Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children, especially in Western countries, with a worldwide prevalence estimated between 3 and 12% in the general pediatric population, with peaks of 70% in obese children. NAFLD pathogenesis is not fully understood, but it is known that many risk factors, both environmental and genetic, contribute to the development and progression of liver injury, which can eventually lead to cirrhosis and hepatocellular carcinoma. The only treatment approved is weight loss through diet and physical activity, but it is very difficult to obtain an optimal compliance by children and their families. Some different pharmacological approaches, such as insulin sensitizers, omega-3 fatty acids, and vitamin D, have been studied, but the results are not univocal, and their applicability is actually limited in the pediatric population. Many other therapeutic strands are currently under investigation, especially in view of the new pathogenetic findings.
†Professor Valerio Nobili suddenly passed away on March 15, 2019, at the age of 52. The editor and the authors of this book wish to honor the memory of the pediatric hepatologist who brought to light the importance of hepatic steatosis in children’s liver disease, an inspired and passionate scientist, and a dear friend.
–Lorenzo D’Antiga
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
NAFLD is the most common liver disease in children and adolescents.
-
Obese and selected overweight children should be routinely screened for NAFLD.
-
Liver biopsy is still the reference standard for diagnosis NAFLD, but it should not be performed in all patients.
-
NAFLD can be associated with other features of metabolic syndrome.
-
Weight loss is the cornerstone of NAFLD therapy.
-
Deepening of our knowledge of pathophysiology of the disease, mainly about gut-liver interactions.
-
Identify a reliable, noninvasive diagnostic tool to diagnose and stage NAFLD/NASH.
-
Evaluate safety and effectiveness of the new pharmacologic treatments.
1 Introduction
NAFLD represents the most common cause of chronic liver disease in children and adolescents; it is characterized by accumulation of fat in the hepatocytes (5%) in the absence of other causes of liver steatosis, such as Wilson’s disease, deficiency of alpha-1-antitripsin, celiac disease, autoimmune hepatitis, HCV infection, metabolic disorders, and alcohol or drug consumption [1].
The simple hepatic steatosis is usually a benign condition, but in some cases, it progresses to more advanced forms of liver injury, characterized by the presence of inflammation and various degrees of fibrosis up to cirrhosis, predisposing to liver failure and/or hepatocellular carcinoma (HCC) [2].
NAFLD is closely associated with insulin resistance; obesity and metabolic syndrome are common underlying factors [3].
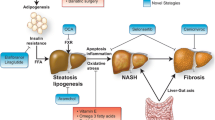
From the first studies, it emerged that the main cause of the accumulation of liver fat is visceral adipose tissue. In fact, the adipose tissue fulfils important endocrine functions, producing pro-inflammatory adipocytokines, such as tumor necrosis factor- α (TNF-α), interleukin 6 (IL-6), leptin, and adiponectin, which are implicated in the clinical manifestation of NAFLD and its progression to NASH and cirrhosis [4]. However, in the last years, other several causes of NAFLD were showed, such as gut-liver axis derangements. In this chapter the epidemiology, pathogenesis, and clinical, diagnostic, and therapeutic strategies of NAFLD currently known will be discussed.
2 Epidemiology
The exact prevalence of pediatric NAFLD is actually unknown, but available data report a prevalence ranging from 3 to 12% in the general pediatric population, with peaks of 70% in obese children. Clinical series of NAFLD children demonstrate the predominance of boys versus girls, with a male to female ratio of 2:1 [5]. In addition to gender, race and ethnicity also play an important role in the development of NAFLD. Fatty liver is more prevalent in children and adolescents of Hispanic ethnicity and less prevalent among black children and adolescents [6]. Ethnic differences may be related to genetic, environmental, or sociocultural factors as well as differences in body composition, insulin sensitivity, and adipocytokine profile. In Western countries, the NAFLD prevalence is estimated to be around 20–46%, while in Asian children, the prevalence is 5–18%. In Asia and Pacific islands, significant difference is reported between urban and rural populations with a prevalence of 16–32% in urban areas versus 9% in rural populations for the prevalence of NAFLD. Obesity-related NAFLD was reported in 77% of Chinese children. In Australia, the prevalence of pediatric NAFLD was estimated to be approximately 10% in the total population and 27.6% among overweight and obese children.
