007). If the IMF exceeds the GEF, a compound is judged as positive.
EVALUATION
Small colony mutants have been shown to predominantly lack the TKb allele as a consequence of structural or numerical alterations or recombinational events whereas large colonies are the consequence of point mutations. Based on the recommendations of the ICH2b the following points should be considered in general for the interpretation of the results:
-
Does the Induced Mutant Frequency (IMF) exceed the Global Evaluation Factor (GEF)? For microwell technique, the GEF has been defined to be equal to 126 × 10−6.
-
Is the increase in response over the negative or solvent control background regarded as a meaningful genotoxic effect for the cells?
-
Is the response concentration-related?
-
For weak/equivocal responses, is the effect reproducible?
-
Is the positive result a consequence of an in vitro specific metabolic activation pathway/in vitro specific active metabolite?
-
Can the effect be attributed to extreme culture conditions that do not occur in in vivo situations, e.g., extremes of pH; osmolality; heavy precipitates especially in cell suspensions?
-
Is the effect only seen at extremely low survival levels/ high cytotoxicity?
In addition, the use of statistical methods is recommended for the evaluation of the MLA. Two methods should be used, one to evaluate the statistical difference between the different groups and, more importantly, the statistical significance of the dose-response curve. For the evaluation of the results the consideration of the biological relevance is the most important. A detailed discussion of parameters indicating a biological relevance is published in the ICH2B Guideline (1997) and in the recommendation of the International Working Group of Genotoxicity Testing (IWGT; Moore et al. 2003).
CRITICAL ASSESSMENT OF THE METHOD
It is apparent that the MLA has some advantages compared to other mutation assays including: (a) rapid growth in suspension culture to high cell density, which provided for the very large numbers of cells necessary for a statistically valid test and (b) the relatively short time (48 h) required for the expression of newly induced mutants.
However, the assay is only really capable of detecting large increases in mutation frequencies. This is because very small increases in mutation frequencies can often be seen at high cytotoxicity levels. At very low survival levels in mammalian cells, mechanisms other than direct genotoxicity per se can lead to “positive” results that are related to cytotoxicity and not genotoxicity (e.g., events associated with apoptosis, endonuclease release from lysosomes, etc.). It has long been recognized for all in vitro systems that mutations induced under these circumstances would not normally occur in vivo. Thus, these responses, while perhaps statistically significant by some methods, are not considered to be biologically relevant. A careful discussion of such responses is needed.
In addition, while the spectrum of mutations detected by the assay is very broad, it should be noted that not all such events are always detected with equal efficiency following treatment with particular test substances. Especially, for the detection of structural aberrations and aneugenicity the recommended conditions of the protocol should be kept in detail.
MODIFICATIONS OF THE METHOD
The test could be performed with different cell lines (e.g., TK6 cells) and different metabolic activation systems.
References and Further Reading
Clark L, Hart D, Vojta P, Harrington-Brock K, Barrett J, Moore M, Tindall K (1998) Identification and chromosomal assignment of two heterozygous mutations in the Trp53 gene in L5178Y/Tk + \–3.7.2 C mouse lymphoma cells. Mutagenesis 13: 427–439
Clive D, Flamm W, Patterson J (1972) A mutational assay system using the thymidine kinase locus in mouse lymphoma cells. Mutat Res 16: 77
Cole J, Muriel W-J, Bridges B-A (1986) The mutagenicity of sodium fluoride to L5178Y [wild-type and TK+/– (3. 7.2c)] mouse lymphoma cells. Mutagenesis 1: 157–167
ICH S2 (2011) International conference on harmonization. Guidance on genotoxicity testing and data interpretation for phamaceuticals intended for human use S2(R1) 9 Nov 2011. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step2/S2_R1__Guideline.pdf. Accessed 3 Sep 2011
Moore MM, Clive D, Hozier JC, Barry E, Howard BE, Batson AG, Turner NT, Sawyer J (1985) Analysis of trifluorothymidine-resistant (TFTr) mutants of L5178Y/TK+/- mouse lymphoma cells. Mutat Res 151: 161–174
Moore M-M, Doerr C-L (1990) Comparison of chromosome aberration frequency and small-colony TK-deficient mutant frequency in L5178Y/TK(+/–)-3.7.2 C mouse lymphoma cells. Mutagenesis 5: 609–614
Moore MM, Honma M, Clements J, Awogi T, Douglas GR, van Goethem F, Gollapudi B, Kimura A, Muster W, O’Donovan M, Schoeny R, Wakuri S (2011) Suitable top concentration for tests with mammalian cells: mouse lymphoma assay workgroup Mutat Res 723(2): 84–86. Epub 2011 Apr 14. PubMed PMID: 21514400
Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Clay P, Doppalapudi R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Muster W, Pant K, Kidd DA, Lorge E, Lloyd M, Myhr B, O’Donovan M, Riach C, Stankowski LF Jr, Thakur AK, Van Goethem F, Mouse Lymphoma Assay Workgroup, IWGT (2007) Mouse lymphoma thymidine kinase gene mutation assay: meeting of the International Workshop on Genotoxicity Testing, San Francisco, 2005, recommendations for 24-h treatment. Mutat Res 627(1): 36–40. Epub 2006 Dec 6. PubMed PMID: 17157054
Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Lloyd M, Majeska J, Myhr B, O’Donovan M, Omori T, Riach C, San R, Stankowski LF Jr, Thakur AK, Van Goethem F, Wakuri S, Yoshimura I (2006) Mouse lymphoma thymidine kinase gene mutation assay: follow-up meeting of the International Workshop on Genotoxicity Testing--Aberdeen, Scotland, 2003 – Assay acceptance criteria, positive controls, and data evaluation. Environ Mol Mutagen 47(1):1–5. PubMed PMID: 15991242
Moore M-M, Honma M, Clements J, Bolcsfoldi G, Cifone M, Delongchamp R, Fellows M, Gollapudi B, Jenkinson P, Kirby P, Kirchner S, Muster W, Myhr B, O’Donovan M, Oliver J, Omori T, Ouldelhkim MC, Pant K, Preston R, Riach C, San R, Stankowski LF Jr, Thakur A, Wakuri S, Yoshimura I (2003) Mouse Lymphoma Assay Workgroup mouse lymphoma thymidine kinase gene mutation assay: international workshop on genotoxicity tests workgroup report – Plymouth, UK 2002. Mutat Res 540(2): 127–140
OECD Guideline 476, In Vitro Mammalian Cell Mutation Test, adopted 21 July 1997
Sofuni T, Wilcox P, Shimada H, Clements J, Honma M, Clive D, Green M, Thybaud V, San R-H., Elliott B-M and Muller L (1997) Mouse lymphoma workshop: Victoria, British Columbia, Canada, March 27, 1996. Protocol issues regarding the use of the microwell method of the mouse lymphoma assay. Environ Mol Mutagen 29: 434–438
Micronucleus Test In Vitro
PURPOSE/RATIONALE
The purpose of the micronucleus test in vitro is to evaluate the potential of compounds to induce micronuclei (formation of small membrane-bound DNA fragments) in different cell lines or primary cultures, with and without metabolic activation by S9 liver homogenate.
