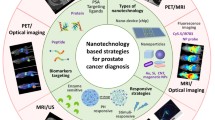
Abstract
Prostate cancer (PCa) is the most common type of cancer in men with high morbidity and mortality. However, the current treatment with drugs often leads to chemotherapy resistance. It is known that the multi-disciplines research on molecular imaging is very helpful for early diagnosing, staging, restaging and precise treatment of PCa. In the past decades, the tumor-specific targeted drugs were developed for the clinic to treat prostate cancer. Among them, the emerging nanotechnology has brought about many exciting novel diagnosis and treatments systems for PCa. Nanotechnology can greatly enhance the treatment activity of PCa and provide novel theranostics platform by utilizing the unique physical/chemical properties, targeting strategy, or by loading with imaging/therapeutic agents. Herein, this chapter focuses on state-of-art advances in imaging and diagnosing PCa with nanomaterials and highlights the approaches used for functionalization of the targeted biomolecules, and in the treatment for various aspects of PCa with multifunctional nanoparticles, nanoplatforms and nanodelivery system.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
In 1851, Adams first described prostate cancer (PCa) through histological examination [1]. At that time, peopled defined the case of prostate cancer as a rare disease. But to date, prostate cancer becomes the most common type of cancer for males, particularly in the developed countries [2]. Additionally, the incidence of prostate cancer has kept increasing. In 2012, more than 1.1 million cases were diagnosed with prostate cancer and 307,000 died [3]. To reduce the public health impact of PCa, research has been focused on developing detection and treatment strategies for PCa [4, 5]. CT and MRI technologies are very useful to diagnosis of prostate cancer in clinic. However, to date these technologies haven’t been applied for the intraoperative imaging [6]. Fluorescence imaging would be an ideal approach to detect PCa and the image-guided surgery due to its high sensitivity, real-time, noninvasive and high compatibility [7, 8]. On the other hand, the main clinical treatments for PCa include surgery, radiotherapy and chemotherapy [9,10,11]. Chemotherapy with drugs is the primary clinical treatment to prolong patient survival [12]. Unfortunately, the serious toxicity of chemotherapeutics to normal tissues, poor penetration into deeper tumor tissues and the chemotherapy resistance limited their efficacy [13, 14]. Recently, the emerging nanotechnology has brought about many exciting novel diagnosis and treatment systems for PCa. Utilizing the unique physical/chemical properties and targeting strategies, or loading with imaging/therapeutic agents, nanotechnology can greatly enhance the treatment activity of PCa and provide a diagnosis/theranostics platform for cancer. Nanotechnology can greatly enhance the treatment activity of PCa and provide novel theranostics platform by utilizing the unique physical/chemical properties, targeting strategy, or by loading with imaging/therapeutic agents. Nanotechnology is promising to diagnosis and treatment of cancer by using the unique properties of engineered nanoparticles [15]. We can benefit a lot from nanotechnology, such as delivery of poorly water-soluble drugs, improving the targeting of drugs, increasing of cell permeability, construction of innovative therapeutic and diagnostic probes [16]. In this review, we focus on the bench-to-bed advances of nanotechnologies for fluorescence diagnosis and treatment of PCa.
6.2 Nanotechnologies for Fluorescence Diagnosis of PCa in vivo
Diagnostic can offer phenotype, and stage of cancer and aid in guiding treatment. The multi-disciplines research on molecular imaging is helpful for the early diagnosing, staging, restaging and precise treatment of PCa. Nanotechnology used in diagnostic provides imaging with high sensitivity, resolution, specificity, and reliability. With the developing of nanotechnology and imaging technology, we can detect cancer biomarkers at the molecular and evaluate therapeutic outcomes in vivo. Although CT and MRI are commonly used to diagnosis of prostate cancer in clinic, this section we will focus on fluorescence imaging, due to the high sensitivity, real-time, noninvasive and high compatibility. To realize early detection and imaging in current therapies PCa, various biomarkers of PCa have been discovered, such as prostate specific antigen (PSA), [17, 18] prostate specific membrane antigen (PSMA), [19] hepsin [20] and matriptase [21].
6.2.1 Targeted PSA Nanoprobe for Imaging PCa
PSA is produced by the prostate gland that is a 33 kDa androgen-regulated serine protease. Nowadays, diagnosis of PCa is often relied on the usage of biomarkers, especially PSA. It has been applied as an organ-specific biomarker for a long time and has been one of the most commonly diagnosis index for PCa, leading to the obvious enhanced detection at earlier stage and helping to decrease the number of metastatic patients.
In 2001 Lövgren reported a detection technology based on a europium (III) nanoparticles and successfully demonstrated the concentration detection and visualization of PSA molecules by a time-resolved microscope [22]. They first washed and activated the commercially available europium chelate (β-diketone)-incorporated polystyrene nanoparticles by phosphate buffer and Fluka. Then, 15 mmol/L streptavidin was added into the activated nanoparticles buffer for 2 h incubation. Finally, the particles were loaded with streptavidin. Biotinylated PSA was incubated with streptavidin-coated 107-nm nanoparticles, with a small volume of 30 μL in order to make PSA direct react on the bottom of the plate for detection. The detection limit was 0.38 ng/L, or 10 fmol/L of PSA molecules correspondence. The nanoparticle loaded with streptavidin was more than ten-fold sensitive than the previously reported molecule probe in a microtiter plate-based PSA assay [23]. In addition, the nanoparticles could achieve an obvious visible in a 45-s exposure time to PSA, indicating a good future in clinical application.
In 2006, Lee designed a hybrid probe with artificial tag molecules by combining particle and peptides, which have high specificity to PSA [24]. After the digestion reaction by with PSA, the peptide was cleaved, leading an individual surface enhanced Raman scattering (SERS) of nanoparticles signal change. The probe could achieve PSA proteolytic reactions imaging. The probe was prepared starting from evaporating nanoscale Au layer on polystyrene nanoparticles. Meanwhile, peptides were preparation by the PSA specific substrate sequence (HSSKLQ) and were ended by a Raman tag molecule. The peptides were linked through a Au-S bond of the nanocrescents to the Au surface at last. During the peptide digestion experiments, the peptide-conjugated nanoparticles were incubated with PSA molecules for 2 h on a 37 °C thermal plate. Monitored by the SERS spectra on the peptide digestion experiments, the peaks of the Raman tag molecules, such as 525 cm−1 from biotin almost disappeared completely after the digestion reaction had finished. The results indicated that such peptide-conjugated nanoparticles could be applied as a specific probe on the concentration of the cancer biomarker PSA image.
Fluorescent probes with multiplexing capability and improved brightness are in great need for low abundance targets analysis in bioassays and clinical cases. QDs are found to be 20–50 times brighter than single dye molecules, and were of vital importance to various applications owing to their desirable optical properties. Gao developed a new strategy of nanoparticles probe design, successfully demonstrated a sensitive detection of human prostate specific antigen (PSA) probe in 2009 [25]. They developed a new method for preparation of QD based on nanoparticle-amphiphilic polymer complexes self-assembly in homogeneous solution. QDs coupled with polymaleic anhydride-octadecene via multivalent hydrophobic interactions are highly soluble in tetrahydrofuran but form aggregates in polar solvents. As a new approach of the formation mechanism, a great deal of QDs can be loaded into a nanocore and the embedded nanoparticles space distribution could be manipulated. In 2016, Chen reported the application of novel sub-5 nm Lu6O5F8:Eu3+ nanoprobe for the successful detection of PSA in clinical cases [26]. They have developed inorganic lanthanide fluoride nanoparticles based on dissolution-enhanced luminescent bioassay technique, leading to amplified signal and improving the detection sensitivity. They synthesized monodisperse and ultra-small Ln3+ doped lutetium oxyfluoride nanoparticles via a modified thermalde composition route. Ln3+-NPs were activated with EDC and NHS. Then, the activated NPs were purified by centrifuging at 13,600 rpm and incubated with avidin in phosphate buffered saline. Biotinylated anti-PSA monoclonal antibody was added to each well and the plate was incubated. Thereafter, avidin-conjugated Lu6O5F8:Eu3+ NPs was added to each well and the plate was incubated. The buffer was measured at room temperature under the kinetic and time-resolved detection mode on a multimodal microplate reader. The limit of detection for PSA was as low as 0.52 pg/mL, almost a 200-fold sensitivity to that of a commercial DELFIA kit, which indicated a highly promising for the early diagnosis of PCa.
6.2.2 Targeted Prostate-Specific Membrane Antigen (PSMA) Nanoprobes for Imaging PCa
The PSMA is expressed in both the benign, and the neoplastic prostatic epithelial cells, and in other tissues, such as kidney, liver and brain. It is up-regulation in metastatic disease and in hormone-resistant states. It is a transmembrane with 750 amino acid and type II glycoprotein which is primarily expressed in normal human prostate epithelium while overexpressed in PCa cells. PSMA is a very significantly target for PCa imaging and therapy because it is expressed by virtually all PCa cells and its expression is further increased in poorly differentiated, metastatic and hormone-refractory carcinomas [27].
Research work indicated that biotinylated anti-PSMA antibody conjugated to streptavidin-labeled iron oxide nanoparticles would be used as the unique probe for detection and diagnosis of PCa cells. In 2013 Berkman exhibited the first AuNPs system for targeting PSMA expressing level in PCa cells with conjugation of a small molecule peptidomimetic inhibitor [28]. The construction of the PSMA-targeted AuNPs was generated by commercially available 5 nm AuNPs coated with streptavidin and incubating the biotinylated PSMA inhibitor. The PSMA-targeted AuNPs was generated by commercially available 5 nm, and the AuNPs was coated with streptavidin and incubated by the biotinylated PSMA inhibitor. After centrifugal filtration to remove redundant biotinylated PSMA inhibitor, the PSMA-targeted nanoparticles has been composed in suspension and characterized by transmission electron microscopy. The PSMA inhibitor-mediated binding test indicated that the PSMA-targeted nanoparticles have a superior significant binding ability to LNCaP cells, compared to non-targeted AuNPs nanoparticles. The results suggested that the unique targeting of PSMA-targeted AuNPs is better than over non-targeted non-specificity AuNPs, and for the first time it demonstrated that AuNPs can be used to target PSMA by the employment of small molecule inhibitors.
6.3 Nanotechnologies for Prostate Cancer Treatment
6.3.1 Treatment of Prostate Cancer via Chemotherapy with Nanomaterials
In clinical practices, current treatments of prostate cancer are predominantly systemically administered chemotherapy, surgery and radiotherapy [29]. Chemotherapy with drugs, such as paclitaxel (PTX), doxorubicin and docetaxel (DTX) is effective to prolong survival and improve quality of life for patients. However, chemotherapeutics can cause many side-effects, such as body weight, hair loss, nausea, cardiac, liver and kidney toxicity and a destructive “bystander” effect to neighboring cells [30, 31]. In addition, due to the poor penetration of drugs into tumor tissues, the therapeutic efficacy is limited [32]. In order to overcome the systemic toxicity and low therapeutic efficacy, many technologies, such as drug analogs, prodrugs and nanomaterials, have been developed for clinical applications [33, 34]. In recent year, nanomaterial has been one of the most promising tools to significantly enhance antitumor efficacy because of their unique intrinsic physical and chemical properties, [35] and more and more studies were devoted to the treatment of prostate cancer via chemotherapy with nanomaterials to increase drug efficacy, decrease drug toxicity, and maintain a relatively high concentration of drug at the site of interest.
As known, poly(d,l-lactic-co-glycolic acid) (PLGA) is an excellent controlled release polymer because of their safety in clinic. In 2008, Farokhzad’s group reported a unique nanotechnology to deliver cisplatin to prostate cancer cells [36]. In their strategy, platinum (IV) compound c,t,c-[Pt(NH3)2(O2CCH2CH2CH2CH2CH3)2Cl2], as a cisplatin-prodrug, was encapsulated in nanoparticles to deliver cisplatin, and the prostate-specific membrane antigen targeting aptamers (Apt) was introduced to decorate the surface of the nanoparticles and target to the prostate cancer cell. The nanoparticles were derived from PEG-functionalized PLGA and used as a controlled release polymer system to deliver and release drugs to target cells with high safety and low clearance. Through the intrastrand cross-links, the cisplatin could be reductive released from the nanoparticles forms. Cell experiments demonstrated that the curative effect of aptamer-derivatized Pt(IV)-encapsulated nanoparticles was better than cisplatin or nontargeted nanoparticles significantly. The in vivo result demonstrated that system was efficacious in reducing prostate tumors at a significantly low dose of platinum [37]. Further, they codelivered cisplatin and docetaxel to prostate cancer cells through a self-assembled polymeric nanoparticle platform in 2010 [38]. The self-assembled polymeric NPs could target to PSMA through the A10 aptamer on the surface with an outstanding efficacy on PCa. In addition, since NPs size could affect the penetration and distribution of tumor cells through the enhanced permeability and retention effect, more and more studies focused on the size to enhance the drugs to tumor sites and improve the efficacy. For example, C. Furman group designed and synthesized a paclitaxel-loaded small PLGA NPs [39]. The size of NPs was between in 45 and 95 nm. Their results showed that the small paclitaxel-loaded PLGA NPs have better efficacy than the free drug and larger NPs. Besides the PLGA and related materials mentioned above, there are many other materials, such as carboxymethylcellulose (Cellax) NPs, [40] polyethylene glycol hyperbranched polymers [41] and so on, could be applied as the vehicle to deliver chemical drugs to prostate cancer cell. Recently, magnetic nanoparticles (MNPs) have been attracted more attention due to its advantages such as chemical stability, low toxicity, good biocompatibility. Usually, MNPs refer to the nanomaterials containing cobalt (Co) or iron (Fe) as well as their oxides and alloys. They become superparamagnetic at room temperature when its size is below a critical value. Additionally, MNPs have been treated as promising drug delivery vehicles for therapeutic applications. For instance, Masatoshi’ group designed and synthesized a MgNPs-Fe3O4 nanoprobe to carry drugs to prostate cancer cell, and founded that the nanoprobe could significantly increase ROS production in prostate cancer cell lines and induce oxidative DNA damage [42]. Compared with the chemical drugs alone, the combination of MgNPs-Fe3O4 and a low dose of drug have a superior efficacy on prostate cancer cell in vitro. In 2015, Wang and co-workers reported a magnetic nanoparticle clusters (MNCs) loading chemotherapeutic agent of DOX and developed the combination of photothermal therapy (PTT) and chemotherapy for destruction of PC3 cells [43]. Due to the near-infrared property of MNCs, DOX@MNCs could be used as both photothermal mediators and drug vehicles, and could be applied in the combination of PTT and drug delivery for therapy of prostate cancer. The in vitro results showed that a higher therapeutic efficacy could be obtained by the chemophotothermal therapy of DOX@MNCs. Recently, gold nanoparticles were also considered as ideal drug delivery platforms due to their nonimmunogenicity and nontoxicity. Moreover, they were synthesized easily, and the high surface area could increase drug density. For example, Liang and co-workers developed a targeted drug delivery strategy based on GSH-stabilized gold NPs (Au@GSH NPs) consisting of a platinum (IV) drug and a receptor targeting peptide CRGDK [44]. Their results indicated that the cytotoxicity and uptake efficiency of this NPs is superior to that of Au@GSH and Au@Pt(IV) systems, and further demonstrated potent cytotoxicity against prostate cancer cells that overexpress Nrp-1 receptors.
In the recent years, some anti-cancer compounds have been confirmed to have the potential to improve effectiveness of current cancer chemotherapies. For example, some natural products such as curcumin [45, 46] epigallocathechin-3-gallate (EGCG), [47] resveratrol taxanes [48] have been encapsulated or loaded in nanoparticles and exhibited significant efficacy against prostate cancer. Moreover, the vascular disruptive agents (VDAs) have been known to synergistically enhance radiation and chemotherapy. Bischof’s group designed and synthesized a gold nanoparticle conjugated VDA to significantly improve VDA tumor specific action in combination with locally applied thermal therapy in prostate cancer [49].
6.3.2 Treatment of Prostate Cancer via Gene Delivery with Nanomaterials
As one of the most effective approach in cancer cure, gene delivery has caused wide concern over the recent years. To realize cancer gene therapy, toxic genes need to be diverted to cancer cells and toward cells death steadily and accurately [50]. As a significant regulator for various conditions including developmental, physiological, and pathological, microRNA (miRNA), an endogenously expressed non-coding RNA molecule, have been regarded as potential therapeutic targets in many disease [51, 52]. While, because of the existence of cell membranes and other obstacles, naked genes cannot realize cancer gene therapy alone. Therefore, an adequate vector that can divert the genes efficiently and preserve it from degradation in the blood stream should be designed at once [53]. Recently, non-viral gene delivery systems, including lipids, polymers and nanomaterials, have been developed for siRNA delivery [54, 55]. Frank’s group reported the delivery of small interfering RNA (siRNA) through LbL-assembled microcapsules [56]. In his report, based on the LbL(layer-by-layer) assembly of a crosslinked poly(methacrylic acid) film, two different types of microcapsules were used to deliver an siRNA targeting survivin and the expression of the anti-apoptotic protein was observed. The function of this film is to maintain capsule integrity in the oxidizing bloodstream and in the extracellular environment, thereby, protecting the siRNA from denaturation and make sure the siRNA was released in the reducing intracellular environment. Similarly, Joseph’s group reported the fabrication of poly(lactic acid-co-glycolic acid)/siRNA nanoparticles coated with lipids by a unique soft lithography particle molding process named particle replication in nonwetting templates (PRINT) [57]. Combining polymers and lipids, hybrid NPs with high drug encapsulation yields, tunable and sustained drug release profiles, and excellent serum stabilities could makes it applicable drug delivery platform [58].
Polycationic monodispersed poly(l-lysine) (PLL) is a promising carrier among the variety of polymers designed for gene delivery as the result of controllable size, shape, and the feasible for flexible chemical modification [59, 60]. Nevertheless, the relatively low transfection efficiency limited the application of PLL-based polyplexes in clinical treatment [61]. Through PEGylation of poly-l-lysine-cholic acid (PLL-CA), a kind of amphiphilic polycations have been synthesized [62]. With ‘stealth’ capacity, the benzoic imine linker between PEG and PLL-CA is stable at physiological pH. It is cleavable at lower pH especially in the extracellular environment of tumours and the interior of endosomes/lysosomes. It was reported that the solid lipid PEI hybrid nanocarrier has various advantages including the high silencing efficiency in vitro and in vivo, and the low poisonousness and immunogenicity. As one of the most popular polycationic polymers, polyethylenimine (PEI) was widely used as nonviral gene carriers [63]. Because of the high charge density, PEI molecules can form well-condensed complexes with nucleic acids and can strengthen the interaction with cell surfaces [64]. Furthermore, nucleic acids can be released efficiently from the endosomes through proton sponge effect [65]. Those outstanding properties make contribution to the high transfection efficiency of PEI among nonviral gene carriers. Wang’s group reported a lipid PEI hybrid nanocarrier (LPN) which combining linear PEI with hydrophobic, hexadecyl groups (hydrophobic hexadecylated polyethylenimine (H-PEI)) [66]. The LPN would solved or improved several key issues of siRNA/PEI systems. It includes physical encapsulation of the siRNA rather than coating them on carrier surface, reduction of the loss of siRNA and easiness of controlled, continuing intracellular siRNA release, prevented cells from quick exposure to a high level of unencapsulated PEI molecules, provided more sites for grafting cell-targeting [67,68,69,70]. While, the severe cytotoxicity of PEI caused by the high density of positive charge was discovered and limited the application of PEI [71]. Contrapose this phenomenon, a kind of non-viral cationic polymer vector mPEG-PEI nanoparticles was used as a carrier and the shRNA plasmid was rebuilt [72]. With the engrafted of moieties polyethylene glycol, PEI polymers showed a lower cytotoxicity and better stability. To further increase cell biocompatibility, disulfide linkage was introduced in the branched PEI (SSPEI) containing multiple amine backbone [73]. SSPEI polymer labeled with poly-arginine (R11) which has the highest uptake by different prostate cancer cell lines compared with other four cell permeable peptide was used to deliver miR-145 to the prostate cancer. Moreover, SSPEI polymer introduced a polyethylene glycol chain linker which could enhance biocompatibility and extend circulation time in the bloodstream [74, 75]. The result showed that the R11-SSPEI/FAM-miR-145 complex could dramatically inhibit tumor growth and prolong survival time. To build a better gene delivery system, the ability of target is significant.
With the development of gene therapy technology, therapeutic effects of single gene-targeted therapy was regard as limited, and multiple gene silencing was proposed. Recently, the combinatorial RNAi technology and simultaneous multiple gene silencing have been attempted to cancer therapy and received a big success [76,77,78,79]. Therefore, a new class of dual-genes targeted two different sequences of siRNA (vascular endothelial growth factor (VEGF) and B-cell lymphoma 2 (Bcl-2)) and its their delivery systems for efficient cancer treatment was were developed [80]. Carrying glycol chitosan nanoparticles, the dual-poly-siRNA encapsulated thiolated glycol chitosan (tGC) nanoparticles (dual-NPs) can provided efficient and controlled dual-poly-siRNA delivery and achieved multi-gene silencing with synergistic effects of cancer therapy. Recently, researches showed that the suppression of crucial gene products such as REV1, REV3L can resistant the sensibility of tumors to chemotherapy reduce the drug resistance of relapsed tumors during the errorprone translation DNA synthesis pathway. Based on those researches, a promising strategy which combining siRNA based therapeutics with traditional DNA-damaging chemotherapy was proposed for treating patients with malignancies [81, 82]. A versatile nanoparticle platform was developed to deliver REV1/REV3L-specific siRNAs and a cisplatin prodrug to the same tumor cells simultaneously. Obviously, the result showed a better therapeutic efficacy both in vitro and in vivo than signal single cisplatin prodrug or REV1/REV3L-specific siRNAs [83]. To overcome the accumulation of chemotherapeutic agent in tumor tissue, a synergistic and selective inhibition of cancer cell proliferation platform was reported [84]. With high positive charges on the surface, the DTX-encapsulated bovine serum albumin-polyethylenimine layer-by-layer (LBL) nanoparticles (DTX/BSA-PEILBL NPs) can could adsorb the negative charged p44/p42 MAPK siRNA efficiently. And then, branched polyethylenimine (bPEI) was adsorbed on the surface to form DTX/BSA-PEILBL/siRNA NPs. The result reported a less values of IC50 and a higher median survival, provided a promising synergistic delivery system for clinical treatment of PCa. To realize the application of RNAi therapeutic regimen in clinical treatment of prostate cancer, an approach to evaluate the siRNA delivery at the intended site of action is significant. Therefore, theranostics nanoparticles that associated imaging with therapeutic features was proposed and developed [85]. A nice platform for theranostic imaging of prostate cancer was designed and developed [86]. This theranostic nanoparticle was combined by three core components including the prodrug-activating enzyme bacterial cytosine deaminase (βCD), the imaging carrier poly-l-lysine which traced with a near-infrared fluorescent probe Cy5.5 and the carrier which is not only for siRNA delivery but also for labeling with [111In]DOTA for SPECT imaging. Their results verified the feasibility of the platform for associate detection and treatment. Later, a multimodal theranostic lipid-nanoparticle was reported. The probe was constitutive by a near-infrared (NIR) fluorescent core, covered by phospholipid monolayer, instituted with siRNA payloads with ultra-small particle size (<30 nm) [87]. The siRNA delivery with the orthotopic tumor model was evaluated by image co-registration of computed tomography and fluorescence molecular tomography, achieving efficacious RNAi therapy.
6.3.3 Treatment of Prostate Cancer via Cancer Immunotherapy with Nanomaterials
Cancer immunotherapy is an approach of triggering lymphocyte reaction of cancer related antigen [88]. With the development of tumor-specific therapies, treatments such as peptide-TAAs, protein-TAAs, or cell-based vaccination approaches, was reported and they were potentially capable of stimulating pre-existing antitumor immunity or of inducing de novo antigenic responses. However, after decades of intensive pursuit, this remains a challenging goal. Classical vaccination approaches have been extensively tested and found to be largely inefficient [89, 90]. Whereas, current vaccine design paradigms can effectively generate prophylactic and therapeutic immunities against foreign pathogens, they maybe ill-suited as platforms with which to build cancer fighting vaccines. Compared to conventional approaches, nanoparticles can protect the payload (antigen/adjuvant) from the surrounding biological milieu, increase its half-life, minimize its systemic toxicity, promote its delivery to APCs, or even directly trigger the activation of TAA-specific T-cells. The application of nanomedicine in cancer immunotherapy is currently one of the most challenging areas in cancer therapeutic intervention. Development of nanovaccine formulations was mainly from two directions, nanoparticles as vehicles for drug delivery and nanoparticle-based approaches to elicit antitumor immunity. During the last two decades, several nanoparticle-based compounds delivering encapsulated or conjugated cytotoxic drugs had reached the clinical trial stage [91, 92]. On the other hand, nanoparticle-based delivery TAAs to professional APCs were reported as a potential nanovaccine formulation. It has been shown that certain nanoparticle designs possess immunostimulatory properties, and that antigens delivered by these nanoparticle types can induce T- and B-cell responses in the absence of exogenously added adjuvants [93, 94]. Efficient and targeted delivery of immunomodulatory and immunostimulatory molecules to appropriate cells is vital to the successful development of nanovaccine formulations [95].
Recently, nanoparticles were used as vehicles for drug delivery. For example, Lee had established an interesting platform for effective chemoimmunotherapy. He described a delivery system based on a dendrimer and a single-strand DNA-A9 PSMA RNA aptamer hybrid, and was designed to overcome the drawbacks of conventional cancer therapies. Employing these vehicles, they researchers had demonstrated the promising possibility of this chemoimmunotherapeutic system against prostate cancer both in in vitro and in vivo models. The system has many advantages including cancer-targeting ability, immune-stimulating function, and drug delivery for chemotherapy. The drug-loaded conjugate showed excellent antitumor efficacy and target specificity in an in vivo prostate tumor model due to the high drug-loading capacity and enhanced stability of oligonucleotides in vivo. This proof-of-concept demonstrates the potential value of this nanostructure system (the high drug-loading capacity and enhanced in vivo stability) as a new combination approach for improving cancer treatments [96]. Sun designed a redox-responsive immunostimulatory polymeric prodrug carrier, PSSN10, for programmable co-delivery of an immune checkpoint inhibitor NLG919 (NLG) and a chemotherapeutic doxorubicin. In his work, the prodrug carrier could achieve synergistic therapeutic efficacy, prevent cancer relapse, and combined chemotherapy with immunotherapy as a new modality for tumor treatment. NLG-containing PSSN10 prodrug polymers were self-assembled into nano-sized micelles that served as a carrier to load DOX (DOX/PSSN10 micelles). The PSSN10 carrier dose-dependently enhanced T-cell immune responses in the lymphocyte-Panc02 co-culture experiments, and significantly inhibited tumor growth in vivo. DOX/PSSN10 micelles showed potent cytotoxicity in vitro against 4T1.2 mouse breast cancer cells and PC-3 human prostate cancer cells comparable to that of DOX [97]. Successful treatment requires delivery of critical amounts of drug into the cancerous tissue [98,99,100]. As a model, Jankun and coworkers used LnCAP human prostate cancer cells targeted by antibody (against prostate-specific membrane antigen) to conjugate with hematoporphyrin (HP) through protein-based nanotechnology. Their results suggested that mAb/HP conjugates could deliver HP to the tumor cells and then result in considerably less HP in the circulation and, therefore, lower the delivery of HP to normal tissue, and fewer side effects [101]. Nanoparticle-based approaches can elicit antitumor immunity. Regulating molecular interactions in the T-cell synapse to prevent autoimmunity or, conversely, to boost anti-tumor immunity has long been a goal in immunotherapy. However, delivering therapeutically meaningful doses of immune-modulating compounds into the synapse is still a major challenge [102]. For this purpose, Stephan and coworkers reported a male imide-functionlized nanoparticles by covalent coupling to free thiol groups on T-cell membrane proteins. It could efficient delivery of compounds into the T-cell synapse. They had demonstrated that surface-linked NPs are rapidly polarized toward the nascent immunological synapse (IS) at the T-cell/APC contact zone during antigen recognition. Combination of NSC-87877-the loaded NPs on the surface of tumor specific T cells can cause the tumor site to produce a large number of T cell proliferations before cancer cells to adoptive transfer in mice. Relative to the other of the same drug intake system, nanoparticle-based can improve survival rate of the treated animals [103].
6.4 Conclusion and Future Trends
Molecular imaging probes represent an important, growing class of chemical compound for biology, pharmaceutical sciences, preclinical and clinic studies and further application. In conjunction with the nanoparticle, the identification of molecular imaging targets and the development of new labeled molecular probes for those targets are crucial for expanding the capability of in vivo molecular imaging for biological research, molecular diagnostics and drug discovery.
Various nanomaterials, such as PLGA NPs, cellax NPs, MNPs, AuNPs and etc., have been developed owing to their unique properties. Compared with traditional chemotherapy, the nanomaterial drug/anti-cancer compound delivery system has a better targeting and a lower toxicity, and thus they could exhibit a high therapeutic efficacy for prostate cancer. There is still a long way to realize the application of RNAi therapeutic regimen in clinical treatment of prostate cancer. Too much work need to be done, such as novel miRNA and siRNA with higher efficiency to kill cancer cells, better siRNA delivery system with no cytotoxicity, low accumulation in in tumor tissue, better targeting and other properties that can improve therapeutic efficiency and make patients feel more comfortable, better evaluating and monitoring system toward all aspects of RNAi therapeutic regimen. Combination therapy may be another practicable strategy to get a better therapeutic efficiency. Nanoparticle-based tumor immunotherapy is still in its infancy, but apparently this is a method with great prospects for development. Some researchers have reported various nanoparticles as vehicles for drug delivery to tumor antigens and immune stimulating molecules to DCs and other professional APC type. Compared to conventional approaches, nanoparticles can protect the payload (antigen/adjuvant) from the surrounding biological milieu, increase its half-life, and minimize its systemic toxicity. Similarly, nanoparticle-based approaches aimed at regulating molecular interactions in the T-cell synapse to prevent autoimmunity or, conversely, to boost anti-tumor immunity have also provided preliminary evidence of efficacy.
The design, synthesis and application of dual- and multi-modality probes will be a hot research area, which may be the next generation of probes. The combination of different functional modality undoubtedly will improve the accuracy of diagnosis and analysis to prostate cancer. On the other hand, a targeted gene-therapy approach is also being developed to activate the immune system to recognize prostate cancer cells. To discovery nanoprobes based on labeled gene and related macromolecule and these types of approaches might provide a new direction of prostate cancer therapies. We believe that such imaging probes will play a vital role in further understanding of prostate cancer, for PCa’s early detection and more effective treatment.
References
Adams J (1853) The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. Lancet 1(1):393–393
Ito K (2014) Prostate cancer in Asian men. Nat Rev Urol 11(4):197–212
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Thompson IM Jr, Cabang AB, Wargovich MJ (2014) Future directions in the prevention of prostate cancer. Nat Rev Clin Oncol 11(1):49–60
Ravindranathan P, Lee T-K, Yang L, Centenera MM, Butler L, Tilley WD, Hsieh J-T, Ahn J-M, Raj GV (2013) Peptidomimetic targeting of critical androgen receptor-coregulator interactions in prostate cancer. Nat Commun 4:1923–1934
Picchio M, Mapelli P, Panebianco V, Castellucci P, Incerti E, Briganti A, Gandaglia G, Kirienko M, Barchetti F, Nanni C (2015) Imaging biomarkers in prostate cancer: role of PET/CT and MRI. Eur J Nucl Med Mol Imaging 42(4):644–655
Nguyen QT, Tsien RY (2013) Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat Rev Cancer 13(9):653–662
Komljenovic D, Wiessler M, Waldeck W, Ehemann V, Pipkorn R, Schrenk H-H, Debus J, Braun K (2016) NIR-cyanine dye linker: a promising candidate for isochoric fluorescence imaging in molecular cancer diagnostics and therapy monitoring. Theranostics 6(1):131–142
Klotz L, Emberton M (2014) Management of low risk prostate cancer [mdash] active surveillance and focal therapy. Nat Rev Clin Oncol 11(6):324–334
Mohiuddin JJ, Baker BR, Chen RC (2015) Radiotherapy for high-risk prostate cancer. Nat Rev Urol 12(3):145–154
Yasufuku T, Arakawa S, Fujisawa M, Shigemura K, Matsumoto O (2010) Combination chemotherapy with weekly paclitaxel or docetaxel, carboplatin, and estramustine for hormone-refractory prostate cancer. J Infect Chemother 16(3):200–205
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15):1502–1512
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E (2010) Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328(5981):1031–1035
Hambley TW (2009) Is anticancer drug development heading in the right direction? Cancer Res 69(4):1259–1262
Kim BY, Rutka JT, Chan WC (2010) Nanomedicine. N Engl J Med 363(25):2434–2443
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5(3):161–171
Constantinou J, Feneley MR (2006) PSA testing: an evolving relationship with prostate cancer screening. Prostate Cancer Prostatic Dis 9(1):6–13
Esfahani M, Ataei N, Panjehpour M (2015) Biomarkers for evaluation of prostate cancer prognosis. Asian Pac J Cancer Prev 16(7):2601–2611
Chang SS (2004) Overview of prostate-specific membrane antigen. Rev Urol 6(Suppl 10):S13
Kelly KA, Setlur SR, Ross R, Anbazhagan R, Waterman P, Rubin MA, Weissleder R (2008) Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res 68(7):2286–2291
Saleem M, Adhami VM, Zhong W, Longley BJ, Lin C-Y, Dickson RB, Reagan-Shaw S, Jarrard DF, Mukhtar H (2006) A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev 15(2):217–227
Härmä H, Soukka T, Lövgren T (2001) Europium nanoparticles and time-resolved fluorescence for ultrasensitive detection of prostate-specific antigen. Clin Chem 47(3):561–568
Ferguson RA, Yu H, Kalyvas M, Zammit S, Diamandis EP (1996) Ultrasensitive detection of prostate-specific antigen by a time-resolved immunofluorometric assay and the immulite immunochemiluminescent third-generation assay: potential applications in prostate and breast cancers. Clin Chem 42(5):675–684
Liu GL, Chen FF, Ellman JA, Lee LP (2006) Peptide-nanoparticle hybrid Sers probe for dynamic detection of active cancer biomarker enzymes. Conf Proc IEEE Engl Med Biol Soc 1:795–798
Gao XH (2009) QD barcodes for biosensing and detection. Annu Int Conf IEEE Eng Med Biol Soc 2009:6372–6373
Xu J, Zhou S, Tu D, Zheng W, Huang P, Li R, Chen Z, Huang M, Chen X (2016) Sub-5 nm lanthanide-doped lutetium oxyfluoride nanoprobes for ultrasensitive detection of prostate specific antigen. Chem Sci 7(4):2572–2578
O’Keefe DS, Bacich DJ, Heston WD (2004) Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate 58(2):200–210
Kasten BB, Liu T, Nedrowbyers JR, Benny PD, Berkman CE (2013) Targeting prostate cancer cells with PSMA inhibitor-guided gold nanoparticles. Bioorg Med Chem Lett 23(2):565–568
Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V (2011) Eau guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59(1):61–71
Yazdan MS, Naghmeh N, Oshani D, Tan A, Seifalian AM (2011) A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomedicine 6(1):2963–2980
Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C (2006) Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J 35(5):446–450
Jia J, Zhu F, Ma X, Cao ZW, Li YX, Chen YZ (2009) Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov 8(2):111–128
Cheng L, Wang C, Feng L, Yang K, Liu Z (2014) Functional nanomaterials for phototherapies of cancer. Chin J Clin Oncol 114(21):10869–10939
Ryu JH, Koo H, Sun IC, Yuk SH, Choi K, Kim K (2012) Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy. Adv Drug Deliv Rev 64(13):1447–1458
Shapira A, Livney YD, Broxterman HJ, Assaraf YG (2011) Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat 14(3):150–163
Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ (2009) Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized pt(iv) prodrug-plga-peg nanoparticles. Proc Natl Acad Sci U S A 2009(45):157–158
Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC (2011) Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A 108(5):1850–1885
Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ (2010) Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci U S A 107(42):17939–17944
Broc-Ryckewaert DL, Carpentier R, Lipka E, Daher S, Vaccher C, Betbeder D (2013) Development of innovative paclitaxel-loaded small plga nanoparticles: study of their antiproliferative activity and their molecular interactions on prostatic cancer cells. Int J Pharm 454(2):712–719
Hoang B, Ernsting MJ, Murakami M, Undzys E, Li S (2014) Docetaxel–carboxymethylcellulose nanoparticles display enhanced anti-tumor activity in murine models of castration-resistant prostate cancer. Int J Pharm 471(1–2):224–233
Pearce AK, Simpson JD, Fletcher NL, Houston ZH, Fuchs AV, Russell PJ (2017) Localised delivery of doxorubicin to prostate cancer cells through a PSMA-targeted hyperbranched polymer theranostic. Biomaterials 141(1):330–339
Sato A, Itcho N, Ishiguro H, Okamoto D, Kobayashi N, Kawai K (2013) Magnetic nanoparticles of Fe3O4 enhance docetaxel-induced prostate cancer cell death. Int J Nanomedicine 8:3151–3160
Zhang W, Zheng X, Shen S, Wang X (2015) Doxorubicin-loaded magnetic nanoparticle clusters for chemo-photothermal treatment of the prostate cancer cell line pc3. Biochem Biophys Res Commun 466(2):278–282
Kumar A, Huo S, Zhang X, Liu J, Tan A, Li S, Jin S, Xue X, Zhao Y, Ji T, Han L, Liu H, Zhang X, Zhang J, Zou G, Wang T, Tang S, Liang XJ (2014) Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(iv) drug for prostate cancer treatment. ACS Nano 8(5):4205–4220
Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N (2014) Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 35(30):8635–8648
Yallapu MM, Dobberpuhl MR, Maher DM, Jaggi M, Chauhan SC (2012) Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr Drug Metab 13(1):120–128
Sanna V, Singh CK, Jashari R, Adhami VM, Chamcheu JC, Rady I (2017) Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci Rep 7:41573–41588
Narayanan NK, Nargi D, Randolph C, Narayanan BA (2009) Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in pten knockout mice. Int J Cancer 125(1):1–8
Shenoi MM, Iltis I, Choi J, Koonce NA, Metzger GJ, Griffin RJ (2013) Nanoparticle delivered vascular disrupting agents (vdas): use of tnf-alpha conjugated gold nanoparticles for multimodal cancer therapy. Mol Pharm 10(5):1683–1694
Soltani F, Sankianm HA, Ramezani M (2013) Development of a novel histone H1- based recombinant fusion peptide for targeted non-viral gene delivery. Int J Pharm 441(1–2):307–315
Barbato C, Ruberti F, Cogoni C (2009) Searching for MIND: microRNAs in neurodegenerative diseases. J Biomed Biotechnol 2009(1):871313–871321
Hwang DW, Son S, Jang J, Youn H, Lee S, Lee D (2011) A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microrna. Biomaterials 32(21):4968–4975
Li L, Wei Y, Gong C (2015) Polymeric nanocarriers for non-viral gene delivery. J Biomed Nanotechnol 11(5):739–770
Park TG, Ji HJ, Kim SW (2006) Current status of polymeric gene delivery systems. Adv Drug Deliv Rev 58(4):467–486
Jing GJ, Fu ZG, Dan B, Lin LR, Yang TC, Shi SL (2010) Development and evaluation of a novel nano-scale vector for sirna. J Cell Biochem 111(4):881–888
Becker AL, Orlotti NI, Folini M, Cavalieri F, Zelikin AN, Johnston AP, Zaffaroni N, Caruso F (2011) Redox-active polymer microcapsules for the delivery of a survivin-specific sirna in prostate cancer cells. ACS Nano 5(2):1335–1344
Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, Tian S, Napier ME, Pohlhaus PD, Rolland JP, Desimone JM (2012) Delivery of multiple sirnas using lipid-coated plga nanoparticles for treatment of prostate cancer. Nano Lett 12(1):287–292
De MI, Imbertie L, Rieumajou V, Major M, Kravtzoff R, Betbeder D (2000) Proofs of the structure of lipid coated nanoparticles (smbv) used as drug carriers. Pharm Res 17(7):817–824
Walsh M, Tangney M, O’Neill MJ, Larkin JO, Soden DM, Mckenna SL, Darcy R, O’Sullivan GC, O’Driscoll CM (2006) Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: implications for cancer gene therapy. Mol Pharm 3(6):644–653
Watanabe K, Harada-Shiba M, Suzuki A, Gokuden R, Kurihara R, Sugao Y, Mori T, Katayama Y, Niidome T (2009) In vivo siRNA delivery with dendritic poly(Llysine) for the treatment of hypercholesterolemia. Mol BioSyst 5(11):1306–1310
Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C (2011) Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics. Biotechnol Adv 29(4):402–417
Guo J, Cheng WP, Gu J, Ding C, Qu X, Yang Z, Yang Z, O’Driscoll C (2012) Systemic delivery of therapeutic small interfering rna using a PH-triggered amphiphilic poly-l-lysine nanocarrier to suppress prostate cancer growth in mice. Eur J Pharm Sci 45(5):521–532
Jere D, Jiang HL, Arote R, Kim YK, Choi YJ, Cho MH, Akaike T, Cho CS (2009) Degradable polyethylenimines as DNA and small-interfering RNA carriers. Expert Opin Drug Deliv 6(8):827–834
Demeneix B, Behr JP (2005) Polyethylenimine (PEI). Adv Genet 53(1):217–230
Dehshahri A, Oskuee RK, Shier WT, Hatefi A, Ramezani M (2009) Gene transfer efficiency of high primary amine content, hydrophobic, alkyl-oligoamine derivatives of polyethylenimine. Biomaterials 30(25):4187–4194
Xue HY, Narvikar M, Zhao JB, Wong HL (2013) Lipid encapsulation of cationic polymers in hybrid nanocarriers reduces their non-specific toxicity to breast epithelial cells. Pharm Res 30(2):572–583
Xu Z, Chen L, Gu W, Gao Y, Lin L, Zhang Z, Xi Y, Li Y (2009) The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials 30(2):226–232
Pozo-Rodríguez AD, Pujals S, Delgado D, Solinís MA, Gascón AR, Giralt E, Pedraz JL (2009) A proline-rich peptide improves cell transfection of solid lipid nanoparticle-based non-viral vectors. J Control Release 133(1):52–59
Wang MT, Jin Y, Yang YX, Zhao CY, Yang HY, Xu XF (2010) In vivo biodistribution, anti-inflammatory, and hepatoprotective effects of liver targeting dexamethasone acetate loaded nanostructured lipid carrier system. Int J Nanomedicine 5(1):487–497
Stevens PJ, Sekido M, Lee RJ (2004) A folate-receptor-targeted lipid nanoparticle formulation for a lipophilic paclitaxel prodrug. Pharm Res 21(12):2153–2157
Huang W, Lv M, Gao Z (2011) Polyethylenimine grafted with diblock copolymers of polyethylene glycol and polycaprolactone as sirna delivery vector. J Control Release 152(Suppl 1):e143–e145
Wu Y, Yu J, Liu Y, Yuan L, Yan H, Jing J, Xu G (2014) Delivery of EZH2-shrna with mpeg-PEI nanoparticles for the treatment of prostate cancer in vitro. Int J Mol Med 33(6):1563–1569
Son S, Hwang DW, Singha K, Jeong JH, Park TG, Lee DS, Kim WJ (2011) Rvg peptide tethered bioreducible polyethylenimine for gene delivery to brain. J Control Release 155(1):18–25
Zhang T, Xue X, He D, Hsieh JT (2015) A prostate cancer-targeted polyarginine-disulfide linked PEI nanocarrier for delivery of microRNA. Cancer Lett 365(2):156–165
Tarokh Z, Naderi-Manesh H, Nazari M (2016) Towards prostate cancer gene therapy: development of a chlorotoxin-targeted nanovector for toxic (melittin) gene delivery. Eur J Pharm Sci 99:209–218
Tai W, Qin B, Cheng K (2010) Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol Pharm 7(2):543–556
Shibata MA, Morimoto J, Shibata E, Otsuki Y (2008) Combination therapy with short interfering RNA vectors against VEGF-c and VEGF-α suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther 15(12):776–786
Han L, Zhang AL, Xu P, Yue X, Yang Y, Wang GX, Jia ZF, Pu PY, Kang CS (2010) Combination gene therapy with PTEN and EGFR siRNA suppresses U251 malignant glioma cell growth in vitro and in vivo. Med Oncol 27(3):843–852
Grimm D, Kay MA (2007) Combinatorial RNAi: a winning strategy for the race against evolving targets. Mol Ther 15(5):878–888
Lee SJ, Yook S, Yhee JY, Yoon HY, Kim MG, Ku SH, Kim SH, Park JH, Jeong JH, Kwon IC, Lee S, Lee H, Kim K (2015) Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J Control Release 220(Pt B):631–641
Wang Y, Gao S, Ye WH, Yoon HS, Yang YY (2006) Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater 5(10):791–796
Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC (2012) Interactions of nanomaterials and biological systems: implications to personalized nanomedicine. Adv Drug Deliv Rev 64(13):1363–1384
Xu X, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, Shi J, Wu J, Kantoff PW, Lippard SJ, Langer R, Walker GC, Farokhzad OC (2013) Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc Natl Acad Sci U S A 110(46):18638–18643
Pang ST, Lin FW, Chuang CK, Yang HW (2017) Co-delivery of docetaxel and p44/42 mapk sirna using PSMA antibody-conjugated BSA-PEI layer-by-layer nanoparticles for prostate cancer target therapy. Macromol Biosci 17(5):1600421
Tandon P, Farahani K (2011) Nci image guided drug delivery summit. Cancer Res 71(2):314–317
Chen Z, Penet MF, Nimmagadda S, Li C, Banerjee SR, Winnard PT, Artemov JD, Glunde K, Pomper MG, Bhujwalla ZM (2012) PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano 6(9):7752–7762
Lin Q, Jin CS, Huang H, Ding L, Zhang Z, Chen J, Zheng G (2014) Nanoparticle-enabled, image-guided treatment planning of target specific RNAi therapeutics in an orthotopic prostate cancer model. Small 10(15):3072–3082
Shao K, Singha S, Clementecasares X, Tsai S, Yang Y, Santamaria P (2015) Nanoparticle-based immunotherapy for cancer. ACS Nano 9(1):16–30
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10(9):909–915
Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D (2004) Part I: vaccines for solid tumours. Lancet Oncol 5(11):681–689
Cho K, Wang X, Nie S, Chen ZG, Shin DM (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14(5):1310–1316
Taurin S, Nehoff H, Greish K (2012) Anticancer nanomedicine and tumor vascular permeability; where is the missing link? J Control Release 164(3):265–275
Zolnik BS, Asadrieh GF (2010) Nanoparticles and the immune system. Endocrinology 151(2):458–465
Dwivedi PD, Tripathi A, Ansari KM, Shanker R, Das M (2011) Impact of nanoparticles on the immune system. J Biomed Nanotechnol 7(1):193–194
Leleux J, Roy K (2013) Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv Healthc Mater 2(1):72–94
Lee IH, An S, Yu MK, Kwon HK, Im SH, Jon S (2011) Targeted chemoimmunotherapy using drug-loaded aptamer–dendrimer bioconjugates. J Control Release 155(3):435–441
Sun JJ, Chen YC, Huang YX, Zhao WC, Liu YH, Venkataramanan R, Lu BF, Li S (2017) Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol Sin 38(6):823–834
Allison RR, Mota HC, Bagnato VS, Sibata CH (2008) Bio-nanotechnology and photodynamic therapy-state of the art review. Photodiagnosis Photodyn Ther 5(1):19–28
Jankun J, Keck RW, Skrzypczak-Jankun E, Lilge L, Selman SH (2005) Diverse optical characteristic of the prostate and light delivery system: implications for computer modelling of prostatic photodynamic therapy. BJU Int 95(9):1237–1244
Mitton D, Ackroyd R (2008) A brief overview of photodynamic therapy in Europe. Photodiagnosis Photodyn Ther 5(2):103–111
Jankun J (2011) Protein-based nanotechnology: antibody conjugated with photosensitizer in targeted anticancer photoimmunotherapy. Int J Oncol 39(4):949–953
Carreño LJ, González PA, Bueno SM, Riedel CA, Kalergis AM (2011) Modulation of the dendritic cell-t-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy 3(4):6–11
Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ (2012) Synapse-directed delivery of immunomodulators using t-cell-conjugated nanoparticles. Biomaterials 33(23):5776–5787
Acknowledgement
We are grateful for the financial supports from National Natural Science Foundation of China (81741134, 81671756 and 81271634), and Key Research Project of Science and Technology Foundation of Hunan Province (2017SK2093).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gao, T., Bi, A., Yang, S., Liu, Y., Kong, X., Zeng, W. (2018). Applications of Nanoparticles Probes for Prostate Cancer Imaging and Therapy. In: Schatten, H. (eds) Molecular & Diagnostic Imaging in Prostate Cancer. Advances in Experimental Medicine and Biology, vol 1126. Springer, Cham. https://doi.org/10.1007/978-3-319-99286-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-99286-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99285-3
Online ISBN: 978-3-319-99286-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)