Abstract
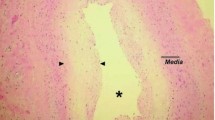
Cardiac allograft vasculopathy (CAV) – formerly termed chronic rejection – was described already in 1969, 2 years after the first heart transplant [1]. Soon after the phenomenon was described in details on cadaveric hearts as an obliterative intimal proliferation of the coronary arteries [2]. It became clear that CAV is a disease of the coronary circulation of transplanted hearts distinct from conventional arteriosclerosis. It is a very important complication after heart transplantation as it affects 30–40% of recipients after 5 years and since it is the dominating cause of graft failure and a common cause of death late after transplant [3]. CAV involves not only epicardial arteries but also intramyocardial small arteries and arterioles as well as the cardiac venous vessels (Fig. 21.1). Rapidly, the diagnosis moved from being pathologic-anatomic to become radiologic as coronary angiography was introduced as a routine examination in heart transplant recipients. Typically CAV presents on angiography as diffuse coronary disease with distal arterial obliteration and often also significant proximal stenosis (Fig. 21.2). Further insight to the natural history of CAV was obtained from intravascular ultrasound (IVUS) studies, which have been used primarily for research. Recent developments include use of optical coherence tomography, MRI and CT angiography.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Definition
Cardiac allograft vasculopathy (CAV) – formerly termed chronic rejection – was described already in 1969, 2 years after the first heart transplant [1]. Soon after the phenomenon was described in details on cadaveric hearts as an obliterative intimal proliferation of the coronary arteries [2]. It became clear that CAV is a disease of the coronary circulation of transplanted hearts distinct from conventional arteriosclerosis. It is a very important complication after heart transplantation as it affects 30–40% of recipients after 5 years and since it is the dominating cause of graft failure and a common cause of death late after transplant [3]. CAV involves not only epicardial arteries but also intramyocardial small arteries and arterioles as well as the cardiac venous vessels (Fig. 21.1). Rapidly, the diagnosis moved from being pathologic-anatomic to become radiologic as coronary angiography was introduced as a routine examination in heart transplant recipients. Typically CAV presents on angiography as diffuse coronary disease with distal arterial obliteration and often also significant proximal stenosis (Fig. 21.2). Further insight to the natural history of CAV was obtained from intravascular ultrasound (IVUS) studies, which have been used primarily for research. Recent developments include use of optical coherence tomography, MRI and CT angiography.
2 Incidence and Prognostic Importance
CAV can be very aggressive and be present already 1 year after transplantation. In the ISHLT registry the overall prevalence of CAV diagnosed by angiography in survivors at 1, 5, and 10 years after transplantation was 8%, 30%, and 50%, respectively. Higher prevalence is found if the diagnosis is made by IVUS. Prognosis in patients with CAV appears to be improved slightly over time, however, almost one third of the patients dying more than 5 years post transplantation die from CAV [3]. The prognosis clearly also depends on the severity of CAV. Indeed, 5 year mortality in patients with severe CAV occurred has been reported to be >50% [4].
3 Risk Factors
Several risk factors for CAV, both relating to the donor and the recipient, have been identified [5] (Table 21.1). Recipient factors may be immunological or non-immunological. Recurrent rejection, especially humoral rejection and the development of donor specific HLA antibodies, appears to accelerate CAV [6]. Rejection, however, is not sufficient to induce CAV, as it is well described that a calcineurin inhibitor free, proliferation signal inhibitor based immunosuppressive regimen, which is associated with increased rates of acute rejection episodes, results in a slower progression of CAV early after transplantation [7]. Infection has been proposed to play a role in development of CAV, in particular CMV [8]. Indeed, CMV D+/R- recipients have an increased risk of CAV and CMV infection has been shown to predispose to CAV. In observational studies, aggressive CMV prophylaxis, resulting in lower rates of CMV infection was associated with lower rates of CAV [9].
Classical risk factors for development of arteriosclerosis such as diabetes, hyperlipidemia and hypertension are very common among heart transplant recipients [10]. It has been clearly shown that these factors significantly accelerate CAV development and that intervention against hyperlipidemia (statins) lower the risk of development of vascular disease [11]. Smoking, although a contraindication to transplantation, is resumed in some patients and is a potent risk factor for CAV [12].
4 Pathophysiology
CAV is characterized by concentric intimal hyperplasia of the coronary arteries and severe medial hypertrophy of coronary resistance vessels [2]. The processes are confined to the vessels of the transplanted heart and not part of a generalized vascular disease. The endothelial cells of the coronary circulation appear to play a significant role in initiating the process and several circulating and locally derived factors appear to be implicated, such as platelet derived growth factor, vascular endothelial growth factor, TGF-beta and endothelin-1. T-cell derived cytokines upregulate endothelial factors promoting growth and microthrombosis such ICAM-1 and VCAM −1 as well a P-selectin. As CAV progresses it leads to myocardial ischemia or infarction, arrhythmia and graft failure.
5 Diagnosis and Surveillance
Unlike conventional coronary arteriosclerosis, CAV may cause uniform remodeling of the coronary vessels which may be difficult to detect by routine angiography (Fig. 21.3). In patients with angiographically normal corornary arteries, IVUS may uncover severe CAV by demonstrating significant intima thickening. Despite this shortcoming of angiography, the current definition of CAV is predominantly based on this technique. An angiographic definition has now been published by ISHLT (Table 21.2) and constitutes the nomenclature to be used for CAV [13].
Due to cardiac denervation, even patients with severe CAV rarely develop classical angina pectoris, but more often present with more unspecific symptoms of dyspnea, fluid retention, palpitations or syncope. Given the lack of specificity of these symptoms and the high prevalence of CAV, surveillance is recommended. ISHLT guidelines recommend annual or biannual angiography to screen for CAV. In patients without early aggressive CAV (i.e. no angiographic evidence for vasculopathy after 3–5 years), non-invasive screening using dobutamine stress echocardiography or myocardial perfusion scintigraphy may be used in asymptomatic patients. These diagnostic modalities may also be preferred as screening tool in patients with significant renal dysfunction in whom contrast use should be minimized [14].
6 Prevention and Treatment
At the current time preventive strategies based on statin therapy and immunosuppression based on an mTOR inhibitor (sirolimus, everolimus) have proven effective. Treatment with pravastatin within 2 weeks from transplantation has been demonstrated to significantly lower the rate of CAV, documented both on angiography and IVUS [11]. A similar effect has been documented with simvastatin and both trials of pravastatin and simvastatin showed effect on survival despite the fact that they were moderately sized [15]. Caution must be paid to interaction between statins and immunosuppressants, but statins are recommended for all heart transplant recipients (including children), irrespective of cholesterol levels based on these trials.
Use of sirolimus and everolimus in de novo heart transplant recipients has been associated with lower rates of CAV. Together with a calcineurin inhibitor, both sirolimus and everolimus, have in randomized trials been proven superior to azathioprine [16] and everolimus has also been associated with smaller increase in intimal thickness on IVUS compared with mycophenolate mofetil [17]. Finally, everolimus together with mycophenolate has recently been shown to result in less intima thickness 1 year after transplantation compared with a conventional regimen containing a calcineurin inhibitor and mycophenolate, indicating that the presence of the mTOR inhibitor rather than the absence of another immunosuppressant is the deciding factor for slowing CAV early after transplantation [18].
Later after transplantation switch to an mTOR inhibitor based regimen may slow progression of CAV but the effect is much less pronounced and has not been documented in all studies [19, 20]. When CAV has developed, therapy concentrates on prevention of complications including aspirin for prophylaxis against coronary thrombosis and heart failure therapy if graft dysfunction occurs. Localized coronary stenosis in proximal vessels without obliterated periphery may be treated with percutaneous coronary intervention (PCI) and stenting or very occasionally by coronary artery bypass surgery. The use of prophylactic defibrillators is highly controversial in this setting, since overall prognosis in terms of non-arrhythmic death is difficult to predict in this population.
When advanced CAV develops, and especially when complicated by graft failure, re-transplantation should be considered. CAV is the most common indication for re-transplantation which may yield acceptable results in selected patients [21, 22].
References
Thompson JG. Atheroma in a transplanted heart. Lancet. 1969;2(7633):1297.
Bieber CP, Stinson EB, Shumway NE, Payne R, Kosek J. Cardiac transplantation in man. VII. Cardiac allograft pathology. Circulation. 1970;41(5):753–72.
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34(10):1244–54.
Costanzo MR, Naftel DC, Pritzker MR, Heilman JK III, Boehmer JP, Brozena SC, et al. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac transplant research database. J Heart Lung Transplant. 1998;17(8):744–53.
Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant. 2012;31(10):1052–64.
Nath DS, Angaswamy N, Basha HI, Phelan D, Moazami N, Ewald GA, et al. Donor-specific antibodies to human leukocyte antigens are associated with and precede antibodies to major histocompatibility complex class I-related chain a in antibody-mediated rejection and cardiac allograft vasculopathy after human cardiac transplantation. Hum Immunol. 2010;71(12):1191–6.
Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Radegran G, Gude E, et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: a randomized trial. Am J Transplant. 2014 Aug;14(8):1828–38.
Delgado JF, Reyne AG, de Dios S, Lopez-Medrano F, Jurado A, Juan RS, et al. Influence of cytomegalovirus infection in the development of cardiac allograft vasculopathy after heart transplantation. J Heart Lung Transplant. 2015;34(8):1112–9.
Potena L, Grigioni F, Magnani G, Lazzarotto T, Musuraca AC, Ortolani P, et al. Prophylaxis versus preemptive anti-cytomegalovirus approach for prevention of allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant. 2009;28(5):461–7.
Valantine H, Rickenbacker P, Kemna M, Hunt S, Chen YD, Reaven G, et al. Metabolic abnormalities characteristic of dysmetabolic syndrome predict the development of transplant coronary artery disease: a prospective study. Circulation. 2001;103(17):2144–52.
Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333(10):621–7.
Arora S, Aukrust P, Andreassen A, Simonsen S, Gude E, Grov I, et al. The prognostic importance of modifiable risk factors after heart transplantation. Am Heart J. 2009;158(3):431–6.
Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29(7):717–27.
Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56.
Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Steinbeck G, et al. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation. 1997;96(5):1398–402.
Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-Von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847–58.
Eisen HJ, Kobashigawa J, Starling RC, Pauly DF, Kfoury A, Ross H, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13(5):1203–16.
Arora S, Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Botker HE, et al. The effect of Everolimus initiation and Calcineurin inhibitor elimination on cardiac allograft vasculopathy in De novo recipients: one-year results of a Scandinavian randomized trial. Am J Transplant. 2015;15(7):1967–75.
Ruygrok PN, Webber B, Faddy S, Muller DW, Keogh A. Angiographic regression of cardiac allograft vasculopathy after introducing sirolimus immunosuppression. J Heart Lung Transplant. 2003;22(11):1276–9.
Masetti M, Potena L, Nardozza M, Prestinenzi P, Taglieri N, Saia F, et al. Differential effect of everolimus on progression of early and late cardiac allograft vasculopathy in current clinical practice. Am J Transplant. 2013;13(5):1217–26.
Goldraich LA, Stehlik J, Kucheryavaya AY, Edwards LB, Ross HJ. Retransplant and medical therapy for cardiac allograft vasculopathy: International Society for Heart and Lung Transplantation registry analysis. Am J Transplant. 2016;16(1):301–9.
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):996–1008.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gustafsson, F. (2019). Cardiac Allograft Vasculopathy. In: Feldman, D., Mohacsi, P. (eds) Heart Failure. Cardiovascular Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-98184-0_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-98184-0_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98182-6
Online ISBN: 978-3-319-98184-0
eBook Packages: MedicineMedicine (R0)