Abstract
The human skin itself hinders the widespread use of transdermal drug delivery (TDD) for administration of medications. Despite the different strategies devised and employed to reversibly overcome the skin barrier, this noninvasive delivery mode is restricted to potent, low molar mass therapeutic agents. As most drugs would not be able to penetrate the skin in a sufficient quantity to reach the desired therapeutic level, chemical penetration enhancers (CPE) are commonly used to breach the skin barrier and increase drug permeation. Over the years, extensive screening and testing have identified different classes of chemicals as potential adjuvants. Among these, terpenes, which are constituents of plant essential oils, have been widely investigated as skin penetration enhancers for both hydrophilic and hydrophobic drugs. Their enhancing effects on human skin and interactions with skin lipids have been extensively studied. The mechanisms of action of terpenes on excised human skin as determined by several analytical techniques were found to be the extraction and phase separation of stratum corneum intercellular lipids. The enhancing efficacies of terpenes with various physicochemical properties for lipophilic and hydrophilic drugs could be compared and ranked, while their enhancing effects on the skin were found to be reversible and the in vitro permeability of skin recovered once they were removed from the excised skin. Terpenes have been incorporated as adjuvants in the form of penetration enhancers or sorption promoters for improved drug delivery from various dosage forms including solutions, gels, and transdermal therapeutic systems.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
The human skin barrier hinders the widespread use of transdermal drug delivery (TDD) for drug administration. Despite the range of strategies devised and employed to reversibly overcome the skin barrier, TDD is restricted to potent and low molar mass therapeutic agents. Nonetheless, this noninvasive delivery mode offers advantages such as sustained and controlled drug release, reduced side effects, improved bioavailability, better patient acceptance and compliance, and easy termination of drug therapy. The use of chemical penetration enhancers (CPE) is the conventional approach to modify the skin structure and lower its resistance, so as to allow sufficient drug to reach desired therapeutic levels for systemic effects. Over the years, extensive screening and testing have identified different classes of chemicals as potential adjuvants. Among these, terpenes, the natural volatile oils extracted from plant sources, have been widely used as CPE by permeating into the human skin and reversibly decreasing its barrier resistance.
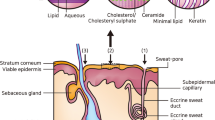
2 Structure of the Human Skin
The skin, with a surface area of ca. 1.72 m2, is the most accessible organ of the human body and continues to be the preferred site for the application of topical dosage forms [1]. It consists of three main histological layers: the epidermis, dermis, and subcutis as show in Fig. 124.1 [2]. The stratum corneum (SC) of the epidermis, the outermost part of the skin [3, 4] is a remarkable transport barrier which effectively retards the diffusion of exogenous and endogenous moieties into and out of the host and may be regarded as the rate-limiting layer [5]. Superficial SC is formed in the final stage of differentiation from several layers of dead cells embedded in a lipid matrix, and its morphology resembles a “brick and mortar” array [6].
Cross-sectional view of the uppermost layers of human skin (stratum corneum, viable epidermis, and dermis) (Adapted from Venkatraman and Gale [2])
The protective quality of the skin is firmly attributed to the unique composition and structural configuration of the intercellular SC lipids. Lipid bilayers of ceramides, fatty acids, cholesterol, and cholesterol esters are arranged into a continuous semicrystalline and crystalline interconnecting domain [7–9]. As sweat glands and hair follicles occupy a fractional skin area of 0.1% [3], absorption across the SC layer can be geometrically subdivided into transcellular and intercellular routes. Tortuous intercellular pathway is supposedly the principal route [10–12].
3 Human Skin Permeation and Transdermal Drug Delivery
The continuous SC has been the usual target in TDD. In recent years, researchers have employed physical and chemical methods to temporarily disrupt its elegant molecular structure. Physical methods such as low-frequency ultrasound (sonophoresis) and electrical current (iontophoresis and electroporation) may facilitate absorption of drug molecules by physically altering the skin morphology [13]. Ultrasound causes cavitation, and the shock waves from the collapsing vacuum bubbles increase the free volume space within the lipid lamellae [14, 15]. Iontophoresis electrically drives or repels ionized drug molecules and peptides through current-induced defects [16, 17]. Skin electroporation creates transient aqueous pores in the lipid bilayers.
