Abstract
Human pluripotent stem cells (hPSCs) provide unprecedented access to the earliest stages of retinogenesis that remain inaccessible to investigation, thereby serving as powerful tools for studies of retinal development. Additionally, the ability to derive hPSCs from patient sources allows for the modeling of retinal degenerative diseases in vitro, with the potential to facilitate cell replacement strategies in advanced stages of disease. For these purposes, many studies over the past several years have directed the differentiation of hPSCs to generate retinal cells using stochastic methods of differentiation, yielding all major cell types of the retina. In particular, these studies have favored the derivation of RPE, photoreceptors, and more recently retinal ganglion cells for disease modeling, drug screening as well as cell replacement purposes. More recently, advances in retinal differentiation methods have led to the generation of three-dimensional retinal organoids that recapitulate key developmental and morphological features of the retina, including the stratified organization of retinal cells into a tissue-like structure. This review provides an overview of retinal differentiation from hPSCs and their potential use for studies of retinogenesis as well as diseases that affect the retina.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
The human retina is a multilayered tissue composed of an intricate network of several types of retinal neurons that function in an integrated manner to convert the incoming light stimulus into an electrical impulse , which will be propagated to the brain to be converted into an image. Consequently, any disease or injury affecting retinal neurons disrupts this visual circuit, resulting in blindness. Hence, a thorough understanding of the development and functions of the human retina will facilitate the development of successful therapies for retinal degenerative diseases. However, studies of the human retina are especially challenging as retinogenesis occurs early in gestation and remains largely inaccessible to investigation [1]. In this regard, human pluripotent stem cells (hPSCs), including human embryonic stem cells [2] and human induced pluripotent stem cells [3,4,5], provide a unique in vitro model capable of recapitulating the growth and diversification of developing retinal neurons.
hPSCs are self-renewing cells analogous to the inner cell mass/blastocyst stage of human development, which possess the ability to generate all cell types of the body. Therefore, hPSCs can be used to study even the earliest events of retinogenesis and generate limitless numbers of retinal neurons for translational applications [6,7,8,9,10,11,12,13,14,15]. While advancements in hPSC-retinal differentiation protocols over the last decade have led to the successful generation of all types of retinal neurons [9, 16,17,18,19,20,21,22,23,24,25,26,27], these cells have traditionally been differentiated in a manner that lacked the ability to assemble into a multilayered retinal-like structure. This lack of cellular organization not only affects the ability to faithfully recapitulate the events of retinogenesis as an in vitro model, but may also impact the quality and functionality of retinal cells generated for future translational applications, including disease modeling and cell replacement.
More recently, a fundamental shift in retinal differentiation protocols has developed which allows for the organization of hPSC-derived retinal neurons into an organized, multi-layered retinal-like structure [21, 28,29,30]. These resultant populations, known as retinal organoids, are composed of retinal neurons arranged in a stratified manner that recapitulates the spatial and temporal patterning of native retinal tissue [21, 23, 28,29,30,31,32,33,34,35,36,37,38,39]. Thus, such hPSC-derived retinal organoids will likely serve as more effective in vitro models with which to recapitulate earliest events of retinogenesis. Furthermore, these retinal organoids may enhance the application of hPSCs for disease modeling and cell replacement. To serve in these capacities, however, refinements in the differentiation of retinal organoids will be needed, with improvements in these protocols likely to be inspired by our growing understanding of the regulatory factors at play in the developing retina in vivo.
2.2 Development and Organization of the Vertebrate Retina
The retina is a complex multilayered tissue that originates from the developing diencephalon and consists of six neuronal cell types that work in a coordinated fashion to perceive and interpret incoming visual information [40, 41]. Based on the orientation of retinal cells, the retina can be broadly classified into three layers: (1) the outer nuclear layer consisting of the photoreceptor cells, including the rods and cones, (2) the inner nuclear layer consisting of the interneurons, namely bipolar cells, amacrine cells, and horizontal cells, and (3) the ganglion cell layer consisting of the retinal ganglion cells whose axons extend to form the optic nerve [42, 43]. In addition to these neuronal cell types, Muller glia are the primary glial cells of the retina, with cell bodies in the inner nuclear layer and processes traversing the length of the retina, providing necessary architectural and functional support. Additionally, photoreceptors are supported and nourished by the retinal pigmented epithelium (RPE), a sheet-like layer of epithelium located below the photoreceptor layer. Retinal neurons are intricately connected through a network of synapses, with connections between the photoreceptors, bipolar cells, and horizontal cells, referred to as the outer plexiform layer. Similarly, the inner plexiform layer represents the dense fibrils between the ganglion cells, bipolar cells, and amacrine cells.
This structure forms a highly regulated pathway for visual transduction, which is critical to the functioning of the retina [44]. Briefly, incoming light is focused onto the retina via the cornea and lens, where it first interacts with the photoreceptors in the outermost layers of the retinal tissue . These photoreceptors convert the visual light into an electrical stimulus via the phototransduction pathway, which is then transmitted to the retinal ganglion cells via the interneurons of the retina. Finally, the ganglion cells extend their long axons via the optic nerve and synapse with their postsynaptic targets, including the superior colliculus and the lateral geniculate nucleus. Relays to cortical areas responsible for signal integration enable vision. Overall, the function of the retina depends on all its components working in a sequential manner to integrate and transmit the visual information to the brain. Consequently, any disruption in this visual circuit due to injury or disease results in loss of vision or blindness. As such, the use of hPSCs provides a powerful tool with which to study the development of the retina, as well as disruptions to retinal function resulting in vision loss [45]. However, modeling the functions of the retina and its pathophysiology requires the differentiation and organization of these cells in a manner which closely recapitulates the native retina, necessitating a thorough understanding of mechanisms associated with retinal development in vivo.
Retinal development is determined by the combinatorial actions of growth factors as well as transcription factors, which not only specify retinal cell types but also determine their spatio-temporal location. Retinogenesis begins early in gestation and the first morphological evidence of the retina is seen during neurulation [46]. As the developing neural plate forms the neural tube, optic grooves emerge on either side of the diencephalon. These grooves, now known as optic vesicles, evaginate toward the surface ectoderm, resulting in reciprocal signaling between these structures. This reciprocal exchange of signals leads to the induction of the retina from the distal optic vesicle and the formation of the lens placode from the ectoderm . Consequently, the proximal optic vesicle is induced by the surrounding mesenchyme to form the retinal pigmented epithelium (RPE). Following specification of the optic vesicle, these cells acquire a multipotent progenitor identity and will subsequently multiply and differentiate into all cell types of the retina (Fig. 2.1). Retinal cell genesis is specified in an evolutionally-conserved order, which is dictated by the competence of retinal progenitors, and a combination of exogenous signaling gradients as well as endogenous transcriptional regulation [47,48,49]. Based on this model, studies of retinal development in model systems have demonstrated that ganglion cells, horizontal cells, and cone photoreceptors are the earliest-born retinal cell types. Amacrine cells are specified slightly later in development, followed by rod photoreceptors, while bipolar and muller glia cells are the last cell types to develop in the retina. Retinal development and maturation continues throughout gestation and visual synapses continue to mature after birth. Overall, the specification of the retina from its early diencephalic origins follows a tightly conserved order of events. Likewise, to properly and reliably direct the differentiation of hPSCs to a retinal fate, knowledge and application of these developmental events to cellular differentiation protocols is essential.
hPSCs generate retinal cells using stochastic methods of differentiation. hPSCs were directed to a retinal fate using a stepwise protocol, where retinal neurons were specified in a conserved, temporal sequence. Within 70 days of differentiation, photoreceptors were readily identified by the expression of photoreceptor-specific markers CRX/RECOVERIN (a), while ganglion cells expressed BRN3 and extended MAP2 positive neurites (b). hPSCs-derived RPE demonstrate characteristic pigmentation and hexagonal morphology, seen via bright field microscopy (c). Additionally, hPSC-derived interneurons such as horizontal cells and amacrine cells could be identified via PROX1 and AP2α expression respectively. Scale bars equal 40 μm
2.3 In Vitro Studies of Retinal Development Using hPSCs
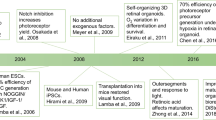
The development of the human retina is initiated at some of the earliest stages of gestation , making the study of these critical cell fate determination events difficult. Given their pluripotent nature, hPSCs may provide a unique and novel tool for the study of these early developmental events by serving as comprehensive models of the major stages of human retinogenesis, even at stages that would be otherwise inaccessible to investigation in the embryo. With the resultant retinal cells, the potential then exists for their translational application, including cell replacement approaches as well as the ability to model and study retinal degenerative diseases in a dish when derived from specific patient sources. In order to serve in this capacity, however, these cells must be directed to differentiate toward a retinal lineage in a step-wise process that faithfully recapitulates the major stages of retinogenesis in vivo [50]. As such, numerous efforts have been made over the last decade focused on the derivation of retinal cells from hPSCs, often adopting critical principles of developmental biology to guide the differentiation process (Table 2.1). Initial work in this field focused upon the differentiation of retinal cells by inhibiting BMP and WNT signaling in the presence of IGF-1 [9, 19, 68]. Similarly, other groups have been successful in achieving retinal differentiation through the inhibition of WNT and Nodal signaling [17, 18, 24, 25, 69]. Subsequently, efforts relying upon the default adoption of a rostral neural fate in the absence of specific morphogenic factors led to the development of discrete retinal progenitor cell populations, which would later give rise to retinal neurons in a temporally appropriate manner [21,22,23, 26, 27, 63].
While these protocols provided the ability to generate all the major cell types of the retina, most of the early focus has favored the generation of RPE and photoreceptor cells as many retinal diseases primarily affect these outer retinal cells, resulting in their degeneration and subsequent loss of vision. Additionally, RPE and photoreceptors possess unique phenotypic markers and functional properties that enables their ease of identification in vitro, which is often lacking for other cell types of the retina. Among the earliest retinal differentiation studies, RPE was first observed to be spontaneously differentiated from hPSCs in relatively high numbers [51]. These cells were initially identifiable in cultures of differentiating stem cells due to the accumulation of melanin pigment within these cells that could be readily visualized. Further confirmation of RPE differentiation was provided by their characteristic hexagonal shape and upon isolation of these cells, they commonly expressed a full complement of RPE-associated features [21, 52, 54,55,56, 59, 60, 70,71,72,73,74,75,76,77]. Similarly, photoreceptors were among the first retinal neurons to be identified due to the large number of photoreceptor-specific markers that have been previously identified in retinal development studies [9, 19, 20, 22, 24, 25, 78].
More recently, some efforts have focused upon the differentiation of retinal ganglion cells from hPSCs. These cells have been somewhat more difficult to definitively identify in differentiating cultures as they lack any truly specific markers to separate them from some other neuronal populations. However, the ability to identify these cells has been facilitated in recent years by following their differentiation through a retinal progenitor intermediary or via the use of fluorescent reporters [58, 62, 63, 65, 79,80,81,82]. Additionally, some studies have demonstrated the ability to derive all the major neuronal cell types of the retina, including interneurons, although these cells have not been extensively characterized to date [21, 22, 25,26,27, 29, 61, 63, 68, 69, 83].
While the above methodologies have been highly successful for the derivation of all of the major types of retinal neurons, this differentiation often occurred as a somewhat heterogenous population of retinal cells. This differentiation allows for the ability to study many features within individual cells, but does not account for the critical interactions between neurons of the retina which are necessary for their proper maturation and function. Furthermore, many disorders of the retina result from the loss of connectivity between cells, making the study of these disorders more difficult in heterogeneously arranged cultures. To overcome these shortcomings, efforts have been directed toward the differentiation of retinal cells from hPSCs in a manner which closely mimics the development and three-dimensional organization of the retina. Initially, studies described the ability of hPSCs to differentiate toward a retinal lineage in a step-wise fashion, yielding three-dimensional structures closely resembling the optic vesicle and optic cup stages of retinogenesis [21,22,23, 29]. Subsequently, further efforts expanded upon these early results to generate three-dimensional structures termed retinal organoids that were found to effectively recapitulate the spatial and temporal organization of the various neuronal cell types of the retina, resulting in a stratified, multilayered structure [28, 30, 34,35,36,37,38].
2.4 Applications of Retinal Organoids for Modeling Human Development
With the goal of effectively recapitulating the complex organization and interplay between the different types of neurons of the retina, studies within the past few years have described the ability of hPSCs to differentiate toward a retinal lineage in a step-wise fashion [17, 22, 68]. The resultant populations of cells have yielded structures that closely resemble the developing optic cup, with enriched populations of retinal progenitor cells discretely arranged into a cup-like structure (Fig. 2.2). Subsequent efforts have expanded upon these early results to generate retinal organoids that effectively recapitulate the spatial and temporal organization of the various neuronal cell types of the retina (Table 2.2). As a result, these retinal organoids provide a powerful and novel tool for studies of the earliest stages of human retinal development.
hPSCs can be directed to generate retinal organoids using three-dimensional differentiation approaches. hPSCs were directed to generate retinal organoids in a stepwise manner analogous to major stages of retinogenesis. Optic vesicle-like retinal organoids expressed retinal progenitor markers, CHX10, PAX6, and cell proliferation marker Ki67 after 1 month of differentiation (a–d) while photoreceptor marker RECOVERIN and ganglion cells marker BRN3 were seldom seen at this early stage of development. After 2 months of differentiation, retinal organoids acquired a cup-like appearance and retinal cells were arranged in a stratified manner. Photoreceptor markers (CRX, RECOVERIN, OTX2, and ND1) occupied apical layers of the organoids (e–i), horizontal and amacrine cells (PROX1, AP2α) in the middle and ganglion cells (BRN3, SV2) occupied basal layers within the organoids (e–j). Scale bars equal 150 μm for a–d and 100 μm for e–j
As compared to early methods of retinal differentiation from pluripotent cells, retinal organoids offer several advantages as an in vitro model of retinogenesis. Importantly, these organoids can self-assemble into discrete three-dimensional structures with major classes of retinal neurons arranged into distinct layers similar to their organization within the retina [21, 28,29,30,31, 33, 35,36,37,38,39]. The differentiation of these retinal organoids progresses through all the major stages of retinogenesis, including stages analogous to the eye field, optic vesicle, and optic cup, thereby allowing for the ability to visualize some of the earliest events of human retinal development. Similar to embryonic retinogenesis [50], differentiation of resultant cells within retinal organoids has been demonstrated to follow a conserved sequence of events, with early-born cell types such as RGCs among the first retinal neurons to be specified, while later-born cell types such as rod photoreceptors among the last [21, 23, 28,29,30,31, 33, 34, 37, 85].
Retinal cells occupy strategic positions within the adult retina, with ganglion cells residing in the innermost layers of the retina, whereas photoreceptor cells closely associate with RPE and form the outermost layers. The spatial arrangement of retinal neurons and their synaptic connections linking them together are critical to their proper function and as such, retinal cells derived from hPSCs should similarly recapitulate this level of organization. While traditional methods of differentiation have allowed for the successful generation of all the major cell types of the retina, these approaches have lacked the ability of retinal cells to assemble into a layered structure. These shortcomings of traditional approaches have been overcome by the development of retinal organoids, which allow for the maintenance of cell–cell contacts between retinal neurons [21, 23, 28,29,30, 32, 33, 35, 37, 38, 85]. These organoids formed a pseudostratified epithelium-like structure which allows the retinal cells to mature in both a temporal and spatial fashion, with ganglion cells specified in basal laminae of the organoids while photoreceptors occupy apical regions .
The three-dimensional nature of organoids also likely aids in the functional maturation of retinal neurons, which has been largely limited in retinal cells derived using traditional differentiation methods. While these retinal cells differentiated by traditional approaches commonly express a variety of features associated with all of the major cell types of the retina, these cells lacked the structural and functional differentiation typically associated with more mature retinal neurons. The use of three-dimensional retinal organoids allows for the acquisition of more advanced features of differentiation within these cells including enhanced outer segments and the ability to respond to light stimuli, presumably due to their ability to interact and self-organize with neighboring cells. Further refinements of these organoid cultures have also involved the addition of external signaling molecules in long-term cultures to further guide their differentiation [28, 30, 32]. This has been particularly true for photoreceptors, which have been the most extensively studied cell type derived within retinal organoids. The experimental manipulation of critical signaling pathways within retinal organoids has led to refinements in photoreceptor differentiation, including accelerated differentiation as well as increased expression of phototransduction proteins. Photoreceptors derived in this fashion exhibited characteristic bulb-like structures at their tips, demonstrated membranous disc-like structures in regions resembling outer segments, and occasionally displayed electrophysiologic responses to light stimuli [30, 38].
2.5 Application of hPSC-Derived Cells for Retinal Disease Modeling
Beyond the applications of hPSCs for modeling retinal development, these cells also serve as powerful and unique platforms for the study of human retinal degenerative diseases. Due to the degeneration of specific populations of retinal neurons, these diseases are characterized by loss of vision and eventual blindness. Retinal degenerative diseases can be most readily classified into diseases that affect cells of the outer retina or those affecting the inner retina, most notably age-related macular degeneration and glaucoma, respectively [86, 87]. Traditionally, the ability to study the progression of these disease states has been limited to animal models. While these animal models have led to significant advances in our understanding of retinal disease progression [88,89,90,91,92,93,94], important differences exist between the retinas of animal models and humans, including the prevalence of rods and cones as well as the presence of a macula in humans. Furthermore, studies in humans have been largely limited to postmortem retinal tissue or to retinal imaging approaches that lack the resolution to examine individual cells. While these studies have been informative about the end-result of disease pathology, the approach necessarily limits the ability to better understand disease progression within individual cells.
In order to overcome these shortcomings for studies of retinal degenerative diseases, recent research has focused on the use of hPSCs to model and understand disease progression (Table 2.3). When generated from patients with a known genetic basis for retinal degeneration, hPSCs provide an infinite supply of cells for the derivation of the affected cell type, and can thereby serve as powerful tools to study the disease phenotype [85, 110, 111]. Over the last several years, studies have utilized hPSCs for studies of degenerative diseases of the retina, with a particular focus on those diseases that affect RPE and photoreceptors [11, 15, 21, 56, 60, 70,71,72, 95, 96, 98, 101, 105, 109, 112,113,114,115,116,117,118,119]. These cells are often affected in retinal degenerative diseases such as age-related macular degeneration, and the derivation of these cells has been extensively characterized through hPSC retinal differentiation protocols. Such approaches have helped to demonstrate the improper function and/or reduced survival of RPE and photoreceptors in patient-derived cells, thereby providing insight into potential mechanisms underlying the loss of these retinal cell types [11, 21, 53, 56, 101, 105, 115, 116]. Furthermore, patient-derived hPSCs have also been utilized to identify novel genetic variants underlying retinal degeneration, highlighting the potential to target this area for the development of therapies [96, 98].
While diseases affecting cells of the outer retina have been extensively studied with hPSCs, studies related to diseases affecting inner retinal neurons have been largely limited. Of the diseases affecting inner retinal neurons, the most common is glaucoma with a current incidence of greater than 60 million people worldwide [120, 121]. Glaucoma results in the degeneration of retinal ganglion cells (RGCs), leading to a decreased connectivity between the eye and the brain and subsequent loss of vision. The ability to derive RGCs from hPSCs has been a more recent area of investigation, which now allows for the application of these cells for studies of retinal degenerative diseases affecting the inner retina [58, 62, 63, 81, 82, 97, 99, 122]. Recently, efforts have focused on the use of hPSCs from patients with genetic determinants of degenerative diseases that directly affect the RGCs, such as gene mutations underlying some forms of normal tension glaucoma and dominant optic atrophy. Interestingly, upon the differentiation of these cells, RGCs from patient sources exhibited increased apoptosis, thereby allowing for subsequent studies of disease mechanisms leading to degeneration of RGCs [63, 106].
While traditional retinal differentiation protocols have been highly successful in modeling certain features of some retinal degenerative diseases, the resultant retinal cells differentiate in a manner that lacks any three-dimensional organization that mimics how cells are arranged into retinal tissue. Retinal organoids may serve as an improved model for studies of retinal disease modeling, allowing for the interaction between different cell types and therefore providing the ability to assess the effects of degeneration on the entire tissue. While such an ability has yet to be demonstrated for retinal organoids, the use of cerebral organoids for disease modeling has provided an important proof of principle and have been particularly successful for some of the effects of cerebral diseases , such as microcephaly and lissencephaly [123,124,125]. In the near future, it is likely that retinal organoids will be applied for the study of retinal degenerative diseases. As recent studies have demonstrated the successful organization and maturation of photoreceptors within retinal organoids [21, 29, 30, 34, 38, 76], disease-modeling approaches will most likely be applied for outer retinal diseases. Recent studies utilizing hPSC-derived retinal organoids have primarily utilized a genetic basis versus an idiopathic basis for retinal degenerative diseases [39, 76, 84]. Additionally, further improvements to retinal organoids will likely be necessary to be able to apply them to a wide variety of retinal degenerative diseases. For example, hPSC-derived retinal organoids do not demonstrate a macula-like region or a functioning RPE layer, they are currently suited to model diseases that affect peripheral photoreceptors [39, 76, 84]. Further improvements in the differentiation methods to also include the characterization and maturation of inner retinal neurons will enable the study of diseases to affect ganglion cells with retinal organoids .
2.6 Drug Screening with hPSC-Derived Retinal Cells
When derived from individual patient populations, particularly those with a known genetic basis underlying retinal disease, hPSCs possess the ability to recreate certain features of the disease phenotype and model the degeneration associated with retinal diseases. With the resulting data accumulated from such studies, these cells can then be utilized for the development of therapeutic approaches for retinal degenerative diseases [111, 119, 126,127,128,129]. Following the directed differentiation of patient-derived hPSCs to a retinal fate, drug screening efforts can be targeted to an affected retinal cell type, providing a platform for assessing the ability of candidate compounds to rescue the disease phenotype.
The use of patient-derived hPSCs for drug screening has been particularly successful for degenerative diseases that affect the outer retina, whose cells have been routinely derived and extensively characterized from hPSCs [6, 11, 21, 76, 84, 102,103,104, 107, 108, 116, 117, 130]. Photoreceptors and RPE are the most common cell types affected in many retinal degenerative diseases such as age-related macular degeneration (AMD) , where the loss of photoreceptors combined with dysfunctions in RPE leads to loss of vision. As patient-derived RPE has been shown to recapitulate some of the hallmark features of AMD, including elevated expression of inflammatory factors and defective oxidative stress responses, recent studies have utilized hPSC-derived RPE as a platform for the screening of candidate drugs to assess the ability to improve their survival [56, 59, 76, 100, 131]. The results of these studies have enabled the identification of select compounds as potential neuroprotective agents that can alleviate RPE degeneration [107]. Similarly, hPSC-derived retinal cells have also been utilized for drug screening purposes as a means to alleviate photoreceptor loss due to retinitis pigmentosa, with results indicating that hPSC-derived photoreceptors were able to recapitulate the disease phenotype and upregulate markers of oxidative stress, lipid oxidation, and apoptosis [11, 57, 102, 103, 108]. Treatment of the degenerating rod photoreceptors with antioxidant vitamins effectively increased photoreceptor survival .
While hPSC differentiation strategies initially emphasized the cells of the outer retina, recent refinements in differentiation protocols have enabled the stepwise differentiation and identification of inner retinal neurons, particularly RGCs [58, 62, 63, 79, 81, 82, 106]. RGCs serve as the critical connection between the eye and the brain to transmit visual information, and their degeneration is part of a spectrum of diseases known as optic neuropathies, resulting in vision loss and eventual blindness. RGCs differentiated from hPSCs, particularly when derived from patient-specific sources, allow for the ability to screen new drug compounds and develop personalized treatment profiles for optic neuropathies [63, 106]. As a proof of principle, recent studies have successfully demonstrated the ability to faithfully recapitulate some of the degenerative processes associated with optic neuropathies in hPSC-derived RGCs, with subsequent drug screening approaches enabling the identification of neurotrophic factors capable of rescuing RGC degeneration [63].
While a number of studies have successfully demonstrated the ability to screen compounds for their neuroprotective effects on hPSC-derived retinal cells, these approaches have focused on isolated cells lacking any three-dimensional organization reminiscent of retinal tissue. With the advent of retinal organoids, hPSCs can be directed to differentiate in a manner that recapitulates the architecture, spatial connectivity and functioning of the retina, and may therefore be better suited for drug screening purposes. Given the more detailed demonstration to date of photoreceptor differentiation and organization in the outer layers of retinal organoids, these cells are likely better suited for drug screening applications for photoreceptor diseases. In contrast to outer retinal diseases, retinal organoids can also be used to test and develop therapies for inner retinal neurons such as RGCs, which are primarily affected in optic neuropathies.
2.7 hPSC-Derived Retinal Cells as a Vehicle for Cell Replacement
While early stages of retinal degenerative diseases may be effectively studied with hPSCs, and subsequently drug screening approaches may aid in the neuroprotection of these degenerating cells, the irreversible loss of retinal neurons in later stages renders such measures ineffective, resulting in severe vision loss and blindness. In such cases, attempts to replace degenerated cells through transplants of healthy retinal cells constitute the only remaining effective option to restore some visual function [129, 132]. The transplantation of cells into the retina represents a more feasible option for cell replacement when compared to other cells of the nervous system, as the relative ease of accessibility of the retina and its reduced immunological response will likely facilitate cell replacement [133, 134]. To aid in this goal, hPSCs can serve as a renewable source of stem cells for the differentiation of retinal cells for a variety of translational approaches to retinal repair. Transplants of hPSC-derived retinal cells can assist in neuroprotection, particularly at earlier stages of the disease process, and can lead to potential delay in disease progression. At later stages of the degenerative process, hPSC-derived retinal cells can serve as a source for repopulation of the retina following the loss of host neurons.
Several studies have examined the use of hPSC-derived photoreceptors for cell replacement in diseases that affect the outer retina, with the goal to replace the degenerating neurons with their functional equivalents [9, 39, 64, 135, 136]. Initial studies focused on transplantation of undifferentiated retinal stem cells into animal models, which could integrate into many layers of the retina and exhibit neuronal morphologies [137,138,139]. However, these cells were often limited in number and their ability to be expanded, and rarely exhibited any ability to give rise to photoreceptor cells . As an alternative, more recent efforts have focused upon the ability of hPSC-derived photoreceptor cells for cell replacement. Upon transplantation, several groups have demonstrated the ability of these cells to integrate into the host retina and form connections with other retinal neurons, in some cases leading to improved visual function and restoration of light sensitivity [135, 140, 141]. Further investigations into the transplantation of hPSC-derived photoreceptors have demonstrated the use of immunodeficient mouse models to improve survival of hPSC-derived photoreceptors [136].
As the RPE provides essential support for photoreceptors, similar approaches for cell replacement have also been developed for RPE loss in retinal degenerative diseases, often associated with the secondary loss of photoreceptors. hPSC-derived RPE has been utilized in the development of cell replacement strategies for diseases such as age-related macular degeneration [15, 21, 76, 107, 109, 114, 131]. In this capacity, the transplant of RPE cells has been accomplished by either subretinal injection as a cell suspension or as RPE sheet transplantation [142,143,144,145,146,147,148,149,150,151]. The latter approach may offer numerous advantages, as the cells retain their polarization and are arranged in a discrete monolayer, allowing better integration within the host retina. The success of the above-named transplantation strategies has paved the way for hPSC-derived RPE in clinical trials for AMD and Stargardt’s disease, where transplanted cells were shown to improve visual acuity in patients, illustrating the ability of hPSCs to rescue visual defects in retinal degenerative diseases [14, 152].
Many of the cell replacement strategies developed to date have focused on the transplantation of RPE and/or photoreceptors due to their ease of differentiation and more limited need of these cells to extend neurites to form synaptic connections, which will likely make replacement efforts easier. However, the development of replacement strategies for inner retinal neurons such as RGCs is more complicated, largely due to their more elaborate nature and need to extend long axonal projections to form synaptic connections in the optic tectum [153,154,155]. As such, pharmacologic strategies to combat RGC degeneration have focused on early stages of the disease process where neuroprotection is feasible [63, 106]. The goal is both to improve RGC survival, as well as potentially regrow axons to reestablish central synaptic connections. Similar efforts have not been widely adopted yet for hPSC-derived RGCs, although early studies have demonstrated the ability of hPSC-derived RGCs to survive following intravitreal transplantation [80]. Further studies into the use of hPSC-derived RGCs are certainly warranted, as several recent reports have demonstrated the differentiation and enrichment of RGCs from hPSCs in vitro [63, 66, 67, 80, 82].
Efforts for cell replacement to date have often focused on the transplantation of a single type of retinal neuron. At late stages of retinal degeneration, other retinal neurons are often damaged and lost, leading to the need to replace multiple types of cells. Retinal organoids represent an exciting option for cell replacement at these late stages of retinal degeneration, as these organoids possess the relevant retinal cells pre-assembled into a stratified structure, and can serve as “mini-retinas” for replacement of retinal tissue [21, 23, 28,29,30, 32, 39, 63]. Early attempts at these strategies have recently been demonstrated in mouse models of retinitis pigmentosa, where retinal organoids were transplanted and retained transplants of retinal organoids in mice led to the retention of their three-dimensional architecture and formed presumptive synaptic connections with host bipolar cells [142]. Similar experiments have also been conducted in nonhuman primates, with the transplantation of hPSC-derived retinal organoids resulting in increased visual acuity [156].
2.8 Conclusions and Future Directions
Overall, research over the past several years has established hPSCs as a powerful tool for studying some of the earliest stages of human development that would otherwise remain inaccessible to investigation [19, 22, 23, 25, 27, 37]. This has encouraged the establishment of efficient differentiation protocols to generate all major cell types of the retina, including photoreceptors, RPE, and retinal ganglion cells [9, 20, 21, 30, 53, 55, 58, 61, 63, 70, 78, 81, 82, 113, 157, 158]. These hPSC-derived retinal cells have assisted in modeling retinal degenerative diseases, especially when generated from patients with inherited retinal dystrophies. For this purpose, patient-derived hPSCs have helped in understanding disease progression and mechanisms, and have subsequently enabled the identification of candidate neuroprotective factors to combat the degeneration of retinal neurons [11, 21, 39, 56, 57, 63, 76, 84, 98, 100, 103, 106, 115, 116, 128, 148]. However, these measures have limited utility at late-stage disease, where the loss of multiple retinal cell types is irreversible, resulting in severe loss of vision. As a source of cell replacement therapies, hPSC-derived retinal cells have been shown to integrate within the host retina, form synaptic connections as well as demonstrate functional rescue. Such strategies have been extensively studied in the context of RPE and photoreceptor degeneration [6, 7, 10, 39, 136, 141, 142, 149, 156, 159,160,161], and is finding application in current clinical trials in AMD and Stargardt’s disease using hPSC-derived RPE [14, 152].
While tremendous progress has been made in the differentiation of retinal neurons from hPSCs [17, 19, 20, 22, 26, 53, 63, 69, 71, 81, 162], these cells often fail to fully differentiate into functionally relevant phenotypes which would better mimic the structure and functionality of the retina. Therefore, recent advances have led to the development of a three-dimensional approach to retinal differentiation, where hPSCs are directed to yield discrete populations closely analogous to the developing optic cup and eventually giving rise to a pseudostratified structure resembling the retina [21, 28,29,30,31, 33, 35,36,37,38,39]. With these advances, retinal organoids follow predicted stages of retinal development, and have led to enhanced differentiation and maturation of photoreceptors, facilitating the application of these approaches for studies of retinal development and pathogenesis in both normal and diseased states.
Patient-derived organoids may be best suited for assessing the effects of disease-related neurodegeneration on specific retinal cell types, as well as their interactions with each other. Currently, retinal organoids are likely better suited for studies of photoreceptor diseases, as photoreceptor development and maturation has been extensively characterized in retinal organoids, leading to rod-dominant retinal domains similar to peripheral regions of the retina [29, 30, 35, 38, 39]. Therefore, rod-cone dystrophies like retinitis pigmentosa, which begins as a peripheral retinal degeneration, can be most effectively modeled with retinal organoids, with the goal of developing neuroprotective strategies. Moreover, future efforts to characterize inner retinal neurons within retinal organoids will help to model and develop therapies for RGC degeneration in optic neuropathies. In addition to studies of retinal development and disease, the most exciting feature of retinal organoids may be their ability to serve as a replacement for retinal tissue in severely degenerated retinas. The interconnected structure composed of multiple retinal neurons may facilitate integration and replacement of multiple cell types within the degenerated retina.
References
Barnstable, C. J. (1987). A molecular view of vertebrate retinal development. Molecular Neurobiology, 1, 9–46.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872. https://doi.org/10.1016/j.cell.2007.11.019
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. https://doi.org/10.1016/j.cell.2006.07.024
Yu, J., Vodyanik, M.A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J.L., Tian, S., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920. https://doi.org/10.1126/science.1151526
Burnight, E. R., Wiley, L.A., Drack, A.V., Braun, T.A., Anfinson, K.R., Kaalberg, E.E., et al. (2014). CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Therapy, 21, 662–672. https://doi.org/10.1186/1756-6606-7-45
Carr, A. J., Vugler, A.A., Hikita, S.T., Lawrence, J.M., Gias, C., Chen, L.L., et al. (2009). Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One, 4, e8152. https://doi.org/10.1371/journal.pone.0008152
Gonzalez-Cordero, A., Kruczek, K., Naeem, A., Fernando, M., Kloc, M., Ribeiro, J., et al. (2017). Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors. Stem Cell Reports, 9(3), 820–837. https://doi.org/10.1016/j.stemcr.2017.07.022
Lamba, D. A., McUsic, A., Hirata, R. K., Wang, P. R., Russell, D., & Reh, T. A. (2010). Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One, 5, e8763. https://doi.org/10.1371/journal.pone.0008763
Li, Y., Tsai, Y.T., Hsu, C.W., Erol, D., Yang, J., Wu, W.H., et al. (2012). Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Molecular Medicine, 18, 1312–1319. https://doi.org/10.2119/molmed.2012.00242
Lukovic, D., Artero Castro, A., Delgado, A.B., Bernal Mde, L., Luna Pelaez, N., Diez Lloret, A., et al. (2015). Human iPSC derived disease model of MERTK-associated retinitis pigmentosa. Scientific Reports, 5, 12910. https://doi.org/10.1016/j.trsl.2015.08.007
Pearson, R. A. (2014). Advances in repairing the degenerate retina by rod photoreceptor transplantation. Biotechnology Advances, 32, 485–491. https://doi.org/10.1016/j.biotechadv.2014.01.001
Rowland, T. J., Buchholz, D. E., & Clegg, D. O. (2012). Pluripotent human stem cells for the treatment of retinal disease. Journal of Cellular Physiology, 227, 457–466. https://doi.org/10.1002/jcp.22814
Schwartz, S. D., Hubschman, J.P., Heilwell, G., Franco-Cardenas, V., Pan, C.K., Ostrick, R.M., et al. (2012). Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet, 379, 713–720. https://doi.org/10.1016/s0140-6736(12)60028-2
Tsai, Y., Lu, B., Bakondi, B., Girman, S., Sahablan, A., Sareen, D., et al. (2015). Human iPSC-derived neural progenitors preserve vision in an amd-like model. Stem Cells (Dayton, OH), 33, 2537–2549. https://doi.org/10.1002/stem.2032
Banin, E., Obolensky, A., Idelson, M., Hemo, I., Reinhardtz, E., Pikarsky, E., et al. (2006). Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem cells. Stem Cells (Dayton, OH), 24, 246–257. https://doi.org/10.1634/stemcells.2005-0009
Hirami, Y., Osakada, F., Takahashi, K., Okita, K., Yamanaka, S., Ikeda, H., et al. (2009). Generation of retinal cells from mouse and human induced pluripotent stem cells. Neuroscience Letters, 458, 126–131. https://doi.org/10.1016/j.neulet.2009.04.035
Ikeda, H., Osakada, F., Watanabe, K., Mizuseki, K., Haraguchi, T., Miyoshi, H., et al. (2005). Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 102, 11331–11336. https://doi.org/10.1073/pnas.0910012107
Lamba, D. A., Karl, M. O., Ware, C. B., & Reh, T. A. (2006). Efficient generation of retinal progenitor cells from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 103, 12769–12774. https://doi.org/10.1073/pnas.0601990103
Mellough, C. B., Sernagor, E., Moreno-Gimeno, I., Steel, D. H., & Lako, M. (2012). Efficient stage specific differentiation of human pluripotent stem cells towards retinal photoreceptor cells. Stem Cells (Dayton, OH). https://doi.org/10.1002/stem.1037
Meyer, J. S., Howden, S. E., Wallace, K. A., Verhoeven, A. D., Wright, L. S., Capowski, E. E., et al. (2011). Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells (Dayton, OH), 29, 1206–1218. https://doi.org/10.1002/stem.674
Meyer, J. S., Shearer, R. L., Capowski, E. E., Wright, L. S., Wallace, K. A., McMillan, E. L., et al. (2009). Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America, 106, 16698–16703. https://doi.org/10.1073/pnas.0905245106
Ohlemacher, S. K., Iglesias, C. L., Sridhar, A., Gamm, D. M., & Meyer, J. S. (2015). Generation of highly enriched populations of optic vesicle-like retinal cells from human pluripotent stem cells. Current Protocols in Stem Cell Biology, 32, 1H 8 1–1H 8 20. https://doi.org/10.1002/9780470151808.sc01h08s32
Osakada, F., Ikeda, H., Mandai, M., Wataya, T., Watanabe, K., Yoshimura, N., et al. (2008). Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nature Biotechnology, 26, 215–224. https://doi.org/10.1038/nbt1384
Osakada, F., Ikeda, H., Sasai, Y., & Takahashi, M. (2009). Stepwise differentiation of pluripotent stem cells into retinal cells. Nature Protocols, 4, 811–824. https://doi.org/10.1038/nprot.2009.51
Sridhar, A., Ohlemacher, S. K., Langer, K. B., & Meyer, J. S. (2016). Robust differentiation of mRNA-reprogrammed human induced pluripotent stem cells toward a retinal lineage. Stem Cells Translational Medicine, 5, 417–426. https://doi.org/10.5966/sctm.2015-0093
Sridhar, A., Steward, M. M., & Meyer, J. S. (2013). Nonxenogeneic growth and retinal differentiation of human induced pluripotent stem cells. Stem Cells Translational Medicine, 2, 255–264. https://doi.org/10.5966/sctm.2012-0101
Nakano, T., Ando, S., Takata, N., Kawada, M., Muguruma, K., Sekiguchi, K., et al. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell, 10, 771–785. https://doi.org/10.1016/j.stem.2012.05.009
Phillips, M. J., Wallace, K. A., Dickerson, S. J., Miller, M. J., Verhoeven, A. D., Martin, J. M., et al. (2012). Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Investigative Ophthalmology & Visual Science, 53, 2007–2019. https://doi.org/10.1167/iovs.11-9313
Zhong, X., Gutierrez, C., Xue, T., Hampton, C., Vergara, M. N., Cao, L. H., et al. (2014). Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications, 5, 4047. https://doi.org/10.1038/ncomms5047
Kaewkhaw, R., Kaya, K. D., Brooks, M., Homma, K., Zou, J., Chaitankar, V., et al. (2015). Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and gene networks. Stem Cells (Dayton, OH), 33, 3504–3518. https://doi.org/10.1002/stem.2122
Kuwahara, A., Ozone, C., Nakano, T., Saito, K., Eiraku, M., & Sasai, Y. (2015). Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nature Communications, 6, 6286. https://doi.org/10.1038/ncomms7286
Lowe, A., Harris, R., Bhansali, P., Cvekl, A., & Liu, W. (2016). Intercellular adhesion-dependent cell survival and ROCK-regulated actomyosin-driven forces mediate self-formation of a retinal organoid. Stem Cell Reports, 6, 743–756. https://doi.org/10.1016/j.stemcr.2016.03.011
Mellough, C. B., Collin, J., Khazim, M., White, K., Sernagor, E., Steel, D. H., et al. (2015). IGF-1 signaling plays an important role in the formation of three-dimensional laminated neural retina and other ocular structures from human embryonic stem cells. Stem Cells (Dayton, OH), 33, 2416–2430. https://doi.org/10.1002/stem.2023
Reichman, S., Slembrouck, A., Gagliardi, G., Chaffiol, A., Terray, A., Nanteau, C., et al. (2017). Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells (Dayton, OH). https://doi.org/10.1002/stem.2586
Singh, R. K., Mallela, R. K., Cornuet, P. K., Reifler, A. N., Chervenak, A. P., West, M. D., et al. (2015). Characterization of three-dimensional retinal tissue derived from human embryonic stem cells in adherent monolayer cultures. Stem Cells and Development, 24, 2778–2795. https://doi.org/10.1089/scd.2015.0144
Volkner, M., Zschatzsch, M., Rostovskaya, M., Overall, R. W., Busskamp, V., Anastassiadis, K., et al. (2016). Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Reports, 6, 525–538. https://doi.org/10.1016/j.stemcr.2016.03.001
Wahlin, K. J., Maruotti, J. A., Sripathi, S. R., Ball, J., Angueyra, J. M., Kim, C., et al. (2017). Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Scientific Reports, 7, 766. https://doi.org/10.1038/s41598-017-00774-9
Wiley, L. A., Burnight, E. R., DeLuca, A. P., Anfinson, K. R., Cranston, C. M., Kaalberg, E. E., et al. (2016). cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Scientific Reports, 6, 30742. https://doi.org/10.1038/srep30742
Chow, R. L., & Lang, R. A. (2001). Early eye development in vertebrates. Annual Review of Cell and Developmental Biology, 17, 255–296. https://doi.org/10.1146/annurev.cellbio.17.1.255
Fuhrmann, S. (2010). Eye morphogenesis and patterning of the optic vesicle. Current Topics in Developmental Biology, 93, 61–84. https://doi.org/10.1016/b978-0-12-385044-7.00003-5
Kolb, H., Nelson, R., Ahnelt, P., & Cuenca, N. (2001). Cellular organization of the vertebrate retina. Progress in Brain Research, 131, 3–26.
Neves, G., & Lagnado, L. (1999). The retina. Current Biology, 9, R674–R677.
Dowling, J. E., & Werblin, F. S. (1971). Synaptic organization of the vertebrate retina. Vision Research, 3, 1–15.
Reynolds, J., & Lamba, D. A. (2014). Human embryonic stem cell applications for retinal degenerations. Experimental Eye Research, 123, 151–160. https://doi.org/10.1016/j.exer.2013.07.010
Lamb, T. D., Collin, S. P., & Pugh, E. N. (2007). Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nature Reviews. Neuroscience, 8, 960–976. https://doi.org/10.1038/nrn2283
Andreazzoli, M. (2009). Molecular regulation of vertebrate retina cell fate. Birth Defects Research. Part C, Embryo Today, 87, 284–295. https://doi.org/10.1002/bdrc.20161
Cepko, C. L., Austin, C. P., Yang, X., Alexiades, M., & Ezzeddine, D. (1996). Cell fate determination in the vertebrate retina. Proceedings of the National Academy of Sciences of the United States of America, 93, 589–595.
Livesey, F. J., & Cepko, C. L. (2001). Vertebrate neural cell-fate determination: Lessons from the retina. Nature Reviews. Neuroscience, 2, 109–118. https://doi.org/10.1038/35053522
Zaghloul, N. A., Yan, B., & Moody, S. A. (2005). Step-wise specification of retinal stem cells during normal embryogenesis. Biology of the Cell, 97, 321–337. https://doi.org/10.1042/bc20040521
Klimanskaya, I., Hipp, J., Rezai, K. A., West, M., Atala, A., & Lanza, R. (2004). Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning and Stem Cells, 6, 217–245. https://doi.org/10.1089/clo.2004.6.217
Carr, A. J., Vugler, A., Lawrence, J., Chen, L. L., Ahmado, A., Chen, F. K., et al. (2009). Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Molecular Vision, 15, 283–295. https://doi.org/10.5966/sctm.2012-0163
Idelson, M., Alper, R., Obolensky, A., Ben-Shushan, E., Hemo, I., Yachimovich-Cohen, N., et al. (2009). Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell, 5, 396–408. https://doi.org/10.1016/j.stem.2009.07.002
Buchholz, D. E., Hikita, S. T., Rowland, T. J., Friedrich, A. M., Hinman, C. R., Johnson, L. V., et al. (2009). Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells (Dayton, OH), 27, 2427–2434. https://doi.org/10.1002/stem.189
Singh, R., Phillips, M. J., Kuai, D., Meyer, J., Markin J. M., Smith, M. A., et al. (2013). Functional analysis of serially expanded human iPS cell-derived RPE cultures. Investigative Ophthalmology & Visual Science, 54, 6767–6778. https://doi.org/10.1093/hmg/dds469
Singh, R., Shen, W., Kuai, D., Martin, J. M., Guo, X., Smith, M. A., et al. (2013). iPS cell modeling of best disease: Insights into the pathophysiology of an inherited macular degeneration. Human Molecular Genetics, 22, 593–607. https://doi.org/10.1016/j.stem.2016.03.021
Tucker, B. A., Mullins, R. F., Streb, L. M., Anfinson, K., Eyestone, M. E., Kaalberg, E., et al. (2013). Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. eLife, e00824, 2. https://doi.org/10.1038/mt.2014.100
Riazifar, H., Jia, Y., Chen, J., Lynch, G., & Huang, T. (2014). Chemically induced specification of retinal ganglion cells from human embryonic and induced pluripotent stem cells. Stem Cells Translational Medicine, 3, 424–432. https://doi.org/10.5966/sctm.2013-0147
Ferrer, M., Corneo, B., Davis, J., Wan, Q., Miyagishima, K. J., King, R., et al. (2014). A multiplex high-throughput gene expression assay to simultaneously detect disease and functional markers in induced pluripotent stem cell-derived retinal pigment epithelium. Stem Cells Translational Medicine, 3, 911–922. https://doi.org/10.1167/iovs.11-9313
Maruotti, J., Sripathi, S. R., Bharti, K., Fuller, J., Wahlin, K. J., Ranganathan, V., et al. (2015). Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America, 112, 10950–10955. https://doi.org/10.1073/pnas.1422818112
Zhou, S., Flamier, A., Abdouh, M., Tetreault, N., Barabino, A., Wadhwa, S., et al. (2015). Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFbeta and Wnt signaling. Development, 142, 3294–3306. https://doi.org/10.1242/dev.125385
Sluch, V. M., Davis, C. H., Ranganathan, V., Kerr, J. M., Krick, K., Martin, R., et al. (2015). Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Scientific Reports, 5, 16595. https://doi.org/10.1038/srep16595
Ohlemacher, S. K., Sridhar, A., Xiao, Y., Hochstetler, A. E., Sarfarazi, M., Cummins, T. R., et al. (2016). Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells (Dayton, OH). https://doi.org/10.1002/stem.2356
Barnea-Cramer, A. O., Wang, W., Lu, S. J., Singh, M. S., Luo, C., Huo, H., et al. (2016). Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Scientific Reports, 6, 29784.
Gill, K. P., Hung, S. S., Sharov, A., Lo, C. Y., Needham, K., Lidgerwood, G. E., et al. (2016). Enriched retinal ganglion cells derived from human embryonic stem cells. Scientific Reports, 6, 30552. https://doi.org/10.1038/srep30552
Sluch, V. M., Chamling, X., Liu, M. M., Berlinicke, C. A., Cheng, J., Mitchell, K. L., et al. (2017). Enhanced stem cell differentiation and immunopurification of genome engineered human retinal ganglion cells. Stem Cells Translational Medicine, 6, 1972–1986. https://doi.org/10.1002/sctm.17-0059
Langer, K. B., Ohlemacher, S. K., Phillips, M. J., Fligor, C. M., Jiang, P., Gamm, D. M., et al. (2018). Retinal ganglion cell diversity and subtype specification from human pluripotent stem cells. Stem Cell Reports, 10, 1282–1293. https://doi.org/10.1016/j.stemcr.2018.02.010
Reh, T. A., Lamba, D., & Gust, J. (2010). Directing human embryonic stem cells to a retinal fate. Methods in Molecular Biology, 636, 139–153. https://doi.org/10.1007/978-1-60761-691-7_9
Osakada, F., Jin, Z. B., Hirami, Y., Ikeda, H., Danjyo, T., Watanabe, K., et al. (2009). In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. Journal of Cell Science, 122, 3169–3179. https://doi.org/10.1242/jcs.050393
Bharti, K., Miller, S. S., & Arnheiter, H. (2011). The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell & Melanoma Research, 24, 21–34. https://doi.org/10.1111/j.1755-148X.2010.00772.x
Buchholz, D. E., Pennington, B. O., Croze, R. H., Hinman, C. R., Coffey, P. J., & Clegg, D. O. (2013). Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Translational Medicine, 2, 384–393. https://doi.org/10.1038/nbt.3070
Capowski, E. E., Simonett, J. M., Clark, E. M., Wright, L. S., Howden S. E., Wallace, K. A., et al. (2014). Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Human Molecular Genetics, 23, 6332–6344. https://doi.org/10.1007/978-1-4614-3209-8_20
Clarke, L., Ballios, B. G., & van der Kooy, D. (2012). Generation and clonal isolation of retinal stem cells from human embryonic stem cells. The European Journal of Neuroscience, 36, 1951–1959. https://doi.org/10.1111/j.1460-9568.2012.08123.x
Maeda, T., Lee, M. J., Palczewska, G., Marsilli, S., Tesar, P. J., Palczewski, K., et al. (2013). Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. The Journal of Biological Chemistry, 288, 34484–34493. https://doi.org/10.5966/sctm.2014-0038
Okamoto, S., & Takahashi, M. (2011). Induction of retinal pigment epithelial cells from monkey iPS cells. Investigative Ophthalmology & Visual Science, 52, 8785–8790. https://doi.org/10.1167/iovs.11-8129
Singh, R., Kuai, D., Guziewicz, K. E., Meyer, J., Wilson M., Lu, J., et al. (2015). Pharmacological modulation of photoreceptor outer segment degradation in a human iPS cell model of inherited macular degeneration. Molecular Therapy, 23, 1700–1711. https://doi.org/10.1093/hmg/ddu053
Vugler, A., Carr, A. J., Lawrence, J., Chen, L. L., Burrell, K., Wright A., et al. (2008). Elucidating the phenomenon of HESC-derived RPE: Anatomy of cell genesis, expansion and retinal transplantation. Experimental Neurology, 214, 347–361. https://doi.org/10.1016/j.expneurol.2008.09.007
Boucherie, C., Sowden, J. C., & Ali, R. R. (2011). Induced pluripotent stem cell technology for generating photoreceptors. Regenerative Medicine, 6, 469–479. https://doi.org/10.2217/rme.11.37
Deng, F., Chen, M., Liu, Y., Hu, H., Xiong, Y., Xu, C., et al. (2016). Stage-specific differentiation of iPSCs toward retinal ganglion cell lineage. Molecular Vision, 22, 536–547.
Li, K., Zhong, X., Yang, S., Luo, S., Li, K., Liu, Y., et al. (2017). HiPSC-derived retinal ganglion cells grow dendritic arbors and functional axons on a tissue-engineered scaffold. Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2017.02.032
Tanaka, T., Yokoi, T., Tamalu, F., Watanabe, S., Nishina, S., & Azuma, N. (2015). Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Scientific Reports, 5, 8344. https://doi.org/10.1038/srep08344
Teotia, P., Chopra, D. A., Dravid, S. M., Van Hook, M. J., Qiu, F., Morrison, J., et al. (2017). Generation of functional human retinal ganglion cells with target specificity from pluripotent stem cells by chemically defined recapitulation of developmental mechanism. Stem Cells (Dayton, OH), 35, 572–585. https://doi.org/10.1002/stem.2513
Jin, Z. B., & Takahashi, M. (2012). Generation of retinal cells from pluripotent stem cells. Progress in Brain Research, 201, 171–181. https://doi.org/10.1016/b978-0-444-59544-7.00008-1
Parfitt, D. A., Lane, A., Ramsden C. M., Carr, A. J., Munro, P. M., Jovanovic, K., et al. (2016). Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell, 18, 769–781.
Wiley, L. A., Burnight, E. R., Songstad, A. E., Drack, A. V., Mullins, R. F., Stone, E. M., et al. (2015). Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases. Progress in Retinal and Eye Research, 44, 15–35. https://doi.org/10.1016/j.preteyeres.2014.10.002
Congdon, N., O'Colmain, B., Klaver, C. C., Klein, R., Muñoz, B., Friedman, D. S., et al. (2004). Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology, 122, 477–485. https://doi.org/10.1001/archopht.122.4.477
Veleri, S., Lazar, C. H., Chang, B., Sieving, P. A., Banin, E., & Swaroop, A. (2015). Biology and therapy of inherited retinal degenerative disease: Insights from mouse models. Disease Models & Mechanisms, 8, 109–129. https://doi.org/10.1242/dmm.017913
Fletcher, E. L., Jobling, A. I., Greferath, U., Mills, S. A., Waugh M., Ho, T., et al. (2014). Studying age-related macular degeneration using animal models. Optometry and Vision Science, 91, 878–886. https://doi.org/10.1097/opx.0000000000000322
Fletcher, E. L., Jobling, A. I., Vessey, K. A., Luu, C., Guymer, R. H., & Baird, P. N. (2011). Animal models of retinal disease. Progress in Molecular Biology and Translational Science, 100, 211–286. https://doi.org/10.1016/b978-0-12-384878-9.00006-6
Iglesias, A. I., Springelkamp, H., Ramdas, W. D., Klaver, C. C., Willemsen, R., & van Duijn, C. M. (2015). Genes, pathways, and animal models in primary open-angle glaucoma. Eye (London, England), 29, 1285–1298. https://doi.org/10.1038/eye.2015.160
Jones, M. K., Lu, B., Girman, S., & Wang, S. (2017). Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Progress in Retinal and Eye Research. https://doi.org/10.1016/j.preteyeres.2017.01.004
Kostic, C., & Arsenijevic, Y. (2016). Animal modelling for inherited central vision loss. The Journal of Pathology, 238, 300–310. https://doi.org/10.1002/path.4641
Niwa, M., Aoki, H., Hirata, A., Tomita, H., Green, P. G., & Hara, A. (2016). Retinal cell degeneration in animal models. International Journal of Molecular Sciences, 17. https://doi.org/10.3390/ijms17010110
Pennesi, M. E., Neuringer, M., & Courtney, R. J. (2012). Animal models of age related macular degeneration. Molecular Aspects of Medicine, 33, 487–509. https://doi.org/10.1016/j.mam.2012.06.003
Jin, Z. B., Okamoto, S., Osakada, F., Homma, K., Assawachananont, J., Hirami, Y., et al. (2011). Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One, 6, e17084. https://doi.org/10.1371/journal.pone.0017084
Tucker, B. A., Scheetz, T. E., Mullins, R. F., DeLuca, A. P., Hoffmann, J. M., Johnston, R. M., et al. (2011). Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America, 108, E569–E576. https://doi.org/10.1073/pnas.1108918108
Minegishi, Y., Iejima, D., Kobayashi, H., Chi, Z. L., Kawase, K., Yamamoto T., et al. (2013). Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Human Molecular Genetics, 22, 3559–3567. https://doi.org/10.1093/hmg/ddt210
Lustremant, C., Habeler, W., Plancheron, A., Goureau, O., Grenot, L., de la Grange, P., et al. (2013). Human induced pluripotent stem cells as a tool to model a form of Leber congenital amaurosis. Cellular Reprogramming, 15, 233–246. https://doi.org/10.1089/cell.2012.0076
Tucker, B. A., Solivan-Timpe, F., Roos, B. R., Anfinson, K. R., Robin, A. L., Wiley L. A., et al. (2014). Duplication of TBK1 stimulates autophagy in iPSC-derived retinal cells from a patient with normal tension glaucoma. Journal of Stem Cell Research and Therapy, 3, 161. https://doi.org/10.4172/2157-7633.1000161
Yang, J., Li, Y., Chan, L., Tsai, Y. T., Wu, W. H., Nguyen, H. V., et al. (2014). Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Human Molecular Genetics, 23, 3445–3455.
Cereso, N., Pequignot, M. O., Robert, L., Becker, F., De Luca, V., Nabholz, N., et al. (2014). Proof of concept for AAV2/5-mediated gene therapy in iPSC-derived retinal pigment epithelium of a choroideremia patient. Molecular Therapy – Methods & Clinical Development, 1, 14011. https://doi.org/10.1038/mtm.2014.11
Yoshida, T., Ozawa, Y., Suzuki, K., Yuki, K., Ohyama, N., Akamatsu, W., et al. (2014). The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Molecular Brain, 7, 45.
Li, Y., Wu, W. H., Hsu, C. W., Nguyen, H. V., Tsai, Y. T., Chan, L., et al. (2014). Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Molecular Therapy, 22, 1688–1697. https://doi.org/10.1186/1756-6606-7-45
Schwarz, N., Carr, A. J., Lane, A., Moeller, F., Chen, L. L., Aguilà, M., et al. (2015). Translational read-through of the RP2 Arg120stop mutation in patient iPSC-derived retinal pigment epithelium cells. Human Molecular Genetics, 24, 972–986. https://doi.org/10.1371/journal.pone.0017084
Moshfegh, Y., Velez, G., Li, Y., Bassuk, A. G., Mahajan, V. B., & Tsang, S. H. (2016). BESTROPHIN1 mutations cause defective chloride conductance in patient stem cell-derived RPE. Human Molecular Genetics, 25, 2672–2680. https://doi.org/10.1093/hmg/ddw126
Chen, J., Riazifar, H., Guan, M. X., & Huang, T. (2016). Modeling autosomal dominant optic atrophy using induced pluripotent stem cells and identifying potential therapeutic targets. Stem Cell Research & Therapy, 7, 2. https://doi.org/10.1186/s13287-015-0264-1
Saini, J. S., Corneo, B., Miller, J. D., Kiehl, T. R., Qang, Q., Boles, N. C., et al. (2017). Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.12.015
Ramsden, C. M., Nommiste, B., R Lane, A., Carr, A. F., Powner, M. B., J K Smart, M., et al. (2017). Rescue of the MERTK phagocytic defect in a human iPSC disease model using translational read-through inducing drugs. Scientific Reports, 7, 51. https://doi.org/10.1038/s41598-017-00142-7
Hallam, D., Collin, J., Bojic, S., Chichagova, V., Buskin, A., Xu, Y., et al. (2017). An induced pluripotent stem cell patient specific model of complement factor H (Y402H) polymorphism displays characteristic features of age-related macular degeneration and indicates a beneficial role for UV light exposure. Stem Cells (Dayton, OH), 35, 2305–2320. https://doi.org/10.1002/stem.2708
Comyn, O., Lee, E., & MacLaren, R. E. (2010). Induced pluripotent stem cell therapies for retinal disease. Current Opinion in Neurology, 23, 4–9. https://doi.org/10.1097/WCO.0b013e3283352f96
Wu, S. M., & Hochedlinger, K. (2011). Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature Cell Biology, 13, 497–505. https://doi.org/10.1038/ncb0511-497
Chichagova, V., Hallam D., Collin, J., Buskin, A., Saretzki, G., Armstrong, L., et al. (2017). Human iPSC disease modelling reveals functional and structural defects in retinal pigment epithelial cells harbouring the m.3243A > G mitochondrial DNA mutation. Scientific Reports, 7, 12320. https://doi.org/10.1038/s41598-017-12396-2
Croze, R. H., & Clegg, D. O. (2014). Differentiation of pluripotent stem cells into retinal pigmented epithelium. Developments in Ophthalmology, 53, 81–96. https://doi.org/10.1159/000357361
Du, H., Lim, S. L., Grob, S., & Zhang, K. (2011). Induced pluripotent stem cell therapies for geographic atrophy of age-related macular degeneration. Seminars in Ophthalmology, 26, 216–224. https://doi.org/10.3109/08820538.2011.577498
Howden, S. E., Gore, A., Li, Z., Fung, H. L., Nisler, B. S., Nie, J., et al. (2011). Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proceedings of the National Academy of Sciences of the United States of America, 108, 6537–6542. https://doi.org/10.5966/sctm.2012-0163
Jin, Z. B., Okamoto, S., Xiang, P., & Takahashi, M. (2012). Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Translational Medicine, 1, 503–509. https://doi.org/10.2119/molmed.2012.00242
Leach, L. L., Buchholz, D. E., Nadar, V. P., Lowenstein, S. E., & Clegg, D. O. (2015). Canonical/beta-catenin Wnt pathway activation improves retinal pigmented epithelium derivation from human embryonic stem cells. Investigative Ophthalmology & Visual Science, 56, 1002–1013. https://doi.org/10.1167/iovs.14-15835
Phillips, M. J., Perez, E. T., Martin, J. M., Reshel S. T., Wallace, K. A., Capowski, E. E., et al. (2014). Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells (Dayton, OH), 32, 1480–1492. https://doi.org/10.1093/hmg/ddu351
Yvon, C., Ramsden, C. M., Lane, A., Powner, M. B., da Cruz, L., Coffey, P. J., et al. (2015). Using stem cells to model diseases of the outer retina. Computational and Structural Biotechnology Journal, 13, 382–389. https://doi.org/10.1016/j.csbj.2015.05.001
Cedrone, C., Mancino, R., Cerulli, A., Cesareo, M., & Nucci, C. (2008). Epidemiology of primary glaucoma: Prevalence, incidence, and blinding effects. Progress in Brain Research, 173, 3–14. https://doi.org/10.1016/s0079-6123(08)01101-1
Tham, Y. C., Li, X., Wong, T. Y., Quigley, H. A., Aung, T., & Cheng, C. Y. (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology, 121, 2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Maekawa, Y., Onishi, A., Matsushita, K., Koide, N., Mandai, M., Suzuma, K., et al. (2016). Optimized culture system to induce neurite outgrowth from retinal ganglion cells in three-dimensional retinal aggregates differentiated from mouse and human embryonic stem cells. Current Eye Research, 41, 558–568. https://doi.org/10.3109/02713683.2015.1038359
Bershteyn, M., Nowakowski, T. J., Pollen, A. A., Di Lullo, E., Nene, A., Wynshaw-Boris, A., et al. (2017). Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia cell. Stem Cell, 20, 435–449 e434. https://doi.org/10.1016/j.stem.2016.12.007
Qian, X., Nguyen, H. N., Jacob, F., Song, H., & Ming, G. L. (2017). Using brain organoids to understand Zika virus-induced microcephaly. Development, 144, 952–957.
Qian, X., Nguyen, H. N., Song, M. M., Hadiono, C., Ogden S. C., Hammack, C., et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell, 165, 1238–1254. https://doi.org/10.1242/dev.140707
Grskovic, M., Javaherian, A., Strulovici, B., & Daley, G. Q. (2011). Induced pluripotent stem cells—opportunities for disease modelling and drug discovery. Nature Reviews. Drug Discovery, 10, 915–929. https://doi.org/10.1038/nrd3577
Gunaseeli, I., Doss, M. X., Antzelevitch, C., Hescheler, J., & Sachinidis, A. (2010). Induced pluripotent stem cells as a model for accelerated patient- and disease-specific drug discovery. Current Medicinal Chemistry, 17, 759–766.
Wahlin, K. J., Maruotti, J., & Zack, D. J. (2014). Modeling retinal dystrophies using patient-derived induced pluripotent stem cells. Advances in Experimental Medicine and Biology, 801, 157–164. https://doi.org/10.1007/978-1-4614-3209-8_20
Wright, L. S., Phillips, M. J., Pinilla, I., Hei, D., & Gamm, D. M. (2014). Induced pluripotent stem cells as custom therapeutics for retinal repair: Progress and rationale. Experimental Eye Research, 123, 161–172. https://doi.org/10.1016/j.exer.2013.12.001
Chang, Y. C., Chang, W. C., Hung, K. H., Yang, D. M., Cheng, Y. H., Liao, Y. W., et al. (2014). The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Frontiers in Aging Neuroscience, 6, 191. https://doi.org/10.3389/fnagi.2014.00191
Kokkinaki, M., Sahibzada, N., & Golestaneh, N. (2011). Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells (Dayton, OH), 29, 825–835. https://doi.org/10.1002/stem.635
Santos-Ferreira, T. F., Borsch, O., & Ader, M. (2016). Rebuilding the missing part-A review on photoreceptor transplantation. Frontiers in Systems Neuroscience, 10, 105.
Streilein, J. W. (2003). Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. Journal of Leukocyte Biology, 74, 179–185. https://doi.org/10.1083/jcb.201006020
Sung, C. H., & Chuang, J. Z. (2010). The cell biology of vision. The Journal of Cell Biology, 190, 953–963.
Lamba, D. A., Gust, J., & Reh, T. A. (2009). Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell, 4, 73–79. https://doi.org/10.1016/j.stem.2008.10.015
Zhu, J., Cifuentes, H., Reynolds, J., & Lamba, D. A. (2017). Immunosuppression via loss of IL2rgamma enhances long-term functional integration of hESC-derived photoreceptors in the mouse retina cell. Stem Cell, 20(374–384), e375. https://doi.org/10.1016/j.stem.2016.11.019
Coles, B. L., Angénieux, B., Inoue, T., Del Rio-Tsonis, K., Spence, J. R., McInnes, R. R., et al. (2004). Facile isolation and the characterization of human retinal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 101, 15772–15777.
Czekaj, M., Haas, J., Gebhardt, M., Müller-Reichert, T., Humphries, P., Farrar, J., et al. (2012). In vitro expanded stem cells from the developing retina fail to generate photoreceptors but differentiate into myelinating oligodendrocytes. PLoS One, 7, e41798.
Klassen, H. J., Ng, T. F., Kurimoto, Y., Kirov, I., Shatos, M., Coffey, P., et al. (2004). Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Investigative Ophthalmology & Visual Science, 45, 4167–4173. https://doi.org/10.1167/iovs.04-0511
Eberle, D., Kurth, T., Santos-Ferreira, T., Wilson, J., Corbeil, D., & Ader, M. (2012). Outer segment formation of transplanted photoreceptor precursor cells. PLoS One, 7, e46305.
Lund, R. D., Wang, S., Klimanskaya, I., Holmes, T., Ramos-Kelsey R., Lu, B., et al. (2006). Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning and Stem Cells, 8, 189–199. https://doi.org/10.1089/clo.2006.8.189
Assawachananont, J., Mandai, M., Okamoto, S., Yamada, C. Eiraku, M., Yonemura, S., et al. (2014). Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports, 2, 662–674.
Chao, J. R., Lamba, D. A., Klesert, T. R., Torre, A., Hoshino, A., Taylor R. J., et al. (2017). Transplantation of human embryonic stem cell-derived retinal cells into the subretinal space of a non-human primate. Translational Vision Science & Technology, 6, 4. https://doi.org/10.1167/tvst.6.3.4
da Cruz, L., Chen, F. K., Ahmado, A., Greenwood, J., & Coffey, P. (2007). RPE transplantation and its role in retinal disease. Progress in Retinal and Eye Research, 26, 598–635. https://doi.org/10.1016/j.preteyeres.2007.07.001
Falkner-Radler, C. I., Krebs, I., Glittenberg, C., Povazay, B., Drexler, W., Graf, A., et al. (2011). Human retinal pigment epithelium (RPE) transplantation: Outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. The British Journal of Ophthalmology, 95, 370–375. https://doi.org/10.1136/bjo.2009.176305
Kamao, H., Mandai, M., Okamoto, S., Sakai, N., Suga, A., Sugita, S., et al. (2014). Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports, 2, 205–218. https://doi.org/10.5966/sctm.2014-0038
Lakowski, J., Gonzalez-Cordero, A., West, E. L., Han, Y. T., Welby E., Naeem, A., et al. (2015). Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells (Dayton, OH), 33, 2469–2482
Mandai, M., Watanabe, A., Kurimoto, Y., Hirami, Y., Morinaga, C., Daimon, T., et al. (2017). Autologous induced stem-cell-derived retinal cells for macular degeneration. The New England Journal of Medicine, 376, 1038–1046. https://doi.org/10.1056/NEJMoa1608368
Park, U. C., Cho, M. S., Park, J. H., Kim, S. J., Ku, S. Y., Choi, Y. M., et al. (2011). Subretinal transplantation of putative retinal pigment epithelial cells derived from human embryonic stem cells in rat retinal degeneration model. Clinical and Experimental Reproductive Medicine, 38, 216–221. https://doi.org/10.5653/cerm.2011.38.4.216
Petrus-Reurer, S., Bartuma, H., Aronsson, M., Westman, S., Lanner, F., & Kvanta, A. (2018). Subretinal transplantation of human embryonic stem cell derived-retinal pigment epithelial cells into a large-eyed model of geographic atrophy. Journal of Visualized Experiments. https://doi.org/10.3791/56702
Radtke, N. D., Aramant, R. B., Petry, H. M., Green, P. T., Pidwell, D. J., & Seiler, M. J. (2008). Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. American Journal of Ophthalmology, 146, 172–182. https://doi.org/10.1016/j.ajo.2008.04.009
Schwartz, S. D., Regillo, C. D., Lam, B. L., Eliott, D., Rosenfeld, P. J., Gregori, N. Z., et al. (2015). Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet, 385, 509–516. https://doi.org/10.1016/S0140-6736(14)61376-3
Cooke, J. A., & Meyer, J. S. (2015). Human pluripotent stem cell-derived retinal ganglion cells: Applications for the study and treatment of optic neuropathies. Current Ophthalmology Reports, 3, 200–206.
Lebrun-Julien, F., & Di Polo, A. (2008). Molecular and cell-based approaches for neuroprotection in glaucoma. Optometry and Vision Science, 85, 417–424. https://doi.org/10.1097/OPX.0b013e31817841f7
Sluch, V. M., & Zack, D. J. (2014). Stem cells, retinal ganglion cells and glaucoma. Developments in Ophthalmology, 53, 111–121.
Shirai, H., Mandai, M., Matsushita, K., Kuwahara, A., Yonemura, S., Nakano, T., et al. (2016). Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America, 113, E81–E90.
Boucherie, C., Mukherjee, S., Henckaerts, E., Thrasher, A. J., Sowden, J. C., & Ali, R. R. (2013). Brief report: Self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells (Dayton, OH), 31, 408–414. https://doi.org/10.1002/stem.1268
Viczian, A. S., Solessio, E. C., Lyou, Y., & Zuber, M. E. (2009). Generation of functional eyes from pluripotent cells. PLoS Biology, 7, e1000174. https://doi.org/10.1371/journal.pbio.1000174
Lu, B., Malcuit, C., Wang, S., Girman, S., Francis, P., Lemieux, L., et al. (2009). Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells (Dayton, OH), 27, 2126–2135. https://doi.org/10.1002/stem.149
MacLaren, R. E., Pearson, R. A., MacNeil, A., Douglas, R. H., Salt, T. E., Akimoto, M., et al. (2006). Retinal repair by transplantation of photoreceptor precursors. Nature, 444, 203–207. https://doi.org/10.1038/nature05161
Song, W. K., Park, K. M., Kim, H. J., Lee, J. H., Choi, J., Chong, S. Y., et al. (2015). Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Reports, 4, 860–872. https://doi.org/10.1038/srep12910
Rowland, T. J., Blaschke, A. J., Buchholz, D. E., Hikita, S. T., Johnson, L. V., & Clegg, D. O. (2013). Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. Journal of Tissue Engineering and Regenerative Medicine, 7, 642–653. https://doi.org/10.1002/term.1458
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sridhar, A. et al. (2018). Human Pluripotent Stem Cells as In Vitro Models for Retinal Development and Disease. In: Ballios, B., Young, M. (eds) Regenerative Medicine and Stem Cell Therapy for the Eye. Fundamental Biomedical Technologies. Springer, Cham. https://doi.org/10.1007/978-3-319-98080-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-98080-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98079-9
Online ISBN: 978-3-319-98080-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)