Abstract
Superior semicircular canal dehiscence syndrome (SCDS) is a clinical entity resulting in a myriad of audiological and vestibular symptoms. Pressure and/or sound-induced vertigo/nystagmus, autophony, conductive hearing loss, and conductive hyperacusis are commonly seen in patients with SCDS. The physiologic mechanism of this syndrome is thought to be due to the dehiscence creating a low-impedance outlet for fluid waves in the labyrinth, commonly referred to as a third window. This shunts flow from the cochlea to the labyrinth, which both activates the vestibular system and decreases pressure driving the traveling fluid wave in the cochlea. Diagnosis of SCDS can be difficult and requires both radiographic evidence of a dehiscence and clinical evidence supporting SCDS as the etiology for a patient’s symptoms. Testing for SCDS includes high-resolution CT imaging, audiogram, cervical and ocular VEMP testing, head impulse testing, and visualization of sound- or pressure-induced eye movements in the plane of the affected superior canal. Traditional surgical repair of SCDS is via the middle fossa approach. This approach allows for the dehiscence to be seen directly and for it to be both plugged and resurfaced. This approach is preferred in the vast majority of patients, but in certain situations a transmastoid approach can offer benefits over the middle fossa approach. Surgical outcomes for repair of SCDS are quite favorable, with patients having improvement of autophony, imbalance, and vertigo. Patients with predominately audiological symptoms have the highest likelihood of postoperative improvement. The majority of patients report an improvement in their quality of life postoperatively. Complications are rare but do occur in both the middle fossa and transmastoid approaches to SCDS repair. The most common complication is hearing loss, which is typically mild but can be profound in a low percentage of patients. The importance of proper patient selection and preoperative counseling on the risks of surgery cannot be overstated to ensure good surgical outcomes in SCDS repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Superior semicircular canal dehiscence
- Vertigo
- Conductive hearing loss
- Superior semicircular canal dehiscence syndrome
- SSCD
- SCDS
- Middle fossa craniotomy
Background
Superior canal dehiscence syndrome (SCDS) was first described by Minor in 1998 [1]. It is a disease characterized by the clinical findings of sound-induced vertigo and eye movements, chronic disequilibrium, conductive hearing loss (CHL), and decreased hearing thresholds for bone-conducted sounds. Conductive hyperacusis may lead to autophony (hearing their own voice) and pulsatile tinnitus or hearing their eye movements. The presence of a dehiscence creates a mobile third window within the labyrinth, leading to physiologic stimuli causing excitatory ampullofugal or inhibitory ampullopetal deflection of the cupula [1].
Symptoms caused by abnormal openings into the labyrinth have been known for decades. Fenestration of the semicircular canals was known to produce eye movements in response to sound in animals as early as 80 years ago [2]. The Tullio phenomenon, or eye movements in response to loud sound, was initially identified in humans suffering from advanced syphilis secondary to gummatous osteomyelitis and labyrinthine fistulae [3]. Subsequent reports have identified the Tullio phenomenon in perilymphatic fistula [4], head trauma [5], and cholesteatoma with semicircular canal erosion and fenestration [6]. The Hennebert sign (eye movement induced by pressure in the external auditory canal) is also often present in cases of abnormal openings into the labyrinth. These symptoms can be present in SCDS and helped to lead to the understanding of the constellation of symptoms encompassing this syndrome.
The exact mechanism for which SCDS causes its audiological and vestibular symptoms is still under investigation, but it is generally accepted that the dehiscence of the superior semicircular canal functions as a mobile “third window” in the bony labyrinth. This third window allows a low-impedance outlet for fluid waves in the labyrinth to shunt flow from the cochlea to the labyrinth, which both activates the vestibular system and decreases pressure driving the traveling fluid wave in the cochlea. Cadaveric and animal models have supported this, showing measurable fluid velocities across the dehiscence, a decrease in intracochlear pressures in the scala tympani and scala vestibuli, a decrease in cochlear differential pressure, and a decrease in round window velocity, most notably in lower frequencies [7,8,9].
Epidemiology
The anatomic prevalence of superior canal dehiscence within a temporal bone library consisting of 1000 specimens revealed a 0.5% prevalence of complete dehiscence of the superior canal into the middle fossa or superior petrosal sinus [10]. In 1.4% of specimens, the bone was 0.1 mm or thinner. The prevalence of SCDS is not known with certainty, but it is likely that only a subset of patients with SCD actually experience symptoms. Re et al. found a SCD prevalence rate of 5.8% on temporal bone CT in a series of 191 consecutive patients scanned for all causes. Individuals identified with SCD then underwent otoneurological examinations. Of those identified with SCD on CT imaging, only 0.5% had symptoms or signs consistent with SCDS [11].
The effect of dehiscence size on the clinical manifestation of SCDS is currently debated in the literature. Small case series have found dehiscences greater than or equal to 2.5 mm often present with both vestibular and cochlear symptoms, whereas those less than 2.5 mm often present with either vestibular or cochlear symptoms, but not both [12]. However, in multivariate analysis, the length of the dehiscence was only shown to correlate with the size of the air-bone gap [13]. Assessments of the surface area of SSCDs have shown that larger dehiscences are associated with larger cVEMP and oVEMP amplitudes [14] Cadaveric models of SSCD have shown that larger dehiscences decrease intracochlear pressure and decrease the cochlear drive at low frequencies. This effect seems to saturate around 3 mm in length. Paradoxically at higher frequencies, pinpoint dehiscences appear to cause a decrease in the cochlear drive, while larger dehiscences do not appear to effect at these frequencies [7, 15]. Cadaveric studies have also shown that the location of the SSCD along the arc of the canal does not have a major effect on intracochlear pressures. This is consistent with clinical studies showing that the location of the SCD did not correlate with the amount of hearing loss, although dehiscences located closer to the ampulla were found to be commonly seen in patients with auditory symptoms [16].
Diagnostic Evaluation
Patients with SCDS generally present with a primary complaint of dizziness, and when evaluating a patient with this complaint, a thorough history is the most effective diagnostic tool. Vertigo symptoms related to SCDS are usually induced by loud sound or pressure changes and are brief in duration. Dizziness or oscillopsia induced by loud sound are present in 90% of SCDS patients [17]. Vestibular symptoms induced by pressure changes such as coughing or straining are present in 73% of patients, with 67% exhibiting both pressure- and sound-related symptoms [17]. Chronic disequilibrium and cognitive impairment (“brain fog”) may also be attributed to SCDS.
In addition to dizziness, auditory symptoms are also a common feature of SCDS. Autophony, defined as the hyperperception of one’s own voice, breathing, or other internal sounds, is present to varying degrees in up to 60% of patients [17]. Hyperacusis for bone-conducted sound [18] is present in 52% of SCDS patients [17]. Hyperacusis symptoms include patients hearing their own pulse, eye movements, or the impact of the feet during walking. Patients with SCDS can occasionally hear in the affected ear a 512 Hz tuning fork placed against the foot or ankle [19]. Pulsatile tinnitus is present in about one-third of patients seen at our institution.
Evoked eye movements in the plane of the superior canal are the hallmark of SCDS [20]. The eyes should be examined under Frenzel lenses, infrared video goggles, or by some other means to eliminate the effect of visual fixation. Using an audiometer, pure tones at levels up to 110 dB nHL should be delivered in one ear at a time covering the frequency range of 125–4000 Hz. Sound-evoked eye movements at one or more frequencies were noted in 82% of SCDS patients using such stimuli [17]. Among our patient population, eye movements can also be induced with Valsalva maneuvers (34%) or pressure in the external auditory canal (23%).
Depending on the type of stimulus, either excitation or inhibition of the superior canal may occur as shown in Fig. 17.1. Valsalva against pinched nostrils, pressure in the external auditory canal (e.g., tragal compression), or sound will produce excitatory affects (ampullofugal deflection of cupula). Valsalva against a closed glottis, jugular venous compression, or negative external canal pressure will produce inhibitory secondary to ampullopetal cupula deflection. Pressure- or sound-evoked eye movements almost always occur in the plane of the superior canal as shown in Fig. 17.2. In the case of larger dehiscences, eye movements may be shifted out of the superior canal plane [21]. However if eye movements are not in this direction, the diagnosis of SCDS should be questioned, and alternative diagnoses of posterior canal dehiscence [22] or horizontal canal fistula [23] must be considered.
Route of excitatory and inhibitory pressure changes causing stimulation of the superior canal ampulla in SCDS. Superior canal excitation is caused by ampullofugal displacement of the cupula (green arrow) typically by positive external auditory canal pressure, nasal Valsalva, or sound. Superior canal inhibition is caused by ampullopetal displacement of the cupula (red arrow) from negative external auditory canal pressure or glottic Valsalva maneuver, which transiently increases intracranial pressure
Direction of the slow phase of eye movements with superior canal excitation. Eye movement occurs in the plane of the superior canal regardless of the direction of gaze. There are both vertical and torsional components when the patient is looking directly ahead (center gaze). The torsional and vertical components can be separated by having the patient look to the right or left during stimulation
The audiogram (Fig. 17.3) is an important part of the SCDS evaluation. A minority of patients have auditory symptoms in the absence of any vestibular signs or symptoms [17, 19, 24, 25]. Conductive hearing loss and bone conduction thresholds less than 0 dB nHL (conductive hyperacusis) are often greatest at lower frequencies [24, 25]. It is important to consider SCDS in patients with CHL and normal otologic exam, as case reports exist of SCDS being misdiagnosed as otosclerosis [19]. The key differences between SCDS and otosclerosis are (1) that conductive hyperacusis does not occur in otosclerosis and (2) that the acoustic stapedial reflex, which is often normal in superior canal dehiscence should be absent in an ear affected with otosclerosis.
Electrocochleography (ECochG) has been used in the past for diagnosing SCDS. Initial studies of ECochG showed elevated summating potential (SP) to action potential (AP) ratios >0.4 which were reported in all (n = 21) patients with unilateral SCDS, with normalization of the SP/AP ratio postoperatively [26]. More recent studies though have failed to reproduce the postoperative results [27].
Cervical vestibular-evoked myogenic potentials or cVEMPs are inhibitory electromyographic (EMG) signals measured over the contracted sternocleidomastoid muscle (SCM) ipsilateral to the ear being stimulated by multiple loud clicks or tone bursts (Fig. 17.4). It is thought that cVEMPs are activated through the stapes footplate to the saccule and vestibular nerve [28]. In SCDS, abnormally low thresholds and enlarged peak-to-peak amplitudes are demonstrated [4, 17]. The theory is that a dehiscent semicircular canal lowers the impedance of the vestibular system, resulting in a lower resistance for pressure and sound transmission [18, 19]. Thus, cVEMP signals are enhanced with lower thresholds in patients with SCDS. For air-conducted 500 Hz tone bursts, for example, we have found that cVEMP thresholds were 80–95 dB SPL for 13 patients with SCDS (83.85 ± 1.40 dB SPL, mean ± SD), 20–30 dB lower than in normal control subjects (110.25 ± 1.28 dB SPL) [29]. It has been argued that cVEMP is better with 90% sensitivity and specificity for SCD [30], while other series have found the sensitivity and specificity closer to 80% [31]. The cVEMP is not measurable in all patients and is especially likely to be absent in patients who have had previous middle ear surgery. The cVEMP threshold may also be decreased in other conditions such as enlarged vestibular aqueduct syndrome [32].
Typical cervical vestibular-evoked myogenic potential (cVEMP) results in a patient with right-sided SCDS and an intact left side. The cVEMP is initially measured with clicks at 95 dB nHL, and the stimulus amplitude is decreased until the response is no longer measurable. In the left ear, the patient has a cVEMP response at 95 dB but not with lower amplitude stimuli. In the right ear, the amplitude of the cVEMP is much larger at 95 dB, and the response continues to be detectable at amplitudes as small as 60 dB. Thus, in this example, the cVEMP threshold is 95 dB nHL on the left and 60 dB nHL on the right
Ocular VEMP (oVEMP) is also used for the diagnostic evaluation of suspected SCDS. An excitatory EMG response is obtained from the contralateral inferior oblique muscle with the pathway thought to be a result of utricular activation. We have demonstrated oVEMP results in response to air-conducted sound provide greater sensitivity and specificity than cVEMP for diagnosing SCDS [33]. In 29 patients with surgically confirmed SCDS, a peak-to-peak amplitude greater than 17.1 μV corresponded to 100% sensitivity and 98% specificity. The performance of oVEMP is also less time-consuming compared to cVEMP. oVEMPs may also be a good screening test for SCDS. In a prospective study, SCDS patients were more likely to have abnormal oVEMPs when compared to healthy controls [34].
For the diagnosis of SCD to be considered, imaging of the temporal bone using computed tomography (CT) must show the absence of bone over the superior canal. If the superior canal appears surrounded with bone on CT, the diagnosis of SCDS is effectively excluded; however, the appearance of a dehiscence on CT does not rule out thin bone covering the SC below the resolution of the scanner. Thus, CT is a highly sensitive test for SCD, but it is not specific [31].
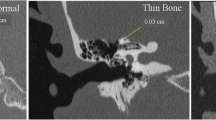
Optimal imaging uses high-resolution CT (HRCT) formatted in the plane of the superior canal [31, 35]. Unfortunately, the term “high-resolution” has been applied to a wide variety of CT scanning parameters which continue to change as technology is updated. In a review of temporal bone CT scans done in the general population, 9% of scans had apparent SCD with one observer calling as many as 12% [36]. Many of these are likely false dehiscences caused by the limits of resolving thin bone. In scans with greater than 1 mm thickness, thin structures are subjected to partial volume artifacts. Furthermore, with bone structures less than 0.1 mm thickness, volume artifacts can give the impression bone is absent thus leading to a higher rate of dehiscence [11].
A properly done scan should have a resolution near 0.2 mm. This requires attention to a number of parameters. The most important of these is slice thickness. Collimation of the x-ray beam to 0.5 mm allows the data to be represented by nearly isotropic voxels, so that the images can be reformatted in any plane without distortion.
Helical CT scanning, in which the table moves along the z-axis while the gantry rotates and scans, may lead to some loss of resolution. The “step, scan, and repeat” mode is preferred. The field of view used to reconstruct the images of the inner ear should be of the smallest size possible, so that the labyrinth is displayed to maximal resolution over the fixed size of the image matrix (usually 512 × 512 pixels). Image filters should be set for bone edge detection, as those filters producing less “noisy” images are likely to filter out a thin layer of bone that might remain over the canal. Images should be reconstructed in the plane of the superior canal as well as orthogonal to it so that any dehiscence can be definitively demonstrated (Fig. 17.5). Parallel (Pöschl position) and perpendicular (Stenver) reformatted planes can allow for more accurate assessment. In 1 study of 850 patients (1700 temporal bones), the prevalence of any semicircular canal dehiscence decreased from 7% to 2.5% when use of HRCT was combined with a semicircular canal evaluation whereby dehiscence was confirmed in two perpendicular planes [19]. However, even optimized scans are not without the risks of false-positive findings, so the diagnosis of SCD must never be based on a CT scan alone. The authors cannot stress enough that a finding of SCD on CT should be considered in the context of findings on physical exam, cVEMP or oVEMP, audiogram, and the patient’s symptoms before concluding that the patient has SCDS.
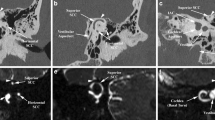
CT scan demonstrating SCD. Panel A: CT image is reformatted in the plane of the superior canal. An area of dehiscence between the superior canal and middle fossa is present. Panel B: Orthogonal reconstructions are performed at 3 degree intervals for 180° around the superior canal. These planes of reconstruction are shown as white lines. Panel C: An orthogonal reconstruction demonstrating SCD. The region of the reconstruction is shown in small view in the lower left
Differential Diagnosis
In assessing a patient with possible SCDS, it is important to consider other possible diagnoses, such as otosclerosis, Meniere’s disease, patulous Eustachian tube, perilymphatic fistula, and vertiginous migraine.
The CHL component of SCDS can appear similar to otosclerosis because both occur in adulthood in ears that appear normal on physical exam [19]. The audiograms differ in that SCDS patients often have conductive hyperacusis, and if there is no previous history of middle ear surgery, the acoustic reflex is often intact. Otosclerosis is also not associated with decreased cVEMP or oVEMP thresholds, vertigo symptoms, or CT findings of SCD.
Meniere’s disease is characterized by the triad of low-frequency hearing loss, vertigo, aural fullness, and tinnitus [37]. Although the hearing loss in Meniere’s disease is classically sensorineural hearing loss, CHL has also been described [38]. The attacks of vertigo associated with Meniere’s disease usually are severe and last hours with normal periods between attacks. The dizziness associated with SCDS can be chronic, but distinct vertigo attacks are often shorter and associated with exposure to noise or pressure changes.
Autophony is often the predominant symptom in patients with a patulous Eustachian tube [39], but it can also be the most disturbing symptom in SCDS. One distinguishing feature between the two conditions is that patients with patulous Eustachian tube typically have autophony for their own breath sounds, whereas patients with SCDS usually do not [39]. A history of vertigo symptoms and hyperacusis of bone-conducted sound is not typical of a patulous Eustachian tube. The audiogram, VEMP testing, and CT will typically differentiate a patulous Eustachian tube from SCDS.
A perilymphatic fistula, along with fenestrations of other semicircular canals, is often considered in the differential diagnosis of SCDS [40]. A perilymphatic fistula is a leak of perilymph within the vestibular labyrinth and generally is used to describe a fistula involving the round or oval window. The leak creates an abnormal compliance that allows fluid to move and stimulate the vestibular end organs in response to sound or pressure changes. The diagnosis of a perilymphatic fistula diagnosis should be considered in the context of a recent stapes surgery, temporal bone fracture, or barotrauma injury. In these cases acute vertigo is usually accompanied by a sensorineural hearing loss. A fistula in the horizontal canal can be acquired in cases of cholesteatoma or prior mastoidectomy [41]. Spontaneous perilymphatic fistula is a controversial diagnosis and should be considered as a diagnosis of exclusion [42].
One of the most common causes of spontaneous (non-positional) vertigo is migraine-associated vertigo and should be considered in the differential with SCDS [43]. The incidence of migraine is 17.6% of females and 5.7% of males [44], and approximately 25% of migraine patients report some vertigo [45]. Thus migraine is much more common than SCDS, and inevitably we have found some patients with radiographically apparent SCD whose symptoms were non-specific and better explained by migraine. Particularly challenging are those patients who have specific symptoms of both SCDS and migraine. For example, it may be difficult to determine if their sound sensitivity is due to one more than the other. Their chronic disequilibrium may be related to migraine, or it may be due to the constant transmission of intracranial pressure pulsations through the dehiscence to the labyrinth. Moreover, the physiological disturbances of the labyrinth caused by SCDS could serve as triggers to exacerbate migraine in susceptible individuals. However, the neurotologist must also consider that failure to recognize and treat coexistent migraine can lead to disappointing results in SCDS surgery.
Preoperative Decision-Making
The decision to undergo surgery for SCD plugging is often more difficult than settling on the diagnosis. The physician must help the patient weigh the severity of symptoms against the risks and benefits of surgery. In the authors’ institution, approximately 72% with SCDS opt to have surgical SC plugging, with the remaining patients opting to live with their symptoms or making lifestyle changes to avoid situations which exacerbate the symptoms like loud noise. This number may be a reflection of the referral pattern of patient seeking care at our institution.
The dizziness handicap inventory (DHI) [46] is an instrument which may be helpful in gauging vestibular symptom severity. This questionnaire grades dizziness symptoms on a scale from 0 to 100. It has previously been validated for surgical treatment of benign paroxysmal positional vertigo (BPPV) [47], acoustic neuroma surgery [48, 49], and ablative procedures for Meniere’s disease [50]. We measured the DHI in 19 patients with SCDS before they underwent SCD repair via a middle fossa approach. The average pre-op DHI score was 44 ± 24 (mean ± SD) [51]. This compares with the handicap caused by untreated primary benign paroxysmal positioning vertigo, in which the DHI score averaged 38.5 in one series [52], and with the handicap caused by active Meniere’s disease, in which the DHI score averaged 39.6 ± 21.1 in another series [53]. The comparisons indicate a high degree of dizziness handicap for SCDS patients who seek surgical treatment.
Auditory symptoms are the primary complaint in a significant number of SCDS patients [25]. Autophony or conductive hyperacusis can often be quite disabling, especially in patients for whom singing or speaking is important. There is no medical treatment for autophony symptoms due to SCDS, as the sound transmission is via bone, not the Eustachian tube. Thus, for SCDS patients who are significantly disturbed by autophony or conductive hyperacusis, surgery is the only option for relief.
CHL is a common symptom in SCDS [54]. It is often limited to low frequencies and usually only affects one ear, so many patients do not have a significant disability. In most patients, the CHL improves with surgery [54], and resolution of a large sensorineural hearing loss has even been reported [55]. However, plugging of SCD does carry a risk of hearing loss, and this risk is greater in patients who have had previous inner ear surgery, including stapes surgery [54]. In a retrospective review of 43 cases of SCDS who underwent repair via middle fossa approach with plugging, 25% developed a mild high-frequency hearing loss [56]. Long-term follow-up of 242 patients who have undergone repair at our institution shows 2.5% of patients ultimately developing a profound sensorineural hearing loss [57]. Patients should be carefully counseled on these risks, and those with hearing loss as their primary symptom of SCDS should strongly consider nonsurgical options such as a hearing aid.
As part of the preoperative decision-making process, it is important to provide patients counseling on their likely postoperative course and possible complications of SCD. Although dizziness symptoms are often the motivation for surgery, it is common for imbalance symptoms to be worse during the immediate postoperative period. At the author’s institution, all patients are evaluated by inpatient physical therapy postoperatively to determine if continued inpatient or outpatient therapy is required.
In the initial postoperative period, there are often decreased VOR gains in all ipsilateral canals. Whether this is due to labyrinthine inflammation or loss of perilymph is not clear [58]. This is typically transient in the horizontal and posterior canal, but plugging of the superior canal will cause a permanent vestibular sensory deficit due to the hydrodynamic insufficiency of the canal. This can be seen as decreased VOR in the superior canal plane (rotating the head to align the superior canal in the vertical orientation and quickly thrusting the patients head down in that vertical plan) [3]. However, patients can adapt very well to this single-canal insufficiency. Low-frequency, low-acceleration head movements will generate useful inhibitory signals from the contralateral posterior canal, and recent studies of video head impulse testing postoperatively have shown evidence of central compensation within 1 week [58]. Vestibular physical therapy can take advantage of the contralateral posterior canal’s function and of other gaze-stabilizing mechanisms in promoting compensation for the loss caused by SCD plugging. In our experience, the compensated state after SCD plugging allows the patient to lead a much more active lifestyle than did the SCDS condition.
Complications of SCD plugging are rare but can be serious. The most common complication is postoperative BPPV, which occurs in 4–24% of all patients [57, 59]. It is important to monitor for this in the early postoperative period, as it is easy to dismiss as normal vestibular hypofunction. A Dix-Hallpike maneuver looking at the posterior and horizontal canal should be performed in patients with abnormal bouts of vertigo with head positioning. As mentioned above, hearing loss is a real risk of SCD repair. As noted above, one-fourth of patients undergoing MFC repair at our institution developed mild high-frequency SNHL [56]. While fortunately, this hearing loss is mild, profound SNHL does occur in 2–3% of patients at our institution [57]. Surgery for SCD via a middle fossa approach shares the risk of perioperative complications common to any craniotomy [60]. Cerebrospinal fluid (CSF) leak may occur if the dura is violated, especially if air cells into the mastoid are exposed during surgery or if there is a tegmen dehiscence. Intracranial hematoma is a rare postoperative complication that can occur after any middle fossa surgery. In 220 primary middle fossa approaches at our institution, epidural hematoma occurred 1.4% of the time [57]. The patient’s mental status should be closely monitored during the acute postoperative period, and the onset of unusually severe pain should also be a warning sign. If this complication occurs, the patient must be quickly returned to the operating room for hematoma evacuation to prevent more serious sequela.
The age and general state of health of the patient should also be considered in the decision to undergo surgery. In older patients it is more difficult to elevate the middle fossa dura without tearing the dura and causing CSF leak [61]. Language impairment due to damage of the dominant temporal lobe must be considered. Postoperative vestibular adaptation and recovery can be a longer and more difficult process in older patients.
Outcomes
In the properly selected patient, the vast majority of patients have improvement of symptoms following surgical repair. In a study of 93 postoperative patients, 95% of patients reported that their symptoms had improved postoperatively. Importantly this reported benefit did not seem to decrease the further outpatients were from surgery, implying that patients can expect longevity of their surgical repair. It was also noted in this study that auditory symptoms, such as tinnitus, autophony, and sensitivity to sound were noted to have the greatest improvement in patient-reported outcomes. Symptoms such as headaches, imbalance, dizziness, and cognitive impairment were noted to have a lower reported improvement by patients. This is important to consider when managing patient expectations preoperatively. For patients with headache or cognitive impairment as a major symptom, we routinely treat patients for migraine-related imbalance prior to consideration of any surgical intervention [62].
Bilateral Dehiscences
At our institution 38% of individuals diagnosed with SCDS have the appearance of bilateral SCD on high-resolution CT scan. Fortunately, one side is usually responsible for most of the symptoms and can be readily identified by the patient. In some cases, symptoms and signs can be elicited from both ears, including decreased VEMP thresholds, conductive hyperacusis, and sound- or pressure-induced eye movements. In such patients that do have bilateral SCDS, every effort should be made to identify the more symptomatic ear and operate on that side first. In most cases, symptoms will either resolve after operating on the more symptomatic side or abate to the point that contralateral surgery is not required. While exceedingly rare, some patients do ultimately require bilateral surgery. We recommend that the second side should only be considered for plugging surgery after at least 6 months have passed since the initial operation. Plugging of both superior canals significantly impairs the ability to sense downward head rotation in the vertical plane, so these patients are at risk of developing vertical oscillopsia during ambulation, particularly while walking down stairs.
Revision Surgery
While the majority of patients have improvement postoperatively, some patients will ultimately need revision surgery. This can be due to a variety of reasons. In a review of 23 patients undergoing revision surgery, the majority of patients were found to have a canal plug in the correct location, but that was not entirely covering the dehiscence. Results of these revision surgeries did not show a significant increase in complication rates, or hearing loss, but did show a decreased rate of resolution of symptoms compared with patients undergoing initial surgery [63]. We have found success in both revision middle fossa approaches and transmastoid approaches and decide the best approach on a case-by-case basis.
Operative Technique
Since the initial description of SCDS, much has been learned about the pathophysiology and treatment outcomes. Multiple surgical approaches have been described and recently reviewed by Shaia and Diaz [64]. The middle cranial fossa approach was described first [1], and the technique is detailed in the following paragraphs. Since the initial description of surgical treatment of SCDS, several alternative approaches have been described. Most notable are transmastoid SCD plugging, transmastoid resurfacing, or endoscopic-assisted middle cranial fossa resurfacing [66,67,67].
Advocates of the transmastoid approach have noted that it avoids a craniotomy, involves no temporal lobe retraction, and may lead to better stability of the canal plug. Moreover, most otolaryngologists are more familiar with the transmastoid anatomy [68, 69]. Case series using transmastoid plugging have reported success rates of 94% [65]. A modification of the original middle fossa approach has been made with the introduction of intraoperative endoscopy. The technique allows for a smaller, 2 cm diameter craniotomy. This method permits resurfacing, but exposure adequate for canal plugging is not attained. Others have described the use of a mini-craniotomy (2 × 3 cm) and angled rigid endoscopes for enhanced visualization of more medial defects [67].
We favor the middle fossa approach over the transmastoid approach for the vast majority of patients. There are several reasons for this. First, the transmastoid approach does not allow direct visual confirmation of the dehiscence. This presents several problems, and transmastoid plugging of a superior canal that was later found to be intact has been described [68]. Furthermore, without direct access to the dehiscence, the transmastoid approach requires drilling, irrigation, and suctioning on the bony canal. Once the canal is opened, these manipulations could contaminate or remove perilymph and cause collapse of the membranous labyrinth or serous labyrinthitis. Anatomically, the transmastoid approach is not always possible in patients with a low-hanging dura or extensive tegmen dehiscences [68]. In the transmastoid approach, the plug is also placed closer to the sensory epithelia of the ampulla and the utricle. This may be more traumatic to these structures, risking disturbance of their baseline firing rates. Furthermore, opening the superior canal distal to the dehiscence may place the plug into the common crus, causing loss of sensory function of the posterior canal as well [70].
Transmastoid Repair
Despite these drawbacks, we do perform transmastoid repairs in select cases. Patients with multiple medical comorbidities requiring anticoagulation, and those that have undergone prior MFC repair, are often best approached via the mastoid. We have also found that patients whose dehiscences are located medially along the canal are often difficult to access via the MFC [71].
The transmastoid approach is set up with electrophysiological monitoring and image navigation in a similar fashion to the middle fossa craniotomy approach described below. A cortical mastoidectomy is performed, with care taken to thin the tegmen to allow for maximum exposure of the canal. Once the canal is clearly identified, image navigation is used to determine the location of the dehiscence along the arc of the canal. Once the location of the dehiscence has been confirmed with navigation, two small labyrinthotomies are made with a 1 mm diamond burr on low speed. One is made on the ampullopetal side of the dehiscence, and the other is made on the ampullofugal side of the dehiscence.
Plugging is performed in a manner similar to the middle fossa craniotomy approach described below. Care must be taken when plugging the canal via a transmastoid approach to not place an excessive amount of material into to the labyrinth. Due to the need to isolate the dehiscence, which cannot be directly visualized via this approach, the labyrinthotomies are placed closer toward the cupula (ampullopetally) and closer to the common crus (ampullofugally). Excessive plugging could lead to deflection of the cupula, which can cause long-term vestibular dysfunction, or accidental plugging of the posterior canal, which can cause a reduction in function of that canal as well.
When performing the transmastoid repair, we switch to a basic salt solution irrigation when opening the bony labyrinth. Basic salt solution’s electrolyte composition is the most similar to perilymph of all commercially available solutions. The goal of switching the basic salt solution is to limit changes in the electrolyte composition of the exposed perilymph, with the goal of reducing injury to the inner ear. After completion of plugging, a titanium plate is placed over the mastoid bowl at the end of the case to limit patient sensitivity to mastoid pressure inducing vertigo or auditory distortions.
Middle Fossa Craniotomy
Our preferred technique for SCD repair is to plug the canal via a middle cranial fossa approach. The approach has evolved to involve a smaller craniotomy and the use of CT-guided image navigation to aid in both craniotomy planning and localization of the dehiscence. We routinely utilize image guidance to minimize the risk of applying suction to the dehiscence when attempting to identify the dehiscence, in what is often a field of tegmen dehiscences. On the day of or prior to surgery, the patient undergoes a CT scan. We use the LandmarX® image guidance system (Medtronic Corporation, Minneapolis, MN), which allows us to fuse the low-resolution, whole-head dataset with a high-resolution scan of the temporal bone. The latter is invaluable for precise localization of the dehiscence.
The navigation system allows placement of the craniotomy for optimum exposure to the superior canal while avoiding mastoid air cells. The precise placement of the craniotomy centered over the trajectory of the dehiscence also allows for a smaller craniotomy. Craniotomy size less than 3 × 3 cm have been performed with excellent access to the dehiscence for plugging and resurfacing (Fig. 17.6).
Navigation and placement of craniotomy. Panel A: Surgeon positioned at head of bed with navigation on left side. Panel B: Trajectory view mode is used to “sight” a line from the surface of the skull to the dehiscence. Panel C: The lower border of craniotomy (marked in purple) is centered here on the skull
On the day of surgery, after the anesthesiologist has intubated the patient and placed any necessary lines and monitors, the table is rotated 180° so that the head faces the surgeon. The head is placed on a horseshoe head rest. Positioning of the head should ensure no strain is placed on the neck and to minimize significant rotation of the neck. Additionally, the contralateral ear should be centered within the head rest to avoid bending of the neuromonitoring equipment.
The surgeon is positioned at the head of the bed, and thus it serves the scrub technologists to be located on the right side of the patient, while the CT navigation system is on the left (Fig. 17.6a). Given this design, both arms of the patient should be tucked and secured. Care should be made to ensure pressure points are protected and no undo traction is placed on the shoulder that could lead to brachial plexus injury.
Dexamethasone 0.1 mg/kg and appropriate prophylactic antibiotics are given intravenously. Mannitol dosed at 0.5 g/kg should be prepared to be administered just prior to making the craniotomy.
An area of the scalp away from the area of the middle fossa approach incision is prepped and sterilely draped for placement of the reference frame. In positioning of the reference frame, the surgeon should anticipate the position of the eventual incision, the location of the surgeon’s hands during surgery, the location of microscope, the location of the navigation system, as well as the patient’s anatomy, including the thickness of the bone and the location of the superior sagittal sinus (for the right) or mastoid emissary vein (for the left). For right-sided surgery, we position the reference frame in a parasagittal orientation. For left-sided approaches, the reference frame is placed in the postauricular region. When the site is chosen, a 1 cm incision is made, and a small patch of periosteum is cleared from the bone. The reference frame is anchored (Fig. 17.7a). The reference frame is then registered with surface point mapping to allow navigation during surgery. Typically, the precision of the navigation registration is ≤1 mm. The surgeon should be cognizant of any tape used to secure the endotracheal tube and its potential for distorting the skin during point mapping, leading to inaccuracy during navigation system registration.
After registration is complete, the neuromonitoring team places the necessary sound probes and electrodes for facial nerve monitoring, somatosensory-evoked potential monitoring, ECochG, and auditory brainstem response (ABR). ECochG is performed using gold foil-tip electrodes (Etymotic Research Inc., Elk Grove, IL, USA), which are placed adjacent to the tympanic membrane in the external auditory canal. The electrodes are placed under otomicroscopic visualization by the surgeon, with conductive gel placed in the EAC leading onto the tympanic membrane. Bone wax is placed at the external auditory meatus to prevent surgical prep solution from entry into the external canal. The ECochG compression fittings, output cables, and ground electrode are secured to the pinna with water-tight Tegaderm™ adhesive dressing and tape.
The incision is then marked on the scalp extending from the helical root around the helix to a location over the external auditory canal and then superiorly (Fig. 17.7b). The exact orientation of the incision is determined with aid from the image guidance system to allow for the optimal trajectory and position of the craniotomy to access the dehiscence. Hair around the area of the planned incision is shaved, and the area is infiltrated with 1% lidocaine with 1:100,000 epinephrine. The skin is sterilized widely enough to include the previously placed reference frame in the field and to be prepared for the rare case in which a craniotomy may need to be enlarged in order to control bleeding or evacuate a hematoma. After the skin incision is completed, bleeding is controlled using Raney clips along the skin edges. A large piece of true temporalis fascia is harvested for later use in plugging the superior canal, repair of any tegmen defects, or cerebrospinal fluid leak that may occur (Fig. 17.8). Afterward, the temporalis muscle is divided, and the area of the craniotomy is exposed. Fish hook and cerebellar retractors are used to improve visualization of the proposed craniotomy site.
The intraoperative navigation system is used to plan the craniotomy. The trajectory view mode is used to “sight” a line from the surface of the skull to the dehiscence (Fig. 17.6b), and the craniotomy is centered here on the skull (Fig. 17.6c). The trajectory and craniotomy should be oriented in a position that allows for comfortable positioning of the microscope and the surgeon. The lower border of the craniotomy is placed just high enough to avoid the mastoid air cells. If a navigation system is not used, the craniotomy should be centered on the external auditory canal. This is slightly different from the placement used for drilling of the internal auditory canal (IAC), where the craniotomy is placed with its center anterior to the external auditory canal because of the more anterior location of the IAC relative to the labyrinth.
The width and height of the craniotomy is enough to accommodate a Fisch retractor, typically 3 cm wide by 4 cm high (Figs. 17.6c). Care is taken to ensure the anterior and posterior cuts of the craniotomy are parallel to facilitate stable placement of the Fisch retractor. Once the craniotomy is marked, the bone is opened by drilling troughs around the borders beginning with a 4 mm cutting burr. As the bone is thinned, a 4 mm diamond burr is used to drill until an eggshell layer of bone remains over the dura. This is fractured with a blunt instrument. During the drilling of the cortex, bone dust is collected and placed in sterile saline for later use during plugging of the dehiscence.
Penfield instruments are used to elevate the bone flap away from the dura. The bone flap is placed in saline for later cranioplasty. Bleeding from branches of the middle meningeal artery, which traverses the field, often must be controlled with bipolar cautery. The dura is slightly further elevated from the edges of the craniotomy to accommodate the retractor. The sharp edges of the craniotomy are removed using 2 mm and 1 mm Kerrison rongeurs, and the bone chips created in this process are saved for later use as plugs for the superior canal.
The initial dural dissection is accomplished with the use of large, saline-soaked cotton balls with strings. We find that the large cotton balls soaked in saline are the least traumatic means for the dural elevation. A hemostatic agent such as (Floseal®) or gelatin powder (Gelfoam®) mixed as a paste with thrombin is generously applied in advance of the cotton balls. The Fisch middle cranial fossa retractor is then placed and used to gently elevate the dura off of the floor of the middle fossa (Fig. 17.9). Retraction of the temporal lobe is minimized and the distal end of the retractor is most often in contact with the petrous bone. Extradural retraction is felt to distribute the pressure to the dura as opposed to the underlying brain parenchyma [72]. Dura of the middle fossa can be very thin, especially if tegmen dehiscences are also present. The image navigation system is frequently useful during the exploration to identify the precise location of the superior canal and its dehiscence. The surgeon is careful to only suction on the cotton balls and not to directly suction the area of the dehiscence. This minimizes removing excessive perilymph or tearing the membranous labyrinth, which could cause sensorineural hearing and vestibular loss. More recently, irrigation fluid has been changed from saline to warm basic salt solution to more closely represent the electrolyte composition of perilymph.
Once the superior canal dehiscence has been identified, attention is immediately shifted toward plugging the dehiscence (Figs. 17.10 and 17.11). The uncovering of the dehiscence and the subsequent plugging of the anterior and posterior limbs of the canal are communicated with the intraoperative monitoring technician to facilitate close monitoring of the ECochG. Copious amounts of irrigation are used once the dehiscence has been uncovered (Fig. 17.11a, b) to limit the risk of perilymph aspiration. From the harvested temporalis fascia, small moist pieces of fascia are slid into the two open lumens of the bony superior canal with gentle pressure from a curved pick (Figs. 17.10 and 17.11c). Several pieces are placed in each end so as to push the plugs several millimeters beyond the dehiscence. Bone dust is also used to reinforce the fascia and aid in plugging. This is done so as to prevent a recurrence should further bone erosion occur from the ends of the present dehiscence. Note that hydraulic pressure tends to push previously placed pieces of fascia out of one end of the dehiscence while the other is being packed. In fact, we look for this as the final confirmation that the correct holes are being plugged. To prevent fascia from becoming displaced, bone chips matching the diameter of the canal are firmly lodged so as to “cork” each end of the dehiscence (Figs. 17.10 and 17.11d). Any degradation of the ECochG response serves as a warning that too much pressure may be built up within the inner ear.
Following plugging of both sides of the dehiscence, the middle fossa floor is resurfaced using hydroxyapatite bone cement (HydroSet, Stryker®). All cotton balls used during the dissection are removed prior to placement. The bone cement is allowed to set for 2 min in warm lactated Ringer’s solution. The remaining harvested temporalis fascia is placed over the bone cement followed by fibrin glue
Closure is achieved by anchoring the previously harvested bone flap in place using titanium plates (Fig. 17.12). A burr may be used to recess the plates into the bone so that they are not palpable postoperatively, or the plates and screws may be covered with hydroxyapatite bone cement. The temporalis muscle is approximated with absorbable sutures, and the skin is closed with staples and/or nylon suture. A drain is not typically used. The wound is cleaned, and a formal mastoid dressing is applied.
Postoperative Care
Patients are closely monitored in the postanesthesia care unit for 4 h prior to transfer to the surgical ward, with frequent neurological checks overnight due to the risk of epidural hematoma. Postoperative patients are treated with intravenous dexamethasone generally dosed 6 mg IV every 6 h with a taper beginning on the second postoperative day. Longer courses may be considered for patients who experience postoperative sensorineural hearing loss or loss of sensory function in the horizontal or posterior canals as manifested on head thrust testing. Patients are encouraged to be out of bed in a chair and ambulating starting on the first postoperative day. An oral diet can be started the day after surgery. The typical hospitalization lasts a total of 2 or 3 days.
Patients frequently experience nausea during the initial hours after surgery. This is best controlled using intravenous promethazine. Due to the risk of sedation, low doses should be given initially, starting at 6.25 mg and increasing up to 25 mg dosing every 4–6 h. There are also many other medications available to control nausea, some of which may be traditionally preferred in neurosurgical patients due to the risk of sedation associated with promethazine. However, for nausea related to simulation of the vestibular end organs, we have found superior results with promethazine.
Postoperative pain is usually not severe and is localized to the area of the incision. The pain is mostly due to division of the temporalis muscle and is often worse with chewing. Routine postoperative analgesics are sufficient to control the pain. If the patient is experiencing intense pain, an epidural hematoma may be the cause, and an immediate head CT should be considered. Any change in mental status or consciousness should also raise concerns of intracranial bleeding.
Long-Term Results
In our experience most patients are extremely satisfied with the surgery. Relief of dizzy symptoms has recently been documented by measuring the dizziness handicap inventory (DHI) [46] prior to SCD plugging surgery and 3 months afterward. On average DHI improved by 26 points, with patients with more severe dizziness (preoperative DHI ≥ 30) improving by an average of 39 points [51]. This improvement is greater than the mean improvement seen after surgical labyrinthectomy for Meniere’s disease, which decreased DHI score by 17, and after vestibular neurectomy, which decreased DHI score by 16 [50].
We have found that when patients have significant autophony or hyperacusis, these symptoms are frequently much improved immediately after surgery. Occasionally some autophony symptoms will take time to resolve, which is likely due to fluid collecting in the middle ear during the immediate postoperative period and causing conductive hearing loss. Utilizing a created autophony index, Crane et al. found a statically lower mean score with 94% of patients reporting plugging improved their autophony symptoms [73].
The results for improving hearing with SCD surgery are less clear. Dramatic results have been observed in individual patients [55], but are not common. In a series of 6 patients with an air-bone gap prior to SCD plugging who had no previous history of ear surgery, 4 (66%) had at least partial closure of the air-bone gap after surgery [54]. However, in patients with previous middle cranial fossa or stapes surgery, the risk of hearing loss was high in this series. In a study from our institution, the average patient experienced a 10 dB improvement in air conduction hearing, although individual results varied from a 45 dB gain to a 45 dB hearing loss [31]. There has even been a report of improvement in sensorineural hearing loss after SCD surgery [55]. However, as discussed earlier, there is a risk of mild high-frequency sensorineural hearing loss with 25% of patients suffering permanent loss [56].
Balance can be significantly impaired in the immediate postoperative setting. Hypofunction of the canals can be assessed with head thrust testing in the plane of the canal. Agrawal et al. [73] noted that 1 mm increases in dehiscence length increased the odds of immediate postoperative hypofunction 2.6-fold (95% confidence interval, 1.3–5.1). The prevalence of vestibular hypofunction was significantly higher in the early compared with the late postoperative period. Despite this, even patients with large dehiscences have recovery of dynamic and static measures of balance [74]. Patients should undergo fall risk assessment, and involvement of vestibular physical therapy in the inpatient postoperative period is beneficial.
Summary
The diagnosis of SCDS is based on an appropriate patient history, physical exam findings including eye movements in response to sound or pressure, and other supporting studies including the audiogram, VEMPs, and CT scanning. The spectrum and severity of SCDS symptoms vary significantly between individuals, and one must carefully weigh the potential benefit of surgery against the risks and probability of success in each patient.
Superior canal dehiscence plugging may be performed via a transmastoid or middle fossa approach, with the authors preferring the middle fossa for the majority of patients. Overall, patients experience an improvement in dizziness, autophony, and hyperacusis symptoms. Although there is often an improvement in hearing after surgery, this must be carefully weighed against the risk of hearing loss, which is significant in patients who have had previous middle fossa or stapes surgery.
References
Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–58.
Tullio P. Das ohr und die entstehung de sprache und schrift. Berlin: Urban Scharzenberg; 1929.
Mayer O, Fraser JS. Pathological changes in the ear in late congenital syphilis. J Laryngol Otol. 1936;51:683–714.
Fox EJ, Balkany TJ, Arenberg IK. The Tullio phenomenon and perilymph fistula. Otolaryngol Head Neck Surg. 1988;98:88–9.
Kacker SK, Hinchcliffe R. Unusual Tullio phenomena. J Laryngol Otol. 1970;84:155–66.
Ishizaki H, Pyykko I, Aalto H, Starck J. Tullio phenomenon and postural stability: experimental study in normal subjects and patients with vertigo. Ann Otol Rhinol Laryngol. 1991;100:976–83.
Pisano DV, Niesten MEF, Merchant SN, Nakajima HH. The effect of superior semicircular canal dehiscence on intracochlear sound pressures. Audiol Neurootol. 2012;17(5):338–48. https://doi.org/10.1159/000339653.
Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25(3):323–32.
Chien W, Ravicz ME, Rosowski JJ, Merchant SN. Measurements of human middle- and inner-ear mechanics with dehiscence of the superior semicircular canal. Otol Neurotol. 2007;28(2):250–7. https://doi.org/10.1097/01.mao.0000244370.47320.9a.
Carey JP, Minor LB, Nager GT. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg. 2000;126:137–47.
Re M, Gioacchini FM, Salvolini U, et al. Multislice computed tomography overestimates superior semicircular canal dehiscence syndrome. Ann Otol Rhinol Laryngol. 2013;122:625–31.
Pfammatter A, Darrouzet V, Gartner M, et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol. 2010;31:447–54.
Chien WW, Janky K, Minor LB, Carey JP. Superior canal dehiscence size: multivariate assessment of clinical impact. Otol Neurotol. 2012;33:810–5.
Hunter JB, O’Connell BP, Wang J, et al. Correlation of superior canal dehiscence surface area with vestibular evoked myogenic potentials, audiometric thresholds, and dizziness handicap. Otol Neurotol. 2016;37(8):1104–10. https://doi.org/10.1097/MAO.0000000000001126.
Niesten MEF, Stieger C, Lee DJ, et al. Assessment of the effects of superior canal dehiscence location and size on intracochlear sound pressures. Audiol Neurootol. 2015;20(1):62–71. https://doi.org/10.1159/000366512.
Niesten MEF, Hamberg LM, Silverman JB, et al. Superior canal dehiscence length and location influences clinical presentation and audiometric and cervical vestibular-evoked myogenic potential testing. Audiol Neurootol. 2014;19(2):97–105. https://doi.org/10.1159/000353920.
Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–27.
Watson SR, Halmagyi GM, Colebatch JG. Vestibular hypersensitivity to sound (Tullio phenomenon): structural and functional assessment. Neurology. 2000;54:722–8.
Halmagyi GM, Aw ST, McGarvie LA, et al. Superior semicircular canal dehiscence simulating otosclerosis. J Laryngol Otol. 2003;117:553–7.
Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55:1833–41.
Minor LB, Cremer PD, Carey JP, Della Santina CC, Streubel SO, Weg N. Symptoms and signs in superior canal dehiscence syndrome. Ann N Y Acad Sci. 2001;942:259–73.
Krombach GA, DiMartino E, Schmitz-Rode T, et al. Posterior semicircular canal dehiscence: a morphologic cause of vertigo similar to superior semicircular canal dehiscence. Eur Radiol. 2003;13:1444–50.
Sheehy JL, Brackmann DE. Cholesteatoma surgery: management of the labyrinthine fistula—a report of 97 cases. Laryngoscope. 1979;89:78–87.
Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282–9.
Mikulec AA, McKenna MJ, Ramsey MJ, et al. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004;25:121–9.
Adams ME, Kileny PR, Telian SA, et al. Electrocochleography as a diagnostic and intraoperative adjunct in superior semicircular canal dehiscence syndrome. Otol Neurotol. 2011;32:1506–12.
Wenzel A, Ward BK, Ritzl EK, et al. Intraoperative neuromonitoring for superior semicircular canal dehiscence and hearing outcomes. Otol Neurotol. 2015;36(1):139–45. https://doi.org/10.1097/MAO.0000000000000642.
Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;64:1682–8.
Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70:464–72.
Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol. 2007;28:920–6.
Crane BT, Minor LB, Carey JP. Three-dimensional computed tomography of superior canal dehiscence syndrome. Otol Neurotol. 2008;29:699–705.
Sheykholeslami K, Schmerber S, Habiby Kermany M, Kaga K. Vestibular-evoked myogenic potentials in three patients with large vestibular aqueduct. Hear Res. 2004;190:161–8.
Zuniga MG, Janky KL, Nguyen KD, Welgampola MS, Carey JP. Ocular versus cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol Neurotol. 2013;34:121–6.
Janky KL, Nguyen KD, Welgampola M, Zuniga MG, Carey JP. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol Neurotol. 2013;34:127–34.
Belden CJ, Weg N, Minor LB, Zinreich SJ. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology. 2003;226:337–43.
Williamson RA, Vrabec JT, Coker NJ, Sandlin M. Coronal computed tomography prevalence of superior semicircular canal dehiscence. Otolaryngol Head Neck Surg. 2003;129:481–9.
Minor LB. Meniere’s disease and migraine. Arch Otolaryngol Head Neck Surg. 2005;131:460.
Muchnik C, Hildesheimer M, Rubinstein M, Arenberg IK. Low frequency air-bone gap in Meniere's disease without middle ear pathology. A preliminary report. Am J Otol. 1989;10:1–4.
Poe DS. Diagnosis and management of the patulous eustachian tube. Otol Neurotol. 2007;28:668–77.
Minor LB. Labyrinthine fistulae: pathobiology and management. Curr Opin Otolaryngol Head Neck Surg. 2003;11:340–6.
Hakuba N, Hato N, Shinomori Y, Sato H, Gyo K. Labyrinthine fistula as a late complication of middle ear surgery using the canal wall down technique. Otol Neurotol. 2002;23:832–5.
Friedland DR, Wackym PA. A critical appraisal of spontaneous perilymphatic fistulas of the inner ear. Am J Otol. 1999;20:261–76. discussion 276-269.
Eggers SD. Migraine-related vertigo: diagnosis and treatment. Curr Pain Headache Rep. 2007;11:217–26.
Tepper SJ. A pivotal moment in 50 years of headache history: the first American migraine study. Headache. 2008;48:730–1. discussion 732.
Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107(Pt 4):1123–42.
Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–7.
Shaia WT, Zappia JJ, Bojrab DI, LaRouere ML, Sargent EW, Diaz RC. Success of posterior semicircular canal occlusion and application of the dizziness handicap inventory. Otolaryngol Head Neck Surg. 2006;134:424–30.
Tufarelli D, Meli A, Labini FS, et al. Balance impairment after acoustic neuroma surgery. Otol Neurotol. 2007;28:814–21.
Humphriss RL, Baguley DM, Moffat DA. Change in dizziness handicap after vestibular schwannoma excision. Otol Neurotol. 2003;24:661–5.
Badke MB, Pyle GM, Shea T, Miedaner J. Outcomes in vestibular ablative procedures. Otol Neurotol. 2002;23:504–9.
Crane BT, Minor LB, Carey JP. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118:1809–13.
O'Reilly RC, Elford B, Slater R. Effectiveness of the particle repositioning maneuver in subtypes of benign paroxysmal positional vertigo. Laryngoscope. 2000;110:1385–8.
Perez N, Martin E, Garcia-Tapia R. Dizziness: relating the severity of vertigo to the degree of handicap by measuring vestibular impairment. Otolaryngol Head Neck Surg. 2003;128:372–81.
Limb CJ, Carey JP, Srireddy S, Minor LB. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969–80.
Wilkinson EP, Liu GC, Friedman RA. Correction of progressive hearing loss in superior canal dehiscence syndrome. Laryngoscope. 2008;118:10–3.
Ward BK, Agrawal Y, Nguyen E, et al. Hearing outcomes after surgical plugging of the superior semicircular canal by a middle cranial fossa approach. Otol Neurotol. 2012;33:1386–91.
Xie Y, Sharon JD, Pross SE, et al. Surgical complications from superior canal dehiscence syndrome repair: two decades of experience. Otolaryngol Head Neck Surg. 2017;157(2):273–80. https://doi.org/10.1177/0194599817706491.
Mantokoudis G, Saber Tehrani AS, Wong AL, Agrawal Y, Wenzel A, Carey JP. Adaptation and compensation of vestibular responses following superior canal dehiscence surgery. Otol Neurotol. 2016;37(9):1399–405. https://doi.org/10.1097/MAO.0000000000001196.
Barber SR, Cheng YS, Owoc M, et al. Benign paroxysmal positional vertigo commonly occurs following repair of superior canal dehiscence. Laryngoscope. 2016;126(9):2092–7. https://doi.org/10.1002/lary.25797.
Sanna M, Taibah A, Russo A, Falcioni M, Agarwal M. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004;25:379–86.
Oghalai JS, Buxbaum JL, Pitts LH, Jackler RK. The effect of age on acoustic neuroma surgery outcomes. Otol Neurotol. 2003;24:473–7.
Alkhafaji MS, Varma S, Pross SE, et al. Long-term patient-reported outcomes after surgery for superior canal dehiscence syndrome. Otol Neurotol. 2017;38(9):1319–26. https://doi.org/10.1097/MAO.0000000000001550.
Sharon JD, Pross SE, Ward BK, Carey JP. Revision surgery for superior canal dehiscence syndrome. Otol Neurotol. 2016;37(8):1096–103. https://doi.org/10.1097/MAO.0000000000001113.
Shaia WT, Diaz RC. Evolution in surgical management of superior canal dehiscence syndrome. Curr Opin Otolaryngol Head Neck Surg. 2013;21:497–502.
Beyea JA, Agrawal SK, Parnes LS. Transmastoid semicircular canal occlusion: a safe and highly effective treatment for benign paroxysmal positional vertigo and superior canal dehiscence. Laryngoscope. 2012;122:1862–6.
Deschenes GR, Hsu DP, Megerian CA. Outpatient repair of superior semicircular canal dehiscence via the transmastoid approach. Laryngoscope. 2009;119:1765–9.
Carter MS, Lookabaugh S, Lee DJ. Endoscopic-assisted repair of superior canal dehiscence syndrome. Laryngoscope. 2014;124:1464–8.
Agrawal SK, Parnes LS. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008;29:363–7.
Crovetto M, Areitio E, Elexpuru J, Aguayo F. Transmastoid approach for resurfacing of superior semicircular canal dehiscence. Auris Nasus Larynx. 2008;35:247–9.
Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28:356–64.
Cheng YS, Kozin ED, Lee DJ. Endoscopic-assisted repair of superior canal dehiscence. Otolaryngol Clin N Am. 2016;49(5):1189–204. https://doi.org/10.1016/j.otc.2016.05.010. Review. PubMed PMID: 27565386.
Driscoll CL, Jackler RK, Pitts LH, Banthia V. Extradural temporal lobe retraction in the middle fossa approach to the internal auditory canal: biomechanical analysis. Am J Otol. 1999;20:373–80.
Agrawal Y, Migliaccio AA, Minor LB, Carey JP. Vestibular hypofunction in the initial postoperative period after surgical treatment of superior semicircular canal dehiscence. Otol Neurotol. 2009;30:502–6.
Janky KL, Zuniga MG, Carey JP, Schubert M. Balance dysfunction and recovery after surgery for superior canal dehiscence syndrome. Arch Otolaryngol Head Neck Surg. 2012;138:723–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Creighton, F.X., Carey, J.P. (2019). Surgical Treatment of Superior Semicircular Canal Dehiscence Syndrome. In: Babu, S., Schutt, C., Bojrab, D. (eds) Diagnosis and Treatment of Vestibular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-97858-1_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-97858-1_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-97857-4
Online ISBN: 978-3-319-97858-1
eBook Packages: MedicineMedicine (R0)