The conventional strategy of disrupting the skin barrier and improving the skin permeation of poorly absorbed drugs is to incorporate CPE into drug preparations [10, 18]. Lipid-protein-partitioning (LPP) model [19] theorizes that CPE intensifies TDD by increasing drug solubility in the donor formulation, increasing drug partitioning between the formulation and the skin, or disrupting the intercellular SC lipids [13, 20]. At clinically acceptable concentrations, most CPE interact with the lipoidal domains. These interactions include lipid fluidization, polarity alteration, phase separation, and lipid extraction as illustrated in Fig. 124.2 [10, 20]. Compounds such as sulfoxides, pyrrolidones, fatty acids, terpenes, and alcohols are potential enhancers. The ideal CPE would be pharmacologically inert, nontoxic, nonirritating, nonallergenic, and compatible with other components of the transdermal formulation and device as well as upon removal, the skin barrier properties would be restored rapidly and fully. In addition, the onset and duration of the action of a CPE should be predictable and reproducible [21].
Action of chemical penetration enhancers within the intercellular lipid domain (Adapted from Williams and Barry [20])
4 Terpenes
4.1 Source, Nomenclature, Classification, and Uses of Terpenes
Terpenes are constituents of essential oils which are the volatile and fragrant substances present in flowers, fruits, and leaves of plants. They are named “terpenes” after “turpentine” as turpentine oil is a mixture of these compounds [22, 23]. They are usually named after the plants from which they were first isolated. Some terpenes share the same composition by percentage, and some have even the same molecular weights and similar boiling points. However, they smell different, have different optical properties, and behave differently in chemical reactions, they are not identical.
The term “terpene” is used to describe a compound, which is a constituent of an essential oil containing carbon and hydrogen or carbon atoms, hydrogen, and oxygen atoms, and is not aromatic in character [24, 25]. This definition is usually extended to include other compounds called terpenoids, which are not of natural occurrence but are very closely related to the natural terpenes. Most terpenes, which include terpenoids, are invariably hydrocarbons, alcohols, aldehydes, ketones, or oxides, and they may be solids or liquids. Terpene hydrocarbons are usually liquids, while terpenes of higher molecular weights, mostly obtained from the natural gums and resins of plants and trees, are not steam volatile.
Terpenes are defined and classified by the so-called isoprene rule, introduced by Wallach in 1887 [22]. Two isoprene units make one “terpene unit.” They are grouped according to the number of isoprene units, for example, monoterpenes [C10], sesquiterpenes [C15], diterpenes [C20], triterpenes [C30], and tetraterpenes [C40] contain 2, 3, 4, 6, and 8 isoprene units, respectively. A subsidiary classification is based on the number of carbon rings present in the terpene; monoterpenes, for example, may be acyclic, monocyclic, or bicyclic. Each group is further broken down into chemical divisions of hydrocarbons, alcohols, oxides (ethers), and ketones.
Terpenes have been utilized for a number of therapeutic purposes, such as in antispasmodics, carminatives, and perfumery. They have been found to be useful when incorporated into topical and transdermal pharmaceutical formulations as they act as chemical penetration enhancers facilitating the permeation of drugs through the skin barrier, both healthy and diseased in comprehensive reviews [26, 27].
4.2 Terpenes as Chemical Penetration Enhancers
Terpenes, in particular monoterpenes (Fig. 124.3), are generally recognized as safe (GRAS) adjuvants as indicated by high percutaneous enhancement [28–30], reversible effect on skin lipids, and mild cutaneous irritancy at low concentrations (1–5%w/v) [31, 32]. No skin irritation or sensitization was noted in humans for formulations containing hydrophobic limonene [31] and oxygen-containing anethole, linalool, carvacrol, thymol, and menthol [33, 34]. Sesquiterpenes (Fig. 124.4), probably due to a more bulky molecular structure, tend to be less effective and favorable. They are less active [21] and have a longer duration of action, implying poor reversibility, that is, they do wash out of the skin readily [29]. However, in a recent work by Nokhodchi et al. [35], sesquiterpenes (farnesol and nerolidol) were found to be more potent enhancers than monoterpenes (carvone, limonene oxide) for diclofenac sodium.
The activity of terpenes is dependent on terpene concentration, but there is no clear activity-concentration relationship. Kunta et al. [32] examined the influence of terpene concentration on propranolol permeation. The terpenes investigated were carvacrol, linalool, menthol, and limonene. In general, propranolol transport initially increased and subsequently remained constant with terpene concentration. This phenomenon was also documented by Krishnaiah et al. [34], for menthol in the delivery of nicardipine hydrochloride. Probable reasons are limited terpene solubility in the vehicle and terpene saturation in the skin. In a study by Kararli et al. [33], the permeation flux of zidovudine dropped significantly as thymol and carvacrol concentrations increased from 5% to 10%w/w. Similarly, Babu and Pandit [36] noted a decrease in bupranolol permeation as menthol concentration increased from 2% to 5%w/v. No clear explanations were given for the reduced activities with terpene concentration. Many researchers have chosen a terpene concentration of around 5%w/v in their preparations, and at this level, significant improvements in drug permeation were obtained [28, 37–40]. The bottom line is that the enhancer concentration should be high enough to produce an adequate skin penetration but not high enough to elicit an irritating cutaneous response.
