Abstract
Around the beginning of the nineteenth century, Unna isolated Corynebacterium acnes (now known as Propionibacterium acnes) from acne lesions in patients, establishing the link between acne and local P. acnes infection. However, later when P. acnes was also isolated from normal, healthy skin [1], its concept as a pathogen greatly declined. In 1963, Kirschbaum and Kligman [2] re-confirmed P. acnes as a factor involved in the complex pathogenesis of acne by showing that an injection of viable P. acnes into sterile steatocystomas (as a model for sterile acne comedones) could convert these quiescent cysts into inflammatory lesions. Since then research has revealed that P. acnes influences inflammation through a wide range of pathways, ranging from neutrophil chemotaxis by P. acnes lipase [3] to direct induction of Toll-like receptors in keratinocytes [4]. However, the question remains whether this commensal is capable of initiating inflammation in the sebaceous gland and if so, why colonization does not always result in inflammation. In other words, what triggers P. acnes to play its part in acne? The answer to this question may very well be found using the concept of microbial biofilms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Core Messages-
Biofilms are the natural form of microbial growth.
-
Biofilms have different properties than planktonic (free-floating) cells.
-
A biofilm consists of three essential components: the microbial cells, a surface onto which these cells adhere, and an extracellular polymeric matrix, in which cells are embedded and can form larger communities.
-
Biofilms are notoriously resistant to antimicrobial therapies.
-
Factors considered to be responsible for this increased resistance in biofilms include restricted penetration of antimicrobials, decreased growth rate, expression of resistance genes, and the presence of resistant “persister” cells.
-
Propionibacterium acnes can form a biofilm in acne, although it is difficult to demonstrate as it cannot be “explanted” and analyzed.
1 Propionibacterium acnes and Acne
Around the beginning of the nineteenth century, Unna isolated Corynebacterium acnes (now known as Propionibacterium acnes) from acne lesions in patients, establishing the link between acne and local P. acnes infection. However, later when P. acnes was also isolated from normal, healthy skin [1], its concept as a pathogen greatly declined. In 1963, Kirschbaum and Kligman [2] re-confirmed P. acnes as a factor involved in the complex pathogenesis of acne by showing that an injection of viable P. acnes into sterile steatocystomas (as a model for sterile acne comedones) could convert these quiescent cysts into inflammatory lesions. Since then research has revealed that P. acnes influences inflammation through a wide range of pathways, ranging from neutrophil chemotaxis by P. acnes lipase [3] to direct induction of Toll-like receptors in keratinocytes [4]. However, the question remains whether this commensal is capable of initiating inflammation in the sebaceous gland and if so, why colonization does not always result in inflammation. In other words, what triggers P. acnes to play its part in acne? The answer to this question may very well be found using the concept of microbial biofilms.
2 Biofilms
Costerton and Cheng first used the term “biofilm” in the 1970s [5]. Although since then the definition of a biofilm has been changed multiple times, there are three essential components: the microbial cells, a surface onto which these cells adhere, and an extracellular polymeric matrix, in which cells are embedded and can form larger communities [6]. Over the last years, biofilm research has expanded considerably and revealed that biofilms are probably the most common form of microbial growth in nature and that the planktonic (free-floating) phenotype of microorganisms, the original subject of microbiological research over the last 100 years, could be an in vitro artifact.
Dental plaque, one of the oldest known examples of a biofilm consists of a well-defined surface (dental enamel), a matrix of polysaccharides (mainly dextran), and microbial cells (e.g., Streptococcus mutans) [7]. However, not all biofilms fit the biofilm definition that easily. For “mucosal” biofilms in, e.g., cystic fibrosis lungs [8] and otitis media [9], the term “surface” has a wider interpretation. In these biofilms the thick mucous layer, which is essentially abiotic (i.e., nonliving material), provides anchorage for the microbial cells and acts as a surface for biofilm formation.
One of the most important properties of the microbial cells (sessile cells) in a biofilm is that they are phenotypically different from their planktonic counterparts [10]. Depending on the microenvironment, microorganisms can regulate the expression of certain genes, allowing them to adapt to changing conditions. Although alterations in gene expression patterns can influence a large number of phenotypical properties, the increased resistance toward antimicrobial agents is one of the most remarkable. Factors considered to be responsible for this increased resistance in biofilms include restricted penetration of antimicrobials, decreased growth rate, expression of resistance genes, and the presence of resistant “persister” cells [11, 12]. This increased resistance allows biofilms to survive in various environments (including the human host) and for infection to persist after treatment. Many chronic infections are now thought to be biofilm related, which would help explain their chronic nature. Antimicrobial treatment kills off a large number of the microbial cells, thereby reducing the symptoms. However, the biofilm, in total, may persist and regrow, and cause reoccurrence of the symptoms.
Another important aspect of biofilms is the ability of sessile cells to communicate with each other using various communication systems. This process, called quorum sensing, is cell-density dependent and allows bacteria to coordinate gene expression by producing signal molecules, i.e., quorum-sensing molecules [13]. By using quorum sensing, microorganisms increase their chances of successfully infecting their host by delaying the production of virulence factors until the population has reached a certain threshold density (quorum), high enough to overwhelm the immune system [14].
3 P. Acnes Biofilm
P. acnes is an aerotolerant anaerobic, gram positive, and relatively slow growing commensal of the human skin. It produces extracellular lipases that hydrolyze triglycerides in the sebum into glycerol and fatty acids. P. acnes can use this glycerol as energy source and the end product of the fermentation process is propionic acid (hence the name Propionibacterium). The free fatty acids are thought to play a role in the pathogenesis of acne, eliciting an inflammatory response [4].
The complete genome of P. acnes has been sequenced [15] and detailed analyses showed it contains several genes that may be relevant for biofilm formation. These genes include the glucosyltransferase (GTF) genes (Open reading frame (ORF) 125–134, 145–150, 1692–1700, 1185, 1791, 2181), responsible for the production of the extracellular polysaccharide matrix, genes for the production of adhesion proteins [16], and the LuxS homolog gene (ORF 405), responsible for the production of the quorum sensing molecule autoinducer-2 (AI-2) [17]. Other genes that encode enzymes for degrading skin and proteins, like hyaluronate lyase (ORF 380), endoglycoceramidases (ORF 644, ORF 2106), and sialidases (ORF 1560, ORF 1569) may contribute to the immunogenic properties of P. acnes.
Apart from this indirect genomic proof of P. acnes as a potential biofilm former, there are several reports of P. acnes biofilms in vivo. The first report of P. acnes biofilm formation was published over 20 years ago when Passerini et al. discovered this organism in biofilms from right heart flow-directed catheters [18]. Later studies have detected P. acnes biofilms on prosthetic hip implants [19], polymethylmetacrylate bone cement and different titanium alloys used in orthopedic materials [20], cerebrospinal shunts, surgical steel and silicone [21], prosthetic heart valves [22], and intraocular lenses [23]. Often the cells inside these biofilms produce an exopolymer similar in appearance to that of Staphylococcus epidermidis [21], a well-known biofilm former. Sessile P. acnes cells could also be implicated in endophthalmitis after cataract surgery [23] and in chronic prostatitis [24]. Qi et al. recently showed that P. acnes isolates causing fatal bacterial granuloma after trauma are also capable of biofilm formation [25].
4 The Acne Biofilm Fits in with the Clinical Picture of Acne
The ability of P. acnes isolates to form biofilms has already been extensively demonstrated. However, the question remains: does P. acnes form a biofilm in acne? Unfortunately, mucosal biofilms or biofilms without a clear-cut abiotic surface in general, as would be the case in acne, are difficult to demonstrate as they cannot be “explanted” and analyzed. Burkhart and Burkhart first suggested that P. acnes resides within the pilosebaceous unit as a biofilm [26], based on circumstantial evidence. They surmised that the failure of antibiotic treatment in acne vulgaris could well be caused by the high resistance of sessile P. acnes cells. This high resistance to many commercially available antibiotics (including clindamycin and penicillin) and disinfectants (including benzoylperoxide) was indeed demonstrated in several in vitro studies [17, 20, 27].
Coenye et al. also demonstrated that P. acnes biofilms produce significantly more lipase than their planktonic counterparts and that this was most pronounced in isolates from acne patients [17]. Lipase is a known virulence factor of P. acnes [3] and inhibition of lipase activity has been suggested as a possible treatment of acne [28]. Farrar et al. showed that P. acnes in stationary phase induces higher levels of cytokines in keratinocytes than P. acnes in exponential phase [29]. This is in line with the biofilm theory, as sessile cells are more comparable to stationary phase cells than exponential phase cells.
It was also shown that cells in young and mature P. acnes biofilms produce more of the quorum sensing molecule AI-2 than planktonic cells [17]. AI-2 is a boronated signaling molecule used by bacteria to coordinate gene expression and it is thought to be important in the regulation of virulence of biofilms [30].
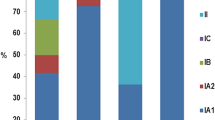
The difference between the occurrence of P. acnes as a skin commensal in healthy hosts or as a pathogen in acne lesions could be related to phenotypical differences associated with biofilm formation (see Fig. 19.1). These phenotypical switches do not occur until a certain “quorum” of microbial cells is reached. It could be possible that in healthy pilosebaceous units, P. acnes has not reached the required “acne-quorum.” As to why in certain lesions P. acnes reaches the “acne-quorum” is at present unclear.
Conclusions
-
Biofilms are ubiquitous and notoriously difficult to eradicate.
-
Biofilms are associated with chronic infections.
-
P. acnes can form biofilms.
-
Acne conditions may favor P. acnes biofilm formation.
-
P. acnes biofilm fits in with the clinical picture of acne.
-
An “acne-quorum” could explain why P. acnes occurs in both healthy hosts and acne lesions.
References
Lovejoy ED, Hastings TW. Isolation and growth of the Acne Bacillus. J Cutan Dis. 1911;80–80.
Kirschbaum JO, Kligman AM. The pathogenic role of Corynebacterium acnes in acne. Arch Dermatol. 1963;88:832–3.
Lee WL, Shalita AR, Suntharalingam K, Fikrig SM. Neutrophil chemotaxis by Propionibacterium acnes lipase and its inhibition. Infect Immun. 1982;35:71–8.
Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, Dreno B. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–13.
Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238:86–95.
Davey ME, O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–67.
Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93.
May TB, Shinabarger D, Maharaj R, Kato J, Chu L, Devault JD, Roychoudhury S, Zielinski NA, Berry A, Rothmel RK, Misra TK, Chakrabarty AM. Alginate synthesis by Pseudomonas aeruginosa – a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991;4:191–206.
Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–94.
Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209.
Keren I, Kaldalu N, Spoering A, Wang YP, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13–8.
Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007.
Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–99.
Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46.
Bruggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, Hujer S, Durre P, Gottschalk G. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–3.
Burkhart CN, Burkhart CG. Genome sequence of Propionibacterium acnes reveals immunogenic and surface-associated genes confirming existence of the acne biofilm. Int J Dermatol. 2006;45:872.
Coenye T, Peeters E, Nelis HJ. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol. 2007;158(4):386–92.
Passerini L, Phang PT, Jackson FL, Lam K, Costerton JW, King EG. Biofilms on right heart flow-directed catheters. Chest. 1987;92:440–6.
Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37:3281–90.
Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221–7.
Bayston R, Ashraf W, Barker-Davies R, Tucker E, Clement R, Clayton J, Freeman BJC, Nuradeen B. Biofilm formation by Propionibacterium acnes on biomaterials in vitro and in vivo: impact on diagnosis and treatment. J Biomed Mater Res A. 2007;81A:705–9.
Guio L, Sarria C, Sala M, Sanchez Madrid F, McDowell A, Las Cuevas C, Gamallo C, Duarte J. Demonstration of biofilm in vitro propionibacterium acnes prosthetic valve endocarditis. Clin Res Cardiol. 2007;96:446–7.
Lai JY, Chen KH, Lin YC, Hsu WM, Lee SM. Propionibacterium acnes DNA from an explanted intraocular lens detected by polymerase chain reaction in a case of chronic pseudophakic endophthalmitis. J Cataract Refract Surg. 2006;32:522–5.
Alexeyev OA, Marklund I, Shannon B, Golovleva I, Olsson J, Andersson C, Eriksson I, Cohen R, Elgh F. Direct visualization of Propionibacterium acnes in prostate tissue by multicolor fluorescent in situ hybridization assay. J Clin Microbiol. 2007;45:3721–8.
Qi X, Gao J, Sun D, Liang W, Wan Y, Li C, Xu X, Gao T. Biofilm formation of the pathogens of fatal bacterial granuloma after trauma: potential mechanism underlying the failure of traditional antibiotic treatments. Scand J Infect Dis. 2008;40:221–8.
Burkhart CN, Burkhart CG. Microbiology’s principle of biofilms as a major factor in the pathogenesis of acne vulgaris. Int J Dermatol. 2003;42:925–7.
Bayston R, Nuradeen B, Ashraf W, Freeman BJC. Antibiotics for the eradication of Propionibacterium acnes biofilms in surgical infection. J Antimicrob Chemother. 2007;60:1298–301.
Higaki S. Lipase inhibitors for the treatment of acne. J Mol Catal B Enzym. 2003;22:377–84.
Farrar MD, Ingham E. Acne: inflammation. Clin Dermatol. 2004;22:380–4.
Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002;99:3129–34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Honraet, K., Rossel, B., Coenye, T. (2014). The Acne Biofilm. In: Zouboulis, C., Katsambas, A., Kligman, A. (eds) Pathogenesis and Treatment of Acne and Rosacea. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-69375-8_19
Download citation
DOI: https://doi.org/10.1007/978-3-540-69375-8_19
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-69374-1
Online ISBN: 978-3-540-69375-8
eBook Packages: MedicineMedicine (R0)