Abstract
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease that affects an increasing number of individuals worldwide. The underlying cause(s) of the late-onset form (LOAD) of the disease is still a matter of intensive investigations. The possibility that infectious organisms are involved in the etiology of AD has been gaining momentum. Furthermore, a wealth of data has suggested the involvement of the immune system in AD. In this context, properties of cells of the innate immune system such as natural killer (NK) cells specialized in destroying virus-infected cells have recently been investigated in amnestic mild cognitive impairment (aMCI) subjects and patients with a mild form (mAD) of the disease and data compared to healthy elderly individuals. These studies revealed an absence of differences in immune senescence between the three experimental groups. However, there were differential phenotypic changes in aMCI and mAD patients. This was the case of TLR2 and TLR9 (both decreased in AD) and NKG2A (decreased in aMCI). However, functional assays revealed an absence of modification of killing and degranulation activity in the cohort despite increased CD95 receptor, granzyme B expression, and upregulation of TNFα and IFNγ production. Furthermore, decreased chemotactic activity to CCL19 but not to CCL21 was observed in aMCI and mAD patients. Increased CD16 expression was observed in mAD patients. The bulk of these data suggested that NK cells of aMCI subjects were in a state of activation state in response of an as-yet-unidentified challenge.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
NK Cells
NK cells are lymphocytes that belong to the innate immune system. NK lymphocytes along with innate lymphoid cells 1 (ILC1) constitute the innate lymphoid group 1 (Lanier 2013; Walker et al. 2013). As innate lymphoid cells, NK cells are effectors and regulators of innate immunity and tissue modeling and repair. As a member of group 1 of ILC, they are characterized by their capacity to produce γ-interferon (IFNγ) (Spits et al. 2016). NK cells take part in the major processes of the immune system which are maintenance of homeostasis, surveillance against malignancy, immunosenescence (Iannello and Raulet 2013; Sagiv and Krizhanovsky 2013), and pathogen protection, specifically destruction of virus-infected cells (Lam and Lanier 2017). Systemic NK cell subsets can be distinguished according to expression of characteristic cell surface markers. For instance, CD56dimCD16+ NK cells possess distinct phenotype and function and account for approximately 90% of total NK cells, whereas CD56brightCD16− NK cells amount to approximately 10%. These subsets possess different homing property, the majority of CD56brightCD16− cells being located in the secondary lymphoid organs, whereas CD56dimCD16+ NK cells are largely found in the blood (Carrega and Ferlazzo 2012). NK cells can also be found in the brain in the case of experimental autoimmune encephalomyelitis (EAE), although the mechanism of recruitment of NK cells to the CNS under normal physiological conditions remains unclear (Poli et al. 2013). These two NK cell subpopulations also possess characteristic functions. For example, CD56dimCD16+ NK cells are more cytotoxic and express more CD16 (FcγRIII) and immunoglobulin-like receptors, whereas CD56brightCD16− NK cells mainly produce type I pro-inflammatory cytokines such as tumor necrosis factor α (TNFα) and IFNγ (Cooper et al. 2001). Activation of NK cells and production of IFNγ can be triggered by IL-12, IL-15, or IL-18 stimulation (Long et al. 2013). NK cells can also produce other immune factors such as immunosuppressive cytokine IL-10, growth factors such as granulocyte macrophage colony-stimulating factor (GM-CSF) and granulocytes colony-stimulating factor (G-CSF), and a series of chemokines such as CCL2, CCL3, CCL4, CCL5, and CXCL8 (Narni-Mancinelli et al. 2013). NK cells also act as regulatory cells to influence various other cell types of the innate and adaptive system such as monocytes/macrophages, DCs, T cells, B cells, and endothelial cells. NK cells also exert a negative feedback on macrophage activation following microbial infection (Vivier et al. 2008).
Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) is characterized by memory loss and cognitive decline, often associated with behavioral disorders (Scheltens et al. 2016). As neurodegenerative progresses, dead neurons are primarily found in hippocampal structures until it involves the whole brain (Selkoe 2011; Serrano-Pozo et al. 2011). The pathological characteristics of AD present extracellular accumulation of amyloid-β peptides (Aβ) that aggregate into plaques and intraneuronal hyperphosphorylation of the Tau protein which generates neurofibrillar tangles (DNF) (Querfurth and LaFerla 2010). According to recent surveys, 900 million of people worldwide are older than 60 years, and it is projected that 5% to 8% of these individuals will be afflicted with dementia during their lifetime (Prince et al. 2015). Up to 60% to 80% of the cases of dementia are of the Alzheimer type (Prince et al. 2009). Current estimates are that 47.5 million of people are AD sufferers in the world (Prince et al. 2015). According to the World Health Organization, this number could increase to 135.5 million individuals by the year 2050 (Prince et al. 2015). There are two forms of AD. A genetic early onset form (EOAD) characterized by identified genetic mutations that lead to an overproduction of Aβ in the patient’s AD brain. The second form of AD has a sporadic onset, and progression occurs over a period of several years to culminate in neurodegeneration in the elderly. This form of AD is referred to as late-onset AD (LOAD). In the case of LOAD, the main risk factor is age. Women are more affected than men (Carter et al. 2012). It is now well accepted that the first pathological changes are initiated as early as 20 years before the appearance of the clinical symptoms. One obligatory but not exclusive, stage of the progression to full-blown AD is the clinical stage of amnestic mild cognitive impairment (aMCI). aMCI patients display alterations in their cognitive ability which can be clearly identified by neuropsychological tests (Petersen 2009). Approximately 50% of aMCI individuals progress to AD within 3–4 years following their diagnostic (Ganguli et al. 2004). Thus, aMCI subjects represent a particular interest in the study of the progression to AD. Whereas neuroinflammation is well described in the AD brain, its detection in the periphery is less clear. The main factor identified to date is high baseline levels of TNFα in sera that is associated with a fourfold increase in the rate of cognitive decline (Holmes et al. 2009; Lyman et al. 2014; Calsolaro and Edison 2016). Changes in peripheral innate and adaptive immune systems have been associated with AD. In addition, the possibility that microbial infection may have an implication in AD pathogenesis has been suggested by several investigators (Holmes and Cotterell 2009; Miklossy 2011; Monastero et al. 2014; Itzhaki et al. 2016). The microbial culprits may be of bacterial, viral, fungal, and protozoan origins (Harris and Harris 2015; McNamara and Murray 2016).
NK Cells and Aging
NK Cell Alterations with Aging
Cellular senescence is one of the nine hallmarks of aging (López-Otín et al. 2013). Senescence was originally described by Hayflick’s group following quantification of the number of passages that cultured human fibroblasts could undergo before dying (Hayflick and Moorhead 1961). Cellular senescence is defined as a stable arrest of the cell cycle associated with specific phenotype features (Collado et al. 2007; Kuilman et al. 2010). It has been widely assumed that senescence contributes to aging due to the fact that the amount of senescent cells increases with aging. Senescence can be viewed as a beneficial compensatory response to prevent propagation of damaged or potentially oncogenic cells (López-Otín et al. 2013). In this context, senescence should be detected and regulated by the immune system (Sagiv and Krizhanovsky 2013). However, decreased efficiency of the immune system with aging (immunosenescence) ought also to be taken into account. Immunosenescence consists in a progressive alteration of immune functions through aging which affects all of the components of the system whether it involves the innate, adaptive, humoral, or cellular compartments. Consequently, NK lymphocytes are stricken by senescence as well.
In healthy elderly individuals, an increase in NK cell frequency and number has been reported (Solana et al. 1999; Gayoso et al. 2011). NK cells compartment also remodels with aging (Solana et al. 2012, 2014; Hazeldine and Lord 2013). There is an increased proportion of CD56dimCD16+ NK cells (Campos et al. 2014a; Solana et al. 2014) and a decreased in CD56bright NK subset (Chidrawar et al. 2006). These observations suggest that the increase in NK cell frequency with aging originates from accumulation of CD56dimCD16+ NK cells. This subset is prone to be more mature (Krishnaraj and Svanborg 1992; Hazeldine and Lord 2013). Furthermore, a minor population of NK cells characterized by a CD56−CD16+ phenotype has been reported to be increased in healthy elderly patients (Björkström et al. 2010a; Campos et al. 2014b; Solana et al. 2014). This subset expresses low levels of NCR or NKG2A and high levels of inhibitory receptors, compared to classical NK cells (Mavilio et al. 2005; Gonzalez et al. 2009). The function of this NK subset remains to be defined, but it has been suggested to be less effective in NK function assays (Milush et al. 2013; Solana et al. 2014). It is possible that redistribution of this NK cell subset contributes to the deregulation of the adaptive and innate immune responses. CD56brightCD16− NK cells produce cytokines and are critical for activation of dendritic cells and promotion of monocyte-dependent inflammation (Strowig et al. 2008; Michel et al. 2012). Chronic low levels of inflammation associated with aging (a condition defined as inflamm-aging) could be linked to NK cell subset remodeling since the proportion of CD56bright NK cells has been inversely correlated to C-reactive protein (CRP) levels (Campos et al. 2014b; Solana et al. 2014).
NK cell functions are altered with aging (Camous et al. 2012; Solana et al. 2012; Manser and Uhrberg 2015; Pera et al. 2015). Phenotype changes include a decrease in CD69 (Borrego et al. 1999; Camous et al. 2012) and CD62L (Juelke et al. 2009) expression, which are, respectively, a C-type lectin and a L-selectin whose expression increases following activation. An additional marker of phenotype changes is KLRG1 which is a lectin-like receptor that shows a decrease in expression on NK cells. KLRG1 is a bona fide marker of senescence, whose functional significance on NK cells is still unclear (Hayhoe et al. 2010). The diversity of activating and inhibitory receptors expressed on NK cells surface allows the precise control of activation and inhibition of NK cells, but some of these receptor expression is altered with aging (Biassoni 2008). Cytotoxicity-activating receptors NKp30 and NKp46 expression is decreased with aging (Garff-Tavernier et al. 2010; Gayoso et al. 2011; Almeida-Oliveira et al. 2011), whereas expression of the NKG2C receptor is increased (Gayoso et al. 2011). Conflicting data concerning steady-state levels or decreased expression of NKG2A and his co-receptor CD94 have been reported (Lutz et al. 2005; Garff-Tavernier et al. 2010), whereas NKG2D expression was unchanged (Gayoso et al. 2011; Solana et al. 2012). Inhibitory KIR expression was maintained or increased (Gayoso et al. 2011; Almeida-Oliveira et al. 2011; Lutz et al. 2011; Solana et al. 2012; Manser and Uhrberg 2015). The expression of HLA-DR, MHC class-I (Borrego et al. 1999), and the senescence marker CD57 are increased on NK cells of elderly patients (Lutz et al. 2005, 2011; Almeida-Oliveira et al. 2011; Hazeldine et al. 2012). In addition, the expression of the DNAX accessory molecule-1 (DNAM-1), an activating co-stimulatory receptor belonging to the immunoglobulin superfamily, is decreased on NK cells of elderly patients (Garff-Tavernier et al. 2010; Almeida-Oliveira et al. 2011; Sanchez-Correa et al. 2012; Solana et al. 2012).
Changes in the function of NK cells with aging alter their ability to proliferate, particularly in response to IL-2 stimulation (Borrego et al. 1999). This alteration has been suggested to be related to telomere shortening (Mariani et al. 2003). By and large, the cytotoxic capacity of NK cells is unchanged with aging. However, since CD56dim and CD56bright NK cells remodel with age, single-cell cytotoxic capacity is prone to decrease (Hazeldine et al. 2012) (Solana and Mariani 2000; Garff-Tavernier et al. 2010; Solana et al. 2012). Decreased expression or defective function of activating receptors has been suggested (Solana et al. 2012), but defect in target binding (Pera et al. 2015) or in CD107 (Hazeldine et al. 2012) and perforin (Mariani et al. 1996) expression was not altered.
CD16 expression (Solana and Mariani 2000; Lutz et al. 2005; Garff-Tavernier et al. 2010; Hazeldine and Lord 2013; Solana et al. 2014; Manser and Uhrberg 2015) and ADCC function (Solana and Mariani 2000; Solana et al. 2012; Manser and Uhrberg 2015) are unchanged in NK cells with aging. Production of cytokines, chemokines, and IFNγ as well as response to cytokines and chemokines remains unchanged in NK cells with aging (Garff-Tavernier et al. 2010). However, production of IFNα and IFNγ decreases, whereas production of IL-1, IL-4, IL-6, IL-10, and TNFα increases (Rink et al. 1998; Camous et al. 2012) in response to IL-2 stimulation, but response to cytokines tends to decrease (DelaRosa et al. 2006). Chemokine production by CD56bright NK cells decreases in NK cells exposed to IL-2 or IL-12 (Solana et al. 2012). NK cells function alterations in elderly patients have been associated with an increased incidence of infections in aged individuals (Ogata et al. 2001). Accordingly, increased mortality has been linked to lower numbers of NK cells in these patients compared to patients with a normal number of NK cells. Evidence for this interpretation is the fact that healthy, nonagerians, and centenarians possess normal numbers of NK cells (Gayoso et al. 2011).
Senescence of NK Cells and AD
The involvement of senescence of NK cells with AD has been investigated within the framework of (1) aging as being the main risk factor of AD (Farrer et al. 1997; Ballard et al. 2011), (2) the emergence of the microbial hypothesis, and (3) the microglial senescence hypothesis (Streit and Xue 2014). Expression of the cell surface marker CD57 (also called HNK-1, LEU-7, or L2) (Abo and Balch 1981) has been studied to identify terminally differentiated senescent NK cells (Kared et al. 2016). CD57 is a 100–115 kDa protein with terminally sulfated carbohydrate epitopes that is expressed on senescent T and NK lymphocytes (Kared et al. 2016). A gradual shift from CD56bright via CD56dimCD57− to CD56dimCD57+ NK cells occurs with aging (Gayoso et al. 2011). This cell subpopulation can amount to 60% of the NK cell pool (Garff-Tavernier et al. 2010). This NK subset has been suggested to be associated with reduce proliferative capacity (Lopez-Vergès et al. 2010; Kared et al. 2016), decreased response to cytokines (Björkström et al. 2010a; Campos et al. 2014b; Solana et al. 2014), high cytotoxicity potential (Kared et al. 2016), and increased sensitivity to stimulation via CD16 (Lopez-Vergès et al. 2010). However, a recent study has reported an absence of changes in CD57 expression on NK cells of aMCI and mAD patients compared to healthy elderly controls (Le Page et al. 2015).
The two main NK cell subsets in circulation are composed of two distinct states with different degree of maturation. CD56dimCD16+-positive cells correspond to the more mature cells, whereas CD56brightCD16−-negative cells are less mature. An analysis of NK cell subset distribution in healthy elderly individuals, aMCI and mAD patients revealed the absence of significant differences between these three groups (Le Page et al. 2015). Two subpopulations have been identified among CD56dim NK cells. One subpopulation was CD56dimCD94high positive, whereas the other subpopulation was CD56dimCD94low positive and corresponded to a more mature phenotype associated with the loss of CD94 expression (Yu et al. 2010). CD94 is a transmembrane adapter glycoprotein related to the C-type lectin superfamily (Chang et al. 1995). CD94 forms heterodimeric disulfide bonds with five different members of the NKG2 family (NKG2A, B, C, E, and H) but not with NKG2D, resulting in the assembly of activating and inhibitory complex, respectively (Borrego et al. 2006; Long et al. 2013). The natural ligand for these heterodimers in humans is the non-classic MHC class-I molecule HLA-E (Borrego et al. 2006). CD94 and NKG2A expression has been shown to decrease with age (Lutz et al. 2005; Hayhoe et al. 2010). However, a study of a cohort of healthy individuals, aMCI and mAD patients revealed that the percentage of CD56dimCD94high- and CD56dimCD94low-positive cells was similar in the three groups of subjects and that there were no differences in expression of CD94 and NKG2A (Le Page et al. 2015).
NK Cells and AD
Subpopulation Distribution
The percentages of NK cells defined by the CD56+CD3− phenotype in the total population of lymphocytes are similar in the case of healthy individuals and aMCI and mAD patients (Le Page et al. 2015). Similar results have been reported in the case of the number of CD56+CD16+-positive NK cells in AD patients (Richartz-Salzburger et al. 2007). An absence of differences has been noted with respect to the CD56dimCD16+ and CD56brightCD16− subsets in the case of healthy individuals and aMCI and mAD patients (Le Page et al. 2015). Furthermore, the distribution between NKbright and NKdim populations was not affected in mAD patients.
Expression of TLR
In humans, NK cells express different levels of the ten known TLRs (Chalifour et al. 2004; Hart et al. 2005; Lauzon et al. 2006). TLR2 and TLR4 recognize bacterial peptidoglycans and lipopolysaccharide, respectively (Della Chiesa et al. 2014). Of particular interest within the context of AD is the fact that TLR2 and TLR4 recognize Aβ (Udan et al. 2008; Liu et al. 2012). MFI analysis of the expression of TLR2 and TLR4 on NK cells has been investigated in healthy individuals and aMCI and mAD patients. Data showed that TLR2 expression was significantly lower in mAD patients with respect to healthy controls but expression in aMCI subjects was similar to controls. However, expression of TLR4 was similar in the three groups. One assumption based on these observations was that TLR2 recognition of Aβ could be compromised in mAD patients, further contributing to alteration in Aβ clearing. In this context, the maintenance of TLR4 expression may compensate for lower TLR2 levels. In accordance with the infection hypothesis of AD (Monastero et al. 2014; Itzhaki et al. 2016), a number of groups have studied endosomal TLRs whose function is to recognize intracellular viruses. In this connection, TLR3 recognizes double-stranded viral RNA, whereas TLR9 ligates unmethylated CpG islands on DNA (Ishii and Akira 2006; Amarante and Watanabe 2010). TLR3 expression in NK cells of healthy individuals and aMCI and mAD patients was similar, whereas TLR9 expression was significantly decreased in mAD subjects compared to healthy controls and aMCI patients (Le Page et al. 2015). This observation suggested a correlation with AD progression as well as an altered capacity to defend against pathogens that was already present at the aMCI stage. Low TLR9 levels on NK cells may be an important step in the pathogenesis of AD, and modulation of TLR9 signaling may represent a novel approach in the management of AD.
Activating and Inhibitory Receptors
Inflammation associated with AD could affect NK cell functions. As a mean to protect the innate immune system, NK cell activation is repressed under physiological conditions. Activation of NK cells depends on a balance between multiple activating and inhibitory receptors (Pegram et al. 2011). For instance, the activating receptor NKG2D can trigger signaling by itself, whereas NKG2A needs to recruit the transplasma membrane adaptor glycoprotein CD94 (Long et al. 2013). It has been reported that NKG2D expression remains unchanged on NK cells of aMCI and mAD patients as compared to healthy individuals. In contrast, expression of the NKG2A inhibitory receptor has been shown to be severely lower on NK cells of aMCI patients than healthy subjects and mAD patients. However, expression of CD94 was unchanged and similar in the three experimental groups. In view of the fact that the balance between activator and inhibitory receptors determines NK cell function status, it can be suggested that a decrease in expression of an inhibitory receptor is a determining factor which is consistent with a state of activation of NK cells present in aMCI patients. However, this state of activation is lost in mAD patients and, presumably, in full-blown AD.
Cytotoxic Activity
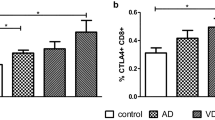
The cytotoxic activity of NK cells toward infected cells is crucial to stop pathogen spreading and to allow resolution of the infection. Cytotoxic effector function of NK cells is achieved by two distinct pathways. The granule-dependent pathway generates the release of perforin and granzyme B that form lytic pores in the membrane of infected targeted cells (Pardo et al. 2009). This process of degranulation can be followed by analysis of CD107a (also called LAMP1) upregulation of expression, a lysosomal-associated marker protein 1 (Alter et al. 2004). NK cell capacity to kill target cells in vitro does not appear to be altered in mAD subjects as reported in a comparative study of healthy individuals and aMCI and mAD patients (Le Page et al. 2015). Furthermore, expression of CD107a was similarly conserved among these three experimental groups and was not upregulated in IL-12-stimulated NK cells. The other cytotoxic pathway involves death receptors of the TNF family such as recognition between CD95 (Fas) and CD95L (FasL) (Chávez-Galán et al. 2009). High levels of granzyme B and CD95 have been reported in aMCI subjects compared to healthy individuals and mAD patients, suggesting that this pathway was upregulated in these individuals. Increases in CD95 expression have also been reported in the case of T lymphocytes of AD patients, and it has been suggested to be associated with increased apoptosis and caspase-3 expression (Frey et al. 2006). The bulk of these observations suggest that there is a commitment of NK cells at the aMCI state to be poised to defend against immune challenges although this commitment is lost at the mAD stage.
Cytokine Receptors and Cytokine Production
Within the context of inflamm-aging that affects elderly individuals, AD is associated with a chronic inflammation status (Sardi et al. 2011). NK cells, T cells and monocytes express the cytokine receptors IL-18 β-chain and the IL-12 β1- and β2-chains (Parihar et al. 2002). Furthermore, an increase in expression of the IL-18 receptor has been shown in PBMCs of aMCI and AD patients (Salani et al. 2013). However, an analysis of IL-12β1, IL-12β2, and IL-18β receptors on NK cells of aMCI and mAD patients has not revealed changes compared to healthy elderly controls (Le Page et al. 2015). This observation suggested that NK cells did not respond to the elevated levels of IL-12 and IL-18 by receptor downregulation.
NK cells of AD patients have been reported to upregulate cytokine production in in vitro response to IL-2 (Solerte et al. 2000). A recent detailed analysis of TNFα and IFNγ production by NK cells of aMCI and mAD patients stimulated by a combination of IL-12 and MHC1-negative K562 cells revealed that production of these cytokines was low in cells of healthy individuals and mAD patients, whereas it was increased more than tenfold in aMCI subjects (Le Page et al. 2015). These results suggested that NK cells may contribute to the inflammatory status in aMCI patients by increasing production of TNFα and IFNγ. The involvement of NK cells in inflammation and in neuroinflammation has also been suggested (Camous et al. 2012). One possibility is that NK cells could play a protective role against AD development by decreasing TH17 differentiation (Jiang et al. 2013), as reported in the case of rheumatoid arthritis and HIV infection (Aggarwal et al. 2013; Jiang et al. 2013).
Chemokine Receptors and Chemotaxis
NK subsets express distinct chemokine receptors in relationship with their protective functions (Maghazachi 2010). For instance, CX3CR1 is expressed by the CD56dimCD16+ NK subset and is associated with ligation of the CX3CL1 chemokine (fractalkine). The study of CX3CR1 within the context of neurological diseases is of particular interest because it has been implicated in the recruitment of NK cells through the BBB in patients with multiple sclerosis (Infante-Duarte et al. 2005; Huang et al. 2006; Hamann et al. 2011). It is tempting to suggest that CX3CR1 may play an influential role in AD pathology. CCR5 is the receptor for CCL5 (Rantes), CCL3 (macrophage inflammatory protein, MIP1α), and CCL4 (MIP1β) (Maghazachi 2010). CCL5 has been reported to be upregulated in the AD brain (Tripathy et al. 2010). Furthermore, CCR5 expression has been shown to be increased on Th1 cells and DCs in AD patients (Goldeck et al. 2013). However, recent data have shown that CCR5 expression on NK cells was similar in a cohort of healthy individuals and aMCI and AD patients (Le Page et al., 2015). Enhanced expression of chemokines receptors have been found on AD leucocytes. In particular, an increase of CCR6 expression on leucocytes and an increase in CCR4 expression on Th2 cells have been identified on AD patients’ immune cells (Goldeck et al. 2013). CCR6 is expressed on CD56brightCD16− NK cells, whereas CCR4 is found on the CD56dimCD16+ subset (Berahovich et al. 2006; Maghazachi 2010). CCR2 participate in migration of monocytes/macrophages across the BBB in the AD brain (Saresella et al. 2014) and is also expressed on NK cells (Grégoire et al. 2007). CD56brightCD16− NK cells express CCR7 which is an important chemokine receptor for the function of this NK cell subset (Maghazachi 2010). CCR7 facilitates the homing of the CD56brightCD16− NK cell subset to lymph nodes where it interacts with monocytes and dendritic cells. In this respect, CCR7 is an important participant in initiation of the adaptive immune response (Griffith et al. 2014). CCR7 expression has been shown to be upregulated in NK cells of aMCI subjects, whereas its expression in mAD patients was similar to healthy controls (Le Page et al. 2015). CCL19 and CCL21 are chemokines abundantly secreted by stromal cells of secondary lymphoid organs. Their sole ligand is CCR7 that is one of the key ligands of the homing mechanism of immune cell migration to secondary lymphoid organs and subsequent defined compartments (Förster et al. 2008). The influence of this process in AD has been addressed in a series of in vitro investigations of NK cell chemotaxis and migration in response to CCL19 and CCL21 (Le Page et al. 2015). These experiments revealed that migration of NK cells of aMCI and mAD patients was impaired in response to CCL19 but not in response to CCL21. These observations suggested a clear defect in the pathway following CCR7/CCL19 recognition.
Expression of CD16
CD16 (FcRγIII) is a low-affinity receptor for the Fc fragment of immunoglobulins G (Rosales 2017). Whereas no changes in CD16 expression have been reported with aging, it has been shown that CD16 expression was increased in mAD patients but not in aMCI subjects (Le Page et al. 2015). Furthermore, the ratio of CD16 expression was tilted in favor of the CD16high subset with respect to the CD16low subset in mAD patients compared to healthy controls. Taken together, these observations suggested that changes in ADCC occurred in AD patients. One likely implication is the lower effective IgG-mediated opsonization of infected cells, in support of the implication of viral pathogens in AD.
Perspectives
Studies of the role of NK cell subsets in AD are still relatively limited and much efforts are needed to understand the involvement of these cells in AD pathology. In particular, investigation of activator and inhibitor receptors expression and analysis of CCR6, CCR4, and CCR2 chemokine receptors and their respective ligand ought to be done to unravel the mechanisms of NK cell activation, migration, and functions within the context of AD development and progression. Studies in aMCI and mAD patients have paved the way to unravel the complex implication of NK cells in AD (Table 1). Of interest is the report that inhibition of HMG-CoA reductase activity by treatment with Atorvastatin® attenuates the increase in NK cells number in the brain of rats challenged with Aβ by limiting the production of IFNγ, MCP-1, and IP-10 (Lyons et al. 2011). In this context, modulation of IFNγ production by NK cells could limit microglia activation and interfere with AD progression. This observation may provide an important clue in the clinical treatment of AD.
Conclusions
An obvious challenge with respect to NK cell involvement in AD is to relate brain viral infection to NK cell activation. Promising therapeutics strategies may come from this approach.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- BBB:
-
Blood-brain barrier
- CD:
-
Cluster of differentiation
- CNS:
-
Central nervous system
- EOAD:
-
Early onset of Alzheimer’s disease
- IFN:
-
Interferon
- KIR:
-
Killer-cell immunoglobulin-like receptor
- LOAD:
-
Late onset of Alzheimer’s disease
- MHC:
-
Major histocompatibility complex
- NK:
-
Natural killer
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
References
Abo T, Balch CM (1981) A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol 127:1024–1029
Aggarwal A, Sharma A, Bhatnagar A (2013) Bi(o)communications among peripheral blood fractions: a focus on NK and NKT cell biology in rheumatoid arthritis. Autoimmunity 46(4):238–250. https://doi.org/10.3109/08916934.2012.755959
Almeida-Oliveira A, Smith-Carvalho M, Porto LC et al (2011) Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol 72:319–329. https://doi.org/10.1016/j.humimm.2011.01.009
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294(1–2):15–22
Amarante MK, Watanabe MA (2010) Toll-like receptor 3: involvement with exogenous and endogenous RNA. Int Rev Immunol 29(6):557–573. https://doi.org/10.3109/08830185.2010.525723
Ballard C, Gauthier S, Corbett A et al (2011) Alzheimer’s disease. Lancet 377:1019–1031. https://doi.org/10.1016/S0140-6736(10)61349-9
Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ (2006) Evidence for NK cell subsets based on chemokine receptor expression. J Immunol 177(11):7833–7840
Biassoni R (2008) Natural killer cell receptors. Adv Exp Med Biol 640:35–52. https://doi.org/10.1007/978-0-387-09789-3_4
Björkström N, Ljunggren H-G, Sandberg J (2010a) CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 31:401–406. https://doi.org/10.1016/j.it.2010.08.003
Björkström N, Riese P, Heuts F et al (2010b) Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 116:3853–3864. https://doi.org/10.1182/blood-2010-04-281675
Borrego F, Alonso MC, Galiani MD et al (1999) NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol 34:253–265
Borrego F, Masilamani M, Marusina A et al (2006) The CD94/NKG2 family of receptors: from molecules and cells to clinical relevance. Immunol Res 35:263–278. https://doi.org/10.1385/IR:35:3:263
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12(6):719–732. https://doi.org/10.1016/j.jalz.2016.02.010
Camous X, Pera A, Solana R, Larbi A (2012) NK cells in healthy aging and age-associated diseases. J Biomed Biotechnol 2012:195956. https://doi.org/10.1155/2012/195956
Campos C, Pera A, Lopez-Fernandez I et al (2014a) Proinflammatory status influences NK cells subsets in the elderly. Immunol Lett 162:298–302. https://doi.org/10.1016/j.imlet.2014.06.015
Campos C, Pera A, Sanchez-Correa B et al (2014b) Effect of age and CMV on NK cell subpopulations. Exp Gerontol 54:130–137. https://doi.org/10.1016/j.exger.2014.01.008
Carrega P, Ferlazzo G (2012) Natural killer cell distribution and trafficking in human tissues. Front Immunol 3:347. https://doi.org/10.3389/fimmu.2012.00347. eCollection 2012
Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A (2012) Sex and gender differences in Alzheimer’s disease: recommendations for future research. J Womens Health (Larchmt) 21(10):1018–1023. https://doi.org/10.1089/jwh.2012.3789
Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N’Guyen T, Thieblemont N, Delneste Y (2004) Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 104(6):1778–1783
Chang C, Rodríguez A, Carretero M et al (1995) Molecular characterization of human CD94: a type II membrane glycoprotein related to the C-type lectin superfamily. Eur J Immunol 25:2433–2437. https://doi.org/10.1002/eji.1830250904
Chávez-Galán L, Arenas-Del Angel MC, Zenteno E, Chávez R, Lascurain R (2009) Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol 6(1):15–25. https://doi.org/10.1038/cmi.2009.3
Chidrawar S, Khan N, Chan T et al (2006) Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing 3:1–8. https://doi.org/10.1186/1742-4933-3-10
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130:223–233. https://doi.org/10.1016/j.cell.2007.07.003
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22(11):633–640
DelaRosa O, Pawelec G, Peralbo E et al (2006) Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology 7:471–481. https://doi.org/10.1007/s10522-006-9062-6
Della Chiesa M, Marcenaro E, Sivori S, Carlomagno S, Pesce S, Moretta A (2014) Human NK cell response to pathogens. Semin Immunol 26(2):152–160. https://doi.org/10.1016/j.smim.2014.02.001
Farrer L, Cupples A, Haines J et al (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. J Am Med Assoc 278:1349–1356. https://doi.org/10.1001/jama.1997.03550160069041
Förster R, Davalos-Misslitz AC, Rot A (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8(5):362–371. https://doi.org/10.1038/nri2297
Frey C, Bonert A, Kratzsch T, Rexroth G, Rösch W, Müller-Spahn F, Maurer K, Müller WE, Eckert A (2006) Apolipoprotein E epsilon 4 is associated with an increased vulnerability to cell death in Alzheimer’s disease. J Neural Transm (Vienna) 113(11):1753–1761
Ganguli M, Dodge HH, Shen C, DeKosky ST (2004) Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 63(1):115–121
Garff-Tavernier M, Béziat V, Decocq J et al (2010) Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 9:527–535. https://doi.org/10.1111/j.1474-9726.2010.00584.x
Gayoso I, Sanchez-Correa B, Campos C et al (2011) Immunosenescence of human natural killer cells. J Innate Immun 3:337–343. https://doi.org/10.1159/000328005
Goldeck D, Larbi A, Pellicanó M, Alam I, Zerr I, Schmidt C, Fulop T, Pawelec G (2013) Enhanced chemokine receptor expression on leukocytes of patients with Alzheimer’s disease. PLoS One 8(6):e66664. https://doi.org/10.1371/journal.pone.0066664
Gonzalez V, Falconer K, Björkström N et al (2009) Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol 183:6612–6618. https://doi.org/10.4049/jimmunol.0901437
Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T (2007) The trafficking of natural killer cells. Immunol Rev 220:169–182
Griffith JW, Sokol CL, Luster AD (2014) Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32:659–702. https://doi.org/10.1146/annurev-immunol-032713-120145
Hamann I, Unterwalder N, Cardona AE, Meisel C, Zipp F, Ransohoff RM, Infante-Duarte C (2011) Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology 133(1):62–73. https://doi.org/10.1111/j.1365-2567.2011.03409.x
Harris SA, Harris EA (2015) Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer’s disease. J Alzheimers Dis 48(2):319–353. https://doi.org/10.3233/JAD-142853
Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM (2005) TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol 175(3):1636–1642
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Hayhoe RP, Henson SM, Akbar AN, Palmer DB (2010) Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol 71:676–681. https://doi.org/10.1016/j.humimm.2010.03.014
Hazeldine J, Lord JM (2013) The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev 12:1069–1078. https://doi.org/10.1016/j.arr.2013.04.003
Hazeldine J, Hampson P, Lord JM (2012) Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell 11:751–759. https://doi.org/10.1111/j.1474-9726.2012.00839.x
Holmes C, Cotterell D (2009) Role of infection in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 12:993–1002. https://doi.org/10.2165/11310910-000000000-00000
Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH (2009) Systemic inflammation and disease progression in Alzheimer disease. Neurology 73(10):768–774. https://doi.org/10.1212/WNL.0b013e3181b6bb95
Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM (2006) The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J 20(7):896–905
Iannello A, Raulet D (2013) Immune surveillance of unhealthy cells by natural killer cells. Cold Spring Harb Symp Quant Biol 78:249–257. https://doi.org/10.1101/sqb.2013.78.020255
Infante-Duarte C, Weber A, Krätzschmar J, Prozorovski T, Pikol S, Hamann I, Bellmann-Strobl J, Aktas O, Dörr J, Wuerfel J, Stürzebecher CS, Zipp F (2005) Frequency of blood CX3CR1-positive natural killer cells correlates with disease activity in multiple sclerosis patients. FASEB J 19(13):1902–1904
Ishii KJ, Akira S (2006) Innate immune recognition of, and regulation by, DNA. Trends Immunol 27(11):525–532
Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Sue W, Griffin T, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lỏovheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA (2016) Microbes and Alzheimer’s disease. J Alzheimers Dis 51:979–984
Jiang Y, Chen O, Cui C, Zhao B, Han X, Zhang Z, Liu J, Xu J, Hu Q, Liao C, Shang H (2013) KIR3DS1/L1 and HLA-Bw4-80I are associated with HIV disease progression among HIV typical progressors and long-term nonprogressors. BMC Infect Dis 13:405. https://doi.org/10.1186/1471-2334-13-405
Juelke K, Killig M, Thiel A et al (2009) Education of hyporesponsive NK cells by cytokines. Eur J Immunol 39:2548–2555. https://doi.org/10.1002/eji.200939307
Kared H, Martelli S, Ng T et al (2016) CD57 in human natural killer cells and T-lymphocytes. Cancer Immunol Immunother 65:441–452. https://doi.org/10.1007/s00262-016-1803-z
Krishnaraj R, Svanborg A (1992) Preferential accumulation of mature NK cells during human immunosenescence. J Cell Biochem 50:386–391. https://doi.org/10.1002/jcb.240500407
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24:2463–2479. https://doi.org/10.1101/gad.1971610
Lam VC, Lanier LL (2017) NK cells in host responses to viral infections. Curr Opin Immunol 44:43–51. https://doi.org/10.1016/j.coi.2016.11.003
Lanier LL (2013) Shades of grey – the blurring view of innate and adaptive immunity. Nat Rev Immunol 13:73–74
Lauzon NM, Mian F, MacKenzie R, Ashkar AA (2006) The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol 241(2):102–112
Le Page A, Bourgade K, Lamoureux J, Frost E, Pawelec G, Larbi A, Witkowski JM, Dupuis G, Fülöp T (2015) NK Cells are Activated in Amnestic Mild Cognitive Impairment but not in Mild Alzheimer’s Disease Patients. J Alzheimers Dis 46(1):93–107. https://doi.org/10.3233/JAD-143054
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rübe CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K (2012) TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J Immunol 188(3):1098–1107. https://doi.org/10.4049/jimmunol.1101121
Long E, Kim H, Liu D et al (2013) Controlling natural killer cell responses: integration of signals for activation and inhibition. Immunology 31:227–258. https://doi.org/10.1146/annurev-immunol-020711-075005
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Lopez-Vergès S, Milush J, Pandey S et al (2010) CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 116:3865–3874. https://doi.org/10.1182/blood-2010-04-282301
Lutz C, Moore M, Bradley S et al (2005) Reciprocal age related change in natural killer cell receptors for MHC class I. Mech Ageing Dev 126:722–731. https://doi.org/10.1016/j.mad.2005.01.004
Lutz CT, Karapetyan A, Al-Attar A et al (2011) Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. J Immunol 186:4590–4598. https://doi.org/10.4049/jimmunol.1002732
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D (2014) Neuroinflammation: the role and consequences. Neurosci Res 79:1–12. https://doi.org/10.1016/j.neures.2013.10.004
Lyons A, Murphy KJ, Clarke R, Lynch MA (2011) Atorvastatin prevents age-related and amyloid-β-induced microglial activation by blocking interferon-γ release from natural killer cells in the brain. J Neuroinflammation 8:27. https://doi.org/10.1186/1742-2094-8-27
Maghazachi AA (2010) Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol 341:37–58. https://doi.org/10.1007/82_2010_20
Manser A, Uhrberg M (2015) Age-related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol Immunother 65:417–426. https://doi.org/10.1007/s00262-015-1750-0
Mariani E, Sgobbi S, Meneghetti A et al (1996) Perforins in human cytolytic cells: the effect of age. Mech Ageing Dev 92:195–209
Mariani E, Meneghetti A, Formentini I et al (2003) Different rates of telomere shortening and telomerase activity reduction in CD8 T and CD16 NK lymphocytes with ageing. Exp Gerontol 38:653–659
Mavilio D, Lombardo G, Benjamin J et al (2005) Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A 102:2886–2891. https://doi.org/10.1073/pnas.0409872102
McNamara J, Murray TA (2016) Connections between herpes simplex virus type 1 and Alzheimer’s disease pathogenesis. Curr Alzheimer Res 13(9):996–1005
Michel T, Hentges F, Zimmer J (2012) Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol 3:403. https://doi.org/10.3389/fimmu.2012.00403
Miklossy J (2011) Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med 13:e30. https://doi.org/10.1017/S1462399411002006
Milush J, López-Vergès S, York V et al (2013) CD56negCD16+NK cells are activated mature NK cells with impaired effector function during HIV-1 infection. Retrovirology 10:1–13. https://doi.org/10.1186/1742-4690-10-158
Monastero R, Caruso C, Vasto S (2014) Alzheimer’s disease and infections, where we stand and where we go. Immun Ageing 11:26
Narni-Mancinelli E, Ugolini S, Vivier E (2013) Tuning the threshold of natural killer cell responses. Curr Opin Immunol 25(1):53–58. https://doi.org/10.1016/j.coi.2012.11.005
Ogata K, An E, Shioi Y et al (2001) Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol 124:392–397
Pardo J, Aguilo JI, Anel A, Martin P, Joeckel L, Borner C, Wallich R, Müllbacher A, Froelich CJ, Simon MM (2009) The biology of cytotoxic cell granule exocytosis pathway: granzymes have evolved to induce cell death and inflammation. Microbes Infect 11(4):452–459. https://doi.org/10.1016/j.micinf.2009.02.004
Parihar R, Dierksheide J, Hu Y, Carson WE (2002) IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest 110(7):983–992
Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH (2011) Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89(2):216–224. https://doi.org/10.1038/icb.2010.78
Pera A, Campos C, López N et al (2015) Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 82:50–55. https://doi.org/10.1016/j.maturitas.2015.05.004
Petersen RC (2009) Early diagnosis of Alzheimer’s disease: is MCI too late? Curr Alzheimer Res 6(4):324–330
Poli A, Kmiecik J, Domingues O, Hentges F, Bléry M, Chekenya M, Boucraut J, Zimmer J (2013) NK cells in central nervous system disorders. J Immunol 90(11):5355–5362. https://doi.org/10.404
Prince MJ, Acosta D, Castro-Costa E, Jackson J, Shaji KS (2009) Packages of care for dementia in low- and middle-income countries. PLoS Med 6(11):e1000176. https://doi.org/10.1371/journal.pmed.1000176
Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, Yusuf S (2015) The burden of disease in older people and implications for health policy and practice. Lancet 385(9967):549–562. https://doi.org/10.1016/S0140-6736(14)61347-7
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. https://doi.org/10.1056/NEJMra0909142
Richartz-Salzburger E, Batra A, Stransky E, Laske C, Köhler N, Bartels M, Buchkremer G, Schott K (2007) Altered lymphocyte distribution in Alzheimer’s disease. J Psychiatr Res 41(1–2):174–178
Rink L, Cakman I, Kirchner H (1998) Altered cytokine production in the elderly. Mech Ageing Dev 102:199–209
Rosales C (2017) Fcγ receptor heterogeneity in leukocyte functional responses. Front Immunol 8:280. https://doi.org/10.3389/fimmu.2017.00280
Sagiv A, Krizhanovsky V (2013) Immunosurveillance of senescent cells: the bright side of the senescence program. Biogerontology 14:617–628. https://doi.org/10.1007/s10522-013-9473-0
Salani F, Ciaramella A, Bizzoni F, Assogna F, Caltagirone C, Spalletta G, Bossù P (2013) Increased expression of interleukin-18 receptor in blood cells of subjects with mild cognitive impairment and Alzheimer’s disease. Cytokine 61(2):360–363. https://doi.org/10.1016/j.cyto.2012.11.001
Sanchez-Correa B, Gayoso I, Bergua JM et al (2012) Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol 90:109–115. https://doi.org/10.1038/icb.2011.15
Sardi F, Fassina L, Venturini L, Inguscio M, Guerriero F, Rolfo E, Ricevuti G (2011) Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev 11(2):149–153. https://doi.org/10.1016/j.autrev.2011.09.005
Saresella M, Marventano I, Calabrese E, Piancone F, Rainone V, Gatti A, Alberoni M, Nemni R, Clerici M (2014) A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J Alzheimers Dis 38(2):403–413. https://doi.org/10.3233/JAD-131160
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet 388(10043):505–517. https://doi.org/10.1016/S0140-6736(15)01124-1
Selkoe DJ (2011) Alzheimer’s disease. Cold Spring Harb Perspect Biol 3(7). https://doi.org/10.1101/cshperspect.a004457
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189. https://doi.org/10.1101/cshperspect.a006189
Solana R, Mariani E (2000) NK and NK/T cells in human senescence. Vaccine 18:1613–1620. https://doi.org/10.1016/S0264-410X(99)00495-8
Solana R, Alonso M, Peña J (1999) Natural killer cells in healthy aging. Exp Gerontol 34:435–443. https://doi.org/10.1016/S0531-5565(99)00008-X
Solana R, Tarazona R, Gayoso I et al (2012) Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 24:331–341. https://doi.org/10.1016/j.smim.2012.04.008
Solana R, Campos C, Pera A, Tarazona R (2014) Shaping of NK cell subsets by aging. Curr Opin Immunol 29:56–61. https://doi.org/10.1016/j.coi.2014.04.002
Solerte SB, Cravello L, Ferrari E, Fioravanti M (2000) Overproduction of IFN-gamma and TNF-alpha from natural killer (NK) cells is associated with abnormal NK reactivity and cognitive derangement in Alzheimer’s disease. Ann N Y Acad Sci 917:331–340
Spits H, Bernink JH, Lanier L (2016) NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol 17(7):758–764. https://doi.org/10.1038/ni.3482
Streit W, Xue Q-S (2014) Human CNS immune senescence and neurodegeneration. Curr Opin Immunol 29:93–96. https://doi.org/10.1016/j.coi.2014.05.005
Strowig T, Brilot F, Münz C (2008) Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol 180:7785–7791
Tripathy D, Thirumangalakudi L, Grammas P (2010) RANTES upregulation in the Alzheimer’s disease brain: a possible neuroprotective role. Neurobiol Aging 31(1):8–16. https://doi.org/10.1016/j.neurobiolaging.2008.03.009
Udan ML, Ajit D, Crouse NR, Nichols MR (2008) Toll-like receptors 2 and 4 mediate Abeta(1-42) activation of the innate immune response in a human monocytic cell line. J Neurochem 104(2):524–533
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells. Nat Immunol 9(5):503–510
Walker JA, Barlow JL, McKenzie AN (2013) Innate lymphoid cells – how did we miss them? Nat Rev Immunol 13:75–87. https://doi.org/10.1038/nri3349
Yu J, Mao H, Wei M et al (2010) CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood 115:274–281. https://doi.org/10.1182/blood-2009-04-215491
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Le Page, A., Dupuis, G., Fulop, T. (2019). Natural Killer Cells and Alzheimer’s Disease. In: Fulop, T., Franceschi, C., Hirokawa, K., Pawelec, G. (eds) Handbook of Immunosenescence. Springer, Cham. https://doi.org/10.1007/978-3-319-99375-1_146
Download citation
DOI: https://doi.org/10.1007/978-3-319-99375-1_146
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99373-7
Online ISBN: 978-3-319-99375-1
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences