Abstract
The mesenteric small bowel is the portion of the gastrointestinal tract which begins at the duodenojejunal flexure and ends at the ileocecal valve. It is 3 m long and it is divided into jejunum and ileum. It is attached to the posterior abdominal wall by fan-shaped mesentery which contains the blood vessels, lymphatics and nerves.
There are multiple diseases which can present during the neonatal period, such as atresias, meconium abnormalities, cysts, and malrotation. In this chapter we summarise these diseases, with their clinical presentation, imaging modality of choice, and radiological findings.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Normal Anatomy of the Mesenteric Small Bowel
The small intestine is that portion of the GI tract between the pyloric sphincter of the stomach and the ileocecal valve that opens into the large bowel. It is positioned in the central and lower portions of the abdominal cavity and is supported, except for the first portion, by the mesentery.
The purpose of this chapter is to study the mesenteric portion of the small bowel, which extends from the ligament of Treitz to the ileocecal valve and consists of the jejunum and ileum, which are completely intraperitoneal and are attached posteriorly to a mesentery (Ryan et al. 2011).
The small bowel develops embryologically from the yolk sac. The midgut elongates rapidly so that, at the beginning of the sixth week, it forms a U-shaped ventral loop that projects into the extraembryonic coelom. The midgut proximal to the apex of the loop is small bowel, and the distal to the apex is colon. While the bowel is in the extraembryonic coelom, it undergoes a 90° counterclockwise rotation around the superior mesenteric artery.
During this rotation, the jejunum and ileum grow more rapidly than the colon, producing small bowel loops. The bowel returns to the abdominal cavity by the tenth week. The small bowel enters first, passes posterior to the superior mesenteric artery, and finishes up occupying the central part of the abdomen.
The mesenteries of the ascending and descending colon fuse with the posterior abdominal wall, as does that of the duodenum. As a result, the small bowel mesentery becomes fan shaped with a broad-based attachment (Sadler 2000).
The mesentery permits movement of the small intestine but leaves little chance for it to become twisted or kinked. Enclosed within the double-folded mesentery are blood vessels, nerves, and lymphatic vessels that supply and drain the intestinal wall.
The attached border, the root of the mesentery, extends obliquely from the point of termination of the duodenum, at the lower border of the pancreas on the left side of the second lumbar vertebra, to the cecum in the right iliac fossa near the right sacroiliac articulation (Meyers 2005).
The small intestine is approximately 3 m long and 2.4 cm wide. The small intestine is the body’s major digestive organ and the primary site of nutrient absorption.
The small intestine is innervated by the superior mesenteric plexus. The branches of the plexus contain sensory fibers, postganglionic sympathetic fibers, and preganglionic parasympathetic fibers.
The arterial blood supply to the small intestine is through the superior mesenteric artery which arises from the aorta at L1 vertebral level. This artery passes anterior to the second part of the duodenum to enter the mesentery of the small intestine. About 4–5 branches to the jejunum and up to 12 ileal arteries arise from the left of the main trunk of the superior mesenteric artery. These branches link with each other in a series of arcades, which are usually single in the jejunum and progressively smaller and more multiple in the ileum. The branches that enter the intestinal wall, the vasa recta, are terminal arteries (Fig. 34.1).
The venous drainage is through the superior mesenteric vein. This vein unites with the splenic vein to form the hepatic portal vein, which carries nutrient-rich blood to the liver (Fig. 34.2).
Lymphatic drainage of the small intestine is via vessels that travel with the arteries. Drainage is first to juxtaintestinal mesenteric nodes at the level of the primary arterial arcades. From here, lymph drainage is to preaortic nodes called the superior mesenteric nodes (Netter 1996).
Regions of the Small Intestine
On the basis of function and histological structure, the small intestine is divided into three regions.
-
1.
The duodenum, the first 25 cm retroperitoneal portion of the intestine extends from the pyloric sphincter of the stomach to the duodenojejunal flexure. (see Chap. Stomach and Duodenum)
-
2.
The jejunum is an intraperitoneal organ, which extends from the duodenum to the ileum, is approximately 1 m long. It has a slightly larger lumen than the ileum. The jejunum has prominent crescentic folds of mucosa and submucosa, the valvulae conniventes (also called the plicae circulares, folds of Kerckring, or simply, small bowel mucosal folds). These folds are thicker in the jejunum than the ileum and are more numerous – 10–15 folds per centimeter in jejunum compared to 8–12 per centimeter in the ileum (Fig. 34.3).
The prominence of folds and wall thickness depends on age (more in younger) and degree of bowel distention, and varies in numerous systemic and small bowel disease states.
-
3.
The ileum completes the remaining 2 m of the mesenteric small intestine. The terminal portion of the ileum empties into the medial side of the cecum through the ileocecal valve. Lymph nodules, called mesenteric (Peyer’s) patches, are abundant in the walls of the ileum, especially in the distal ileum in children. Mucosal folds are flatter, thinner, and fewer than in the jejunum (Ryan et al. 2011).
Functional Anatomy
The products of digestion are absorbed across the epithelial lining of the intestinal mucosa. Absorption occurs primarily in the jejunum, although some also occurs in the duodenum and ileum. Absorption occurs at a rapid rate as a result of four specializations that increase the intestinal surface area.
-
1.
The 3 m length of the small intestine.
-
2.
The plicae circulares are large macroscopic folds of mucosa.
-
3.
The intestinal villi are finger like macroscopic folds of the mucosa that project into the lumen of the small intestine.
-
4.
The microvilli are microscopic projections formed by the folding of each epithelial cell membrane. In a light microscope, the microvilli display a somewhat vague brush border on the edges of the columnar epithelium. The terms “brush border” and “microvilli” are often used interchangeably in describing the small intestine.
The intestinal villi are covered with columnar epithelial cells, among which are interspersed the mucus-secreting goblet cells. The lamina propria, which forms the connective tissue core of each intestinal villus, contains numerous lymphocytes, blood capillaries, and a lymphatic vessel called the lacteal.
Absorbed monosaccharides and amino acids enter the blood capillaries; absorbed fatty acids and cholesterols enter the lacteals. Intestinal villi are considered the functional units of the digestive system because absorption through these structures is how digested molecules enter the blood or lymph.
Epithelial cells at the tips of the intestinal villi are continuously shed and are replaced by cells that are pushed up from the bases of the intestinal villi. The epithelium at the base of the intestinal villi invaginates downward at various points to form narrow pouches that open through pores into the intestinal lumen. These structures are called the intestinal crypts (crypts of Lieberkühn) (Netter 1996).
Pearls to Remember: Anatomy
-
The mesenteric small bowel begins at the duodenojejunal flexure and ends at ileocecal valve.
-
It is approximately 3 m long, the luminal diameter is approximately 2.5 cm.
-
It is attached to the posterior abdominal wall by fan-shaped mesentery which contains the blood vessels, lymphatics, and nerves.
-
It is divided into:
-
Jejunum or proximal 60% of the small bowel characterized by the valvulae conniventes or small bowel folds (4–7 per inch).
-
Ileum or distal 40% of small bowel, the mucosal folds are fewer and flatter, there are prominent submucosal lymphoid follicles, especially in terminal ileum.
-
Small Bowel Congenital Abnormalities
Jejunal Atresia
Jejunal atresia is caused by an ischemic injury to the developing intestine. It presents in the newborn period with small bowel obstruction characterized by vomiting and abdominal distention.
A rare form of inherited jejunal atresia is the “apple peel” small bowel. In this condition, the jejunal atresia is associated with partial absence of the mesentery, absence of the distal superior mesenteric artery, and shortening of the small bowel distal to the atresia. The shortened small intestine spirals around its vascular supply and resembles an apple peel. The condition presents like other jejunal atresia with high intestinal obstruction but is associated with a very poor prognosis.