Oxygen-containing terpenes were more potent for hydrophilic drugs (Table 124.1) such as 5-fluorouracil [41], propranolol [32, 49], and zidovudine [33, 45]. Hydrocarbon terpenes apparently gave a better enhancement for lipophilic drugs (Table 124.1) such as ketoprofen [1] and indomethacin [20]. These collectively give rise to the general rule of thumb – the use of hydrophilic terpenes for polar drugs and the use of hydrocarbon terpenes for nonpolar drugs. Evidently, there are exceptions to the rule. Font et al. [37] and Ueda et al. [50] showed that hydrophobic limonene improved the deliveries of hydrophilic sumatriptan succinate (Table 124.1) and aminopyrine through excised animal skins the most as compared to oxygen-containing terpenes. The generic trend acts as a guideline for the screening of terpenes, but it does not provide any information on the enhancement level.
The use of terpenes in conjunction with physical enhancers aims to further enhance drug permeation. Ueda and coworkers explored the coupled effect of ultrasound and several chemical enhancers on the percutaneous delivery of aminopyrine [50]. A greater enhancement in aminopyrine flux was obtained when ultrasound was used with monoterpenes (menthol, carvone, and limonene); the coupled effect was much greater than the sum of its individual effects. Rastogi and Singh [51] demonstrated a similar synergistic effect when iontophoresis was combined with limonene pretreatment in the percutaneous absorption of insulin through porcine epidermis.
4.3 Mechanisms of Skin Permeation of Terpenes
Penetration enhancers are applied onto the skin in an amount that is many times more than the skin lipid molecules beneath the area of administration. This means that there are possibly multiple mechanisms taking place at the same time, making it difficult to determine the relative importance and contribution of each mechanism [52, 53].
4.3.1 Techniques to Investigate the Mechanisms of Skin Permeation of Terpenes
Analytical techniques are used in elucidating changes in lipid conformation and composition caused by skin enhancers [54]. Differential scanning calorimetry (DSC), Fourier transform infrared (FTIR), and isothermal calorimetry (ITC) are techniques which have been utilized to examine macroscopic and molecular transitions of the skin.
DSC is sensitive to the thermal effects accompanying phase changes or transitions of the components of the SC layer. DSC thermograms of human SC, extracted lipids, and protein residue showed three endotherms at ca. 65 °C, 75 °C, and 95 °C [55]. The first is due to the melting of lipids. The second is believed to be the melting of lipid-protein complexes and the last represents protein denaturation. A decrease in lipid melting transition of the enhancer-treated skin denotes a plausible phase separation [39, 56, 57]. Phase separation involves the formation of interfacial defects in the SC lamellae, resulting in decreased diffusional path length or resistance. Based on the lowered lipid endotherms, Vaddi and our fellow researchers suggested that alcoholic terpenes in ethanol-water could exist as pools of fluid within the intercellular lipids [39]. The presence of fluid terpenes at physiological temperatures presumably aids molecular transport. Moghimi et al. reported some degree of phase transformation, the replacement of the lamellae by a reversed viscous isotropic phase, in limonene-treated matrices [57]. These macroscopic defects within the lipid structure may lead to enhanced drug permeation.
To better understand the effects of the terpenes on the permeability of a drug through the skin, the interactions of the terpenes with SC intercellular lipids were studied using the isothermal titration (ITC) method by our research group [58]. Cholesterol, palmitic acid, and stearic acid were found to be the most soluble among all the lipids in propylene glycol, and they were further significantly solubilized upon the addition of farnesol. The interactions between farnesol and four representative lipids, that is, cholesterol, behenic acid, ceramide 3, and ceramide 9 were studied using the ITC method. The binding ratios of farnesol to cholesterol, behenic acid, ceramide 3, and ceramide 9 were found to be 1, 2, 2, and 2, respectively. All were endothermic and entropy driven except for that between farnesol and behenic acid, which was exothermic and enthalpy driven. Hydrogen bonding may be the driving force of these interactions. The results suggested that the skin permeation enhancement mechanism of farnesol, the terpene of interest, could be due to lipid extraction and/or triggering lipid phase transition of the SC lamella. This finding was also consistent with the permeation study results, which showed the permeability coefficients of the drug increased as the lipophilicities of monoterpene and sesquiterpenes increased. It is perceivable that terpenes with high lipophilicities will have more interactions with skin lipids.
