Abstract
-
Histology: Each acinus is made up of an irregular cluster of pyramidal secretory cells with apices projecting toward the lumen of a small central duct.
-
Endocrine secretions enter the blood by way of a fine reticular network of fenestrated capillary beds surrounding the islets of Langerhans, which consist of groups of endocrine cells of various sizes.
-
Symptoms: Pancreatic exocrine dysfunction and malabsorption are the most commonly reported complications following pancreatic radiation.
-
Patients may more rarely develop an abnormal glucose tolerance test. Patients may or may not also demonstrate accompanying abnormal random or fasting plasma glucose levels.
-
Radiology: Imaging of the pancreas following radiation is often normal but sometimes radiographic changes consistent with chronic pancreatitis can be observed.
-
Multiple Fractions: At 45 Gy, pancreatic exocrine insufficiency with or without malabsorption has been reported.
-
Hypofractionation. The estimate risk of diabetes is 16% in childhood cancer survivors who have received at least 10 Gy to the tail of the pancreas, with a dose response up to 20 to 29 Gy, and a plateau at higher radiation doses. Stereotactic body radiosurgery has recently been utilized to deliver a range of hypofractionated radiation treatments to patients with pancreatic tumors. Up to 25 Gy delivered in a single fraction has been used for treatment, including some patients who received prior chemotherapy and fractionated external beam radiation.
No pancreatic complications were reported in these studies though the number of evaluable patients is small.
-
Recommended dose-volume: As radiation-induced damage to pancreatic function is rarely life threatening and is usually well treated with medical management, dose-volume constraints have not been formally evaluated as with other critical normal tissues.
-
Management: Because pancreatic exocrine deficiency can be managed with replacement therapy, pancreatic injury has not routinely been considered a serious complication compared to other normal tissue damage that may occur from abdominal radiation, such as radiation enteritis. Nonetheless, the risks associated with diabetes and the association with pancreatic tail irradiation in childhood cancer survivors may significantly contribute to public health issues. Hence, the pancreas should be identified as a critical organ for radiation planning purposes, especially in children receiving abdominal irradiation. Long-term follow-up of patients receiving abdominal irradiation should include diabetic screening.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Radiation therapy is often incorporated into the multimodality treatment for a number of abdominal malignancies, including gastric, hepatobiliary, intra-abdominal lymphomas, retroperitoneal sarcomas, and intra-abdominal metastases. Many of the normal tissues in the gastrointestinal tract are made up of rapidly multiplying mucosal epithelial cells, rendering them susceptible to acute radiation injury. In contrast, both exocrine and endocrine cells of the pancreas have very low cell turnover rates (Fajardo and Berthrong 1981; Hauer-Jensen et al. 1995; Hellerstrom et al. 1976; Rubin and Casarett 1968), and acute radiation injuries, including pancreatitis and pancreatic endocrine and exocrine insufficiency, have been rarely reported. For this reason, many believe that pancreatic tissue is radioresistant. There have been a few reports of chronic pancreatic complications as a result of late radiation injury. Radiation can result in progressive changes in the microenvironment and fibrovascular tissue in the treatment area, which manifests as ischemia. Such changes can contribute to damage to the pancreas and resultant clinical manifestations.
Unlike other organs, such as lung, where a variety of dose-volume parameters have been linked to radiation injury and provide a rationale for clinical guidelines, there are very limited data for radiation-induced pancreatic damage. In addition, where predictive models for RT-induced injury are suboptimal for most tissues, they are not at all defined for the pancreas. This is partially due to the lack of objective measures of pancreatic injury and the difficulty in separating tumor effects from the effects of the therapy. In this chapter, we will review the data for radiation-induced pancreatic injury and provide dose/volume guidelines for the clinician. The bio-continuum of adverse early and late effects are shown in Fig. 1.
Bio-continuum: Pancreatic acinar cells are known to be radioresistant to irradiation, even with larger doses and chemoradiation combo-based regiments, since modern radio techniques (IMRT, SERT) tend to spare a significant volume of this elongated organ, acute pancreatitis has not been reported clinically. Malabsorption has been reported but is transitory. With experimental radiation injury to the exocrine pancreas, islet of Langerhans appear to be spared, and although late diabetes has not been reported, it has been observed as a late effect in long-term survivors of childhood malignancies who have received abdominal irradiation. Oncogenesis with second malignant causes have been observed (with permission from Rubin and Casarett 1968)
2 Anatomy and Histology
2.1 Gross Anatomy
The pancreas arises embryogenically from endoderm. It is covered by a loose connective tissue capsule that penetrates the pancreas and separates the parenchyma into lobules. The pancreas is a long gland that lies transversely across the central posterior abdomen and extends from the duodenum to the splenic hilum. The organ is divided into three parts; the head with a small uncinate process, the body, and the tail. The head of the pancreas lies in the curve of the duodenum, while the body lies posterior to the transverse colon and greater curvature of the stomach. The pancreas lies anterior to the upper abdominal aorta, the first and second lumbar vertebral bodies, and the superior poles of the kidneys (Fig. 2).
Anatomy of pancreatic area (with permission from Tillman 2007)
Rich vascular and lymphatic networks surround the pancreas. Regional lymph node basins are associated with specific locations within the pancreas. The pancreatic head region drains primarily to the lymph nodes along the common bile duct, common hepatic artery, porta hepatis, anterior and posterior peri-pancreatic soft tissues, and the superior mesenteric chain. The pancreatic body and tail may drain to the common hepatic artery, celiac axis, splenic artery, and splenic hilum. The posterior pancreas also drains to the para-aortic lymph nodes.
2.2 Histology and the Functional Subunit
The exocrine component of the pancreas consists of closely packed secretory acini, which drains into a branched ductal system. Each acinus is made up of an irregular cluster of pyramidal secretory cells with apices projecting toward the lumen of a small central duct. The acinar cells are typically protein-secreting (zymogenic) cells with the apices packed with zymogen secretory granules, which secrete into the central duct in response to cystokinin, produced by enteroendocrine glands of the duodenum. In addition, a bicarbonate-rich fluid produced by terminal intercalated ducts located in the acinar cell walls is secreted into the central duct in response to secretin, also from the duodenal enteroendocrine glands. This secretion serves to neutralize acidic gastric chyme. (Fig. 3a, b).
The small central ducts are lined with cuboidal epithelium, that becomes stratified as the ducts become progressively larger in size. The dense connective tissue that comprises the wall of the ducts becomes thicker as they increase in size, and the main pancreatic duct contains smooth muscle. The branched ductules join together and drain into the main pancreatic duct, which joins the common bile duct and drains into the duodenum via the ampulla of Vater through the duct of Wirsung and the more proximal duct of Santorini.
Endocrine secretions enter the blood by the way of a fine reticular network of fenestrated capillary beds surrounding the islets of Langerhans, which consist of groups of endocrine cells of various sizes. During embryologic development the endocrine cells migrate from the duct system and aggregate around capillaries to form isolated clumps of cells scattered throughout the exocrine pancreas. Islets of Langerhans are most numerous in the tail. Each islet is supplied by as many as three arterioles, which branch into the fine capillary network and is drained by about 6 venules passing between the exocrine acini into the interlobular veins.