Imaging Findings
Antenatal Ultrasound
The presence of polyhydramnios and dilated bowel loops can suggest the diagnosis. Relatively few dilated loops but more than the double bubble of duodenal atresia, are seen.
Radiography
The first imaging of choice is a radiograph of the abdomen. The typical appearance in jejunal atresia is that of a few upper abdominal dilated small bowel loops with air-fluid levels on the decubitus view (Fig. 34.4).
Contrast Studies
The patient usually requires no further radiologic investigation, although contrast enema may be performed to exclude second and third areas of atresia lower in the bowel. In isolated proximal atresia of the jejunum, the colon is normal in size because the remaining small bowel distal to the atresia produces sufficient intestinal secretions to produce a normal caliber colon. The finding of jejunal atresia and a microcolon indicates the presence of at least one additional distal atresia (Donoghue and Twomey 2005).
Ileal Atresia
Ileal atresia presents as distal small bowel obstruction with abdominal distension and failure to pass meconium.
There are several types of atresia but these have similar clinical and radiographic presentations:
-
Type 1: Membranous or web-like.
-
Type 2: Atresia with a fibrous cord connecting the atretic bowel ends.
-
Type 3: Total atresia with an associated V-shaped defect in the mesentery.
-
Type 4: Multiple atresias.
Imaging Findings
Antenatal Ultrasound
The presence of polyhydramnios and multiple dilated fluid-filled bowel loops can suggest the diagnosis. The bowel content in ileal atresia is less echogenic than the bowel content in ileal obstruction due to meconium ileus.
Radiography
The first imaging of choice is a radiograph of the abdomen and the typical appearance is of multiple dilated small bowel loops throughout the abdomen with air-fluid levels on the decubitus view (Fig. 34.5a). The presence of fluid levels in the horizontal beam view makes ileal atresia more likely than meconium ileus as a cause of distal obstruction. The bowel loop proximal to the ileal atresia may be very distended and may be visible as a dominant loop on the radiograph (Fig. 34.5b). The decubitus view may show two large fluid levels, one due to fluid and gas in the stomach and the other in the pre-atresia dominant loop.
Contrast Enema
In distal ileal obstruction, a water-soluble contrast enema is performed to distinguish ileal atresia from meconium ileus and colonic obstruction, principally Hirschsprung’s disease. In ileal atresia the colon tends to be small, because of the disuse. The ileocecal valve is usually not competent at birth, so the contrast will move freely to the level of, but not proximal to the atresia (Donoghue and Twomey 2005).
Meconium Ileus
Meconium ileus is a mechanical obstruction of the distal ileum by impaction of abnormal meconium. It is usually the first manifestation of cystic fibrosis and it is the presenting feature of the disease in 5–20% of the cases. It presents with abdominal distention, bilious vomiting, and failure to pass meconium during the first 24 h of life. Volvulus, perforation, and peritonitis are possible complications.
Imaging Findings
Ultrasound
Ultrasound can show multiple distended bowels with echogenic content, in contrast with the more echofree fluid pattern seen in the ileal atresia (Fig. 34.6).
Radiography
Multiple distended bowel loops are seen. The thick meconium mixed with gas can simulate the fecal pattern giving a mottled or “soap-bubble” appearance (Fig. 34.7a). Normally, there is no gas in the rectum. The absence of fluid levels in the horizontal beam view is characteristic of meconium ileus as a cause of distal obstruction (Fig. 34.7b).
Contrast Enema
A contrast enema with water-soluble contrast media is the modality of imaging of choice. The colon is small and the distal ileum is distended with solid appearing meconium (Figs. 34.8a, b).
Meconium ileus in same newborn as Fig. 34.7 with cystic fibrosis. (a) Contrast enema showing microcolon and filling defects in terminal ileum. (b) Contrast enema with further filling of the ileum shows filling defects in multiple small intestinal loops some of which are quite distended. (c) Radiograph 20 h after gastrografin enema shows opacification of a further hugely dilated small intestinal loop in the right lower abdomen with clearing of much of remainder of the meconium and contrast. Reduction was successful with all of inspissated meconium passed and obstruction resolved over next 12 h
Therapeutic Enema
Once a satisfactory diagnosis of meconium ileus is made a therapeutic enema is done, with gastrografin. Half-strength gastrografin is passed via the rectum until it reaches the distended loops of small intestine proximal to the inspissated meconium. The procedure is considered successful if, after the procedure, the baby passes meconium and the abdominal distension resolves. If the first attempt is not successful, a further attempt may be made after 6–12 h or even a third attempt after 24 h provided the baby remains well hydrated and hemodynamically stable (Fig. 34.8c) (Donoghue and Twomey 2005).
Meconium Peritonitis and Meconium Cyst
Meconium peritonitis is a chemical inflammatory process of the peritoneum due to an intrauterine perforation of bowel. Perforation is usually secondary to obstruction which is usually due to ileal atresia or meconium ileus. The acute inflammatory process in the peritoneum can cause fibrosis and calcification. This calcification may be detectable on antenatal or postnatal imaging. If a section of the peritoneal cavity becomes separated and fluid accumulates within this, a cyst can form. Such a cyst is called a meconium cyst though typically it does not contain meconium.
Imaging Findings
Antenatal Ultrasound
Multiple linear or round calcifications can be seen in the fetal abdomen. The distended obstructed bowel loops may also be detectable. A meconium cyst is less commonly seen.
Radiography
The typical appearance is of curvilinear or speckled peritoneal calcifications within the abdomen, along the abdominal wall, above or below the liver, or in the scrotum (Fig. 34.9). The localized form or meconium cyst can be seen on radiographs as curvilinear thin calcification associated with a mass effect (Figs. 34.10a, b).
Ultrasound
In the neonatal ultrasound, multiple linear or round calcifications can be seen within the abdomen. As on the radiograph, these may be along the abdominal wall, above or below the liver or spleen, or in the scrotum. A unilocular or multilocular cyst (meconium cyst) can be seen with contents of variable echogenicity (Berrocal et al. 1999).
Rotational Anomalies of the Midgut. Malrotation and Volvulus
Malrotation is a rotational abnormality due to an abnormality in the embryologic development of the gut. Malrotation includes a wide variety of anomalies of mesenteric fixation.
From the sixth to tenth gestational week, the duodenojejunal loop rotates 180° counterclockwise, ending inferior to the superior mesenteric artery (SMA). During this period, the cecocolic loop rotates 90° also counterclockwise, ending at the left of the SMA.
At the tenth gestational week, the midgut returns to the peritoneal cavity and the duodenojejunal loop rotates additional 90° counterclockwise, giving the definitive appearance, the duodenojejunal flexure to left of spine, the jejunum in the left upper quadrant (LUQ), and the ileum in the right lower quadrant (RLQ). Simultaneously the cecocolic loop rotates 180° and the cecum is placed in the RLQ. Normally the small bowel mesentery is broad base from LUQ to RLQ.
Complete nonrotation is featured by the small bowel on the right side of the abdomen and the large bowel on the left side of the abdomen, as a result of the midgut returning to the peritoneal cavity without rotation. An incomplete rotation is seen when the duodenojejunal loop fails to complete the final 90° rotation.
A reversed rotation is a rare anomaly seen in which the duodenum and colon rotates clockwise about the SMA. This results with the duodenum anterior to the mesenteric vessels and the transverse colon posterior.
Because of the short mesenteric attachment, malrotation carries the risk of volvulus. Forty percent of children with malrotation will have volvulus by 2 weeks of age and 80% by 2 months. The possibility of malrotation and volvulus must be considered in any child with acute abdominal pain and bilious vomiting. It is particularly important to consider this possibility in a neonate with bilious vomiting (Federle et al. 2004).
Malrotation can be associated with abnormal bands of peritoneum, Ladd’s bands that may become obstructive even in the absence of volvulus. In approximately 10% of children with malrotation, an intrinsic duodenal atresia or web is found.
Malrotation occurs as an integral part of diaphragmatic hernia, gastroschisis, and omphalocele.
Imaging Findings
Radiography
The presence of partial duodenal obstruction – dilatation of the stomach and proximal duodenum and a small amount of gas in the distal loops in a neonate with bilious vomiting must suggest the diagnosis of volvulus. When the obstruction is complete, a “double-bubble” sign, resulting from a distended stomach and duodenal bulb can be seen. These findings are seldom seen however and the plain radiograph is seldom diagnostic and can almost never exclude the diagnosis of volvulus. The role, if any, of the radiograph is to suggest that another diagnosis is more likely. A radiograph that shows multiple dilated fluid-filled loops of bowel in a newborn with vomiting, for example, is more suggestive of Hirschsprung’s disease than volvulus (Fig. 34.11a).
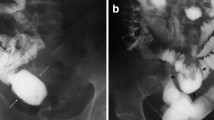
Malrotation and volvulus. (a) Supine radiograph of abdomen in a 2-day-old baby with bilious vomiting is nonspecific without dilated loops. (b) Contrast meal in the same baby shows dilation to mid third part of the duodenum (black arrow), low position of the DJ flexure (white arrow), and corkscrew appearance of volvulus of jejunum (v). (c) Operative photograph of the same baby shows volvulus with 72° turn but bowel was still viable and the baby made a good recovery
Contrast Studies
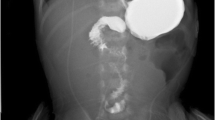
If volvulus is being considered, an upper gastrointestinal contrast examination is essential. In complete nonrotation, it will show the small bowel on the right side of the abdomen and the colon on the left.
The location of the duodenojejunal (DJ) flexure (ligament of Treitz) is critical. A true anterior-posterior view of the abdomen with the first pass of the contrast through the beginning of the small bowel is essential.
An abnormally placed DJ flexure is the most accurate indicator of malrotation. The normal position is at the left of the left pedicle of L2 at the level of the duodenal bulb. In malrotation, the DJ flexure is seen projected over the spine or to the right, and lower than the duodenal bulb.
The normal duodenum is a retroperitoneal structure, which in a lateral view must be behind the level of the stomach, a malrotated duodenum may be anterior to the stomach.
A malrotated jejunum is usually placed on the right abdomen, but this characteristic is not specific because the jejunum is mobile in the newborn, and can be seen on the right without pathology.
Intestinal obstruction at the level of the second or third part of the duodenum, associated with abnormal position of DJ flexure, could be due to obstruction by Ladd’s bands or volvulus. Often the characteristic spiral of a volvulus (corkscrew appearance) is demonstrated in the bowel loop distal to the incomplete obstruction (Figs. 34.11b, c) (Donoghue and Twomey 2005).
Ultrasound
Malrotation may be suspected on ultrasound if the superior mesenteric vein is seen on left of the superior mesenteric artery, the reverse of the normal relationship (Figs. 34.12a, b). This sign is not sensitive, because one-third of the patients with malrotation have a normal relation between the mesenteric vein and the artery. It is therefore used as part of general abdominal ultrasound screening for recurrent abdominal pain rather than in the assessment of acute bilious vomiting that could be due to volvulus (De Bruyn 2005).
Malrotation and position of mesenteric vessels. (a) Ultrasound of upper abdomen showing normal orientation of the superior mesenteric artery left of the superior mesenteric vein. (b) Ultrasound of upper abdomen in another child showing abnormal orientation of these vessels with the vein anterior and left of the artery. A aorta, I inferior vena cava, a superior mesenteric artery, v superior mesenteric vein
The volvulus itself may be visible however on ultrasound. The superior mesenteric vein and its mesentery are seen to rotate clockwise about the artery on color Doppler ultrasound (Fig. 34.13). This is called the “whirlpool” sign and in a symptomatic patient indicates malrotation with volvulus (Pracros et al. 1992).
It has been suggested that the ultrasound or CT definition of the relative positions of the superior mesenteric artery and the transverse part of the duodenum are more important than the position of the DJ flexure. Yousefzadeh has said that the normal third part of the duodenum is posterior to the superior mesenteric artery whereas it is always anterior to the superior mesenteric artery in malrotation. The value of this finding has not been validated in the setting of possible acute volvulus (Yousefzadeh et al. 2010).
Enteric Duplication Cyst
The small intestine is the commonest site of enteric duplication cysts. This is typically a unilocular or multilocular rounded cystic mass with thick walls, an epithelial lining, and a muscular wall. The cysts typically do not communicate with the normal bowel, but communication can be seen. The commonest location is in the distal ileum in its mesenteric border, followed by the esophagus or duodenum. A duplication cyst may have ectopic gastric or pancreatic tissue and this may be associated with bleeding, ulceration, or perforation. Duplication cysts in the ileum may become lead points for an intussusception (Fig. 34.14).
Imaging Findings
Antenatal Ultrasound
The finding of a cyst in the abdomen is a relatively commonly fetal ultrasound finding. In a female fetus, this is much more likely to be an ovarian cyst than a duplication cyst though ultrasound differentiation is not usually possible.
Radiography
Large duplication cysts may be visible as a dense mass on an abdominal radiograph, with or without signs of small bowel obstruction (Donoghue and Twomey 2005).
Ultrasound
Ultrasound shows a well-defined rounded mass, which may have a characteristic gut signature. The “gut signature” refers to a double-lined wall, an inner echogenic layer (mucosa), and an outer hypoechoic layer (smooth muscle). This helps to differentiate a duplication cyst from an ovarian cyst – the main differential for an abdominal cyst in a girl. Sometimes, debris can be seen inside the cyst due to hemorrhage or mucosal secretion (Fig. 34.15) (De Bruyn 2005).
Technetium 99 m Sodium Pertechnetate Scan
This can be useful in the presence of gastric or pancreatic mucosa in uncertain cases.
Gastroschisis
Gastroschisis is a herniation of abdominal contents through a nonmidline, usually right-sided, defect in the anterior abdominal wall (Fig. 34.16). More than half of the newborns are premature. Associated gastrointestinal abnormalities include malrotation, shortened bowel, small bowel atresia, dismotility, reflux, and increased risk of necrotizing enterocolitis (De Bruyn 2005).
Omphalocele
In omphalocele, abdominal viscera herniate through the base of the umbilical cord. The herniated structures are covered by an amniotic and peritoneal sac. Occasionally, the liver can herniate with some intestinal loops (Fig. 34.17). Malposition of the viscera and malrotation of the loops are part of the same entity.
No radiologic studies are usually necessary though associated anomalies such as cardiac anomalies, chromosomal anomalies, or Beck-Wiedemann syndrome, may require radiological evaluation (De Bruyn 2005). The mortality in omphalocele is higher than in gastroschisis, due to associated anomalies.
Omphalomesenteric Duct Remnants
The omphalomesenteric duct in the embryo is a communication between the yolk sac and the primitive midgut. This duct begins to close between the eighth and ninth week of gestation and disappears soon after. If there is an incomplete closure, various anomalies can occur. Meckel’s diverticulum is the most frequent of these anomalies.
Other anomalies include a fibrous band attached to the ileum or the tip of the diverticulum. The other end of the band may be attached to the umbilicus, may lay free in the peritoneum, or be fixed to another structure. Obstruction associated with this fibrous band is not uncommon.
The duct can also persist as a fistula or sinus tract to the umbilicus or can form a cyst when the central part of the duct remains patent and both ends of the duct close.
Meckel’s diverticulum is an outpouching along the antimesenteric border of the distal ileum, due to an incomplete involution of the omphalomesenteric duct. It is the most prevalent congenital malformation of the small bowel. Meckel’s diverticulum is a true diverticulum that contains all layers of intestinal wall and has its own blood supply.
The features of Meckel’s diverticulum are sometimes described as the so-called rule of 2 s: It is seen in the 2% of the population, it is detected between the first 2 ft proximal to the ileocecal valve, it is about 2 in. long, it may include two types of mucosa (pancreatic or gastric), and it is symptomatic usually during the first 2 years of life. These are not always strictly true however.
The diverticulum can contain gastric or pancreatic mucosa and may present with ulceration and bleeding. This is the most frequent presentation in children. The diverticulum may become inflamed and present as a diverticulitis or may be associated with small bowel obstruction. These complications are more frequent in adults. A tumor, most commonly a carcinoid tumor, arising from the diverticulum is rare (Levy and Hobbs 2004).
Imaging Findings
Radiography
A Meckel’s diverticulum may rarely be visible on a radiograph as a rounded gas collection can be seen in the right inferior quarter with single or multiple calcifications (enteroliths). When a fibrous band or Meckel’s diverticulum causes small bowel obstruction, the appearance is commonly that of low small bowel obstruction, with or without air in the colon.
Ultrasound
When a Meckel’s diverticulum is inflamed, a hypoechoic mass can be seen in the right lower quadrant with or without an intraluminal calculus with shadowing – an appearance that is seldom differentiated preoperatively from acute appendicitis.
Sinogram
The direct injection of contrast is often helpful to study the extension of the omphalomesenteric duct (Fig. 34.18).
Scintigraphy
In children with persistent gastrointestinal bleeding of unknown cause, a Meckel’s diverticulum must be excluded. A technetium 99 m pertechnetate scan with serial images every 10 minutes, for one hour, is a simple and accurate technique, looking for ectopic focus of accumulation of the radioisotope separate from the stomach. A focus of radiopharmaceutical uptake in the lower abdomen is consistent with Mecklel’s diverticulum in this clinical setting (Fig. 34.19). Giving Cimetidine prior to the scan reduces the secretion of acid and pertechnetate into the gastric lumen and reduces the false-positive rate. Giving gastrin or pentagastrin prior to the scan enhances uptake of the agent by gastric mucosa. There can be false negatives in the absence of ectopic mucosa.
CT
Meckel’s diverticulum may appear as a fluid or air-fluid blind structure that arises from the antimesenteric side of the distal ileum. A noncomplicated Meckel’s diverticulum is difficult to distinguish from a normal bowel on CT. When Meckel’s diverticulum or an omphalomesenteric band is complicated with obstruction, the findings are similar to those of isolated small bowel obstruction. When Meckel’s diverticulitis is seen, inflammatory changes are seen around a cystic mass with or without a calcification (enteroliths) within the right iliac fossa (Levy and Hobbs 2004).
Megacystis-Microcolon-Malrotation-Intestinal Hypoperistalsis Syndrome (MMMIHS)
This rare syndrome affects the newborn, usually females, and consists of a distended unobstructed bladder-megacystis, dilated small bowel-microcolon, and decreased or absent intestinal peristalsis. The exact etiology is unknown. It may have an autosomal-recessive pattern of inheritance, although most cases are sporadic. It has a fatal outcome in most of cases, with only 6% survival after the first year.
Sequence of Imaging Studies
Antenatal Ultrasound
The earliest sign may be hydronephrosis. Dilatation of the urinary bladder after 20 weeks gestation, in the absence of oligohydramnios should raise the suspicion of the diagnosis.
Radiograph
Abdominal distention is caused by a massively distended urinary bladder more than the dilatation of the bowel (Bloom and Slovis 2008).
Pearls to Remember: Congenital Anomalies of the Small Bowel
-
Jejunal atresia
-
Most common signs/symptoms: Vomiting and abdominal distention in a newborn.
-
Best diagnostic clue: Decubitus radiograph, few upper abdominal dilated small bowel loops with air-fluid levels.
-
-
Ileal atresia
-
Most common signs/symptoms: Abdominal distention and failure to pass meconium in a newborn.
-
Best diagnostic clue: Decubitus radiograph, multiple distended small bowel loops with air-fluid levels. One dominant loop may be more dilated than the others. Contrast enema can be helpful to distinguish from other distal obstructions, including meconium ileus and Hirschsprung’s disease.
-
-
Meconium ileus
-
Most common signs/symptoms: Abdominal distention, bilious vomiting, and failure to pass meconium in a newborn.
-
Best diagnostic clue: Contrast enema with water-soluble contrast media shows a small colon and distended distal ileum containing solid appearing meconium. Therapeutic enema with gastrografin may clear the obstruction.
-
-
Meconium peritonitis/cyst
-
Most common signs/symptoms: Asymptomatic or intestinal obstruction.
-
Best diagnostic clue: Radiograph or ultrasound, curvilinear and speckled peritoneal calcifications.
-
-
Malrotation/volvulus
-
Most common signs/symptoms: Abdominal pain and bile-stained vomiting in a young infant.
-
Best diagnostic clue: Upper GI contrast study, localizing the duodenojejunal flexure on the left of the left pedicle of L2. Volvulus could be seen as a spiral appearance in the bowel distal to the incomplete obstruction. Ultrasound can be also helpful.
-
-
Enteric duplication cyst
-
Most common signs/symptoms: Normally it is an incidental finding. It can be a lead point for an intussusception.
-
Best diagnostic clue: Ultrasound reveals a well-defined round mass with a characteristic gut signature.
-
-
Gastroschisis/omphalocele
-
Most common signs/symptoms: Herniation of abdominal contents.
-
Best diagnostic clue: No imaging is necessary.
-
-
Omphalomesenteric duct anomalies
-
Most common signs/symptoms: Bleeding of unknown cause.
-
Best diagnostic clue: Scintgraphy, an ectopic uptake in the Lower abdomen can be seen.
-
-
MMMIHS
-
It is a rare syndrome with a poor prognosis.
-
References
Berrocal T, et al. Congenital anomalies of the small intestine, colon and rectum. Radiographics. 1999;19:1219–36.
Bloom DA, Slovis TL. Congenital anomalies of the gastrointestinal tract. In: Slovis TL, editor. Caffey’s pediatric diagnostic imaging, vol. II. 11th ed. Philadelphia: Elsevier; 2008. p. 188–235.
De Bruyn R. Pediatric ultrasound. How, why and when. London: Elsevier; 2005.
Donoghue V, Twomey E. The neonatal gastrointestinal tract. In: Carty H et al., editors. Imaging children. New York: Elsevier; 2005. p. 1305–65.
Federle MP, et al. Diagnostic imaging. Abdomen. Manitoba: Amirsys; 2004.
Levy AD, Hobbs CM. From the archives of the AFIP: Meckel diverticulum: radiologic features with pathologic correlation. Radiographics. 2004;24:565–87.
Meyers MA. Dynamic radiology of the abdomen. New York: Springer; 2005.
Netter FH. Atlas of human anatomy. 2nd ed. East Hanover, New Jersey: ICON; 1999.
Pracros JP, Sann L, Genin G, Tran-Minh VA, et al. Ultrasound diagnosis of midgut volvulus: the “whirlpool” sign. Pediatr Radiol. 1992;22:18–20.
Ryan S, McNicholas M, Eustace S. Anatomy for diagnostic imaging. 3rd ed. Philadelphia: Elsevier; 2011.
Sadler TW. Langman’s medical embriology. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
Yousefzadeh DK, Kang L, Tessicini L. Assessment of retromesenteric position of the third portion of the duodenum: an US feasibility study in 33 newborns. Pediatr Radiol. 2010;40:1476–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Caro, P., Ryan, S. (2013). Small Bowel Normal Anatomy and Congenital Anomalies. In: Hamm, B., Ros, P.R. (eds) Abdominal Imaging. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-13327-5_42
Download citation
DOI: https://doi.org/10.1007/978-3-642-13327-5_42
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-13326-8
Online ISBN: 978-3-642-13327-5
eBook Packages: MedicineReference Module Medicine