Infrared spectra bands of the SC can be attributed to the lipid or protein molecular vibrations. The hydrocarbon chains of SC lipids give rise to asymmetric and symmetric CH2 stretching vibrations at 2,920 and 2,850 cm−1, respectively [39]. The shift of these bands to a higher frequency occurs when the methylene groups change from a trans to a more energetic gauche conformation, and this shift is associated with lipid fluidization. The absorbance of the CH2 stretching bands is proportional to the amount of SC lipids, and thus, the extraction of lipids by a skin enhancer results in a decrease in absorbance [5, 39, 53, 59]. SC proteins give rise to CN stretching and NH bending vibrations at 1,550 cm−1, and C = O stretching vibration at 1,650 cm−1. The shift of these bands to either a higher or lower frequency signifies a change in the protein conformation [45, 53]. FTIR findings of several research groups identified partial lipid extraction as a dominant mechanism for alcoholic, oxide, and hydrocarbon terpenes [39, 40, 60, 61] and showed that delipidization was consistent with enhanced drug permeation.
After the permeation behaviors of the model drug and the enhancers have been investigated by studying the interactions between skin lipids and terpene enhancers, the modeling of drug permeation process through excised skin using both Franz cell and flow-through cell was used to compare the effects of terpenes on drug permeation [62]. A mathematical solution based on finite outflow volume was derived from Fick’s law. It can serve as a statistical model to estimate the permeability coefficient from in vitro skin permeation study with the accumulation of penetrants in the receptor compartment of the static diffusion cells. The model is suitable to describe the in vitro drug or chemical permeation studies using Franz cells. However, the flow-though cells have infinite outflow volume, so a different model that could enable the parameter estimation without impairing the integrity and quality of the original permeation data was proposed. The nonlinear regression model derived from Fick’s law is appropriate. Bootstrap sampling is useful for checking the precision of parameter inference based on the large-sample theory. For the in vitro permeation study that we conducted with flow-through cells, the method proved to be robust. The estimates of permeated drug/chemical are important in that, unlike in vivo environment where stratum corneum is replenished by the adjacent live stratum granulosum through keratinization, the excised stratum corneum, though composed of dead corneocytes, will deteriorate after days in contact with solvents, which will cause overhydration of stratum corneum that can destroy the lamella and decomposition that will leave highly permeable passages in the stratum corneum. The predictions are relevant for transdermal drug delivery, the cosmetic industry, and regulatory risk assessment on dermal exposure to toxic substances.
4.3.2 Comparison of the Skin Permeation Effects of Terpenes
The effects of a terpene as a CPE are very much dependent on its physicochemical properties and molecular structure. Increases in the skin transports of propranolol [32, 45], haloperidol [39], and zidovudine [33] were partially attributed to the hydrogen-bonding ability of oxygen-containing monoterpenes. The ether and hydroxyl groups of terpenes interact with the polar head groups of skin ceramides and fatty acids, thereby disrupting the lateral/traverse hydrogen bonding of the lipid bilayers [45, 49]. On the contrary, hydrocarbon terpenes would reside preferentially and cause structural perturbation in the alkyl tail regions of the lipid bilayers [61].
Cornwell and Barry [29] obtained a positive, linear relation between skin conductivity ratio and 5-fluorouracil enhancement ratio for eight terpenes (limonene, terpineol, carvone, pulegone, nerolidol, menthone, ascaridole, and cineole) with oxygen-containing cineole and hydrocarbon limonene positioned at the higher and lower ends of the curve, respectively. Increases in both skin conductivity and permeability after treatment with terpenes suggest the creation of new polar channels through which both ions and polar molecules traverse. These polar routes would be confined in the aqueous regions near the head groups of SC lipids [45], and their formation is possibly linked to the hydrogen-bonding potential of hydrophilic terpenes.
The enhancing effects of 49 terpenes were compared by in vitro drug permeation studies of haloperidol through excised human epidermis by our research group [62]. The derived multiple linear regression models which provided estimations of the permeability coefficients of a drug or chemical through the human skin were found to be useful for the preliminary screening of CPE. The authors reported that for monoterpenes and sesquiterpenes, the permeability coefficients of haloperidol increased as the lipophilicities of terpenes increased. For all terpenes studied, their enhancing abilities decreased as their molecular weights increased. Melting points and boiling points of terpenes were negatively correlated with the permeability coefficients of haloperidol. Sesquiterpenes were better than monoterpenes when only their enhancing effects were considered. The effects of the skin permeation enhancement by terpenes were ranked as follows: ester > aldehyde > oxide > hydrocarbon > alcohol > ketone > phenol > acid (Table 124.2).
4.4 Reversible Effects of Terpenes
In addition to the enhancing effects, the reversibility of the effects of terpenes on the skin is also an important characteristic of an ideal CPE. The permeability of the pretreated epidermis was comparable to that of the control, so the insult to the barrier function of the skin caused by the enhancers was restored as reported by our research group [43]. As an in vitro study was performed, the recovery of the epidermal barrier function could not be due to the physical barrier being restored via cellular regeneration of the horny layer. The mechanism for this reversible enhancement would be attributed to the insertion of these enhancers within the SC intercellular lipid lamella. The disruptions in the lipid lamella eased the permeation of the lipophilic drug through the tortuous pathway, hence resulting in enhancement of drug permeation. Likewise, once the enhancers were removed, bonds between the lipids could start to re-form, and the depletion of the enhancers could allow the packing of the lipids to revert back to its original alignment. Of the terpenes studied, (R) – (−) carvone had a much faster elution profile out of the epidermis than eucarvone. The results also showed that (R) – (−) carvone, rather than eucarvone, retained more drug, haloperidol, within the epidermis, which suggests that (R) – (−) carvone could be useful as an enhancer for depot HP therapy. Both (R) – (−) carvone and eucarvone were shown to be effective and reversible enhancers for the in vitro permeation of haloperidol through the human epidermis.
4.5 Terpenes in Transdermal Formulations
The transdermal drug formulations could be in the form of a suspension, solution, gel, ointment, or multilayer transdermal patch. A controlled drug release from the patch is usually achieved by changing the properties of either the rate-controlling membrane or the drug matrix.
Lim et al. [42] demonstrated a controlled drug release by varying the gelator content of an organogel vehicle which presented different degrees of resistance to drug diffusion. He also went on to show the effect of CPE, such as terpenes, on the physical, rheological, and chemical characteristics of a model pharmaceutical formulation for topical and transdermal drug delivery [63] by examining the effects of three terpenes (linalool, cineole, limonene) on the rheology and chemical stability of an organogel composed of dibutyllauroylglutamide (GP1) and propylene glycol (PG). At a given GP1 concentration, oxygen-containing linalool and cineole decreased gel moduli (elastic and viscous) and brittleness, and the reverse was obtained for hydrocarbon limonene. Probably, linalool and cineole interfered with hydrogen bonding between GP1 molecules, while limonene could have initiated a phase separation-mediated gelation, changing the gel morphology. Microcalorimetry detected minute heat endotherms for gels (with and without terpenes) subjected to accelerated heat testing. These heat changes could arise from a small degree of structural disruption of the gel network. Heat endotherms normalized with respect to GP1 content were used to assess gel chemical stability. Although the terpenes altered rheology, they did not significantly affect the chemical stability of the gels. This is the first literature report on the effect of penetration enhancers, such as terpenes, on the physical, rheological, and chemical characteristics of a model pharmaceutical formulation for topical and transdermal drug delivery.
The enhancing effect of a selected enhancer, farnesol, incorporated into gels containing small molecule gelling agents (SMGA), was reported by our research group [38]. The SMGA gels developed for application on the skin retained their characteristic aesthetic and rheological properties with the incorporation of the drug and enhancer. These in vitro human skin permeation studies showed that the gels possessed desirable properties for both topical and transdermal delivery. The translucent lipophilic gels with ISA were stable and the permeation of the drug reached the pseudo steady state in less time compared to the PG-based gel. The latter, opaque white in color, delivered the drug at a faster rate with the addition of the enhancer. The gelator, GP1, did not influence the drug permeation rate but increased its permeation lag-time.
Experimental transdermal patches have also been fabricated by flanking a solution or gel containing the test drug and other excipients between an impermeable backing laminate and a rate-controlling membrane. A pressure-sensitive adhesive coated on the membrane ensures an intimate patch-skin contact. The patch is kept in a sealed aluminum pouch to minimize solvent loss [31, 62, 64].
Krishnaiah et al. [31] designed a transdermal therapeutic system (TTS) for nimodipine. The drug reservoir was an ethanolic gel with 4%w/w limonene as the penetration enhancer. A copolymer film (rate-controlling membrane) was coated with a pressure-sensitive adhesive. In vivo study performed on human volunteers showed that the TTS provided a steady-state nimodipine plasma level with minimal fluctuations for around 20 h and a much-improved bioavailability relative to a tablet dosage form of nimodipine. The intersubject variation in the drug plasma level was observed to be significantly lower for the transdermal route than for the oral route. Hepatic first-pass metabolism, differences in gastric emptying, and gastrointestinal absorption among the subjects are the key causes of a low bioavailability and a less reproducible pharmacokinetics, commonly associated with oral administration. Also, the absence of any local irritation at the application sites of the volunteers demonstrated that the components of the TTS patch (drug, terpene, vehicle, and adhesive) were well tolerated by the skin. Similar findings and conclusions were obtained for the TTS for nicardipine hydrochloride and nicorandil with menthol and nerolidol as the penetration enhancer, respectively [34, 65, 66].
5 Conclusions
Terpenes, as constituents present in plant essential oils, have been investigated as chemical enhancers in transdermal drug delivery. In particular, monoterpenes were generally more efficacious probably due to their small molecular sizes. The main mechanisms of action of terpenes on the skin as determined by several analytical techniques were lipid extraction and phase separation. Terpenes such as limonene and menthol in experimental patch formulations were demonstrated to be safe on the skin and effective in improving drug delivery across the skin.
Abbreviations
- CPE:
-
Chemical penetration enhancers
- DSC:
-
Differential scanning calorimetry
- FTIR:
-
Fourier transform infrared
- GP1:
-
Dibutyllauroylglutamide
- ITC:
-
Isothermal titration
- LPP:
-
Lipid-protein-partitioning
- PG:
-
Propylene glycol
- SC:
-
Stratum corneum
- SMGA:
-
Small molecule gelling agents
- TDD:
-
Transdermal drug delivery
- TTS:
-
Transdermal therapeutic system
References
Gupta P, Garg S (2002) Recent advances in semisolid dosage forms for dermatological application. Pharm Technol 26:144–161
Venkatraman S, Gale R (1998) Skin adhesives and skin adhesion 1. Transdermal drug delivery systems. Biomaterials 19:1119–1136
Downing TD (1992) Lipid and protein structures in the permeability barrier of mammalian epidermis. J Lipid Res 33:301–313
Moss GP, Dearden JC, Patel H, Cronin MTD (2002) Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicol In Vitro 16:299–317
Hadgraft J, Lane ME (2005) Skin permeation: the years of enlightenment. Int J Pharm 305:2–12
Suhonen TM, Bouwstra JA, Urtti A (1999) Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J Control Rel 59:149–161
Bouwstra JA, Dubblelaar FER, Gooris GS, Ponec M (2000) The lipid organization in the skin barrier. Acta Derm Venereol Supp 208:23–30
Jager MWD, Gooris GS, Dolbnya IP, Ponec M, Bouwstra JA (2004) Modelling the stratum corneum lipid organisation with synthetic lipid mixtures: the importance of synthetic ceramide composition. Biochim Biophys Acta 1684:132–140
Wertz PW (2000) Lipids and barrier function of the skin. Acta Derm Venereol Supp 208:7–11
Barry BW (2004) Breaching the skin’s barrier to drugs. Nat Biotechnol 22:165–167
Nemanic MK, Elias PM (1980) In situ precipitation: a novel cytochemical technique for visualization of permeability pathways in mammalian stratum corneum. J Histochem Cytochem 28:573–578
Yamashita F, Hashida M (2003) Mechanistic and empirical modeling of skin permeation of drugs. Adv Drug Deliv Rev 55:1185–1199
Walker RB, Smith EW (1996) The role of percutaneous penetration enhancers. Adv Drug Deliv Rev 18:295–301
Lavon I, Kost J (2004) Ultrasound and transdermal drug delivery. Drug Discov Today 9:670–676
Merino G, Kalia YN, Guy RH (2003) Ultrasound-enhanced transdermal transport. J Pharm Sci 92:1125–1137
Green PG (1996) Iontophoretic delivery of peptide drugs. J Control Rel 41:33–48
Naik A, Kalia YN, Guy RH (2000) Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today 3:318–326
Hashida M, Yamashita F (1995) Terpenes as penetration enhancers. In: Smith EW, Maibach HI (eds) Percutaneous penetration enhancers. CRC Press, Boca Raton, pp 309–321
Barry BW (1991) Lipid-protein-partitioning theory of skin penetration enhancement. J Control Rel 15:237–248
Williams AC, Barry BW (2004) Penetration enhancers. Adv Drug Deliv Rev 56:603–618
Godwin DA, Michniak BB (1999) Influence of drug lipophilicity on terpenes as transdermal penetration enhancers. Drug Dev Ind Pharm 25:905–915
Wallach O (1910) Alicylic compounds. Nobel lecture, December 12, 1910. http://nobelprize.org/chemistry/laureates/1910/wallach-lecture.pdf. Accessed 1 July 2005
Montelius O (1910) The Nobel prize in chemistry 1910. http://nobelprize.org/chemistry/laureates/1910/press.html. Accessed 1 July 2005
Newman AA (ed) (1972) Chemistry of terpenes and terpenoids. Academic, London/New York
Pinder AR (1961) The chemistry of the terpenes. Chapman & Hall, London, p 223
Lim PFC, Liu XY, Chan SY (2009) A review on terpenes as skin penetration enhancers in transdermal drug delivery. J Essent Oil Res 21:423–428
Aqil M, Ahad A, Sultana Y, Ali A (2007) Status of terpenes as skin penetration enhancers. Drug Discov Today 12:1061–1067
Almirall M, Montana MJ, Escribano E, Obach R, Berrozpe JD (1996) Effect of d-limonene, α-pinene and cineole on in vitro transdermal human skin penetration of chlorpromazine and haloperidol. Drug Res 46:676–680
Cornwell PA, Barry BW (1994) Sesquiterpene components of volatile oils as skin penetration enhancers for the hydrophilic permeant 5-fluorouracil. J Pharm Pharmacol 46:261–269
Yamane MA, Williams AC, Barry BW (1995) Effects of terpenes and oleic acid as skin penetration enhancers towards 5-fluorouracil as assessed with time; permeation, partitioning and differential scanning calorimetry. Int J Pharm 116:237–251
Krishnaiah YSR, Bhaskar P, Satyanarayana V (2004) Formulation and evaluation of limonene-based membrane-moderated transdermal therapeutic system of nimodipine. Drug Deliv 11:1–9
Kunta JR, Goskonda VR, Brotherton HO, Khan MA, Reddy IK (1997) Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J Pharm Sci 86:1369–1373
Kararli TT, Kirchhoff CF, Penzotti SC (1995) Enhancement of transdermal transport of azidothymidine (AZT) with novel terpene and terpene-like enhancers: in vivo-in vitro correlations. J Control Rel 34:43–51
Krishnaiah YSR, Satyanarayana V, Bhaskar P (2003) Influence of menthol and pressure-sensitive adhesives on the in vivo performance of membrane-moderated transdermal therapeutic system of nicardipine hydrochloride in human volunteers. Eur J Pharm Biopharm 55:329–337
Nokhodchi A, Sharabiani K, Rashidi MR, Ghafourian T (2007) The effect of terpene concentrations on skin penetration of diclofenac sodium. Int J Pharm 335:97–105
Babu RJ, Pandit JK (2005) Effect of penetration enhancers on the transdermal delivery of bupranolol through rat skin. Drug Deliv 12:165–169
Font AF, Fernandez CB, Merino V, Rodilla V, Castellano AL (2005) Effect of chemical enhancers on the in vitro percutaneous absorption of sumatriptan succinate. Eur J Pharm Biopharm 61:50–55
Kang L, Liu XY, Sawant PD, Ho PC, Chan YW, Chan SY (2005) SMGA gels for the skin permeation of haloperidol. J Control Rel 106:88–98
Vaddi HK, Ho PC, Chan YW, Chan SY (2002) Terpenes in ethanol: haloperidol permeation and partition through human skin and stratum corneum changes. J Control Rel 81:121–133
Zhao K, Singh J (2000) Mechanism(s) of in vitro percutaneous absorption enhancement of tamoxifen by enhancers. J Pharm Sci 89:771–780
Cornwell PA, Barry BW (1993) The routes of penetration of ions and 5-fluorouracil across human skin and the mechanisms of action of terpene skin penetration enhancers. Int J Pharm 94:189–194
Lim PFC, Liu XY, Kang L, Ho PCL, Chan YW, Chan SY (2006) Limonene GP1/PG organogel as a vehicle in the transdermal delivery of haloperidol. Int J Pharm 311:157–164
Kang L, Poh AL, Fan SK, Ho PCL, Chan YW, Chan SY (2007) Reversible effects of permeation enhancers on human skin. Eur J Pharm Biopharm 67:149–155
Vaddi HK, Ho PC, Chan SY (2002) Terpenes in propylene glycol as skin-penetration enhancers: permeation and partition of haloperidol, Fourier transform infrared spectroscopy and differential scanning calorimetry. J Pharm Sci 91:1639–1651
Narishetty STK, Panchagnula R (2004) Transdermal delivery of zidovudine: effect of terpenes and their mechanism of action. J Control Rel 95:367–379
Howard PH, Meylan WM (eds) (1997) Handbook of physical properties of organic chemicals. CRC Press, Boca Raton
DrugBank. http://redpoll.pharmacy.ualberta.ca/drugbank/index.html. Accessed 13 Dec 2007
Syracuse Research Corporation. http://www.syrres.com/esc/est_kowdemo.htm. Accessed 13 Dec 2007
Amnuaikit C, Ikeuchi I, Ogawara K, Higaki K, Kimura T (2005) Skin permeation of propranolol from polymeric film containing terpene enhancers for transdermal use. Int J Pharm 289:167–178
Ueda H, Isshiki R, Ogihara M, Sugibayashi K, Morimoto Y (1996) Combined effect of ultrasound and chemical enhancers on the skin permeation of aminopyrine. Int J Pharm 143:37–45
Rastogi SK, Singh J (2005) Effect of chemical penetration enhancers and iontophoresis on the in vitro percutaneous absorption enhancement of insulin through porcine epidermis. Pharm Dev Tech 1:97–104
Engstrom S, Ekelund K, Engblom J, Eriksson L, Sparr E, Wennerstrom H (2000) The skin barrier from a lipid perspective. Acta Derm Venereol Supp 208:31–35
Wang MY, Yang YY, Heng PWS (2004) Role of solvent in interactions between fatty acids-based formulations and lipids in porcine stratum corneum. J Control Rel 94:207–216
Knutson K, Potts RO, Guzek DB, Golden GM, McKie JE, Lambert WJ, Higuchi WI (1985) Macro- and molecular physical-chemical considerations in understanding drug transport in the stratum corneum. J Control Rel 2:67–87
Jain AK, Thomas NS, Panchagnula R (2002) Transdermal drug delivery of imipramine hydrochloride. I. Effect of terpenes. J Control Rel 79:93–101
Ongpipattanakul B, Burnette RR, Potts RO, Francoeur ML (1991) Evidence that oleic acid exists in a separate phase with stratum corneum lipids. Pharm Res 8:350–354
Moghimi HR, Williams AC, Barry BW (1997) A lamellar matrix model for stratum corneum intercellular lipids. V. Effects of terpene penetration enhancers on the structure and thermal behaviour of the matrix. Int J Pharm 146:41–54
Kang L, Ho PC, Chan SY (2006) Interactions between a skin penetration enhancer and the main components of human stratum corneum lipids isothermal titration calorimetry study. J Ther Anal Calor 83:27–30
Panchagnula R, Desu RH, Jain A, Khandavilli S (2005) Feasibility studies of dermal delivery of paclitaxel with binary combinations of ethanol and isopropyl myristate: role of solubility, partitioning and lipid bilayer perturbation. Farmaco 60:894–899
Krishnaiah YSR, Satyanarayana V, Bhaskar P (2002) Effect of limonene on the in vitro permeation of nicardipine hydrochloride across the excised rat abdominal skin. Pharmazie 57:842–847
Vaddi HK, Ho PC, Chan YW, Chan SY (2003) Oxide terpenes as human skin penetration enhancers of haloperidol from ethanol and propylene glycol and their modes of action on stratum corneum. Biol Pharm Bull 26:220–228
Kang L, Yap CW, Lim PFC, Chen YZ, Ho PC, Chan YW, Wong GP, Chan SY (2007) Formulation development of transdermal dosage forms: quantitative structure-activity relationship model for predicting activities of terpenes that enhance drug penetration through human skin. J Control Rel 120:211–219
Lim PFC, Liu XY, Kang L, Ho PCL, Chan SY (2008) Physicochemical effects of terpenes on organogel for transdermal drug delivery. Int J Pharm 358:102–107
Valiveti S, Hammell DC, Earles DC, Stinchcomb AL (2004) In vitro/in vivo correlation studies for transdermal Δ8-THC development. J Pharm Sci 93:1154–1164
Panchagnula R, Bokalial R, Sharma P, Khandavilli S (2005) Transdermal delivery of naloxone: skin permeation, pharmacokinetic, irritancy and stability studies. Int J Pharm 293:213–223
Krishnaiah YSR, Al-Saidan SM, Chandrasekhar DV, Satyanarayana V (2005) Bioavailability of nerodilol-based transdermal therapeutic system of nicorandil in human volunteers. J Control Rel 106:111–122
Wang MY, Yang YY, Heng PWS (2005) Skin permeation of physostigmine from fatty acids-based formulations: evaluating the choice of solvent. Int J Pharm 290:25–36
Acknowledgments
Research scholarships from the National University of Singapore to support Vaddi Haranath Kumar, Lifeng Kang, Fung Chye Perry Lim, and Han Hui Cheong for their PhD programs.
Academic Research Fund from the Ministry of Education, Singapore for Sui Yung Chan as the principal investigator of research projects R-148-000-044-112 and R-148-000-044-112.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Kang, L., Kumar, V.H., Lim, P.F.C., Cheong, H.H., Chan, S.Y. (2013). Terpenes and Improvement of Transdermal Drug Delivery. In: Ramawat, K., Mérillon, JM. (eds) Natural Products. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22144-6_160
Download citation
DOI: https://doi.org/10.1007/978-3-642-22144-6_160
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-22143-9
Online ISBN: 978-3-642-22144-6
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics