Abstract
Cancer anorexia-cachexia syndrome is a condition characterized by muscle wasting and lack of appetite for food. Cancer anorexia-cachexia syndrome is a very common occurrence in patients suffering from cancer and is associated with many adverse outcomes. Presented here is information regarding cancer anorexia-cachexia’s diagnosis, pathophysiology, and treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cancer anorexia-cachexia syndrome is a combination of anorexia, the lack of or loss of appetite for food, and cachexia, weakness and muscle wasting usually due to a chronic disease. It is estimated that 50% of cancer patients suffer from cancer anorexia-cachexia syndrome and it is the cause of death in 20% of cancer patients. Further, cancer anorexia-cachexia syndrome is associated with decreased survival, decreased quality of life, and more adverse effects from cancer therapies [1]. Cancer is the predominant risk factor for cancer anorexia-cachexia syndrome, however, the type of cancer is a significant predictor of the incidence of weight loss. Patients with pancreatic, gastric, and esophageal cancer have the highest incidence of weight loss at 83%, 83%, 79% respectively while those with breast cancer have the lowest incidence at 10–35% [2]. Because of the far-reaching consequences of cancer anorexia-cachexia syndrome, a developed understanding of its diagnosis, pathogenesis, and treatment is essential. It is essential for providers treating patients with cancer pain to understand the impact of this syndrome on the care they provide for their patients and the interaction with medications and procedures.
Diagnosis
Historically anorexia was diagnosed based on reduction in appetite. Currently however, many clinicians are using a combination of questionnaires and visual analog scales to diagnose anorexia. These questionnaires focus on symptoms that interfere with food intake and are predictive of anorexia such as early satiety, taste alterations, smell alterations, meat aversion, and nausea/vomiting [2].
Cachexia is challenging to diagnose as it can be indolent for years without showing clinical manifestations. Many clinicians use body weight as the primary marker of nutritional status and cachexia should be suspected in any patient with a cancer diagnosis that experiences a decrease in body weight greater than 5% over a 6 month period [1].
Pathogenesis
Cancer anorexia-cachexia syndrome is a complex phenomenon and its pathogenesis spans several biochemical and neurochemical pathways. While the precise mechanism of this syndrome is not known, several pathways have been proven to be important in its development.
Changes in Gastrointestinal System
Many patients with cancer have abnormalities of the gastrointestinal system which make adequate nutrition intake and absorption difficult. Some of these abnormalities include things such as mucositis, enteritis, dysphagia, odynophagia, and mechanical obstructions. These abnormalities can be the direct effect of the underlying cancer or a consequence of chemotherapy and or radiation therapy. Regardless of the cause, these abnormities make it difficult for patient with cancer to maintain adequate nutrition and can contribute to the development of cancer anorexia-cachexia syndrome.
Improper Hypothalamic Response
Under normal physiologic conditions intake is controlled primarily by the hypothalamus. There is consistent data showing that in patient that develop anorexia the hypothalamus does not respond appropriately to peripheral signals which should stimulate increased energy intake [2].
Leptin
Leptin is an anorexigenic hormone produced by the adipose tissue. Under normal conditions weight loss leads to low leptin levels. These low leptin levels in turn cause a decrease in energy expenditure, decrease in anorexigenic signals, and an increase in areas of the hypothalamus that increase appetite. In patients suffering from cancer anorexia-cachexia syndrome leptin levels remain high despite significant weight loss [1, 2]. The consequences of these elevated leptin levels are continued protein breakdown, muscle wasting, and weight loss.
Changes in Carbohydrate Metabolism
Many tumors produce large amounts of lactate which is subsequently converted into glucose in the liver via the Cori cycle. This method of glucose production is very inefficient and uses a considerable amount of adenosine triphosphate (ATP). In patients with cancer anorexia cachexia syndrome, the end result of this process is increased energy expenditures resulting in an increased resting metabolic rate which further exacerbates weight loss and muscle breakdown.
Altered Neuropeptide Y Regulation
Neuropeptide Y (NPY) is a powerful appetite stimulating peptide. NPY is a key component of an appetite stimulating pathway which includes galanin, opioid peptide, melanin-concentrating hormone, orexin, and agouti-related peptide [1, 3]. Animal studies have shown that NPY is dysfunctional in anorexic mice with tumors and also that the level of NPY produced is reduced. The importance of NPY in the development of cancer anorexia is a subject of ongoing research.
Dysfunctional Lipid Metabolism
Several abnormalities in lipid metabolism have been identified in patients with cancer anorexia-cachexia syndrome; however, two key lipid changes are changes in lipoprotein lipase activity and changes in lipid mobilization factor.
Lipoprotein lipase (LPL) is an enzyme found in adipose, cardiac, and skeletal tissues. The function of LPL is to breakdown triglycerides of lipoprotein and store them as three fatty acids and one glycerol [4]. The net result of an appropriately functioning LPL system is fat storage. In cancer anorexia-cachexia syndrome cytokines inhibit LPL resulting in increased lipids in the circulation and decreased fat storage [1, 4].
Lipid mobilizing factor (LMF) causes the release of free fatty acids and glycerol from the adipose tissue [5]. LMF has been isolated from the urine of cancer patients that are losing weight and from tumors that induce cachexia in animal models [5]. Many investigator believe that LMF is at least partially responsible for the weight loss seen in patients with cancer anorexia-cachexia syndrome.
Changes in Protein Metabolism
Under normal physiologic conditions the body seeks to maintain an equilibrium between protein breakdown and protein synthesis. In cancer anorexia-cachexia syndrome this equilibrium is shifted and protein degradation outweighs protein synthesis. The cause of this increased protein degradation is likely multifactorial; however, one key mediator is proteolysis-inducing factor (PIF). Interestingly, PIF is found in the urine of cancer patients with cachexia but not in patient with similar tumors but without cachexia further highlighting its importance in the pathogenesis of cachexia [1].
Treatment
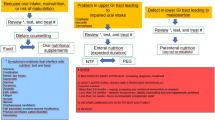
The most effective treatment for cancer anorexia-cachexia syndrome would be curing the underlying cancer. Unfortunately this is often not possible and instead cancer anorexia-cachexia syndrome is managed with a combination of dietary counseling and pharmacologic therapy. Even with appropriate management, cancer anorexia-cachexia syndrome is very difficult to treat and many patients continue to experience anorexia and cachexia despite therapy.
Dietary Counseling
Patient suffering from cancer anorexia-cachexia syndrome should be advised to [2].:
-
Consume frequent small meals.
-
Avoid extremes in taste and smell.
-
Eat foods low in fat.
-
Consume energy dense foods.
-
Eat in environments that bring them happiness and enjoyment.
-
Insure that food is aesthetically pleasing.
-
Take oral supplements.
Pharmacologic Therapies
Progestans, corticosteroids, and cannabinoids are the most widely used classes of medication to treat cancer anorexia-cachexia syndrome. Other less frequently used medications that have shown some utility include cyproheptadine, branched chain amino acids, prokinetic agents, ghrelin, and omega-3 fatty acids. Finally some emerging therapies include melatonin, beta-2 agonist, and thalidomide.
Progestans
-
First line agents for cancer anorexia-cachexia syndrome.
-
Two most common agents are megestrol acetate and medroxyprogesterone.
-
Precise mechanism of action unknown.
-
Megestrol has been shown to have positive effects on appetite, calorie intake, and body weight (mostly through and increased fat and water retention) [1].
-
Key adverse side effects: thromboembolic phenomena, hyperglycemia, hypertension, adrenal suppression, and adrenal insufficiency with abrupt discontinuation.
Corticosteroids
-
Commonly used agents include prednisone, prednisolone, methylprednisolone, and dexamethasone.
-
Some studies have shown positive effects on appetite and food intake.
-
Have significant anti-nausea effects.
-
No significant impact on body weight.
-
All glucocorticoids are equivalent in ability to stimulate appetite.
-
Less hypothalamic pituitary axis suppression may occur with intermediate acting steroids (prednisone, prednisolone, methylprednisolone) compared with long acting (dexamethasone) [1].
-
Key adverse effects: osteoporosis, hyperglycemia, delirium, gastric ulcers, hypertension, and weakness.
Cannabinoids
-
Can improve appetite and body weight slightly.
-
Dronabinol is the chemical responsible for appetite changes and body weight changes.
-
Key adverse effects: dizziness, drowsiness, and lightheadedness.
Cyproheptadine
-
Antiserotonergic and antihistamine effects.
-
Improves appetite slightly, no effect on body weight.
Branched Chain Amino Acids (BCAAs)
-
Theoretically serve as substrates for muscle metabolism and gluconeogenesis.
-
Oral BCAAs have been used to decrease severity of anorexia [1].
Prokinetic Drugs
-
Used primarily for patients with conditions such as gastric stasis and delayed gastric emptying which can lead to nausea and vomiting.
-
Metoclopramide is a common agent.
-
Slow release preferred over immediate release to help provide continuous stimulation.
Ghrelin
-
Ghrelin stimulate appetite.
-
IV ghrelin infusion have been shown to increase energy intake.
-
Key adverse effect is that they can cause increased tumor growth [2].
Omega-3 Fatty Acids
-
Eicosapentanoic acid is a common agent.
-
Interferes with LMF and PIF.
-
Has been shown to have positive effects on weight gain and body mass [1].
High Yield Points
-
Cancer anorexia-cachexia syndrome is associated with decreased survival and decreased quality of life.
-
Key components of pathophysiology include gastrointestinal changes, high leptin levels, inefficient of lactate to glucose through the Cori cycle, dysfunctional neuropeptide Y, dysfunctional lipid metabolism, and excessive protein breakdown.
-
Curing underlying cancer is best treatment for cancer anorexia cachexia syndrome.
-
Given that curing cancer is not possible best approach to treatment is a combination of dietary changes and pharmacologic therapies such and progestans, corticosteroids, and cannabinoids.
-
Providers dealing with cancer pain patients should understand the interaction between this syndrome and its treatments with modalities used for treating chronic pain, for example, if patient is on oral steroids for treating anorexia-cachexia syndome then use of steroids in pain procedures may cause more risk than usual and alternatives may be more appropriate.
Differential Diagnosis
-
Chronic organ failure.
-
AIDS.
-
Chronic severe infection.
-
Advanced cardiac, pulmonary or renal disease.
-
Psychiatric disorder.
-
Systemic inflammatory disorder.
-
Substance abuse.
-
Endocrine disease.
Questions
-
1.
67 year old male with history of stage IIB esophageal cancer presents because of weight loss. In the past 2 months his weight has decreased from 170 lbs. to 130 lbs. When asked about his weight loss he says that he “just has not felt hungry.” He was seen by his primary care physician 1 month ago and since that meeting he has been attempting to eat small frequent meals, has been consuming a low fat diet, and has been eating all his meals in his garden. What is the most effective next step in this patient’s management?
-
A.
Melatonin
-
B.
Eicosapentanoic acid
-
C.
Megestrol acetate
-
D.
Metoclopramide
Answer: C
-
A.
-
2.
73 year female with history of non-small cell lung cancer presents due to decreased appetite and a 20 lb. unintentional weight loss in the last 3 months. She takes over 20 medications daily and is not interested in taking more medications at this time. What is the most effective next recommendation for this patient?
-
A.
Fasting to increase appetite
-
B.
Consuming several small meals throughout the day
-
C.
Megestrol acetate
-
D.
Corticosteroids
Answer: B
-
A.
-
3.
80 year old male present with known diagnosis of pancreatic cancer. Since his diagnosis 3 months ago he has lost 30lbs and often goes several days without eating. Of the following which is NOT involved in the pathogenesis of his weight loss and decreased appetite?
-
A.
Excessive protein breakdown
-
B.
Elevated leptin levels
-
C.
Excessive lipid metabolism
-
D.
Low resting metabolic rate
Answer: D
-
A.
References
Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. Can J Clin. 2002;52(2):72–91.
Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome – when all you can eat is yourself. Nat Clin Pract Oncol. 2005;2(3):158–65.
Tatemoto K. Neuropeptide Y: history and overview. Handb Exp Pharmacol. 2004;162:1–21.
Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80(12):753–69.
Sanders PM, Tisdale MJ. Role of lipid-mobilising factor (LMF) in protecting tumor cells from oxidative damage. Br J Cancer. 2004;90(6):1274–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jackson, M., Abd-Elsayed, A. (2019). Cancer Anorexia-Cachexia Syndrome. In: Abd-Elsayed, A. (eds) Pain. Springer, Cham. https://doi.org/10.1007/978-3-319-99124-5_210
Download citation
DOI: https://doi.org/10.1007/978-3-319-99124-5_210
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99123-8
Online ISBN: 978-3-319-99124-5
eBook Packages: MedicineMedicine (R0)