Abstract
Dugongs (Dugong dugon), also known as ‘sea cows’, have captured the imagination of the general public ever since they were first scientifically named in the 18th century. Much of the research on dugongs has been undertaken in Australia and SE Asia and publications are rarely dedicated specifically to the Red Sea population of dugongs and their conservation status. This is a reflection of the relatively poor state of knowledge of Red Sea dugongs—a situation that has changed marginally in the case of Egypt through research work undertaken by the second author. Methods employed to count dugongs, in order to estimate the size of a particular population, vary according to the general nature of their habitats (e.g., close to shore in sheltered bays or over deeper water further offshore), the frequency of sightings and facilities available to the surveying team, both in terms of observation platforms (e.g., helicopter, fixed-wing aeroplane, drone, boat or car) and time that can be allotted to the task. Given the seasonal nature of their behaviour, it would seem necessary that surveys in particular areas extend over at least 12 months and preferably longer. Research on this species in the Red Sea began with largely anatomical and physiological work on dugongs that were accidentally killed or purposely netted. Today, they are protected throughout the region so studies have shifted, largely to observations of live animals in the wild and to data that can be collected from stranded carcasses. Meanwhile, much of the data on their distribution, both in the Red Sea and elsewhere, is based on tapping into the local knowledge of fishermen and, more recently, dive guides at marine resorts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The dugong (Dugong dugon—Müller 1776) is the only herbivorous mammal which is strictly marine and is the only existing species in the order Sirenia, family Dugongidae (Domning 1999; Marsh et al. 2002a, b; Bakkar et al. 2016). The dugong is a charismatic species (Cullen-Unsworth et al. 2017) that feeds mainly on seagrass (Preen 1992; Rajamani 2009; Marsh et al. 2012; Hossain et al. 2016). Moore et al. (2017) reported that dugongs could probably be considered as a keystone species in tropical seagrass ecosystems in the Indo-Pacific region. The dugong is listed as vulnerable in the IUCN Red List (Marsh and Sobtzick 2015) and in CITES Appendix I (UNEP-WCMC 2015). Marsh et al. (2002a, b) reported that the dugong is vulnerable to extinction because it feeds only on seagrass in constrained habitats in coastal waters and has a low reproductive output.
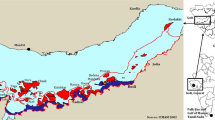
The dugong or “sea cow” has a range spanning waters of 48 countries, from the tropical and subtropical shallow coastal habitats of East Africa to the Red Sea and Arabian Gulf, and eastward to the Indo-Pacific region as far as Australia (Nishiwaki and Marsh 1985; Marsh 2008). They are usually recorded in the shallow coastal areas of the Indian and Western Pacific Oceans (Marsh et al. 2002a, b; D’Souza et al. 2013; Ponnampalarm et al. 2014; Pilcher et al. 2017). Within the western Indian Ocean their range extends from Madagascar and Mozambique northward along the East African coast to the Red Sea, Gulf of Aden and Arabian Gulf. Their range extends eastward from there, along the south coast of Asia, including India and Malaysia, to the western, northern and eastern coasts of Australia (Bertram and Bertram 1973; Husar 1975, 1978; FAO 1979; Sobtzick et al. 2012) and as far as Micronesia (Figs. 18.1 and 18.2).
Gohar’s often quoted scientific paper on dugongs of the Red Sea, published by the Institute of Oceanography at Cairo University (Gohar 1957), has a bibliography comfortably occupying a single page, with just 17 references spanning the period 1833–1957. Contrast that with a digital search for “Dugong dugon” on Google Scholar which presently (on 20 March, 2018) returns 17,700 positive results, 6780 of which are from the current decade and 878 from the 2017 alone. It is clear that this fascinating marine mammal retains its appeal, both at a scientific level and to members of the general public, amongst whom it is often referred to as the ‘original mermaid’. The dugong is known among fishermen in the Red Sea and Gulf of Aden region by various local Arabic titles among which are Al-Gild (leather), Arus Al-Bahar (bride of the sea), Baqar Al-Bahar (sea cow), and Al-Egle or Egle Al-Bahr (sea cow) (Gohar 1957).
In addition to Gohar’s work, descriptions of their anatomy, ecology and distribution have been given by Hill (1945), Kingdon (1971), Bertram and Bertram (1973), Husar (1978), Marsh et al. (1984a, b, c, d), Nishiwaki and Marsh (1985), Thornback and Jenkins (1982), Preen (1989) and Marsh (2014). The food of the Red Sea dugongs was investigated by Lipkin (1975), and the conservation of sirenians, in general, was reviewed by Bertram (1974), PERSGA/GEF (2004), Marsh et al. (2012), and Woinarski et al. (2014). Research that has recently been undertaken in Egypt has thrown new light on the northern Red Sea dugong population. Behavioural ecology of the dugong, for instance, was studied by the second author (Shawky 2018) during the period December 2015–October 2017 using snorkelling and SCUBA diving techniques (Figs. 18.3 and 18.4).
Studies about many aspects of dugong biology are based on specimens accidentally drowned in shark nets or killed by native hunters in northern Australia and Papua New Guinea (Marsh 1980, 1986; Marsh et al. 1984a, b, c, d). In Arabian waters, aerial surveys were conducted providing a basis for estimating the dugong population. These visual surveys were complemented by interviews with fishermen and fish sellers at 29 locations in Saudi Arabia, Bahrain, Qatar, the United Arab Emirates and Yemen between 1986 and 1988 (Preen 1989). In the Red Sea and Gulf of Aden region, fishermen were interviewed during March/April 1994 at Suez, Hurghada, Al-Wejh, Rabigh, Tuwwal, Jeddah, Al-Lith, Gizan, Farasan Islands, Hodaidah and the Aden area. The information gathered from such interviews confirmed the findings cited by many workers in various aspects of dugong activities such as distribution, feeding and movement (Marsh 2002).
It is not the purpose of this chapter to provide a comprehensive review of this considerable body of work, but it is worth mentioning that there are still many gaps in our knowledge. Relatively little research has been undertaken in the Red Sea itself, and much of our knowledge comes from interviews with local fishermen, who are ‘out in the field’ at the remote sites favoured by these shy mammals, far more than most scientists. Indeed, this source of local knowledge has been a significant ingredient of the research programme in the Red Sea and we wish to acknowledge both the skills and generous cooperation of artisanal fishermen in all areas (Figs. 18.5 and 18.6).
Population in the Red Sea
Most of our knowledge of dugongs in the Red Sea stems from skulls and other skeletal remains (Mitchell 1973) or from animals accidentally caught and drowned in large-mesh shark-nets. Field observations (aerial, surface and underwater) indicate that they occur as isolated individuals or in small family groups. Aerial surveys of the Arabian Gulf carried out in 1985–1986 under the aegis of Saudi Arabia’s Marine Environment Protection Agency, MEPA, estimated the Arabian Gulf population at 7310 ± 1,380 dugongs. MEPA produced aerial photographs of a herd off Bahrain that included at least 674 dugongs, 12% of which were calves (Anthony Preen, pers. comm., and Vine 1986). A very crude estimate of the dugong population of Sudan was mentioned by Ormond (1978) to be 20–40 animals, and accordingly, he suggested that the total population of the Red Sea was around 200 animals.
Saudi Arabia remains an area of global significance in terms of its dugongs (PERSGA/GEF 2001). Surveys by Preen (1989) estimated there were 4000 dugongs within the Red Sea and 2000 (1818 ± 382) of these occur in Saudi Arabian waters. In 1987–1988, key populations were still present around the Tiran Islands (under joint administration with Egypt) at the southern entrance to Gulf of Aqaba, Al Wejh and Sharm Munaibira (south of Al Wejh), Farasan Islands, near Qishran Island, especially Ash Sharifa (25 km north of Al Lith), and from Khawr Ja’afirah (north of Gizan) to the Saudi-Yemen border (this population continued into Yemen as far as Al Hudaydah) (Preen 1989; Marsh 2002; Sheppard et al. 1992; PERSGA/GEF 2003). As one moves north, up the Red Sea’s eastern shoreline, the first area where dugongs are likely to be encountered is about 35 km north of Hudaydah and from there onward, moving toward Gizan, the population increases gradually. An aerial survey conducted in September 1993 reported 27 dugongs in the Gizan area at Ras At Tarfa (W. Gladstone, pers. comm.). Data on which to base a current estimate of the Red Sea’s local and regional populations of dugongs is deficient, but it can be assumed that numbers have decreased in all areas where fishing nets are employed or where coastal development has involved habitat destruction (Figs. 18.7 and 18.8).
Within Yemen, dugongs occur over seagrass beds as far south as Al Hudaydah. Crossing over the Red Sea to its coastline with Africa, there is a concentration of dugongs living among seagrass beds of the Dahlak archipelago, particularly near Marsa Fatma (Marsa is a bay with sandy access) between Massawa and the Sudanese border. Moving north, through Sudanese waters, dugongs may be found in Suakin harbour and archipelago (south of Port Sudan) and in the various wadis to the north of Port Sudan such as Marsa Halot, Marsa Arous, Marsa Arikiyai and off Mohammed Quol, which lies south of the wide entrance to Dungonab Bay, where dugongs have also been recorded. According to Marsh et al. (2002a, b), only two calves were sighted in 1997 at Abou Galum. Between 1999 and 2000, only two dugongs were sighted at the mangrove area of Nabq Protected Area. On separate occasions dugongs were encountered in the Red Sea at Suakin harbour and archipelago, Mersa Halot and Dungonab in Sudan, and at Tuwwal in Saudi Arabia (D. Nasr and P. J. Vine, pers. comm.).
Research aimed at assessing the current status of dugongs in the Red Sea is ongoing and largely based on interviews with fishers. A study of dugongs in the Egyptian Red Sea, undertaken in 2000–2003 (Hanafy et al. 2006), focused on the distribution and relative abundance of dugongs along the coast from Hurghada, immediately south of the Gulf of Suez, to Shalateen, about 200 km north of the Sudan border. A low-density population occurred throughout the area, but the maximum and minimum numbers reported were 17 in 2002 and 12 each in 2001 and 2003. The lowest sightings in winter were attributed to either migration to warmer waters or greater difficulty in observation during winter months (Figs. 18.9 and 18.10).
Recent studies by the second author on the Egyptian population of dugongs included recognition of 30 individuals that were observed and identified using underwater photo identification techniques (Photo ID). In what is believed to be the first study (Shawky et al. 2018) to document dugongs along the180 km of shoreline between Marsa Alam and Wadi El Gemal National Parks (WGNP), 16 individuals were followed at Marsa Alam and 14 at WGNP. The sex ratio among these individuals was 7:1 (males:females). Shawky et al. (2018) used photo identification to keep track of individual dugongs, concentrating mainly on permanent notches of their tail flukes and flippers rather than scars which can heal in a couple of months or more. Dugongs may show aggressive behaviour, so seemingly mildly injurious scars from the small tusks of males are commonly observed on their backs (Marsh et al. 2012). A Photo ID catalogue was prepared for the different dugong individuals with records of their occurrence among sites. Shawky et al. (2018) confirmed the presence of particular dugongs in specific locations. The success of these techniques suggests that further efforts, including observations at offshore sites, would yield valuable data (Fig. 18.11 and 18.12).
Research conducted by the second author in Egyptian waters throws more light on the country’s population of dugongs. A total of 207 questionnaires were carried out using the standardised dugong catch/bycatch questionnaire developed by CMS-UNEP Dugong MOU (Pilcher et al. 2017). More than 97% of the respondents were 15–75 years old, and the largest age group was 26–50 years (77%) with a mean age of around 35 ± 6 years. The majority of the respondents (98%) were aware of dugongs. Dugong encounters mostly occurred during fishing (27%) or in transit to fishing areas (>39%). Sixty-one percent of respondents estimated the dugong population to be about 2–10 individuals in key areas. Nearly 89% of the fishermen claimed that the trend in the net capture of dugongs was decreasing. Most of the respondents (96%) had encountered a dugong at least once in the past year. More than 72% of dugongs were released alive, and 13% were reported as being eaten. Most of the fishermen (66%) stated that dugongs were not hunted in their village, but more than 25% claimed that they were captured in other villages.
Dugongs were sighted at approximately 92 sites in the western coast of the Egyptian Red Sea, including nine in Hurghada, three in Safaga, 11 in Qosseir, 31 in Marsa Alam, 17 in Wadi El Gemal National Parks (WGNP), four in Ras Banaas and 17 in Shalateen, Abou Ramaad and Halayeb regions. A total of 1322 sightings of dugong were recorded between 1980 and 2016. Dugong sightings, fishing areas and seagrass distribution were represented on GIS maps (Geographic Information Systems). 34% of the respondents claimed that the trend in dugong populations displayed a decline. More than 79% believed that dugongs could be extinct in the future and >90% affirmed that they are playing an essential role in the marine ecosystem (Figs. 18.13 and 18.14).
Habitat
Several investigators suggested that dugongs preferred habitats sheltered from rough winds and heavy waves with shallow to medium depth and warm waters (Bertram and Bertram 1973; Marsh et al. 1982; Preen 1995; Marsh et al. 2002a, b, 2012; Spiegelberger and Ganslosser 2005). Abu El-Regal et al. (2012) evaluated the status of dugongs in Marsa Abou Dabbab, western coast of Egyptian Red Sea, and recorded that the mean water temperature varied between 30.4 and 33.4 ºC in 2010. As a result of the seawater current the salinity values fluctuated between 41.61 and 42.72‰. With the exception of the Gulf of Suez, the temperature of the Red Sea falls within the optimal range reported for dugong habitats. From April through December water temperatures in the Gulf of Suez are above 20 ˚C; only at the head of the Gulf of Suez do water temperatures fall below 20 ˚C (Preen 1989).
Seagrass beds are an essential element since dugongs feed almost exclusively on these throughout their life-cycle (Marsh et al. 2012; Collier et al. 2012; Hossain et al. 2016). Dugongs play an important role in the seagrass communities, affecting their structure, distribution, species composition, productivity and nutrient status (Aragones et al. 2006; Hines et al. 2012; Ebrahim et al. 2014; Bessey et al. 2016; Mizuno et al. 2017). In particular, dugongs can play a vital role in the potential for seed and propagule dispersion (Kendrick et al. 2012).
The distribution and abundance of suitable seagrass habitats may be the most important factor influencing dugong distribution, migration and abundance (Preen 1989). Seagrasses tend to occur on soft-bottomed substrates in the lower intertidal and shallow sublittoral in the Red Sea and Gulf of Aden (IUCN/UNEP 1985). However, such soft-bottomed substrates suitable for seagrasses are restricted in the northern Red Sea by the extensive fringing reefs that drop off steeply into deep water (IUCN/MEPA 1987; Preen 1989). Thus, the most extensive seagrass beds are restricted to the shallow, soft bottom areas of sharms and mersas or to intertidal and submarine wadi outwash plains (IUCN/MEPA 1987) (Figs. 18.15 and 18.16).
Sheppard et al. (2010) investigated dugong habitat use in relation to seagrass nutrients, tides and diel cycles. They found that dugongs tend to focus on seagrass patches with high nitrogen concentrations “except during the day at low tides when the animals had fewer habitat choices, and their space use was centred over high seagrass biomass”. They commented that dugongs prefer high energy foods such as seagrasses high in starch. Their model of dugong resource selection pointed to nitrogen as “the primary limiting nutrient for dugong populations”. Tidal ranges in the Red Sea are at their maximum in the south and north compared to very small daily tidal fluctuations near the nodal point, around 20˚N. Whilst these observations are relevant to intertidal seagrass beds in the north and south, seagrasses in the central Red Sea are more affected by a seasonal rise in winter sea level, providing shallow access to areas that are only just submerged in summer months (Figs. 18.17 and 18.18).
By contrast, in the southern Red Sea, the continental shelf is both wider and shallower, and the sedimentary substrates which favour the development of extensive seagrass communities are more abundant (Preen 1989; El Shaffai 2011, 2016). Thus, the distribution of dugongs matches very well with the availability of seagrass beds, where they tend to occur in isolated pockets of suitable habitats in the north and to be continuous in the south where there is a greater freshwater input and the inshore environment is more sedimentary (Preen 1989).
The information collected from fishermen interviewed at Al-Wejh and Rabigh during March/April 1994 confirmed these findings. At the Al-Wejh area, they reported that dugongs are relatively more numerous at Wadi Al-Myiah (about 15 km south of Al-Wejh) where there is a greater rain water input creating a sedimentary bottom. At Rabigh, on the other hand, dugongs were more frequent when freshwater input was greater, and they disappeared with the scarcity of rain water runoff in the area (Figs. 18.19 and 18.20).
Migration
The degree to which local populations of dugongs migrate depends on local geography, environmental conditions and behaviour patterns of different populations. Migrations may be influenced by seasonal temperatures, rainfall or wind conditions. In Australia, long-distance movements along the Queensland coast are well-documented (Sheppard et al. 2006), but regular migratory patterns do not appear to be established (Sheppard et al. 2006; Marsh et al. 2012; Gredzens et al. 2014). Cope et al. (2015) used pedigree analysis based on individual genetic markers to infer the movements of dugongs between locations in southeast Queensland, including Moreton Bay and Hervey Bay. They discovered that approximately 30% of assigned parents had at least one offspring found in a different locality, implying recent movement of the parent or offspring. This analysis suggested markedly more movement between localities than was detected through repeated direct sampling of individuals (Seddon et al. 2014) or through telemetry (Sheppard et al. 2006).
It is clear from various studies that dugongs can move over considerable distances for a number of reasons, generally associated with weather, food or reproduction but also likely to be linked to environmental disturbance. Apart from major migrations, there is some regular daily movement between feeding grounds and deeper waters (Husar 1978; Preen 1989) and tidal changes are the suspected triggers for this movement (Jonklass 1961; Jarman 1966; Kingdon 1971). Whilst such aerial surveys confirm that dugongs migrate, they may also occur as local residents (Heinsohn and Wake 1976). Evidence for seasonal migrations is less clear for Red Sea-based individuals than for the large aggregations that have been recorded off Somalia (Travis 1967) and in the Arabian Gulf (Preen 1989). It has been observed that shallow waters are used as sites for calving, minimising the risk of predation whilst deep waters may provide a thermal refuge from cooler waters closer to the shore during winter (Marsh 2002) (Figs. 18.21 and 18.22).
In the Egyptian Red Sea, the residence pattern as indicated by the photo-ID technique was determined along the study sites (Shawky et al. 2018). The identified dugongs were not observed moving among the different regions between Marsa Alam and WGNP. In the Marsa Alam region, out of the sixteen identified dugongs, only seven were re-sighted at different locations with a mean distance travelled of 16.6 ± 14.0 km within the home range. One individual travelled a distance of 3 km between Marsa Assalaya and Marsa Egla while another individual travelled 36 km from Marsa Alam Port to Marsa Abou Dabbab. This finding might imply that each dugong shows an implicit preference for one site over the others. The absence of some individuals from the sites for more time might be explained by their movement to alternative feeding areas to avoid disturbance. High site fidelity to areas of key habitat has been recorded for Florida manatees (Weigle et al. 2001; Deutsch et al. 2003). Their movements appeared to be affected by regional and seasonal fluctuations in biomass and nutritional content of their principal forage plants (Sheppard et al. 2006) (Fig. 18.23).
Feeding
As mentioned above, dugongs feed almost exclusively on seagrasses (Gohar 1957; Lipkin 1975; Marsh et al. 1982, 2012; Preen 1992; Collier et al. 2012; Cullen-Unsworth et al. 2014; Hossain et al. 2016; Marsh et al. 2018). They have also been reported to occasionally consume algae and soft-bodied invertebrates such as ascidians, hydrozoans and holothurians (Spain and Heinsohn 1973; Wake 1975; Best 1981; Preen 1995; Marsh et al. 2012; Tol et al. 2016; Dentzien-Dias et al. 2018). These may fufill the role of beneficial dietary supplements (FAO 1979). Seagrass species such as Halophila ovalis and Halodule uninervis are often selected by dugongs for their low-fibre and high-nitrogen content (Jones 1967; Heinsohn and Birch 1972; Preen 1995; De Iongh et al. 2007; Marsh et al. 2012; Hines et al. 2012; Shawky et al. 2016). Dugongs usually feed on the entire seagrass plant, including shoots, rhizomes and roots. In doing so they create meandering sandy tracks known as feeding trails which provide confirmation of dugong feeding (Preen 1995; Marsh et al. 2012; Anand 2012). Their feeding trails have a 50–87% lower shoot density, and 51–75% reduction in belowground biomass (Bakker et al. 2016a, b).
Recent studies were conducted on the feeding ecology of live dugongs in Marsa Alam and Wadi El Gemal National Park (WGNP) at the western coast of the Egyptian Red Sea (Shawky 2018). The study included the characteristics of feeding sites, feeding trail dimensions, the amount of seagrass removed from the feeding trails and grazing intensity on seagrass species composition. Dugong fed mostly on a sandy bottom in depths that ranged from 1 to 9 m (average 3 ± 2 m). In Marsa Alam, the median shoot density of seagrass abundance was almost double that of WGNP (2585 shoots/m2 and 1095 shoots/m2 respectively). According to the site, Halophila stipulacea and Halophila ovalis were the most dominant species in Marsa Assalaya (2232 ± 454 and 2024 ± 572 shoots/m2, respectively) in Marsa Alam. Halodule uninervis was the most dominant in Ras Baghdady (558 ± 58 shoots/m2) in WGNP. Cymodocea rotundata was the most dominant in Shams Alam (66 ± 20 shoots/m2) in WGNP.
According to Shawky (2018) the dugongs in Marsa Alam left feeding trails with a mean length of 3.2 ± 1.4 m (range 0.6–3.1 m) and of 3.3 ± 1.3 m in WGNP (range 1.2–8.6 m). Dugongs left feeding trails with a mean width of 17.2 ± 4 cm in Marsa Alam (range 9–26 cm) and of 17.6 ± 1.3 cm in WGNP (range 7–26 cm). Shawky et al. (2016) recorded small feeding trails of 7 cm and 11 cm width in Ras Baghdady and Wadi El Gemal Island respectively. The dugong sizes were estimated from feeding trail widths, where 53% of the individuals were adults, and 47% were young. With regard to the Marsa Alam sites, the density of feeding trails was the highest in Marsa Abou Dabbab (2 ± 0.2 trails/m2) and lowest in Marsa Mobarak (1.2 ± 0.1). As for the WGNP sites, the density was the highest in Shams Alam (3.3 ± 0.3 trails/m2) and the lowest in Ras Baghdady (1.2 ± 0.1).
In the study sites, 88% of the seagrass shoots was grazed along the feeding trails (Shawky 2018). In Marsa Alam, the maximum recorded was in Marsa Assalaya site (4110 shoots/m2, 92%) and the minimum was in Marsa Abou Dabbab site (1775 shoots/m2, 89%). Dugongs removed 94% of shoots of Halophila ovalis in Marsa Mobarak and only 40% in Marsa Assalaya from the same species. In WGNP, the maximum recorded was in Shams Alam (3984 shoots/m2, 90%) and the minimum was in Ras Baghdady (2792 shoots/m2, 87%). In Ras Baghdady, dugongs removed 90% and 92% of shoots of Halodule uninervis and Halophila stipulacea respectively.
Changes in shoot density (shoots/m2) of four species of seagrass were studied for one year under three treatments (no-grazing, low-grazing and high-grazing) (Shawky 2018). In the no-grazing area, the abundance of Halophila stipulacea increased over the monitoring period while that of Halophila ovalis decreased. At the low-grazing area, Halophila stipulacea colonised the “feeding trails” first. By the end of the experiment, Halophila stipulacea and Cymodocea rotundata had recovered to pre-treatment levels. At high-grazing, Halophila ovalis showed significantly greater recovery than Halophila stipulacea or Cymodocea rotundata, increasing its relative and absolute abundance within an 80–100 day period respectively.
Dugongs are reasonably flexible and adaptable regarding their choice of seagrasses, basically making use of whatever is available and most closely tallies with their preferences. Lipkin (1975) studied stomach contents of four dugongs. Three animals were from the Gulf of Aqaba, and one from the western shore of the Gulf of Suez. One stomach contained three kinds of seagrass, Halodule uninervis, Syringodium isoetifolium and, to a lesser extent, Halophila stipulacea; another contained mainly Halodule uninervis with small amounts of Halophila stipulacea and fragments of an alga (Stypopodium zonale); a third was almost exclusively composed of Halophila stipulacea; and the last contained almost equal amounts of Halophila stipulacea and Halodule uninervis, with a little Thalassodendron ciliatum and Cymodocea rotundata. The stomach contents of each animal agreed with the seagrass resources available where it was captured. Apparently, Red Sea dugongs prefer soft and delicate seagrasses but are not as fastidious as once thought.
Elsewhere, they are reported to feed on seagrass beds at around 10 m (Fox 1999), but this depends on local topography and the availability of seagrasses. The first author regularly observed feeding channels left by dugongs at two or three metres deep in Suakin Harbour. In areas where the continental shelf remains shallow dugongs have been known to travel more than 10 km (6 miles) from the shore, descending to as deep as 37 m where seagrasses such as Halophila spinulosa are found (Marsh 2002).
Behaviour
Detailed information on dugong behaviour is scarce due to difficulties involved in direct observation of the animals in the wild (Maitland et al. 2006; Marsh et al. 2012). Their behaviour has been difficult to study since they spend little time at or near the surface and usually occur in turbid waters throughout most of their range (Barnett and Johns 1976; Anderson 1982a, b; Chilvers et al. 2004; Marsh et al. 2012). Previous studies on dugong behaviour were based on visual observations from boats (Anderson and Birtles 1978; Anderson 1982a, 1998; Marsh and Ruthbun 1990; Whiting 2002); using aerial blimp cameras for diurnal behaviour (Hodgson 2004); diving profiles using satellite tagging (Chilvers et al. 2004); observing the effect of tourism activities on dugongs (Bode 2009); observing its feeding behaviour (Wongsuryrat et al. 2011); its unusual behaviour (Hobbs and Willshaw 2015); and behavioural budget (Shawky et al. 2016).
Bode (2009), for instance, studied the behaviour of the dugong and the influence of tourists snorkeling and diving at Marsa Alam (Egypt) by surface and underwater observations. The forage time averaged 473 ± 66 s. Around 97% of the dugong dives were feeding dives of which 84% were feeding on Halodule uninervis. The dugong took a mean of 3 ± 1 breaths between the dives. The time spent in water deeper than 1.5 m averaged 79% of one hour, 21% of which was spent at the surface in a depth less than 1.5 m. The number of snorkelers per day averaged 178. During 88% of the encounters with tourists, the tourists could approach the dugong to within less than 3 m. During 40% of the underwater observation time, the dugong swam out of the bay and during 30% of this time the observed individual moved into deeper waters as a reaction to the tourist approaching within a radius of 10 m. The short-term effect of tourist activities on behavioural patterns such as dive times, forage times, distances between foraging lots and number of breaths between dives could not be ascertained, but a long-term influence on the dugong cannot be excluded. Bode’s study raises some queries since the methodology for data analysis was not described and it refers to ‘foraging’ which means the time spent eating as well as the movements made in search of food rather than feeding.
Recent studies in 2015, 2016 and 2017 were conducted by Shawky (2018) on the diurnal behaviour of dugongs at the western coast of the Egyptian Red Sea. According to Hodgson (2004), dugong behaviour was represented as a time budget for eight behavioural categories including feeding, resting, travelling, surfacing, rolling, socializing (or approaching), fleeing, and playing.
Feeding may be with or without visible sediment plumes, with the body resting on a substrate with slow movement forward and nose pressed to the substrate. Travelling by swimming forward using the tail may be slow, cruising or fast. Resting is by floating without moving at all, or above the bottom with no movement or pumping the tail and nose not pressed to substrate, and resting may occur at the surface, at the mid-water column or on the bottom. Surfacing starts by ascending to the surface to exhale and inhale at the surface, and descending by pumping the tail to reach the bottom to feed. Rolling involves rotating the body horizontally or vertically at the substrate or mid-water column. Socializing includes approaching and all contact and non-contact interactions between dugong individuals. Fleeing is a fast backward movement where a dugong turns and swims rapidly away from vessels or swimmers, and playing is free swimming with other animals such as turtles and dolphins.
The previous study was conducted for four different individuals, one in WGNP and three in Marsa Alam. Four sites were selected including Marsa Egla, Marsa Abou Dabbab, Marsa Mobarak and Shams Alam. The selection of these sites was based on the exposure of dugongs to different human activities. Dugong behaviour was studied by observation using snorkelling and SCUBA diving with video recording underwater. Dugongs spent most of their time (54.3%) in a feeding manner, 14.2% travelling, 11.9% surfacing and 10% resting. The remaining four behavioural categories, that is, rolling (1.7%), approaching (3.8%), fleeing (0.3%) and playing (3.9%) were exhibited during a relatively small proportion of the observations (Shawky 2018).
The data on the dugong dive cycle varied among individuals; however, a mean of 122 submergence intervals, and 164 surface intervals was recorded. Dive depth ranged from 1–18 m (mean 5.3 ± 3.2 m) and submergence intervals ranged from <3–287 s, while the mean time across all dives recorded was 57 ± 8 s. The overall mean surfaced interval was 2.2 ± <1 s with a range of <1–4 s. The overall mean dive rate was approximately 45 complete dive cycles per hour. The second author (Shawky et al. 2016) studied the time budget for a male dugong calf in the Egyptian Red Sea. The different behavioural activities comprising travelling, surfacing, and resting were recorded before and after approaching the shore. The change in the calf behavioural activities after coming ashore was observed regarding increased resting and decreased travelling by almost 30% for both.
Behavioural studies typically require a long-term research commitment to obtain meaningful data (Marsh et al. 2012). Understanding the environmental pressures that have influenced the behavioural strategies employed by a species enables researchers to predict whether these strategies are flexible enough to cope with novel circumstances. Information on dugong behaviour is critical for their conservation and management (Marsh et al. 2012). Conservationists and managers emphasise the need for studies that have direct application, while scientists interested in animal behaviour typically advocate fundamental research. Behavioural studies are an essential tool which, when combined with studies of population trends and demographics, will enhance our ability to inform strategies to protect sirenian populations.
Social Behaviour
Although they are social animals, having been recorded in Arabian waters in large herds of hundreds of individuals (Preen 1989), in the Red Sea they are usually found as solitary animals, or in small family groups with their distribution determined by the extent of their food resource in the form of seagrass beds (Fox 1999; Anderson 1984; Shawky 2018).
Reproduction
When an oestrous female is located in mating herds, the number of males can grow substantially, at times involving vigorous ‘cavorting’ with substantial rolling, pushing and mouthing of the body surfaces of the female and each other for hours at a time (Hartman 1979; Marsh et al. 2012). Females have been known to enter very shallow water in apparent attempts to discourage males (Hartman 1979; Reynolds 1981).
The age when a female first gives birth varies between ten and eighteen years (Marsh 2002). Despite the longevity of the dugong, which may live for 70 years or more (Marsh 1980, 1995; Marsh et al. 1984d; Kwan 2002), females give birth only a few times during their life, and invest considerable parental care in their young (Anderson 1984; Marsh and Kwan 2008). The time between births is unclear, with estimates ranging from 2.4 to 7 years. The calf nurses for 14–18 months, although it begins to eat seagrasses soon after birth (Marsh 2002). A calf will only leave its mother once it has matured (Fox 1999).
Dugongs are vulnerable to predation while giving birth and consequently seek refuge in areas such as very shallow sandbanks that are inaccessible to large sharks and killer whales (Marsh et al. 2012). On at least two occasions, cows calving in very shallow or sheltered waters have been aground but awash, behaviour that has been interpreted as a predator-avoidance strategy (MacMillan 1955; Marsh et al. 1984c). In the Egyptian Red Sea, several refuge areas with very shallow sandbanks have been identified. In these areas, small feeding trails of 7, 9 and 11 cm width were recorded beside wide trails of 24 and 26 cm width (Shawky et al. 2016; Shawky 2018). These results confirmed the presence of dugong calves with their mothers in a specific habitat.
Communication
Dugongs use vocalization for communication (Tanaka et al. 2017). With their small eyes and poor eyesight, dugongs often use smell to locate edible plants. Dark at night and often murky by day, the aquatic environment requires visual senses adapted more to locating patches of vegetation at a distance than fine discrimination (Marsh et al. 2012). They also have a strong tactile sense, and feel their surroundings with their long sensitive bristles (Fox 1999). Visual communication is mainly used for activities such as courtship. Mothers and calves are in almost constant physical contact, and calves have been known to reach out and touch their mothers with their flippers, apparently for reassurance (Fox 1999; Anderson 1984). Sound also provides an important communication medium for dugong social behaviour (Marsh et al. 2012). Anatomical specializations of dugong ears have been described and reviewed by Robineau (1969) and Ketten et al. (1992).
Several fishermen at Hodaidah, Tuwwal and Al-Wejh report hearing vocalizations produced by dugongs (generally at night-time). A number of authors have reported vocalizations of dugongs during nighttime via acoustic monitoring systems (Ichikawa et al. 2006; Tsutsumi et al. 2006; Amamoto et al. 2009). Such dugong vocalisations have been heard by the first author at night in Dungonab Bay (Sudan) in 1973 (together with the staff of the Fisheries Research Section and Professor Bertram who was visiting the area looking for dugongs); also soon after dawn at Marsa Halot, north of Port Sudan, after accidental drowning of a dugong in a fishing net during the previous evening (Vine, pers. comm.).
They have been reported to whistle (Kingdon 1971), bleat like a lamb (Troughton 1947), trill, bark, chirp and quack, but none of these sounds provides long distant communications (Kingdon 1971). A paper by Parsons et al. (2013) provides a recent study of dugong vocalizations based on recordings of tagged individuals. The authors make the point that dugongs are essentially shallow-water dwellers spending over 72% of their time in less than 1 m depth. Sound transmission at such levels is susceptible to interference and communication ranges are substantially reduced to between 10 m and 100 m. In total, five kinds of calls were categorized, all at relatively low frequencies and unlike those of dolphins or larger cetaceans that employ echolocation. In the Egyptian Red Sea, a calf dugong was recorded swimming in circles before ascending to the surface to take a quick breath and continued touching its muzzle with its flippers producing a chirping like sound (Shawky et al. 2016).
Utilisation
Outside the Arabian region dugongs have played a role in legends in Kenya where the animal is known there as the “Queen of the Sea”. Body parts are used as food, medicine, and decorations. In the Gulf states, dugongs served not only as a source of food, but their tusks were used as sword handles. Dugong oil was used as a preservative and conditioner for wooden boats, whilst people living in and around the Gulf of Kutch in India believe its meat is an aphrodisiac. Dugong ribs were used to make carvings in Japan. In southern China dugongs were traditionally regarded as a “miraculous fish”, and it was bad luck to catch them. In the Philippines, dugongs are thought to bring bad luck, and parts of them are used to ward against evil spirits. In areas of Thailand, it is believed that the dugong’s tears form a powerful love potion, while in parts of Indonesia they are considered reincarnations of women. In Papua New Guinea they are seen as a symbol of strength (Marsh 2002).
At present, dugongs are not deliberately caught by fishermen in Egypt, Saudi Arabia or Yemen, but if they are accidentally caught in gill nets, their meat is eaten, mostly by fishermen. However, fishermen at Tuwwal in Saudi Arabia do not eat dugong meat. Dugongs captured accidentally in their nets are either released if they are still alive or thrown back into the sea if they are dead or given to universities for study and/or display.
In Sudan, dugongs have in the past been hunted from small boats with a spear, but since their tough skin made them difficult to kill wooden plugs were apparently hammered into their nostrils to suffocate them. Their meat was appreciated as a nutritious addition to the local diet, and everything was utilized, including the skin. When this dries out, it became rock hard and was used by Beja tribesmen to make shields.
In Egypt dugong skin was utilized in making shoes (Gohar 1957) with skins from dugongs caught at Al-Wejh, Saudi Arabia being sold in Egypt for shoe-making (Preen 1989). Bertram and Bertram (1973) also noted the use of dugong skin for sandals and shields in the Red Sea countries. Some people in the Egyptian Red Sea used the thick skin as protective armour (Shawky 2018). Similarly, skins from dugongs caught in northern parts of Yemen were sold in Aden and Djibouti where they were turned into shields and soldiers’ helmets (Preen 1989). Dugong oil was recommended as a substitute for cod-liver oil in Egypt (Gohar 1957) and was used for a variety of purposes in Gizan (Saudi Arabia), including cooking and massage (Preen 1989). Gizani fishermen also used dugong meat to treat kidney failure and for the relief of stomach gases, while the bones were used to treat rheumatism (Preen 1989).
Archaeological and Historical Context
Archaeological evidence confirms the exploitation of dugongs by early humans. As long as 6000 years ago, dugongs on the small island of Akab in the present day United Arab Emirates (UAE) were hunted for food (Méry et al. 2009). Excavations in the Torres Strait, Australia, have revealed that dugong hunting in this region dates back at least 4000 years and possibly up to 7000 years (Crouch et al. 2007) and that the harvest has been substantial for at least 400–500 years (McNiven and Bedingfield 2008). Despite a long history of utilization (Bibby 1970), their dietary and cultural importance to the people of the Arabian Peninsula has declined (Bertram and Bertram 1973; Husar 1978; Nishiwaki et al. 1979).
Population Decline
Al-Abdulrazzak and Pauly’s (2017) paper in the April 2017 edition of Zoology in the Middle East attempts to reconstruct historical baselines for the Arabian Gulf’s population of dugongs, providing evidence of much larger numbers in the past. The situation in the Red Sea is also critical and, disturbingly, there is evidence to suggest that some protection strategies are proving ineffectual. A recent study of dugong protection in the northern Red Sea, focused on a Marine Park specifically established to conserve marine life, reached a negative conclusion regarding the prognosis for Red Sea dugongs: “the lack of detail about current threats to dugongs and turtles in the Red Sea is of international concern given that these animals are threatened worldwide and that populations in the Red Sea may be isolated from other populations. The closest dugong populations are in southern Somalia and the Arabian Gulf, both about 1600 km from the northern Red Sea. Due to this isolation, population declines of dugongs and sea turtles in the Red Sea are unlikely to be reversed rapidly or could even result in local extinction” (Rouphael et al. 2013).
Summarising the basis for this depressing outlook, Rouphael and colleagues concluded that “Elba National Park is not affording complete protection to dugongs and sea turtles because these animals form by-catch to local fishers”. Recommending designation of IUCN Category 1a Protected Areas, they acknowledge that this will not be straightforward as fishers operating in Elba National Park are highly dependent on marine resources. The strategies adopted in such areas need to have community support “in order to maximise compliance and minimise social impacts” (Rouphael et al. 2013).
The relatively poor protection afforded by designated conservation areas and marine parks is by no means unique to the Elba National Park, or to other marine parks in the Red Sea. In a bid to improve this situation, Grech and Marsh (2008) developed a rapid approach to assess the risk to dugongs and “evaluate options to ameliorate that risk”. They looked at dugong environments in terms of their exposure to five types of risk and then developed management plans appropriate to the particular issues faced by dugongs in different areas. The main risk factors were “netting, indigenous hunting, trawling, vessel traffic, and poor-quality terrestrial runoff” and the key habitats were classified as ‘urban’ or ‘remote’. They found that “commercial netting or indigenous hunting had to be reduced in remote areas and the effects of vessel traffic, terrestrial runoff, and commercial netting had to be reduced in urban areas”. This work suggests a powerful approach to prioritizing management issues affecting dugongs in the Red Sea.
There are reports of dugongs accidentally captured on occasion in fishing nets in Djibouti and the Farasan Islands (Saudi Arabia) (Gladstone 2000; PERSGA/GEF 2001). Preen (2004a) observed a decline in numbers of dugong between 1987 and 1993 in the vicinity of Gizan and the Farasan Islands (Saudi Arabia) and suggested that this indicated the level of accidental net drowning of dugongs was unsustainable. A major issue for future conservation of dugongs is the paucity of information on dugong populations, particularly on the western coast of the Red Sea. There is an urgent need for regular and repeated population surveys (Preen 2004a). The recent development of standard survey methods will facilitate the acquisition of population data and monitoring (Preen 2004a).
The fate of dugongs in the nearby Arabian Gulf is of relevance to the Red Sea population. According to Preen (2004b), the Gulf supported a population of about 5800 dugongs, which was the largest known outside Australia. “The most important habitats occur (1) around Murawah Island (UAE), (2) between Qatar and Bahrain and (3) between Qatar and the UAE. Surveys of the UAE were repeated 13 years apart. The two estimates of the dugong population in that area were not significantly different, suggesting a stable population of ca. 3000 between 1986 and 1999” (Preen 2004b). However, a dramatic decline in the abundance of dugongs since then (Preen 2004b; Marsh 2002) is a cause for great concern. The decline emphasizes the need for systematic monitoring to detect the beginnings of such declines. The declines also emphasise the global conservation value of the remaining dugong populations within the Red Sea, especially on the African coast.
We still do not know enough about Red Sea based dugongs or the issues they face. Marsh et al. (2012) undertook a regional assessment of the dugong and concluded that the population of the Red Sea and Gulf of Aden Region is ‘Data Deficient’. The writing is, however ‘on the wall’, and the decline is by no means confined to Australia and Arabia. The overall dugong population has also been drastically impacted by commercial fishing in Indian and Sri Lankan waters (Bertram and Bertram 1970a, b; Husar 1978). In other areas, such as Borneo and the Philippines, dugongs have been rendered locally extinct (Wycherly 1969; Philip and Fisher 1970).
The opinion that the dugong of the Red Sea is possibly sub-specific has been argued for many years. This should stimulate more research such as analysis of DNA of the dugongs in the Red Sea and Arabian Gulf (J. Gasperetti, pers. comm.). The presence of deep water barriers across the Arabian Sea, the upwelling of very cold Antarctic waters of the Indian Ocean in the area of the Kuria Muria Islands, and the absence of dugong records along the coast of the Arabian Sea in Oman, suggest that there may be limited genetic exchange between the Red Sea and the East African dugongs (J. Gasperetti, in prep.). Preen (1989) has also mentioned the isolation of the Arabian Gulf dugong population.
Counting dugongs in order to assess population size has never been easy, but the introduction of unmanned aerial vehicles (UAVs) has brought new possibilities. A study carried out in Australia using a ScanEagle UAV mounted with a digital SLR camera payload was flown at 500, 750 and 1000 ft. and captured 6243 images, 627 containing dugongs. “Of all possible dugong sightings, 95% (CI = 90%, 98%) were subjectively classed as ‘certain’ (unmistakably dugongs). Neither our dugong sighting rate nor our ability to identify dugongs with certainty, were affected by UAV altitude. Turbidity was the only environmental variable significantly affecting the dugong sighting rate. Our results suggest that UAV systems may not be limited by sea state conditions in the same manner as sightings from manned surveys. The overlap between images proved valuable for detecting animals that were masked by sun glitter in the corners of images and identifying animals initially captured at awkward body angles. This initial trial of a basic camera system has successfully demonstrated that the ScanEagle UAV has great potential as a tool for marine mammal aerial surveys” (Hodgson et al. 2013).
Why are dugongs declining across their range? The numerous threats to their survival have not diminished. Along the urban coast of Queensland, impacts from gill netting, subsistence hunting, habitat loss from extreme weather events that are likely to be exacerbated by climate change, human settlement and agricultural pollution that continue to challenge local marine life, especially dugongs. But things could be worse! The magnitude of these threats is likely to be greater in most other parts of the dugong’s range than in Queensland. The Queensland coast supports a low human population density relative to most other parts of the dugong’s range and has a well- developed system of marine parks and pro-active management. Despite this, there is evidence of an ongoing decline in dugong numbers (Sobtzick et al. 2012) along the urban coast of the Great Barrier Reef Region, largely attributed to habitat loss associated with extreme weather events.
Marsh et al. (2012) undertook a regional assessment of the dugong and concluded that the East African population was likely ‘Endangered’; Red Sea and Gulf of Aden ‘Data Deficient’; Arabian Gulf ‘Data Deficient’; Indian sub-continent and Andaman and Nicobar Islands ‘Endangered’; continental South-East Asia ‘Endangered’; archipelagic East and South-East Asia ‘Data Deficient’; Western Pacific Islands ‘Data Deficient’; and Palau and the Japan (Ryukyus) as ‘Critically Endangered’. The most recent assessment of the Australian population (Woinarski et al. 2014) concluded it was ‘Near Threatened’.
Threats
Dugongs, like other populations of large marine mammals, are under threat of extinction (Levy and Prizzia 2018). They are very sensitive creatures, easily panicked, rapidly weakened and vulnerable to shocks causing potentially lethal injuries by forced encounters with unfamiliar environments or being trapped in dry conditions (Adulyanukosol et al. 2009). Acknowledging that the population is likely to be under a variety of threats, Halpern et al. (2008) “consider anthropogenic impacts on the Red Sea as medium and medium-to-high, and impacts on the Gulf of Aden as medium-to-high”. The main threats to the Red Sea’s dugongs, as noted by Marsh et al. (2012) are artisanal fishing, coastal development and shipping, with gill nets posing a particular threat. The behaviour of a dying calf in the Egyptian Red Sea has been described by Shawky et al. (2016).
By-catch is one of the main threats that affects the dugong population (Marsh et al. 2002a, b, 2012; Pilcher et al. 2017). There are reports of dugongs accidentally captured on occasion in fishing nets in Djibouti and the Farasan Islands (Saudi Arabia) (Gladstone 2000; PERSGA/GEF 2001). Preen (2004a) observed a decline in numbers of dugongs between 1987 and 1993 in the vicinity of Gizan and the Farasan Islands and suggested this indicated the level of accidental net drownings of dugongs was unsustainable. There is no doubt that entanglement in fishing nets has caused many deaths, although there are no precise statistics. In the Gizan area, 20–30 accidental gill net captures per year were estimated.
In Egypt, 35% of interviewees believed that fishing nets are the main reason for the dugong decline (Shawky 2018). The author recorded that 11% of fishermen indicated that the accidental capture of dugongs in fishing nets had increased, where 72% of fishermen release them back to the sea. During the last 50 years, a total of 25 dead dugongs was recorded. The highest percentage was recorded in Hurghada and Ras Banas (13%), and the lowest values (2%) were encountered in Wadi El Gemal National Park and Elba Protected Area, while no dead dugongs were recorded in Qosseir and Marsa Alam.
In Sudan, although hand-lining is the most common fishing method, large mesh nets used to catch sharks sometimes drown dugongs (Ormond 1976, 1978; IUCN/MEPA 1984). At present, fishermen in Sudan set their gill nets in shallow seagrass areas receiving rain water run-off to catch fish, whilst dugongs accidentally drown in their nets. Similar cases occur throughout the dugong’s range in the Red Sea, including Yemen, Saudi Arabia, Egypt, Sudan, Djibouti and Eritrea (Robineau and Rose 1982).
Monofilament nylon fish nets are considered to be much more destructive than are multifilament nets (FAO 1979). Bertram and Bertram (1973) state that the serious progressive decrease of dugongs in waters off Sri Lanka was a result of accidental drowning in commercial fish nets. Preen (1989) has described the behaviour of dugongs when they are entangled in fishing nets: “In large meshed nets the dugong’s snout, flippers or fluke tips can become caught, and, because these nets are made from stronger material, it is less likely that the dugong can break free. In its effort to escape the dugong often twists along its longitudinal axis and this can cause the net to roll up. Once the net is rolled enough to lift the lead-line off the bottom, its weight eventually pulls the dugong away from the surface, and the dugong drowns”. Hunting has historically been a problem too, although in most areas they are no longer hunted, with the exception of certain indigenous communities. In areas such as northern Australia, hunting remains the greatest impact on the dugong population (Marsh 2002). In many countries, the legislation does not exist to protect dugongs, and if it does it is not enforced (Marsh 2002).
The major drivers for incidental capture in fishing gear and illegal hunting are poverty and declining fish stocks. Dugongs are legally protected in most of their range. However, enforcement is typically weak or non-existent. In Egypt, more than 50% of the fishermen said that there is no enforcement and penalties are not imposed (Shawky 2018). The imperative of artisanal fishers to break the law is increased by the opportunity to sell both dugong meat (Robards and Reeves 2011) and valuable commodities such as swim bladders and shark fins (Marsh et al. 2012). Artisanal coastal and riverine fisheries are vital to the livelihoods and food security of coastal peoples, especially in the tropics (Batista et al. 2014), including in most dugong ranges. On a global scale, such fisheries catch the same amount of fish for human consumption as commercial fisheries, yet employ some 25 times the number of fishers (over 12 million people; Chuenpagdee and Pauly 2008; Batista et al. 2014). Gill nets pose a significant threat to many marine mammals including dugongs (Pilcher and Nasr 2003; Read et al. 2006; Read 2008; Moore et al. 2010). Some dugong declines coincide with the introduction of monofilament nylon gill nets (Muir and Kizka 2012). However, it is often very difficult to convince the fishers or the fisheries managers to take the capture of dugongs seriously. When dugong population sizes are low, their capture in fisheries is a rare event, which becomes rarer as the dugong population declines.
Habitat loss and degradation represents another important driver (Marsh et al. 2002a, b). Significant seagrass loss by mining, boat propeller and coastal development leads to dugongs having reduced food resources, delayed reproduction and starvation (Marsh et al. 2012). Food shortages can be caused by many factors, such as a loss of habitat, death and decline in the quality of seagrass, and a disturbance of feeding caused by human activity. Sewage, detergents, heavy metals, hypersaline water, herbicides, and other waste products all negatively affect seagrass meadows (Marsh et al. 2002a, b). Seagrass beds are often targets for trawler fishing (Marsh et al. 2002a, b). Trawling has a detrimental effect on seagrass beds where the trawl chain and net, which are dragged across the seagrasses, can strip the plants of their leaves, thereby reducing the available food for dugongs (Preen 1989). Damage to seagrass beds by trawling has been demonstrated by Peres and Picard (1975). The depth distribution of seagrass depends on a number of interrelated factors, the most important being water turbidity (Young and Kirkman 1975; Harris et al. 1980). Any activity that increases turbidity reduces light penetration and limits the growth and survival of seagrasses (Zieman et al. 1984; Marsh 2002). Deposition of sediments has destroyed areas of seagrass beds in Moreton Bay in Australia (Young and Kirkman 1975).
Extreme weather such as cyclones and floods can destroy hundreds of square kilometres of seagrass meadows, as well as washing dugongs ashore (Heinsohn and Spain 1974; Kenyon and Poiner 1987). The recovery of seagrass meadows and the spread of seagrass into new areas, or areas where it has been destroyed, can take over a decade. Most measures for protection involve restricting activities such as trawling in areas containing seagrass meadows, with little to no action on pollutants originating from land. In some areas, water salinity is increased due to wastewater from desalination plants, and it is unknown how much salinity seagrass can withstand (Marsh 2002). Climate change is projected to lead to altered coastal environmental conditions and increases in severe tropical storms and flood events that could affect both dugongs and their seagrass habitats, exacerbating the effects of the other drivers listed above (Marsh et al. 2012; Levy and Prizzia 2018). Abu El-Regal et al. (2012) reported that Marsa Abou Dabbab (one of the popular dive sites in Egypt) was subject to a very severe flood in winter 2010 and the bay bottom was almost covered by sediments and seagrasses were almost lacking for one year. The bay started to be restored again in September 2011, and one dugong was recorded once by some divers.
In some cases, where recreational diving is a regular occurrence, individual dugongs may become acclimated to the presence of divers that are not perceived as a threat. Ecotourism has resulted in the establishment of operations involving dugong-watching cruises in several countries (Marsh et al. 2002a, b). In such instances, such as at Marsa Alam in Egypt, large groups of divers may observe an acclimated dugong for considerable periods of time. A number of scientific studies have been recently undertaken on such animals (Shawky 2018). Dugongs are difficult animals to study, partially because of their shyness and wariness, avoiding contact with boats and people, especially at the surface. Their responses to divers underwater can be slightly less reticent with their poor eyesight often resulting in gradual advances, apparently to take a closer look (P. J. Vine, pers. comm.).
Vessel strikes and the alienation of dugongs from key habitats as a result of harassment are possible adverse impacts. Based on interview surveys in Egypt, Shawky (2018) recorded that 31% of respondents indicated that tourism activities have effects on dugongs. The author was studying the impact of tourism activities on the ecological behaviour of dugongs in Marsa Alam, Egypt. Fleeing behaviour was mainly recorded at the surface when dugongs ascend for breathing. The snorkelers at the surface used to approach one of the dugongs and sometimes touch it. The dugong changed its direction and moved a few metres away from the snorkelers then ascended to take a quick breath. This attitude interferes with the normal breathing of the dugong, which may explain more travelling of dugongs in the presence of a disturbance. This necessitates raising public awareness and development of guidelines for tourists to understand the different behavioural categories of dugongs.
The impact of chemical pollution on dugongs is unquantified (Marsh et al. 2012). Oil spills are a danger to dugongs in some areas (Marsh 2002). At least 37 dugongs were killed by the Nowruz oil spill in 1983 (Preen 1989). Fishermen at Al Ghardaqa attribute the scarcity of dugongs in the area to oil pollution, especially at the production sites. Oil could impact upon dugongs through fouling by direct contact, absorption of dissolved toxic fractions from the water column or through the ingestion of seagrass or sediment particles with absorbed oil (Preen 1989). Inhalation of oil would lead to intense lung tissue reaction and pneumonic symptoms, which are frequently fatal in marine mammals (Gaskin 1982). Respiratory diseases may be common in dugongs (Campbell and Ladds 1981), increasing their vulnerability to irritants such as oil (Preen 1989). Oil can also damage seagrass communities (Zieman et al. 1984) which causes nutritional stress on dugongs.
Acoustic pollution causes indirect effects on dugongs such as injury, habitat damage and social disturbance (Marsh et al. 2002a, b). Seismic surveys may have an impact on dugongs, for example, interfering with the animal’s natural acoustic communication signals, behavioural changes including disturbance reactions, damage to their hearing systems and effects on individuals or populations in the short- or long-term.
Sharks and rays are a source of danger to dugongs and may attack them. Jones (1967) reported a 7 cm long spine of a ray found attached to the peritoneum of a captured dugong. The presence of sharks probably alters dugong behaviour and habitat use through fear (Wirsing et al. 2007). In 2012, one diver reported that he saw two dugongs with a tiger shark feeding on one of them at 30 m depth in Shaab Saleh at the southern east coast of Egyptian Red Sea (Shawky 2018). The lower body part of the dugong was seen inside the shark’s mouth. The other dugong was not seen for more than one month. O’Connell and de Jonge (2014) suggested that tiger sharks (Galeocerdo cuvier) can directly have an influence on the dugong distribution. Dugongs can manage predation risk by modifying the sequence of their behavioural states in spatial and temporal scales (Wirsing and Heithaus 2012).
Conservation
Conservation of dugongs has recently been reviewed by Marsh et al. (2012), covering both manatees and dugongs on a global scale. The monograph includes discussions on both extinct and extant species, their feeding biology, behaviour and habitat use, life history, reproductive biology and population dynamics, threats, conservation status and opportunities together with a comprehensive bibliography. The Red Sea and Gulf of Aden is mentioned on three pages (out of 521) and their comments have been covered elsewhere in this chapter.
The United Nations Environment Programme (UNEP) created the Convention on Biological Diversity (CBD) in response to increasing worldwide biodiversity loss (Marsh et al. 2012; Cleguer 2015). The need for dugong conservation is in agreement with the purpose of this convention as they are essential components of tropical and subtropical marine environments. The Conservation of Migratory Species (CMS) and Memorandum of Understanding (MoU) by UNEP determined the threats and challenges for the Conservation and Management of Dugongs (Dugong dugon) and their seagrass habitats (http://cms.int/dugong/en/node/4251).
Management of coastal marine environments for the conservation of dugongs and seagrass habitat requires a deep understanding of dugong populations, their movements, behaviour and the distribution of the seagrass on which they depend. The task is complicated by the complexity of dugong behaviour and resource use across multiple spatial scales, confusing efforts to define and protect key dugong habitats.
Analysis of interview surveys and the feeding ecology mentioned in this chapter indicate that dugong populations along the Red Sea are distributed according to the distribution of seagrass habitat across a range of spatiotemporal scales (Marsh and Rathbun 1990; Marsh and Lawler 2001, 2002, 2006) and, therefore, dugong management activities should be conducted through various scales and jurisdictional limits. The efficiency of wide-ranging monitoring programs to identify trends in dugong numbers at scales of hundreds of km2 is confounded by the dugong movements on a large scale (Marsh et al. 1994; Gales et al. 2004). As mentioned before in this chapter, dugongs in the western coast of the Egyptian Red Sea were not sighted for a long time from a specific site which reflects that they may have moved long distances out of the study area. Also from the 30 identified dugongs, not one was re-sighted in the other area which suggests that the individuals move north and south or offshore around the islands. Thus, estimates of population size of dugongs can be made at a regional range. This is in agreement with Cleguer (2015), who suggested that the implementation of conservation actions might not be sufficient at smaller scales to confirm the sustainability of dugong populations. The dugongs frequently make large-scale moves between core seagrass habitats.
Recent studies in the Egyptian Red Sea indicated that important dugong habitats occur in Marsa Alam and Wadi El Gemal National Park. A strategy for protecting dugongs in a DPA (Dugong Protected Area) as suggested by Marsh et al. (2002a, b) in Queensland, Australia, is a high priority. In a DPA, the human impacts and netting uses are adapted or forbidden, but this may not be as successful as thought before (Grech and Marsh 2007); the tendency for dugongs to follow the bottom in a wide range of movements may increase their exposure to incidental capture in bottom-set gill nets (Sheppard 2008). Therefore, management needs to be harmonised on an ecological scale related to the dugong by arranging cross-jurisdictional management between local official authorities. However, the results reported in this chapter indicate the necessity to implement dugong conservation actions at local scales as well.
In Egypt, many activities were conducted by the second author and his team to raise public awareness for dugong conservation. Several workshops focusing on dugong monitoring were conducted for 15 persons. The ‘Egyptian Dugong Team (EDT)’ (Fig. 18.24) has been involved in all field activities reported in this chapter in addition to their involvement in conducting public awareness campaigns among local communities and training of diving and tourists guides. To enhance public awareness, the second author, certified as a PADI Master Instructor, has created a PADI approved ‘Dugong Conservation Distinctive Specialty Diver Course’ (PADI course approval on January 25, 2018) (Fig. 18.25).
Legal Protection
Rouphael et al. (2013) discuss the role of marine protected areas in the Red Sea in terms of their efficacy in protecting dugongs and turtles. They concluded that the activities of fishermen in Elba National Park (Egypt) were leading to deaths of dugongs caught in their nets and that this was likely to lead to local extinction with little prospect of population recovery. They state that part of the issue relates to the level of protected status and a lack of awareness of the extreme fragility of the region’s dugong population.
Referring to the marine park, they state “…it is managed solely as an IUCN Category VI Protected Area; fishers lack awareness of laws pertaining to these animals, and fishers are highly resource dependent. Potential management strategies to reduce bycatch include the establishment of IUCN Category 1a Protected Areas in important dugong and sea turtle habitat, encouraging fishers to adopt fishing gear that poses less risk to megafauna and raising awareness among fishers of the protected status of dugongs and sea turtles” (Rouphael et al. 2013).
Outside of the Red Sea, the United Arab Emirates has banned all hunting of dugongs within its waters, as has Bahrain. The UAE has additionally banned drift net fishing. India and Sri Lanka ban the hunting and selling of dugongs and their products. Japan has listed dugongs as endangered and has banned intentional kills and harassment. Hunting, catching and harassment is banned by the People’s Republic of China. The first marine mammal to be protected in the Philippines was the dugong, although monitoring this is difficult. Palau has legislated to protect dugongs, although this is not well enforced and poaching persists. The dugong is a national animal of Papua New Guinea, which bans all except traditional hunting. Vanuatu and New Caledonia ban hunting of dugongs. Dugongs are protected throughout Australia, although the rules vary by state; in some areas, indigenous hunting is allowed (Marsh 2002).
In accordance with Article III of the Jeddah Convention and Protocol of 1982, PERSGA formulated two additional protocols: (a) Protocol concerned with the Protection of the Marine Environment from Land-Based Activities in the Red Sea and Gulf of Aden (September 2005), and (b) Protocol concerned with the Conservation of Biological Diversity and the Establishment of a Network of Protected Areas in the Red Sea and Gulf of Aden (December 2005). The two Protocols were signed by plenipotentiaries during 2005. Saudi Arabia, Sudan, Djibouti and Jordan ratified the two protocols, and ratification is in the process for other countries. Yemen and Jordan have developed final National Programmes of Action (NPAs). PERSGA supported Egypt, Sudan, Djibouti and Saudi Arabia to develop NPAs during 2008–2009 (PERSGA 2006).
According to Khalil (2010), the total area of the already defined 8 MPAs in the Network is around 11,000 km2. However, there are still 5 MPAs in the Network of undefined areas. On the other hand, there are more national protected areas in countries not yet included in the Network (Khalil 2010). Limited technical capacity and experience in MPA management together with lack of surveillance and enforcement in MPAs are the main constraints in the region. The MPAs and Biodiversity Programmes administered by PERSGA undertake projects and activities addressing capacity building, networking and assistance in MPA assessments and planning (Khalil 2010; PERSGA 2016). The ecological and social parameters guiding management of marine protected areas in the Red Sea were discussed by Gladstone (2000).
PERSGA and UNEDO implemented a GEF funded project for developing a regional strategy for reducing unintentional emissions of Persistent Organic Compounds in coastal zones of the Red Sea and Gulf of Aden (PERSGA 2006). At a regional level PERSGA has taken the initiative to conserve key habitats and key species in the region, taking several steps toward their protection. The first step was developing a set of Standard Survey Methods (SSM). The second step involved training regional specialists in these methods.
PERSGA joined efforts with the World Bank for implementation of the GEF funded project “Strategic Ecosystem-Based Management of the Red Sea and the Gulf of Aden”, also known as the SEM project. The SEM project focuses on improving the management of marine resources in the Red Sea and the Gulf of Aden through building the capacity for resource protection, implementing incentive-based systems for communities and harmonization of the knowledge base of marine resources between the PERSGA member countries. This is being achieved through institutional and technical assistance with on‐ground activities in selected MPAs, including awareness of the participatory approach in using marine resources and applying Ecosystem-Based Management principles (EBM). The SEM project has three technical components and a management component. Its implementation phase began in January 2014.
Focusing on issues concerning the protection of the Farasan Islands in the southern sector of the Saudi Arabian Red Sea, surveys were undertaken to assess the state of the coastal and marine resources and the issues associated with human activities. Stakeholders were interviewed about issues and their attitudes toward the proposed protected area as well as possible constraints to planning and management. Here, as elsewhere in the Red Sea, the most immediate threat to the marine resources was over-exploitation by fisheries. The nature of management activities appropriate for the MPA, and the scale of management, were constrained by a number of unique and important factors: declines in national financial support for conservation efforts, a lack of trained personnel, difficulties in attracting staff to this remote location, loss of community support, the absence of a tourist base from which economic instruments could be developed, and the lack of local non-governmental organizations. Management initiatives recommended for the ‘Farasan Islands Marine-Protected Area’ included zoning, community participation in management, public awareness and training as a first step, followed by site-specific management actions, research and monitoring, and infrastructure development.
Conclusion
A global overview of issues relating to conservation of dugongs was published in 2002 (Marsh 2002), and this had a section specifically devoted to the Red Sea. Marsh based this section of her country by country report primarily on the work of Anthony Preen (Preen 1989). Reliable data for much of Yemen, Eritrea, Sudan and Egypt was sparse, and this situation remains the case today, with the possible exception of the Egyptian coastline and the Eilat Coral Reserve where recent surveys have been undertaken. Although legal protection exists in many parts of the dugong’s range, problems of law enforcement and education remain (FAO 1979).
In terms of dugong conservation actions in the Red Sea, Marsh et al. (2012) note that protection measures have been introduced into legislation in most of the countries bordering the Red Sea including establishment of Marine Protected Areas (MPAs). Declaration of Marine Protected Areas does not mean that dugongs are adequately protected in these areas. MPAs in the Red Sea have been described as ‘paper parks’ where implementation of protection measures is not enforced. Recent efforts, largely coordinated by PERSGA, have been made to counter this criticism and create credible and effective safe habitats for Red Sea dugongs. Hopefully, it is not too late to support the establishment of sustainable dugong populations in the region.
References
Abu El-Regal MA, El-Moselhy K, El-Saman MM (2012) Evaluation of threats to the rare and endangered inhabitants of the seagrass beds in the Red Sea: a case study at Abu Dabbab bay, Marsa Alam, Egypt. J Egypt Acad Soc Environ Develop 7(1):1–15
Adulyanukosol K, Dulyanukosol K, Prasittipornkul C, Man-anansap SO, Boukaew P (2009) Stranding records of dugong (Dugong dugon) in Thailand. In: Proceedings of 4th international symposium on SEASTAR2000 and Asian bio-logging science (The 8th SEASTAR2000 workshop)
Al-Abdulrazzak D, Pauly D (2017) Reconstructing historical baselines for the Persian/Arabian Gulf Dugong, Dugong dugon (Mammalia: Sirena). Zool Middle East 63(2):95–102
Amamoto N, Ichikawa K, Arai N, Akamatsu T, Shinke T, Hara T, Adulyanukosol K (2009) Seasonal characterization of dugong feeding and biomass utilization on selected sites in Talibong Island. In: Proceedings of 4th international symposium on SEASTAR2000 and Asian bio-logging science (The 8th SEASTAR2000 workshop), pp 41–43
Anand Y (2012) First record of feeding trails of dugongs in Gulf of Kachchh (GoK), Gujarat. Indian Forester 138(10):968–969
Anderson PK (1982a) Studies of dugongs at Shark Bay, Western Australia II. Surface and subsurface observations. Aust Wildlife Res 9:85–100
Anderson PK (1982b) Studies of dugongs at Shark Bay, Western Australia I. Analysis of population size, composition, dispersion and habitat use on the basis of aerial survey. Aust Wildlife Res 9:69–84
Anderson PK (1984) Dugong. In: Macdonald D (ed) The encyclopedia of mammals. Facts on File, New York, pp 298–299
Anderson PK (1998) Shark Bay dugongs (Dugong dugon) in summer: II. Foragers in a Halodule-dominated community. Mammalia 62:409–425
Anderson PK, Birtles A (1978) Behaviour and ecology of the dugong, Dugong dugon (Sirenia): observations in Shoalwater and Cleveland Bays, Queensland. Aust Wildlife Res 5:1–23
Aragones LV, Lawler IR, Foley WJ, Marsh H (2006) Dugong grazing and turtle cropping: grazing optimization in tropical seagrass systems? Oecologia 149:635–647
Bakker ES, Pagès JF, Arthur R, Alcoverro (2016a) Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39:162–179
Bakker ES, Wood KA, Pagès JF, Veen GF, Christianen MJA, Santamaría L, Bart A, Nolet BA, Hilt S (2016b) Herbivory on freshwater and marine macrophytes: a review and perspective. Aquat Bot 135:18–36
Barnett C, Johns D (1976) Underwater observations of dugong in northern Queensland, Australia, with notes on dugong hunting and recommendations for future research. In: FAO scientific consultation on marine mammals, advisory committee on marine resources research, Bergen, Norway
Batista VS, Fabré N, Malhado ACM, Ladle RJ (2014) Tropical artisanal coastal fisheries: challenges and future directions. Rev Fisheries Sci Aquaculture 22(1). https://doi.org/10.1080/10641262.2013.822463
Bertram GCL (1974) Conservation of Sirenia. Current status and perspectives for action. IUCN Occas Paper 12:1–120
Bertram GCL, Bertram CKR (1970a) The dugongs of Ceylon. Loris 12:53–55
Bertram GCL, Bertram CKR (1970b) Dugongs in Ceylon. Oryx 10:362–364
Bertram GCL, Bertram CKR (1973) The modern Sirenia: their distribution and status. Biol J Linn Soc 5:297–338
Bessey C, Heithaus MR, Fourqurean JW, Gastrich KR, Burkholder DA (2016) Importance of teleost macrograzers to seagrass composition in a subtropical ecosystem with abundant populations of megagrazers and predators. Marine Ecology Prog Ser 553:81–92
Best RC (1981) Foods and feeding habits of wild and captive Sirenia. Mammal Rev 11:3–29
Bibby G (1970) Looking for Dilmun. Penguin, Middlesex, UK, pp 21–30
Bode M (2009) The behaviour of Dugong dugon and the influence of tourism on the Dugong in Abu Dabab in Marsa Alam, a popular dive site in Egypt. Field report on BSc study. Georg-August University Göttingen, Germany
Campbell RSF, Ladds PW (1981) Diseases of dugongs in northeastern Australia: a preliminary report. In: Marsh H (ed) The Dugong. Proceedings of seminar/workshop. James Cook University, North Queensland, pp 100–102
Chilvers BL, Delean S, Gales NJ, Holley DK, Lawler IR, Marsh H, Preen AR (2004) Diving behaviour of dugongs, Dugong dugon. J Exp Marine Biol Ecol 304:203–224
Chuenpagdee RA, Pauly DA (2008) Small is beautiful? A database approach for global assessment of small-scale fisheries: preliminary results and hypotheses. Am Fish Soc Symp 49(1):575
Cleguer C (2015) Informing dugong conservation at several spatial and temporal scales in New Caledonia. Biodiversity and ecology. PhD thesis, Université Pierre et Marie Curie, Paris (France), James Cook University, Townsville (Australia)
Collier C, Waycott M, McKenzie LJ (2012) Light thresholds derived from seagrass loss in the coastal zone of the northern Great Barrier Reef, Australia. Ecol Ind 23:211–219
Cope RC, Pollett PK, Lanyon JM, Seddon JM (2015) Indirect detection of genetic dispersal (movement and breeding events) through pedigree analysis of dugong populations in southern Queensland, Australia. Biol Conserv 181:91–101
Crouch J, McNiven IJ, David B, Rowe C, Weisler MI (2007) Marine resource specialization and environmental change in Torres Strait during the past 4000 years. Archaeol Ocean 42:49–64
Cullen-Unsworth LC, Nordlund LM, Paddock J, McKenzie LJ, Unsworth RKF (2014) Seagrass meadows globally as a coupled social-ecological system: implications for human wellbeing. Marine Pollut Bull 83:387–397
Cullen-Unsworth LC, Jones BL, Seary R, Newman R, Unsworth RKF (2017, in press) Reasons for seagrass optimism: local ecological knowledge confirms presence of dugongs. Marine Pollut Bull
D’Souza E, Patankar V, Arthur R, Alcoverro T, Kelkar N (2013) Long-term occupancy trends in a data-poor dugong population in the Andaman and Nicobar archipelago. PLoS ONE 8:e76181
De Iongh HH, Kiswara W, Kustiawan W, Loth PE (2007) A review of research on the interactions between dugongs (Dugong dugon Müller 1776) and intertidal seagrass beds in Indonesia. Hydrobiologia 591:73–83
Dentzien-Dias P, Carrillo-Briceño JD, Francischini H, Sánchez R (2018) Paleoecological and taphonomical aspects of the Late Miocene vertebrate coprolites (Urumaco Formation) of Venezuela. Paleogeog Paleoclimat Paleoecol 490:590–603
Deutsch CJ, Reid JP, Bonde RK (2003) Seasonal movements, migratory behavior, and site fidelity of West Indian manatees along the Atlantic Coast of the United States. Wildlife Monogr 151:1–77
Domning DP (1999) Sirenia. In: Maglio VJ, Cooke HBS (eds) Evolution of African mammals. Harvard University Press, Cambridge, pp 573–581
Ebrahim A, Olds AD, Maxwell PS, Pitt KA, Burfeind DD, Connolly RM (2014) Herbivory in a subtropical seagrass ecosystem: separating the functional role of different grazers. Marine Ecol Prog Ser 511:83–91
El Shaffai A (2011) Studies on the seagrass ecosystems in ‘Wadi El Gemal National Park’, Red Sea. MSc thesis, Suez Canal University, Ismailia
El Shaffai A (2016) Field Guide to Seagrasses of the Red Sea. In: Rouphael A, Abdulla A (eds) IUCN, Gland, Switzerland and total foundation. Courbevoie, France, 2nd edn. 56 p
FAO (1979) Mammals in the sea. Pinniped species summaries and report on sirenians in cooperation with UNEP. FAO Fisheries 5(2):151
Fox DL (1999) Dugong dugon: information. Animal diversity web. University of Michigan Museum of Zoology
Gales N, Mccauley RD, Lanyon JM, Holley D (2004) Change in abundance of dugongs in Shark Bay, Ningaloo and Exmouth Gulf, Western Australia: evidence for large-scale migration. Wildlife Res 31:283–290
Gaskin DE (1982) The ecology of whales and dolphins. Heinemann, London, p 459
Gladstone W (2000) Ecological and social basis for management of a Red Sea marine protected area. Ocean Coast Manag 43:1015–1032
Gohar HAF (1957) The Red Sea dugong. Publ Marine Biol Station Al Ghardaqa 9:3–49
Grech A, Marsh H (2007) Prioritising areas for dugong conservation in a marine protected area using a spatially explicit population model. Appl GIS 3:1–14
Grech A, Marsh H (2008) Rapid assessment of risks to a mobile marine mammal in an ecosystem-scale marine protected area. Conserv Biol 22(3):711–720
Gredzens C, Marsh H, Fuentes MM, Limpus CJ, Shimada T, Hamann M (2014) Satellite tracking of sympatric marine megafauna can inform the biological basis for species co-management. PLoS ONE 9(6):e98944
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Hanafy M, Gheny MA, Rouphael AB, Salam A, Fouda A (2006) The dugong, Dugong dugon, in Egyptian waters: distribution, relative abundance and threats. Zool Middle East 39(1):17–24
Harris M, King RJ, Ellis J (1980) The eelgrass Zostera capricomi in Illawarra Lake, New South Wales. Proc Linn Soc NSW 104:23–33
Hartman DS (1979) Ecology and behaviour of the manatee (Trichechus manatus) in Florida. Am Soc Mammalogists Spec Publ 5:1–153
Heinsohn GE (1972) A study of dugongs (Dugong dugon) in northern Queensland, Australia. Biol Conserv 4(3):205–13
Heinsohn GE, Birch WR (1972) Foods and feeding habits of the dugong, Dugong dugon, in northern Queensland, Australia. Mammalia 36(3):414–422
Heinsohn GE, Spain AV (1974) Effects of a tropical cyclone on littoral and sub-littoral biotic communities and on a population of dugongs (Dugong dugon (Muller)). Biol Cons 6:143–152
Heinsohn GE, Wake J (1976) The importance of the Fraser Island region to dugongs. Paper presented to the ACMRR (FAO) scientific consultation on the conservation and management of marine mammals and their environment. Bergen, Norway, 31 August–9 September 1976. Rome, FAO, ACMRR/MM/SC/WG4.11. Operculum 5(1):15–8
Hill WCO (1945) Notes on the dissection of the dugongs. J Mammal 26:153–175
Hines E, Reynolds J, Mignucci A, Aragones L, Marmontel M (eds) (2012) Sirenian conservation: issues and strategies in developing countries. University of Florida Press, Gainesville
Hobbs JPA, Willshaw K (2015) Unusual behavior and habitat use of a solitary male dugong inhabiting coral reefs at the Cocos (Keeling) Islands. Marine Biodivers. https://doi.org/10.1007/s12526-015-0360-6
Hodgson AJ (2004) Dugong behaviour and responses to human influences. PhD thesis, James Cook University, Townsville
Hodgson A, Kelly N, Peel D (2013) Unmanned aerial vehicles (UAVs) for surveying marine fauna: a dugong case study. PLoS ONE 8(11):e79556
Hossain MS, Bujang SJ, Zakaria MH, Hashim M (2016) Marine and human habitat mapping for the coral triangle initiative region of Sabah using landsat and Google earth imagery. Marine Policy 72:176–191
Husar SL (1975) A review of the literature of the Dugong (Dugong dugon). Paper presented to the ACMRR (FAO) scientific consultation on the conservation and management of marine mammals and their environment. Bergen, Norway, 31 August–9 September 1976. Rome, FAO, ACMRR/MM/SC/WG4.2. US Dept Int Fish Wildl Serv Rept 4, 30 p
Husar SL (1978) Dugong dugon. Mammalian species. Am Soc Mammal 88:1–7
Ichikawa K, Tsutsumi C, Arai N, Akamatsu T, Shinke T, Hara T, Adulyanukosol K (2006) Dugong (Dugong dugon) vocalization patterns recorded by automatic underwater sound monitoring systems. J Acoustical Soc Am 119(6):3726–33
IUCN/MEPA (1984) Report on the distribution of natural habitats and species in the Saudi Red Sea. Saudi Arabia Marine conservation programme 4(1/2), 274 p
IUCN/MEPA (1987) Red Sea, Saudi Arabia: an analysis of coastal and marine habitats of the Red Sea. MEPA coastal and marine management Series. Report no 1, 250 p
IUCN/UNEP (1985) Management and conservation of renewable marine resources in the Red Sea and Gulf of Aden region. UNEP regional seas reports studies no 64, 83 p
Jarman PJ (1966) The status of the dugong (Dugong dugon Muller); Kenya, 1961. East African Wildl J 4:82–88
Jones S (1967) The Dugong—its present status in the seas round India with observations on its behaviour in captivity. Internat Zool Yearbook 7:215–220
Jonklass R (1961) Some observations on Dugongs (Dugong dugon Erxleben). Loris 9:1–8
KendricK GA, Waycott M, Carruthers TJB, Cambridge MI, Hovey R, Krauss SI, Lavery PS, Les DH, Lowe JR, Vidal OMI, Ooi JIS, Orth RJ, Rivers OD, Montoya LR, Sinclair EA, Statton J, Dijk JKV, Verduin JJ (2012) The central role of dispersal in the maintenance and persistence of seagrass populations. Bioscience 62:56–65
Kenyon R, Poiner I (1987) Seagrass and cyclones in the western Gulf of Carpentaria. CSIRO Marine Laboratory Information Sheet, February 1987
Ketten DR, Odell DK, Domning DP (1992) Structure, function, and adaptation of the manatee ear. In: Thomas JA, Kastelein RA, Supin AY (eds) Marine mammal sensory systems. Plenum Press, New York, pp 77–95
Khalil AS (2010) Pressures, status and response to marine and coastal biodiversity in the Red Sea and Gulf of Aden. Report to PERSGA in accordance with the contract “Compiling data and information for biodiversity outlook report in the Regional Seas” in accordance with the set of indicators developed by MCEB. Jeddah, Kingdom of Saudi Arabia
Kingdon J (1971) East African mammals, vol. 1. An Atlas of evolution in Africa. Academic Press, New York, 457 p
Kwan D (2002) Towards a sustainable fishery for dugongs in Torres Strait: a contribution of empirical data and process. PhD thesis, James Cook University
Levy J, Prizzia R (2018) From data modeling to algorithmic modeling in the big data era: Water resources security in the Asia-Pacific Region under conditions of climate change. In: Masys AJ, Lin LSF (eds) Asia-pacific security challenges: managing black swans and persistent threats. Springer International Publishing, pp 197–220. https://doi.org/10.1007/978-3-319-61729-9_9
Lipkin Y (1975) Food of the Red Sea dugong (Mammalia: Sirenia) from Sinai. Israel J Zool 24:81–98
MacMillan L (1955) The dugong. Walkabout 21:17–20
Maitland RN, Lawler IR, Sheppard JK (2006) Assessing the risk of boat strike on dugongs Dugong dugon at Burrum Heads, Queensland, Australia. Pac Conserv Biol 12:321–326
Marsh H (1980) Age determination of the Dugong, Dugong dugon (Müller) in northern Australia and its biological implications. Rept Internat Whaling Comm (3):181–201
Marsh H (1986) The status of the Dugong in Torres Strait. In: Haines AK, Williams GC, Coates D (eds) Torres strait fisheries seminar. Port Moresby, 11–14 February 1985. Australian Govt Publish Service, Canberra, pp 53–76
Marsh H (1995) The life history, pattern of breeding, and population dynamics of the dugong. In: O’Shea TJ (ed) Proc workshop on manatee population biology. U.S. Fish and wildlife service technical report, pp 75–83
Marsh H (2002) Dugong: status report and action plans for countries and territories. UNEP/Earth print Nairobi, 162 p
Marsh H (2008) Dugong dugon. IUCN Red List of Threatened Species v. 2012.2. http://www.iucnredlist.org [accessed 10 November 2012]
Marsh H (2009) Dugong: Dugong dugon. In: Würsig B, Perrin W, Thewissen JGM (eds) Encyclopedia of Marine mammals, 2 edn. Academic Press, pp 332–335
Marsh HD (2014) Family dugongidae (Dugong). In: Wilson DE, Mittermeier RA (eds) Handbook of the mammals of the world: 4: sea mammals. Lynx Edicions, Barcelona, Spain, pp 564–573
Marsh H, Lawler IR (2001) Dugong distribution and abundance in the Southern Great Barrier Reef Marine Park and Hervey Bay: results of an aerial survey in October–December 1999. Research Publication Great Barrier Reef Marine Park Authority, Townsville
Marsh H, Lawler IR (2002) Dugong distribution and abundance in the Northern Great Barrier Reef Marine Park—November 2000. GBRMPA Research Publication 77, Townsville.http://www.gbrmpa.gov.au
Marsh H, Lawler IR (2006) Dugong distribution and abundance in the Southern Great Barrier Reef Marine Park and Hervey Bay: results of an aerial survey in 2005. Great Barrier Reef Marine Park Authority, Townsville
Marsh H, Kwan D (2008) Temporal variability in the life history and reproductive biology of female dugongs in Torres Strait: the likely role of seagrass dieback. Cont Shelf Res 28(16):2152–2159
Marsh H, Rathbun GB (1990) Development and application of conventional and satellite radio-tracking techniques for studying dugong movements and habitat usage. Aust Wildlife Res 17:83–100
Marsh H, Sobtzick S (2015) Dugong dugon. IUCN Red list of threatened species. https://doi.org/10.2305/iucn.uk.2015-4.rlts.t6909a43792211.en
Marsh H, Heinsohn GE, Channells PW (1984a) Changes in the ovaries and uterus of the Dugong, Dugong dugon (Sirenia: dugongidae), with age and reproductive activity. Aust J Zool 32:743–766
Marsh H, Heinsohn GE, Glover TD (1984b) Changes in the male reproductive organs of the Dugong, Dugong dugon (Sirenia: dugongidae), with age and reproductive activity. Aust J Zool 32:721–742
Marsh H, Heinsohn GE, Marsh LM (1984c) Breeding cycle, life history and population dynamics of the Dugong, Dugong dugon (Sirenia: dugongidae). Aust J Zool 32:767–788
Marsh H, Heinsohn GE, Marsh LM (1984d) Life history, breeding cycle and population dynamics of the dugong, Dugong dugon (Sirenia, Dugongidae). Aust J Zoology 32:767–788
Marsh H, O’Shea TJ, Reynolds JE (2012) Ecology and conservation of the Sirenia: dugongs and Manatees. Cambridge University Press, Cambridge, UK, p 521
Marsh H, Chanells PW, Heinsohn GE, Morrissey J (1982) Analysis of stomach contents of dugongs from Queensland. Aust Wildl Res 9:55–67
Marsh H, De’ath G, Gribble N, Lane B (2001) Shark control records hindcast serious decline in dugong numbers off the urban coast of Queensland. Great Barrier Reef Marine Park Authority/James Cook University
Marsh H, Penrose H, Eros C, Hugues J (2002a) Dugong: status report and action plans for countries and territories. UNEP early warning assessment report series, vol 1
Marsh H, Penrose H, Eros C, Hugues J (2002b) The Dugong (Dugong dugon) status reports and action plans for countries and territories in its range. Final report, United Nations Environment Programme, Nairobi, Kenya
Marsh H, Prince RIT, Saalfeld WK, Shepherd R (1994) The distribution and abundance of dugongs in Shark Bay. Wildlife Res 21:149–161
McNiven IJ, Bedingfield AC (2008) Past and present marine mammal hunting rates and abundances: Dugong (Dugong dugon) evidence from Dabangai Bone Mound, Torres Strait. J Archaeol Sci 35(2):505–515
Méry S, Charpentier V, Auxiette G, Pelle E (2009) A Neolithic ritual site in Umm al-Quwain, United Arab Emirates: the dugong bone mound of Akab. Antiquity 83:696–708
Mitchell J (1973) Determination of relative age in the dugong Dugong dugon (Müller) from a study of skulls and teeth. Zool J Linnean Soc 53:1–23
Mizuno K, Asada A, Matsumoto Y, Sugimoto K, Fujii T, Yamamuro M, Fortes MD, Sarceda M, Jimenez LA (2017) A simple and efficient method for making a high-resolution seagrass map and quantification of dugong feeding trail distribution: a field test at Mayo Bay, Philippines. Ecol Inform 38:89–94
Moore JE, Cox TM, Lewison RL, Read AJ, Bjorkl R, McDonald SL, Crowder LB, Aruna E, Ayissi I, Espeut P, Joynson-Hicks C, Pilcher N, Poonian CNS, Solarin B, Kiszka J (2010) An interview-based approached to assess marine mammal and sea turtle captures in artisanal fisheries. Biol Conserv 143:795–805
Moore AM, Ambo-Rappe R, Ali Y (2017) “The Lost Princess (putri duyung)” of the Small Islands: dugongs around Sulawesi in the Anthropocene. Frontiers Marine Sci 4:284
Muir C, Kiszka J (2012) East African dugongs. In: Hines E, Reynolds J, Mignucci-Giannoni A, Aragones L, Marmonental M (eds) Sirenian conservation: issues and strategies in developing countries. University Press of Florida, Gainesville
Nishiwaki M, Marsh H (1985) Dugong: Dugong dugon (Muller, 1776). In: Ridgway SH, Harrison R (eds) Handbook of marine mammals, vol 3. Academic Press, London, pp 1–31
Nishiwaki M, Kasuya T, Miyazaki N, Tobayama T, Kataoka T (1979) Present distribution of the Dugong in the world. Sci Rep Whales Res Inst 3:133–141
O’Connell CP, de Jonge VN (2014) Integrating the findings from this special issue and suggestions for future conservation efforts: a brief synopsis. Ocean Coast Manag 97:58–60
Ormond RFG (1976) The Red Sea. Promotion of the establishment of marine parks and reserves in the northern Indian Ocean including the Red Sea and Persian Gulf. IUCN Publications New Series no 35, pp 115–123
Ormond RFG (1978) Requirements and progress in marine conservation in the Red Sea. In: Gamble JC, Yorke RA (eds) Progress in underwater science, vol 3. Pentech, London, pp 167–176
Parsons MJG, Holley D, McCauley RD (2013) Source levels of Dugong (Dugong dugon) vocalizations recorded in Shark Bay. J Acoust Soc Am 134(3):2582–2588. https://doi.org/10.1121/1.4816583
Peres JM, Picard J (1975) Causes de la rarefaction et de la disparition des herbiers, de Posidonia oceanica sur les cotes Francaises de la Mediterranee. Aquatic Bot 1:133–139
PERSGA (2006) State of marine environment in the Red Sea and Gulf of Aden. PERSGA, Jeddah
PERSGA (2016) Guideline for planning and management of Marine Protected Areas using mapping techniques. Report prepared for PERSGA through Component 1 of the GEF/World Bank project “Strategic Ecosystem Management (SEM) of the Red Sea and Gulf of Aden”, PERSGA, Jeddah
PERSGA/GEF (2001) Strategic action programme for the Red Sea and Gulf of Aden, Country Reports. PERSGA, Jeddah, and World Bank, Washington, 205 p
PERSGA/GEF (2003) Coral Reefs in the Red Sea and Gulf of Aden. Surveys 1990 to 2000: summary and recommendations. PERSGA technical series no 7, PERSGA, Jeddah
PERSGA/GEF (2004) Survey of the proposed marine protected area at Dungonab Bay and Mukawwar Island, Sudan. Report for PERSGA, Jeddah
Philip Prince (Duke of Edinburgh), Fisher J (1970) Wildlife Crisis. Cowles Book Co, New York, 256 p
Pilcher N, Nasr D (2003) The status of coral reefs in Sudan. In: Coral reefs in the Red Sea and Gulf of Aden, Surveys 1990–2000: summary and recommendations. PERSGA technical series no. 7, PERSGA, Jeddah
Pilcher NJ, Adulyanukosol K, Das H, Davis P, Hines E, Kwan D (2017) A low-cost solution for documenting distribution and abundance of endangered marine fauna and impacts from fisheries. PLoS ONE 12(12):e0190021
Ponnampalarm L, Adulyanukosol K, Ooi JLS (2014) Aligning conservation and research priorities for proactive species and habitat management: the case of dugongs Dugong dugon in Johor, Malaysia. Oryx, 7 p. https://doi.org/10.1017/s0030605313001580
Preen A (1989) The status and conservation of dugongs in the Arabian region. MEPA report no 10, 200 p
Preen AR (1992) Interactions between dugongs and seagrasses in a subtropical environment. PhD thesis. James Cook University of North Queensland, Townsville, Australia
Preen A (1995) Impacts of dugong foraging on seagrass habitats: observational and experimental evidence for cultivation grazing. Marine Ecol Prog Ser 124:201–213
Preen A (2004a) Marine Mammals. In: Standard survey methods for key habitats and key species in the Red Sea and Gulf of Aden. PERSGA technical series, PERSGA, Jeddah, 10:267–309
Preen A (2004b) Distribution, abundance and conservation status of dugongs and dolphins in the southern and western Arabian Gulf. Biol Conserv 118:205–218
Rajamani L (2009) The conservation biology of the Dugong (Dugong dugon) and its seagrass habitat in Sabah, Malaysia: a basis for conservation planning. PhD thesis, Malaysia Sabah University
Read AJ (2008) The looming crisis: interactions between marine mammals and fisheries. J Mammal 89(3):541–548
Read AJ, Drinker P, Northridge S (2006) Bycatch of marine mammals in US and global fisheries. Conserv Biol 20(1):163–169
Reynolds JE (1981) Aspects of the social behaviour and herd structure of a semiisolated colony of West Indian manatees, Trichechus manatus. Mammalia 45:431–451
Robards MD, Reeves RR (2011) The global extent and character of marine mammal consumption by humans: 1970–2009. Biol Conserv 144(12):2770–2786
Robineau D (1969) Morphologie externe du complexe osseux temporal chez les sirinens. Mémoire du Muséum National d’Histoire Naturelle (Paris). Ser A Zool 60:1–32
Robineau D, Rose JM (1982) Le Dugong (Dugong dugon (Muller, 1776) Sirenia, dugongidae) en Republique de Djibouti. Biol Conserv 24:233–238
Rouphael T, Abdulla A, Attum O, Marshall N, Ghazali U (2013) Do marine protected areas in the Red Sea afford protection to dugongs and sea turtles? J Biodivers Endanger Species 1:1–6
Seddon JM, Ovenden J, Sneath H, Broderick D, Dudgeon C, Lanyon J (2014) Fine scale population structure of dugongs (Dugong dugon) implies low gene flow along the southern Queensland coastline. Conserv Genet 15(6):1381–1392
Shawky AM (2018) Ecological and behavioural studies on the Dugong Dugong dugon inhabiting Marsa Alam Egyptian Red Sea. Unpublished PhD thesis, Suez Canal University, Ismailia, Egypt
Shawky AM, Sallam WS, Alwany MA, Mohammad DA, Mohamed SZ (2016) Stranding of a neonatal dugong calf in Wadi El Gemal National Park: implications for Dugong conservation in Egypt. Al Azhar Bull Sci 27(2):1–11
Shawky AM, Sallam WS, Alwany MA, Mohammad DA, Mohamed SZ (2018, in press) Photo identification of Dugongs in Marsa Alam and Wadi El Gemal National Park, western Egyptian coast of the Red Sea
Sheppard JK (2008) The Spatial Ecology of Dugongs: applications to Conservation Management. PhD thesis, James Cook University, Townsville (Australia)
Sheppard C, Price A, Roberts C (1992) Marine ecology of the Arabian region: patterns and processes in extreme tropical environments. Academic Press, London
Sheppard JK, Preen AR, Marsh H, Lawler I, Whiting S, Jones R (2006) Movement heterogeneity of dugongs, Dugong dugon (Müller), over large spatial scales. J Exp Marine Biol Ecol 344:64–83
Sheppard JK, Marsh H, Jones RE, Lawler IR (2010) Dugong habitat use in relation to seagrass nutrients, tides and diel cycles. Marine Mammal Sci 26(4):855–879
Sobtzick S, Hagihara R, Grech A, Marsh H (2012) Aerial survey of the urban coast of Queensland to evaluate the response of the dugong population to the widespread effects of the extreme weather events of the summer of 2010–11. Final report to the Australian marine mammal centre and the national environmental research program. Reef and Rainforest Research Centre Ltd., Cairns, 62 p
Spain AV, Heinsohn GE (1973) Cyclone associated feeding changes in the dugong (Mammalia: Sirenia). Mammalia 37(4):678–680
Spiegelberger T, Ganslosser U (2005) Habitat analysis and exclusive bank feeding of the Antillean manatee (Trichechus manatus manatus L. 1758) in the Coswine Swamps of French Guiana, South America. Trop Zool 18:1–12
Tanaka K, Ichikawa K, Nishizawa H, Kittiwattanawong K, Arai N, Mitamura H (2017) Differences in vocalisation patterns of dugongs between fine-scale habitats around Talibong Island. Acoust Aust, Thailand. https://doi.org/10.1007/s40857-017-0094-7
Thornback J, Jenkins M (1982) Dugong, Dugong dugon (Muller 1776) Order Sirenia, family dugongidae. In: Thornback J, Jenkins M (eds) IUCN mammal Red Data book, part 1. Gland, Switzerland, pp 417–427
Tol SJ, Coles RG, Congdon CB (2016) Dugong dugon feeding in tropical Australian seagrass meadows: implications for conservation planning. PeerJ 4:e2194. https://doi.org/10.7717/peerj.2194
Travis W (1967) The voice of the turtle. Allen and Unwin, London
Troughton EL (1947) Furred animals of Australia. Charles Scribner’s Sons, New York, p 374
Tsutsumi C, Ichikawa K, Arai N, Akamatsu T, Shinke T, Hara T, Adulyanukosol K (2006) Feeding behavior of wild dugongs monitored by a passive acoustical method. J Acoust Soc Am 120(3):1356–60
Vine PJ (1986) Pearls in Arabian Waters. Immel Publishing, London, p 59
Wake J (1975) A study of habitat requirements and feeding biology of the Dugong, Dugong dugon, (Muller). BSc thesis, James Cook University, Townsville
WCMC (2015) https://www.unep-wcmc.org/resources-and-data/unep-wcmc-annual-report-2015
Weigle B, Wright IE, Ross M, Flamm R (2001) Movements of radio-tagged manatees in Tampa Bay and along Florida’s west coast 1991–1996. Technical Report TR-7/ISSN 1092-194X, St. Petersburg, Florida
Whiting SD (2002) Dive times for foraging dugongs in the Northern Territory. Aust Mammal 23:167–168
Wirsing AJ, Heithaus RM (2012) Behavioural transition probabilities in dugongs change with habitat and predator presence: implications for sirenian conservation. Marine Freshwater Res 63:1069–1076
Wirsing AJ, Heithaus MR, Dill LM (2007) Can you dig it? Use of excavation, a risky foraging tactic, by dugongs is sensitive to predation danger. Anim Behav 74:1085–1091
Woinarski JC, Burbidge AA, Harrison PL (2014) A review of the conservation status of Australian mammals. Therya 6(1):155–66
Wongsuryrat M, Chunkao K, Prabuddham P, Daungsavat M (2011) Distribution, abundance and conservation status of dugong around Koh Talibong, Trang Province, Thailand. J Sustain Dev 3(4):118–124
Wycherley PR (1969) Conservation in Malaysia. IUCN Publ, suppl paper 22, pp 113–114
Young PC, Kirkman H (1975) The seagrass communities of Moreton Bay, Queensland. Aquatic Bot 1:191–202
Zieman JC, Orth R, Phillips RC, Thayer G, Thorhaug A (1984) The effects of oil on seagrass ecosystems. In: Cairns J, Buikema AL (eds) Restoration and management of marine ecosystems impacted by oil. Butterworth, Boston, pp 37–64
Acknowledgements
We wish to acknowledge the cooperation of the many biologists who have studied the Red Sea’s dugongs in recent years. Some, but not all, of their work is referenced in this review. Institutional cooperation with, and interest in, dugongs has been sustained, and mention must be made of The Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden (PERSGA) and the United Nations Environment Programme (UNEP). The recent dugong studies in Egypt by Ahmed Shawky would not have been possible without the financial assistance of Rufford Small Grant (RSG: 17553-1, 21354-2), which is gratefully acknowledged. Special thanks go to PADI for approving the ‘Dugong Conservation Distinctive Specialty Diver Course’.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nasr, D., Shawky, A.M., Vine, P. (2019). Status of Red Sea Dugongs. In: Rasul, N., Stewart, I. (eds) Oceanographic and Biological Aspects of the Red Sea. Springer Oceanography. Springer, Cham. https://doi.org/10.1007/978-3-319-99417-8_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-99417-8_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99416-1
Online ISBN: 978-3-319-99417-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)