Abstract
Many immunologists understand the importance of mucosal immunology, an area that is distinctly regulated from its systemic counterpart. Despite extensive current studies and their outcomes, it still remains for us to fill major gaps in our knowledge of the mucosal immune system in the aged, often described as mucosal immunosenescence. It is well established that pathogen-specific secretory immunoglobulin A (SIgA) antibody (Ab) is the major player for host defense from various pathogens at mucosal surfaces. Alterations in the mucosal immune system occur in advanced aging which ultimately results in a failure to elicit pathogen-specific SIgA Ab responses in order to protect the host from infectious diseases. Symptoms of mucosal immunosenescence were initially detected in the gastrointestinal (GI) immune system, especially in the gut-associated lymphoid tissues (GALT), i.e., the Peyer’s patches (PPs). Thus, a diminished size of PP tissues as well as reduced numbers of naïve CD4+ T cells, follicular dendritic cells (DCs), and antigen (Ag) uptake or microfold (M) cells were noted during the aging process. In contrast, immunological functions of nasopharyngeal-associated lymphoid tissues (NALT) remain intact during aging with notable signs of immunosenescence seen only in the elderly (2-year-old mice). To overcome the effects of immunologic aging in mucosal immunity, it is essential to develop novel immunologic strategies for health in the elderly including vaccines and immune therapies to combat pathogens. In this regard, it has been shown that stem cell transfer as well as several mucosal adjuvant and delivery systems for activation of and deposition of Ag to mucosal DCs or targeting M cells, respectively, are attractive and effective immunologic intervention approaches.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction: The Mucosal Immune System
Mucosa-associated cells, lymphoid and effector molecules (e.g., antibody; Ab) creates an integrated network as the mucosal immune system of higher mammals. Immunoglobulin A (IgA) plays a central role in this sophisticated immune system. Along with cytokines, chemokines and their receptors involved in IgA induction and regulation, the IgA isotype appears to function in synergy with the innate immune system including epithelial cells, macrophages, innate lymphoid cells and their derived cytokines, and anti-microbial peptides (Fujihashi et al. 2013; Kiyono et al. 2008). In order to induce antigen (Ag)-specific immune responses at these mucosal barriers, one must consider the common mucosal immune system (CMIS), which consists of functionally distinct but highly interconnected mucosal IgA inductive and effector tissues (Fujihashi et al. 2013; Kiyono et al. 2008). In the mammalian host, organized secondary lymphoid tissues have evolved in the upper respiratory (UR) and gastrointestinal (GI) tracts to facilitate Ag uptake, processing, and presentation for the initiation of Ag-specific immune responses. These tissues are termed nasopharygeal-associated lymphoid tissue (NALT) and gut-associated lymphoid tissue (GALT), respectively. Collectively, NALT and GALT in humans and mice comprise a mucosa-associated lymphoid tissue (MALT) network. This MALT serves as the major mucosal inductive sites. In general, individual components of MALT are assumed to share the molecular and cellular characteristics of well-characterized Peyer’s patches (PPs). PPs are covered by a follicle associated epithelium (FAE) interspersed with Ag-sampling microfold (M) cells and well-organized microarchitectures, such as a subepithelium (dome) containing Ag-presenting cells (APCs) enriched in dendritic cells (DCs) and macrophages, and a B cell zone with germinal centers (GCs), and adjacent T cell areas as well as high endothelial venules (HEVs). We know that naïve, recirculating B and T lymphocytes enter MALT via the HEVs (Fujihashi et al. 2013; Kiyono et al. 2008). For the initiation of Ag-specific mucosal immune responses through MALT (or PPs and NALT), the FAE M cell plays a crucial role by sampling Ags from the lumen of the gut or nasal passages and transporting the intact form of Ag to the underlying APCs for subsequent processing and presentation of the peptide Ag. Further, APCs (e.g., PP DCs) induce necessary mucosal imprinting of the molecules CCR9 and α4β7 on Ag-specific lymphocytes (Fujihashi et al. 2013; Kiyono et al. 2008). Following this Ag-presentation and activation process, Ag-specific B and T cell populations then emigrate from the mucosal inductive environment via lymphatic drainage, circulate through the bloodstream, and home to mucosal effector sites where they conduct effector functions including the differentiation of PP originating B cells into IgA producing plasma cells. Effector sites for mucosal immune responses include the numerous subsets of lymphoid cells in the lamina propria (LP) of the gastrointestinal (GI), upper respiratory (UR), and reproductive tracts, as well as secretory glandular tissues (Fujihashi et al. 2013; Kiyono et al. 2008). Resident in these mucosal effector sites, which are characterized by more diffuse connective tissues, are the Ag-specific CD4-positive (CD4+) Th1 cells, Th17 cells, and CD8+ cytotoxic T lymphocytes (CTLs) responsible for cell-mediated immunity (CMI)/CTL functions, as well as CD4+ Th2 cells, IgA-committed B lymphocytes, and IgA-producing plasma cells for humoral mucosal immunity. Mucosal surfaces are protected by SIgA which is mainly produced in local effector tissues through the cellular cooperation between polymeric IgA producing plasma cells and poly Ig receptor expressed by columnar epithelial cells (Fujihashi et al. 2013; Kiyono et al. 2008). Since the effector sites of mucosal surfaces play a central role as the first line of host defense, these tissues contain relatively high numbers of activated T and B cells, expressing a memory phenotype in order to be ready for an immediate immune responses to mucosally invading, undesired pathogens (Fujihashi et al. 2013; Kiyono et al. 2008). Further, regulatory T (Treg) cells and CD4+ Th17 cells, which control the suppression and protection/inflammation phases of the GALT immune system, respectively, have been identified in the intestinal LP region (Fujihashi et al. 2013; Kiyono et al. 2008). More recent evidence showed that newly identified type 3 innate lymphoid cells (ILC3) in the intestinal LP play key roles in the regulation of epithelial cell repair and glycosylation in order to assist mucosal protection (Goto et al. 2014).
Despite extensive current studies and their outcomes which provide a better understanding of mucosal immune system, we still do not have a clear view of the age-associated alterations which occur in this sophisticated immune system, which is termed mucosal immunosenescence. In this review, we will focus on the changes exhibited in both GI and UR tracts with advanced aging and introduce potential strategies for the restoration of mucosal immunosenescence in order to describe progress toward development of effective mucosal vaccines which are most needed in the elderly.
Rationale for Mucosal Immunization
The successful induction of mucosal immune responses has been shown to require the use of mucosal adjuvants together with appropriate forms of Ag-delivery vehicles displaying purified Ags (Fujihashi et al. 2013; Kiyono et al. 2008). Further, live, attenuated viruses, bacteria, or their microbial substructures have been shown to be effective immunization platforms. Co-administration of adjuvant offers the additional advantage of supporting and eliciting parenteral immune responses leading to double layers of protective immunity against invasive mucosal pathogens. Two bacterial enterotoxins (native cholera toxin [CT] from Vibrio cholerae and native heat-labile toxin [LTx] from Escherichia coli) are well-established mucosal adjuvants for the induction of both mucosal and systemic immunity (e.g., SIgA and serum IgG, respectively) to co-administered protein Ags (Marinaro et al. 1995; Xu-Amano et al. 1993) in experimental animal models. Attenuated pathogenic bacteria such as recombinant Salmonella (rSalmonella) have also been used as an effective mucosal delivery platform for the induction of SIgA Ab responses to expressed recombinant Ags (Curtiss et al. 1988; Okahashi et al. 1996; VanCott et al. 1996). The nature of the recombinant delivery system, as well as the route of immunization, influences the type of CD4+ Th cell subsets induced and thereby predisposes the host towards systemic or mucosal immunity, or both. For example, oral administration of vaccine protein together with CT or nontoxic mutants of CT (mCTs) tends to induce CD4+ Th2-type cells with characteristic plasma IgG1, IgG2b, IgE, and IgA, as well as mucosal SIgA Ab responses (Marinaro et al. 1995; Xu-Amano et al. 1993). In contrast, oral immunization using recombinant bacteria, e.g., rSalmonella-expressing proteins, tends to induce not only CD4+ Th1-type cells for CMI responses but also characteristic CD4+ T cells which produce cytokines such as IFN-γ and IL-10, which are thought to also support mucosal SIgA Ab responses (Okahashi et al. 1996; VanCott et al. 1996).
Nasal delivery of Ag plus mucosal adjuvant has emerged as perhaps the most effective route for induction of both mucosal and peripheral immunity. Again, most studies can be divided into those which use soluble vaccine components with mucosal adjuvants such as CT (as well as protein-CT-B conjugates) and those which use attenuated microbial vectors such as rSalmonella. A bacterial protein Ag given via the nasal route with or coupled to CT-B subunit induced Ag-specific mucosal SIgA Ab responses (Wu and Russell 1994). Further, nasal immunization with trivalent influenza vaccines in the presence of CT-B containing a trace amount of CT provided cross-protection against a broad range of influenza viruses (Tamura et al. 1992). It was also shown that nasal immunization with influenza vaccine together with the B subunit of LTx (containing a trace amount of LTx) induced Ag-specific immune responses in humans (Hashigucci et al. 1996). Since use of a native enterotoxin-based adjuvant given nasally elicited induced central nervous system (CNS) toxicity leading to Bell’s palsy syndrome, CT, and LTx are unacceptable for human use and native enterotoxin-based adjuvants have been withdrawn from the market. In order to avoid potential toxicity of CT, mCTs have been developed and nasal delivery with protein Ag showed significant enhancement of Ag-specific SIgA Ab responses in the UR tract (Yamamoto et al. 1997, 1998). Further, nasal vaccines containing tetanus toxoid (TT) and a nontoxic mCT spurred the generation of tetanus toxin-specific neutralizing Abs, thereby affording protective immunity (Hagiwara et al. 2003; Kweon et al. 2002). These findings show that an appropriate nasal vaccine can provide effective mucosal and systemic immunity against infections. Indeed, different types of adjuvants, including ligands for toll-like receptors, cytokines/chemokines, chemicals, nonliving systems, and plasmid DNA, have been used in nasal vaccines. As with oral immunization, a rSalmonella delivery system was also employed as a nasal immunization strategy. Nasal delivery of rSalmonella encoding a hybrid form of the hepatitis B virus core Ag (HBc) induced Ag-specific IgA Ab responses in external secretions including the UR tract (Hopkins et al. 1995; Schodel et al. 1996). In addition, rSalmonella strains encoding papillomavirus-like particles (Nardelli-Haefliger et al. 1997), urease A and B subunits of Helicobacter pylori (Corthesy-Theulaz et al. 1998), hepatitis B Ag (Nardelli-Haefliger et al. 2001), and the protective Ag of anthrax toxin (Galen et al. 2009) successfully elicited Ag-specific protective immunity. To this end, nasal immunization with rSalmonella expressing PsaA, a conserved Ag important for Streptococcus pneumoniae adhesion to and invasion into nasopharyngeal epithelia, resulted in protection from nasal colonization by S. pneumoniae (Wang et al. 2010). In addition to this bacterial delivery system, viral vectors such as the adenovirus have been commonly used in order to induce mucosal immunity in the UR tract. For example, nasal or intratracheal delivery of the replication-deficient adenovirus 5 (Ade5) vector induced elevated mucosal IgA Ab responses to adenovirus and β-galactosidase in the lungs, the lower respiratory lymph nodes and nasal LP (Van Ginkel et al. 1995). Others showed that nasal delivery of adenovirus expressing herpes simplex virus Ag induced long-lived, Ag-specific cytotoxic T lymphocyte memory in mucosal tissues (Gallichan and Rosenthal 1996). In addition, a poxvirus and avipoxvirus vector system was developed and some of these vectors were employed as a nasal delivery strategy (Rothenthal et al. 2015).
The Potential Regulation of Mucosal Immunosenescence by the Intestinal Microbiota
The density of bacteria in mammalian large intestine can reach up to 1012 bacteria per gram of intestinal contents (Macpherson et al. 2008; Tsuji et al. 2008). In this regard, 50 genera/several hundred species which represent more genes in the gut microflora than is seen in the human genome, was found in the human gut microbiota (Kurokawa et al. 2007). In order to maintain appropriate homeostatic conditions, the normal microbiota protects from potential pathogenic bacteria colonization by producing antimicrobial peptides. Further, this intestinal microbiota provides energy in the form of short-chain fatty acids and nutrients (vitamin K and B12) (Tremaroli and Backhed 2012; Tsuji et al. 2008). Furthermore, mucosal tissue development and the host immune system development including SIgA Ab synthesis were closely regulated by the intestinal microbiota (Cebra 1999; Macpherson et al. 2008; Suzuki and Fagarasan 2008). For example, hypoplasia of PPs, reduced numbers of IgA plasma cells, and CD4+ T cells have been reported in germ free (GF) mice (Cebra 1999; Macpherson and Harris 2004; Macpherson et al. 2008). When GF mice were exposed to normal mice or mice monoassociated with E. coli, these mice developed a mature mucosal immune system (Klaasen et al. 1991; Shroff et al. 1995). Although GF mice failed to establish tolerance to orally fed Ags, oral treatment with lipopolysaccharide converted GF mice to sensitivity to oral tolerance induction (Wannemuehler et al. 1982). Further, IgA2 subclass switching was preferentially supported by bacterial stimulation of human intestinal epithelial cells (He et al. 2007). Conversely, the absence of mucosal IgA Abs induced dysbiosis in the intestine by allowing bacterial population changes to occur. Thus, activation-induced cytidine deaminase (AID)-deficient mice which lack an appropriate molecular environment for IgA class switching showed aberrant expansion of segmented filamentous bacteria (Suzuki et al. 2004). Further, opportunistic bacteria, largely Alcaligenes species , specifically inhabit GALT and isolated lymphoid follicles (ILFs), with the associated preferential induction of Ag-specific SIgA Abs in the GI tract (Obata et al. 2010; Sato et al. 2015). Recent studies showed that diverse and select IgA Abs contribute to the maintenance of a diversified and balanced microbiota, which in turn facilitates the expansion of Foxp3 T cells, induction of GCs, and SIgA Ab responses in the gut through a symbiotic regulatory loop (Kawamoto et al. 2014). Based upon these findings, one could predict that alterations in the intestinal microflora may lead to a dysregulation of the immune system in the GI tract as major age associated-changes occur. Indeed, it has been reported that significant changes in the intestinal microflora were noted in the elderly (>65 years old) (Claesson et al. 2011; Woodmansey 2007). In addition, other human microbiome analyses showed that the centenarians exhibited increased inflammatory cytokine responses (“inflammaging,” see discussion below) associated with significant changes in their microbiota when compared with those seen in young adults (Biagi et al. 2010).
Alterations in the GI Tract Immune System
Immunologic analyses of the GI tract during aging have provided extensive evidence for dysregulation and an overall decline in mucosal immunity (Fujihashi and Kiyono 2009). The most common method for assessing mucosal immune responses is perhaps to test external secretions for the presence of SIgA Abs. It has been shown that total IgA Ab levels in mucosal secretions in humans were either increased or remained unchanged in aging. Thus, studies have shown that elderly subjects had significantly higher concentrations of salivary SIgA Abs than did younger ones (Arranz et al. 1992). The same report also showed that whole gut lavages of aged and young subjects contained similar Ab levels (Arranz et al. 1992). Others have also reported similar total IgA Ab responses in the serum of aged humans (Ammann et al. 1980; Buckley et al. 1974). Further, studies in aged mice and rats also revealed total IgA Ab levels resembling those seen in humans (Ebersole et al. 1985; Finkelstein et al. 1984; Kawanishi and Kiely 1989; Senda et al. 1988). Similarly, our previous study showed that fecal extracts from 1-year-old mice contained essentially the same levels of IgA Abs as those seen in young adult mice (Koga et al. 2000). These results indicate that age does not impair total IgA Ab responses in external secretions. To support these findings, when B cells from the PPs of aged mice were stimulated with autoreactive T cell hybridoma-derived B cell stimulatory factors, significantly higher levels of IgA Abs were produced than were noted in identically treated PP B cells from young adult mice (Kawanishi et al. 1989). In contrast, in vitro Ab production in B cells from aged PPs and MLNs were depressed when T cell-dependent B cell mitogens were employed (Kawanishi and Kiely 1989). These findings suggest that T cells which are involved in the induction of Ag-specific immune responses are more susceptible than B cells to immunosenescence in the mucosal compartment. Furthermore, it is possible that natural IgA Ab responses in aged mice could be due to increased levels of low affinity, T cell-independent IgA Ab production.
Ag-specific IgA B cell responses are known to play a central role in the induction of mucosal immunity to infectious diseases (Kiyono et al. 2008). The GI tract in the elderly is particularly susceptible to infectious diseases, suggesting that Ag-specific mucosal immunity is also affected in aging (Powers 1992; Schmucker et al. 1996). Indeed, despite intact overall IgA Ab levels in aging, Ag-specific immunity in the elderly and in experimental animals are significantly diminished when compared with their younger counterparts. For example, intestinal lavages from aged rats given oral CT were shown to contain significantly lower titers of anti-CT-B IgA Abs than did those from identically immunized young rats (Schmucker et al. 1988). Furthermore, the numbers of Ag-specific IgA Ab forming cells (AFCs) in the intestinal LP were also reduced in aged rats (Schmucker et al. 1988; Thoreux et al. 2000) and rhesus macaques (Taylor et al. 1992) given oral CT. When aged mice were orally immunized with the hemagglutinin (HA) from influenza virus along with CT as mucosal adjuvant, reduced levels of HA-specific SIgA Ab responses were noted when compared with those seen in young adult mice (Enioutina et al. 2000). These results clearly indicate that Ag-specific mucosal SIgA Ab responses are diminished in aged animals, especially those associated with the GALT immune system. Of importance, our previous studies showed that age-associated dysregulation of the GI tract mucosa existed as early as 12–14-months of age in mice (Koga et al. 2000) (Fig. 1). Thus, when 1-year-old mice were orally immunized with ovalbumin (OVA) plus CT, reduced levels of OVA- and CT-B-specific mucosal and peripheral immune responses were noted which resembled those seen in aged (2-year old) mice given the same oral vaccine (Koga et al. 2000). In contrast, 1-year-old mice given OVA plus CT via the subcutaneous route failed to reveal CT adjuvanticity (essentially no OVA-specific Ab responses) but maintained its antigenicity for Ab responses to CT-B (Koga et al. 2000). It has been shown that CT enhanced CD86 expression by APCs and these effects were not influenced by CD40-CD40L interactions. Thus, age-associated alterations in CD40L expression by splenic CD4+ T cells could be the reason for impaired OVA-specific immunity, which require CD40-CD40L interactions. Based upon these studies, one could suggest that the parenteral immune system in 1-year-old mice may be in a transitional stage between a normal and age-associated deficiency. Thus, we would conclude that age-associated alterations may arise in the mucosal immune system of the GI tract earlier (1 year of age) than in the parenteral immune compartment.
Mucosal aging effects on GALT versus NALT. Reduced induction of Ag-specific intestinal SIgA Ab responses were noted in 1-year-old mice. Peyer’s patches (PPs) exhibited a reduced size and lower numbers of PPs were present. Reduced numbers of naïve CD4+ T cells and follicular dendritic cells (FDCs) were already seen in PP of 1-year old mice. In contrast, NALT functions remained intact during aging with notable signs of mucosal immunosenescence (loss of Ag-specific SIgA Ab responses) seen only in 2-year-old mice
Involvement of GALT in GI Tract Immunosenescence
As indicated above, since PPs are the major mucosal inductive tissues in the GI tract, lack of PPs can result in impaired Ag-specific-SIgA Ab responses when oral CT adjuvant or rSalmonella delivery systems are employed (Hashizume et al. 2008; Yamamoto et al. 2000). Thus, one could easily predict that this impaired Ag-specific Ab response was due to age-associated alteration in the PPs. Indeed, a substantial senescence-associated decline in numbers of lymphoid cells was found in the GALT, specifically in PPs and mesenteric lymph nodes (MLNs) (Kawanishi and Kiely 1989). Further, a significant size reduction in PPs was seen in 1-year-old mice along with reduced Ag-specific mucosal Ab responses (Koga et al. 2000) (Fig. 1). Although the ratio of CD4+ and CD8+ T cells and B cells were unchanged (Kato et al. 2003; Koga et al. 2000), the actual numbers of lymphocyte counts in PPs of 1-year-old mice were significantly lower than those seen in young adult mice (6–8 weeks old) (Kato et al. 2003; Koga et al. 2000) (Fig. 1). Indeed, Ag-stimulated CD4+ T cells from 1-year-old mice given oral OVA plus CT resulted in reduced Th2-type cytokine (e.g., IL-4) production (Koga et al. 2000). Further, it was reported that Ag-specific T cell regulatory/helper functions in PPs were diminished by aging (Kato et al. 2003; Kawanishi and Ajitsu 1991). These findings clearly suggest that the development of effector T cells is influenced by senescence. Indeed, it has been shown that age-associated alterations closely parallel increases in memory type and loss of the naïve T cell phenotype during aging (Fujihashi and Kiyono 2009). In this regard, when the actual cell numbers of naïve CD4+ T cells between young adult (6–8 weeks old) and aging (1-year old) mice were compared, PPs of aging mice showed significant reductions in CD4+, CD45RB+ naïve T cell frequencies in addition to total cell numbers (Hagiwara et al. 2003) (Fig. 1).
Potential Roles for M Cells in Gut Aging
M cells play a central role in an Ag-sampling system which takes up luminal Ags from the gut lumen into the GALT (Kiyono et al. 2008). M cells have different morphological features when compared with normal intestinal epithelial cells. In this regard, their apical sides show relatively short-irregular microvilli and their basolateral sides form a pocket structure which containing enfolds of lymphocytes and APCs. To this end, M cells can effectively transport luminal Ags from the gut lumen to underlying MALT lymphocytes (Kiyono et al. 2008). Based upon this evidence, M cell-targeting strategies have been developed and successfully used to elicit mucosal immunity. It has been shown that reovirus protein sigma one (pσ1) specifically bind to M cells (Wu et al. 2001). In this regard, M cell-targeting DNA vaccine complexes consisting of plasmid DNA and the covalently attached reovirus pσ1 to poly-L-lysine (PL) induced significant mucosal SIgA Ab responses in addition to systemic immunity (Wu et al. 2001). Further, it has been shown that a novel M cell-specific monoclonal antibody (NKM 16-2-4) which recognizes the unique glycosylation moiety of the M cells conjugated with botulinum toxoid as a M cell-targeting mucosal vaccine provided significant protection when challenged with a lethal dose of botulinum neurotoxin (Nochi et al. 2007). Oral delivery of Ag combined with the M cell-targeting peptide ligand (Co1, selected from a phage display library panning against the in vitro M-cell co-culture system) resulted in enhanced Ag-specific immune responses (Kim et al. 2010). Since no in vitro M cell systems have been developed, only limited information is available about how Ag sampling actually occurs. However, glycoprotein 2 (GP2) expressed by M cells has been reported to be M cell-specific molecule which acts as a binding receptor for FimH-expressed E. coli and Salmonella spp. to elicit effective uptake of and induction of specific immune responses (Hase et al. 2009; Terahara et al. 2008). Of interest, it was also reported that a transition of FAE enterocytes into M cells was induced by Salmonella enterica serovar Typhimurium (S. Typhimurium) type III effector protein SopB (Tahoun et al. 2012).
Recently, it has been shown that Spi-B, which is one of the E26 avian leukemia oncogene transformation-specific (Ets) family transcription factors, is required for the functional and structural differentiation of M cells (de Lau et al. 2012; Kanaya et al. 2012; Sato et al. 2013). M cells differentiate from leucine rich repeat containing G protein coupled receptor 5-positive (Lgr5+) intestinal epithelial stem cells as with all other intestinal epithelial cell lineages (de Lau et al. 2012). Receptor activator of nuclear factor-κB ligand (RANKL) signal stimulation from the subepithelial stromal cells in the FAE region (Knoop et al. 2009), triggers the expression and activation of Spi-B in M cell precursors, and subsequently upregulate several Spi-B-target genes including glycoprotein 2 (GP2), which is considered to be a mature M cell marker (Hase et al. 2009). Importantly, aged mice have significantly decreased numbers of GP2+, mature GALT M cells (Kobayashi et al. 2013) (Fig. 1). Through an unknown mechanism, the numbers of Spi-B-positive cells are significantly reduced in the FAE region of aged mice although the expression of RANKL and RANK and their signaling pathways are intact in aged mice. In agreement with reduced numbers of mature M cells, aged mice failed to transport latex particles into the PPs. Furthermore, T cell activation by orally delivered S. Typhimurium is markedly reduced due to the absence of M cell-intrinsic Spi-B (Kanaya et al. 2012). Therefore, reduced numbers of M cells may be one of the causes of impaired GI tract immunity in the elderly. Forced Spi-B activation and/or expression may be a potential target strategy for the development of effective mucosal vaccines in the elderly.
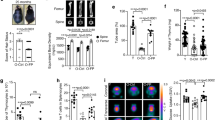
Mesenchymal Stem Cells for Restoration of Ag-Specific SIgA Ab Responses in the GI Tract
Adipose tissue-derived mesenchymal stem cells (AMSCs) are attractive candidates for cell replacement therapies since they can be obtained and expanded relatively easily. It has been shown that AMSCs can differentiate into adipocytes, chondrocytes, and osteoblasts (Tobita et al. 2011). In addition, various clinical trials have shown the regenerative capacity of AMSCs (Garcia-Olmo et al. 2008; Miyahara et al. 2006; Psaltis et al. 2008). Previous studies suggested a therapeutic potential for AMSCs for treatment of Alzheimer’s syndrome (Katsuda et al. 2013) and periodontal disease (Tobita et al. 2013). In this regard, the potential of AMSCs to restore mucosal immunosenescence in the GI tract was investigated by adoptively transferring AMSCs into aged mice. Both OVA and CT-B-specific SIgA Ab responses were significantly increased in aged mice (12–14 months old and over 18 months of age) adoptively transferred with AMSCs when orally immunized with OVA and CT (Aso et al. 2016) (Fig. 2). The induction of Ag-specific SIgA Ab responses was supported by increased levels of IL-4 production in mucosal tissues of aged mice which was achieved by pretreatment with AMSCs (Aso et al. 2016). Of importance, Ag-specific SIgA Abs in aged mice restored by AMSC transfer were functional. Thus, fecal extracts containing CT-B-specific SIgA Abs exhibited neutralizing activity against CT intoxication (Aso et al. 2016) (Fig. 2). This finding contrasts with previous studies generally showing that MSCs downregulate various immunocompetent cells. For example, MSCs inhibited both CD4+ and CD8+ T cell proliferation following co-culture and polyclonal stimulation (Cuerquis et al. 2014; Dorronsoro et al. 2014; Malcherek et al. 2014). Other in vitro studies showed reduced Abs in mixed lymphocyte cultures (Comoli et al. 2008) as well as reduced B cell proliferation and Ab synthesis in the presence of MSCs (Corcione et al. 2006). Finally, co-culture of MSCs with splenic B cells induced regulatory B cells producing IL-10 that ameliorated autoimmunity and aberrant Ab synthesis (Park et al. 2015). The major difference between these opposite studies is that one assessed AMSC functions by adoptive transfer in vivo in a mouse model instead of in vitro systems. Since adoptively transferred AMSCs were generated by serum-free medium, adoptively transferred AMSCs and their soluble products may totally differ from the in vitro studies by others and result in upregulation of various immune competent cells.
Potential mechanisms for stem cell transfer to overcome GALT aging. One of the features of immunosenescence is an increased threshold of inflammation known as “inflammaging.” Thus, chronic inflammatory responses may hamper induction of Ag-specific immune responses when active immunization is initiated, since it is essential to induce transient inflammatory innate immune responses in order to trigger subsequent acquired immunity. It is possible that AMSCs could reduce inflammaging and facilitate the subsequent restoration of Ag-specific protective immunity
One of the features of immunosenescence is an increased threshold of inflammation known as “inflammaging ” (Franceschi et al. 2007). Thus, chronic inflammatory responses may hamper induction of Ag-specific immune responses when active immunization is initiated, since it is essential to induce a transient inflammatory innate immune response in order to elicit subsequent acquired immunity (Iwasaki and Medzhitov 2015). It has been shown that MSCs exhibited potential roles for anti-inflammatory functions (Ho et al. 2015). Thus, MSCs have been employed as therapeutic strategies for various immune disorders including graft-versus-host disease (GVHD) (Le Blanc et al. 2008; Perez-Simon et al. 2011), organ transplantation (Casiraghi et al. 2013), autoimmune diseases (Figueroa et al. 2012), and inflammatory bowel diseases (Forbes et al. 2014; Knyazev et al. 2013). Indeed, MSCs interact with T cells to reduce their pro-inflammatory cytokines (Di Nicola et al. 2002; Krampera et al. 2003), while increasing their production of anti-inflammatory cytokines, including IL-4 and IL-10 (Kong et al. 2009; Prevosto et al. 2007). In this regard, it is possible that adoptive transfer of AMSCs into aged mice could reduce inflammaging and facilitate the subsequent restoration of Ag-specific immune responses when mice were orally immunized with OVA and CT (Fig. 2). Of importance, AMSC adoptive transfer studies revealed increased numbers of IL-4 producing CD4+ T cells with increased levels of OVA-induced IL-4 production by CD4+ T cells in PPs (Aso et al. 2016). Since IL-4 is an essential Th2-type cytokine for adjuvant activity of CT (Okahashi et al. 1996; Vajdy et al. 1995), these results clearly indicate that AMSCs enhanced IL-4 production in aged mice, which could also potentially down-regulate inflammatory responses and simultaneously allow CT to enhance OVA-specific Ab responses (Fig. 2). Taken together, the AMSC transfer system would be a potent novel strategy in order to overcome mucosal immunosenescence.
Distinct Features of NALT Versus GALT
It has been shown that PPs and NALT share common features; however, it is also clear that both tissues possess unique features reflecting their local environments. For example, a compartmentalization occurs between the GALT and NALT immune systems for the induction of Ag-specific immune responses (Holmgren and Czerkinsky 2005; Kiyono et al. 2008). Thus, oral immunization mainly elicits Ag-specific immune responses in the small intestine, in the proximal part of the large intestine, mammary, and salivary glands, whereas nasal immunization induces mucosal immunity in the UR tract, nasal, and oral cavities as well as in the cervicovaginal mucosa (Holmgren and Czerkinsky 2005). Further, NALT and GALT organogenesis and lymphocyte trafficking are distinctly regulated (Kunisawa et al. 2008). For example, PPs develop during embryonic days 14–17 in an IL-7-IL-7Rα- and LTα1β2-LTβR-dependent manner, whereas NALT organogenesis occurs postnatally without involvement of either of these cytokine pathways (Fukuyama et al. 2002; Kunisawa et al. 2008). In addition, Peyer’s patch inducer cells require both Id2 and RORγt transcripts for their development; however, NALT inducer cells only require Id2 (Fukuyama et al. 2002). It has been shown that activated T and B cells in GALT preferentially express α4β7 and CCR9 as gut-homing receptors which help guide their migration back into the intestinal LP (iLP) (Fujihashi et al. 2013; Kiyono et al. 2008). In contrast, CD62L, α4β1 and CCR10 preferentially regulate the trafficking of T and B cells from NALT into the UR tract effector tissues (Csencsits et al. 2001; Kunisawa et al. 2008; Pascual et al. 2008). Finally, others and our recent studies have shown that the NALT immune system represents a unique CMIS compartment which supports the induction of SIgA Ab responses in the submandibular glands (SMGs) and saliva (Csencsits et al. 2001; Sekine et al. 2008). These findings clearly show some common as well as distinct compartmentalization occurs in GI and UR tract immune systems in an otherwise framework of the CMIS.
Advantages of a Delayed NALT Aging Process That Differs from GALT
In addition to the progression during organogenesis and lymphocyte trafficking, the aging process in NALT is also distinctly regulated when compared with that of GALT. When the frequencies of naïve CD4+ T cells in NALT and GALT (i.e., the PPs) were compared in young adult and 1-year-old mice, reduced frequencies of CD4+, CD45RB+ T cells were seen in aged mice (Fujihashi and Kiyono 2009; Hagiwara et al. 2003). On the other hand, the actual cell counts of naïve CD4+ T cells in NALT of 1-year-old mice were higher than those seen in young adult mice (Fig. 1). The size as well as total lymphocyte count in NALT increases approximately fivefold to ninefold during the aging process through the first year (Fujihashi and Kiyono 2009; Hagiwara et al. 2003) (Fig. 1). Although the total lymphocyte count is reduced by 2 years of age, NALT contains approximately twice the number of total lymphocytes (Fujihashi and Kiyono 2009; Hagiwara et al. 2003). Thus, the overall numbers of naïve CD4+, CD45RB+ T cells in the NALT were comparable between aged and young adult mice. These results suggest that the continuous generation of this naïve T cell populations in NALT plays a pivotal role in maintaining young adult mouse levels for the induction of both systemic and mucosal immune responses to nasally administered Ags in aged mice. Based upon these findings, one could easily predict that nasal immunization of 1-year-old mice would reveal an intact mucosal immune response. In contrast to oral immunization, nasal immunization with OVA plus CT indeed effectively induced Ag-specific mucosal and systemic immune responses in 1-year-old mice (Hagiwara et al. 2003) (Fig. 1). Thus, equivalent levels of OVA-specific Ab responses in plasma and external secretions and Ag-specific Ab forming cells (AFCs) in the nasal cavity were seen (Hagiwara et al. 2003). These results clearly show that both mucosal and systemic immunity occurred in 1-year-old mice following nasal immunization (Fig. 1). Further, 1-year-old mice given nasal tetanus toxoid vaccine were protected from tetanus intoxication (Hagiwara et al. 2003). These results suggest that a distinct immune aging process is occurring in NALT versus GALT that mediates Ag-specific Ab induction accounting for differences in the induction of Ag-specific mucosal SIgA and parenteral IgG Ab responses (Fig. 1).
It is generally agreed that experimental mice should be at least 18 months of age or older to be suitable and equivalent models for evaluating immunological aging effects in order to provide useful information for the understanding of immunosenescence in humans. In this regard, when 2-year-old mice were thus immunized nasally with OVA and CT as adjuvant, the mice failed to undergo induction of Ag-specific SIgA Ab responses (Fukuiwa et al. 2008; Hagiwara et al. 2003). However, these mice underwent OVA-specific, peripheral immune responses which were essentially identical to the responses seen in young adult mice (Fukuiwa et al. 2008; Hagiwara et al. 2003). Similarly, OVA-specific CD4+ T cell proliferative as well as Th1 and Th2 cytokine responses in spleens of 2-year-old mice were comparable to those of young adult mice when CT was used as nasal adjuvant (Hagiwara et al. 2003). These results further agree with the findings that mucosal immunosenescence takes place prior to systemic immune dysregulation (Koga et al. 2000), even though the process of NALT immunosenescence was less than that seen in GALT in 2-year-old mice (Hagiwara et al. 2003).
To consider the control of infectious diseases in the elderly, one must overcome this mucosal immunosenescence and seek to develop novel immune modulators which can maintain appropriate mucosal immunity in 2-year-old mice. Further, as we described above, although the numbers of M cells in GALT were reduced in aged mice, a change in the density of mature M cells in NALT FAE with aging has not been reported (Sato et al. 2015). Thus, it remains possible that one of the reasons for the slower process of immunosenescence in NALT of aged mice could be due to intact numbers of mature and functional M cells on NALT FAE. The evidence indicates another advantage of using the NALT immune system for eliciting mucosal immunity in aging. Thus, an M cell-targeting nasal delivery system would be a potent strategy for inducing mucosal immunity in the elderly (Fig. 3).
Nasal vaccines given with DC- and M cell-targeting systems elicit protective mucosal immunity in aging. Nasal immunization with virulence compartments plus pFL and CpG ODN induces specific SIgA Ab responses in aged mice. Thus, nasal challenge with pathogens (influenza virus or S. pneumoniae) resulted in complete protection of the nasal mucosa of aged mice. In addition, nasal delivery of GP2-ligand-conjugated-Ag (M cell targeting) also induced pathogen-specific immunity
Compensation for Immunosenescence
Humans of advanced age significantly more sensitive to infection and mortality caused by influenza virus and the bacterial pathogen S. pneumoniae (the pneumococcus) (Thompson et al. 2004; Webster 2000). Although vaccines which can prevent these two respiratory pathogens are available, they are less effective in the elderly and thus a need exists to develop safe and improved vaccines (Fujihashi and Kiyono 2009). Thus far, it has been shown that adjuvant systems are required in order to improve influenza vaccines in the elderly (Galli et al. 2009; Jackson et al. 2012). Thus, when MF59 was employed as adjuvant for an H5N1 vaccine, broadly cross-reactive Abs and long-lived memory B cells were rapidly elicited (Galli et al. 2009). Further, immune responses to inactivated 2009 H1N1 influenza vaccine in both healthy adults (18–64 years) and older adults (> 65 years) were successfully enhanced by the AS03 adjuvant system (Squalene, DL-a-tocopherol and polysorbate 80, GlaxoSmithKline) (Jackson et al. 2012). In addition to these injectible influenza vaccines, poly I:C as an adjuvant enhanced the effectiveness of an influenza virus-like particle nasal vaccine in aged mice (Schneider-Ohrum et al. 2011). Despite these successful reports, one must carefully consider adjuvant selection as well as vaccine and delivery method since mice given detergent split-influenza Ag [A/Uruguay716/2007 (H3N2)] plus purified monophosphoryl lipid A (MPL) in liposomes via the nasal route showed transient weight-loss which was induced by Th17-mediated immune responses (Maroof et al. 2014).
As a more general approach, CpG ODN as vaccine adjuvant has been shown to restore Ag-specific immune responses to OVA, diphtheria toxoid, hepatitis B, pneumococcal polysaccharides, amyloid β, and tumor cells in aged mice and rats (Fujihashi and Kiyono 2009). Oral immunization with OVA (considered tobe a weak immunogen) plus CpG ODN induced equally increased levels of Ag-specific SIgA and IgG Ab responses in mucosally normal (3 month old) as well as mucosally aged (18 month old) mice (Alignani et al. 2005). These studies clearly show the potential of CpG ODN as adjuvant to compensate for the reduced immune responses seen in aging.
In addition, strategies to restore the ratio of naïve to memory CD4+ T cell subsets have successfully compensated for the altered immune responses in aging since increased numbers of memory-type cells and decreased numbers of naïve CD4+ T cells are associated with immunosenescence (Fujihashi and Kiyono 2009). In this regard, aged Fas-CD2 transgenic mice (overexpressing the Fas gene regulated by the CD2 promoter) resulted in reduced numbers of memory-type T cells and rejuvenated immune responses which resembled those of young adult mice (Zhou et al. 1995). Further, exogenous IL-2 delivery effectively restored development of effector cells from naïve precursors in aged mice (Haynes et al. 1999). Similarly, mucosal IL-2 treatment reversed age-impaired mucosal immune responses by enhancing mucosal immunity or by abrogating tolerance in aged mice (Fayad et al. 2004). Additional studies showed that keratinocyte growth factor or IL-7 treatment prevented thymic atrophy and thus resulted in a continuous supply of naïve T cells (Henson et al. 2004; Min et al. 2007). These studies suggest that continuous supply of naïve T cell populations is a critical factor for the maintenance of an appropriate immunological state including the induction of Ag-specific immunity in aged mice. Both IL-2 and IL-7 are common γ chain cytokine receptor-related interleukins, therefore IL-15 treatment also restored impaired DC function in mesenteric lymph nodes of aged mice (Moretto et al. 2008).
A DC-Targeting Mucosal Immunization Strategy for Restoring Immunity in Aging
It has been shown that mice can survive an otherwise lethal challenge of influenza virus by production of pathogen-specific systemic IgG without mucosal IgA responses; however, these mice became sick and showed significant weight loss (Harriman et al. 1999). Similarly, since new adjuvant systems for influenza vaccines for the elderly described above would fail to induce protective SIgA Ab responses at the UR tract mucosa, it is possible that influenza virus infection would still elicit flu symptoms and delay recovery of elderly patients. Indeed, it is essential to have pathogen-specific SIgA Ab responses in order to provide a first line of defense against major respiratory pathogens (i.e., influenza virus and S. pneumoniae) at their entry site (Asanuma et al. 2012; Fukuyama et al. 2010). These findings clearly suggest that Ag-specific SIgA Abs are the most important component for effective protection. However, as we have discussed thus far, Ag-specific mucosal SIgA Ab responses are diminished in aged animals and presumably in humans despite slower development of immunosenescence in the UR tract (Fujihashi and Kiyono 2009; Fujihashi and McGhee 2004). To explore new avenues for effective mucosal immunization strategies which can induce pathogen-specific protective SIgA Ab responses, investigators have begun to target mucosal tissues and immune cells for vaccine delivery. To this end, mucosal DC-targeting Ag delivery systems have been shown to induce Ag-specific SIgA responses (Fukuiwa et al. 2008; Kataoka et al. 2004; Sekine et al. 2008).
The unmethylated CpG motifs are recognized by the innate immune system via the toll-like receptor 9 (TLR9), expressed by B cells and plasmacytoid DCs (pDCs) (Hemmi et al. 2000). Thus, CpG DNA induced the maturation and stimulation of professional pDCs as well as the subsequent Ag-specific Th1 cell and CTL responses (Klinman et al. 2004; Wagner 1999). Further it has been show that CpG ODN acts as an effective adjuvant for the induction of Ag-specific immunity (Klinman et al. 1999). Indeed, CpG ODN enhanced both Ab and CMI responses to OVA in mice (Klinman 1998). Further, when viral or toxoid vaccines were given with CpG ODN, significantly increased levels of Ag-specific Ab and CTL responses were seen (Brazolot Millan et al. 1998; McCluskie and Davis 1998; Moldoveanu et al. 1998). Mucosal delivery of CpG ODN plus formalin-inactivated influenza virus or hepatitis B virus surface Ag successfully induced Ag-specific Ab responses in both external secretions and plasma of mice (McCluskie and Davis 1998; Moldoveanu et al. 1998). In addition, mice given nasal recombinant protective antigen (PA) of the anthrax lethal toxin plus CpG ODN exhibited high levels of PA-specific IgG2a and IgA Ab responses in both plasma and external secretions (Boyaka et al. 2003). Importantly, these PA-specific Abs neutralized the lethal toxin in vitro (Boyaka et al. 2003).
The Flt3 ligand (FL), which binds to the fms-like tyrosine kinase receptor Flt3/Flk2, is a growth factor that dramatically increases the numbers of DCs in vivo without inducing their activation (Brasel et al. 1996; Maraskovsky et al. 1996). Treatment of mice by systemic FL injection induced marked increases in the numbers of DCs in both systemic (i.e., spleen) and mucosal lymphoid tissues (i.e., intestinal LP, PPs and MLNs) (Viney et al. 1998). Other studies have now shown that FL treatment also favors the induction of immune responses after mucosal (Williamson et al. 1999), systemic (Pisarev et al. 2000), or cutaneous (Baca-Estrada et al. 2002) vaccine delivery. In addition, plasmid DNA encoding FL (pFL) has been systemically co-administered with plasmids encoding protein Ags or linked to the Ag itself. These studies support the use of FL as adjuvant to induce both IgG Ab- and CMI-responses (Hung et al. 2001; Moore et al. 2002). When pFL was employed as a nasal adjuvant, it induced significant expansion of mature-type CD8+ DCs in NALT which contributed to IL-4 production by CD4+ T cells and enhancement of co-administered Ag-specific SIgA Ab responses (Kataoka et al. 2004).
Although DC-targeting adjuvants have shown promising outcomes, conflicting reports concerning functional DC subsets in aged mice have been put forth (Kovacs et al. 2009; Shaw et al. 2010; Tesar et al. 2006). Those suggesting impaired DC effects have included the reduced expression of CCR7 involved in cell tracking, interferon (IFN)-α production after herpes simplex virus-2 infection and IFN regulatory factor-7 (IRF-7) synthesis following CpG ODN activation (Kovacs et al. 2009; Shaw et al. 2010). In contrast, myeloid type DCs were shown to exhibit intact APC functions and TLR expression in aging (Tesar et al. 2006). Nevertheless, in order to broadly stimulate potentially weakened DC functions in aging and to avoid polarized Th1 (inflammatory)- or Th2 (allergic)-type immune responses in the elderly, a double adjuvant system has been developed using a combination of pFL and CpG ODN. In this regard, it has been shown that aged mice given nasal OVA plus a combined nasal adjuvant consisting of a plasmid encoding the Flt3 ligand cDNA (pFL) and CpG ODN showed significantly increased levels of Ag-specific, mucosal SIgA and plasma IgG Ab responses (Fukuiwa et al. 2008). It is important to note that a balanced Th1- and Th2-type cytokine response with essentially no potential inflammatory IL-17 responses were induced by this double adjuvant system (Fukuiwa et al. 2008) (Fig. 3).
In order to assess whether this double adjuvant system could successfully induce bacterial Ag-specific SIgA Ab responses in the UR tract mucosa for prevention of both S. pneumoniae carriage and infection in the elderly, aged mice were nasally immunized with pneumococcal surface protein A (PspA) plus a combination of pFL and CpG ODN. Vaccinated aged mice showed elevated levels of PspA-specific SIgA Ab responses in external secretions and plasma which were comparable to those seen in young adult mice (Fukuyama et al. 2011) (Fig. 3). Significant levels of PspA-induced CD4+ T cell proliferative and PspA-induced Th1- and Th2- but not Th17-type cytokine responses were noted in NALT and cervical lymph nodes of aged mice (Fukuyama et al. 2011). In addition, increased numbers of mature-type CD8- or CD11b-expressing DCs were detected in mucosal tissues of aged mice as a result of the DC-targeting pFL and CpG ODN delivery (Fukuyama et al. 2011). Importantly, aged mice given PspA plus a combination of pFL and CpG ODN showed protective immunity against nasal S. pneumoniae colonization (Fukuyama et al. 2011) (Fig. 3). In contrast, both aged and young adult mice given nasal PspA alone failed to provide sufficient protection after nasal challenge. Thus, high numbers of S. pneumoniae CFUs were seen in nasal washes (NWs) and nasal passages (NPs) of both groups of mice when compared with mice nasally immunized with PspA plus the double adjuvant. Further, aged mice given PspA plus pFL or CpG ODN (single nasal adjuvant regimen) revealed high numbers of bacterial CFUs in both NWs and NPs. The numbers of S. pneumoniae CFUs were essentially the same as seen in mice given PspA alone (Fukuyama et al. 2011). These results demonstrate that nasal delivery of a combined DNA adjuvant offers an attractive possibility for the induction of necessary Ag-specific immune responses (e.g., PspA-specific SIgA and plasma IgG) for protection against S. pneumoniae in the elderly (Fig. 3).
As we have emphasized above, influenza virus is a major human respiratory pathogen in addition to S. pneumoniae and a significant cause of morbidity and death in the elderly. To this end, our study was next designed to assess whether a nasal influenza vaccine together with our double adjuvant system pFL and CpG ODN would enhance influenza virus-specific immunity for the prevention of influenza virus infection in aged mice. A double adjuvant system plus A/Puerto Rico/8/34 (PR8)-HA induced increased numbers of CD11b+ CD11c+ DCs and both CD4+ Th1- and Th2-type responses in mucosal inductive tissues and subsequently elicited PR8-HA-specific SIgA Ab responses in the UR tract of aged mice (Asanuma et al. 2012) (Fig. 3). Thus, when mice were challenged with PR8 virus via the nasal route, both aged and young adult mice given the double adjuvant nasal vaccine exhibited complete protection (Asanuma et al. 2012) (Fig. 3). It should be emphasized that the influenza vaccine given with the double adjuvant system induced high titers of influenza-specific SIgA and plasma IgG Ab responses which provided protective immunity in fully aged mice. These results support the potential use of a double adjuvant system for future human studies (Fig. 3).
Future Prospects for Mucosal Vaccines for the Elderly
In addition to oral and nasal delivery systems, one should consider targeting other mucosal inductive tissues which would potentially induce mucosal immune responses. To this end, it has been shown that vaccine delivery through eye-drops effectively induced Ag-specific SIgA Ab responses (Nagatake et al. 2009; Seo et al. 2010). Further, sublingual application of influenza virus vaccine successfully elicited protective mucosal immunity (Park et al. 2012; Song et al. 2008). It was demonstrated that a nanometer-sized hydrogel (nanogel) consisting of a cationic cholesteryl group-bearing pullulan (cCHP) is also an effective nasal vaccine delivery vehicle for the induction of protective immunity without co-administration of a biologically active adjuvant (Kong et al. 2013; Nochi et al. 2010). Although these alternative mucosal immunization routes and nasal delivery vehicles have been shown to be effective for the induction of Ag-specific immune responses in both mucosal and systemic compartments, it remains to be determined whether they are also applicable to and effective under immunosenescence situations. Conversely, it would be of great benefit to the aged population if one could use an innate adjuvant system alone, without Ag to enhance mucosal SIgA responses, since the elderly should possess preexisting, pathogen-specific memory responses against past respiratory infections. Nevertheless, we still need to understand the precise cellular and molecular mechanisms for mucosal immunosenescence in order to develop novel mucosal vaccines for the elderly which can overcome their age-associated immunodeficiency.
References
Alignani D, Maletto B, Liscovsky M et al (2005) Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J Leukoc Biol 77(6):898–905. https://doi.org/10.1189/jlb.0604330
Ammann AJ, Schiffman G, Austrian R (1980) The antibody responses to pneumococcal capsular polysaccharides in aged individuals. Proc Soc Exp Biol Med 164(3):312–316
Arranz E, O’Mahony S, Barton JR et al (1992) Immunosenescence and mucosal immunity: significant effects of old age on secretory IgA concentrations and intraepithelial lymphocyte counts. Gut 33(7):882–886
Asanuma H, Zamri NB, Sekine S et al (2012) A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine 30(4):803–812. https://doi.org/10.1016/j.vaccine.2011.10.093
Aso K, Tsuruhara A, Takagaki K et al (2016) Adipose-derived mesenchymal stem cells restore impaired mucosal immune responses in aged mice. PLoS One 11(2):e0148185. https://doi.org/10.1371/journal.pone.0148185
Baca-Estrada ME, Ewen C, Mahony D et al (2002) The haemopoietic growth factor, Flt3L, alters the immune response induced by transcutaneous immunization. Immunology 107(1):69–76
Biagi E, Nylund L, Candela M et al (2010) Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5(5):e10667. https://doi.org/10.1371/journal.pone.0010667
Boyaka PN, Tafaro A, Fischer R et al (2003) Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol 170(11):5636–5643
Brasel K, McKenna HJ, Morrissey PJ et al (1996) Hematologic effects of flt3 ligand in vivo in mice. Blood 88(6):2004–2012
Brazolot Millan CL, Weeratna R, Krieg AM et al (1998) CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci (USA) 95(26):15553–15558
Buckley CE 3rd, Buckley EG, Dorsey FC (1974) Longitudinal changes in serum immunoglobulin levels in older humans. Fed Proc 33(9):2036–2039
Casiraghi F, Perico N, Remuzzi G (2013) Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant 18(1):51–58. https://doi.org/10.1097/MOT.0b013e32835c5016
Cebra JJ (1999) Influences of microbiota on intestinal immune system development. Am J Clin Nutr 69(5):1046S–1051S
Claesson MJ, Cusack S, O'Sullivan O et al (2011) Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci (USA) 108(Suppl 1):4586–4591. https://doi.org/10.1073/pnas.1000097107
Comoli P, Ginevri F, Maccario R et al (2008) Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant 23(4):1196–1202. https://doi.org/10.1093/ndt/gfm740
Corcione A, Benvenuto F, Ferretti E et al (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107(1):367–372. https://doi.org/10.1182/blood-2005-07-2657
Corthesy-Theulaz IE, Hopkins S, Bachmann D et al (1998) Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun 66(2):581–586
Csencsits KL, Walters N, Pascual DW (2001) Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: alpha(E) versus L-selectin. J Immunol 167(5):2441–2445
Cuerquis J, Romieu-Mourez R, Francois M et al (2014) Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy 16(2):191–202. https://doi.org/10.1016/j.jcyt.2013.11.008
Curtiss R 3rd, Goldschmidt RM, Fletchall NB et al (1988) Avirulent Salmonella typhimurium delta cya delta crp oral vaccine strains expressing a streptococcal colonization and virulence antigen. Vaccine 6(2):155–160
de Lau W, Kujala P, Schneeberger K et al (2012) Peyer’s patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol Cell Biol 32(18):3639–3647. https://doi.org/10.1128/MCB.00434-12
Di Nicola M, Carlo-Stella C, Magni M et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
Dorronsoro A, Ferrin I, Salcedo JM et al (2014) Human mesenchymal stromal cells modulate T-cell responses through TNF-alpha-mediated activation of NF-kappaB. Eur J Immunol 44(2):480–488. https://doi.org/10.1002/eji.201343668
Ebersole JL, Smith DJ, Taubman MA (1985) Secretory immune responses in ageing rats. I. Immunoglobulin levels. Immunology 56(2):345–350
Enioutina EY, Visic VD, Daynes RA (2000) Enhancement of common mucosal immunity in aged mice following their supplementation with various antioxidants. Vaccine 18(22):2381–2393
Fayad R, Zhang H, Quinn D et al (2004) Oral administration with papillomavirus pseudovirus encoding IL-2 fully restores mucosal and systemic immune responses to vaccinations in aged mice. J Immunol 173(4):2692–2698
Figueroa FE, Carrion F, Villanueva S et al (2012) Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res 45(3):269–277. https://doi.org/10.4067/S0716-97602012000300008
Finkelstein MS, Tanner M, Freedman ML (1984) Salivary and serum IgA levels in a geriatric outpatient population. J Clin Immunol 4(2):85–91
Forbes GM, Sturm MJ, Leong RW et al (2014) A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol 12(1):64–71. https://doi.org/10.1016/j.cgh.2013.06.021
Franceschi C, Capri M, Monti D et al (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128(1):92–105. https://doi.org/10.1016/j.mad.2006.11.016
Fujihashi K, Kiyono H (2009) Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol 30(7):334–343. https://doi.org/10.1016/j.it.2009.04.004
Fujihashi K, McGhee JR (2004) Mucosal immunity and tolerance in the elderly. Mech Ageing Dev 125(12):889–898. https://doi.org/10.1016/j.mad.2004.05.009
Fujihashi K, Boyaka PN, McGhee JR (2013) Host defenses at mucosal surfaces. In: Rich RT, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM (eds) Clinical immunology, 4th edn. Mosby Elsevier, Philadelphia, pp 287–304
Fukuiwa T, Sekine S, Kobayashi R et al (2008) A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine 26(37):4849–4859. https://doi.org/10.1016/j.vaccine.2008.06.091
Fukuyama S, Hiroi T, Yokota Y et al (2002) Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3(-)CD4(+)CD45(+) cells. Immunity 17(1):31–40
Fukuyama Y, King JD, Kataoka K et al (2010) Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J Immunol 185(3):1755–1762. https://doi.org/10.4049/jimmunol.1000831
Fukuyama Y, King JD, Kataoka K et al (2011) A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J Immunol 186(4):2454–2461. https://doi.org/10.4049/jimmunol.1002837
Galen JE, Chinchilla M, Pasetti MF et al (2009) Mucosal immunization with attenuated Salmonella enterica serovar Typhi expressing protective antigen of anthrax toxin (PA83) primes monkeys for accelerated serum antibody responses to parenteral PA83 vaccine. J Infect Dis 199(3):326–335. https://doi.org/10.1086/596066
Galli G, Hancock K, Hoschler K et al (2009) Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci (USA) 106(19):7962–7967. https://doi.org/10.1073/pnas.0903181106
Gallichan WS, Rosenthal KL (1996) Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med 184(5):1879–1890
Garcia-Olmo D, Garcia-Arranz M, Herreros D (2008) Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Expert Opin Biol Ther 8(9):1417–1423. https://doi.org/10.1517/14712598.8.9.1417
Goto Y, Obata T, Kunisawa J et al (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345(6202):1254009. https://doi.org/10.1126/science.1254009
Hagiwara Y, McGhee JR, Fujihashi K et al (2003) Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J Immunol 170(4):1754–1762
Harriman GR, Bogue M, Rogers P et al (1999) Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol 162(5):2521–2529
Hase K, Kawano K, Nochi T et al (2009) Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 462(7270):226–230. https://doi.org/10.1038/nature08529
Hashigucci K, Ogawa H, Ishidate T et al (1996) Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine 14(2):113–119
Hashizume T, Togawa A, Nochi T et al (2008) Peyer’s patches are required for intestinal immunoglobulin A responses to Salmonella spp. Infect Immun 76(3):927–934. https://doi.org/10.1128/IAI.01145-07
Haynes L, Linton PJ, Eaton SM et al (1999) Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med 190(7):1013–1024
He B, Xu W, Santini PA et al (2007) Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26(6):812–826. https://doi.org/10.1016/j.immuni.2007.04.014
Hemmi H, Takeuchi O, Kawai T et al (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408(6813):740–745. https://doi.org/10.1038/35047123
Henson SM, Pido-Lopez J, Aspinall R (2004) Reversal of thymic atrophy. Exp Gerontol 39(4):673–678. https://doi.org/10.1016/j.exger.2003.10.030
Ho MS, Mei SH, Stewart DJ (2015) The immunomodulatory and therapeutic effects of mesenchymal stromal cells for acute lung injury and sepsis. J Cell Physiol. https://doi.org/10.1002/jcp.25028
Holmgren J, Czerkinsky C (2005) Mucosal immunity and vaccines. Nat Med 11(4 Suppl):S45–S53. https://doi.org/10.1038/nm1213
Hopkins S, Kraehenbuhl JP, Schodel F et al (1995) A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun 63(9):3279–3286
Hung CF, Hsu KF, Cheng WF et al (2001) Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res 61(3):1080–1088
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16(4):343–353. https://doi.org/10.1038/ni.3123
Jackson LA, Chen WH, Stapleton JT et al (2012) Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J Infect Dis 206(6):811–820. https://doi.org/10.1093/infdis/jis427
Kanaya T, Hase K, Takahashi D et al (2012) The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat Immunol 13(8):729–736. https://doi.org/10.1038/ni.2352
Kataoka K, McGhee JR, Kobayashi R et al (2004) Nasal Flt3 ligand cDNA elicits CD11c+CD8+ dendritic cells for enhanced mucosal immunity. J Immunol 172(6):3612–3619
Kato H, Fujihashi K, Kato R et al (2003) Lack of oral tolerance in aging is due to sequential loss of Peyer’s patch cell interactions. Int Immunol 15(2):145–158
Katsuda T, Tsuchiya R, Kosaka N et al (2013) Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3:1197. https://doi.org/10.1038/srep01197
Kawamoto S, Maruya M, Kato LM et al (2014) Foxp3(+) T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41(1):152–165. https://doi.org/10.1016/j.immuni.2014.05.016
Kawanishi H, Ajitsu S (1991) Correction of antigen-specific T cell defects in aged murine gut-associated lymphoid tissues an immune intervention by combined adoptive transfer of an antigen-specific immunoregulatory CD4 T cell subset and interleukin 2 administration. Eur J Immunol 21(12):2907–2914
Kawanishi H, Kiely J (1989) Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig Dis Sci 34(2):175–184
Kawanishi H, Senda S, Ajitsu S (1989) Aging-associated intrinsic defects in IgA production by murine Peyer’s patch B cells stimulated by autoreactive Peyer’s patch T cell hybridoma-derived B cell stimulatory factors (BSF). Mech Ageing Dev 49(1):61–78
Kim SH, Seo KW, Kim J et al (2010) The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol 185(10):5787–5795
Kiyono H, Kunisawa J, McGhee JR et al (2008) The mucosal immune system. In: Paul WE (ed) Fundamental immunology, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 983–1030
Klaasen HL, Koopman JP, Van den Brink ME et al (1991) Mono-association of mice with non-cultivable, intestinal, segmented, filamentous bacteria. Arch Microbiol 156(2):148–151
Klinman DM (1998) Therapeutic applications of CpG-containing oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev 8(2):181–184. https://doi.org/10.1089/oli.1.1998.8.181
Klinman DM, Barnhart KM, Conover J (1999) CpG motifs as immune adjuvants. Vaccine 17(1):19–25
Klinman DM, Currie D, Gursel I et al (2004) Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev 199:201–216. https://doi.org/10.1111/j.0105-2896.2004.00148.x
Knoop KA, Kumar N, Butler BR et al (2009) RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol 183(9):5738–5747. https://doi.org/10.4049/jimmunol.0901563
Knyazev OV, Parfenov AI, Shcherbakov PL et al (2013) Cell therapy of refractory Crohn’s disease. Bull Exp Biol Med 156(1):139–145
Kobayashi A, Donaldson DS, Erridge C et al (2013) The functional maturation of M cells is dramatically reduced in the Peyer’s patches of aged mice. Mucosal Immunol 6(5):1027–1037. https://doi.org/10.1038/mi.2012.141
Koga T, McGhee JR, Kato H et al (2000) Evidence for early aging in the mucosal immune system. J Immunol 165(9):5352–5359
Kong QF, Sun B, Bai SS et al (2009) Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol 207(1–2):83–91. https://doi.org/10.1016/j.jneuroim.2008.12.005
Kong IG, Sato A, Yuki Y et al (2013) Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect Immun 81(5):1625–1634. https://doi.org/10.1128/IAI.00240-13
Kovacs EJ, Palmer JL, Fortin CF et al (2009) Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol 30(7):319–324. https://doi.org/10.1016/j.it.2009.03.012
Krampera M, Glennie S, Dyson J et al (2003) Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood 101(9):3722–3729. https://doi.org/10.1182/blood-2002-07-2104
Kunisawa J, Nochi T, Kiyono H (2008) Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol 29(11):505–513. https://doi.org/10.1016/j.it.2008.07.008
Kurokawa K, Itoh T, Kuwahara T et al (2007) Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res 14(4):169–181. https://doi.org/10.1093/dnares/dsm018
Kweon MN, Yamamoto M, Watanabe F et al (2002) A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J Infect Dis 186(9):1261–1269. https://doi.org/10.1086/344526
Le Blanc K, Frassoni F, Ball L et al (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–1586. https://doi.org/10.1016/S0140-6736(08)60690-X
Macpherson AJ, Harris NL (2004) Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4(6):478–485. https://doi.org/10.1038/nri1373
Macpherson AJ, McCoy KD, Johansen FE et al (2008) The immune geography of IgA induction and function. Mucosal Immunol 1(1):11–22. https://doi.org/10.1038/mi.2007.6
Malcherek G, Jin N, Huckelhoven AG et al (2014) Mesenchymal stromal cells inhibit proliferation of virus-specific CD8(+) T cells. Leukemia 28(12):2388–2394. https://doi.org/10.1038/leu.2014.273
Maraskovsky E, Brasel K, Teepe M et al (1996) Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 184(5):1953–1962
Marinaro M, Staats HF, Hiroi T et al (1995) Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol 155(10):4621–4629
Maroof A, Yorgensen YM, Li Y et al (2014) Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection. PLoS Pathog 10(1):e1003875. https://doi.org/10.1371/journal.ppat.1003875
McCluskie MJ, Davis HL (1998) CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol 161(9):4463–4466
Min D, Panoskaltsis-Mortari A, Kuro OM et al (2007) Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood 109(6):2529–2537. https://doi.org/10.1182/blood-2006-08-043794
Miyahara Y, Nagaya N, Kataoka M et al (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12(4):459–465. https://doi.org/10.1038/nm1391
Moldoveanu Z, Love-Homan L, Huang WQ et al (1998) CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16(11–12):1216–1224
Moore AC, Kong WP, Chakrabarti BK et al (2002) Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J Virol 76(1):243–250
Moretto MM, Lawlor EM, Khan IA (2008) Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol 181(11):7977–7984
Nagatake T, Fukuyama S, Kim DY et al (2009) Id2-, RORγt-, and LTβR-independent initiation of lymphoid organogenesis in ocular immunity. J Exp Med 206(11):2351–2364. https://doi.org/10.1084/jem.20091436
Nardelli-Haefliger D, Roden RB, Benyacoub J et al (1997) Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun 65(8):3328–3336
Nardelli-Haefliger D, Benyacoub J, Lemoine R et al (2001) Nasal vaccination with attenuated Salmonella typhimurium strains expressing the Hepatitis B nucleocapsid: dose response analysis. Vaccine 19(20–22):2854–2861
Nochi T, Yuki Y, Matsumura A et al (2007) A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med 204(12):2789–2796. https://doi.org/10.1084/jem.20070607
Nochi T, Yuki Y, Takahashi H et al (2010) Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater 9(7):572–578. https://doi.org/10.1038/nmat2784
Obata T, Goto Y, Kunisawa J et al (2010) Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci (USA) 107(16):7419–7424. https://doi.org/10.1073/pnas.1001061107
Okahashi N, Yamamoto M, Vancott JL et al (1996) Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun 64(5):1516–1525
Park HJ, Ferko B, Byun YH et al (2012) Sublingual immunization with a live attenuated influenza A virus lacking the nonstructural protein 1 induces broad protective immunity in mice. PLoS One 7(6):e39921. https://doi.org/10.1371/journal.pone.0039921
Park MJ, Kwok SK, Lee SH et al (2015) Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant 24(11):2367–2377. https://doi.org/10.3727/096368914X685645
Pascual DW, Riccardi C, Csencsits-Smith K (2008) Distal IgA immunity can be sustained by αEβ7+ B cells in L-selectin-/- mice following oral immunization. Mucosal Immunol 1(1):68–77. https://doi.org/10.1038/mi.2007.2
Perez-Simon JA, Lopez-Villar O, Andreu EJ et al (2011) Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 96(7):1072–1076. https://doi.org/10.3324/haematol.2010.038356
Pisarev VM, Parajuli P, Mosley RL et al (2000) Flt3 ligand enhances the immunogenicity of a gag-based HIV-1 vaccine. Int J Immunopharmacol 22(11):865–876
Powers DC (1992) Immunological principles and emerging strategies of vaccination for the elderly. J Am Geriatr Soc 40(1):81–94
Prevosto C, Zancolli M, Canevali P et al (2007) Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92(7):881–888
Psaltis PJ, Zannettino AC, Worthley SG et al (2008) Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26(9):2201–2210. https://doi.org/10.1634/stemcells.2008-0428
Rothenthal KL, Jeyanathan M, Xing Z (2015) Filling the immunological gap: recombinant viral vectors for mucosal vaccines. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H (eds) Mucosal immunology, 4th edn. Elsevier, Waltham, pp 1291–1306
Sato S, Kaneto S, Shibata N et al (2013) Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer’s patch M cells. Mucosal Immunol 6(4):838–846. https://doi.org/10.1038/mi.2012.122
Sato S, Kiyono H, Fujihashi K (2015) Mucosal immunosenescence in the gastrointestinal tract: a mini-review. Gerontology 61(4):336–342. https://doi.org/10.1159/000368897
Schmucker DL, Daniels CK, Wang RK et al (1988) Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology 64(4):691–695
Schmucker DL, Heyworth MF, Owen RL et al (1996) Impact of aging on gastrointestinal mucosal immunity. Dig Dis Sci 41(6):1183–1193
Schneider-Ohrum K, Giles BM, Weirback HK et al (2011) Adjuvants that stimulate TLR3 or NLPR3 pathways enhance the efficiency of influenza virus-like particle vaccines in aged mice. Vaccine 29(48):9081–9092. https://doi.org/10.1016/j.vaccine.2011.09.051
Schodel F, Kelly S, Tinge S et al (1996) Hybrid hepatitis B virus core antigen as a vaccine carrier moiety. II. Expression in avirulent Salmonella spp. for mucosal immunization. Adv Exp Med Biol 397:15–21
Sekine S, Kataoka K, Fukuyama Y et al (2008) A novel adenovirus expressing Flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated lymphoreticular tissue dendritic cell migration. J Immunol 180(12):8126–8134
Senda S, Cheng E, Kawanishi H (1988) Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand J Immunol 27(2):157–164
Seo KY, Han SJ, Cha HR et al (2010) Eye mucosa: an efficient vaccine delivery route for inducing protective immunity. J Immunol 185(6):3610–3619. https://doi.org/10.4049/jimmunol.1000680
Shaw AC, Joshi S, Greenwood H et al (2010) Aging of the innate immune system. Curr Opin Immunol 22(4):507–513. https://doi.org/10.1016/j.coi.2010.05.003
Shroff KE, Meslin K, Cebra JJ (1995) Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun 63(10):3904–3913
Song JH, Nguyen HH, Cuburu N et al (2008) Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci (USA) 105(5):1644–1649. https://doi.org/10.1073/pnas.0708684105
Suzuki K, Fagarasan S (2008) How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol 29(11):523–531. https://doi.org/10.1016/j.it.2008.08.001
Suzuki K, Meek B, Doi Y et al (2004) Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci (USA) 101(7):1981–1986. https://doi.org/10.1073/pnas.0307317101
Tahoun A, Mahajan S, Paxton E et al (2012) Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe 12(5):645–656. https://doi.org/10.1016/j.chom.2012.10.009
Tamura S, Ito Y, Asanuma H et al (1992) Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol 149(3):981–988
Taylor LD, Daniels CK, Schmucker DL (1992) Ageing compromises gastrointestinal mucosal immune response in the rhesus monkey. Immunology 75(4):614–618
Terahara K, Yoshida M, Igarashi O et al (2008) Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J Immunol 180(12):7840–7846
Tesar BM, Walker WE, Unternaehrer J et al (2006) Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell 5(6):473–486. https://doi.org/10.1111/j.1474-9726.2006.00245.x
Thompson WW, Shay DK, Weintraub E et al (2004) Influenza-associated hospitalizations in the United States. JAMA 292(11):1333–1340. https://doi.org/10.1001/jama.292.11.1333
Thoreux K, Owen RL, Schmucker DL (2000) Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology 101(1):161–167
Tobita M, Orbay H, Mizuno H (2011) Adipose-derived stem cells: current findings and future perspectives. Discov Med 11(57):160–170
Tobita M, Uysal CA, Guo X et al (2013) Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy 15(12):1517–1526. https://doi.org/10.1016/j.jcyt.2013.05.007
Tremaroli V, Backhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489(7415):242–249. https://doi.org/10.1038/nature11552
Tsuji M, Suzuki K, Kinoshita K et al (2008) Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin Immunol 20(1):59–66. https://doi.org/10.1016/j.smim.2007.12.003
Vajdy M, Kosco-Vilbois MH, Kopf M et al (1995) Impaired mucosal immune responses in interleukin 4-targeted mice. J Exp Med 181(1):41–53
Van Ginkel FW, Liu C, Simecka JW et al (1995) Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum Gene Ther 6(7):895–903. https://doi.org/10.1089/hum.1995.6.7-895
VanCott JL, Staats HF, Pascual DW et al (1996) Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 156(4):1504–1514
Viney JL, Mowat AM, O'Malley JM et al (1998) Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol 160(12):5815–5825
Wagner H (1999) Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol 73:329–368
Wang S, Li Y, Shi H et al (2010) Immune responses to recombinant pneumococcal PsaA antigen delivered by a live attenuated Salmonella vaccine. Infect Immun 78(7):3258–3271. https://doi.org/10.1128/IAI.00176-10
Wannemuehler MJ, Kiyono H, Babb JL et al (1982) Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol 129(3):959–965
Webster RG (2000) Immunity to influenza in the elderly. Vaccine 18(16):1686–1689
Williamson E, Westrich GM, Viney JL (1999) Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol 163(7):3668–3675
Woodmansey EJ (2007) Intestinal bacteria and ageing. J Appl Microbiol 102(5):1178–1186. https://doi.org/10.1111/j.1365-2672.2007.03400.x
Wu HY, Russell MW (1994) Comparison of systemic and mucosal priming for mucosal immune responses to a bacterial protein antigen given with or coupled to cholera toxin (CT) B subunit, and effects of pre-existing anti-CT immunity. Vaccine 12(3):215–222
Wu Y, Wang X, Csencsits KL et al (2001) M cell-targeted DNA vaccination. Proc Natl Acad Sci (USA) 98(16):9318–9323. https://doi.org/10.1073/pnas.161204098
Xu-Amano J, Kiyono H, Jackson RJ et al (1993) Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med 178(4):1309–1320
Yamamoto S, Kiyono H, Yamamoto M et al (1997) A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci (USA) 94(10):5267–5272
Yamamoto M, Briles DE, Yamamoto S et al (1998) A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol 161(8):4115–4121
Yamamoto M, Rennert P, McGhee JR et al (2000) Alternate mucosal immune system: organized Peyer’s patches are not required for IgA responses in the gastrointestinal tract. J Immunol 164(10):5184–5191
Zhou T, Edwards CK 3rd, Mountz JD (1995) Prevention of age-related T cell apoptosis defect in CD2-fas-transgenic mice. J Exp Med 182(1):129–137
Acknowledgments
Portions of the work described in this review chapter was supported by National Institutes of Aging (NIA) grant AG025873 (to KF) and research funding from BioMimetics Sympathy Inc. (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Fujihashi, K., McGhee, J.R., Kiyono, H. (2019). Mucosal Vaccination Challenges in Aging: Understanding Immunosenescence in the Aerodigestive Tract. In: Fulop, T., Franceschi, C., Hirokawa, K., Pawelec, G. (eds) Handbook of Immunosenescence. Springer, Cham. https://doi.org/10.1007/978-3-319-99375-1_114
Download citation
DOI: https://doi.org/10.1007/978-3-319-99375-1_114
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99373-7
Online ISBN: 978-3-319-99375-1
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences