Abstract
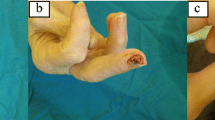
The management of ulcers is a real challenge for physicians and nurses. In systemic sclerosis (SSc), the treatment of digital ulcers (DU) should improve tissue integrity and viability, promote ulcer healing, and reduce the formation of new DU. Wound healing is a complex process regulated by a pattern of events including coagulation, inflammation, formation of granulation tissue, epithelialization, and tissue remodeling. The observation of the ulcer characteristics is fundamental to choose the strategy, and a multidisciplinary approach is required, including both systemic and local treatment, using a combination of non-pharmacological care, antibiotics if an infection is suspected, and surgical intervention in most severe cases. Furthermore, educational aspects are of paramount importance.
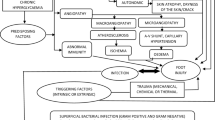
The structured approach to management of chronic wounds in SSc is represented by wound bed preparation (WBP) that includes different steps as detersion and debridement (passive debridement, active debridement, selective debridement, nonselective debridement, and maintenance debridement). The removal of foreign material and devitalized or contaminated tissue from or adjacent to the lesion is important because it is well known that tissue necrosis and slough may release cytokines that can frequently determine pain and worsen the status of DU. Debridement can be achieved through surgical, enzymatic, autolytic, mechanic, or biological methods. New research on wound care has focused on the “advanced” dressings that can help the operator with difficult/chronic lesions. These products are able to trigger the healing process of a lesion during the different phases, keeping a moist environment in the lesion. There are more than 1000 different dressings to choose in the different stages of the ulcers including transparent film dressings, barrier cream, oil solution fatty acids, hydrogel, non-adherent dressing, hydrocolloid dressing, polyurethane foams, alginate, hydrophilic dressing, collagen and cellulose dressing, hyaluronic acid dressing, modulators of protease metal, hydrophobic dressings for controlling bacterial charge, and dressings with silver. Obviously, infections necrosis and gangrene can complicate the scenario. For this reason, the physician must foster vascularization as much as possible, while the nurse will choose the most appropriate dressings for the wound characteristics. This combined approach may significantly accelerate wound healing and improve the quality of life of SSc patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Wound healing is a complex process regulated by a pattern of events including coagulation, inflammation, formation of granulation tissue, epithelialization, and tissue remodeling. The first injury damages blood vessels, triggers coagulation, and provokes an acute local inflammatory response. It is followed by mesenchymal cell recruitment, proliferation, and extracellular matrix generation which allow scar formation [1]. In systemic sclerosis (SSc), the reduction of the flow through the microcirculation involves a state of chronic tissue hypoxia, which slows down the wound healing process, affecting quality of life (QoL) and potentially leading to therapeutic failure. The most common skin lesions are represented by digital ulcers (DUs) that develop in poorly oxygenated tissues, compounded by the presence of infection, epidermal thinning, and tightly stretched skin and contractures [2]. In SSc, the treatment of DUs should improve tissue integrity and viability, promote ulcer healing, and reduce the formation of new DU. The healing of the digital ulcerations may lead to scarring and/or digital resorption; most seriously, chronic ulcers can become infected and can be complicated by osteomyelitis and/or gangrene needing amputation [2,3,4] requiring hospitalizations for aggressive treatment with high socioeconomic cost [5, 6].
A multidisciplinary approach to management of DUs is required, including both systemic and local treatment, using a combination of non-pharmacological care, antibiotics if an infection is suspected [7,8,9], and surgical intervention in most severe cases. Furthermore, educational aspects are of paramount importance to make patient active in the healing process of DU [10,11,12].
Systemic therapy is crucial both for disease treatment, with immunosuppressive therapy that should help to control immune system dysregulation, and for improving vascular dilatation with the use of vasoactive drugs as calcium channel blockers, phosphodiesterase type 5 (PDE-5) inhibitors, prostanoids (iloprost), and endothelin receptor antagonist (ERA) (bosentan) [13].
The local treatment of ulcers is based on a methodological approach that considers the type of lesion and any variables (dimension, depth, presence of exudates, smell and/or other signs of infection) that can be present at baseline or it can occur subsequently, as reported in Chap. 18. Besides an accurate evaluation of DU characteristics, it is fundamental to assess local pain in the area of wound and surrounding tissue. Patients with skin wounds almost invariably need analgesic treatment for long lasting because of chronic pain as well as procedural pain management caused by local wound treatment [14] such as removal and replacing dressing and bandages. In particular, extensive and in-depth debridement of slough and necrotic tissue is an extremely painful procedure [15, 16].
A topical anesthetic drug suitable for use in skin ulcer debridement should have a documented evidence of clinical efficacy, low systemic toxicity and potential for sensitization, and no adverse effects on healing process [17].
Wound Bed Preparation
The structured approach to management of chronic wounds (a chronic wound is defined as a wound which lasts more than 6 weeks) in SSc is represented by wound bed preparation (WBP), as indicated in Chap. 18, and its definition “the management of an ulcer in order to accelerate endogenous healing or to facilitate the effectiveness of other therapeutic measures” well summarized the characteristic and the aim of local therapy of DUs in SSc.
The first step is detersion – defined as the mechanical removal of dirt, cellular debris, necrotic tissue, and other wastes present on the wound bed [18]. It can be performed irrigating with a warm (37 °C) saline solution (NaCl 0.9%) and using a 35 ml syringe and 19 G needle for lower limbs ulcers and a 10 ml syringe and a 19G needle cannula for all the other types of ulcers. The second step is debridement, recommended in all types of SSc wounds, mainly consists in the removal of nonviable material, foreign bodies, and necrotic tissue from a wound, that is a fundamental step to foster healing, prevent chronicity, and reduce the risk of bacterial infection [19]. The removal of foreign material and devitalized or contaminated tissue from or adjacent to the lesion is important because it is well known that tissue necrosis and slough may release cytokines that can frequently determine pain and worsen the status of DU. Debridement can be achieved through surgical, enzymatic, autolytic, mechanic, or biological methods. When the removal of devitalized tissue in DU is performed using scalpels and surgical instruments, the procedure is usually painful. Therefore, it is essential to carefully remove necrotic tissue while maintaining the highest patient comfort possible [17].
There are five types of debridement (passive debridement, active debridement, selective debridement, nonselective debridement, and maintenance debridement) as reported in Chap. 18.
Debridement can be mechanical, via curette or scalpel, or chemical, via enzyme-debriding agents [20]. Autolitic debridement, using endogenous proteolytic enzymes, takes advantage of the moist and warm environment present on the interface between the wound bed and the dressing. This kind of debridement does not cause pain to the patient, but it is a slow method of nonviable tissue removal. The most common dressings used are hydrogel and hydrocolloids as reported in Chap. 18.
Hydrogel includes hydrated carboxymetil-cellulose polymer dressings, containing 90% water in a gel base, which helps regulate fluid exchange from the wound surface. Hydrogel is used in association of sharp debridement.
Hydrocolloids are occlusive or semiocclusive dressings composed by carboxymetil-cellulose, pectin, and elastomers. They jellify absorbing the wound exudate. This type of dressing is rarely used in SSc, owing to its occlusive nature. Hydrocolloids may cause discomfort and harm perilesional sclerotic skin.
Wound Dressing in the Different Healing Phase
New research on wound care has focused on the “advanced” dressings that can help the operator with difficult/chronic lesions. These products are able to trigger the healing process of a lesion during the different phases, keeping a moist environment in the lesion.
In presence of:
-
Necrosis or fibrin : proteolytic enzymes, maggots, silvers dressings, and alginate may be used.
-
Granulation : foams and hydrogels should be used.
-
Epithelization : hydrocolloid, foams, and impregnated gauzes are helpful.
There are more than 1000 different dressings to choose in the different stages of the ulcers:
-
Transparent film dressings provide a moist, healing environment, promote autolytic debridement, protect the wound from mechanical trauma and bacterial invasion, and act as a blister roof or “second skin.” Because they’re flexible, these dressings can conform to wounds located in awkward locations such as the elbow. The transparency makes it easy to visualize the wound bed. Transparent film dressings are waterproof and impermeable to bacteria and contaminants. Although these dressings can’t absorb fluid, they’re permeable to moisture – allowing one-way passage of carbon dioxide and excess moisture vapor away from the wound. Indicated for partial-thickness wounds with little or no exudate, wounds with necrosis, and as both primary and secondary dressing. Also used to cover IV sites, donor sites, lacerations, abrasions, and second-degree burns. Available in a wide variety of sizes, both sterile and bulk.
-
Barrier cream : Protect perilesional skin from maceration due to excess exudate.
-
Oil solution fatty acids : Includes hyperoxygenated essential fatty acids (EFA) that help to maintain skin elasticity and donate moisture to promote skin repair. Pleasant odor. Quick absorption. Easy spray application.
-
Hydrogel : The amorphous gel may contain CMC, calcium alginate versus sodium, starch polyglycosides, and sodium chloride. It is used for surface wounds or cavities or in combination with other dressings.
-
Non-adherent dressing (medicazioni non aderenti): Impregnated gauze with gel, vaseline, paraffin, and silicone. Gauzes are useful in avoiding pain from trauma during dressing removal.
-
Hydrocolloids dressing: The active surface of the dressing is coated with a cross-linked adhesive mass containing a dispersion of gelatin, pectin, and carboxy-methylcellulose together with other polymers and adhesives forming a flexible wafer. In contact with wound exudate, the polysaccharides and other polymers absorb water and swell, forming a gel. The gel may be designed to drain or to remain within the structure of the adhesive matrix. The moist conditions produced under the dressing are intended to promote fibrinolysis, angiogenesis, and wound healing, without causing softening and breaking down of tissue. The gel, which is formed as a result of the absorption of wound exudate, is held in place within the structure of the adhesive matrix. Most hydrocolloid dressings are waterproof, allowing normal washing and bathing. They can be used in poorly exuding lesions to favor an autolytic debridement.
-
Polyurethane foams : Multilayer absorbing dressing with or without adhesive edges, with or without adherent contact, may have a gelificant component. This type of dressing could be useful in certain anatomic sites (heels) and for mild/moderate exudate.
-
Alginate : Medications based on calcium or sodium salts of alginic acid, a polysaccharide extract from seaweed. They are used as dressing of cavity lesions with moderate to abundant exudate that need debridement.
-
Hydrophilic dressing : Dressings made of carbohydrate methyl cellulose pure sodium with a high degree of absorption, gelling in contact with the exudate by holding it without releasing it. They are used for superficial or deep lesions, with high exudate, under bandage.
-
Collagen and cellulose dressing : Matrix based on collagen packed in tampons, particles, and gel; they are useful in presence of granulation tissue or mild exudate, and they should be associated to a subocclusive medication.
-
Hyaluronic acid dressing : Dressings are as cream, spray, transparent film, and microgranules. Medications are useful in lesions with difficulty in healing, requiring debridement, or lesions with granulation tissue with moderate exudate.
-
Modulators of protease metal : Medications made up of an oxidized cellulose matrix and collagen favoring the formation and organization of new collagen fibers modulating growth factors. They are used on superficial and deep, well-detached, deep-fractured lesions with delay in healing.
-
Hydrophobic dressings for controlling bacterial charge: Medications made up of acetate gauze and a hydrophobic compound. They are used as primary dressings for critically colonized lesions, even cavities or infestations, in intolerant antiseptic patients.
-
Dressings with silver : Of various technologies, polyurethane foam, hydrocolloid, alginate, and hydrophobic with silver addition in ionic form or nanocrystals or antiseptic. They are used in lesions with mild and moderate exudate, smelly; they can also be used under bandage.
Semiocclusive wound dressings prevent evaporation and water loss thus retaining warmth, which improves wound healing [19]. Antiseptics should be avoided because of the known cytotoxic effects on cells, and local antibiotics may induce the emergence of resistance to the entire class of antibiotics used topically. Ulcers must be cleaned with physiologic water. The use of systemic antibiotics should be reserved only for clinically infected ulcers and not for bacterial colonization [19].
Conclusions
The management of ulcers is a real challenge for physicians and nurses. The observation of the ulcer characteristics is fundamental to choose the strategy which will drive the WBP in the effort to heal the wound as soon as possible. Obviously, infections necrosis and gangrene can complicate the scenario. For this reason, the physician must foster vascularization as much as possible, while the nurse will choose the most appropriate dressings for the wound characteristics. This combined approach may significantly accelerate wound healing and improve the quality of life of SSc patients.
References
Goss JR. Regeneration versus repair. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound healing biochemical and clinical aspects. Philadelphia: WB Saunders; 1992. p. 40–62.
Korn JH, Mayes M, Matucci Cerinic M, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–9.
Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine. 2002;81:139–53.
Denton CP, Korn JH. Digital ulceration and critical digital ischaemia in scleroderma. Scleroderma Care Res. 2003;1:12–6.
Botzoris V, Drosos AA. Management of Raynaud's phenomenon and digital ulcers in systemic sclerosis. Joint Bone Spine. 2011;78:341–6.
Barr WG, Robinson JA. Systemic sclerosis and digital gangrene without scleroderma. J Rheumatol. 1988;15:875–7.
Chung L, Fiorentino D. Digital ulcers in patients with systemic sclerosis. Autoimmun Rev. 2006;5:125–8.
Korn JH. Scleroderma: a treatable disease. Cleve Clin J Med. 2003;70:954–8.
Denton CP, Black CM. Scleroderma and related disorders: therapeutic aspects. Baillieres Best Pract Res Clin Rheumatol. 2000;14:17–35.
Steen V, Denton CP, Pope JE, Matucci-Cerinic M. Digital ulcers: overt vascular disease in systemic sclerosis. Rheumatology (Oxford). 2009;48(Suppl 3):iii19–24.2.
Abraham S, Steen V. Optimal management of digital ulcers in systemic sclerosis. Ther Clin Risk Manag. 2015;11:939–47.
Schiopu E, Impens AJ, Phillips K. Digital ischemia in scleroderma spectrum of diseases. Int J Rheumatol. 2010;2010:1.
Kowal-Bieleck O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327–39.
Giuggioli D, Manfredi A, Vacchi C, Sebastiani M, Spinella A, Ferri C. Procedural pain management in the treatment of scleroderma digital ulcers. Clin Exp Rheumatol. 2015;33:5–10.
Moffatt CJ, Franks PJ, Hollinworth H. Understanding wound pain and trauma: an international perspective. EWMA Position Document: Pain at wound dressing changes; 2002.
Hollinworth H. Pain and wound care. Wound care society educational leaflet. Wound Care Society: Huntingdon; 2000.
Braschi F, Bartoli F, Bruni C, Fiori G, Fantauzzo C, Paganelli L, De Paulis A, Rasero L, Matucci-Cerinic M. Lidocaine controls pain and allows safe wound bed preparation and debridement of digital ulcers in systemic sclerosis: a retrospective study. Clin Rheumatol. 2017 Jan;36(1):209–12.
International consensus. Per un uso corretto delle medicazioni all’argento nelle ferite. Consenso di un panel di esperti. London: Wounds International; 2012. http://www.woundsinternational.com/media/issues/588/files/content_10495.pdf
Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206.
Fiori G, Galluccio F, Braschi F, Amanzi L, Miniati I, Conforti ML, et al. Vitamin E gel reduces time of healing of digital ulcers in systemic sclerosis. Clin Exp Rheumatol. 2009;27:51–4.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bellando-Randone, S., Pucci, T., Rasero, L., Denton, C.P., Matucci-Cerinic, M. (2019). Wound Dressing for Digital Ulcers in Systemic Sclerosis. In: Matucci-Cerinic, M., Denton, C. (eds) Atlas of Ulcers in Systemic Sclerosis. Springer, Cham. https://doi.org/10.1007/978-3-319-98477-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-98477-3_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98475-9
Online ISBN: 978-3-319-98477-3
eBook Packages: MedicineMedicine (R0)