The test system allows to discriminate between a clastogenic and aneugenic potential of a test compound by using an immunochemical labeling of the kinetochores or staining the DNA fragments with FISH (fluorescence in situ hybridization) technique.
PROCEDURE
The cells are treated with the test article in 96-well microplates for a short treatment period with metabolic activation (e.g., 3 h) and for a long treatment period without metabolic activation (e.g., 24 h) and harvested 24 h (recovery time) after the end of the treatment. Cells are then fixed and stained. The cytotoxicity of the test compound is evaluated by the relative cell growth, expressed as a percentage of the negative control. The highest evaluated concentration should produce approximately 50% cell survival or should be the first concentration where precipitation is observed. Duplicated cultures should be performed at each dose level. A very detailed description of the protocol, the validity criteria, and the evaluation was published by the IWGT in 2003 (Kirsch-Volders et al. 2003).
EVALUATION
Structural/numerical chromosome damage is evaluated by the increase in the number of micronucleated cells, scored out of 1,000 cells in three analyzable concentrations. The compound is considered positive if either:
-
The increase of micronucleated cells is statistically significant compared to the negative (solvent or untreated) control, or
-
The number of micronuclei is dose dependent and showed a biological relevance compared to the negative control.
The positive control must show a clear statistical significant effect compared to the negative control.
CRITICAL ASSESSMENT OF THE METHOD
The micronucleus test in vitro is easy and rapid to perform, inexpensive, and needs much less test compound compared to the mammalian chromosome aberration test. In addition no detailed training of the personnel for the light microscopical evaluation of the slides is necessary. In fact, the micronucleus test in vitro is currently the only in vitro test which allows the differentiation between a clastogenic or aneugenic effect, and is becoming ever more important in the genotoxic testing strategy. The finalization of the OECD Guideline for this assay and the implementation into the ICH S2R Guidance was key to use this assay for regulatory purposes. As the evaluation of micronuclei is much easier and less time consuming, this assay will replace the chromosome aberration assay in vitro in the future.
MODIFICATIONS OF THE METHOD
An alternative method for the evaluation of the induced micronuclei is measurement by Flow cytometry. This method allows the discrimination between micronuclei that contain one or several acentric chromosome fragments (clastogenic action) or one or several whole chromosomes (aneugenic action, interference with the mitotic spindle apparatus) or even a combination of both. To discriminate between the actions an additional staining of the micronuclei with, e.g., CREST antibodies is necessary.
References and Further Reading
Kirsch-Volders M (1997) Towards a validation of the micronucleus test. Mutat Res 392: 1–4
Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, Ishidate M, Kirchner S, Lorge E, Morita T, Norppa H, Surrales J, Vanhauwaert A, Wakata A (2003) Report from the in vitro micronucleus assay working group. Mutat Res 540: 153–163
Nüsse M, Beisker W, Kramer J, Miller B, Schreiber G, Viaggi S, Weller W-M, Wessels J-M(1994) Measurement of micronuclei by flow cytometry. Methods Cell Biol 42: 149–158
OECD Guideline for testing of chemicals, draft proposal for a new guideline 487 in vitro micronucleus test, adopted 22 July 2010
Micronucleus Test In Vivo
PURPOSE/RATIONALE
The in vivo mammalian micronucleus test is used to assess the mutagenic hazard in consideration of factors like in vivo metabolism, pharmacokinetics, and DNA repair processes, although these may vary among species and among tissues. This assay is an important part of the gentox testing battery, applied for pharmaceuticals and chemicals.
During erythropoiesis, nuclei are expelled during the formation of polychromatic erythrocytes while micronuclei are retained in the cells. This fact is used for the detection of micronuclei. A significant increase in the number of micronucleated polychromatic erythrocytes is usually considered as indicative of structural and/or numerical chromosome damage caused by exposure to a clastogenic and/or aneugenic substance. The identification of the presence or absence of a kinetochore or centromeric DNA in the micronuclei is needed to discriminate a clastogenic effect from an aneugenic effect. The incidence of micronuclei in the polychromatic erythrocytes is scored 24 h after the end of treatment to take into account the time interval between the last mitosis and the formation of polychromatic erythrocytes (at least 8 h) and the lifespan of polychromatic erythrocytes, which is approximately 24 h.
PROCEDURE
The bone marrow of rodents (rats and mice) is routinely used in this test since polychromatic erythrocytes are produced in that tissue, it is a highly vascularized tissue and it contains a population of rapidly cycling cells that can be readily isolated and processed. The assay was developed by Schmid (1975) and modified by Salamone et al. (1980). Recent protocols and recommendations are published in the OECD Guideline 474 and Hayashi et al. 2000. The measurement of micronucleated immature (polychromatic) erythrocytes in peripheral blood is equally acceptable in any species in which the inability of the spleen to remove micronucleated erythrocytes has been demonstrated. The evaluation of micronuclei in peripheral blood is an easy method integrated the micronucleus assay into General toxicity studies (Rothfuss et al. 2011).
The route of administration of the test compound should ensure a relevant target exposure and in the case of pharmaceuticals it should consider the application route in humans. Each treated and control group must include at least five analyzable animals per sex. It is possible to use only one gender if it could be demonstrated that no substantial differences in metabolism, toxicity, and pharmacokinetics between genders was observed. Test substances could be administered as a single treatment. Repeated treatment up to 28 days could is also possible. If a single treatment is used the sampling time should be between 24 and 48 h. If repeated treatment is used, the sampling time is 24 h after the last treatment.
Bone marrow cells are usually obtained from the femurs or tibias immediately following sacrifice. Usually, cells are removed from femurs or tibias in a suitable medium such as fetal serum, and are prepared and stained using established methods. DNA specific stains (e.g., acridine orange) are preferred instead of conventional stains like Giemsa.
The likelihood that the test substance or its metabolites reach the general circulation or the target tissue (e.g., systemic toxicity) should be demonstrated. Preferably, experimental evidence of systemic or target tissue exposure should be presented (e.g., blood level or bone marrow concentration analysis), especially in the case of a negative result with an agent that does not induce observable toxicity.
EVALUATION
The proportion of immature among total (immature + mature) erythrocytes as measure for target organ toxicity is determined for each animal by counting a total of at least 200 erythrocytes for bone marrow and 1,000 erythrocytes for peripheral blood. At least 2,000 immature erythrocytes per animal are scored for the incidence of micronucleated immature erythrocytes to determine the clastogenic/aneugenic potential of the test compound. The automatic analysis of micronucleated erythrocytes was developed by Romagna and Staniforth (1989).
For a discussion of the result of the micronucleus assay a comparison of the data from the treatment group versus concurrent negative control data and historical control data as well as a statistical analysis of the experimental data using trend analysis or pair-wise comparison (treatment group versus control) need to be considered. It is also recommended to check the variance between the animals and gender. However, for the final assessment, biological relevance of the results should be considered.
CRITICAL ASSESSMENT OF THE METHOD
There are compounds for which standard in vivo tests do not provide additional useful information. This is particularly true for compounds for which data from studies on toxicokinetics or pharmacokinetics indicate that they are not systemically absorbed and therefore are not available for the target tissues. Examples of such compounds are some radioimaging agents, aluminum-based antacids, and some dermally applied pharmaceuticals. In those cases other tests systems should be considered to be more relevant.
In addition, parameters like decreased body temperature may lead to an indirect induction of micronuclei that is not biologically relevant.
References and Further Reading
Frieauff W, Romagna, F (1994) Technical aspects of automatic micronucleus analysis in rodent bone marrow assays. Cell Biol Toxicol 10: 283–289
Hayashi M, MacGregor J-T, Gatehouse D-G, Adler I-D, Blakey D, Dertinger S-D, Krishna G, Morita T, Russo A, Sutou S (2000) In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments, integration with toxicity testing and automated scoring. Environ Mol Mutagen 35: 234–252
ICH S2 (2011) International conference on harmonization. Guidance on genotoxicity testing and data interpretation for phamaceuticals intended for human use S2(R1) 9 Nov 2011. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step2/S2_R1__Guideline.pdf. Accessed 3 Sep 2011
OECD Guideline 475, Mammalian Bone Marrow Chromosome Aberration Test, adopted 21 July 1997
Romagna F, Staniforth C-D (1989) The automated bone marrow micronucleus test. Mutat Res 213: 91–104
Rothfuss A, Honma M, Czich A, Aardema MJ, Burlinson B, Galloway S, Hamada S, Kirkland D, Heflich RH, Howe J, Nakajima M, O’Donovan M, Plappert-Helbig U, Priestley C, Recio L, Schuler M, Uno Y, Martus HJ (2011) Improvement of in vivo genotoxicity assessment: combination of acute tests and integration into standard toxicity testing. Mutat Res 723(2): 108–120. Epub 2010 Dec 21
Salamone M, Heddle J, Stuart E, Katz M (1980) Towards an improved micronucleus test. Mutat Res 74: 347–356
Schmid W (1975) The micronucleus test. Mutat Res 31: 9–15
Mammalian Chromosome Aberration Test In Vitro
PURPOSE/RATIONALE
This in vitro cytogenetic test is a clastogenicity test system for the detection of chromosomal aberrations in cultured mammalian cells or primary cultures. Chromosomal aberrations may be either structural or numerical. However, because cytogenetic assays are usually designed to analyze cells at their first posttreatment mitosis and numerical aberrations require at least one cell division to be visualized, this type of aberration is generally not observed in a routine cytogenetic assay. The best estimate of aberration frequency is the first cell division after the start of treatment. Structural aberrations are of two types: chromosome or chromatid aberrations.
Chromosome-type aberrations are induced when a compound acts in the G1 phase of the cell cycle. Chromatid-type aberrations are induced when a chemical acts in the S or G2 phase of the cell cycle.
-
Chromosome-type aberrations are structural chromosome damages expressed as breakage, or breakage and reunion, of both chromatids at an identical site
-
Chromatid-type aberrations are structural chromosome damages expressed as breakage of single chromatids or breakage and reunion between chromatids
-
Numerical aberrations are changes in the number of chromosomes of the normal number characteristic of the animals utilized
The best estimate of aberration frequency is the first cell division after the start of treatment.
PROCEDURE
In this test, cultured cells are seeded onto slides and the cells, which have been treated with and without metabolic activation for a short-time period (e.g., 3 h). Where negative or equivocal results are obtained, an independent experiment is conducted in which cells are treated for long-time period (e.g., 20 h) in the absence of metabolic activation alone and then sampled and examined for chromosome analysis. In both experiments the cells are sampled 20 h after the start of treatment as are the concurrent solvent and positive control cultures. Colcemid is added to each culture 2 h before sampling in order to arrest cell division. Chromosome preparations are made, fixed, stained, and examined. However, if clearly positive results are obtained in the first experiment, those from the second assay are not examined. If equivocal or negative results are obtained in the first experiment, modifications to the testing procedure are included in order to clarify the result.
EVALUATION
The set of chromosomes is examined for completeness and the various chromosomal aberrations are assessed and classified. The metaphases are examined for the following aberrations: chromatid gap, chromosome gap, chromatid break, chromosome break, chromatid acentric fragment, chromosome acentric fragment, chromatid deletion, chromosome deletion, chromatid exchanges including intrachanges, chromosome exchanges including intrachanges, dicentrics, pulverization, and ring formation. Metaphases including five or more break events are scored as multiple aberrant. Furthermore the incidence of polyploid metaphases is determined for each cell culture. The quantity of cells is determined by counting the number of cells in, e.g., 10 fields of vision per slide as an indicator of toxicity. The survival of cells is expressed as a percentage. Additionally the mitotic index should be determined by counting the number of cells undergoing mitosis in a total of, e.g., 1,000 cells. The mitotic index is also expressed as a percentage. For each experiment the results from the dose groups is compared with those of the control group and the positive control at each sampling time.
The assay is considered valid if both of the following criteria are met:
-
The solvent control data are within the laboratory’s normal control range for the number of cells carrying structural chromosomal aberrations
-
The positive controls induce increases in the number of cells carrying structural aberrations which are statistically significant and within the laboratory’s normal range.
A test substance is classified as non-clastogenic if either of the following is met:
-
The number of induced structural chromosome aberrations in all evaluated dose groups is in the range of our historical control data.
-
No significant increase of the number of structural chromosome aberrations is observed.
A test substance is classified as clastogenic if both the following are met:
-
The number of induced structural chromosome aberrations is not in the range of our historical control data.
-
Either a concentration-related or a significant increase of the number of structural chromosome aberrations is observed.
When evaluating the findings, both biological and statistical significance should be considered together. If the criteria mentioned above for the test item are not clearly met, the classification with regard to the historical data and the biological relevance should be discussed and/or a confirmatory experiment should be performed.
CRITICAL ASSESSMENT OF THE METHOD
Care should be taken to avoid conditions (pH, high cytotoxicity, osmolality, etc) that would lead to positive results but which do not reflect intrinsic mutagenicity. Mammalian carcinogens are often positive in this test. Nevertheless, there is not a perfect correlation between this test and carcinogenicity, which depends on the chemical class. Some chemicals may test positive in this test because they appear to act through other mechanism than direct DNA damage, e.g., apoptosis.
MODIFICATIONS OF THE METHOD
The test could be performed with different cell lines (permanent and primary) and different metabolic activation systems.
References and Further Reading
Ames BN, McCann J, Yamasaki E (1975) Methods for detecting carcinogens and mutagens with the Salmonella/mammalian microsome mutagenicity test. Mutat Res 31: 347–364
Bradley MO, Bhuyan B, Francis MC, Langenbach R, Peterson A, Huberman E (1981) Mutagenesis by chemical agents in V79 Chinese hamster cells: a review and analysis of the literature. A report of the gene-tox program. Mutat Res 87: 81–142
Bridge BA (1976) Short term screening tests for carcinogens. Nature 261: 195–200
EEC Directive 2000/32/EC, L 136, B.10., Mutagenicity – in vitro mammalian chromosome aberration test, adopted 19 May 2000
Howard-Flanders P (1981) Mutagenesis in mammalian cells. Mutat Res 86: 307–327
Mitelman F (ed) (1995) An international system for human cytogenetic nomenclature recommendations of the international standing committee on human cytogenetic nomenclature. S. Karger, Basel
OECD Guideline For Testing Of Chemicals, 473 “Genetic Toxicology: In Vitro Mammalian Chromosome Aberration Test”, adopted 21 Jul 1997
U.S. EPA: OPPTS 870.5375 (1998) Health effects test guidelines in vitro mammalian chromosome aberration test
Mammalian Chromosome Aberration Test In Vivo
PURPOSE/RATIONALE
Like the in vivo micronucleus assay, the in vivo mammalian chromosome aberration assay is especially relevant for assessing the mutagenic hazard while taking into consideration factors like in vivo metabolism, pharmacokinetics, and DNA repair processes, although these may vary among species and among tissues. In the gentox testing battery this assay is mainly used for further investigation of mutagenic effects detected by an in vitro test. In addition, the assay can be used for the detection of compounds that induce polyploidies. An increase in the number of polyploidy cells may indicate that a compound has the potential to induce numerical aberrations.
The induction of structural chromosome aberrations is classified in two types, chromosome or chromatid aberrations. The majority of induced aberrations are of the chromatid-type, but chromosome-type aberrations also occur. Chromosomal mutations and related events are the cause of many human genetic diseases and there is evidence that chromosomal mutations and related events are involved in cancer development.
In addition, the assay can be used for the detection of compounds that induce polyploidies. An increase in the number of polyploidy cells may indicate that a compound has the potential to induce numerical aberrations.
PROCEDURE
Rodents (rat, mice, Chinese hamster) are routinely used in this test. Although chromosome aberrations can be detected in various tissues, the most common methodologies are available for investigations of bone marrow (Preston et al. 1987), peripheral blood, and female and male germ cells (Russo 2000). Bone marrow is the normally used target tissue in this test, since it is a highly vascularized tissue, and it contains a population of rapidly cycling cells that can be readily isolated and processed (Tice et al. 1994). However, methodologies are available for investigations of other tissues and cells.
The route of administration of the test compound should ensure a relevant target exposure and in the case of pharmaceuticals it should consider the application route in humans. Each treated and control group must include at least five animals that can be analyzed per sex. It is possible to use only one gender if it can be demonstrated that no substantial differences in metabolism, toxicity, and pharmacokinetics between genders was observed. Test substances are preferably administered as a single treatment; however, repeated treatment up to 28 days could also be performed. If a single treatment is used two sample times (12–18 h and 36–44 h) should be used for bone marrow. If repeated treatment is used, the sampling time is 6–24 h after the last treatment. If species other than rodents or other tissues are used, the sampling time must be scientifically justified.
Prior to sacrifice (3–5 h), animals are treated with a metaphase-arresting agent (e.g., colchicine). Chromosome preparations are then made from the respective tissues and stained with an appropriate method. For a better discrimination of the chromosomes and to detect translocations, the FISH technology can be used (Natrajan and Boei 2004). Metaphase cells are microscopically analyzed for the occurrence of structural and numerical chromosome aberrations.
The likelihood that the test substance or its metabolites reach the general circulation or the target tissue (e.g., systemic toxicity) needs to be demonstrated. Preferably, experimental evidence of systemic or target tissue exposure should be presented (e.g., blood level or bone marrow concentration analysis), especially in the case of a negative result with an agent that does not induce observable toxicity.
EVALUATION
At least 100 metaphase plates per animal should be scored per animal based on the use of at least five animals per gender per treatment group. The minimal classes of aberrations to score and categorize would be chromosome type versus chromatid type. Within these two categories gaps, breaks, and rearrangements should be differentiated. To describe the toxicity in the target organ, the mitotic index is determined in at least 1,000 nucleated cells per animal. However, for the detection of polyploidies at least 22 metaphase cells should be scored. Due to differences in the mechanism of development, endoreduplicated cells should be scored separately.
For a discussion of the result of the chromosome aberration assay the following parameters need to be considered: (a) comparison of the data from the treatment group versus concurrent negative control data and historical control data (b) statistical analysis of the experimental data using trend analysis or pair-wise comparison (treatment group versus control). It is also recommended to check the variance between the animals and gender. However, for the final assessment, biological relevance of the results should be considered.
CRITICAL ASSESSMENT OF THE METHOD
There are compounds for which standard in vivo tests do not provide additional useful information. This is particularly true for compounds for which data from studies on toxicokinetics or pharmacokinetics indicate that they are not systemically absorbed and therefore are not available for the target tissues. Examples of such compounds are some radioimaging agents, aluminum-based antacids, and some dermally applied pharmaceuticals. In those cases other tests systems should be considered to be more relevant (p32 Postlabeling, Comet Assay in vivo).
References and Further Reading
ICH S2 (2011) International conference on harmonization. Guidance on genotoxicity testing and data interpretation for phamaceuticals intended for human use S2(R1) 9 Nov 2011. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step2/S2_R1__Guideline.pdf. Accessed 3 Sep 2011
Natarajan AT, Boei JJ (2004) Formation of chromosome aberrations: insights from FISH. Mutat Res 544: 299–304
OECD Guideline 475, Mammalian Bone Marrow Chromosome Aberration Test, adopted 21 July 1997
Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M (1987) Mammalian in vivo cytogenetic assays: analysis of chromosome aberrations in bone marrow cells. Mutat Res 189 (2): 157–165
Richold M, Chandley A, Ashby J, Gatehouse DG, Bootman J, Henderson L (1990) In vivo cytogenetic assays. In Kirkland DJ (ed) Basic mutagenicity tests, UKEMS recommended procedures. UKEMS subcommitee on guidelines for mutagenicity testing. Report. Part I revised. Cambridge University Press, Cambridge/New York/Port Chester/Melbourne/Sydney, pp 115–141
Russo A (2000) In vivo cytogenetics: mammalian germ cells. Mutat Res 455: 167–189
Tice RR, Hayashi M, MacGregor JT, Anderson D, Blakey DH, Holden HE, Kirsch-Volders M, Oleson FB Jr, Pacchierotti F, Preston RJ, Romagna F, Shimada H, Sutou S, Vannier B (1994) Report from the working group on the in vivo mammalian bone marrow chromosome aberration test. Mutat Res 312: 305–312
Comet Assay In Vivo
PURPOSE/RATIONALE
The in vivo Comet assay (single-cell gel electrophoresis assay) is increasingly used in regulatory genotoxicity testing for the evaluation of DNA damage and repair in various tissues of mice and rats that cannot easily be evaluated with other standard tests. Protocols are established for liver, skin, stomach, gut, kidney, retina, and nasal tissues. The Assay is used as follow-up assay for in vitro positive results and is discussed as second endpoint for a combined in vivo protocol (micronuclei in bone marrow/Comet assay in liver) Rothfuss et al. 2010. The advantage of the model is that different target organs can be used to detect a genotoxic potential.
The test is in general conducted based on the method described by Singh et al. (1988) then modified by Hartmann et al. (2003), Tice et al. (2000) and the validation management team of the International Validation of the In Vivo rodent alkaline COMET assay. Protocols for conducting the in vivo Comet assay were developed by different expert panels, e.g., at the 2nd and 4th International Workshops on Genotoxicity Testing, Burlinson et al. (2007) and the 4th International Comet assay Workshop.
PROCEDURE
The Comet assay is used to visualize and measure DNA strand breaks in individual cells by microscopy. Animals are treated between 2 and 28 days. In general, the last compound administration is 3 h before the animals are anesthetized and euthanized. After necropsy of the specific organs, the cells are isolated by organ perfusion or mincing the cell tissues. It is in general recommended that at least three slides will be prepared per tissue and animal.
In the alkaline version, the isolated cells are embedded in an agarose gel on a microscope slide, immersed in a lysis solution to remove lipids and proteins. The slides will remain in the buffer for 20 min. Using the same buffer, electrophoresis will be conducted in general at a constant voltage of 0.7 V/cm. The current at the start of the electrophoresis will be adjusted to 300 mA, a weak electric field to attract broken negatively charged DNA toward the anode.
After electrophoresis, the DNA is stained using a fluorescent dye and viewed using a fluorescence microscope. When viewed under a microscope, the cell containing DNA strand breaks has the appearance of a comet, with a head (the nuclear region) and a tail containing DNA fragments or strands. Individual images are analyzed for quantifying DNA migration parameters such as the percentage of DNA that has migrated, named tail intensity. These measurements give an indication of the incidence of strand breaks present in the cell.
Coded slides must be scored in blind fashion. Slides will be stained with a DNA stain prior to scoring (SYBR-gold, propidium iodide, or any appropriate DNA stain).
EVALUATION
DNA effects will be assessed by the software system by measuring Comet tail migration, % tail intensity, and Olive tail moment. Tail migration is the distance from the perimeter of the Comet head to the last visible point in the tail; % tail intensity the percentage of DNA fragments present in the tail; and Olive tail moment is the product of the amount of DNA in the tail and the mean distance of migration in the tail. For each sample, 150 cells (three slides per sample with 50 cells per slide or two slides per sample with 75 cells per slide, if possible) will be scored for DNA damage.
Generally, all cells including heavily damaged cells are scored as long as the image analysis system can properly discern a head. Cells without discernable head and large diffuse tail which cannot be scored by the image analyzer (ghosts) are excluded from analysis. The frequency of such ghosts should be determined per sample, based on the visual scoring of 100 cells per sample.
As an example Liver Comet Assay data will be accepted if the following criteria are met: Negative control: Means of %DNA in tail are 1–8%.
Positive control: Ratio of means of %DNA in tail between groups of positive and vehicle control is twofold or higher.
Those values need to be verified for each organ, as there is high variability between organs.
CRITICAL ASSESSMENT OF THE METHOD
The assay is rapid and easy to conduct, but the laboratory needs experience in preparation of the cells. The quality of the assay is highly depending on that. The advantage of the assay is that the assay can be applied to any tissue and cell division not required for the detection of DNA damage. The analysis is conducted in individual cells; therefore low number of cells is sufficient. However, the assay is resource intensive and time consuming, in particular the scoring of comets takes time if not automated. Apoptosis/necrosis as indicators of tissue cytotoxicity needs to be controlled in the histopathology part of the study, and positive results in the presence of toxicity are difficult to interpret. At the moment there are no guidelines available. Different statistical methods are available but should be carefully applied for the interpretation of the results.
References and Further Reading
Burlinson B, Tice RR, Speit G, Speit E, Agurell GSY, Collins EAR, Escobar P, Honma M, Kumaravel TS, Nakajima M, Sasaki YF, Thybaud V, Uno Y, Vasquez M, Hartmann A (2007) In vivo comet assay workgroup, part of the fourth international workgroup on genotoxicity testing. Mutat Res 627: 31–35
Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR (2003) Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis 18: 45–51
Rothfuss A, O’Donovan M, De Boeck M, Brault D, Czich A, Custer L, Hamada S, Plappert-Helbig U, Hayashi M, Howe J, Kraynak AR, van der Leede BJ, Nakajima M, Priestley C, Thybaud V, Saigo K, Sawant S, Shi J, Storer R, Struwe M, Vock E, Galloway S (2010) Collaborative study on fifteen compounds in the rat-liver Comet assay integrated into 2- and 4-week repeat-dose studies. Mutat Res 702(1): 40–69. Epub 2010 Jul 23. PubMed PMID: 20656055
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175: 184–191
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35: 206–221
Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells In Vitro
PURPOSE/RATIONALE
The UDS test detects DNA repair synthesis after excision of damaged segments. DNA repair synthesis is demonstrated by autoradiographic measurement of tritium-labeled thymidine incorporation. Liver cells are routinely used for the UDS test because cells in S-phase are rare and easily distinguished from cells undergoing DNA repair and because hepatocytes exhibit a high metabolic activity that enables the detection of pro-mutagens.
PROCEDURE
Liver cells are isolated by a two-step perfusion procedure from anesthetized rats.
A medio-ventral incision is made from the pubic symphisis to the thorax. A catheter is inserted into the portal vein and the liver is perfused with collagenase solution. At the end of the perfusion, the liver is taken out, the liver capsule is removed, and the cells are mechanically dispersed by gentle shaking. After dispersion of the liver cells in medium, the vessels and remaining conjunctive tissue are removed and collagenase activity is neutralized. Cell viability is assessed by the percentage of refringent cells. Only cell suspensions with more than 70% viability should be retained.
Liver cells are then exposed in vitro to the test compound and incubated with tritium-labeled thymidine for about 18 h. At the end of the incubation, the cells are fixed on slides and prepared for autoradiography. For that the slides are first exposed to liquid photographic emulsion, air-dried and following a 7-day exposure in the dark, exposed to developing solution.
EVALUATION
Cells undergoing DNA repair are identified by the increase in the number of silver grains in the nuclei, i.e., the net nuclear grain count. Only normal-appearing nuclei are scored; occasional nuclei blackened by grains are excluded since these are nuclei undergoing replicative DNA synthesis. One hundred cells per concentration and control are analyzed. For each cell, the number of silver grains in the nucleus and the number of silver grains in three adjacent nucleus-equivalent areas on the cytoplasm are measured. For each cell, the following parameters are calculated:
-
The nuclear grain count (N)
-
The mean of the three cytoplasmic grain counts (C)
-
The net nuclear grain count (NG), i.e., the difference between the nuclear grain count and the mean of the three cytoplasmic grain counts
A cell is considered undergoing DNA repair if the value of the net nuclear grain count is greater than five.
For each slide, the following parameters are calculated:
-
The mean and standard deviation of nuclear and cytoplasmic grain counts, and of net nuclear grain count
-
The percentage of cells undergoing DNA repair and mean value of net nuclear grain count for these cells.
The test article is considered positive in the UDS assay if the mean net nuclear grain count is greater than five and if the percentage of cells undergoing DNA repair is greater than 20%.
CRITICAL ASSESSMENT OF THE METHOD
The biological significance has to be taken into consideration for positive evaluation (i.e., cytotoxicity can artefactually lead to an increase in the value of net nuclear grain count). The positive control should induce a clear increase in the mean net nuclear grain count higher than the threshold value of five.
References and Further Reading
Ashby J, Trueman RW, Mohammed R, Barber G (1987) Positive and negative control observations for the in vivo/in vitro rat liver assay for unscheduled DNA synthesis (UDS). Mutagenesis 2 (6): 489–490
Bermudez E, Mirsalis JC, Eales HC (1982) Detection of DNA damage in primary cultures of rat hepatocytes following in vivo and in vitro exposure to genotoxic agents. Environ Mutagen 4: 667–679
Cleaver JE, Thomas GH (1981) Measurement of unscheduled synthesis by autoradiography. In Friedberg EC, Hanawalt PC (eds) DNA repair. A laboratory manual of research procedures. Marcel Dekker, New York, pp 277–287
Mirsalis J, Butterworth BE (1980) Detection of unscheduled DNA synthesis in hepatocytes isolated from rats treated with genotoxic agents: an in vivo/in vitro assay for potential carcinogens and mutagens. Carcinogenesis 1: 621–625
Mirsalis JC, Tyson CK, Butterworth BE (1982) Detection of genotoxic carcinogens in the in vivo-in vitro hepatocyte DNA repair assay. Environ Mutagen 4: 553–562
Mitchell AD, Casciano DA, Meltz ML, Robinson DE, San RHC, Williams GM, Von Halle ES (1983) Unscheduled DNA synthesis tests. A report of the U.S. Environmental Protection Agency. Gene-Tox Program. Mutat Res 123: 363–410
Rogers AW (1973) Technics of autoradiography. Elsevier Scientific, Amsterdam, p 218
Seglen PO (1975) Preparation of isolated rat liver cells. Methods Cell Biol 13: 29–83
Williams GM (1977) Detection of chemical carcinogens by unscheduled DNA synthesis in rat liver primary cell culture. Cancer Res 37: 1845–1851
Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells In Vivo
The principles of this method are the same and the procedure similar as for the above described method “Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells in vitro.” The differences between the in vivo and the in vitro method are related to the test compound exposure. In the in vivo assay, rats are first treated in vivo with the test compound and the liver cells are isolated 14 h after treatment. Then the liver cells are incubated for 4 h with a medium containing tritium-labeled thymidine, followed by 18 h in a medium containing non-labeled thymidine. The incorporation of tritium-labeled thymidine is measured by autoradiography. Cells undergoing repair are identified by the increase in the number of silver grains in the nuclei.
References and Further Reading
Ashby J, Lefèvre PA, Burlinson B, Penman MG (1985) An assessment of the in vivo rat hepatocyte DNA repair assay. Mutat Res 156: 1–18
Butterworth BE, Ashby J, Bermudez E, Casciano D, Mirsalis J, Probst G, Williams GM (1987) A protocol and guide for the in vivo rat hepatocytes DNA repair assay. Mutat Res 189: 123–133
Mirsalis JC, Tyson CK, Steinmetz KL et al. (1989) Measurement of unscheduled DNA synthesis and S-phase synthesis in rodent hepatocytes following In Vivo treatment: testing of 24 compounds. Environ and Mol Mutagen 14: 155–164
OECD Guideline 486, Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells in vivo, adopted 21 July 1997
References and Further Reading
Alvares A-P, Bickers D-R, Kappas A (1973) Polychlorinated biphenyls: a new type of inducer of cytochrome P 448 in the liver. Proc Natl Acad Sci USA 70:1321–1325
Ames B-N, Durston W-W, Yamasaki E, Lee F-D (1973) Carcinogens are mutagens. A simple test system combining liver homogenate for activation and bacteria for detection. Proc Natl Acad Sci USA 70:2281–2285
Ames B-N, McCann J, Yamasaki E (1975) Methods for detecting carcinogens and mutagens with the Salmonella/mammalian microsome mutagenicity test. Mutat Res 31:347–364
Ashby J, Lefèvre PA, Burlinson B, Penman MG (1985) An assessment of the in vivo rat hepatocyte DNA repair assay. Mutat Res 156:1–18
Ashby J, Trueman RW, Mohammed R, Barber G (1987) Positive and negative control observations for the in vivo/in vitro rat liver assay for unscheduled DNA synthesis (UDS). Mutagenesis 2(6):489–490
Bermudez E, Mirsalis JC, Eales HC (1982) Detection of DNA damage in primary cultures of rat hepatocytes following in vivo and in vitro exposure to genotoxic agents. Environ Mutagen 4:667–679
Bradley MO, Bhuyan B, Francis MC, Langenbach R, Peterson A, Huberman E (1981) Mutagenesis by chemical agents in V79 Chinese hamster cells: a review and analysis of the literature. A report of the gene-tox program. Mutat Res 87:81–142
Bridge BA (1976) Short term screening tests for carcinogens. Nature 261:195–200
Burlinson J, Tice RR, Speit G, Speit E, Agurell GSY, Collins EAR, Escobar P, Honma M, Kumaravel TS, Nakajima M, Sasaki YF, Thybaud V, Uno Y, Vasquez M, Hartmann A (2007) In vivo comet assay workgroup, part of the fourth international workgroup on genotoxicity testing. Mutat Res 627:31–35
Butterworth BE, Ashby J, Bermudez E, Casciano D, Mirsalis J, Probst G, Williams GM (1987) A protocol and guide for the in vivo rat hepatocytes DNA repair assay. Mutat Res 189:123–133
Clark L, Hart D, Vojta P, Harrington-Brock K, Barrett J, Moore M, Tindall K (1998) Identification and chromosomal assignment of two heterozygous mutations in the Trp53 gene in L5178Y/Tk + /–3.7.2 C mouse lymphoma cells. Mutagenesis 13:427–439
Cleaver JE, Thomas GH (1981) Measurement of unscheduled synthesis by autoradiography. In: Friedberg EC, Hanawalt PC (eds) DNA repair. A laboratory manual of research procedures. Marcel Dekker, New York, pp 277–287
Clive D, Flamm W, Patterson J (1972) A mutational assay system using the thymidine kinase locus in mouse lymphoma cells. Mutat Res 16:77
Cole J, Muriel W-J, Bridges B-A (1986) The mutagenicity of sodium fluoride to L5178Y [wild-type and TK+/– (3. 7.2c)] mouse lymphoma cells. Mutagenesis 1:157–167
Dearfield KL, Thybaud V, Cimino MC, Custer L, Czich A, Harvey JS, Hester S, Kim JH, Kirkland D, Levy DD, Lorge E, Moore MM, Ouédraogo-Arras G, Schuler M, Suter W, Sweder K, Tarlo K, van Benthem J, van Goethem F, Witt KL (2011) Follow-up actions from positive results of in vitro genetic toxicity testing. Environ Mol Mutagen 52(3):177–204. doi:10.1002/em.20617. Epub 2010 Oct 20. Review
EC Directive 2000/32/EC, L 136, Annex 4D, B.13/B.14
EEC Directive 2000/32/EC, L 136, B.10., “Mutagenicity – in vitro mammalian chromosome aberration test”, adopted 19 May 2000
EMEA (2006) Guideline on the limits of genotoxic impurities. Committee For Medicinal Products for Human Use, The European Medicines Agency, London, 2006, CPMP/SWP/5199/02, EMEA/CHMP/QWP/251344/2006
Flückiger-Isler S, Baumeister M, Braun K, Gervais V, Hasler-Nguyen N, Reimann R, Van Gompel J, Wunderlich H-G, Engelhardt G (2004) Assessment of the performance of the Ames IITM assay: a collaborative study with 19 coded compounds. Mutat Res 558:181–197
Frieauff W, Romagna F (1994) Technical aspects of automatic micronucleus analysis in rodent bone marrow assays. Cell Biol Toxicol 10:283–289
Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR (2003) Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis 18:45–51
Hayashi M, MacGregor J-T, Gatehouse D-G, Adler I-D, Blakey D, Dertinger S-D, Krishna G, Morita T, Russo A, Sutou S (2000) In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments, integration with toxicity testing and automated scoring. Environ Mol Mutagen 35:234–252
Howard-Flanders P (1981) Mutagenesis in mammalian cells. Mutat Res 86:307–327
ICH S2 (2011) International conference on harmonization. Guidance on genotoxicity testing and data interpretation for phamaceuticals intended for human use S2(R1) 9 Nov 2011. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step2/S2_R1__Guideline.pdf. Accessed 3 Sep 2011
Kamber M, Flückiger-Isler S, Engelhardt G, Jaeckh R, Zeiger E (2009) Comparison of the Ames II and traditional Ames test responses with respect to mutagenicity, strain specificities, need for metabolism and correlation with rodent carcinogenicity. Mutagenesis 24:359–366
Kirsch-Volders M (1997) Towards a validation of the micronucleus test. Mutat Res 392:1–4
Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, Ishidate M, Kirchner S, Lorge E, Morita T, Norppa H, Surrales J, Vanhauwaert A, Wakata A (2003) Report from the in vitro micronucleus assay working group. Mutat Res 540:153–163
Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG et al. (2004) Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol 42:65–83
Levin D-E, Hollstein M, Christman M-F, Schwiers E-A, Ames B-N (1982) A new Salmonella tester strain with AT base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci USA 79:7445–7449, Genetics
Maron D-M, Ames B-N (1983) Revised methods for the Salmonella mutagenicity test. Mutat Res 113:173–215
McCann J, Springarn N-E, Kobory J, Ames B-N (1975) Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci USA 72:979–983
McGovern T, Jacobson-Kram D (2006) Regulation of genotoxic and carcinogenic impurities in drug substances and products. Trends Anal Chem 100(1):681–685
Mirsalis J, Butterworth BE (1980) Detection of unscheduled DNA synthesis in hepatocytes isolated from rats treated with genotoxic agents: an in vivo/in vitro assay for potential carcinogens and mutagens. Carcinogenesis 1:621–625
Mirsalis JC, Tyson CK, Butterworth BE (1982) Detection of genotoxic carcinogens in the in vivo-in vitro hepatocyte DNA repair assay. Environ Mutagen 4:553–562
Mirsalis JC, Tyson CK, Steinmetz KL et al. (1989) Measurement of unscheduled DNA synthesis and S-phase synthesis in rodent hepatocytes following In Vivo treatment: testing of 24 compounds. Environ Mol Mutagen 14:155–164
Mitchell AD, Casciano DA, Meltz ML, Robinson DE, San RHC, Williams GM, Von Halle ES (1983) Unscheduled DNA synthesis tests. A report of the U.S. Environmental Protection Agency. Gene-Tox Program. Mutat Res 123:363–410
Mitelman F (ed) (1995) An international system for human cytogenetic nomenclature recommendations of the international standing committee on human cytogenetic nomenclature. S. Karger, Basel
Moore MM, Clive D, Hozier JC, Barry E, Howard BE, Batson AG, Turner NT, Sawyer J (1985) Analysis of trifluorothymidine-resistant (TFTr) mutants of L5178Y/TK+/- mouse lymphoma cells. Mutat Res 151:161–174
Moore M-M, Doerr C-L (1990) Comparison of chromosome aberration frequency and small-colony TK-deficient mutant frequency in L5178Y/TK(+/–)-3.7.2 C mouse lymphoma cells. Mutagenesis 5:609–614
Moore M-M, Honma M, Clements J, Bolcsfoldi G, Cifone M, Delongchamp R, Fellows M, Gollapudi B, Jenkinson P, Kirby P, Kirchner S, Muster W, Myhr B, O’Donovan M, Oliver J, Omori T, Ouldelhkim MC, Pant K, Preston R, Riach C, San R, Stankowski LF Jr, Thakur A, Wakuri S, Yoshimura I (2003) Mouse Lymphoma Assay Workgroup mouse lymphoma thymidine kinase gene mutation assay: international workshop on genotoxicity tests workgroup report – Plymouth, UK 2002. Mutat Res 540(2):127–140
Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Lloyd M, Majeska J, Myhr B, O’Donovan M, Omori T, Riach C, San R, Stankowski LF Jr, Thakur AK, Van Goethem F, Wakuri S, Yoshimura I (2006) Mouse lymphoma thymidine kinase gene mutation assay: follow-up meeting of the International Workshop on Genotoxicity Testing–Aberdeen, Scotland, 2003–Assay acceptance criteria, positive controls, and data evaluation. Environ Mol Mutagen 47(1):1–5
Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Clay P, Doppalapudi R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Muster W, Pant K, Kidd DA, Lorge E, Lloyd M, Myhr B, O’Donovan M, Riach C, Stankowski LF Jr, Thakur AK, Van Goethem F, Mouse Lymphoma Assay Workgroup, IWGT (2007) Mouse lymphoma thymidine kinase gene mutation assay: meeting of the international workshop on genotoxicity testing, San Francisco, 2005, recommendations for 24-h treatment. Mutat Res 627(1):36–40, Epub 2006 Dec 6. PubMed PMID: 17157054
Moore MM, Honma M, Clements J, Awogi T, Douglas GR, van Goethem F, Gollapudi B, Kimura A, Muster W, O’Donovan M, Schoeny R, Wakuri S (2011) Suitable top concentration for tests with mammalian cells: mouse lymphoma assay workgroup. Mutat Res 723(2):84–86, Epub 2011 Apr 14. PubMed PMID: 21514400
Mortelmans K, Zeiger E (2000) The Ames Salmonella/microsome mutagenicity assay. Mutat Res 455:29–60
Müller L, Mauthe RJ, Riley CM, Andino MM, Antonis DD, Beels C, DeGeorge J, De Knaep AG, Ellison D, Fagerland JA, Frank R, Fritschel B, Galloway S, Harpur E, Humfrey CD, Jacks AS, Jagota N, Mackinnon J, Mohan G, Ness DK, O’Donovan MR, Smith MD, Vudathala G, Yotti L (2006) A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regul Toxicol Pharmacol 44(3):198–211, Epub 2006 Jan 18. PubMed PMID: 16412543
Natarajan AT, Boei JJ (2004) Formation of chromosome aberrations: insights from FISH. Mutat Res 544:299–304
Nüsse M, Beisker W, Kramer J, Miller B, Schreiber G, Viaggi S, Weller W-M, Wessels J-M (1994) Measurement of micronuclei by flow cytometry. Methods Cell Biol 42:149–158
OECD Guideline 475, Mammalian Bone Marrow Chromosome Aberration Test, adopted 21 July 1997
OECD Guideline 476, In Vitro Mammalian Cell Mutation Test, adopted 21 July 1997
OECD Guideline 486, Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells in vivo, adopted 21 July 1997
OECD Guideline For Testing Of Chemicals, 471 Bacterial Reverse Mutation Test, adopted 21 July 1997
OECD Guideline For Testing Of Chemicals, 473 “Genetic toxicology: in vitro mammalian chromosome aberration test”, adopted 21 July 1997
OECD Guideline for Testing Of Chemicals, Draft Proposal For A New Guideline 487 In Vitro Micronucleus Test, adopted 22 July 2010
Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M (1987) Mammalian in vivo cytogenetic assays: analysis of chromosome aberrations in bone marrow cells. Mutat Res 189(2):157–165
Richold M, Chandley A, Ashby J, Gatehouse DG, Bootman J, Henderson L (1990) In vivo cytogenetic assays. In: Kirkland DJ (ed) Basic mutagenicity tests, UKEMS recommended procedures. UKEMS subcommitee on guidelines for mutagenicity testing. Report. Part I revised. Cambridge University Press, Cambridge/New York/Port Chester/Melbourne/Sydney, pp 115–141
Rogers AW (1973) Technics of autoradiography. Elsevier Scientific, Amsterdam, p 218
Romagna F, Staniforth C-D (1989) The automated bone marrow micronucleus test. Mutat Res 213:91–104
Rothfuss A, O’Donovan M, De Boeck M, Brault D, Czich A, Custer L, Hamada S, Plappert-Helbig U, Hayashi M, Howe J, Kraynak AR, van der Leede BJ, Nakajima M, Priestley C, Thybaud V, Saigo K, Sawant S, Shi J, Storer R, Struwe M, Vock E, Galloway S (2010) Collaborative study on fifteen compounds in the rat-liver comet assay integrated into 2- and 4-week repeat-dose studies. Mutat Res 702(1):40–69, Epub 2010 Jul 23. PubMed PMID: 20656055
Rothfuss A, Honma M, Czich A, Aardema MJ, Burlinson B, Galloway S, Hamada S, Kirkland D, Heflich RH, Howe J, Nakajima M, O’Donovan M, Plappert-Helbig U, Priestley C, Recio L, Schuler M, Uno Y, Martus HJ (2011) Improvement of in vivo genotoxicity assessment: combination of acute tests and integration into standard toxicity testing. Mutat Res 723(2):108–120, Epub 2010 Dec 21
Russo A (2000) In vivo cytogenetics: mammalian germ cells. Mutat Res 455:167–189
Salamone M, Heddle J, Stuart E, Katz M (1980) Towards an improved micronucleus test. Mutat Res 74:347–356
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Seglen PO (1975) Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Sofuni T, Wilcox P, Shimada H, Clements J, Honma M, Clive D, Green M, Thybaud V, San R-H, Elliott B-M, Muller L (1997) Mouse lymphoma workshop: Victoria, British Columbia, Canada, March 27, 1996. Protocol issues regarding the use of the microwell method of the mouse lymphoma assay. Environ Mol Mutagen 29:434–438
Tice RR, Hayashi M, MacGregor JT, Anderson D, Blakey DH, Holden HE, Kirsch-Volders M, Oleson FB Jr, Pacchierotti F, Preston RJ, Romagna F, Shimada H, Sutou S, Vannier B (1994) Report from the working group on the in vivo mammalian bone marrow chromosome aberration test. Mutat Res 312:305–312
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
U.S. EPA: OPPTS 870.5100 (1998) Health effects test guidelines bacterial reverse mutation test
U.S. EPA: OPPTS 870.5375 (1998) Health effects test guidelines in vitro mammalian chromosome aberration test
UKCOM (2000) Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment. Guidance on a strategy for testing of chemicals for mutagenicity. Department of Health, UK. Environmental and Molecular Mutagenesis. doi:10.1002/em192. Available at http://www.advisorybodies.doh.gov.uk/com/guidance. pdf. Accessed 03 Sep 2011
WHO. 2007. WHO/IPCS (World Health Organisation/International Programme on Chemical Safety). Draft guidance on mutagenicity testing for chemical risk assessment. Available at http://www.who.int/ipcs/methods/harmonization/areas/mutagenicity_testing. Accessed 03 Sep–Oct 2011
Williams GM (1977) Detection of chemical carcinogens by unscheduled DNA synthesis in rat liver primary cell culture. Cancer Res 37:1845–1851
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Stammberger, I., Czich, A., Braun, K. (2013). Genotoxicity. In: Vogel, H.G., Maas, J., Hock, F.J., Mayer, D. (eds) Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-25240-2_57
Download citation
DOI: https://doi.org/10.1007/978-3-642-25240-2_57
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-25239-6
Online ISBN: 978-3-642-25240-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences