Abstract
Hepatic encephalopathy (HE) describes the alteration in brain function that occurs in the setting of advanced liver dysfunction or shunting of blood from the portal to systemic circulation. As such, it represents one of the complications of cirrhosis and portal hypertension . HE can manifest as either subclinical (minimal HE) or overt clinical disease, ranging from mild cognitive impairment to coma. The lifetime risk of overt HE in a cirrhotic patient approaches 30–40%. The presence of HE often has a significant impact on the quality of life of patients and their caregivers, frequently resulting in repeated hospitalizations. Symptoms of liver dysfunction, such as HE, are important as these events signify hepatic decompensation. The degree of HE is incorporated into the Child-Pugh classification of severity of liver disease. However, HE is not a part of the Model for End-Stage Liver Disease (MELD) scoring system , which is now the most common and accepted method to assess severity of liver disease. This chapter will focus on the diagnosis and management of HE in the setting of cirrhosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cirrhosis
- Portal hypertension
- Liver dysfunction
- Circulatory dysfunction
- Asterixis
- Acute liver failure
- Chronic liver disease
- Portosystemic shunting
- Glasgow Coma Scale
- Lactulose
- Neomycin
- Metronidazole
- End-stage liver disease
Introduction
Hepatic encephalopathy (HE) describes the alteration in brain function that occurs in the setting of advanced liver dysfunction or shunting of blood from the portal to systemic circulation. As such, it represents one of the complications of cirrhosis and portal hypertension . HE can manifest as either subclinical (minimal HE) or overt clinical disease, ranging from mild cognitive impairment to coma. The lifetime risk of overt HE in a cirrhotic patient approaches 30–40%. The presence of HE often has a significant impact on the quality of life of patients and their caregivers, frequently resulting in repeated hospitalizations. Symptoms of liver dysfunction, such as HE, are important as these events signify hepatic decompensation. The degree of HE is incorporated into the Child-Pugh classification of severity of liver disease. However, HE is not a part of the Model for End-Stage Liver Disease (MELD) scoring system , which is now the most common and accepted method to assess severity of liver disease. This chapter will focus on the diagnosis and management of HE in the setting of cirrhosis.
Clinical Case Scenario
A 62-year-old female presents to the emergency department. She has a diagnosis of nonalcoholic fatty liver disease and cirrhosis. Her disease has been managed in an outpatient clinic up to this point. Her manifestations of liver disease have included mild fluid overload and ascites which have responded to low doses of furosemide and spironolactone. Her labs reveal total bilirubin of 1.9 mg/dL, international normalized ratio (INR) of 1.4, creatinine of 1.7 mg/dL, and serum sodium of 134 mmol/L. Her MELD-sodium score is 20. She was seen 1 month prior in clinic for chronic disease management with a MELD-Na of 14 and underwent a liver ultrasound which showed no ascites and no evidence of liver cancer. Her husband now brings her to the emergency room and reports that she was a little slow mentally the past few days, often repeating statements. She also has not been eating well recently. Last night she awoke from sleep to use the bathroom, and her husband found her staring at the sink, seemingly unable to turn the faucet on. She only stared with a blank expression when he asked her what was wrong. At the hospital, she is mildly tachycardiac and appears dehydrated. She has no clinical or laboratory findings of infection or bleeding.
Questions
-
1.
How does HE develop?
-
2.
How is HE diagnosed?
-
3.
What are the typical clinical manifestations of HE?
-
4.
How is HE best managed?
-
5.
How should patients with difficult-to-manage HE be approached?
Discussion
Question 1. How does HE develop?
The pathogenesis of HE is complex and incompletely understood. A simplistic way to view the development of HE separates its pathogenesis into factors related to liver dysfunction and factors related to circulatory dysfunction (portal to systemic shunting). Although generally considered reversible, repeated episodes of HE are associated with some degree of chronic and cumulative cognitive deficits, which may persist even after liver transplant.
Liver Dysfunction
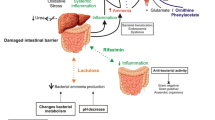
Although the changes of HE cannot solely be attributed to the hyperammonemia of liver dysfunction , the impaired metabolism of ammonia is an important contributor to its pathogenesis. Elevated ammonia levels cause increased GABAergic tone, astrocyte dysfunction, and subsequent impaired uptake of glutamate in the brain, resulting in an imbalance between excitatory and inhibitory neurotransmitters. Decreased clearance of other nitrogenous substances from the colon by a dysfunctional liver may also play a role in the development of HE.
Circulatory Dysfunction
In addition to a poorly functioning liver, patients with cirrhosis often have spontaneous portosystemic shunts, allowing substances such as ammonia, inflammatory cytokines, and endotoxin from the gastrointestinal tract to bypass the liver and directly enter the systemic circulation. The significance of such shunting is evident in the fact that HE occurs in about 30% of patients after transjugular intrahepatic portosystemic shunt (TIPS) . A growing body of evidence also points to the role of gut microbial dysbiosis in liver dysfunction and HE. Specifically, impaired bile acid synthesis by a poorly functioning liver may lead to deleterious changes in the gut microbiome, while portosystemic shunting allows potentially toxic gut-derived products direct access to the systemic circulation and thus the brain. Advancing liver disease may be associated with an unfavorable shift in the ratio of autochthonous (commensal and potentially beneficial bacteria) to non-autochthonous bacteria, which correlates with increasing MELD score and endotoxin levels.
Question 2. How is HE diagnosed?
HE remains a clinical diagnosis . The diagnosis can be reached by using a stepwise approach, as follows:
-
1.
Does the patient have liver disease or portosystemic shunting? Some features of liver disease or portosystemic shunting should be present to make a diagnosis of HE. History, physical examination, and imaging can be helpful in this regard. This can include, but is not limited to, the following:
-
(a)
History: known diagnosis of liver disease, family history of liver disease, risk factors for viral hepatitis, risk factors for fatty liver disease, medication use, substance abuse, alcohol abuse
-
(b)
Physical examination: jaundice, spider angiomata, palmar erythema, splenomegaly, ascites, dilated abdominal wall blood vessels, asterixis (described below)
-
(c)
Laboratories: thrombocytopenia indicative of portal hypertension, hyperbilirubinemia, coagulopathy, elevated aminotransferases (although liver enzymes can be normal in cirrhosis)
-
(d)
Imaging: reversal of flow (hepatofugal flow) in the portal vein on ultrasound, contrast imaging (CT or MRI scan) showing collateral vessels or varices
-
(a)
-
2.
Is there another cause of altered mental status?
-
(a)
History: substance use, alcohol use, medication history (including narcotics, sedatives, anticholinergics, and sleep aides), fever, new-onset focal neurologic symptoms
-
(b)
Physical examination: detailed physical examination with detailed neurologic examination
-
(c)
Laboratory testing: complete blood count, complete metabolic panel, urinalysis, cultures when appropriate, toxicology tests
-
(d)
Other tests: imaging of the central nervous system and electroencephalogram when appropriate
-
(a)
-
3.
Does the patient respond as expected to therapy? Improvement of HE symptoms with treatment is expected and often occurs rapidly. If there is a lack of response to therapy, the healthcare provider should consider if the diagnosis of HE is correct. Other causes of altered mental status such as delirium or Wernicke-Korsakoff syndrome can manifest similarly to HE.
Asterixis is often present with hepatic encephalopathy. Asterixis can be assessed by having the patient maintain their hands in a fully extended position. With asterixis, a flap of the fingers in a downward or more flexed position will occur. Asterixis should not be confused with a resting or intention tremor. However, asterixis can be present in other forms of encephalopathy also, so its presence supports the diagnosis of HE only in the appropriate clinical context.
Measuring ammonia levels is sometimes performed when the diagnosis of HE is in question. In this setting, an elevated ammonia level can support the diagnosis, but ammonia levels should not be sent for the intention of making a diagnosis of HE. Additionally, ammonia levels should not be measured serially or used to guide medical therapy in the setting of overt HE. Current guidelines for the management of HE state that ammonia levels do not add diagnostic, prognostic, or staging information. The guidelines do suggest that a normal ammonia level should prompt reevaluation of the diagnosis of HE. Treating a patient based on ammonia levels alone may result in “over-treating” a patient with normal mentation, which can result in volume depletion and electrolyte abnormalities with subsequent worsening of confusion.
Question 3. What are the typical clinical manifestations of HE?
The manifestations of HE can be classified in different manners (Table 14.1). It is important to note that cerebral edema with the risk of cerebral herniation and brain death can occur in the setting of HE associated with acute liver failure (Type A). However, cerebral edema and its associated morbidity/mortality is not seen with HE related to chronic liver disease or portosystemic shunting (Types C and B).
Minimal HE
It is important to be aware of minimal HE. By definition, there are no easily recognizable changes in mentation in this condition. Patients will present alert and oriented and will not have asterixis . However, despite the absence of overt manifestations, minimal HE can still impact patient well-being. Historical clues that physicians should be aware of include patients reporting decreased performance in usual daily tasks or recent motor vehicle accidents. For example, a patient who works as an accountant may report making unusual errors when preparing tax returns. Evaluation for the presence of minimal HE can be done through neuropsychiatric testing or through computer-based programs. Management could include a medication trial. The assessment for a therapeutic effect of medication is sometimes based on patient-reported symptoms but may require more formal repetitive testing.
Overt HE
Overt HE is more easily recognized and includes both neurologic and psychiatric manifestations. In general, patients can be staged using the West Haven criteria of altered mental state in HE and the Glasgow Coma Scale . Grade I HE is characterized by a decrease in attention span and awareness. Alterations or reversals in the sleep-wake cycle can be common and may be the initial manifestation of HE in some patients. Asterixis may be present in Grade I HE, but is not required. Grade II HE is characterized by more notable changes in awareness, and lethargy may be present. Personality changes and abnormal behaviors can also become prominent. At this point, asterixis should be evident. Grade III HE is notable for a somnolent state where it can be difficult to arouse the patient. He or she may not be able to participate in an examination to display asterixis, but the clinician may note muscle rigidity. As HE progresses, the patient may be unable to safely swallow medications. Grade IV is the onset of coma. The Glasgow Coma Scale scores eye opening, motor response, and verbal response.
Clinical Case: Continued
In the emergency room, the patient is diagnosed with HE in the setting of volume depletion. Fluids are given intravenously and lactulose is started. She has four bowel movements over the next 12 h. After a night in observation, her creatinine and sodium have normalized and she is back to her baseline. She is discharged home on lactulose and instructed to follow up with her outpatient physician. She does well for a few months before returning to the emergency department with recurrent symptoms of HE. Her husband reports that she had been doing well on lactulose until the day prior. She was more sleepy than usual during the daytime, was not eating as well, and refused her lactulose on multiple occasions. On this visit, she is diagnosed with a urinary tract infection. Her HE improves after hydration, treatment of the urinary tract infection, and continued lactulose. She is discharged home after a short stay in the hospital, and rifaximin is added to her outpatient medications.
Question 4. How is HE best managed?
Supportive Treatment
If a reversible underlying etiology that triggers HE is found, such as infection, volume depletion, or electrolyte abnormalities, it should be promptly treated. Evaluation for infection should be thorough and include history, examination, and laboratory findings. Spontaneous bacterial peritonitis is a common infection that can result in worsened HE. Therefore, patients with ascites should undergo a diagnostic paracentesis with, at a minimum, cell count and culture. Urinary tract and respiratory tract infections are other potential sources which should be excluded. An evaluation for gastrointestinal bleeding as a trigger for HE should also be considered. Constipation can also result in HE, and it is important to assess with the patient and caregivers how many bowel movements were occurring prior to the development of clinical symptoms. Medication adherence should be assessed, as lactulose has significant side effects that may limit adherence, but the clinician should avoid the temptation to immediately blame “medication non-compliance” as the culprit for recurrent HE episodes.
Euvolemia should be achieved, and any deranged electrolytes, especially hypokalemia, hypomagnesemia, and hypo- or hypernatremia should be corrected. If the patient is comatose and there is concern for inadequate airway protection and the risk of aspiration, the patient should be monitored in an intensive care unit setting and consideration given to intubation.
There should be a low threshold to initiate a nutritional assessment, and small frequent meals plus a bedtime snack should be recommended. There is no role for protein restriction in the treatment of HE. Rather, a goal of 1.2–1.5 g/kg/day of protein intake should be targeted in these patients, and if this cannot be achieved by standard means, oral branched-chain amino acid supplementation can be considered.
As one of the heralding events of decompensated cirrhosis, the onset of HE in a patient should lead to referral to a liver transplant center.
Pharmacologic Treatment
Lactulose remains the mainstay for management of acute overt HE. Doses should be given with a goal of achieving four to five bowel movements daily to treat active HE requiring hospitalization, followed by maintenance dosing to achieve three to four soft bowel movements daily. If the patient is unable to safely swallow medications, a nasogastric tube should be promptly placed to allow safe medication administration. For grades III–IV HE, rectal lactulose via enemas could also be considered. It is important to recognize that after the treatment of an initial episode of HE, long-term maintenance therapy should be instituted. Rifaximin twice daily could be added to lactulose to prevent a recurrent episode of HE. The role of probiotics for HE is not well defined currently, though emerging evidence suggests that they may be of similar efficacy to lactulose for minimal or low-grade HE. Similarly, zinc supplementation is of uncertain benefit.
Both neomycin and metronidazole are accepted alternative treatment options for overt HE. Neomycin was widely used in the past but has been supplanted by lactulose and rifaximin for most cases as it has known risks of ototoxicity and nephrotoxicity. Similarly, a short course of metronidazole may be used in lieu of the previously discussed medications, but its use is also limited due to ototoxicity, nephrotoxicity, and neurotoxicity. In addition, the well-known disulfiram-like reaction that ingestion of alcohol can elicit with metronidazole must be taken into account, especially in patients with alcohol abuse.
In some settings when liver recovery occurs, it may be appropriate to reduce or stop therapy; however, this must be done cautiously and with careful monitoring for recurrence of HE.
Clinical Case: Continued
Unfortunately, despite home use of lactulose and rifaximin, the patient continues to have hospitalizations for HE. Her husband brings her to all scheduled clinic visits and reports adherence with both lactulose and rifaximin. He has taken a leave of absence from his job and is frustrated as he feels that some healthcare staff members are blaming him for the repeated hospitalizations. He is exhausted and reports that he is afraid to leave the house even to go to the store. In addition, he sets an alarm every night for 3 AM to wake her up and give her lactulose. She comes to the appointment in a wheelchair and has lost considerable muscle mass. She has been evaluated and listed for liver transplant, but has not had MELD scores high enough to result in an organ offer. Her husband is wondering what he should do next.
Question 5. How should patients with difficult-to-manage HE be approached?
Refractory HE, whether a single difficult-to-treat episode, or discrete episodes that recur repeatedly with minimal or no obvious precipitating factor, should prompt an evaluation for an alternative cause of altered mental status. If other etiologies for the clinical presentation are excluded, the presence of portosystemic shunts should be considered. In patients with TIPS in this situation, downsizing should be considered, although there is no universally agreed-upon portal pressure to target, and the risks of recurrent varices or ascites must be considered. For patients without TIPS, imaging such as contrast-enhanced CT or MRI may help discover spontaneous portosystemic shunts (Fig. 14.1). Embolization of these shunts by an interventional radiology team may help reduce the frequency and severity of HE. Ultimately, liver transplantation is the only definitive treatment for refractory HE.
For all cases of HE, patient and provider education on early recognition of symptoms, aggressive outpatient treatment, and knowing when to seek help are of paramount importance.
Conclusions
HE is a common manifestation of end-stage liver disease and is one of the hallmarks of decompensated cirrhosis. Timely diagnosis and a balanced approach to treatment, with close involvement of patient family members and caregivers, are crucial to optimize outcomes and improve patient quality of life. Post-TIPS HE is an expected complication of this procedure and requires aggressive monitoring and treatment; similarly, refractory HE may reflect the presence of spontaneous portosystemic shunts. Once a patient with cirrhosis is diagnosed with HE, strong consideration should be given to referral for transplant evaluation.
Further Reading
Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology. 2013;58(1):325–36. https://doi.org/10.1002/hep.26370.
Amodio P, Del Piccolo F, Petteno E, Mapelli D, Angeli P, Iemmolo R, et al. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol. 2001;35(1):37–45.
Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5(3):397–403. https://doi.org/10.4161/gmic.28684.
Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–40. https://doi.org/10.1053/j.gastro.2010.02.015.
Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–81. https://doi.org/10.1056/NEJMoa0907893.
Blei AT, Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–76. https://doi.org/10.1111/j.1572-0241.2001.03964.x.
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21. https://doi.org/10.1053/jhep.2002.31250.
Garcia-Martinez R, Rovira A, Alonso J, Jacas C, Simon-Talero M, Chavarria L, et al. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17(1):38–46. https://doi.org/10.1002/lt.22197.
Jones EA, Mullen KD. Theories of the pathogenesis of hepatic encephalopathy. Clin Liver Dis. 2012;16(1):7–26. https://doi.org/10.1016/j.cld.2011.12.010.
Ortiz M, Jacas C, Cordoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(Suppl 1):S45–53. https://doi.org/10.1016/j.jhep.2004.11.028.
Saab S, Suraweera D, Au J, Saab EG, Alper TS, Tong MJ. Probiotics are helpful in hepatic encephalopathy: a meta-analysis of randomized trials. Liver Int. 2016;36(7):986–93. https://doi.org/10.1111/liv.13005.
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the study of liver diseases and the European Association for the study of the liver. Hepatology. 2014;60(2):715–35. https://doi.org/10.1002/hep.27210.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kallwitz, E., Lominadze, Z. (2019). Hepatic Encephalopathy. In: Cohen, S., Davitkov, P. (eds) Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-98506-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-98506-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98505-3
Online ISBN: 978-3-319-98506-0
eBook Packages: MedicineMedicine (R0)