Abstract
Interesting optical properties of gold and silver nanoparticles have been known for a fairly long time. Intensive colour of suspensions of such nanoparticles is a consequence of the excitation of a collective oscillation of surface conduction electrons (called surface plasmons) when electromagnetic radiation interacts with metal nanoparticles with a negative real and small positive imaginary dielectric constant (such as nanoparticles of gold or silver). The plasmonic optical properties of metal nanostructures are dependent on their shape and size, the dielectric properties of the metal and the surroundings, and on the possible electromagnetic coupling with the localized surface plasmons in nearby other plasmonic objects. The other important consequence of the excitation of surface plasmons is a local significant enhancement of the electromagnetic field at some places of the illuminated nanoparticles. Mentioned above specific plasmonic properties of gold and silver nanoparticles allowed on development of many sensors for chemical analysis, including sensors dedicated for environmental analysis. Some of these sensors are so sensitive, that recording of the reliable analytical signal even from a single molecule of an analyte is possible.
This chapter describes the most important analytical techniques based on plasmonic properties of gold and silver nanoparticles, which are used for environmental analysis. The basic theoretical background of these techniques including the mechanism of the interaction of the electromagnetic radiation with the plasmonic nanoparticles is also presented. Analytical techniques presented in this review include methods utilizing local enhancement of the intensity of the electromagnetic field induced by plasmons, and hence increase of the efficiency of some optical processes in the proximity of the plasmonic nanoparticles, such as: surface-enhanced Raman scattering, surface enhanced infrared absorption and metal enhanced fluorescence, and methods based on the change of the optical properties of plasmonic nanoparticles caused by the analyte-induced aggregation or by analyte-influenced growth or etching of plasmonic nanostructures. We focus on description of methods used for the detection of compounds important in the environmental analysis, such as: cations of heavy metals, metallo-organic compounds, polycyclic aromatic hydrocarbons, pesticides, nitrite ions, and bacterial cells and bacterial pathogens. The review ends with a perspective of these methods, description of the main challenges to be overcome, and suggestion of areas where the most promising developments are likely to happen in future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Since the begging of the Industrial Revolution in the 18th century in Britain, environmental pollution grown to the paramount issue that the humanity must face and solve. To accentuate this problem, just in China, the total discharging in the water, solid wastes, and gas, of very harmful heavy metals was around 900 kt each year from 2005 to 2011 (Hu et al. 2014). The most of the produced environmental pollutants, such as heavy metals, polycyclic aromatic hydrocarbons, and pesticides, could be harmful for human and other organisms. For example, mercury that can be discharged in the environment through used batteries, pesticides and paper industries, could cause tremors, gingivitis, psychological changes, damage to nervous system and much more adverse effects on human health (Singh et al. 2011). Moreover, heavy metals are, in large part, bio-accumulative. Hence, continuous consumption of even small amounts of heavy metals could be harmful in long term. Additionally, excess production of air environmental pollutants known as greenhouse gases may lead to severe climate changes. Such air pollution is considered responsible for global temperature growth, sea levels increase and the melting of glaciers (Jacobson 2008). As showed above, environmental pollution is very harmful both in term of human health and the condition of our planet, so, there is a great need for good analytical tools for detection of various pollutants with good sensitivity and specificity. One of the ways for detection of these hazardous compounds is use of plasmonic nanoparticles.
All analytical methods utilizing plasmonic nanoparticles are based on their very specific optical properties. While a macroscopic piece of gold is yellow and a macroscopic piece of silver is lustrous white, gold or silver nanoparticles could have all colours from red to violet that depend on their size and shape. This difference in optical properties between plasmonic nanoparticles and bulk size metals is caused by the occurrence of localized surface plasmon resonance on the nanoparticles’ surface during illumination. When electromagnetic radiation interacts with metal nanoparticles having a negative real and small positive imaginary dielectric constant it induces collective oscillations of surface conduction electrons called surface plasmons. As mentioned above, surface plasmons excited by the incident light in the sol of silver and gold nanoparticles are responsible for bright colours of these colloids. Excitation of surface plasmons also leads to strongly enhanced electromagnetic field at some places in the close proximity of the nanoparticles’ surface. The strongest enhancement of the electromagnetic field occurs on sharp apexes and edges of nanoparticles or in slits between them – such places are called “hot spots”. There are many very sensitive analytical tools utilising excitation of surface plasmons in metal nanoparticles and their aggregates. Some of these techniques are so sensitive, that observation of reliable signal even from a single molecule of an analyte is possible.
In this chapter we present the most important analytical techniques based on plasmonic properties of gold and silver metal nanoparticles, which are used in the environmental analysis. We have also very briefly described theoretical basics of the interaction between incident radiation and plasmonic nanopartilces, that is essential to fully understand functioning of the majority of the described nanosensors. Analytical techniques presented in this chapter include methods based on the increase of the efficiency of some optical processes in the proximity of plasmonic nanoparticles, such as: surface enhanced infrared absorption (SEIRA), surface-enhanced Raman scattering (SERS), and metal enhanced fluorescence (MEF), and on the change of the extinction properties of plasmonic nanostructures (usually caused by the analyte-induced aggregation of plasmonic nanoparticles). In this work, we described utilization of these analytical methods based on plasmonic nanoparticles for the detection of the most important environmental pollutants, such as: heavy metals, metallo-organic molecules, pesticides, polycyclic aromatic hydrocarbons, bacteria and viruses. Recently observed increase of the number of applications of techniques utilising surface plasmon resonance for analysis of various environmental samples allows to predict large increase of the significance of these techniques in the near future.
9.2 Surface Plasmon Resonance
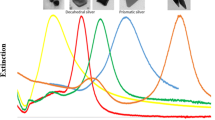
In recent years noble metal nanoparticles have been the subject of intensive scientific interest because of their unique optical, electronic, chemical and mechanical properties (Guzman et al. 2009). The most pronounced difference in properties of nano-sized and bulk noble metals is their optical properties. As bulk silver is grey and bulk gold is yellow colour of Ag and Au nanoparticles can vary from red to violet and depends on their size and shape (see Fig. 9.1) (Liz-Marzan 2004).
Unique optical properties of silver and gold nanoparticles are the result of the excitation of localized surface plasmon resonance. Localized surface plasmon resonance occurs when a small noble metal nanoparticle is irradiated by a light with proper wavelength. Surface plasmon resonance can be understood as a collective oscillation of conduction electrons near nanoparticle’s surface (see Fig. 9.2). Such charge oscillation near metal surface leads to creation of the electric dipole and in consequence to amplification of the electric field in close proximity of the nanoparticle’s surface. For a spherical nanoparticle the electric field near the metal surface (Eout) is a combination of the electric field of the incident electromagnetic wave (E0) and the field generated by surface plasmon resonance, and can be characterized by the following equation:
where: α is the metal polarizability, x, y, z are Cartesian coordinates, r is the radial distance, and \( \overrightarrow{x} \), \( \overrightarrow{y} \), \( \overrightarrow{z} \) are unit vectors. The first part of this equation is the electric field of the incident electromagnetic wave and the second part is a contribution from the surface plasmon resonance (Kelly et al. 2003). As can be seen from this equation, occurring of the surface plasmon resonance can amplify external electric field near the nanoparticle’s surface and impacts on their optical properties. For appearance of the surface plasmon resonance some conditions must be fulfilled. The size of nanoparticle must be smaller than the wavelength of the irradiating light and the imaginary part of the dielectric permittivity must be small (Willets and Van Duyne 2007). When using visible electromagnetic radiation these conditions are fulfilled only by a few metals (Ag, Au, Cu) and from this group silver has the highest capacity to support surface plasmon resonance.
Surface plasmon resonance on the surface of a metal nanoparticle depends on many variables, but size and shape of the nanoparticle are the most important ones. It was shown that nanoparticles with different size and shape have specific and characteristic for them extinction spectra in ultraviolet–visible spectroscopy (see Fig. 9.1) (Bastys et al. 2006; Ma et al. 2010). This situation is the result of the red shift of the surface plasmon resonance band when the size of nanoparticle is increasing and the result of the phenomenon that nanoparticles with different shapes have different plasmon modes (Xue and Mirkin 2007; Stamplecoscie and Scaiano 2012). For small spherical nanoparticles only dipole plasmons are excited whereas for anisotropic nanoparticles also higher order plasmon modes can be excited (Pastoriza-Santos and Liz-Marzan 2008).
As mentioned above, occurrence of surface plasmon resonance on nanoparticle’s surface can significantly enhance electric field in its close proximity. Distribution of electromagnetic field around nanoparticles depends on their size and shape. In the case of size dependence, for small nanoparticles only the dipole mode might be excited, while for bigger nanoparticles also higher plasmon modes can exist (Pastoriza-Santos and Liz-Marzan 2008). For example, Kelly et al. carried out computational simulations of the interaction between electromagnetic wave with smaller (30 nm) and bigger (60 nm) silver nanoparticles (Kelly et al. 2003). Computational results showed, that when nanostuctures are irradiated with light with the wavelength of 358 nm, for bigger nanoparticles a quadrupole mode could be excited, while for smaller structures only dipole modes could be excited. Another important factor that influence the distribution of electric field around nanoparticles is their shape. Nanoparticles with different shape can have very different plasmonic properties (Hao and Schatz 2004). For example, the silver rods and spheroids show two plasmon peaks. One is associated with excitation along long axis, and another one with excitation along short axis. For more anisotropic nanoparticles more plasmon modes can be excited. For example, for triangular nanoprisms four plasmon peaks might be excited: out-of-plane quadrupole, in-plane quadrupole, in-plane dipole and weak out-of-plane dipole (Hao and Schatz 2004).
As shown before, distribution of electromagnetic field strongly depends on size and shape of used nanoparticles. The strongest electromagnetic field is localized on the sharp apexes and edges in the case of isolated nanoparticles and in the slits between two or more nanostructures in the case of aggregates. Such places, where extremely intense electromagnetic field is observed, are called “hot-spots”. As an example, in studies carried out by Hao and Schatz the largest enhancement of the electric field around nanoprisms, nanorods, nanospheroids was observed on their sharp apexes and edges (Hao and Schatz 2004). This enhancement factor was estimated as equal to 3.5 × 103, 4.5 × 103 and 4.7 × 103, respectively (see Fig. 9.3). Electromagnetic “hot spots” are often placed in the narrow slits between nanoparticles. For example, when dimmer of silver nanoprisms positioned with tips directed toward each other are irradiated with 550 nm light the enhancement factor of the electric field between nanoparticles’ tips was estimated as equal to 4.7 × 103, while in the case of using radiation with the wavelength of 932 nm the electric field enhancement was estimated as equal to 4.3 × 104(see Fig. 9.3) (Hao and Schatz 2004). This enormous amplification of the electric field around nanoparticles surface allows to carry out many surface-specific spectroscopic measurements such as: surface-enhanced Raman scattering (SERS), surface-enhanced hyper-Raman scattering (SEHRS), surface-enhanced infrared absorption (SEIRA) or surface-enhanced fluorescence (MEF). As shown in the next sections, using mentioned above spectroscopic techniques, which utilize local enhancement of the intensity of the electromagnetic field induced by surface plasmons, and hence increase of the efficiency of some optical processes in the proximity of plasmonic nanoparticles, one can construct various sensors for the detection of compounds important in the environmental analysis. In this chapter we describe SERS, SEIRA and MEF sensors for detection of: cations of heavy metals, metallo-organic compounds, polycyclic aromatic hydrocarbons, various pesticides, nitrite ions, and bacterial cells and bacterial pathogens.
Distribution of the electric field around monomers of silver nanoparticles with different shape (a) and around dimers of prismatic triangles (b). As can be seen, the strongest enhancement of the external electric field is localized on sharp apexes of nanoparticles (a) or in slits between them (b). Reprinted with permission from (Hao and Schatz 2004). Copyright 2004 American Institute of Physics
Excitation of surface plasmons in silver and gold nanostructures are responsible for bright colours of Ag and Au colloids. As mentioned above the plasmonic properties of various nanostructures depends on their geometrical parameters (shape and size) and the dielectric properties of the plasmonic metal and the surroundings, especially on the presence of other plasmonic object(s) in the close proximity (the electromagnetic coupling with the localized surface plasmons in other nearby plasmonic objects is probably the main factor influencing the extinction band of the actually used plasmonic sols). Therefore, there are also many various analytical techniques based on the analysis of the parameters of the extinction band of the plasmonic metallic nanostructures (such techniques are often called colorimetric methods). In this chapter we present colorimetric methods based on analyte-induced aggregation of plasmonic nanoparticles and also on the analyte-influenced growth or etching of plasmonic nanostructures. Among various species detected using colorimetric methods, we focus on description of sensors for detection of cations of heavy metals, various pesticides, nitrite ions, phosgene, and bacterial pathogens.
9.3 Surface Enhanced Raman Spectroscopy
In 1928 Raman and his co – worker Krishnan for the first time observed non-elastic scattering of photons (Raman and Krishnan 1928). It was a fundamental discovery and already in 1930 Raman, as the first scientist from India, received Nobel Prize in Physics. In typical elastic scattering of light, also known as Rayleigh scattering, photos of scattered and incident light have the same energy. In non-elastic scattering of light, also known as Raman scattering, two kind of bands could be observed. Stokes bands occur when a molecule in lower (usually basic vibrationally) state interacts with light and passes to a virtual excitation state and after this do not come back to the initial basic state but come back to a higher energetic state. As a result, scattered light with lower energy than the incident light is observed. Anti-Stokes bands occur when a molecule in its excited state interacts with light and passes to virtual excitation state and after this do not come back to the initial excited state but drops to basic state (or lower excited state). As a result, scattered light with higher photon energy than the incident light is observed. For both, Stokes and anti-Stokes bands the differences in energy between incident and scattered light is equal to the energy of the oscillation excited in molecule which interact with light. Usually less than one photon per million is non-elastically scattered. Even though that observed phenomenon is very weak Raman’s achievement is very important. In the first years after the discovery of Raman scattering there were many problems with the registration of the spectra because of this poor efficiency of non-elastic scattering. Construction of the laser by Maiman in 1960 (Maiman 1960) contributed to the increased interest of Raman scattering. Continuation of the increase of the importance of Raman spectroscopy is connected with Fleischman. In 1974 Fleischman et al. tried to record Raman spectra of pyridine adsorbed on the electrochemically roughened silver electrode (Fleischmann et al. 1974) and observed very strong Raman signal for such system. They assumed that very strong Raman signal is caused by the increased number of pyridine molecules absorbed on the electrochemically roughened surface of the silver electrode. After three years, two independent research groups correctly explained this experimental observation. First group, Jeanmaire and van Duyne assumed electromagnetic mechanism of this phenomenon (Jeanmaire and van Duyne 1977) when the second group, Albrecht and Creighton, assumed resonance-like chemical mechanism, also known as charge transfer effect, as the reason of this enhancement (Albrecht and Creighton 1974). As confirmed later, both mechanisms contribute to the increased efficiency of Raman scattering for molecules of pyridine adsorbed on the roughened silver surface. The method in which nanostructured surface of the so-called plasmonic metals (e.g., Ag, Au, Cu) is a source of the enhanced Raman signal is called SERS – Surface Enhanced Raman Scattering. In the first mechanism (electromagnetic one), the incident light induces the oscillations of electrons from the conduction band, and as the result of this oscillation large increase of the electromagnetic field is generated near the metal surface. Charge transfer theory assumes the hybridization of the molecular orbital of an analyte molecule with an orbital from the metal surface. The largest enhancement is observe when the energy of photons of the incident light is tuned to the difference between the energy of the Fermi level in metal and non-occupied orbital in the analyte molecule or is tuned to the difference between the energy of the highest occupied orbital in the analyte molecule and the Fermi level in metal. Total averaged enhancement factor in SERS measurements is, in some cases, larger than 106, whereas the local SERS enhancement factor may even excess 10 orders of magnitude. As the result of such enormous SERS enhancement factor, it is possible to record SERS spectra from very diluted samples, and it is even possible to record reliable SERS spectrum from a single molecule (see Fig. 9.4) (Zrimsek et al. 2016).
Raman single molecule spectra of Rhodamine 6G (R6G-d0 and R6G-d4) and crystal violet (CV-d0, and CV d12) measured using silver colloid. The positions of the characteristic peaks of the analyte were marked. As can be seen in this Figure, the position of various bands in the so-called single molecule spectra may be slightly different in various measurements. Reprinted with permission from (Zrimsek et al. 2016). Copyright 2016 American Chemical Society
The contamination of water, air or other samples by pathogens like bacteria or viruses is a significant threat in the present times. Therefore, modern environmental analysis should propose quick and precise methods to detect such peril. Additionally, these methods should reveal very low detection limit. SERS spectroscopy can be considered as very useful tool for such tasks because this method could be used for detection of even a single bacterial cell (Premasiri et al. 2005). For example, Premasiri et al. recorded normal Raman and SERS spectra of Escherichia Coli bacteria. Both samples were prepared in the same way, however, to obtain similar intensity of the normal Raman spectra as the SERS one, the analysed sample have to contain ca. 2 × 104 times more bacteria cells. Premasiri et al. also studied other bacteria and they showed that different bacteria have very different SERS spectra, and hence, recorded SERS spectra could be used as whole-organism fingerprint and can be applied for the bacteria identification (Premasiri et al. 2005). In another study, SERS was used for detection of Salmonella Typhimurium (Ma et al. 2017). This method was based on specific recognition using thiolated Salmonella Typhimurium aptamers immobilised on gold nanoparticles. The amount of bacteria cells were assessed from the intensity of the Raman signal of the 4-mercaptobenzoic acid, which was also deposited on gold nanoparticles and which was used as so called Raman reporter. In similar study, Salmonella Typhimurium cells were detected by label-free SERS aptasensor (Chen et al. 2017). Such aptasensor was made of silver nanorods functionalized with anti-Salmonella Typhimurium DNA aptamer. The detection of Salmonella Typhimurium cells was based on the determination of the ratio of intensities of the bands at 725 and 680 cm−1 – the value of this ratio is significantly higher for Salmonella Typhimurium than for other studied bacteria. As mentioned above, SERS spectroscopy could be also used for detection of viruses. For example, for detection of influenza virus spherical gold nanoparticles functionalized with respective antibody were used (Moon et al. 2016). Influenza viruses were firstly immobilized on glass substrate functionalized with anti-influenza virus antibody, and then specifically modified nanoparticles were added to this system. Afterwards, rhodamine B isothiocyanate solution was added and SERS signal was measured. The intensity of the SERS signal of rhodamine B isothiocyanate depends linearly on the concentration of influenza viruses in range between 103 and 107 tissue culture infective doses per ml. Another interesting example of detection of viruses is detection of Hepatitis B using gold nanoparicles modified with respective antibody and basic fuchsine molecules, which were used as Raman reporters (Kamińska et al. 2015). Such functionalized gold nanoparticles could combine with the studied antigen immobilized on Au-Ag coated GaN surface coated with anti Hepatitis B virus antibodies. Raman signal of basic fuchsine was measured afterwards, and the concentration of Hepatitis B viruses was determined from the intensity of the band at 1178 cm−1. Limit of detection of this method was estimated as equal to 0.01 IU ml−1, with small average deviation of 10%.
The other important class of chemical compounds causing environmental threat are sols and metallo-organic compounds of heavy metals. They are quite often present in water and soil, and generate a significant danger for humans and other organisms. SERS spectroscopy offers highly selective method for detection of heavy metal ions with very low limit of detection. For example, SERS highly sensitive sensor for detection of As3+ ions was developed by Li et al. (2011a). This sensor was based on silver nanoparticles functionalized by glutathione and 4-mercaptopyridine. Figure 9.5 shows schematic depiction of indirect detection of As3+ ions using this method. Briefly, glutathione molecules are attached to the surface of silver nanoparticles through Ag-S bonds. Arsenium ions exhibit high affinity to glutathione ligands and each As3+ ion can bind to three glutathione modified silver nanoparticles through As-O bonds. Also 4-mercaptopyridine molecules could be attached to the surface of silver nanoparticles via Ag-S and Ag-N bonds. Modification of surfaces of silver nanoparticles by glutathione and 4-mercaptopyridine molecules does not affect the stability of nanoparticles. However, addition of As3+ ions to the solution of modified silver nanoparticles and formation of As-O linkage bonds leads to the aggregation of Ag nanoparticles, what could be visually observed by the change of the colour of the solution from yellow to brown. Aggregated silver nanoparticles form very large number of electromagnetic hot-spots, and therefore Raman signal of 4-mercaptopyridine molecules is increased significantly due to the strong SERS effect. It should be mention that only As3+ ions leads to the aggregation of nanoparticles, while different other ions do not induce such aggregation. Limit of detection of As3+ cations was estimated as equal 0.76 ppb, and the linear range of the response of such sensor is observed between 4 and 300 ppb (see Fig. 9.5). This sensor has been applied, for example, for detection of As3+ ions in samples of drinking water. Another interesting method for detection of As3+ ions was developed by Song et al. (2016). They applied Au@Ag nanoparticles modified with molecules of 4-mercaptobenzoic acid and some DNA aptamers. Addition of even small amount of As3+ ions into this suspension lead to specific binding between As3+ ions and aptamer molecules. As a result, aptamers were removed from the surface of nanoparticles leading to their aggregation. During aggregation many hot-spot are created, which causes significant increase of the intensity of measured SERS signal. Such sensor exhibits excellent linear response from 0.5 to 10 ppb. Limit of detection is estimated as equal to 0.1 ppb, which is lower than the maximum limitation for arsenic guided by the United States Environmental Protection Agency and the World Health Organization.
Schematic depiction of the mechanism of As3+ detection with glutathione coated silver nanoparticles. Addition of As3+ ions to stable glutathione coated nanoparticles leads to their agglomeration and creation of many hot-spots between them (a). Raman spectra of glutathione coated nanoparticles, showing increase of the 4-mercaptopyridine signal when concentration of As3+ cations increases. This effect is observed even at very low concentration of As3+ ions. Reprinted with permission from (Li et al. 2011a). Copyright 2011 American Chemical Society
Gold nanoparticles deposited on reduced graphene oxide could be used as SERS sensor for lead cations (Zhao et al. 2016). Addition of lead ions leads to significant decrease of the SERS signal of graphene because of the dissolution of deposited gold nanoparticles in the presence of added thiosulfate. Described decrease in the intensity of SERS signal allows on determination of concentration of Pb2+ ions in the range between 5 nM and 4 μM (Zhao et al. 2016). This SERS sensor was applied for the determination of Pb2+ in water samples with satisfactory results.
Efficient SERS sensors have been also constructed for detection of Hg2+ and Cd2+ ions (Zamarion et al. 2008). Zamarion et al. modified gold nanoparticles by 2,4,6-trimercapto-1,3,5-triazine and observed that after addition of mercury ions to the solution containing 2,4,6-trimercapto-1,3,5-triazine modified gold nanoparticles the intensity of the Raman bands localized at 485 and 432 cm−1(due to the C-S stretching vibrations of various conformers of 2,4,6-trimercapto-1,3,5-triazine) decreases significantly. This is due to the strong binding of the mercury ions to the thiol groups of chemisorbed 2,4,6-trimercapto-1,3,5-triazine. Simultaneously, an increase in the intensity of the peak at 973 cm−1 due to the breathing vibration of the aromatic ring is visible. This indicates the involvement of the heterocyclic N atom in the coordination of the Hg2+ ions. Linear change in the intensity of the mentioned above peaks is observed in the range of the Hg2+ concentration between 2 × 10−7 and 2 × 10−6 M. In the case of Cd2+ ions also strong enhancement of the SERS band 971 cm−1 is observed, however the intensity of the C-S stretching modes at 485 and 432 cm−1 exhibit only small decay as the function of the cadmium ions concentrations. Linear response of this sensor was observed between 2.5 × 10−7 and 3 × 10−6 M. An alternative method for detection of the mercury ions is based on the dimmerisation of gold nanostars (Ma et al. 2013). In this method gold nanostars functionalized by DNA aptamer terminated by thymine were used as nanoresonators, and molecules of 4-aminothiophenol were chosen as SERS reporters. Due to high affinity of thymine to the mercury ions gold nanostar dimmers are created when mercury ions are added to the sol of such gold nanoparticles. Such dimmerisation process creates many SERS hot-spots between Au nanostars, which leads to a significant increase in the SERS signal of 4-aminothiophenol. Ma et al. found that practically only addition of mercury ions leads to the formation of dimmers of gold nanostars. They have also observed that the SERS signal of 4-aminothiophenol is proportion to concentration of Hg2+ ions in the range between 0.002 and 1 ng ml−1. It means that utilising dimmerization of modified gold nanostarts allows on the creation of ultrasensitive and selective SERS platform for detection of Hg2+ ions. Similar system for the detection of mercury ions by the formation of thymine-Hg2+-thymine complexes was developed by Yang et al. (2017). In the first step magnetic CoFe2O4@Ag nanoparticles were modified by the thiol terminated aptamers. Single-stranded DNA aptamer could be stably bind to the single-walled carbon nanotubes due to the stacking between nucleotide bases and the wall of the nanotube, which leads to the wrapping of DNA strand around the surface of the carbon nanotube. In the presence of Hg2+ ions, aptamer can capture Hg2+ ions forming T-Hg2+-T structure, which causes increase in the distances between CoFe2O4@Ag nanoparticles and the carbon nanotube, and hence decrease in the measured Raman signal. Described nanosensor can detect Hg2+ ions in the range from 1 pM to 100 nM (Yang et al. 2017).
Another method for the detection of Hg2+ and Cu2+ ions is based on the agglomeration of silver nanoparticles coated by L-cysteine (Li et al. 2013). In this method 5-dimethoxy-4-(6′-azobenzotriazolyl)phenol is used as Raman reporter, which exhibits two strong Raman bands at 1364 cm−1 and 1618 cm−1 due to the C–C stretching vibrations of the benzene ring. Addition of Hg2+ and Cu2+ ions induces aggregation of L-cysteine modified silver nanoparticles, which leads to the creation of many SERS hot-spot and to the increase of the SERS signal of 5-dimethoxy-4-(6’-azobenzotriazolyl)phenol. The drawback of this method is that mercury ions could be masked by SCN- ions present in the solution because of higher affinity of mercury ions to SCN− ions than to L-cysteine. Limits of detections of this method was estimated as equal 1 pM for Hg2+ and 10 pM for Cu2+.
Highly toxic polycyclic aromatic hydrocarbons can be also detected in very low concentrations using SERS sensors. For example naphthalene, the simplest polycyclic aromatic hydrocarbon, is found in contaminated water and food. The naphthalene contamination is mainly caused by the combustion of motor fuel, so it can be found in crops farmed near busy roads. Naphthalene is also used in chemical synthesis, for example synthesis of some insecticides, so another sources of naphthalene contaminations are some chemical plants. Peron et al. described highly repeatable method of detection of naphthalene in the concentrations range from 1 to 20 ppm (Peron et al. 2011). This method of detection of naphthalene is based on the measurement of the intensity of its characteristic SERS bands. As highly efficient SERS substrate for naphthalene detection, self-assembled layer of polystyrene nanospheres covered with gold nanoparticles is used (such structure generates a lot of hot-spots leading to high SERS enhancement of the naphthalene signal).
Another field of application of SERS spectroscopy in environmental analysis is detection of pesticides. Most of the pesticides are toxic at higher concentrations, so it is important to develop fast and easy method for their routine detection in a variety of samples. For example, Yang et al. reported a very sensitive method for detection of thiram on apple skins (Yang et al. 2014). In this approach Yang et al. used as electromagnetic nanoresonators silica nanospheres coated by silver nanoshells. This SERS substrate gives linear response for the thiram detection in the solution in the concentration range between 10−1 and 10−7 M, while in analysis of the real-samples (fruit peels) the limit of detection was estimated to be 38 ng cm−2. Another SERS method for detection of thiram was proposed by Zhang et al. (2014a). This group used core-shell Au@Ag nanorods as SERS active substrate. Due to covering of gold nanorods by nanometric layer of silver they were able to obtain much more sensitive sensor than when non-modified gold nanorods are used. Using Au@Ag nanorods Zhang et al. were able to detect thiram even at the concentration of 4.6 × 10−7 M. Other widely used pesticide is paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride). Hea et al. developed SERS method using silver nanoparticles for detection of paraquat on the pear skin in the quantities significantly below the permissible level. Another research group reported simple, sensitive, rapid, and fairly quantitative method of detection of thiabendazole on apple peel (Hea et al. 2014). In this case as electromagnetic nanoresonators silver dendrities were used. The lowest concentration of thiabendazole possible to detect using this method was estimated to be 0.1 ppm. This is much below the maximum residue limits designated by the United States Environmental Protection Agency.
Interesting type of SERS spectroscopy is SHINERS – shell-isolated nanoparticle-enhanced Raman spectroscopy. In this method plasmonic metal nanoparticles (which, as mentioned above, could be efficient electromagnetic nanoresonators significantly amplifying Raman signal of the probed molecules) are covered with thin inert layer of SiO2, Al2O3 or MnO2. Deposited inert shells protect surface of the plasmonic metal nanoparticles from direct contact with the analyzed objects and keep them from agglomeration. Scheme of SHINERS measurements was presented in Fig. 9.6. As mentioned above, the main advantage of this method in some applications comes from the use of the inert oxide layer deposited on the plasmonic metal nanoparticles. Therefore, it must be underlined that the thickness of this oxide layer is crucial. As can be seen from Fig. 9.6, the Raman signal is effectively amplified only for very thin protecting oxide layers and the intensity of the recorded SERS signal strongly decreases with increases of the thickness of the shell (Li et al. 2010).
(a) Schematic illustration of SHINERS measurements. Raman signal is collected from the analyte deposited on nanoparticles with dielectric layer. (b) The shell thickness dependence of the pyridine signal intensity in SHINERS measurements (black square) and the corresponding theoretical calculation of this intensity (red triangles). Thicker protecting layer – lower SHINERS intensity. Reprinted with permission from (Li et al. 2010). Copyright 2010 Nature Publishing Group. (c) Raman spectra of (a) solid methyl parathion, (b) skin of the orange fruit, (c) skin of the orange fruit contaminated by methyl parathion, (d) skin of the orange fruit covered with hollow-Ag@SiO2 nanoparticles, and (e) skin of the orange fruit contaminated by methyl parathion and covered with hollow-Ag@SiO2 nanoparticles. As can be seen, deposition of hollow-Ag@SiO2 nanoparticles on the surface of the contaminated orange fruit allows on observation of the Raman band at 1350 cm−1, which is due to the vibration of methyl parathion. Reprinted with permission from (Abdulrahman et al. 2015). Copyright 2015 American Chemical Society
One field of the environmental application of SHINERS spectroscopy is detection of pesticides on the surface of various fruits (Li et al. 2010; Abdulrahman et al. 2016). For example, using Au@SiO2 nanoresonators Li et al. detected methyl parathion (efficient pesticide) on the surface of orange skin (Li et al. 2010). Detection of methyl parathion has been realized by the measurement of the Raman spectra of the orange skin covered with Au@SiO2 nanoresonators. Raman spectrum of the clean (not contaminated) orange skin is dominated by two bands at 1155 and 1525 cm−1, due to the vibrations of the carotenoid molecules present in the skin of the orange fruit, while in the case of pesticide-contaminated surface additional Raman band at 1350 cm−1, due to the vibration of methyl parathion, can be visible in the recorded spectrum (Li et al. 2010). Similar detections of methyl parathion using Ag@SiO2, hollow-Ag@SiO2 and Ag@MnO2 nanoresonators have been carried out by Abdulrahman et al. (see Fig. 9.6) (Abdulrahman et al. 2016).
Silica-protected plasmonic nanoparticles have been also used for detection and determination of other compounds, including water pollutants (Zhang et al. 2013; Gao et al. 2014). For example, Au@SiO2 nanoparticles have been used for determination of the concentration of nitrite ions in tap and lake waters (Zhang et al. 2013). This method is based on the diazotization reaction of p-nitroaniline by nitrites in acidic conditions. Subsequent coupling between diazonium cation and diphenylamine results in synthesis of azo dye with very large SERS cross-section. Concentration of nitrite ions is determined indirectly from the measured intensity of the SERS spectrum of formed azo dye. In this approach the limit of detection of nitrite ions was estimated as equal to 0.07 ppm and the linear response of the sensor is observed in the range between 0.5 and 6.0 ppm. In another approach Gao et al. used Au@SiO2 nanoparticles for direct determination of the concentration of cyanide ions (Gao et al. 2014). The linear response of such sensor is between 1 and 100 μg dm−3, and the limit of detection is lower than 1 μg dm−3. It is worth mentioning that this method showed outstanding selectivity over thiocyanide and other common ions, and hence such method can be applied for analysis of complex solutions as lake water or various food.
Surface-protected plasmonic nanoparticles have been also used for Raman analysis of various organic compounds. For example, Zhang et al. used Au@SiO2 nanoparticles for the detection of traces of formaldehyde in water (Zhang et al. 2014b). Formaldehyde itself doesn’t show strong Raman scattering, therefore, this detection has been carried through the measurement of the Raman signal of formaldehyde azine, which is the product of the reaction between formaldehyde and 3-methyl-2-benzothiazolinone hydrazine. Using such approach low limit of detection of formaldehyde equal to 0.17 μg dm−1 was achieved with very good selectivity. Qian et al. proposed using of Au@(poly-(2-aminothiophenol) nanoparticles for Raman detection of trinitrotoluene (Qian et al. 2012). High selectivity of this method is caused by the formation of so-called Meisenheimer complexes between trinitrotoluene and poly(2-aminothiophenol).
SHINERS spectroscopy could be also used as a very useful tool for surface analysis of complex biological samples. The interaction between the plasmonic metallic surface and the studied sample may cause changes of the structure of many large organic molecules (for example, such interaction may cause denaturation of various proteins). Therefore, in SHINERS measurements very thin layers (for example, from silica) protect plasmonic metallic cores and prevent direct contact between analysed samples and the metallic plasmonic nanoparticles. Interesting example of application of SHINERS for analysis of biological systems is using decahedral silver nanoparticles covered with silica layer for investigation of cell walls of Saccharomyces boulardii (Kołątaj et al. 2017). In this approach yeast cells were covered with decahedral silver nanoparticles protected with the silica layer, and then the Raman spectra were measured. In the obtained spectra bands characteristic for mannoproteins could be detected, whereas such bands could not be noticed in the Raman spectra recorded using standard way.
Surface-enhanced Raman spectroscopy is undoubtedly the most widely used technique in environmental analysis, which utilises local enhancement of the electromagnetic field generated by the plasmonic nanostructures. It is also one of the most sensitive analytical technique, which allows on detection of many very different compounds, such as: cations of heavy metals, polycyclic aromatic hydrocarbons, pesticides, and bacterial cells and bacterial pathogens. Raman spectrum, as other vibrational spectra, can be treated as a compound’s fingerprint. This makes Raman spectroscopy a useful technique for the identification of many compounds including quantitative analysis of atypical samples.
9.4 Surface-Enhanced Infrared Absorption Spectroscopy
In 1980 Harstein et al. found that for some molecules the efficiency of the infrared absorption could be significantly enhanced by the nearby gold or silver nanostructures (Hartstein et al. 1980). Harstein et al. carried out infrared measurements in two different configurations. In the first approach, absorption signal was measured from organic molecules covered with a thin (thinner than 60 Å) gold or silver layer. In the second configuration, thin metallic layer was firstly evaporated on the silicon plate, then organic monolayer was deposited on the obtained surface, and the absorption infrared signal was measured. Observed effect of the enhancement of the infrared signal from molecules in contact with the metallic plasmonic surface was called surface-enhanced infrared absorption (SEIRA). When molecules are adsorbed on gold or silver nanoislands the SEIRA enhancement factor can be even equal to 103(Enders and Pucci 2006; Osawa 2001). Such enhancement factor is not so spectacular as in the case of SERS measurements, in which signal from a single molecule could be measured, but it still enables detection of the monolayer of various molecules (Zrimsek et al. 2016, Johnson and Aroca 1995; Bjerke and Griffiths 2002; Fallah et al. 2016). The SEIRA enhancement factor strongly depends on the type of metal used and on the morphology of its surface. SEIRA measurements are carried out using highly SERS-active metals such as Ag, Au, or Cu, but they could be also carried out using Pt, Pd, Ru, or Rh (Jensen et al. 2000; Kokaislova et al. 2015; Bjerke and Griffiths 1999; Hahn and Melendres 2001; Aroca and Ross 2004). As for other spectroscopic techniques utilizing plasmonic nanoparticles described in this chapter, the SEIRA enhancement factor is mainly connected with the enhanced electric field at the surface of the plasmonic nanoparticles illuminated by the electromagnetic radiation having a proper frequency. The efficiency of the infrared absorption is a function of E2, therefore, the enhanced electric field in the close proximity to the plasmonic nanoparticles leads to the infrared absorption signal amplification, and the SEIRA enhancement factor is roughly proportional the second power of the field enhancement (Osawa 2001).
The sensitivity of SEIRA measurements is significantly lower than that of SERS, therefore, this technique attracted much smaller attention of the chemical analysts (including environmental analysts) than Raman spectroscopy. Although the number of the analytical SEIRA papers is relatively small, some very interesting environmental analysis based on the SEIRA measurements have been already described. For example, SEIRA could be used for detection of polycyclic aromatic hydrocarbons (Carrasco-Flores et al. 2003, 2004; Sánchez-Cortés et al. 2007), which are widespread environmental pollutants produced by the incomplete combustion of coal and other organic fuels (Samanta et al. 2002). Sánchez-Cortés et al. used calix[4]arene as the selective host molecules for trapping polycyclic aromatic hydrocarbons (Sánchez-Cortés et al. 2007). Calix[4]arene molecules were deposited on the gold film, which allowed SEIRA measurements. In this approach detection of polycyclic aromatic hydrocarbons (e.g. pyrene) is based on the observation of the changes in the SEIRA spectrum of calix[4]arene – formation of complexes between host molecules (calixarene) and polycyclic aromatic hydrocarbons induces significant changes in the structure of the host molecules, which significantly influences also their SEIRA spectrum.
Bibikova et al. developed interesting SEIRA sensor for detection of thioglycolic acid in which spherical and star-shaped gold nanoparticles have been used (Bibikova et al. 2017). They estimated that recorded SEIRA signals of thioglycolic acid were about two times stronger when using star-shaped nanoparticles than when spherical nanoparticles were used. The linear response range for this sensor is in the concentrations range of thioglycolic acid between 0.063 and 2 μg ml−1.
Both SEIRA and SERS spectroscopy have been tested as tools for detection of dithiocarbamate fungicides thiram and ziram (Sanchez-Cortes et al. 2001). Interestingly, thiram can be detected on the basis of the intensity of both its SERS and SEIRA spectra, whereas ziram gives very week SERS spectrum and its detection was based only on the measurement of the intensity of its SEIRA spectrum (Sanchez-Cortes et al. 2001).
SEIRA spectroscopy has been also applied in biosensor used for the detection of some bacterial pathogens, such as Salmonella cells (Brown et al. 1998). In this approach Salmonella antibodies were immobilized onto a 10 nm gold film and the respective SEIRA spectra have been measured. SEIRA spectra of Salmonella antibodies deposited on gold surface contain two specific bands at 990 and 1085 cm−1. After addition of the Salmonella cells to this system new band positioned at 1045 cm−1 appears in the measured SEIRA spectrum (see Fig. 9.7). This new band indicating the presence of bacteria cells in the solution is due to the P=O stretching vibration of phospholipids in the bacteria cell walls. Potentially, such SEIRA fingerprint characterization could be conducted also for other bacteria species.
SEIRAS spectra of Salmonella antibodies (anti-SAL) with and without Salmonella cells. In both cases the Salmonella antibodies bands at 990 and 1085 cm−1 are visible. In spectrum with Salmonella cells a new band at 1045 cm−1 due to the P=O stretching vibration of phospholipids in the bacteria cell walls is visible. Appearance of this band allows on detection of Salmonella cells. Reprinted with permission from (Brown et al. 1998). Copyright 1998 American Chemical Society
Surface-enhanced infrared adsorption is relatively seldom used in chemical analysis. This is probably caused by significantly smaller surface-enhancement factors than in SERS spectroscopy: in surface-enhanced infrared adsorption the increase of the efficiency of adsorption is proportional the second power of the electromagnetic field enhancement, whereas in surface-enhanced Raman scattering the increase of the intensity of the measured signal is proportional to the forth power of the field enhancement. However, measured SEIRA spectrum can be treated as a compound’s fingerprint (similarly as SERS spectra). This makes infrared absorption spectroscopy a useful analytical technique for atypical samples.
9.5 Metal Enhanced Fluorescence
Fluorescence spectroscopy is now widely used for biochemical, medical, and environmental analysis (Weiss 1999; Sevick-Muraca et al. 2002; Orrit and Bernard 1990; Stam et al. 2015; Lenhardt et al. 2015; Hambly et al. 2015). In majority of fluorescence analysis increasing of the fluorophore brightness and its photostability would increase the sensitivity of the measurement. Both effects may be achieved by placing fluorophore in the close vicinity (typically less than 10 nm) to the plasmonic metallic surface (Geddes 2013). In such conditions, when so-called metal enhanced fluorescence (MEF) phenomenon is observed, the interaction between excited state of the fluorophore and the surface plasmons occurs and, as the result, fluorescence yield and the photostability of fluorophore is increased. This interaction might be described by two independent mechanisms (Geddes and Lakowicz 2002). The first one is the coupling between the excited state of the fluorophore and a surface plasmon. This interaction may be responsible for the reduction of the lifetime of the excited state which leads to the enhanced fluorophore photostability and also to the increased quantum yield of fluorescence. The other process occurring in the MEF phenomenon is the enhanced absorption of light by the fluorophore due to the increase in the intensity of the electromagnetic field around plasmonic nanostructures. Briefly, when a fluorophore molecule is located in the place where strong enhancement of electromagnetic field occurs, this leads to the considerable enhancement of the absorption cross section of fluorophore and in consequence fluorescence is amplified. Combined contributions of these two mechanisms significantly increase fluorescence yield and photostability of the fluorophore, which makes this kind of fluorescence spectroscopy much more useful. Figure 9.8 presents Jablonski diagram for fluorophore interacting and does not interacting with a plasmonic nanostructure.
Classical Jablonski diagram (left) and Jabloński diagram in case of molecules in the close proximity to the metallic surface. Fluorescence signal is enhanced because of enhanced excitation rate (Em) and increased radiative decay rate (Γm). This Figure has been prepared on the basis of the respective figure from the work of Geddes and Lakowicz (2002)
Because of a significant increase in the fluorescence yield and improved photostability of the fluorophore MEF can find many applications in monitoring of the environmental conditions. An example of such application is detection of various metal cations (Pang et al. 2015; Sui et al. 2014; Cheng et al. 2015; Li et al. 2016; Bondre et al. 2011; Zhou et al. 2013, 2014). For example, Pang et al. prepared Ag@SiO2 nanoparticles with immobilized T-rich DNA adapter, and used such nanoparticles as the fluorescent sensor for highly biotoxic Hg2+ ions. Thiazole orange was selected as the fluorophore in this sensor (Pang et al. 2015). Such sensor utilizes creation of complexes between Hg2+ ions and thymidine from DNA adapter, leading to the creation of hairpin duplex, which enables molecules of thiazole orange to be placed close to the metal surface. In these conditions small fluorescence yield of the free thiazole orange molecules is greatly enhanced by the MEF effect. This enables to obtain very small limit of detection of Hg2+ ions (0.33 nM). Another interesting MEF application in metal ions detection is sensor based on Ag@SiO2 nanoparticles with immobilized DNA-Cy3 aptamer, where Cy3 was used as the fluorophore (Sui et al. 2014). In this approach one is able to detect Hg2+ and Ag+ metal ions due to the formation of base-target-base complexes after addition of the specially designed almost complementary DNA strand with Cy3. This almost complementary DNA strand had three T-T mismatches and two DNA strands couldn’t hybridize without Hg or Ag ions, while in the presence of these ions they can hybridize due to the formation of T-Hg2+-T or T-Ag+-T complexes. In such case fluorophore is placed in the close proximity to metal surface and the fluorescence of Cy3 is greatly enhanced. The limit of detection of Hg or Ag ions using this method is estimated to be 1.4 nM (Sui et al. 2014). Another interesting method for detection of ions of heavy metals is utilization of hybrid magnetic-plasmonic systems (Liang et al. 2017). In this approach ZnFe2O4@Au−Ag nanocomposites, labelled nitrogen sulphur doped carbon dots, and DNA/CeO2 complexes were applied for construction of the Pb2+ sensor. Briefly, the formation of double-stranded DNA brought the labelled nitrogen sulphur doped carbon dots and CeO2 into close proximity, which significantly quenched the fluorescence of nitrogen sulphur doped carbon dots (CeO2 nanoparticles are efficient fluorescence quencher). The presence of Pb2+ led to the breakage of the DNAzyme strand, resulting in the fluorescence signal of Cy3 decreasing, while the fluorescence intensity of nitrogen sulphur doped carbon dots aggrandized. This sensor showed very low detection limit of 3 × 10−13 M and because of its magnetic properties excellent recyclability.
Also aluminium ions can be detected using MEF. Sensor for aluminum ions was produced from Ag-Au nanoclusters stabilized with mercaptosuccinic acid and covered with methoxy-poly(ethylene glycol)-NH2(Zhou et al. 2013). Schematic illustration of such detection method was presented in Fig. 9.9. Mercaptosuccinic acid was used as nanoparticles’ stabilizer and as fluorophore. Ag-Au nanoclusters with methoxy-poly(ethylene glycol)-NH2 protecting layer exhibits high photostability and great fluorescence ability. This fluorescence ability has been used for detection of Al3+ ions. This is due to the enhancement of fluoresce of mercaptosuccinic acid under addition to the sensor’s environment of the aluminum ions. Probably, the mechanism of enhancement of the fluorescence yield is the result of the deposition of Al3+ ions on the surface of Ag-Au nanocluster’s. Under such conditions Al3+ ions reacts with gold forming Al-Au alloy, which enhances MEF activity of the metallic clusters. This MEF sensor shows satisfying sensitivity (detection limit equals to 0.8 μM) and selectivity in analysis of the aluminum ions in aqueous solutions (Zhou et al. 2013).
Schematic illustration of synthesis of Au-Ag clusters functionalized with mercaptosuccinic acid and methoxy-poly(ethylene glycol)-NH2, and mechanism of the detection of Al3+ ions using this system. The mechanism of enhancement of the fluorescence yield is the result of the deposition of Al3+ ions on the surface of Ag-Au nanocluster’s. Under such conditions Al3+ ions reacts with gold forming Al-Au alloy, which enhances MEF activity of the metallic clusters. Reprinted with permission from (Zhou et al. 2013). Copyright 2013 American Chemical Society
MEF has been also used for detection of some pesticides. Fox example, Tuteja et al. immobilized on the silica-coated silver nanoparticles organophosphorus hydrolase and a high quantum yield fluorophore, pyranine (8-hydroxyl pyrene-1,3,6-trisulfonic acid trisodium salt), and used obtained nanocomposite for detection of organophosphate pesticide paraoxon (Tuteja et al. 2014). Organophosphorus hydrolase conducts hydrolysis reaction of the paraoxon causing the local decrease in pH. In this method pyranine is used both as fluorophore with high quantum yield and as a pH sensor. The decrease of pH due to the hydrolysis of the molecules of pesticide leads to the increase of the fluorescence intensity of pyranine. Moreover, proximity of the fluorophore molecules to the silver surface additionally amplifies fluorescence signal. This high fluorescence enhancement enables to obtain very small detection limit of 1 ppb for paraoxon molecules. Another method for detection of organophosphorus pesticides is using of europium doped gold nanoparticles (Dasary et al. 2008). It was observed that Eu3+ fluorescence is enhanced after addition of some organophosphorus pesticides. The pesticides concentration was estimated from the intensity ratio of Eu3+ fluorescence in the presence of pesticides and without pesticides. Using this method one may detect three organophosphorus pesticides: pinacolylmethylphosphonate, methylphosphonic acid and glyphosate. The linear response of the sensor was observed in the concentration range between 1 and 25 μM. MEF might be also utilized for herbicide detection (Sharma et al. 2013). For example, a heterogeneous fluoro-immuno assay has been used for detection of phenylurea herbicide diuron. Utilization of Ag nanoparticles enhances approximately ten times fluorescence signal from used fluorophore (isothiocyanate derivative of fluorescein) and enables obtaining of very low detection limit of diuron equal to ca. 0.01 ng ml−1(Sharma et al. 2013).
An interesting example of application of MEF is detection of polycyclic aromatic hydrocarbons that may be present in water as the result of the oil spills (Zhang et al. 2012). Spherical silver nanoparticles used in this analysis allows for 4.6 fold amplification of the fluorescence of aromatic hydrocarbons in the polluted seawater samples. Another example of the MEF detection of a water pollutant is detection of microcystin-LR (hepatotoxin produced by cyanobacteria that can cause liver damage and even death) (Li et al. 2014).
MEF might be also used for pH measurements. For example, immobilized on silver island films carboxy-semi-naphthofluorescein has been used as the pH sensor (Aslan et al. 2005). The pH of the studied solution was determined from the intensity ratio of the 590 and 552 nm emission peaks due to acidic and basic forms of used carboxy-semi-naphthofluorescein fluorophore. Employment of the metallic islands increases fluorescence approximately 40 times compared to the free carboxy-semi-naphthofluorescein molecules. Because the emissions of acidic and basic forms were enhanced similarly by metal islands, the intensity radio of the 590 and 552 nm emission peaks are not influenced by the presence of the metal surface.
In analyses utilising MEF effect the measured signals are usually not generated by the molecules of analyte (which is quite common in Raman and absorption infrared measurements), but by the added fluorophores (of course there are some exceptions, for example, MEF detection of polycyclic aromatic hydrocarbons). Addition of the analyte may influence on the distance between the fluorophore and the electromagnetic nanoresonator or on the structure of the fluorophore, which can be induced, for example, by its protonation or deprotonation. These effects strongly influence the intensity of the generated fluorescence signal, and hence allows on the detection of various analytes and determination of their concentrations. Fluorescence methods are ones of the most sensitive analytical methods, allowing, in some conditions, on detection of analytical signal even from a single molecule of an analyte.
9.6 Colorimetric Method
Position of the extinction band in the plasmonic systems depends on many factors such as: type of the plasmonic material, size of the plasmonic nanoparticles, their shape, degree of aggregation or agglomeration of plasmonic nanostructures, and electromagnetic permittivity of the surrounding medium. Therefore, the change in one the listed above factors can cause a significant change in colour of the sol of plasmonic nanoparticles. This change in colour can be often easily observed even with the naked eye. From the practical point of view the most important is the change of the optical properties of the sol of plasmonic nanoparticles caused by the nanoparticles’ aggregation. Aggregation of nanoparticles may have many reasons, for example, the change of the charge distribution on the nanoparticles’ surface. Such change of the charge distribution may be caused by the change of the ionic strength of the solution or by the specific adsorption of the analyte on the surface of nanoparticles (which are often specially functionalized by the molecules with high affinity to analite) – see Fig. 9.10. Changes in colour of the sol observed by the naked eye allow only to determine whether the analyte is present in the sample or not. For quantitative analysis the extinction of the sol should be measured using a spectrophotometer. The main advantages of the sensors based on the determination of the position of the extinction plasmonic band (often called localized surface plasmon resonance sensors) are simplicity, sensitivity, speed, and precision of the measurements. These sensors often have low limit of detection and a wide range of the linear response.
Schematic representation of the agglomeration of surface-functionalized nanoparticles after addition of analyte. Because ultraviolet–visible extinction spectra of agglomerated nanoparticles is different than extinction spectra of isolated nanoparticles, one can easily distinguish agglomerated and non-agglomerated plasmonic sols
In many cases aggregation of plasmonic nanoparticles have been used for detection of ions of heavy metals. For example, Kim et al. (2001) used gold nanoparticles functionalized with 11-mercaptoundecanoic acid for detection of mercury and lead cations. Preferential coordination of heavy metal ions to 11-mercaptoundecanoic acid changes the distribution of the negative charge on the noble metal nanoparticles which leads to their aggregation. What is important, this aggregation could be reversed by the addition to the solution of complexing agent, such as ethylenediaminetetraacetic acid. This method provides linear response in the concentration range of mercury and lead cations from 50 to 200 μM.
Aggregation sensor for Pb2+ ions has been developed by Huang et al. (2010). In this sensor gold nanoparticles functionalized by gallic acid are used as the plasmonic medium. Aggregation of Au nanoparticles is probably connected with the multivalent coordination of Pb2+ ions to the phenolic hydroxyl groups of immobilized gallic acid. The most important advantages of this sensor are simplicity, low cost, high sensitivity, high selectivity, and broad linear response range from 10 to 103 nM. Sensor for Pb2+ ions based on the aggregation of gold nanoparticles or SiO2@Au nanocomposites has been also developed by Thatai et al. (2016). Thatai et al. reported that the limit of detection of their sensor when using silica-gold nanocomposites (50 nM) was ten times smaller than when using “standard” gold nanoparticles (500 nM) (Thatai et al. 2016). Aggregation of glutathione-modified Au nanoparticles may be used for detection of cadmium ions (Guo et al. 2014). Glutathione-modified Au nanoparticles are relatively resistant on agglomeration induced by the increased ionic strength, however, added cadmium ions could be coordinated by four molecules of glutathion, which leads to decreasing surface concentration of glutathione on the nanoparticles’ surfaces resulting in their aggregation (see Fig. 9.11). The detection limit for this method was estimated as equal to 5 μM, and the linear response of this sensor is observed in the range from 0.05 to 50 μM. Similar method was developed for detection of Mn2+ ions (He and Zhang 2016). He and Zhang observed that addition of L-arginine to the citrate stabilized silver nanoparticles lead to their aggregation. However, L-arginine exhibits high affinity to Mn2+ ions and the presence of Mn2+ ions can prevent aggregation of silver nanoparticles because of the formation of complexes between Mn2+ ions and L-arginine. Detection limit for this method was estimated as equal to 20 nM, linear response was observed from 20 to 700 nM. Described sensor could be applied for analysis of lake or tap water with good compatibility with other methods.
Schematic depiction of the colorimetric detection of Cd2+ ions due to the analyte-induced agglomeration of glutathione-modified Au nanoparticles. Glutathione-modified Au nanoparticles are relatively resistant on agglomeration induced by the increased ionic strength, however, added cadmium ions could be coordinated by four molecules of glutathion, which leads to decreasing surface concentration of glutathione on the nanoparticles’ surfaces resulting in their aggregation. Aggregation of plasmonic nanoparticles could be easily detected from measurements of respective ultraviolet–visible spectra. Reprinted with permission from (Guo et al. 2014). Copyright 2014 American Chemical Society
Cations of heavy metals could be also detected using plasmonic methods, that are not based on nanoparticles’ aggregation. For example, in order to determine the concentrations of Hg2+ ions, to the analysed sample already prepared gold nanoparticles, hydroxylamine (NH2OH) and chloroauric acid (HAuCl4) have been added (Zhao et al. 2017). In such conditions hydroxylamine reduces AuCl4- and mercury ions on the surfaces of gold clusters. The growth of Au nanoparticles depends on the concentration of Hg2+ ions in the sample. In samples with high mercury concentrations, mercury shell on nanoparticles is thick and this leads to their quicker growth and quicker change in colour of the sol from red to blue. In samples with low mercury concentration, mercury shell is thin and does not accelerate the growth rate of gold nanoparticles. This method is selective for mercury ions, other ions practically do not disturb the analysis. Limit of detection of Hg2+ using this method was estimated as equal to 0.26 nM (Zhao et al. 2017).
Extinction of the sol of plasmonic metal nanoparticles can be also changed by etching of nanoparticles. For example, to detect copper ions etching of silver nanoparticles functionalized by starch has been applied. In this method, in the presence of Cu2+ cations silver nanoparticles are gradually oxidized and becoming smaller. Copper concentration was assessed by measuring the extinction spectra of silver nanoparticles. The linear response of this sensor is observed in the concentration range of Cu2+ between 0.1 and 100 μM (Miao et al. 2013).
Nitrite ions (due to over-fertilization they are quite often contaminant of many water samples) could be also detected by the colorimetric method utilizing gold nanoparticles (Daniel et al. 2009). In the first step two types of gold nanoparticles were prepared: the so-called “aniline” one functionalized by 5-[1,2]dithiolan-3-yl-pentanoic acid [2-(4-amino-phenyl)ethyl]amide and co functionalized with (11-mercapto-undecyl)-trimethyl-ammonium and the so-called “naphthalene” one functionalized by 5-[1,2]dithiolan-3-yl-pentanoic acid [2-(naphthalene-1-ylamino)et-hyl]amide and co functionalized with (11-mercapto-undecyl)-trimethyl-ammonium. Addition of (11-mercapto-undecyl)-trimethyl-ammonium increases the hydrophilic character of nanoparticles and hence increases their solubility. Detection mechanism is based on the Griess reaction: under acidic conditions the nitrite ions transform sulfanilamide into a diazonium salt, which then couples with “naphthalene” nanoparticles. It means that addition of NO2- ions causes the formation of crosslinked nanoparticles, which precipitate rapidly, causing the solution to change from red to colourless. This sensor could be used in analysis of sample contained more than 22 μM of nitrite ions. When nitrite concentration is lower no colour change was observed.
Interesting example of the colorimetric aggregation analysis is detection of pyrethroid pesticides such as lambda-cyhalothrin, which are used as efficient insecticides to control insects in cotton crops (Li et al. 2011b). Li et al. developed colorimetric method based on Ag@SiO2-NH2 nanoparticles allowing detection of lambda-cyhalothrin in concentrations below the European Union maximum residue limit of 1 mg kg−1(median lethal dose for rats is determine as 79–56 mg kg−1). This method is based on strong hydrogen bond interactions between amine groups in Ag@SiO2-NH2 nanoparticles and trifluoromethyl fluorine of lambda-cyhalothrin. Attachment of lambda-cyhalothrin molecules to the nanoparticles surface results in nanoparticles’ aggregation and change in colour of the sol. Experimental results showed that the linear response could be observed in concentration range from 1 × 10−6 to 2 × 10−3 M.
Detection of some gaseous compounds is also an important part of the environmental analysis. For example, Feng et al. developed localized surface plasmon resonance sensor for detection of phosgene. Phosgene (COCl2) is highly toxic, choking gas. Some amount of phosgene is often formed during burning of chlorine containing materials. Localized surface plasmon resonance sensor for phosgene developed by Feng et al. is based on the phosgene-induced aggregation of L-cysteine modified gold nanoparticles (Feng et al. 2010). Plasmon peak of L-cysteine modified gold nanoparticles is located at 520 nm, however, after addition of phosgene, due to the phosgene-induced aggregation of modified gold nanoparticles, the plasmon peak is shifted to ca. 640 nm. Instantaneous change of the colour of the solution from red to purple-blue could be observed even with the naked eye. The limit of detection of this method is 420 nM for phosgene and 140 nM for triphosgene.
In recent years danger of using biological weapons like: toxins, bacteria or viruses significantly increased. One of the potentially dangerous toxins is ricin – a protein consisting two chains and exhibiting strong affinity with some sugars derivatives. Consumption of even a small amount of ricin leads to death. The first symptoms are not characteristic and they may be confused with other diseases. Ricin may be however quickly and very sensitively detected using localized surface plasmon resonance sensors based on gold nanoparticles functionalized by β-lactosyl and β-galactosyl derivatives (Uzawa et al. 2008). Due to strong affinity to sugars derivatives ricin is preferentially adsorbed on surfaces of β-lactosyl and β-galactosyl functionalized nanoparticles, which leads to their aggregation. Such localized surface plasmon resonance sensor allows to detect ricin in a highly sensitive and facile manner (10 pg ml−1, 5 min).
Colorimetric methods are based on analyte-induced aggregation or agglomeration of plasmonic nanoparticles or on the influence of analyte of the process of growth or etching of the plasmonic nanoparticles. These processes strongly influence on the extinction spectra of sols of plasmonic nanoparticles, and therefore, allows detection and quantitative determination of many compounds. Among various colorimetric methods those based on aggregation of plasmonic nanoparticles are the most commonly used. Equipment required for the purpose of performing colorimetric analysis is usually significantly less expensive than equipment required in other analytical methods utilizing plasmonic properties of gold or silver nanoparticles.
9.7 Conclusions
In this chapter we have presented environmental analytical techniques utilising plasmonic properties of Ag and Au nanoparticles. Presented analytical techniques include methods based on the increase of the efficiency of some optical processes in the proximity of plasmonic nanoparticles, such as: surface enhanced infrared absorption, surface-enhanced Raman scattering, and metal enhanced fluorescence, and on the change of the extinction properties of plasmonic nanoparticles (usually caused by their analyte-induced aggregation). As examples of practical analyses carried out using these techniques we described, for example, detection of: cations of heavy metals, metallo-organic compounds, polycyclic aromatic hydrocarbons, pesticides, nitrite ions, and bacterial pathogens, it means detection of compounds important in the environmental analysis. Analytical methods described in this chapter belong to the most sensitive analytical tools. Raman and fluorescence techniques allow in some cases for reliable observation of the signal even from a single molecule. Wide potential field of applications of these techniques in environmental analysis suggests that the number of applications of such methods will significantly increase in the near future. Moreover, one can also expect development of new plasmonic techniques for environmental analysis. For example, one can expect development of methods allowing on very local characterisation of various environmental samples by using only a single plasmonic nanostructure which position will be controlled very precisely, in other words, one can expect practical analytical application of various nanoresonators-enhanced spectroscopy coupled with atomic-force microscopy or scanning tunneling microscopy. In this case a sharp tip made of plasmonic metal will be moved towards the investigated sample and illuminated with the radiation. Since enhancement of the intensity of the electromagnetic field is restricted to the close possibility of the nanoresonator (in this case a tip made from a plasmonic metal), such approach should allow on recording various tip-enhanced spectra with high spatial resolution. Although such approach has not been yet used for carrying out practical environmental analysis, development of such techniques started in 2000 (Stockle et al. 2000; Anderson 2000). The other interesting possibility is utilising plasmonic nanoparticles to enhance efficiency of various chiralooptical effects, it means techniques which detects differences in Raman scattering, infrared absorption, or fluorescence for left and right circularly polarized light (such techniques are called Raman optical activity, vibrational circular dichroism, and circularly polarized fluorescence, respectively). Chiralooptical techniques should allow distinguishing various enantiomers (mentioned above chiralooptical spectra of such isomers are almost mirror images of each other; when at a given frequency the D-isomer exhibits positive peak the L- form should exhibit negative peak). Increase of the number of applications of plasmonic techniques for environmental analysis should be also caused by a significant technical development of various optical devises, which already make possible to construct low-cost, battery-powered, portable spectrometers, which have many of the capabilities of laboratory-based systems. Therefore, one can expect, that almost all described in this chapter examples of environmental analysis should be easily carried out at outside specialised labs.
References
Abdulrahman HB, Krajczewski J, Aleksandrowska D, Kudelski A (2015) Silica-protected hollow silver and gold nanoparticles: new material for Raman analysis of surfaces. J Phys Chem C 119:20030–20038. https://doi.org/10.1021/acs.jpcc.5b03556
Abdulrahman HB, Kołątaj K, Lenczewski P, Krajczewski J, Kudelski A (2016) MnO2-protected silver nanoparticles: new electromagnetic nanoresonators for Raman analysis of surfaces in basis environment. Appl Surf Sci 388:704–709. https://doi.org/10.1016/j.apsusc.2016.01.262
Albrecht MG, Creighton JA (1974) Anomalously intense Raman spectra of pyridine at a silver electrode. J Am Chem Soc 99:5215–5217. https://doi.org/10.1021/ja00457a071
Anderson MS (2000) Locally enhanced Raman spectroscopy with an atomic force microscope. Appl Phys Lett 76:3130–3132. https://doi.org/10.1063/1.126546
Aroca RF, Ross DJ (2004) Surface-enhanced infrared spectroscopy. Appl Spectrosc 58:324–338. https://doi.org/10.1366/0003702042475420
Aslan K, Lakowicz JR, Szmacinski H, Geddes CD (2005) Enhanced ratiometric pH sensing using SNAFL-2 on silver island films: metal-enhanced fluorescence sensing. J Fluoresc 15:37–40. https://doi.org/10.1007/s10895-005-0211-0
Bastys V, Pastoriza-Santos I, Rodríguez-González B, Vaisnoras R, Liz-Marzán LM (2006) Formation of silver nanoprisms with surface plasmons at communication wavelengths. Adv Funct Mater 16:766–773. https://doi.org/10.1002/adfm.200500667
Bibikova O, Haas J, López-Lorente AI, Popov A, Kinnunen M, Ryabchikov Y, Kabashin A, Meglinski I, Mizaikoff B (2017) Surface enhanced infrared absorption spectroscopy based on gold nanostars and spherical nanoparticles. Anal Chim Acta 990:141–149. https://doi.org/10.1016/j.aca.2017.07.045
Bjerke AE, Griffiths PR (1999) Surface-enhanced infrared absorption of CO on platinized platinum. Anal Chem 71:1967–1974. https://doi.org/10.1021/ac981093u
Bjerke AE, Griffiths PR (2002) Surface-enhanced infrared absorption spectroscopy of p-nitrothiophenol on vapor-deposited platinum films. Appl Spectrosc 56:1275–1280. https://doi.org/10.1366/000370202760355226
Bondre N, Zhang Y, Geddes CD (2011) Metal-enhanced fluorescence based calcium detection: greater than 100-fold increase in signal/noise using fluo-3 or fluo-4 and silver nanostructures. Sens Actuators B Chem 152:82–87. https://doi.org/10.1016/j.snb.2010.09.041
Brown CW, Li Y, Seelenbinder JA, Pivarnik P, Rand AG, Letcher SV, Gregory OJ, Platek MJ (1998) Immunoassays based on surface-enhanced infrared absorption spectroscopy. Anal Chem 70:2991–2996. https://doi.org/10.1021/ac980058k
Carrasco-Flores EA, Campos-Vallette M, Leyton P, Diaz G, Clavijo RE, Garcıa-Ramos JV, Inostroza N, Domingo C, Sanchez-Cortes S, Koch R (2003) Study of the interaction of pollutant nitro polycyclic aromatic hydrocarbons with different metallic surfaces by surface-enhanced vibrational spectroscopy (SERS and SEIR). J Phys Chem A 107:9611–9619. https://doi.org/10.1021/jp035242a
Carrasco-Flores EA, Clavijo RE, Campos-Vallette MM, Aroca RF (2004) Vibrational spectra and surface-enhanced vibrational spectra of 1-nitropyrene. Appl Spectrosc 58:555–561. https://doi.org/10.1366/000370204774103381
Chen J, Park B, Huang YW, Zhao Y, Kwon Y (2017) Label-free SERS detection of Salmonella typhimurium on DNA aptamer modified AgNR substrates. Food Measure 11:1773–1779. https://doi.org/10.1007/s11694-017-9558-6
Cheng ZH, Li G, Liu MM (2015) Metal-enhanced fluorescence effect of Ag and Au nanoparticles modified with rhodamine derivative in detecting Hg2+. Sens Actuators B Chem 212:495–504. https://doi.org/10.1016/j.snb.2015.02.050
Daniel WL, Han MS, Lee J-S, ChA M (2009) Colorimetric nitrite and nitrate detection with gold Nnnoparticle probes and kinetic end points. J Am Chem Soc 131:6362–6363. https://doi.org/10.1021/ja901609k
Dasary SSR, Rai US, Yu H, Anjaneyulu Y, Dubey M, Ray PC (2008) Gold nanoparticle based surface enhanced fluorescence for detection of organophosphorus agents. Chem Phys Lett 460:187–190. https://doi.org/10.1016/j.cplett.2008.05.082
Enders D, Pucci A (2006) Surface enhanced infrared absorption of octadecanethiol on wet-chemically prepared Au nanoparticle films. Appl Phys Lett 88:1–4. https://doi.org/10.1063/1.2201880
Fallah MA, Stanglmair C, Pacholski C, Hauser K (2016) Devising self-assembled-monolayers for surface-enhanced infrared spectroscopy of pH-driven poly-L-lysine conformational changes. Langmuir 32:7356–7364. https://doi.org/10.1021/acs.langmuir.6b01742
Feng D, Zhang Y, Shi W, Li X, Ma H (2010) A simple and sensitive method for visual detection of phosgene based on the aggregation of gold nanoparticles. Chem Commun 46:9203–9205. https://doi.org/10.1039/C0CC02703K
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166. https://doi.org/10.1016/0009-2614(74)85388-1
Gao J, Guo L, Wu J, Feng J, Wang S, Lai F, Xie J, Tian Z (2014) Simple and sensitive detection of cyanide using pinhole shell-isolated nanoparticleenhanced Raman spectroscopy. J Raman Spectrosc 45:619–626. https://doi.org/10.1002/jrs.4497
Geddes CD (2013) Metal-enhanced fluorescence. Phys Chem Chem Phys 15:19537–19537. https://doi.org/10.1039/c3cp90129g
Geddes CD, Lakowicz JR (2002) Metal-enhanced fluorescence. J Fluoresc 12:121–129. https://doi.org/10.1023/A:1016875709579
Guo Y, Zhang Y, Shao H, Wang Z, Wang X, Jiang X (2014) Label-free colorimetric detection of cadmium ions in rice samples using gold nanoparticles. Anal Chem 86:8530–8534. https://doi.org/10.1021/ac502461r
Guzman MG, Dille J, Godet S (2009) Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int J Chem Biomol Eng 2:104–111. https://doi.org/10.1080/17458080.2016.1139196
Hahn F, Melendres CA (2001) Anodic oxidation of methane at noble metal electrodes: an ‘in situ’ surface enhanced infrared spectroelectrochemical study. Electrochim Acta 46:3525–3534. https://doi.org/10.1016/S0013-4686(01)00649-1
Hambly AC, Arvin E, Pedersen LF, Pedersen PB, Seredynska-Sobecka B, Stedmon CA (2015) Characterising organic matter in recirculating aquaculture systems with fluorescence EEM spectroscopy. Water Res 83:112–120. https://doi.org/10.1016/j.watres.2015.06.037
Hao E, Schatz GC (2004) Electromagnetic fields around silver nanoparticles and dimers. J Chem Phys 120:357–366. https://doi.org/10.1063/1.1629280
Hartstein A, Kirtley JR, Tsang JC (1980) Enhancement of the infrared absorption from molecular monolayers with thin metal overlayers. Phys Rev Lett 45:3–21. https://doi.org/10.1103/PhysRevLett.45.201
He Y, Zhang X (2016) Ultrasensitive colorimetric detection of manganese(II) ions based on anti-aggregation of unmodified silver nanoparticles. Sens Actuators B Chem 222:320–324. https://doi.org/10.1016/j.snb.2015.08.089
Hea L, Chena T, Labuza TP (2014) Recovery and quantitative detection of thiabendazole on apples using a surface swab capture method followed by surface-enhanced Raman spectroscopy. Food Chem 148:42–46. https://doi.org/10.1016/j.foodchem.2013.10.023
Hu H, Jin Q, Kavan P (2014) A study of heavy metal pollution in China: current status, pollution-control policies and countermeasures. Sustainability 6:5820–5838. https://doi.org/10.3390/su6095820
Huang K-W, Yu C-J, Tseng W-L (2010) Sensitivity enhancement in the colorimetric detection of lead(II) ion using gallic acid–capped gold nanoparticles: improving size distribution and minimizing interparticle repulsion. Biosens Bioelectron 25:984–989. https://doi.org/10.1016/j.bios.2009.09.006
Jacobson MZ (2008) Review of solutions to global warming, air pollution, and energy security. Energy Environ Sci 2:148–173. https://doi.org/10.1039/b809990c
Jeanmaire DL, Van Duyne RP (1977) Surface Raman spectroelectrochemistry part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem 84:1–20. https://doi.org/10.1016/S0022-0728(77)80224-6
Jensen TR, Van Dyune RP, Johnson SA, Maroni VA (2000) Surface-enhanced infrared spectroscopy: a comparison of metal island films with discrete and nondiscrete surface plasmons. Appl Spectrosc 54:371–377. https://doi.org/10.1366/0003702001949654
Johnson E, Aroca R (1995) Surface-enhanced infrared spectroscopy of monolayers. J Phys Chem 99:9325–9330. https://doi.org/10.1021/j100023a004
Kamińska A, Witkowska E, Winkler K, Dzięcielewski I, Weyher JL, Waluk J (2015) Detection of Hepatitis B virus antigen from human blood: SERS immunoassay in a microfluidic system. Biosens Bioelectron 66:461–467. https://doi.org/10.1016/j.bios.2014.10.082
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677. https://doi.org/10.1021/jp026731y
Kim Y, Johnson RC, Hupp JT (2001) Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett 1:165–167. https://doi.org/10.1021/nl0100116
Kokaislova A, Parchansky V, Matejka P (2015) Surface-enhanced infrared spectra of nicotinic acid and pyridoxine on copper substrates: what is the effect of temperature on deposition conditions. J Phys Chem C 119:26526–26539. https://doi.org/10.1021/acs.jpcc.5b08206
Kołątaj K, Krajczewski J, Kudelski A (2017) Silver nanoparticles with many sharp apexes and edges as efficient nanoresonators for shell-isolated nanoparticle-enhanced Raman spectroscopy. J Phys Chem C 121:12383–12391. https://doi.org/10.1021/acs.jpcc.7b02695
Lenhardt L, Bro R, Zekovic I, Dramicanin T, Dramicanin MD (2015) Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem 175:284–291. https://doi.org/10.1016/j.foodchem.2014.11.162
Li JF, Huang YF, Ding Y, Yang ZL, Li SB, Zhou XS, Fan FR, Zhang W, Zhou ZY, Wu DY, Ren B, Wang ZL, Tian ZQ (2010) Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464:392–395. https://doi.org/10.1038/nature08907
Li J, Chen L, Lou T, Wang Y (2011a) Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl Mater Interfaces 3:3936–3941. https://doi.org/10.1021/am200810x
Li Y, Cui Z, Li D, Li H (2011b) Colorimetric determination of pyrethroids based on core–shell Ag@SiO2 nanoparticles. Sens Actuators B Chem 155:878–883. https://doi.org/10.1016/j.snb.2011.01.064
Li F, Wang J, Lai Y, Ch W, Sun S, He Y, Ma H (2013) Ultrasensitive and selective detection of copper(II) and mercury(II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens Bioelectron 39:82–87. https://doi.org/10.1016/j.bios.2012.06.050
Li Y, Sun J, Wu L, Ji J, Sun X, Qian Y (2014) Surface-enhanced fluorescence immunosensor using Au nano-crosses for the detection of microcystin-LR. Biosens Bioelectron 62:255–260. https://doi.org/10.1016/j.bios.2014.06.064
Li Q, Wang J, He Y (2016) Selective chemiluminescent sensor for detection of mercury(II) ions using non-aggregated luminol-capped gold nanoparticles. Sens Actuators B Chem 231:64–69. https://doi.org/10.1016/j.snb.2016.03.007
Liang L, Lan F, Ge S, Yu J, Ren N, Yan M (2017) Metal-enhanced ratiometric fluorescence/naked eye bimodal biosensor for lead ions analysis with bifunctional nanocomposite probes. Anal Chem 89:3597–3605. https://doi.org/10.1021/acs.analchem.6b04978
Liz-Marzan LM (2004) Nanometals: formation and color. Mater Today 7:26–31. https://doi.org/10.1016/S1369-7021(04)00080-X
Ma YW, Wu ZW, Zhang LH, Zhang J (2010) Theoretical studies of optical properties of silver nanoparticles. Chin Phys Lett 27:1–4. https://doi.org/10.1088/0256-307X/27/2/024207
Ma W, Sun M, Xu L, Wang L, Kuang H, Xu C (2013) A SERS active gold nanostar dimer for mercury ion detection. Chem Commun 49:4989–4991. https://doi.org/10.1039/c3cc39087j
Ma X, Xu X, Xia Y, Wang Z (2017) SERS aptasensor for Salmonella Typhimurium detection based on spiny gold nanoparticles. Food Control. https://doi.org/10.1016/j.foodcont.2017.07.016
Maiman TH (1960) Stimulated optical radiation in ruby. Nature 187:493–494. https://doi.org/10.1038/187493a0
Miao LJ, Xin JW, Shen ZY, Zhang YJ, Wang HY, Wu AG (2013) Exploring a new rapid colorimetric detection method of Cu2+ with high sensitivity and selectivity. Sens Actuators B 176:906–912. https://doi.org/10.1021/acs.analchem.6b04978
Moon J, Yi SY, Hwang A, Eom G, Sim J, Jeong J, Lim EK, Chung BH, Kim B, Jung J, Kang T (2016) Facile and sensitive detection of influenza viruses using SERS antibody probes. RSC Adv 6:84415–84419. https://doi.org/10.1039/C6RA13966C
Orrit M, Bernard J (1990) Single pentacene molecules detected by fluorescence excitation in a p-terphenyl crystal. Phys Rev Lett 65:2716–2719. https://doi.org/10.1103/PhysRevLett.65.2716
Osawa M (2001) Surface-enhanced infrared absorption. Topics Appl Phys 81:163–187. https://doi.org/10.1007/3-540-44552-8_9
Pang Y, Rang Z, Xiao R, Wang S (2015) “Turn on” and label-free core-shell Ag@SiO2 nanoparticles-based metal-enhanced fluorescent (MEF) aptasensor for Hg2+. Sci Rep 5:9451. https://doi.org/10.1038/srep09451
Pastoriza-Santos I, Liz-Marzán LM (2008) Colloidal silver nanoplates. State of the art and future challenges. J Mater Chem 18:1724–1737. https://doi.org/10.1039/b716538b
Peron O, Rinnert E, Toury T, Lamy de la Chapelle M, Compere C (2011) Quantitative SERS sensors for environmental analysis of naphthalene. Analyst 136:1018–1022. https://doi.org/10.1039/C0AN00797H
Premasiri WR, Moir DT, Klempner MS, Krieger N, Jones G, Ziegler LD (2005) Characterization of the surface enhanced Raman scattering (SERS) of bacteria. J Phys Chem B 109:312–320. https://doi.org/10.1021/jp040442n
Qian K, Liu H, Yang L, Liu J (2012) Functionalized shell-isolated nanoparticle-enhanced Raman spectroscopy for selective detection of trinitrotoluene. Analyst 137:4644–4646. https://doi.org/10.1039/c2an35947b
Raman CV, Krishnan KS (1928) A new type of secondary radiation. Nature 121:501–502. https://doi.org/10.1038/121501c0
Samanta SK, Singh OV, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20:243–248. https://doi.org/10.1016/S0167-7799(02)01943-1
Sanchez-Cortes S, Domingo C, Garcıa-Ramos JV, Aznarez JA (2001) Surface-enhanced vibrational study (SEIR and SERS) of dithiocarbamate pesticides on gold films. Langmuir 17:1157–1162. https://doi.org/10.1021/la001269z
Sánchez-Cortés S, Guerrini L, García Ramos JV, Domingo C (2007) Functionalization of metal nanoparticles with synthetic and natural hosts for the surface-enhanced spectroscopic detection of polycyclic aromatic hydrocarbons. Opt Pura Apl 40:235–242
Sevick-Muraca EM, Houston JP, Gurfinkel M (2002) Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr Opin Chem Biol 6:642–650. https://doi.org/10.1016/S1367-5931(02)00356-3
Sharma P, Kukkar M, Ganguli AK, Bhasin A, Suri CR (2013) Plasmon enhanced fluoro-immunoassay using egg yolk antibodies for ultra-sensitive detection of herbicide diuron. Analyst 138:4312–4320. https://doi.org/10.1039/c3an00505d
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–253. https://doi.org/10.4103/0253-7613.81505
Song L, Mao K, Zhou X, Hu J (2016) A novel biosensor based on Au@Ag core–shell nanoparticles for SERS detection of arsenic(III). Talanta 146:285–290. https://doi.org/10.1016/j.talanta.2015.08.052
Stam J, Lindqvist C, Hansson R, Ericsson L, Moons E (2015) Fluorescence and UV/VIS absorption spectroscopy studies on polymer blend films for photovoltaics. Proc SPIE 9549. https://doi.org/10.1117/12.2188618
Stamplecoskie KG, Scaiano JC (2012) Silver as an example of the applications of photochemistry to the synthesis and uses of nanomaterials. J Photochem Photobiol 88:762–768. https://doi.org/10.1111/j.1751-1097.2012.01103.x
Stockle RM, Suh YD, Deckert V, Zenobi R (2000) Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem Phys Lett 318:131–136. https://doi.org/10.1016/S0009-2614(99)01451-7
Sui N, Wang L, Yan T, Liu F, Sui J, Jiang Y, Wan J, Liu M, Yu WW (2014) Selective and sensitive biosensors based on metal-enhanced fluorescence. Sensors Actuators 202:1148–1153. https://doi.org/10.1016/j.snb.2014.05.122
Thatai S, Khurana P, Prasad S, Soni SK, Kumar D (2016) Trace colorimetric detection of Pb2+ using plasmonic gold nanoparticles and silica–gold nanocomposites. Microchem J 124:104–110. https://doi.org/10.1016/j.microc.2015.07.006
Tuteja SK, Kukkar M, Kumar P, Paul AK, Deep A (2014) Synthesis and characterization of silica-coated silver nanoprobe for paraoxon pesticide detection. BioNanoSci 4:149–156. https://doi.org/10.1007/s12668-014-0129-6
Uzawa H, Ohga K, Shinozaki Y, Ohsawa I, Nagatsuka T, Seto Y, Nishida Y (2008) A novel sugar-probe biosensor for the deadly plant proteinous toxin, ricin. Biosens Bioelectron 24:923–927. https://doi.org/10.1016/j.bios.2008.07.049
Weiss S (1999) Fluorescence spectroscopy of single biomolecules. Science 12:1676–1683. https://doi.org/10.1126/science.283.5408.1676
Willets KA, Van Duyne RP (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem 58:267–297. https://doi.org/10.1146/annurev.physchem.58.032806.104607
Xue CA, Mirkin CA (2007) pH-switchable silver nanoprism growth pathways. Angew Chem Int Ed 46:2036–2038. https://doi.org/10.1002/anie.200604637
Yang J-K, Kang H, Lee H, Jo A, Jeong S, Jeon S-J, Kim H-I, Lee H-Y, Jeong DH, Kim J-H, Lee Y-S (2014) Single-step and rapid growth of silver nanoshells as SERS-active nanostructures for label-free detection of pesticides. ACS Appl Mater Interfaces 6:12541–1254s. https://doi.org/10.1021/am502435x
Yang X, He Y, Wang X, Yuan R (2017) A SERS biosensor with magnetic substrate CoFe2O4@Ag for sensitive detection of Hg2+. Appl Surf Sci 416:581–586. https://doi.org/10.1016/j.apsusc.2017.04.106
Zamarion VM, Timm RA, Araki K, Toma HE (2008) Ultrasensitive SERS nanoprobes for hazardous metal ions based on trimercaptotriazine-modified gold nanoparticles. Inorg Chem 47:2934–2936. https://doi.org/10.1021/ic800122v
Zhang F, Zhang L, Mao SC, Chen P, Cui JC, Tang YG, Wang K, Lin L, Qi XD (2012) Use of metal-enhanced fluorescence spectroscopy for detection of polycyclic aromatic hydrocarbons in diesel oil emulsions in artificial seawater. Environ Technol 33:2071–2075. https://doi.org/10.1080/09593330.2012.660643
Zhang K, Hu Y, Li G (2013) Diazotization-coupling reaction-based selective determination of nitrite in complex samples using shell-isolated nanoparticle-enhanced Raman spectroscopy. Talanta 116:712–718. https://doi.org/10.1016/j.talanta.2013.07.019
Zhang Y, Wang Z, Wu L, Pei Y, Chen P, Cui Y (2014a) Rapid simultaneous detection of multi-pesticide residues on apple using SERS technique. Analyst 139:5148–5154. https://doi.org/10.1039/c4an00771a
Zhang Z, Zhao C, Ma Y, Li G (2014b) Rapid analysis of trace volatile formaldehyde in aquatic products by derivatization reaction-based surface enhanced Raman spectroscopy. Analyst 139:3614–3621. https://doi.org/10.1039/c4an00200h
Zhao L, Gu W, Zhang C, Shi X, Xian Y (2016) In situ regulation nanoarchitecture of Au nanoparticles/reduced graphene oxide colloid for sensitive and selective SERS detection of lead ions. J Colloid Interface Sci 465:279–285. https://doi.org/10.1016/j.jcis.2015.11.073
Zhao Y, Gui L, Chen Z (2017) Colorimetric detection of Hg2+ based on target-mediated growth of gold nanoparticles. Sens Actuators B Chem 241:262–267. https://doi.org/10.1016/j.snb.2016.10.084
Zhou T, Lin L, Rong M, Jiang Y, Chem X (2013) Silver−gold alloy nanoclusters as a fluorescence-enhanced probe for aluminum ion sensing. Anal Chem 85:9839–9844. https://doi.org/10.1021/ac4023764
Zhou L, Zhang H, Luan Y, Cheng S, Fan LJ (2014) Amplified detection of iron ion based on plasmon enhanced fluorescence and subsequently fluorescence quenching. Nano-Micro Lett 6:327–334. https://doi.org/10.1007/s40820-014-0005-5
Zrimsek AB, Wong NL, Van Duyne RP (2016) Single molecule surface-enhanced Raman spectroscopy: a critical analysis of the bianalyte versus isotopologue proof. J Phys Chem C 120:5133–5142. https://doi.org/10.1021/acs.jpcc.6b00606
Acknowledgements
This work was financed from funds of the National Science Centre, Poland, allocated on the basis of decision number DEC-2017/25/B/ST5/01997. A.K. thanks the Faculty of Chemistry, University of Warsaw for its financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kołątaj, K., Krajczewski, J., Kudelski, A. (2019). Nanosensors for Environmental Analysis Based on Plasmonic Nanoparticles. In: Dasgupta, N., Ranjan, S., Lichtfouse, E. (eds) Environmental Nanotechnology. Environmental Chemistry for a Sustainable World, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-319-98708-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-98708-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98707-1
Online ISBN: 978-3-319-98708-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)