Abstract
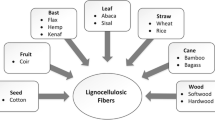
Cellulose is an abundant renewable resource which can be used for a number of applications. It is mainly found in plant cell wall and plays a vital role in maintaining its structure. Besides plants, cellulose is also found in fungi, bacteria, and some tunicates (George and Sabapathi 2015). Cellulose is a fibrous and linear natural polymer consisting of glucose units bound by β-1,4-linkages with a degree of polymerization ranging from 10,000 to 15,000 units depending on the type of biomass (Sjostrom 1993). For more than a century, several applications of cellulose in food, pharmaceuticals, polymer, pulp, and composite industries have been studied (Coffey et al. 1995; de Souza Lima et al. 2003). Agricultural waste such as rice straw, sugarcane bagasse, sawdust, cotton stables, and woody forest residues are the main source of cellulose for various applications in industries. Production of Nano-Crystalline Cellulose (NCC) is one of the products being evaluated for a number of applications in recent years. NCC produced from cellulose fibers are regarded as nanobiomaterial with variety of applications in chemicals, food, pharmaceuticals, etc. (Habibi et al. 2010).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanocrystalline
- Cellulose
- Pretreatment
- Ultrasonication
- Nanocomposite
- Drug delivery
- Enzyme immobilization
- Antimicrobial
17.1 Introduction
Cellulose is an abundant renewable resource which can be used for a number of applications. It is mainly found in plant cell wall and plays a vital role in maintaining its structure. Besides plants, cellulose is also found in fungi, bacteria, and some tunicates (George and Sabapathi 2015). Cellulose is a fibrous and linear natural polymer consisting of glucose units bound by β-1,4-linkages with a degree of polymerization ranging from 10,000 to 15,000 units depending on the type of biomass (Sjostrom 1993). For more than a century, several applications of cellulose in food, pharmaceuticals, polymer, pulp, and composite industries have been studied (Coffey et al. 1995; de Souza Lima et al. 2003). Agricultural waste such as rice straw, sugarcane bagasse, sawdust, cotton stables, and woody forest residues are the main source of cellulose for various applications in industries. Production of Nano-Crystalline Cellulose (NCC) is one of the products being evaluated for a number of applications in recent years. NCC produced from cellulose fibers are regarded as nanobiomaterial with variety of applications in chemicals, food, pharmaceuticals, etc. (Habibi et al. 2010).
NCC is produced by breaking the natural polymer of cellulose and separating the crystalline section. The general steps involved in the production of NCC from various sources of biomass were illustrated in Fig. 17.1. Nanocrystalline celluloses are typically 5–70 nm in width and 100 nm to several micrometers in length. NCCs (referred to as whiskers) can be classified based on their dimensions, functions, and preparation methods. NCC has many useful characteristics compared to cellulose such as its physicochemical properties including high surface area, specific strength, and optical properties (Peng et al. 2011). Various studies in literature have reported the chemical structure, physical and mechanical properties of NCC (de Souza Lima and Borsali 2004; Peng et al. 2011; Huq et al. 2012). The biocompatible and biodegradable nature of NCC makes it ideal for several applications and is thus the focus of considerable research. Developing commercial scale methods to produce nanocrystalline cellulose from the forest-based biomass can certainly contribute to the advancement of biobased industries around the world. In this chapter, we have highlighted the important production methods, modification, applications of NCCs, and the economics of its production.
17.2 Production of Nano-Crystalline Cellulose (NCC)
Traditionally, NCC can be isolated from any cellulose obtained from plants, animals, bacteria, and algae. However, due to high abundance of cellulose in wood and agricultural residues , these biomass sources have become major substrates for the extraction of NCC. Wood-based NCC extraction has also gained importance as these fibers are thinner than the bacterial cellulose (George and Sabapathi 2015). Abraham et al. (2011) have reported that pineapple leaf fiber produce fine NCC than banana and jute (Abraham et al. 2011). However, jute fiber is one of the cheapest materials to make the nanocellulose production cost-effective. Fortunati et al. (2012) have investigated the extraction of micro- and nanocellulose from okra fibers. Nonetheless, the production process of NCC is similar for all cellulosic materials (Fortunati et al. 2012). There are two stages involved in the isolation of NCC for any type of biomass.
First, the raw material is pretreated to separate cellulose from other constituents (lignin and hemicellulose of lignocellulose biomass). Second, the cellulose is treated to break the amorphous regions of the long glycosidic chain polymer. This is usually done by mechanical, chemical, and enzyme methods. Ranby (1951) reported the synthesis of cellulosic nanofibers for the first time using sulfuric acid. When cellulosic material is subjected to either mechanical or chemical treatments, the amorphous regions disintegrate and leave the crystalline regions intact in the form of short crystals. A number of industries are focusing on commercialization of different forms of NCC by evaluating various methods for economically feasible production (Antonio 2014).
17.3 Pretreatment of Biomass
There are different types of pretreatment techniques reported in literature that are used to separate biomass into two major streams, a solid stream containing cellulose and lignin and a liquid stream containing majorly hemicellulose (Dalli and Rakshit 2015). The pretreatment techniques can be categorized into thermal, chemical, or physical. In most cases, the pretreatment has to be optimized and controlled to avoid unwanted by-products. Some of the effective and most studied pretreatment techniques are reported here.
17.3.1 Hydrothermal Pretreatment
This is a widely used method to separate water soluble and insoluble polymers from the biomass (Ma et al. 2014). As the name indicates, water and heat are involved in this process. Mineral acids such as sulfuric acid are used to enhance the efficiency of the process. Though this process is effective using mineral acids like H2SO4, HCl, and H3PO4 (Hendriks and Zeeman 2009), waste disposal is a problem as the acid waste stream can cause environmental pollution. In order to avoid acid treatment, the process can be carried out under pressure (Saha et al. 2013). Hydrolysis does not occur below 100 °C (Abatzoglou et al. 1992). Autohydrolysis, steam explosion, steam extrusion are some of the examples of hydrothermal treatment (Dalli and Rakshit 2015) which are considered to be useful depending on the type of biomass used.
17.3.2 Alkaline Hydrolysis
In the alkaline pretreatment process, biomass is treated with aqueous ammonia, sodium carbonate and hydroxides of sodium, calcium or potassium. Generally, lime and sodium hydroxide are used to hydrolyze the biomass at moderate temperature (Park and Kim 2012). Other types of alkaline treatments include the Ammonia Fiber Explosion (AFEX) and the Ammonia Recycling Percolation (ARP) . Alkaline peroxide is another type of alkaline pretreatment where oxygen or hydrogen peroxide (1–3%) is added to biomass and catalyzed by the addition of lime or NaOH (Carvalheiro et al. 2008). These processes give better results by removing the lignin from the polysaccharides. In the AFEX process, liquid ammonia is added to biomass at a moderate temperature ranging from 40 to 140 °C and under high pressure (250–300 psi) and the reaction is carried out for a shorter time (Teymouri et al. 2004; Keshwani and Cheng 2009). In the ARP process, ammonia is circulated through the biomass in a column reactor. This is more effective with hardwoods and corn stovers than in softwoods and results in high delignification and moderate hemicellulose solubilization of approximately 40–60% (Carvalheiro et al. 2008). In cases where most of the lignin and hemicellulose are separated, cellulose becomes the major component in the solid fraction.
17.3.3 Organosolvent Pretreatment
In this process, organic solvents (e.g. methanol, ethanol, ethylene glycol etc.) with or without acid catalysts (HCl, H2SO4) are used to extract most of the lignin from biomass. Organic solvents dissolve lignin in the presence of acid catalyst and some of the hemicellulosic sugar (Lee et al. 2014). If this pretreatment process is performed under high temperatures (185–210 °C), addition of acids is not necessary because deacetylation from the sugars make the medium acidic . However, when the acids are added externally, this process is more effective in solubilizing lignin and hemicellulose leaving solid cellulose residue, (Zhao et al. 2009). According to Lee et al. (2014), hydroxyl ions from the alcoholic solvents break the bonds of phenolic and polysaccharide linkages in lignin and hemicellulose to dissolve them. This process is has its benefits as it requires low energy to recover the components (Lee et al. 2014). However, it was also observed that organic solvents swell the cellulose fibers and reduce their crystallinity (McDonough 1993). Another demerit of this process is the formation of clumps of lignin while washing the pretreated biomass with water. Therefore, recovery of cellulose often becomes cumbersome and costly process.
17.3.4 Pretreatment Using Ionic Liquids
Pretreatment of lignocellulosics using ionic liquids is a relatively recent technique developed for isolating the cellulosic components from the lignocellulose matrix. Ionic liquids target the β-glycosidic bonds on cellulose polymer and make it soluble by hydrolyzing the linkages (Xiong et al. 2014). However, it is possible to control the concentration of ionic liquids to make cellulose and hemicellulose dissolve without disturbing the chain’s structure (Lee et al. 2014). Ionic liquids are generally composed of an inorganic anion and an organic cation, which can be modified depending on the target compounds. Major benefit involved in this process is the recyclability of ionic liquids (Lee et al. 2014). Some examples of ionic liquids reported in literature are 1-alkyl-3-methylimidazolium [mim]+; 1-alkyl-2,3-dimethylimidazolium [mmim]+; 1-allyl-3-methylimidazolium [Amim]+; 1-allyl-2,3-dimethylimidazolium [Ammim]+; 1-butyl-3-methylpyridinium [C4mPy]+; and tetrabutylphosphonium [Bu4P]+ with n = number of carbons in the alkyl chain (Zavrel et al. 2009; Tadesse and Luque 2011; Lee et al. 2014). Ideally, ionic liquid should possess high dissolution capacity, low melting point, low viscosity, low toxicity and high stability. However, the drawback of using ionic liquids is the cellulose crystallinity would be reduced and become more amorphous. This is because of the tendency of ionic liquids to hydrolyze the hydrogen bonds in the cellulose polymer resulting in bond breaking and subsequent dissolution (Lee et al. 2014). The high costs of the ionic liquids are also an important factor that needs to be taken into account.
17.4 Isolation of Nanocrystalline Cellulose
Though the pretreatment techniques do not produce pure cellulose, most of the lignin and hemicellulose content need to be removed. The solid fraction obtained from the above pretreatment processes contains cellulose and some lignin. This material is then further treated to produce NCC. The purity , physical and mechanical properties of the product depend on the type of method used for the production. Some of the reported methods in literature are mentioned in Table 17.1 and described in detail as follows.
17.4.1 Mechanical Processes
The mechanical process used for nanocrystalline cellulose production from the pretreated cellulose include milling, grinding, cutting, high pressure homogenization (steam explosion), ultrosonication, microfluidization, cryocrushing, etc.(Ng et al. 2015). The shear forces applied in mechanical treatment make the cellulose disintegrate and help in extracting the crystalline cellulose micrfibrils in the form of a uniform powder (de Souza Lima et al. 2003). Common treatments like milling , cutting or grinding are done in Wiley mill or Fritish Pulverisette mills or grinding machines (Ng et al. 2015). Ribbon-like cellulose nanocrystals are usually obtained in this process. These fibers are then sieved in a vibratory sieve to separate fine particulate fibers. The pore size of the mesh used in the vibratory sieves is usually in the range of 50–250 μm. The smaller the size of the fine fibers, higher the activity in subsequent chemical treatments due to the higher availability of the active groups of cellulose to react with the chemical reagents (Ng et al. 2015). The finely ground fibers are washed with water to remove impurities and to make the fibers softer (Frone et al. 2011; Marimuthu and Atmakuru 2015; Ng et al. 2015). Rosa et al. (2012) suggested dewaxing the fibers in a solvent mixture of toluene/ethanol using a soxhlet type extraction (Rosa et al. 2012).
The other components of the lignocellulosic material, hemicellulose and lignin, often make the NCC fibers impure and reduce its crystallinity. Therefore, it is necessary to separate these materials from cellulose fibers. Commonly used techniques for purification include alkali bleaching treatment or mercerization using sodium hydroxide or potassium hydroxide, sodium chlorite, and acetic acid (Ng et al. 2015). Alkali solutions potentially dissolve the other components except cellulose fibers which can be easily filtered out (Acharya et al. 2011; Faruk et al. 2012). For most applications, the fiber concentration should be limited to the optimal range of 4–6% (w/w) during the alkali treatment because the low fiber to alkali ratio lead to chemical degradation, whereas high fiber to alkali ratio might result in inefficient modification by reducing the active sites of reaction (Ng et al. 2015).
17.4.2 Ultrasonication
It has been reported that ultrasonication processes enhance the efficiency of biomass acid hydrolysis (Brinchi et al. 2013). Production of NCC using ultrasonication in water or an organic acid has been reported in the literature (Filson et al. 2009). Although low yields were obtained, it has been proven that ultrasonication helps to increase the NCC yields. Ultrasonication is an advanced technique to isolate micro- and nanocellulose from lignocellulosic material. Ultrasonication associated grinding and homogenization is an effective and efficient method to produce NCC in large volumes (Hielscher Ultrasonics GMBH 2017). It was reported that oxidation of cotton linter pulp using 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) -NaBr-NaClO assisted with ultrasonication led to the production of carboxylated NCC (Qin et al. 2011). Leung et al. (2011) suggested a simple procedure to produce carboxylated NCC using ammonium persulfate instead of TEMPO, at 60 °C (Leung et al. 2011). This method showed relatively high yields of NCC and has the potential for scale up (Brinchi et al. 2013). However, this type of oxidation process is not efficient for the production of pure NCC. Besides producing low yields, use of TEMPO is relatively expensive and toxic to dispose. An advantage in this process is that the raw material can be used directly without any pretreatment steps for the isolation of cellulose.
17.4.3 Chemical Treatment
In the chemical treatment of lignocellulosics for NCC production, acid hydrolysis of cellulose is considered as an efficient method as it consumes less energy and produce rod-like crystal structures of cellulose nanofibers (Sacui et al. 2014). The strong acids easily disintegrate amorphous regions of the cellulose fibers, leaving the crystalline regions unaffected. However, boiling with strong sulfuric acid has its affect only up to a certain period, beyond which it slowly reduces the degree of polymerization of the polymer (George and Sabapathi 2015). The threshold level-off the degree of polymerization (LODP) varies with the type of biomass used to produce NCC. According to George and Sabapathi (2015), crystalline nanocellulose obtained from acid hydrolysis exhibit high polydispersity in molecular weight. This was assumed to be due to the lack of regular distribution of amorphous regions. Several other factors such as temperature, reaction time etc., also affect the quality of NCCs. In case of shorter reaction time is used, amorphous regions of the cellulose are retained in the solution and reduce the quality of the NCC. On the other hand, longer reaction time leads to depolymerization of cellulose crystals and reduces the aspect ratio of nanocrystals. The treatment of biomass at high temperature results in short chains of nanocellulose (Elazzouzi-Hafraoui et al. 2008). Bai et al. (2009) reported the synthesis of NCC whiskers from microcrystalline cellulose (MCC) using sulfuric acid. They have reported that the relative centrifugal force (RCF) also affects the length of NCCs (Bai et al. 2009).
Use of other acids like HCl (Börjesson and Westman 2015), HBr, H3PO4 (Lee et al. 2009) to isolate NCCs from the cellulosic material have been reported in literature. A mixture of acetic acid and nitric acid was used to enhance the production of nanocellulose by Zhang et al. (2014). Aqueous solution of NCCs was synthesized using HCl by grafting technique. It has been reported that grafting on nanocrystals using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) in a procedure known as oxidative carboxylation-amidation prevents the particle aggregation and induces the stability of the colloidal NCC solution.
17.4.4 Enzymatic Treatment
Cellulose treated with enzymes along with mechanical shearing and high pressure homogenization is a relatively new method being developed for NCCs production. Commercial cellulase enzyme is used in these methods to obtain NCCs with better physical properties. Enzymatic treatment is considered better than acid hydrolysis as less waste is produced after the process and its ecofriendly nature (George et al. 2011). Pääkkö et al. (2007) have developed a facile method for the usage of enzymes along with mechanical shearing. They reported that the incorporation of enzymatic hydrolysis reduced the energy consumption and has a drastic effect on the mechanical properties of the NCCs produced. Usually, the synergistic action of endoglucanases and exoglucanases or cellobiohydrolases on biomass leads to disintegration of cellulose at the solid-liquid interface (Lee et al. 2014). This results in a shorter chain length of nanocellulose fibers. Although, the enzymatic treatment provides high yields with high selectivity, the limiting factor is the cost of the enzymes. Cellulose degradation using these enzymes take longer time durations to produce the desired high yields of NCC. The slow rate of enzyme hydrolysis also eventually increases the cost of production.
17.5 Chemical Modifications of NCC
One of the important applications of NCC is its use in engineering products as composite material for reinforcement. The chemical modification of nanocellulose is very helpful in improving the physical properties of polymer matrix in biocomposites (Börjesson and Westman 2015). Some of the chemical modification methods along with the functional agents used are listed in the Table 17.2.
Strengthening of nanocellulose-reinforced composites can be done by modifying the NCCs using peroxide. The hydroxyl groups on glucose molecules in a nanocellulose chain are of great interest to synthetic chemists as they can be modified modify to produce NCCs with different properties. Usually, chemical modification is done to improve the interface between two incompatible phases (Ng et al. 2015). Various compatibilizing agents such as maleic acid (Majeed et al. 2013), lignin, polyvinyl alcohol, polyvinyl acetate have been studied in literature (Ng et al. 2015) to strengthen the polymer containing NCCs. Lignin treatment includes the coating of nanocellulose with kraft lignin which exhibited the compatibility with both hydrophobic and hydrophilic matrices in a composite (Alemdar and Sain 2008). The use of these agents can enhance the interfacial adhesion between nanocrystalline cellulose and the polymer matrix.
Chemical modifications on the surface of NCC can be done by the formation of covalent bonds with the hydroxyl groups on glucose molecules. Several coupling agents have been studied to induce the covalent bonding in the nanocellulose and composite matrix (Majeed et al. 2013). Esterification is beneficial as it requires no solvent and is highly efficient in the conversion of hydroxyl groups and does not affect the crystalline structure of nanocellulose (Ng et al. 2015). The functional properties of the surface of nanocellulose can be altered by esterification. The polar hydroxyl groups are replaced by non-polar carbonyl groups (Panaitescu et al. 2013) which results in altered characteristics.
Acetylation of nanocellulose also one of the commonly employed method to modify the properties of NCC. Acetic acid, propionic acid, alkenyl succinic anhydride (ASA), acetic anhydride and acetyl chloride have been reported as major carriers of acetyl groups which can be easily transferred on to the nanocellulose surface (Majeed et al. 2013; Panaitescu et al. 2013). Dufresne (2010) has reported the studies on a novel modification of nanocellulose by transforming it into a long aliphatic chain. Two approaches such as “grafting-onto” and grafting-from” were used to graft the polymer onto the surface of NCC. In both the approaches, the nanocellulose was suspended in the solvent during grafting (Rebouillat and Pla 2013). In the “grafting-onto” approach, a previously synthesized polymer is directly attached to the available hydroxyl groups on the NCC chain using a coupling agent (e.g. isocyanate or peptide) (Ng et al. 2015). The polymers involved in this type of modification are poly-caprolactone, polyurethane, thermos-responsive polymers etc. (Rebouillat and Pla 2013). The advantage of such methods is that the polymers can be fully characterized before attaching to the NCC chain. However, the reaction time was found to be too long for such modification due to the steric hindrance from the polymer molecules (Rebouillat and Pla 2013).
On the other hand, “grafting-from” is useful as it helps in obtaining long chains of polymers. Unlike grafting-onto, the polymers are built on the surface of the NCC using immobilized initiators via the atom transfer radical polymerization (ATRP) (Rebouillat and Pla 2013). The major bottleneck of this method is that the polymer cannot be characterized before the grafting method. However, the useful factors in this type of surface grafting are the high reaction rates, production of no by-products and high chemical stability (Espino-Perez et al. 2013).
Extensive research is taking place to alter the functional properties of the nanocellulose by reacting it with various reagents having derivative functional groups. Recent reports in literature indicate rapid advancement in improving binding ability and hydrophilicity of NCC (Ng et al. 2015). However, some of the upstream processes for the production of NCCs consume high amount of energy. Some of the recent studies on the energy consumption are discussed below.
17.6 Energy Consumption in Preparing NCC
The energy consumption for NCC production depends mainly on the source of cellulosic fibers and the isolation processes. Different mechanical methods are used to isolate the nanofibrillated cellulose including high pressure homogenization, microfluidization , grinding, cryocrushing and high intensity ultrasonication (Khalil et al. 2014). However, one of the main challenges of all these approaches in nanocellulose production and application results in high energy consumption. Increasing applications of nanocellulose in various field including biomedical uses, food packaging, coating and etc. (George and Sabapathi 2015) requires energy-efficient approaches its production in an industrial scale. There are only a few studies available an energy consumption for a specific pretreatment or property. Further investigation on nanocellulose preparation, modification, production and developing economical approaches are necessary before it can be applied successfully in a large scale.
Production of microfibrillated cellulose is one of the main steps in the NCC isolation. It was reported that, over 25,000 kWh per ton is required for the production of microfibrillated cellulose (MFC) as a result of the multiple passes through the homogenizers (Klemm et al. 2011).
Pretreatments are reported to reduce the energy consumption from 20,000 to 30,000 kWh/ton to 1000 kWh/ton for cellulosic fibers, while improving the production conditions and fiber swelling properties (Zhu et al. 2011). Mechanical and chemical pretreatments are regarded as effective approaches which significantly reduce energy consumption (Stelte and Sanadi 2009). As an example, TEMPO-mediated oxidation of cellulose fibers achieved some level of success in efficiently producing NFC (Saito et al. 2007). Further, it is reported that enzymatic treatment (Henriksson et al. 2007; Janardhnan and Sain 2007) of cellulose prior to the defibrillation facilitate disintegration requires lower energy for producing MFC. Ankerfors (2012) studied three alternative processes for producing microfibrillated cellulose in which pulp fibers where first pretreated and then homogenized. In the first process sulfite pulp were put through two refining steps and an enzymatic pretreatment. Then high pressure (1600 bar) homogenization with 8 passes was used. 33 and 90 kWh/ton pulp was measured for the first and second refining stage and the total energy required was calculated to be 2344 kWh/ton. The paper claimed 91% reduction in energy use, as the energy consumption reported earlier was 27,000 kWh/ton without pretreatments (Klemm et al. 2011). In the second process, chemical pretreatment such carboxymethylation was applied prior to high pressure (1650 bar) homogenizing. Mechanical energy consumption was calculated to be 2221 kWh/ton in this way. The third process associated with combined enzymatic and mechanical pretreatment to facilitate disintegration. Approximately the same amount of energy as in the second process was calculated. It was reported that reduction of required energy to 500 and 1500 kWh/ton for the second and the third process respectively can be obtained by optimization of the parameters of reaction and treatment such as concentration and pressure. In terms of low energy consumption, it is reported that these three processes have the potential for industrial scale production.
An overall assumption is that MFC can be produced with an energy consumption of 500–2300 kWh/ton by these methods. Also, the characterizations of the produced materials have been investigated in different ways and it has been demonstrated that the produced MFC fibrils were approximately 5–30 nm wide and up to several microns long. The number of homogenization steps does matter as well. Even though an increased number of steps are found to improve the quality of the product, each homogenization step costs 2200 kWh/ton. Therefore, the nanocellulose isolation process conditions should be taking into this account (Ankerfors 2012).
Another study by Spence et al. (2011), studied the effect of processing on microfibril and film properties, relative to energy consumption on bleached and unbleached hardwood pulp samples by homogenization, microfluidization , and micro-grinding. Film densities of samples in all three approaches were reported approximately to be 900 kg/m3. However, higher toughness values were reported for microfluidization and micro-grinding with less required energy compare to homogenization.
In fibers processing energy consumption per pass was 3940 kJ/kg by homogenizer and the 620 kJ/kg by micro-grinder. The microfluidizer energy measurements was 200 kJ/kg with operating pressure of 69 MPa, 390 kJ/kg under 138 MPa pressure, and 630 kJ/kg with 207 MPa.
Energy is highly influenced by parameters such as number of passes and flow rate in each processing methods. Homogenization, for instance, had a much slower processing flow rate than the microfluidizer and the micro-grinder, which notably increased the amount of required energy. The processing rates of the suspension were 2 kg/min for micro-grinding, 1 kg/min for microfluidization, and 0.2 kg/min for homogenization. Higher pressures of the microfluidizer also raise the energy required. Generally, the total energy of approximate 9180 kJ/kg for the microfluidizer with pretreatment, 9090 kJ/kg for the grinder with pretreatment, and 5580 kJ/kg for the grinder without pretreatment and 31,520 kJ/kg for the homogenizer is required in processing of MFC films with maximum accessible properties (Table 17.3).
Studies have showed marked increase in tensile index of the MFCs from bleached hardwood fiber after microfluidization and micro-grinding, while homogenization was not considered as an energy-efficient process. However, processing of the unbleached hardwood fibers was more efficient. With overall lower energy consumption, microfluidizer process leads to the highest tensile index (Spence et al. 2011).
From a review of literature, it seems that the following assumptions can be useful for optimizing the processes. Firstly, in microfluidization , even though the increased pressure leads to significant increase in the energy consumption, toughness and tensile index decreases as a result of the microfibrils damage at such high temperature. Secondly, increasing the number of passes would increase the energy consumption, with only a small improvement in properties. It is recommended that 5 steps should be the maximum number of passes in microfluidizing. In terms of tensile strength and toughness, maximum 8 passes are suggested in homogenization including pretreatment (Shahbaz and Lean 2012).
Furthermore, similarity of tensile properties of pretreated and non-pretreated samples shows that no pretreatment is required for micro-grinder processing which leads to great reduction in energy consumption. In general, among the three methods including homogenizer, microfluidization and micro-grinding, nano or micro crystalline cellulose with superior mechanical, optical and physical properties and less energy consumption are produced through microfluidization with a refining pretreatment and the micro-grinding of wood fibers. Finally, unless energy consumption is reduced sufficiently, NCC use at an industrial scale will be limited to some high value applications .
17.7 Applications of Nanocrystalline Cellulose
Celluloses are biocompatible, nontoxic and stable in nature. Even though nanocrystalline cellulose (NCC) are nanometers in dimension, the innate properties of cellulose are retained. Besides the cellulosic characteristics, they possess some unique optical and mechanical properties. Furthermore, nanocrystalline cellulose possess high surface area, better rheological properties, crystallinity, alignment and orientation, etc. Due to these characteristics nanocrystalline cellulose has potential applications in different sectors such as health care, food and beverages, cosmetics, electronics, etc. Its use as a reinforcing agent for nanocomposite materials (Lin and Dufresne 2014) and in the areas of nanomedicine (Dufresne 2013) has been extensively studied.
17.7.1 Use of Nanocomposite Films Production
One of the major applications of NCC is its use as a reinforcing material (or as a filler) during the production of nanocomposite materials such as nanocomposite films. Polymer nanocomposites are multiphasic material made up of polymer and nanomaterials (Jeon and Baek 2010; George and Sabapathi 2015). Because of their nanometric size and large surface area (due to smaller size of the reinforcing materials), they exhibit unique properties. Nanocomposite films obtained using NCC possess characteristics such as low permeability to moisture, gases, aroma, and oil. Due to this, they are commonly used for packaging in food and biomedical field (George and Sabapathi 2015). The properties of nanocomposite films mainly depend on two factors (1) morphology and dimensions of NCC and polymeric matrix and (2) processing method applied. Geometric aspect ratio is one of the factors that dictates the mechanical properties of nanocomposites (Peng et al. 2011). It is defined as the length to diameter (L/d) of the fillers used. Aspect ratio in turn depends on the types of cellulose fibers used and the production conditions involved. Since NCCs have high aspect ratio, they are considered to provide best reinforcing effects (Peng et al. 2011; Dufresne 2013; George and Sabapathi 2015). Good dispersibility of nanocrystals in the polymer matrices are required to enhance the mechanical properties of nanocomposite material. Since, NCCs disperse well on hydrophilic system, they are best suited for water dispersible polymers such as latexes (Hubbe et al. 2008). However, NCCs can also be modified to improve their dispersibility in hydrophobic systems (George and Sabapathi 2015). Thus, some of the commonly used processing methods to produce nanocomposite films includes casting evaporation, electrospinning, extrusion, and impregnation, monolayer films, and multilayer films (Peng et al. 2011).
17.7.2 Biomedical Applications
Due to the safe and natural form of nanocrystalline cellulose, it is gaining increasing attention in biomedical applications. Different scientists have explored its use for the production of biomedical materials suitable for practical clinical applications (Lin and Dufresne 2014). Some of the possible uses of NCCs in biomedical applications have been discussed below.
17.7.2.1 Carrier for Drug Delivery
Toxicity assessment of NCC on human cell lines , insect cells, and aquatic species has been done by various authors (Roman et al. 2009; Kovacs et al. 2010; Male et al. 2012). Most of the results obtained in these studies showed that NCCs were nontoxic to all the samples studied. This makes NCC a potential carrier in targeted drug delivery systems. On the one hand, use of NCC as an excipient in pharmaceutical industries holds considerable potential due to reduced size, hydrophilic nature, and biocompatibility. On the other, with the use of NCC as a carrier for drug delivery, optimal control of dosing can be obtained and large amounts of drugs can be bound to its surface. This is due to its large surface area and possibility of acquiring negative charge during hydrolysis. Surface modification of NCC can also be done to bind nonionized and hydrophobic drugs which normally do not bind to cellulose and its derivatives (George and Sabapathi 2015; Taheri and Mohammadi 2015).
Common forms of nanocellulose-based drug carriers include microspheres (or microparticles), hydrogels (or gels), and membranes (or films) (Lin and Dufresne 2014). Shi et al. (2003) examined the morphology of drug nanoparticles coated onto the cellulosic beads. Similarly, incorporation of the NCC particles into hydrogels has the potential of using such nanocomposite hydrogels as a controlled drug delivery vehicle (Zhang et al. 2010). Some studies carried out by (Clift et al. 2011) have shown that cellulose nanocrystals may slightly induce some dose-dependent cytotoxic and inflammatory effects on human lung cells. Thus, further studies on risk assessment of NCC however should be done before its application in drug delivery system (Peng et al. 2011; Lin and Dufresne 2014).
17.7.2.2 Bioimaging and Biosensor
Nanocellulose can be a very useful material for use in biosensor technologies. It possesses biocompatible transducer surfaces which makes it useful in sensor applications (Edwards et al. 2013). NCC can be derivatized at the hydroxyl regions to make it covalently bound to bioactive molecules. They have chiral neumatic structures which are characteristic for sensitive optical properties (Shopsowitz et al. 2010). Different types of nanocellulose such as nanocomposites , surface grafted molds, and microdialysis membranes were found to show excellent properties at bio-interface of a probe (Edwards et al. 2013). It was reported that cellulose can be used as stimuli responsive mediators by modifying it through radical polymerization (Kang et al. 2013). Such modified celluloses can be used in drug delivery and for bioimaging application (Dong and Roman 2007). These authors reported a method to label NCC with fluorescein-5′-isothiocyanate (FITC). The interaction between labeled NCC with cells where then evaluated using fluorescence techniques.
NCC can be used in biosensing by conjugated it with different biological moieties (such as nucleic acids) or metallic nanoparticles (Lam et al. 2012). Moss et al. (1981) reported a method for producing a DNA-cellulose hybrid suitable for purifying complementary mRNA from total poly(A)-enriched RNA by affinity chromatography. NCC can also be used to produce robust, porous electrodes and sensors. It can be used to replace the cellulose which has been combined with TiO2 nanoparticles (Pang et al. 2016) and chitosan for their potential use as biosensors (Manan et al. 2016).
17.7.3 Enzyme Immobilization
NCC has large surface area and is non-porous in nature which makes it suitable for immobilizing proteins or enzymes (Lam et al. 2012). Yang et al. (2008) reported a model system to remove chlorinated phenolic compounds in aqueous solution by immobilizing peroxidase onto NCC. The obtained immobilized peroxidase showed improved activity compared to its soluble counterpart. The immobilized samples had enzyme activity 594 U/g and stable for 3 months at 5 °C. Furthermore, NCC functionalized with gold nanoparticles has been characterized as a support for immobilization of enzymes (Mahmoud et al. 2009; Lam et al. 2012). For instance, cyclodextrin glycosyl transferase (CGTase) and alcohol oxidase can be immobilized on NCC with high enzyme loading capacity. Activity yield and CGTase loading of 70% and 165 mg/g were, respectively, obtained when such NCC was used as matrices for immobilization. This value is higher than those obtained with other commonly used matrices (Ivanova 2010). Recently, detection of phenol using tyrosinase immobilized in the NCC/Chitosan nanomaterial film has been reported (Manan et al. 2016).
17.7.4 Antimicrobial Application
Use of NCC for antimicrobial applications has been demonstrated by Drogat et al. (2011). The author reported the method used to produce silver colloidal suspension using NCC. The obtained Ag-NCC solutions showed inhibiting activities against E. coli and S. aureus. The inhibiting effect was attributed to the interaction of NCC with the bacterial cell wall. Such Ag nanoparticle NCC suspensions have the potential to be used in antiseptic solutions or wound healing gels. However, detail study involving the long-term toxicity of Ag nanoparticles is yet to be done (Lam et al. 2012). This will confirm the expectations of the possible use of NCC as antimicrobial agents.
17.7.5 Other Applications
In addition to aforementioned applications, use of NCCs as iridescent pigments and biomolecular NMR contrast agent has also been explored (Fleming et al. 2001; Peng et al. 2011). Its use as a reinforcing agent for the production of low thickness polymer electrolytes used in lithium battery has also been reported by some authors (Schroers et al. 2004; Samir et al. 2005). Due to improved crystallinity and interfacial interaction, incorporation of NCCs in polymer nanocomposites can improve the mechanical performance, thermal stability, and optical properties of the same. Thus, biodegradable nanocomposite films with lower permeability to moisture, gases, aromas, and oil can be produced by inclusion of nanocrystalline cellulose. Such obtained films have numerous application in food and biomedical packaging areas (George and Sabapathi 2015). NCCs are biocompatible and their mechanical properties are like natural tissues. Thus, studies involving the use of NCCs as a scaffolding to grow tissue have also been carried out. The use of NCC is being studied in many areas as detailed here. The possible applications of nanocrystalline cellulose in different areas have also been depicted in Fig. 17.2.
17.8 Limitations of NCC Usage
Though NCC was found to be a potential material for several applications, it has several limitations in its upstream processing. For instance, oxidizing the cellulose make the amorphous fibers in NCCs disperse in the aqueous suspension of crystalline cellulose. Separation of these fine fibers is a major hurdle in producing pure NCC. Its usage is limited by factors like its hydrophobic nature and water swelling characteristics in the amorphous regions (Hubbe et al. 2008). Using high concentration sulfuric acid 63.5% (w/w) to produce NCC (Bondeson et al. 2006) also has a major drawback in the perspective of environmental aspects. Despite the use severe conditions, NCC yields have been found to be low (20–40%) (Bondeson et al. 2006; Hamad and Hu 2010). An efficient and reliable process is necessary to maintain the uniform size, aspect ratio, and surface chemistry. It should provide more control in NCC suspensions (Brinchi et al. 2013). Therefore, standardization of the process is necessary to obtain fine NCC fibers. Technological advancements are necessary to develop a method to produce nanocellulose with controlled size, length, and surface properties at low costs. Drying of NCC is necessary to produce in its powder form. It was not listed as one of the limitations by many of the literature reports. However, the conventional drying methods like centrifugation and high temperatures have significant effect on NCC and induce agglomeration (Brinchi et al. 2013). According to Lu and Hsieh (2012), freeze drying and supercritical drying produced a matrix of agglomerates with various sizes of nanocellulose. Therefore, it is important to develop an efficient drying method to produce NCC without altering its structural or functional properties. A number of research studies indicated that the costs and commercial scale production are also the major drawbacks in the production of NCC (Brinchi et al. 2013). However, with the amount of research ongoing on the production, modification, and application of NCC, one can be optimistic about its use in a number of ways in the near future.
References
Abatzoglou N, Chornet E, Belkacemi K, Overend RP (1992) Phenomenological kinetics of complex-systems—the development of a generalized severity parameter and its application to lignocellulosics fractionation. Chem Eng Sci 47:1109–1122
Abraham E, Deepa B, Pothan LA, Jacob M, Thomas S, Cvelbar U, Anandjiwala R (2011) Extraction of nanocellulose fibrils from lignocellulosic fibres: a novel approach. Carbohydr Polym 86:1468–1475
Acharya SK, Mishra P, Mehar SK (2011) Effect of surface treatment on the mechanical properties of bagasse fiber reinforced polymer composite. Bioresources 6(3):3155–3165
Alemdar A, Sain M (2008) Biocomposites from wheat straw nanofibers: morphology, thermal and mechanical properties. Compos Sci Technol 68:557–565
Ankerfors M (2012) Microfibrillated cellulose: energy-efficient preparation techniques and key properties. Innventia, Stockholm
Antonio M (2014) Nanocrystalline cellulose: production, opportunities, and challenges. Available from http://blog.luxresearchinc.com/blog/2014/11/nanocrystalline-cellulose-production-opportunities-and-challenges/. Accessed 12 Jan 2017
Bai W, Holbery J, Li K (2009) A technique for production of nanocrystalline cellulose with a narrow size distribution. Cellulose 16:455–465
Bondeson D, Mathew A, Oksman K (2006) Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 13:171
Börjesson M, Westman G (2015) Crystalline nanocellulose-preparation, modification, and properties. In: Poletto DM (ed) Cellulose—fundamental aspects and current trends. InTech, Rijeka
Brinchi L, Cotana F, Fortunati E, Kenny JM (2013) Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym 94:154–169
Carvalheiro F, Duarte LC, Girio FM (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res 67:849–864
Cherian BM, Leão AL, De Souza SF, Thomas S, Pothan LA, Kottaisamy M (2010) Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr Polym 81:720–725
Clift MJD, Foster EJ, Vanhecke D, Studer D, Wick P, Gehr P, Rothen-Rutishauser B, Weder C (2011) Investigating the interaction of cellulose nanofibers derived from cotton with a sophisticated 3D human lung cell coculture. Biomacromolecules 12:3666–3673
Coffey DG, Bell DA, Henderson A (1995) Cellulose and cellulose derivatives. In: Stephen AM (ed) Food polysaccharides and their applications. Marcel Dekker, New York
Dalli SS, Rakshit SK (2015) Utilization of hemicelluloses from lignocellulosic biomass-potential products. In: Pittman KL (ed) Lignocellulose. Nova, New York, pp 85–113
De Souza Lima MM, Borsali R (2004) Rodlike cellulose microcrystals: structure, properties, and applications. Macromol Rapid Commun 25:771–787
De Souza Lima MM, Wong JT, Paillet M, Borsali R, Pecora R (2003) Translational and rotational dynamics of rodlike cellulose whiskers. Langmuir 19:24–29
Dong S, Roman M (2007) Fluorescently labeled cellulose nanocrystals for bioimaging applications. J Am Chem Soc 129:13810–13811
Drogat N, Granet R, Sol V, Memmi A, Saad N, Klein Koerkamp C, Bressollier P, Krausz P (2011) Antimicrobial silver nanoparticles generated on cellulose nanocrystals. J Nanopart Res 13:1557–1562
Dufresne A (2010) Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals. Molecules 15:4111
Dufresne A (2013) Nanocellulose: a new ageless bionanomaterial. Mater Today 16:220–227
Edwards J, Prevost N, French A, Concha M, Delucca A, Wu Q (2013) Nanocellulose-based biosensors: design, preparation, and activity of peptide-linked cotton cellulose nanocrystals having Fluorimetric and colorimetric elastase detection sensitivity. Engineering 5:20–28
Elazzouzi-Hafraoui S, Nishiyama Y, Putaux J-L, Heux L, Dubreuil F, Rochas C (2008) The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 9:57–65
Espino-Perez E, Bras J, Ducruet V, Guinault A, Dufresne A, Domenek S (2013) Influence of chemical surface modification of cellulose nanowhiskers on thermal, mechanical, and barrier properties of poly(lactide) based bionanocomposites. Eur Polym J 49:3144–3154
Faruk O, Bledzki AK, Fink H-P, Sain M (2012) Biocomposites reinforced with natural fibers: 2000–2010. Prog Polym Sci 37:1552–1596
Filson PB, Dawson-Andoh BE, Schwegler-Berry D (2009) Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem 11:1808–1814
Fleming K, Gray DG, Matthews S (2001) Cellulose crystallites. Chem Eur J 7:1831–1836
Fortunati E, Armentano I, Zhou Q, Iannoni A, Saino E, Visai L, Berglund LA, Kenny JM (2012) Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr Polym 87:1596–1605
Frone AN, Panaitescu DM, Donescu D, Spataru CI, Radovici C, Trusca R, Somoghi R (2011) Preparation and characterization of Pva composites with cellulose nanofibers obtained by ultrasonication. Bioresources 6(1):487–512
George J, Sabapathi SN (2015) Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl 8:45–54
George J, Ramana KV, Bawa AS, Siddaramaiah (2011) Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int J Biol Macromol 48:50–57
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Hamad WY, Hu TQ (2010) Structure–process–yield interrelations in nanocrystalline cellulose extraction. Can J Chem Eng 88:392–402
Hendriks AT, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Henriksson M, Henriksson G, Berglund L, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polym J 43:3434–3441
Hielscher Ultrasonics GMBH (2017) Ultrasonic production of nano-structured cellulose [online]. Hielscher ultrasonics GMBH. Available from https://www.hielscher.com/ultrasonic-production-of-nano-structured-cellulose.htm#62347. Accessed 12 Jan 2017
Hubbe MA, Rojas OJ, Lucia LA, Sain M (2008) Cellulosic nanocomposites: a review. Bioresources 3(3):929–980
Huq T, Salmieri S, Khan A, Khan RA, Le Tien C, Riedl B, Fraschini C, Bouchard J, Uribe-Calderon J, Kamal MR (2012) Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr Polym 90:1757–1763
Ivanova V (2010) Immobilization of cyclodextrin glucanotransferase from Paenibacillus Macerans ATCC 8244 on magnetic carriers and production of Cyclodextrins. Biotechnol Biotechnol Equip 24:516–528
Janardhnan S, Sain MM (2007) Isolation of cellulose microfibrils–an enzymatic approach. Bioresources 1:176–188
Jeon IY, Baek JB (2010) Nanocomposites derived from polymers and inorganic nanoparticles. Materials 3:3654
Kang H, Liu R, Huang Y (2013) Cellulose derivatives and graft copolymers as blocks for functional materials. Polym Int 62:338–344
Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100:1515–1523
Khalil HA, Davoudpour Y, Islam MN, Mustapha A, Sudesh K, Dungani R, Jawaid M (2014) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99:649–665
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466
Kovacs T, Naish V, O'connor B, Blaise C, Gagne F, Hall L, Trudeau V, Martel P (2010) An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 4:255–270
Lam E, Male KB, Chong JH, Leung AC, Luong JH (2012) Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol 30:283–290
Lee SY, Mohan DJ, Kang IA, Doh GH, Lee S, Han SO (2009) Nanocellulose reinforced PVA composite films: effects of acid treatment and filler loading. Fibers Polym 10:77–82
Lee HV, Hamid SBA, Zain SK (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:1–20
Leung ACW, Hrapovic S, Lam E, Liu Y, Male KB, Mahmoud KA, Luong JHT (2011) Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 7:302–305
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Lu P, Hsieh YL (2012) Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr Polym 87:564–573
Ma XJ, Yang XF, Zheng X, Lin L, Chen LH, Huang LL, Cao SL (2014) Degradation and dissolution of hemicelluloses during bamboo hydrothermal pretreatment. Bioresour Technol 161:215–220
Mahmoud KA, Male KB, Hrapovic S, Luong JHT (2009) Cellulose nanocrystal/gold nanoparticle composite as a matrix for enzyme immobilization. ACS Appl Mater Interfaces 1:1383–1386
Majeed K, Jawaid M, Hassan A, Abu Bakar A, Abdul Khalil HPS, Salema AA, Inuwa I (2013) Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater Des 46:391–410
Male KB, Leung AC, Montes J, Kamen A, Luong JH (2012) Probing inhibitory effects of nanocrystalline cellulose: inhibition versus surface charge. Nanoscale 4:1373–1379
Manan FAA, Abdullah J, Nazri NN, Malik INA, Yusof NA, Ahmad I (2016) Immobilization of tyrosinase in nanocrystalline cellulose/chitosan composite film for amperometric detection of phenol. Malay J Anal Sci 20:978–985
Marimuthu TS, Atmakuru R (2015) Isolation and characterization of cellulose nanofibers from the aquatic weed Water Hyacinth: Eichhornia crassipes. In: Pandey JK, Takagi H, Nakagaito AN, Kim HJ (eds) Handbook of polymer nanocomposites. Processing, performance and application, Polymer nanocomposites of cellulose nanoparticles, vol C. Springer, Berlin
McDonough TJ (1993) The chemistry of organosolv delignification. TAPPI J 76(8):186
Moss LG, Moore JP, Chan L (1981) A simple, efficient method for coupling DNA to cellulose. Development of the method and application to mRNA purification. J Biol Chem 256:12655–12658
Ng HM, Sin LT, Tee TT, Bee ST, Hui D, Low CY, Rahmat A (2015) Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos Part B 75:176–200
Pääkkö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941
Panaitescu DM, Frone AN, Nicolae C (2013) Micro- and nano-mechanical characterization of polyamide 11 and its composites containing cellulose nanofibers. Eur Polym J 49:3857–3866
Pang Z, Yang Z, Chen Y, Zhang J, Wang Q, Huang F, Wei Q (2016) A room temperature ammonia gas sensor based on cellulose/TiO2/PANI composite nanofibers. Colloids Surf A Physicochem Eng Aspects 494:248–255
Park YC, Kim JS (2012) Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 47:31–35
Peng BL, Dhar N, Liu HL, Tam KC (2011) Chemistry and applications of nanocrystalline cellulose and its derivatives: a nanotechnology perspective. Can J Chem Eng 89:1191–1206
Qin ZY, Tong G, Chin YCF, Zhou JC (2011) Preparation of ultrasonic-assisted high carboxylate content cellulose nanocrystals by tempo oxidation. Bioresources 6(2):1136–1146
Ranby BG (1951) Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss Faraday Soc 11:158–164
Rebouillat S, Pla F (2013) State of the art manufacturing and engineering of nanocellulose: a review of available data and industrial applications. J Biomater Nanobiotechnol 4:165–188
Roman M, Dong S, Hirani A, Lee YW (2009) Cellulose nanocrystals for drug delivery. polysaccharide materials: performance by design. American Chemical Society, Washington, DC, pp 81–91
Rosa SML, Rehman N, De Miranda MIG, Nachtigall SMB, Bica CID (2012) Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohydr Polym 87:1131–1138
Sacui IA, Nieuwendaal RC, Burnett DJ, Stranick SJ, Jorfi M, Weder C, Foster EJ, Olsson RT, Gilman JW (2014) Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl Mater Interfaces 6:6127–6138
Saha BC, Yoshida T, Cotta MA, Sonomoto K (2013) Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind Crop Prod 44:367–372
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Samir MASA, Alloin F, Sanchez JY, Dufresne A (2005) Nanocomposite polymer electrolytes based on poly(oxyethylene) and cellulose whiskers. Polímeros 15:109–113
Schroers M, Kokil A, Weder C (2004) Solid polymer electrolytes based on nanocomposites of ethylene oxide–epichlorohydrin copolymers and cellulose whiskers. J Appl Polym Sci 93:2883–2888
Shahbaz M, Lean HH (2012) Does financial development increase energy consumption the role of industrialization and urbanization in Tunisia. Energy Policy 40:473–479
Shi HG, Farber L, Michaels JN, Dickey A, Thompson KC, Shelukar SD, Hurter PN, Reynolds SD, Kaufman MJ (2003) Characterization of crystalline drug nanoparticles using atomic force microscopy and complementary techniques. Pharm Res 20:479–484
Shopsowitz KE, Qi H, Hamad WY, Maclachlan MJ (2010) Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 468:422–425
Sjostrom E (1993) Wood chemistry: fundamentals and applications. Academic Press, San Diego, CA
Spence KL, Venditti RA, Rojas OJ, Habibi Y, Pawlak JJ (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18:1097–1111
Stelte W, Sanadi AR (2009) Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind Eng Chem Res 48:11211–11219
Tadesse H, Luque R (2011) Advances on biomass pretreatment using ionic liquids: an overview. Energy Environ Sci 4:3913–3929
Taheri A, Mohammadi M (2015) The use of cellulose nanocrystals for potential application in topical delivery of hydroquinone. Chem Biol Drug Des 86:102–106
Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE (2004) Ammonia fiber explosion treatment of corn stover. Appl Biochem Biotechnol 113-116:951–963
Xiong Y, Zhang Z, Wang X, Liu B, Lin J (2014) Hydrolysis of cellulose in ionic liquids catalyzed by a magnetically-recoverable solid acid catalyst. Chem Eng J 235:349–355
Yang R, Tan H, Wei F, Wang S (2008) Peroxidase conjugate of cellulose nanocrystals for the removal of chlorinated phenolic compounds in aqueous solution. Biotechnology 7:233–241
Zavrel M, Bross D, Funke M, Buchs J, Spiess AC (2009) High-throughput screening for ionic liquids dissolving (ligno-)cellulose. Bioresour Technol 100:2580–2587
Zhang X, Huang J, Chang PR, Li J, Chen Y, Wang D, Yu J, Chen J (2010) Structure and properties of polysaccharide nanocrystal-doped supramolecular hydrogels based on Cyclodextrin inclusion. Polymer 51:4398–4407
Zhang PP, Tong DS, Lin CX, Yang HM, Zhong ZK, Yu WH, Wang H, Zhou CH (2014) Effects of acid treatments on bamboo cellulose nanocrystals. Asia Pac J Chem Eng 9:686–695
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82(5):815
Zhu JY, Sabo R, Luo X (2011) Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem 13:1339–1344
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dalli, S.S., Uprety, B.K., Samavi, M., Singh, R., Rakshit, S.K. (2018). Nanocrystalline Cellulose: Production and Applications. In: Prasad, R., Jha, A., Prasad, K. (eds) Exploring the Realms of Nature for Nanosynthesis. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-99570-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-99570-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99569-4
Online ISBN: 978-3-319-99570-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)