Abstract
Restless legs syndrome (RLS) is a common neurological condition defined by (1) an urge to move the legs, (2) worsening with inactivity, (3) improvement with movement, and (4) worsening at night. It effects women more than men and is most common in Northern Europeans. There is a strong genetic component, but other conditions including systemic iron deficiency, renal failure, and pregnancy also cause RLS symptoms. The main pathology is reduction in brain iron stores, even in the setting of normal serum iron measures. First-line treatments are dopamine agonists and alpha-2-delta blockers. Additional treatments include opioids and intravenous iron.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Case
This 56-year-old Caucasian woman was originally seen 15 years ago but now presents with worsening of restless leg syndrome (RLS). She first noticed a creeping and pulling sensation in both calves during pregnancies in her mid-20s. This seemed to resolve until about the age of 40 when she gradually began to have similar sensations that only occurred after 9 PM. This worsened over 4–5 years to the point where she experienced symptoms most nights, was developing sleep deprivation, and eventually sought medical care. Although she initially denied any family history of RLS, she later met several relatives at a family reunion with similar symptoms. At the initial visit, serum iron studies were normal, and she was placed on ropinirole 1 mg at night, which initially almost completely stopped all RLS symptoms. She returned a year later having increased the ropinirole to 2 mg secondary to breakthrough symptoms, with good results. She then switched care to her local primary care physician.
She now returns 10 years later, with markedly worsened RLS symptoms. They occur from about 11 AM until she finally falls asleep at 2 AM. Her entire legs and now arms are involved. In addition to the original creepy pulling sensation, she now has diffuse aching pain throughout her limbs. Over the last several years, she has increased the ropinirole to 4 mg in the evening and 4 mg at night. After each increase, she would usually demonstrate transient benefit for weeks to months but would then need to further increase the dose. On a couple of nights when she ran out of medicine, she did not sleep at all.
Examination shows an anxious-appearing woman who startles easily. When distracted, there were some arrhythmic stereotype movements in the legs. There was no evidence of neuropathy, and the remainder of her examination was normal. A sleep study showed delayed onset to sleep (2.5 h) and frequent periodic limb movements, some with awakening.
After long discussion about her RLS and the augmentation she is experiencing, we infused high-dose intravenous iron , then 3 weeks later quickly weaned off the ropinirole after starting low-dose methadone. Despite the iron and methadone, she still had a marked exacerbation of RLS for about four nights, including two with no sleep at all, but then rapidly improved. We stopped the methadone and started gabapentin enacarbil 600 mg at 6 PM. Her RLS is now largely controlled with occasional nocturnal breakthrough symptoms.
Discussion
The diagnosis of RLS relies entirely on the subjective report of (1) an urge to move the legs, which may or may not be associated with some other paresthesia; (2) worsening of symptoms with physical inactivity; (3) transient improvement of symptoms with physical activity; (4) worsening of symptoms in the evening and night, with improvement in the morning; and (5) the lack of a better explanation for symptoms. RLS can occur at any age, including children, and affects women somewhat more than men (1.5–2.5: 1). Diagnostic criteria in children are less clear, but there is a consistent association between RLS and attention deficit disorder. RLS is most commonly seen in people from Northern European ancestry, where the prevalence is about 15% of the population. In Asian countries the prevalence is much lower, typically 1–2%. There are currently 19 RLS genes, found mostly on GWAS studies, that confer risk for RLS, but none of them have very high penetrance, so it is not clinically indicated to assess for them.
The differential diagnosis for RLS includes akathisia associated with dopamine-blocking medications, which differs from RLS in that it affects the entire body rather than just the limbs and is not necessarily worse at night. Myalgia and other leg pain are also confused with RLS but can be differentiated from the lack of a true urge to move, even though repositioning may afford temporary relief. Neuropathic pain is typically superficial and worse in the feet, as opposed to RLS, which is usually deep and in the calves. True muscle cramps result in actual chaotic muscle contraction, which is not seen in RLS.
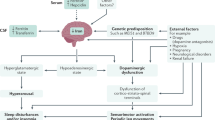
There are several medical conditions associated with RLS, including systemic iron deficiency , uremia, pregnancy, Parkinson’s disease, essential tremor, and probably neuropathy. Of these, uremia is the most robust, as RLS affects about 30% of patients on dialysis, often very severely. Dialysis itself does not improve RLS, but successful kidney transplantation almost immediately and robustly stops symptoms. RLS is seen in about 30% of pregnant women, typically in the last trimester, and resolves within days of delivery in most cases. This can be problematic since most treatments for RLS are not established as safe during pregnancy. RLS is frequently worsened by antihistamine medicines, especially sedating antihistamines, which more readily cross the blood-brain barrier. Dopamine-blocking agents and serotonergic reuptake inhibitors can also potentially worsen RLS.
Evaluation of a typical RLS patient can be fairly minimal. Everyone should have serum iron studies, including ferritin, iron binding percentage, and a complete blood count (CBC). It is important to note that a CBC does not screen for iron deficiency and ferritin may be normal or even high despite reduced iron stores. Other evaluations are only done if clinically indicated. A polysomnogram often demonstrates periodic limb movements of sleep (PLMS) , which are seen in approximately 90% of patients with RLS. Typically these are variations of a triple flexion response that occur every 5–90 s in stage 1 and 2 sleep. They can also occur during drowsiness. However, PLMS are not part of the diagnostic criteria for RLS. Many people have PLMS without RLS, as they are seen in normal aging, sleep apnea, and many neurodegenerative diseases. Therefore polysomnogram is usually reserved for people thought to have other concurrent sleep problems such as sleep apnea. Nerve conduction studies can be done if there is physical examination evidence of neuropathy.
The main pathology of RLS on autopsy studies is reduced brain iron, even in the setting of normal serum iron levels. Therefore, assessment of serum iron is only an indirect measure of what is thought to be truly associated with RLS. There is evidence for altered spinal cord activity, potentially mitigated by descending dopaminergic tracks. There is actually no evidence of overt dopamine deficiency, despite effective treatment with dopaminergic medications. There is evidence of increased dopamine turnover, suggesting increased activity of dopaminergic systems despite reduced functionality. Importantly, there is no evidence that RLS evolves into Parkinson’s disease, although RLS may occur ephemerally as one of many non-motor features of Parkinson’s disease.
Treatments for RLS are effective but not curative, so should be reserved for subjects whose symptoms are sufficient to justify chronic pharmacotherapy, most commonly when sleep deprivation occurs. First-line therapies include dopamine agonists (ropinirole, pramipexole, and rotigotine patch) and alpha-2-delta blockers (gabapentin enacarbil and pregabalin) (Table 76.1). Dopamine agonists work immediately and are especially effective for the pure urge to move and periodic limb movements. The oral drugs should be dosed 1–2 h before typical onset of symptoms and titrated to the lowest effective dose. Although initially very effective, dopaminergics can cause augmentation (earlier onset of symptoms, intensification of symptoms, spread to other parts of the body, and changed quality of symptoms) with chronic use (months to decades) [see case]. This can initially be treated with dose adjustments, usually earlier dosing, but only completely resolves with discontinuation of the dopaminergic, a very difficult process with marked rebound symptom exacerbation for up to 2 weeks, prior to improvement. Other possible side effects include sedation, edema, and impulse control disorders. Hallucinations and hypotension are rarely seen when these drugs are used for RLS.
Alpha-2-delta agonists improve RLS sensory symptoms to a similar degree as dopaminergics but do not improve PLMS as robustly. Unlike dopaminergics, they do improve sleep architecture, increasing deep slow wave sleep, and may improve painful symptoms. They have not been associated with augmentation but can cause sedation, “dizziness” edema, and weight gain.
Second-line therapies include Mu opioid drugs , especially low-dose methadone (5–20 mg/day), which has demonstrated little addiction or dependency in this population, and typically does not require any dose escalation over years. Oral iron is reasonable but poorly absorbed unless there is severe systemic iron deficiency, so not usually very effective. Iron salts are absorbed best on an empty stomach, avoiding other divalent metals such as with a multivitamin. Iron in heme (blood/meat) is the best absorbed form. High-dose intravenous iron , specifically iron dextran or ferric carboxymaltose, may also improve RLS, even in subjects with normal serum iron studies. The peak improvement is seen 4–6 weeks post infusion.
Suggested Reading
Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, Zucconi M, Ferri R, Trenkwalder C, Lee HB. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria-history, rationale, description, and significance. Sleep Med. 2014;15(8):860–73.
Early C, Kuwabara H, Wong D, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36:51–7.
Garcia-Borreguero D, Allen R, Silber M, Winkelman J, Högl B, Bainbridge J, Buchfuhrer M, Hadjigeorgiou G, Inoue Y, Manconi M, Ondo W, Winkelmann J. White paper: summary of recommendations for the prevention and treatment of RLS/WED augmentation: a combined task force of the IRLSSG, EURLSSG and RLS Foundation Sleep Med. 2016. (in press).
Roy M, de Zwaan M, Tuin I, et al. Association between restless legs syndrome and adult ADHD in a German community based sample. J Atten Disord. 2015; https://doi.org/10.1177/1087054714561291.
Silber MH, Becker PM, Earley C, Garcia-Borreguero D, Ondo WG. Willis-Ekbom disease foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin Proc. 2013;88(9):977–86.
Winkelman J, Allen R, Chaudhuri R, Ondo W, Trenkwalder C, Zee P, Zesiewicz T. Practice parameter: treatment of restless legs syndrome. Neurology. 2016. (in press).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ondo, W.G. (2019). Treatment of Restless Legs Syndrome and Periodic Limb Movements. In: Reich, S., Factor, S. (eds) Therapy of Movement Disorders. Current Clinical Neurology. Humana, Cham. https://doi.org/10.1007/978-3-319-97897-0_76
Download citation
DOI: https://doi.org/10.1007/978-3-319-97897-0_76
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-319-97896-3
Online ISBN: 978-3-319-97897-0
eBook Packages: MedicineMedicine (R0)