Both sympathetic and parasympathetic nervous systems innervate the islets of Langerhans, influencing secretions. The endocrine pancreas is composed of four types of secretory cells. The alpha and beta cells mainly secrete polypeptide hormones, glucagon, and insulin, respectively, which mainly regulate carbohydrate metabolism and have a wide variety of other effects on energy metabolism, growth, and development. Insulin promotes the uptake of glucose by liver, skeletal muscle, and adipose tissue, which lowers plasma concentration of glucose. Glucagon has metabolic effects opposite to the actions of insulin, resulting in a balanced carbohydrate metabolism. Generally, the glucagon-secreting alpha cells tend to be distributed at the periphery of the islets. Somatostatin is secreted by the delta cells of endocrine pancreas and has a wide variety of effects on gastrointestinal function, and may also inhibit insulin and glucagon secretion. The F cells secrete pancreatic polypeptide, which is involved in the regulation of endocrine and exocrine secretions.
3 Biology, Physiology, and Pathophysiology
3.1 Biology (Molecular Changes)
As an early event, ionizing radiation produces free radicals that lead to DNA damage and mitotic cell death, which is unlikely to produce sufficient damage for prolonged progression of injury. It is believed that the initial radiation event requires additional mechanisms that lead to late radiation-induced normal tissue damage, including endothelial dysfunction, increased vascular permeability, inflammation, and fibrosis. Although this phenomenon has not been well studied for the pancreas, it has been extensively researched for a number of other tissues.
Oxidative tissue damage and apoptosis plays an important role in abdominal irradiation, as assessed by increased lipid peroxidation, myeloperoxidase activity, and caspase-3 levels in the intestinal and pancreatic tissue. Pancreatic, as well as hepatic and intestinal myeloperoxidase activity and malondialdehyde levels are significantly increased following irradiation in an animal model (Olgac et al. 2006; Erbil et al. 2005). These phenomena may provide an amplifying mechanism for continued oxidative stress after the initial production of reactive oxygen and nitrogen species leading to prolonged progression of oxidative injury. In this study, the imbalance between oxygen-derived free radicals and antioxidant capacity appeared to be mitigated by treatment with octreotide (Olgac et al. 2006) or glutamine (Erbil et al. 2005) prior to, and following radiation, with significantly less elevation in pancreatic myeloperoxidase activity and malondialdehyde levels and accompanying inflammation compared to untreated animals.
It is presumed that an inflammatory process in which various mediators, such as eicosanoids, cytokines, and reactive oxygen metabolites contributes to the pathogenesis of radiation-induced normal tissue injury. Radiation-induced activation of a number of cytokines has been shown to play a key role in the development of chronic normal tissue injury after exposure to radiation in a number of studies. Nuclear Factor Kappa B (NFκ-B) is activated by a wide variety of agents, including hydrogen peroxide, irradiation, reactive oxygen intermediates, interleukin-1 (IL-1), Tumor necrosis factor-alpha (TNF-α), among others, and depends on the cellular redox potential. Once activated, NFκ-B transcriptionally regulates many cellular genes involved in acute phase response, and inflammatory responses (Jobin and Sartor 2000; Schmid and Adler 2000; Schmid et al. 1996).
In the prior studies, elevated pancreatic myeloperoxidase activity and malondialdehyde levels along with histological evidence of inflammation and increased apoptosis of pancreatic tissue following radiation have been correlated with NFκ-B overexpression (Olgac et al. 2006; Erbil et al. 2005) and increased caspase-3 activity (Erbil et al. 2005), both of which may contribute to late radiation-induced injury through apoptosis, and prolonged inflammation. There is no direct evidence regarding long-term complications, as animals were sacrificed 10 days after treatment, however. Both glutamine and octreotide also decreased the overexpression of NFκ-B and increased caspase-3 activity in response to radiation (Olgac et al. 2006; Erbil et al. 2005).
BCL (B Cell Lymphoma)-2 (bcl-2) is the prototype for a family of mammalian genes and the proteins they produce which govern mitochondrial outer membrane permeabilization, and can be either proapoptotic or antiapoptotic. BCL (B Cell Lymphoma)-associated X (bax) promotes apoptosis by competing with Bcl-2. Bcl-2 and bax expression have also been implicated in injury and apoptotic cell death following radiation. Radiation-induced effects on cell proliferation, expression of bcl-2 and bax proteins, and apoptosis in pancreatic tissue have been investigated utilizing a mouse model. Cell proliferation and bcl-2 expression decreased by exposure to a single dose of radiation, whereas the cell apoptosis rate and bax expression were increased (Liu and Zhong 2004).
Although the molecular mechanisms of radiation injury to the pancreas are not well characterized, they appear to be complex and involve prolonged oxidative stress, increased apoptotic death through cytokine activation and disturbed regulation of certain genes. Further investigation is warranted.
3.2 Physiology
The pancreas has two functional unites: the acinar glandular system and the islets of Langerhans, these are exocrine and endocrine components respectively. Pancreatic secretions are under neural and hormonal control (Table 1).
Acinar: Secretin provides high (HCO3) to neutralize the acid from the stomach. Enterokinases are key enzymes that are essential for digestion of starch, fat, and protein in diets. Cholicystokinin allows for reabsorption of bile.
Islet hormones: the islets are scattered and well vascularized aggregates of endocrine cells. There are four major cell types, each secreting one specific peptide hormone. Each are responsible for important physiologic functions. Most widely discussed is insulin, essential for glucogenesis and regulation gylcogenolysis.
3.3 Pathophysiology
Radiation-induced injury has traditionally been divided into an early inflammatory phase, which typically occurs between 6 and 16 weeks after radiation exposure, and a late fibroproliferative phase, which occurs much later and is characterized by diffuse fibrosis. The pathogenesis of radiation injury of the pancreas has been proposed to arise from endothelial cell damage of the microcirculation supplying acinar cells, adding ischemia to direct radiation cell damage. Acute stromal edema as a result of increased capillary permeability also plays a role (Fajardo et al. 2001). Radiation injury to myoepithelial cells and intimal fibroblasts also result in progressive fibrosis and ischemia from arterial and venular lesions and add to long-term pancreatic damage (Fajardo et al. 2001) (Fig. 4a, b, c).
a Normal pancreas. Hematoxylin and eosin (H and E) prepared normal pancreas from postmortem specimen. Most of the field consists of small, dark acini, the exocrine galnds. Three islets of Langerhans are in the upper-left half of the field (thin arrows). A small duct (thick arrow) is in the low center. Various blood vessels are in the right center (arrow heads). b Late radiation injury. Figures 4b and c demonstrate H and E prepared human pancreas several months after exposure to several thousand centigray (cGy) of megavoltage radiation to the pancreas delivered for an adjacent abdominal neoplasm. There is marked loss of the parenchyma, especially of the exocrine glands, with replacement by adipose tissue (fat cells framing the picture) in the midst of atrophic acini. The atrophic acini are dilated and separated from each other. Several islets appear to be intact (arrow heads)
3.3.1 Animal Studies
A few animal studies have focused on radiation-induced vascular and ductal damage. The vascular and connective tissues appear to have similar radiosensitivity as tissues elsewhere in the body. Zook et al. observed moderate radiation-induced occlusive vascular disease in dogs receiving abdominal neutron or photon radiation and postulated that this phenomenon could contribute to acinar and duct cell changes (Zook et al. 1983). In another study, 80 Gy was delivered to rat abdomens and histological changes were observed at intervals following exposure (Kovacs 1976). In the acute phase of radiation injury during the first 2 weeks following radiation, most of the acini remain intact with rare small necrotic foci. Edema, inflammation, and dilatation of efferent ductules with stasis consequently leads to atrophy of the acini associated with the efferent ducts over the next several months. During this period, the number of capillaries diminishes, and the number of necrotic foci increase in areas where no capillaries are visible (Kovacs 1976). These progressive late structural changes are thought to contribute to functional changes over time.
Early radiation-induced pancreatic injury results in the decline in bicarbonate and enzyme secretion shortly following radiation to the pancreas in canines and other animals (Corring et al. 1975; Pieroni et al. 1976; Volk et al. 1966). One canine study utilizing intraoperative pancreatic radiation doses of 17.5–40 Gy resulted in a progressive decrease in exocrine pancreatic function, which was evaluated by measuring N-benzoyl-l-tyrosyl-para-aminobenzoic acid, which correlated with weight loss and percentage of normal appearing acinar cells (Ahmadu-Suka et al. 1988). Autopsies performed on the dogs at 135 days following radiation demonstrated a dose-dependent degree of pancreatic atrophy in the irradiated dogs (Ahmadu-Suka et al. 1988).
In contrast islet cell damage appears to be rapidly repaired without alterations in blood glucose levels (Sarri et al. 1991). Most investigators believe that islet cells are more resistant than the acinar tissue to radiation effects (Fajardo and Berthrong 1981; Cameron and Flecker 1925; Zook et al. 1983; Ahmadu-Suka et al. 1988; Spaulding and Lushbaugh 1955). One study demonstrated that degeneration of beta cells occurs approximately 1–4 days after a single large fraction of pancreatic radiation with complete regeneration at 18 days (Cameron and Flecker 1925). Another investigator delivered single large doses of radiation to monkey pancreas and found necrosis of islet cells at 8 h, with beta cells more resistant that alpha cells (Spaulding and Lushbaugh 1955). In both studies there were no morphologic changes noted in the acinar cells during a short follow-up period (Cameron and Flecker 1925; Spaulding and Lushbaugh 1955). In addition, data suggest that the insulin function of a pancreas in rats exposed to a single 4 Gy dose is maintained at a level sufficient for ensuring adequate regulation of glucose homeostasis and of carbohydrate metabolism (Shkumatov 2004).
Similar studies evaluating histological changes and endocrine function have been performed in primates who underwent irradiation in preparation for pancreatic transplantation. Eight or 10 Gy whole body radiation was delivered over 4–5 weeks, with a maximum of 2 Gy weekly. Although the animals remained normoglycemic, insulin release was significantly reduced in both groups during irradiation and was associated with mild glucose intolerance. Histological changes included necrosis of the islet cells and acinar tissue, and cytocavitary network changes of alpha and beta cells, including degranulation, vacuolization, mitochondrial destruction, and an increase in lysosomes (Du Toit et al. 1987).
Other animal studies utilizing fractionated or single doses of radiation have demonstrated acinar gland damage (Pieroni et al. 1976; Volk et al. 1966; Wellmann et al. 1966). Early ultrastructural changes include acinar cell degeneration, mitochondrial injury, and vesicles in endoplasmic reticulum cisternae, which are evident within 1 h following single dose radiation exposure (50–90 Gy) on electron microscopy and are associated with reduced enzyme secretion (Volk et al. 1966). After 8 days following radiation, numerous membrane-bound cytoplasmic bodies, endoplasmic reticulum containing entrapped degenerating mitochondria, and reduced zymogen granules are observed (Volk et al. 1966).
Acini exposed to radiation are known to exhibit increased autophagy, however, these changes do not appear to be related to radiation-induced lysosomal degradation of intracellular proteins (Somosy et al. 1996; Telbisz et al. 2002). Islet cells show similar degenerative organelle changes that are repaired efficiently and last up to 3 weeks, during which time there is no alteration in blood glucose levels (Cameron and Flecker 1925; Volk et al. 1966; Spaulding and Lushbaugh 1955). Longer follow-up demonstrates a recovery period from 3–5 weeks, consisting of increasing endoplasmic reticulum and gradual disappearance of autophagic remnants. After about 5–12 months, the ultrastructure of the cells returns to normal morphologic appearance with the exception of persistent interstitial fibrosis. Conversely, pancreatic function does not completely recover, and continues to exhibit a persistent decrease in enzyme production compared to pretreatment (Wellmann et al. 1966).
In another early study utilizing fractionated pancreatic radiation (45–50 Gy over 30 days) in nine dogs whose pancreas had been surgically mobilized in the abdomen, Archambeau et al. performed functional studies of blood glucose and pancreatic secretions obtained through fistulae (Archambeau et al. 1966). Samples of the pancreatic head and tail were also obtained at 2, 3, and 6 months after radiation demonstrated progressive interstitial fibrosis with distorted normal pancreatic architecture, and preserved acini and the islet morphology. No changes in secretory volume, serum, and pancreatic electrolytes or blood glucose were detected, but there was an initially elevated serum amylase, which declined progressively (Archambeau et al. 1966).
Injury to small- and medium-sized ducts resulting in occlusion of the lumen by cell debris has been demonstrated after 50 Gy fractionated radiation combined with 25 Gy intraoperative radiation to the pancreas in dogs. Over time, severe interstitial fibrosis was observed and by 135 days small- to medium-sized arteries and veins were narrowed with medial and adventitial fibrosis (Ahmadu-Suka et al. 1988). Others have demonstrated that local radiation impairs microcirculatory blood flow in healthy pancreas in a rat ductal pancreatic tumor model. A single fraction of 15 Gy resulted in increased tumor apoptosis but no change in tumor blood flow, whereas blood flow and functional capillary density in the normal pancreas was impaired 5 days after radiation, resulting in hypoperfusion (Ryschich et al. 2003). These changes are thought to contribute significantly to decline in pancreatic exocrine dysfunction and late parenchymal structural damage.
3.3.2 Human Studies
There is even less data describing radiation damage in humans following radiation to the pancreas. As mentioned earlier, Case and Warthin described atrophy of the acinae and degeneration and necrosis of pancreatic ducts in 3 patients with radiation-induced hepatitis (Case and Warthin 1924). Subsequently, in 1955, Brick observed pancreatic fibrosis during autopsies of three men who received radiation to the para-aortic lymph nodes for testicular cancer (Brick 1955). In another report, autopsies of patients treated with abdominal radiation for lymphoma demonstrated peripancreatic stromal fibrosis, sometimes severe, with myointimal proliferation in arteries. The authors described the appearance as similar to chronic pancreatitis with lack of active inflammation and necrosis (Fajardo 1982). Woodruff et al. performed postmortem studies of 22 patients who received radiation for pancreatic cancer using 50–60 Gy over 30 fractions delivered with helium ions. After a median of 9 months following radiation, approximately 85 % of patients demonstrated radiation injury in the nontumor bearing pancreas consisting of dense fibrosis, mainly located in the exocrine parenchyma. They reported that no patient died of pancreatic insufficiency and only one patient developed hyperglycemia (Cohen et al. 1985; Woodruff et al. 1984).
Because of the paucity of human specimens, very little is known regarding early radiation injury to the human pancreas and we predict early radiation damage by extrapolating from the animal studies previously described. After about 10–20 Gy, acinar injury is observed, similar to animal studies. Inflammatory cells are abundant and the number of secretory granules is reduced and the cytoplasm becomes vacuolated. Similar to other tissues, the amount of cellular damage increases with increasing fraction size. Zonal necrosis and ductule degeneration are observed at 60 Gy. Although larger ducts may only demonstrate mild cell changes and enlargement, smaller ducts develop distended lumens and plugging by cellular debris. Acute injury to arterioles and venules may also be present and associated with edema of the stroma and acute inflammation in regions of early parenchymal necrosis. In contrast, islet capillaries show relatively normal anatomical structure during the early injury phase without endothelial enlargement or fibrin thrombi, and islets of Langerhans appear normal (Fajardo et al. 2001).
Pathologic examination of patient pancreas specimens examined at 6 months or more after treatment with radiation demonstrate shrunken, fibrotic pancreas with thickening of the fibrous capsule and adhesions to the surrounding tissues grossly. Lobulation becomes less prominent, and large ducts may be dilated (Fajardo et al. 2001). Microscopic examination of irradiated pancreas at the same time interval demonstrate fibrotic parenchyma with severe acinar cell loss, abundant collagen, hyaline, and atypical fibroblasts similar in appearance to chronic pancreatitis. Despite these structural changes, islet cells appear normal. Occasionally fat necrosis and calcifications may be found (Case and Warthin 1924; Levy et al. 1993). At this time point, very little inflammation is found with the exception of small collections of lymphocytes trapped within the dense fibrous connective tissue. Arteriole walls are thickened with fibrosis of the intima and narrowed lumens while smaller arteries and veins demonstrate fibrous intimal plaques comprised of foam cells (Berthrong 1986). The number of ductules are reduced or atrophied, and plugged with cellular debris while larger ducts are dilated with enlarged nuclei (Fajardo et al. 2001).
Others have studied pancreatic function following abdominal radiation. In one study, the early and late effects of intraoperative radiation on the exocrine and endocrine functions of the residual pancreas were examined in 54 patients with pancreatic head resection, 20 whom underwent intraoperative radiation, and 34 who did not (Yamaguchi et al. 2000). Fasting blood sugar levels and glucose tolerance tests demonstrated no change at 2 months, but at 6 months the glucose tolerance was compromised compared to the preoperative baselines in both groups, while fasting blood sugar levels remained normal. The total amount of pancreatic juice drainage in the irradiated patients was about half as much as that in nonirradiated group. In addition, univariate and multivariate regression analyses demonstrated that intraoperative radiation was a significant independent factor in the decline of pancreatic exocrine function (N-benzol-L-tyrosyl-p-aminobenzoic acid excretion) in the early postoperative period (Yamaguchi et al. 2000).
4 Clinical Syndromes (Endpoints)
A variety of endpoints can be considered to reflect RT-associated pancreatic dysfunction, as shown in Table 2. It is sometimes useful to categorize the endpoints, albeit somewhat arbitrarily, as shown.
4.1 Detection (Symptoms)
Pancreatic complications are rarely reported following abdominal irradiation. There are reports, however, of exocrine abnormalities, pain, and diabetes occurring after abdominal irradiation.
Pancreatic exocrine dysfunction and malabsorption are the most commonly reported complications following pancreatic radiation. Exocrine dysfunction can present as painful acute episodes or slow, relatively asymptomatic destruction of the pancreas.
Because pancreatic exocrine deficiency can be managed with replacement therapy, pancreatic injury has not routinely been considered a serious complication. Pancreatic lipase is responsible for the hydrolysis of triglycerides and without this enzyme, fat absorption is inhibited and steatorrhea arises. The absence of pancreatic proteases also contributes to protein malabsorption. The evaluation of pancreatic exocrine insufficiency is cumbersome and is rarely performed. Jejunal or duodenal contents detecting pancreatic enzymes or bicarbonate production can be evaluated following a specific meal or hormonal stimulation. A less invasive approach is evaluation of urinary bentiromide, which measures chymotrypsin production by determining whether para-aminobenzoic acid is cleaved off an ingested carrier and excreted through the kidneys. In most cases, a clinical history is obtained that reveals greasy, light-colored, buoyant stools reflective of high lipid content, and continued weight loss despite adequate nutritional intake.
Patients may more rarely develop an abnormal glucose tolerance test. Patients may or may not also demonstrate accompanying abnormal random or fasting plasma glucose levels. Glucose tolerance is tested by ingesting 75 g of glucose after fasting. A 2-h glucose level between 140 and 200 mg/dl, and an intervening value greater than 200 mg/dl is reflective of impaired tolerance. Patients demonstrating impaired tolerance are at increased risk for development of overt diabetes mellitus. It is difficult to determine whether or not glucose intolerance is attributed to radiation in many occasions, as there is often underlying destructive pancreatic pathology present, such as pancreatic cancer, as an underlying etiologic factor contributing to development of endocrine dysfunction.
In addition to radiation-induced pancreatic damage resulting in functional changes, late radiation-induced oncogenesis has been reported, although the mechanism of oncogenesis has not been defined. Low-level radiation has been linked to pancreatic cancer (Cohen 1980). Ductal adenocarcinomas have been found in long-term survivors who have received pancreatic radiation as a late event after a long latent period (Deutsch et al. 1999; Lambert et al. 1998; Travis et al. 1997). A case of adenocarcinoma of the head of the pancreas arising in a previously irradiated volume 14 years after extended field irradiation for Stage II A Hodgkin’s disease has been reported (Deutsch et al. 1999). Similarly, cancers of the pancreas were increased in patients irradiated for peptic ulcer disease with a relative risk of 1.87 (Carr et al. 2002; Griem et al. 1994). Travis et al. reported 1,406 s neoplasms in 28,843 men irradiated to the abdomen for testicular cancer. The pancreas was among the organs at significant risk with an observed: expected ratio of 2.21, higher than many other organs (Travis et al. 1997). In irradiated patients, risk of cancer increased significantly from 1 to 45 Gy (a low to a high-dose level) for stomach and pancreas (Suit et al. 2007).
4.2 Diagnosis (Imaging)
Radiation changes in the pancreas have received little attention because imaging findings are usually nonspecific and may not be of any clinical significance. Imaging of the pancreas following radiation is often normal but sometimes radiographic changes consistent with chronic pancreatitis can be observed. Abdominal X-rays may reveal diffuse calcifications. Because many patients receiving pancreatic radiation have underlying pancreatic carcinoma, it is difficult to distinguish whether the observed radiologic changes are due to the tumor or radiotherapy (Charnsangavej et al. 1994; Hori et al. 1982) (Fig. 5).
Diagnosis: This CT scan demonstrates postradiation changes 6 months after radiotherapy to the head of the pancreas in an asymptomatic patient with cholangiocarcinoma. a CT scan during the bolus phase of IV contrast enhancement shows normal enhancement of the head of the pancreas. b Delayed scan 4 min later shows abnormal hyperdense enhancement of the pancreatic head (arrows) that is indistinguishable from chronic pancreatitis. The uncinate process (arrowhead) is what the pancreatic head “should normally look like” on these 4 min delayed images. Computed tomography images courtesy of Chuslip Charnsangavej M.D., Department of Diagnostic Radiology, University of Texas, M.D. Anderson Cancer Center, Houston, TX
Following radiation to the pancreas, endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography may demonstrate gross ductal changes similar to chronic pancreatitis, with a “chain of lakes”-like appearance.
5 Radiation Tolerance and Predicting Radiation-Induced Injury
5.1 Dose Time Fractionation
The majority of patients that receive curative doses of radiation to the abdomen for malignancy have relatively short survival intervals, therefore there are little data to define the precise dose of radiation that results in 5 % of patients with pancreatic complications at 5 years. Nonetheless, diabetes has been reported as a late effect following radiation, although many factors may contribute to the development of the onset of diabetes in addition to radiation exposure in most settings (Levy et al. 1993). Some argue that even though islets of Langerhans appear histologically intact, function may be altered.
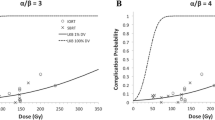
At 45 Gy, pancreatic exocrine insufficiency with or without malabsorption has been reported (Case and Warthin 1924). Recently, evidence of a dose-response relationship between pancreatic irradiation and subsequent risk of diabetes in a retrospective study of childhood cancer survivors. This study demonstrated that the risk of diabetes was strongly correlated with radiation dose to the tail of the pancreas, where islet of Langerhans are concentrated. The cumulative incidence of diabetes was 16% in 511 patients who had received 10Gy or more to the pancreatic tail. A dose response was seen up to 20 to 29 Gy before reaching plateau at higher radiation doses. The relative risk of developing diabetes was 11.5 for patients who received at least 10 Gy to the pancreatic tail compared to patients who did not receive radiation, and was most pronounced for patients under the age of 2 at the time of radiation. This association was not appreciated for radiation to other parts of the pancreas, and results were unchanged after adjustment for body mass index despite a strong independent effect (de Vathaire et al. 2012). There have also been reports of a chronic pancreatitis syndrome following abdominal radiation (Levy et al. 1993; Schoonbroodt et al. 1996). Levy et al. reported on five patients who received abdominal irradiation and developed pseudocysts, pancreatic calcifications, and morphologic changes resembling chronic pancreatitis at a median of 7 years following 36–40 Gy (Levy et al. 1993). There are little data to define a dose response for late radiation-induced pancreatic injury, however, histological evidence of pancreatic injury has been demonstrated with fractionated doses as low as 36 Gy (Levy et al. 1993), and a higher incidence of radiation-induced pancreatic complications has been reported at doses of 60 Gy (Fajardo and Berthrong 1981; Rubin and Casarett 1968; Berthrong 1986).
As targeting and delivery of radiation has improved with newer technologies, investigators have evaluated the potential for dose-escalation in an attempt to improve outcome for patients with intra-abdominal malignancies. One model investigated the use of intensity-modulated radiation therapy (IMRT) optimization for selective dose radiation escalation in patients with pancreatic cancer (Spalding et al. 2007). IMRT using lexicographic ordering, a hierarchical optimization technique, with generalized equivalent uniform dose (gEUD) cost functions, was used to increase the dose to pancreatic tumors and to areas of vascular involvement that precluded surgical resection. IMRT plans optimized by using gEUD-based cost functions resulted in inhomogeneous dose distributions that allowed substantial dose escalation to tumor and areas of vascular involvement compared with 3D conformal radiation treatment plans up to 66 Gy and 85 Gy, respectively, with significant dose reductions in nearby normal tissue organs at risk (Spalding et al. 2007). This model is currently being tested in clinical studies and the effect on normal pancreatic function has not yet been described. It is possible with better tumor control, survival of these patients may be improved, and late pancreatic normal tissue toxicity will be increasingly reported.
5.2 Hypofractionation
A study at Massachusetts General Hospital is currently evaluating the dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment (Kozak et al. 2007). Data for the first nine pancreatic cancer patients treated with neoadjuvant intensity-modulated radiotherapy (1.8 Gy × 28) had treatment plans generated using a 5 CGE × 5 fraction proton regimen. Improved dose conformality provided by the hypofractionated proton regimen resulted in significant reduction in the mean doses to surrounding normal tissue inclusive of kidneys, liver, and small bowel in both high- and low-dose regions (Kozak et al. 2007). The dose to uninvolved pancreas was not evaluated but this treatment planning technique may also be applied. Hypofractionated proton radiotherapy has not yet been tested in a Phase I clinical trial investigation. Similar to dose-escalated IMRT, we may encounter improved tumor control, improved survival, and longer term follow-up resulting in more pancreatic complications.
Stereotactic body radiosurgery has recently been utilized to deliver a range of hypofractionated radiation treatments to patients with pancreatic tumors. Up to 25 Gy delivered in a single fraction has been used for treatment, including some patients who received prior chemotherapy and fractionated external beam radiation. The largest series reported included 77 patients with unresectable adenocarcinoma of the pancreas who received 25 Gy in 1 fraction. The rates of grade two or greater late toxicity were 11 and 25 % and were due to bowel ulceration, stricture, or perforation, but no pancreatic complications were reported (Chang et al. 2009). Several other clinical studies utilizing this technology with a variety of fractionation schedules to treat pancreatic tumors have been reported, but patient numbers remain small, and median follow-up is short. Most studies focus on intestinal toxicity as a major limitation, and there are no reports of pancreatic injury to date.
5.3 Recommended Dose-Volume Constraints
Both the pancreas and salivary glands show many histological and functional similarities. Both originate from endoderm and consist of acinar and ductal epithelial cells, which have similar exocrine function and microenvironments. In addition, investigators have compared two stem cell populations from pancreatic and salivary glandular tissues, which demonstrated similar phenotypes and analogous properties. During embryonic development the two exocrine glands originate from the foregut, which might be the explanation for these similarities (Gorjup et al. 2009). For this reason, some have postulated a similar tolerance to radiation.
As radiation-induced damage to pancreatic function is rarely life threatening and is usually well treated with medical management, dose-volume constraints have not been formally evaluated as with other critical normal tissues. Pancreatic exocrine dysfunction is the side effect most often encountered following pancreatic radiation therapy. If one extrapolates from similarities between salivary and pancreatic exocrine tissue, we may hypothesize similar radiation dose-volume effects. Dose-volume relationships have been well studied for parotid radiation (Blanco et al. 2005; Deasy et al. 2010; Eisbruch et al. 1999; Marmiroli et al. 2005; Roesink et al. 2001; Li et al. 2007; Maes et al. 2002).
Important studies not only evaluated quality of life, but also quantitatively measured salivary function at intervals following radiation and correlated the results with dose-volume data (Blanco et al. 2005). Based on these data, it is estimated that a mean parotid dose of 25.8 Gy, on average, is likely to reduce a single parotid gland’s flow to 25 % of its pretreatment value, and the incidence of xerostomia as a result of radiation-induced exocrine dysfunction was significantly decreased when the mean dose of at least one parotid gland was kept to <25.8 Gy with conventional fractionation. Using this model, salivary function, in each gland, appeared to be lost exponentially at a rate of approximately 5 % per 1 Gy of mean dose (Blanco et al. 2005).
Most recently Deasy et al. reanalyzed available data on dose-volume relationships for salivary glands for the quantitative analyses of normal tissue effects in the clinic (QUANTEC) project (Deasy et al. 2010). Although he concluded that currently available predictive models are imprecise, the risk of severe xerostomia defined as long-term salivary function of <25 % of baseline, may be avoided if at least one parotid gland is spared to a mean dose of less than approximately 20 Gy or if both glands are spared to less than approximately 25 Gy (mean dose) (Deasy et al. 2010). This is somewhat consistent with the limited data published on radiation–induced pancreatic toxicity reporting exocrine dysfunction (Case and Warthin 1924; Brick 1955), and chronic pancreatitis-like syndrome as low as 36 Gy (Levy et al. 1993).
Hence, if we accept the hypothesis that radiosensitivity is similar for parotid exocrine function, we suggest that 50 % of the pancreas not involved by tumor receive a mean dose of less than 25 Gy. However, it should be recognized that radiation-induced pancreatic damage is rarely a limiting factor, which can usually be managed medically and concerns for intestinal or renal toxicity and/or tumor control usually prevail. In general, lower pancreatic mean doses will result in better function. Clinical judgment should play an important role in treatment planning when compliance with dose-volume constraints may not be achievable.
Radiation-induced pancreatic endocrine insufficiency is less commonly encountered but nonetheless, has been reported. Although there are no dose-volume data predicting this rare complication, one should assume that irradiating the entire pancreas to high dose would increase the risk of glucose intolerance, especially since there is a higher proportion of beta cells in the tail region. Analysis of the available literature aforementioned, tolerance dose TD5 = 45 Gy, and the TD55 = 60 Gy is postulated.
6 Chemotherapy
The majority of data presented in this chapter predates the routine use of combined modality therapy, however, we acknowledge that exposure to chemotherapeutic agents may contribute to pancreatic sequelae resulting from treatment. Most current cancer treatment regimens for abdominal malignancies incorporate the use of sequential and/or concurrent chemotherapy in addition to abdominal radiation. To date, there have been only a few sporadic reports of acute pancreatitis associated with certain chemotherapy, mostly in children treated for all who did not receive abdominal radiation (Flores-Calderon et al. 2009; Alvarez and Zimmerman 2000; Adachi et al. 1988; McGrail et al. 1999), and patients receiving alloxan and streptozocin (Scarpelli 1989). To date, there have been no reports of pancreatic toxicity for agents commonly used for the treatment of abdominal malignancies, nor in patients who have received combined chemoradiation for treatment of abdominal malignancies.
7 Special Topics
7.1 Pediatrics
In the course of abdominal RT or TBI, damage to the islet cells of the pancreas can occur and there is now emerging evidence on the increased risk of diabetes mellitus (DM) in long-term survivors of childhood cancer. This may be the result of direct cytotoxicity to pancreatic cells from abdominal RT, but may also be a long-term implication from the metabolic syndrome. In a study of 8599 survivors from the CCSS, DM was reported in 2.5 % of the survivors compared with 1.7 % of the siblings. After adjustment for body mass index, age, sex, race/ethnicity, household income, and insurance, the survivors were 1.8 times more likely than the siblings to report DM. In adjusted models, TBI was associated with a sevenfold increased risk, abdominal irradiation with a 2.7-fold increased risk, alkylating agents with a 1.7-fold increased risk, and age 0–4 years at diagnosis with a 2.4-fold increased risk (Meacham et al. 2009). More recent data demonstrates a dose-response relationship between pancreatic tail irradiation and subsequent risk of diabetes. The cumulative incidence of diabetes was 16% in 511 childhood cancer survivors who had received 10Gy or more to the pancreatic tail. A dose response was seen up to 20 to 29 Gy before reaching plateau at higher radiation doses. The relative risk of developing diabetes was 11.5 for patients who received at least 10 Gy to the pancreatic tail compared to patients who did not receive radiation, and was most pronounced for patients under the age of 2 at the time of radiation (de Vathaire et al. 2012).
8 Prevention
The use of radioprotectant and free-radical scavengers for prevention of radiation-induced pancreatic damage has only been investigated in the laboratory. The first study to investigate this topic was published in 1967 (Volk et al. 1967; Wellmann et al. 1967). In this study dogs were either pretreated with a single small nondiabetogenic dose of alloxan monohydrate (15 mg/kg) or placebo and then received a single dose of 50–60 Gy to a surgically exposed pancreas. In the nontreated dogs, subsequent histological examination of partial pancreatectomy specimens demonstrated widespread focal cytoplasmic degeneration, endoplasmic reticulum and mitochondrial abnormalities, and reduction in size and number of zymogen granules similar to other studies. In addition, these dogs demonstrated marked decrease in amylase, aminopetidase, and lipase activities. In the alloxan treated dogs, the structural changes were not as pronounced, and the marked decrease in enzyme activity was not observed (Volk et al. 1967; Wellmann et al. 1967). A follow-up study evaluated dogs which received a single dose of alloxan at intervals of 2–14 days following radiation, as well as some which receive alloxan both pre- and post-treatment. No severe nor widespread cytological changes were noted in any animals and similar radioprotective effects were observed on enzymatic levels, especially when the dogs received both pre- and post-treatment alloxan (Wellmann et al. 1967). The authors proposed that the protective effect of alloxan may be in part due to interaction with amino acids, which modifies the structure and reduces synthesis of proteins, particularly those with –SH radicals (Doolittle and Watson 1966; Stocker et al. 1965). No studies have reported radioprotective effect of alloxan on the human pancreas.
Amifostine has been evaluated as a radioprotector in the clinical setting for both salivary function preservation in patients receiving radiation for head and neck cancers (Brizel et al. 2000) and as an rectal mucosa protector for patient receiving abdominal or pelvic radiation (Simone et al. 2008). Its effect on pancreatic function has been evaluated in the laboratory setting (Grdina et al. 2009). Amifostine was administered intraperitoneally to mice receiving radiation for transplanted tumors. The use of amifostine produced a delayed radioprotective effect correlating with elevated levels of the antioxidant enzyme, superoxide dismutase (SOD2) activity in selected tissues, including heart, liver, lung, pancreas, small intestine, spleen, and tumor 24 h after amifostine treatment. Other antioxidant enzyme, catalase, and glutathione peroxidase (GPx), activities remained unchanged except for significant elevations in the spleen. GPx was also elevated in the pancreas indicating a radioprotective effect (Grdina et al. 2009). There have been no clinical studies evaluating radioprotective effects of Amifostine in the pancreas in humans.
There have been no other agents formally evaluated as radioprotectants in the pancreas in humans. The only other two agents that demonstrated promising results in animal studies were octreotide and glutamine, which mitigated the radiation-induced changes in myeloperoxidase and caspase activity, and malondialdehyde and NFκ-B levels, as previously described (Olgac et al. 2006; Erbil et al. 2005).
9 Management
Because pancreatic exocrine deficiency can be managed with replacement therapy, pancreatic injury has not routinely been considered a serious complication compared to other normal tissue damage that may occur from abdominal radiation, such as radiation enteritis. In most cases, pancreatic enzyme supplementation is all that is needed to improve intestinal absorption of fats and proteins, reverse weight loss, and normalize stools. Dosage and timing of enteric-coated pancreatic enzymes are important issues in the treatment of malabsorption. Nonenteric-coated enzyme preparations along with acid suppression (histamine-2 blockers or proton-pump inhibitors) are of limited to modest effectiveness in treating pain.
Chronic pain related to progressive fibrosis compressing splanchnic nerves sometimes responds to narcotic pain medication. The role of endoscopic ultrasound-guided celiac plexus block is sometimes useful and is limited to treating those patients whose pain has not responded to other modalities (Abdel Aziz and Lehman 2007). In the setting of pancreatic cancer, such pain is typically associated with tumor progression rather than radiation injury, however.
The management of patients with glucose intolerance and diabetes should ameliorate hyperglycemia and its symptoms and prevent hyperglycemic crises. The burden of such therapy falls on the patient, and education of the patient and/or caregiver is important. Home glucose monitoring is a component of glycemic control and relies on patient compliance. Most patients with glucose intolerance without evidence of hyperglycemia respond to dietary management. Exercise also contributes to weight control, cardiovascular fitness, and increases the body’s sensitivity to insulin. Sulfonylurea oral hypoglycemic agents can sometimes be useful in patients with hyperglycemia to increase endogenous insulin secretion in response to meals and enhance peripheral sensitivity, however, approximately 25 % of patient with Type 2 diabetes will not respond. This number is thought to be higher in patients with radiation injury but the exact proportion of nonresponders is not known.
Most patients who develop hyperglycemia following radiation to the pancreas require insulin therapy. It has been postulated that patients who develop hyperglycemia following pancreatic radiation have blood glucose levels that are more difficult to control than a patient with Type I diabetes who did not receive abdominal radiation. There is convincing evidence that tighter glucose control is accompanied by decreased incidence and progression of chronic complications. Surveillance for early stages of diabetic complications such as retinopathy and neuropathy is important because progression can be delayed with appropriate intervention. Monitoring of hemoglobin A1c may be helpful in predicting risk for these complications.
10 Future Direction and Research
-
a.
The dose–response for reductions in regional pancreatic function (either exocrine or endocrine) are not known. Sophisticated imaging techniques might be able to detect these cellular functions and allow one to quantitatively relate regional RT doses to regional changes in function.
-
b.
The relationship between the three-dimensional dose distribution and changes in global pancreatic function are not well defined. Studies that relate global function (e.g., glucose tolerance testing or fat absorption assessments) to the three-dimensional dose distribution could be performed. The pancreas should be identified as a critical organ for radiation planning purposes, especially in children receiving abdominal irradiation. Long-term follow-up of patients receiving abdominal irradiation should include diabetic screening as a component of survivorship plans.
-
c.
The pancreas is often a target of intra-operative radiation. One could perform biopsies of the normal pancreas immediately before and after such intra-operative radiation to assess the acute molecular events triggered by radiation.
11 History and Literature Landmarks
1924: Case and Warthin: reported first clinical case of patient vigorously irradiated, who died 10 weeks post therapy over epigastrium with atrophy of acinous cells, vacuolar degeneration, and necrosis.
1935: Schurch and Vehlinger reported necrosis of pancreas of a dog following high-dose radium implantation.
1942: Friedman: reviewed literature suggesting islets of Langerhans were more radioresistant than acinar epithelium.
1955: Spalding and Lushbaugh: studied effects of massive radiation dose single exposure of pancreas in monkeys and rats and found pyknosis and necrosis of islet alpha cells which more readily injured than beta cells.
1955: Brick reported first observation of pancreatic fibrosis at autopsy of 3 patients following megavoltage irradiation for para-aortic lymph nodes.
1968: Rubin and Casarett: reviewed the literature concluding the pancreas is relatively radioresistant compared to other abdominal organs, i.e., stomach, intestine, colon, liver, and kidneys and was not dose limiting.
2012: de Vathaire et al. demonstrated a 16% cumulative incidence of diabetes in 511 childhood cancer survivors who had received 10Gy or more to the pancreatic tail, with a dose response identified for up to 20-29 Gy.
Abbreviations
- bcl-2:
-
B cell lymphoma-2
- bax:
-
B cell lymphoma-associated X
- gEUD:
-
Generalized equivalent uniform dose
- GPx:
-
Glutathione peroxidase
- IL-1:
-
Interleukin-1
- IMRT:
-
Intensity modulated radiation therapy
- QUANTEC:
-
Quantitative analyses of normal tissue effects in the clinic
- SOD2:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-alpha
References
Abdel Aziz AM, Lehman GA (2007) Current treatment options for chronic pancreatitis. Curr Treat Options Gastroenterol 10:355–368
Adachi S, Akiyama Y, Takimoto T et al (1988) Aclarubicin-related pancreatitis in a child with AML. Rinsho Ketsueki 29:385–388
Ahmadu-Suka F, Gillette EL, Withrow SJ et al (1988a) Exocrine pancreatic function following intraoperative irradiation of the canine pancreas. Cancer 62:1091–1095
Ahmadu-Suka F, Gillette EL, Withrow SJ et al (1988b) Pathologic response of the pancreas and duodenum to experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys 14:1197–1204
Alvarez OA, Zimmerman G (2000) Pegaspargase-induced pancreatitis. Med Pediatr Oncol 34:200–205
Archambeau J, Griem M, Harper P (1966) The effects of 250-kv x-rays on the dog’s pancreas: morphological and functional changes. Radiat Res 28:243–256
Berthrong M (1986) Pathologic changes secondary to radiation. World J Surg 10:155–170
Blanco AI, Chao KS, El Naqa I et al (2005) Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys 62:1055–1069
Brick IB (1955) Effects of million volt irradiation on the gastrointestinal tract. AMA Arch Intern Med 96:26–31
Brizel DM, Wasserman TH, Henke M et al (2000) Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 18:3339–3345
Cameron G, Flecker H (1925) An inquiry into the behavior of the islets of Langerhans under pathologic conditions. J Metab Res 7:166–182
Carr ZA, Kleinerman RA, Stovall M et al (2002) Malignant neoplasms after radiation therapy for peptic ulcer. Radiat Res 157:668–677
Case J, Warthin A (1924) The occurence of hepatic lesions in patients treated by intensive deep Roentgen irradiation. Am J Roentgenol 12:27–46
Chang DT, Schellenberg D, Shen J et al (2009) Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 115:665–672
Charnsangavej C, Cinqualbre A, Wallace S (1994) Radiation changes in the liver, spleen, and pancreas: imaging findings. Semin Roentgenol 29:53–63
Cohen BL (1980) The low-level radiation link to cancer of the pancreas. Health Phys 38:712–714
Cohen L, Woodruff KH, Hendrickson FR et al (1985) Response of pancreatic cancer to local irradiation with high-energy neutrons. Cancer 56:1235–1241
Corring T, Daburon F, Remy J (1975) Effect of acute irradiation on the exocrine secretion of pancreas in pig. Strahlentherapie 149:417–425
Deasy JO, Moiseenko V, Marks L et al (2010) Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 76(3 Suppl):S58–S63
de Vathaire F, El-Fayech C, Ben Ayed FF, et al (2012) Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol 13(10):1002–1010
Deutsch M, Rosenstein MM, Ramanathan RK (1999) Pancreatic cancer in a young adult after treatment for Hodgkin’s disease. Clin Oncol (R Coll Radiol) 11:280–282
Doolittle DP, Watson JA (1966) Protection from radiation lethality by alloxan. Int J Radiat Biol Relat Stud Phys Chem Med 11:389–391
Du Toit DF, Heydenrych JJ, Smit B et al (1987) The effect of ionizing radiation on the primate pancreas: an endocrine and morphologic study. J Surg Oncol 34:43–52
Eisbruch A, Ten Haken RK, Kim HM et al (1999) Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 45:577–587
Erbil Y, Oztezcan S, Giris M et al (2005) The effect of glutamine on radiation-induced organ damage. Life Sci 78:376–382
Fajardo L (1982) Pathology of Radiation Injury, vol 6. Masson Publishing USA, New York
Fajardo LF, Berthrong M (1981) Radiation injury in surgical pathology. Part III. Salivary glands, pancreas and skin. Am J Surg Pathol 5:279–296
Fajardo L, Bethrong M, Anderson R (2001) Radiation pathology. Oxford University Press, New York
Flores-Calderon J, Exiga-Gonzalez E, Moran-Villota S et al (2009) Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol 31:790–793
Gorjup E, Danner S, Rotter N et al (2009) Glandular tissue from human pancreas and salivary gland yields similar stem cell populations. Eur J Cell Biol 88:409–421
Grdina DJ, Murley JS, Kataoka Y et al (2009) Amifostine induces antioxidant enzymatic activities in normal tissues and a transplantable tumor that can affect radiation response. Int J Radiat Oncol Biol Phys 73:886–896
Griem ML, Kleinerman RA, Boice JD Jr et al (1994) Cancer following radiotherapy for peptic ulcer. J Natl Cancer Inst 86:842–849
Hauer-Jensen M, Skjonsberg G, Moen E et al (1995) Intestinal morphology and cytokinetics in pancreatic insufficiency. An experimental study in the rat. Dig Dis Sci 40:2170–2176
Hellerstrom C, Andersson A, Gunnarsson R (1976) Regeneration of islet cells. Acta Endocrinol Suppl (Copenh) 205:145–160
Hori S, Yoshioka H, Tokunaga K et al (1982) CT detected radiation damage of the liver and pancreas. Nippon Igaku Hoshasen Gakkai Zasshi 42:985–987
Jobin C, Sartor RB (2000) The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol 278:C451–C462
Kovacs L (1976) Histologic studies on pancreas changes, caused by experimental fractionated local roentgen irradiation. Strahlentherapie 152:455–468
Kozak KR, Kachnic LA, Adams J et al (2007) Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys 68:1557–1566
Lambert C, Benk V, Freeman CR (1998) Pancreatic cancer as a second tumour following treatment of Hodgkin’s disease. Br J Radiol 71:229–232
Levy P, Menzelxhiu A, Paillot B et al (1993) Abdominal radiotherapy is a cause for chronic pancreatitis. Gastroenterology 105:905–909
Li Y, Taylor JM, Ten Haken RK et al (2007) The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 67:660–669
Liu HZ, Zhong JY (2004) Inhibitory effect of grape procyanidin on the cell apoptosis induced by radiation. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 22:448–451
Maes A, Weltens C, Flamen P et al (2002) Preservation of parotid function with uncomplicated conformal radiotherapy. Radiother Oncol 63:203–211
Marmiroli L, Salvi G, Caiazza A et al (2005) Dose and volume impact on radiation-induced xerostomia. Rays 30:145–148
McGrail LH, Sehn LH, Weiss RB et al (1999) Pancreatitis during therapy of acute myeloid leukemia: cytarabine related? Ann Oncol 10:1373–1376
Meacham LR, Sklar CA, Li S et al (2009) Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 169:1381–1388
Olgac V, Erbil Y, Barbaros U et al (2006) The efficacy of octreotide in pancreatic and intestinal changes: radiation-induced enteritis in animals. Dig Dis Sci 51:227–232
Pieroni PL, Rudick J, Adler M et al (1976) Effect of irradiation on the canine exocrine pancreas. Ann Surg 184:610–614
Roesink JM, Moerland MA, Battermann JJ et al (2001) Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys 51:938–946
Rubin P, Casarett GW (1968) Clinical radiation pathology, vols I and II. WB Saunders, Philadelphia
Ryschich E, Schmidt J, Loeffler T et al (2003) Different radiogenic effects on microcirculation in healthy pancreas and in pancreatic carcinoma of the rat. Ann Surg 237:515–521
Sarri Y, Conill C, Verger E et al (1991) Effects of single dose irradiation on pancreatic beta-cell function. Radiother Oncol 22:143–144
Scarpelli DG (1989) Toxicology of the pancreas. Toxicol Appl Pharmacol 101:543–554
Schmid RM, Adler G (2000) NF-kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology 118:1208–1228
Schmid SW, Uhl W, Steinle A et al (1996) Human pancreas-specific protein. A diagnostic and prognostic marker in acute pancreatitis and pancreas transplantation. Int J Pancreatol 19:165–170
Schoonbroodt D, Zipf A, Herrmann G et al (1996) Histological findings in chronic pancreatitis after abdominal radiotherapy. Pancreas 12:313–315
Shkumatov LM (2004) Insulin function in rats at early terms after 4 Gy whole-body irradiation. Radiats Biol Radioecol 44:32–37
Simone NL, Menard C, Soule BP et al (2008) Intrarectal amifostine during external beam radiation therapy for prostate cancer produces significant improvements in Quality of Life measured by EPIC score. Int J Radiat Oncol Biol Phys 70:90–95
Somosy Z, Takats A, Bognar G, et al (1996) X-irradiation-induced changes of the prelysosomal and lysosomal compartments and proteolysis in HT-29 cells. Scanning Microsc 10:1079–1090 (discussion 1090–1091)
Spalding AC, Jee KW, Vineberg K et al (2007) Potential for dose-escalation and reduction of risk in pancreatic cancer using IMRT optimization with lexicographic ordering and gEUD-based cost functions. Med Phys 34:521–529
Spaulding J, Lushbaugh C (1955) Radiopathology of islets of langerhans in rats. Fed Proc 14:420
Stocker E, Hauswaldt C, Klinge O (1965) On cellular amino acid incorporation in in- and excretory pancreas under experimental conditions. Experientia 21:512–513
Suit H, Goldberg S, Niemierko A et al (2007) Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat Res 167:12–42
Telbisz A, Kovacs AL, Somosy Z (2002) Influence of X-ray on the autophagic-lysosomal system in rat pancreatic acini. Micron 33:143–151
Tillman BN, Elbermani W (eds) (2007) Atlas of human anatomy, Clinical Edition. 1st edn. New York: Mud Puddle Books Inc
Travis LB, Curtis RE, Storm H et al (1997) Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst 89:1429–1439
Volk BW, Wellmann KF, Lewitan A (1966) The effect of irradiation on the fine structure and enzymes of the dog pancreas. I. Short-term studies. Am J Pathol 48:721–753
Volk BW, Wellmann KF, Lazarus SS (1967) Protection of canine pancreatic ultrastructure against radiation by pretreatment with alloxan. Am J Pathol 51:207–224
Wellmann KF, Volk BW, Lewitan A (1966) The effect of radiation on the fine structure and enzyme content of the dog pancreas. II. Long term studies. Lab Invest 15:100–123
Wellmann KF, Volk BW, Lazarus SS (1967) Protection of canine pancreatic ultrastructure against radiation damage by post-treatment with alloxan. Nature 216:86–87
Woodruff KH, Castro JR, Quivey JM et al (1984) Postmortem examination of 22 pancreatic carcinoma patients treated with helium ion irradiation. Cancer 53:420–425
Yamaguchi K, Nakamura K, Kimura M et al (2000) Intraoperative radiation enhances decline of pancreatic exocrine function after pancreatic head resection. Dig Dis Sci 45:1084–1090
Zook BC, Bradley EW, Casarett GW et al (1983) Pathologic effects of fractionated fast neutrons or photons on the pancreas, pylorus and duodenum of dogs. Int J Radiat Oncol Biol Phys 9:1493–1504
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Russo, S., Ove, R., Fajardo, L., Tepper, J. (2014). Adverse Late Effects of Radiation Treatment in the Pancreas. In: Rubin, P., Constine, L., Marks, L. (eds) ALERT • Adverse Late Effects of Cancer Treatment. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75863-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-540-75863-1_16
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-75862-4
Online ISBN: 978-3-540-75863-1
eBook Packages: MedicineMedicine (R0)