Age is another striking factor: one postmortem study in the USA showed that 17% of teenagers had NAFLD compared to 0.7% of 2–4-year-olds, owing both to a longer period to accumulate steatosis and an increased incidence in adolescents. Despite the diversity of diagnostic criteria used in population-based studies, obesity is the main risk factor for pediatric NAFLD [7]. In fact, the prevalence of NAFLD in obese children increases up to 80% in several obesogenic countries, including the USA, Europe, and Japan. Recent studies reported that in a population of schoolchildren aged 6–12 years, the rates of NAFLD were 3% in the normal weight range, 25% in the overweight range, and 76% in obese children.
NAFLD in children represents a metabolic condition, which is strongly associated with other metabolic features, such as waist circumference >95th percentile, hypertension and insulin resistance increasing the risk of developing type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease at a young age.
3 Pathophysiology
3.1 Multiple-Hit Hypothesis
Several mechanisms may lead to steatosis: dietary habits, environmental and genetic factors, development of insulin resistance, obesity with adipocyte proliferation, and changes in the composition of intestinal microbiota.
Adipose tissue is a metabolically active endocrine organ that causes the release of proinflammatory cytokines, such as TNF-α and IL-6, whereas beneficial adipokines are suppressed. This situation leads to the development of peripheral insulin resistance and hyperinsulinemia and increased fatty acid delivery to the hepatocyte. The disruption of normal insulin signaling in the hepatocyte and increased abundance of fatty acids leads to disordered lipid metabolism, characterized by the over-activation of de novo lipogenesis (DNL) transcriptional factors, causing more fatty acid and glucose products to be shunted into these lypogenetic pathways. Beta-oxidation in the mitochondria is also inhibited, as well as very-low-density lipoprotein (VLDL) packaging and export, leading to buildup of triglycerides in the hepatocytes. Gluconeogenesis is not suppressed despite hyperinsulinemia in the insulin-resistant hepatocyte, and increased glucose levels provide more substrate for DNL in a positive feedback loop [8].
The role of intestinal microbiota has been recently considered within this metabolic dysregulation. A bad diet (rich in fats and lipids) and increase of intestinal bacteria products (i.e., endotoxins, proteins, metabolites, lipopolysaccharides (LPS)) with the subsequent activation of the Toll-like receptor pathway (TLR) may act as inductors of inflammation and progression of hepatic steatosis to NASH and fibrosis. This process seems also be aggravated by the increased intestinal permeability that has been demonstrated in subjects with liver disease, where the gut seems to go through a tight junction disruption process that could be reversed by changes in the microbiota [9].
3.2 Lipogenesis and Lipotoxicity
The role of DNL in the development of hepatic steatosis is a common element in patients with metabolic syndrome and with high consumption of fatty acid. Specific dietary compositions may have different effects. The carbohydrates in the diet will positively influence the amount of DNL in the liver. Simple sugars are converted to fatty acids more easily than complex carbohydrates, and fructose is a more potent inducer of DNL than glucose [10]. Diets rich in saturated fat stimulate DNL by upregulating SREBP-1 (sterol-responsive element-binding protein-1), a key regulator of the lipogenic genes in the liver. Moreover, not all individuals with hepatic steatosis had increased DNL nor upregulated expression of SREBP-1; in fact paradoxical dissociation between hepatic DNL and hepatic fat content due to the PNPLA3 148M allele has been proved [11].
In the pathogenesis of NASH, multiple mechanisms are operative to produce hepatic damage by short-chain fatty acids (SFAs) and free cholesterol from de novo synthesis. The long-chain saturated fatty acids (LCFAs) are transported to mitochondria for 𝛽-oxidation or to be esterified for either excretion in the form of VLDL (very low density lipoproteins) or storage as lipid droplets. Free cholesterol accumulation causes liver injury due to activation of intracellular signaling pathways in Kupffer cells (KCs), hepatic stellate cells (HSCs), and hepatocytes. The activation of KCs and HSCs promotes inflammation and fibrogenesis. Moreover, free cholesterol and SFAs can activate a variety of intracellular responses that causes mitochondrial death pathway activation, resulting in lipotoxic stress in the endoplasmic reticulum and mitochondria. The Toll-like receptor 4 (TLR4) is a receptor that activates a proinflammatory signaling pathway in response to excessive SFAs. This pathway is initiated by recruiting adaptor molecules such as toll/IL-1 receptor domain containing adaptor protein (TIRAP) and myeloid differentiation factor 88(MyD88) that ultimately lead to activation of nuclear factor 𝜅B with production of TNF-𝛼 [12].
Insulin Resistance. Studies have highlighted the fact that insulin resistance is a characteristic feature of NAFLD and is caused by a variety of factors, including release of soluble mediators derived from immune cells and/or adipose tissue, such as TNF-𝛼 and IL-6 [2].
Insulin-resistant subjects with NAFLD show reduced insulin sensitivity mainly in the muscles, liver, and adipose tissue, which can lead to a far more complex metabolic disorder. Serine phosphorylation of insulin receptor substrates by inflammatory signal transducers such as c-jun N-terminal protein kinase 1 (JNK1) or inhibitor of nuclear factor-𝜅B kinase-𝛽 (IKK-𝛽) is considered one of the key aspects that disrupt insulin signaling [13]. It is worth noting that insulin resistance is characterized not only by increased circulating insulin levels but also by increased hepatic gluconeogenesis, impaired glucose uptake by the muscle, and increased release of free fatty acids (FFAs) and inflammatory cytokines from peripheral adipose tissues, which are the key factors promoting accumulation of liver fat and progression of hepatic steatosis.
3.3 Oxidative Stress
In the presence of high concentrations of FFAs in hepatocytes, oxidative stress is due to lipid peroxidation and high levels of reactive oxygen/nitrogen species (ROS/RNS) that are generated during the metabolism of FFAs in microsomes, peroxisomes, and mitochondria [14]. Peroxidation of plasma and intracellular membranes causes direct cell necrosis or apoptosis, while ROS-induced expression of Fas ligand on hepatocytes may induce fratricidal cell death. Recent studies support the idea that oxidative stress may be a primary cause of liver fat accumulation and subsequent liver damage, and ROS may play a part even in fibrosis development. Importantly, these species can initiate lipid peroxidation by targeting polyunsaturated fatty acids (PUFAs), resulting in the formation of highly reactive aldehyde products. These reactive lipid derivatives have the potential to amplify intracellular damage by mediating the diffusion of ROS/RNS into the extracellular space, thus causing tissue injury [15].
3.4 Gut Microbiota
The data of the close connection between the liver and the intestine has been known for a long time. In the intestine occurs the absorption of the nutrients ingested with the diet (vitamins, proteins, carbohydrates, and lipids). These elements reach the liver, through the portal vein and the hepatic artery, where they are metabolized in order to guarantee the main cellular and metabolic functions. Other nutrients such as fats, fat-soluble vitamins, and trace elements (copper, zinc, etc.) need the presence of bile to be absorbed. Finally, the intestine secretes a series of hormones, neuropeptides, and growth factors that influence hepatic functions. The liver receives more than 70% of its blood supply from the intestine, via the portal vein and hepatic artery; therefore, it is the main organ exposed to toxic factors of intestinal origin [16].
In the interaction between the intestine and the liver, a key role is played by the microbiota. A “dysmicrobiosis” or bacterial overgrowth influences the absorption of nutrients and alters the structure of the intestinal walls, compromising their permeability. This favors the passage of macromolecules, bacteria, and bacterial products in the portal and systemic circulation. Probably increased intestinal permeability is the principal pathogenic step in the alteration of the intestinal liver axis, thus favoring the onset or progression of liver diseases [17].
Recent studies have suggested that the intestinal microbiota is responsible for the synthesis of various hepatotoxic bacterial substances (i.e., ammonia, phenols, and ethanol). The main bacterial product involved in the pathogenesis of NASH/NAFLD is lipopolysaccharide (LPS), an active component of bacterial endotoxin. The endogenous production of LPS due to bacterial death induces its translocation through the intestinal capillaries thanks to the TLR4-dependent mechanism [18]. The LPS, through a complex process of association with LPS-binding protein and CD14, activates TLR-4 located on different inflammatory cells, increasing the expression of target genes involved in the synthesis of inflammatory cytokines such as TNF-α and interleukins 1 and 6, promoting thus insulin resistance, hepatic steatosis, hepatic inflammation, and fibrogenesis. In fact, it is now known that TNF-α and tumor growth factor-b1 (TGF-b1) are strongly linked to the progression of NAFLD. The hepatic expression of TNF-α is significantly increased in children with NASH. At the same time, the TGF-b1 concentrations correlate with HSC activation, promoting TLR-4-mediated fibrogenesis. In one of the first pediatric studies, it was shown that the permeability (measured by the lactulose/mannitol ratio) is increased in children with NAFLD compared to healthy controls. This association between increased permeability and NAFLD suggests that bacterial translocation expounds the liver to endotoxins that influence the development of NAFLD and progression in NASH [19, 20].
On the other hand, the microbiota is influenced by many other factors, including bile acids and diet (choline, fructose, etc.). It is possible that changes to the microflora composition alter the intestinal barrier, allowing the intestinal contents to enter in the liver, influencing the development of steatosis and its progression to NASH.
3.5 Bile Acid
Bile acids are synthesized in hepatocytes from cholesterol through enzymatic pathways and then conjugated with glycine or taurine before secretion into bile and release into the small intestine. In the small intestine, conjugated bile acids are not only involved in lipid absorption and transport but have also been increasingly recognized to function as nuclear receptor binders and to have a role in function of microbiota. Bacteria within the intestine can also chemically modify bile acids and thereby alter the composition of the bile acid [21]. Besides the classic role as detergents to facilitate fat absorption, bile acids have also been recognized as important cell signaling molecules regulating lipid and carbohydrate metabolism and inflammatory response. These molecular functions are mediated through their binding and activation of the nuclear hormone receptor, farnesoid X receptor (FXR), and the G protein-coupled cell surface receptor TGR5. Intestinal FXR activity upregulates endocrine FGF19 expression, which inhibits hepatic bile acid synthesis via CYP7A1 signaling [22]. Recently, it was showed that hepatic FXR protein content and plasma FGF19 concentrations in children and adolescents with NASH were decreased compared to levels in children with “simple” NAFLD. Hepatic FXR protein level was positively correlated with serum FGF-19 concentrations, and both FXR and FGF19 concentrations were inversely and independently associated with NASH. This suggests that further studies are needed on the role of FXR in NAFLD [23].
3.6 Genetic
Single nucleotide polymorphisms (SNPs) in the genes involved in lipid metabolism (Lipin 1 (LPIN1), patatin-like phospholipase domain containing protein 3 (PNPLA3)), oxidative stress (superoxide dismutase 2 (SOD2)), insulin signaling (insulin receptor substrate 1 (IRS-1)), and fibrogenesis (Kruppel-like factor 6 (KLF6)) have been associated with the severity of liver damage in NAFLD patients. Moreover, an interesting interaction has recently been reported between genetic risk factors (PNPLA3 I148M), dietary components, and the severity of steatosis [24].
The PNPLA3, also known as adiponectin, is a member of the patatin-like phospholipase family. The rs738409 C>G single-nucleotide polymorphism (SNP), encoding the Ile 148Met variant protein of PNPLA3, is described as genetic determinant of hepatic steatosis. Several studies have established a strong link between PNPLA3 and the development of NAFLD. PNPLA3 is associated with an increased risk of advanced fibrosis among patients with a variety of liver diseases and is an independent risk factor for hepatocellular carcinoma among patients with NASH [25]. Moreover, the polymorphism rs738409 is not only associated with NASH but also with the severity of necroinflammatory changes independent of metabolic factors. NASH was more frequently observed in GG than CC homozygous; in fact rs738409 GG genotype versus the CC genotype was associated with a 28% increase in serum ALT levels and had 3.24-fold greater risk of higher necroinflammatory scores and 3.2-fold greater risk of developing fibrosis compared to CC homozygous [26].
Recently, additional SNPs of genes implicated in NASH pathogenesis have been shown to influence liver damage and fibrosis progression in patients. These include genetic variants regulating insulin receptor activity, ectoenzyme nucleotide pyrophosphate phosphodiesterase 1 (ENPP1), and the insulin receptor substrate-1 (IRS-1), thus underscoring the causal role of IR in the progression of liver damage in NAFLD. The manganese superoxide dismutase (SOD2), regulating SOD2 mitochondrial import and antioxidant activity, and the Kruppel-like factor 6 (KLF6), regulating alternative splicing isoforms of the transcription factor KLF6, are involved in the regulation of metabolism in hepatocytes and fibrogenesis in hepatic stellate cells [11].
The investigation of relevant genetic variants associated with pediatric NAFLD can be useful for the disease both in childhood and in adulthood to better understand their role in the pathogenesis of NAFLD.
4 Diagnosis
NAFLD is the most common liver disease in children, so it is essential to identify it as soon as possible in order to intervene promptly, changing the natural evolution of the disease and preventing complications.
The main problem in the diagnostic approach to the disease is represented by the poverty of suggestive clinical signs. In fact, diagnosis of NAFLD is often posed following the occasional finding of hypetransaminasemia and/or ultrasound abnormalities when these exams are performed for other reasons.
The categories most at risk of developing NAFLD are:
-
Obese (BMI > 95th percentile) or overweight (BMI > 85th percentile) children
-
Children with cardiometabolic risk factors (insulin-resistance, hypertension, dyslipidemia, cardiac and respiratory complications, and elevated waist circumference)
-
Children with familiarity for NAFLD
In literature, there is a lack of uniformity on what screening tool is more effective to identify the pathology in subjects at risk. The European Society of Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) suggests using both the measurement of serum alanine aminotransferase (ALT) concentration and liver ultrasound (US) as a screening method; the North American Society of Pediatric Gastroenterology Hepatology and Nutrition (NASPGHAN) indicates the use of ALT and not US. The National Institute for Health and Care Excellence (NICE) suggests using US and not ALT. Regardless of the method used, the finding of ALT > 30–40 IU/L or a US score ≥2 guarantees a specificity ≥90%, but the sensitivity of these tests, individually, does not exceed 50%, with an intrinsic risk of false negatives. It is also useful to remember that these methods do not provide any information on the degree of inflammation and liver fibrosis. Screening should be initiated between 9 and 11 years of age in obese or overweight children with a cardiometabolic risk factor and repeated every 2–3 years if the risk factors remain unchanged [1, 27, 28].
Hepatic steatosis is not synonymous for NAFLD: there are other pathologies that can lead to an increase in liver fat content (Table 17.1). The differential diagnosis of these conditions must take into account the patient’s anamnestic and clinical characteristics, as well as the outcome of laboratory tests, imaging studies, and liver biopsy.
5 Clinical Features
Diagnostic work-up starts with the evaluation of patient’s medical history followed by physical examination.
Medical History. A complete collection of data on the patient’s past and present clinical history is required. Two aspects need to be focused:
-
Familiar history of NAFLD or other liver diseases, metabolic syndrome, and obesity.
-
Evaluation of patient’s dietary habits, physical activity, lifestyle, and drug consumption.
Physical Examination. As previously stated, specific signs and symptoms do not characterize NAFLD, but it is possible to look for signs of comorbidity.
-
Measurement of waist circumference and calculation of BMI permit diagnosis and staging of overweight/obesity.
-
Blood pressure should be measured in all children and compared with age and sex-matched centiles.
-
The presence of striae rubrae or striae albae (atrophic purple or white linear bands of skin) is common in obese patients. Acanthosis nigricans is a hyperpigmentation of the flexural areas of the body (mainly cervical, axillary, and inguinal) suggesting of insulin resistance.
-
Hepatomegaly (palpable liver edge 2 cm below the right costal margin) is a common feature in patients with NAFLD, while signs of chronic hepatic disease (e.g., spider nevi, ascites, and splenomegaly) are rare in pediatric NAFLD. Some children with NAFLD suffer from fatigue or discomfort in the right upper abdomen quadrant.
6 Laboratory Tests
In the laboratory assessment of children with suspected NAFLD/NASH, it is possible to identify two test categories:
-
Routine laboratory test: widespread and accessible tests that allow an initial assessment and exclusion of other major liver disease
-
Novel biomarkers: second-level tests that allow, once diagnosed with NAFLD, a noninvasive estimation of the severity of the disease
Routine Laboratory Test:
-
Liver function: alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyltransferase (GGT). Increase in transaminases or GGT should be considered a warning sign for hepatic disease, but it is noteworthy that advanced liver fibrosis may have normal or mild elevation of these enzymes. Moreover, they do not correlate with NAFLD severity. Measurement of blood albumin, INR, and coagulation tests should be included in liver evaluation.
-
Glucose metabolism: fasting insulin and glucose should be assessed in all children. HOMA-IR is an index of insulin resistance and can be calculated as follows: fasting insulin (mU/L) × fasting glucose (μmol/L)/22.5. A value ≥2.5 is suggestive of insulin resistance. In selected patients (obese, presence of acanthosis nigricans, abnormal fasting insulin or glucose), oral glucose tolerance test (OGTT) and hemoglobin A1c dosage should be considered.
-
Lipid metabolism: triglycerides; total, LDL-, and HDL-cholesterol; and lipoproteins should be performed. The severity of liver disease is associated with a more atherogenic lipid profile.
-
Uric acid: a diet rich in fructose may increase uric acid concentrations. Hyperuricemia (UA ≥5.9 mg/dL) is an independent factor associated with a higher risk to develop NASH in NAFLD patients.
-
Others: blood counts, urea, electrolytes, thyroid function tests, and morning cortisol.
-
Differential diagnosis: depending on history and clinical information, it is possible to request specific test for possible causes of hepatic steatosis/hypertransaminasemia other than NAFLD (Table 17.2).
Novel Biomarkers. Once the diagnosis of NAFLD is made, one of the most difficult challenges is to estimate the severity of the disease in a noninvasive manner. Higher levels of C-reactive protein and proinflammatory cytokines, such as TNF-α, IL-1, and IL-6, have been associated with evolution from NAFLD to NASH, while two adipokines (adiponectin and retinol-binding protein 4) have been inversely correlated to the degree of liver damage; however all these markers lack of specificity for NAFLD.
Recently Citokeratin-18 (a marker of hepatocyte apoptosis) fragment levels and Cathepsin-D (a lysosomal protease) were identified as reliable markers of NASH. In fact, it has been demonstrated that Cathepsin-D has a high diagnostic value to distinguish pediatric patients with hepatic inflammation from children with steatosis, while Citokeratin-18 correlates significantly with hepatic fibrosis and with NAFLD severity. Actually, the main limitation of these markers is the applicability in community-based practices [29, 30].
7 Imaging Techniques
7.1 Ultrasonography
Because of its availability, security, and relatively low cost, ultrasound (US) is the most common imaging technique used to diagnose NAFLD. Hepatic steatosis is characterized by a liver echotexture more reflective (hyperechoic) when compared to that of the right kidney (“bright liver”); moreover the liver may appear increased in size. The assessment of hepatic echogenicity and liver vessels and diaphragm visualization allow the US grading of steatosis (Table 17.3).
US has some limitations: it is unable to distinguish NAFLD from NASH and to detect liver fibrosis. Moreover, it may be unreliable in evaluating severely obese patients (BMI ≥ 40 kg/m2) or when liver fatty liver infiltration is <30%. Finally, US is an operator- and machine-dependent technique, so its results may change depending on the operator’s experience and training [31].
7.2 Elastography
The term elastography refers to a series of imaging techniques that allow the estimation of liver tissue rigidity by measuring the propagation of specific elastic waves (shear waves, S-waves) emitted by the probe.
Elastography imaging can be US-based (e.g., transient elastography, FibroScan®) or magnetic resonance-based (magnetic resonance elastography, MRE).
These methods have proven to be effective in distinguishing NAFLD from NASH and in highlighting the presence and severity of fibrotic infiltration in the liver, especially in studies conducted in the adult population. The absence of certain cutoff values and the relatively poor diffusion of the equipment currently limit their use. Moreover, MRE is an expensive exam, and to date its application is limited mainly for clinical research purposes.
7.3 Other Imaging Techniques
Computed tomography (CT) should not be routinely used for the assessment of NAFLD in pediatrics because it has a nonacceptable cost-effectiveness ratio due to unjustified exposure to ionizing radiation in relation to information that can be obtained. Moreover, CT is unable to identify mild steatosis (sensitivity for steatosis detection estimated between 46 and 72%).
Magnetic resonance imaging (MRI) is actually considered the most accurate imaging technique to assess liver fat storage in NAFLD patients because it can differentiate tissues containing only water from those containing both fat and water.
MR spectroscopy (MRS) is a novel variant of classical MRI which quantifies triglycerides accumulation within hepatocyte through the measurement of acyl groups within the selected liver region of interest. MRS can discriminate healthy patients from those with NAFLD with a sensitivity of 92.6% and a specificity of 95.7% [32].
In younger children the correct execution of MR requires sedation of the patient. This element, associated with relatively high cost and the need of specific expertise for MRS, limits the application of MRI in everyday clinical practice.
8 Liver Biopsy
Liver biopsy (LB) is actually considered the gold standard for NAFLD diagnosis as it is the only method which can distinguish NAFLD from NASH and provide a reliable scoring system designed to estimate the severity of the disease. In addition, it may help in the differential diagnosis work-up and in detecting coexisting liver diseases. LB should not be performed in all children. In 2015 the ESPGHAN Hepatology Committee defined indications for LB in NAFLD pediatric patients (Fig. 17.1): it is important to highlight that LB should not be considered a screening method [33].
Before performing LB, a careful coagulation study is required because abnormal coagulation and/or thrombocytopenia may preclude LB execution. Furthermore, LB is contraindicated in case of ascites, biliary dilatation, peliosis, hemangioma, and anatomic variation such as abdominal situs inversus.
LB is burdened with some limitations: it is an invasive technique with minor and major complication risks. Pain and bleeding are the most common complications (84% and 2.8%, respectively); other reported complications are infections, visceral perforation, arteriovenous fistula, pneumothorax, hemothorax, and death (0.6%). Another limitation of LB is represented by sampling errors; in fact biopsy specimen could not be representative of whole-liver status. Finally, LB is an operator-dependent technique.
Due to limitations and invasiveness in recent years, new research strands have been developed to identify safe and equally effective methods for diagnosing and staging NAFLD, but to date the biopsy remains the “imperfect reference standard.”
9 Histopathology
By definition, NAFLD is characterized by the presence of hepatic steatosis in at least 5% of hepatocytes.
In relation to the histological features, NASH can be classified into three subtypes [34]:
-
Type 1: steatosis with ballooning degeneration and/or perisinusoidal fibrosis, without
-
portal involvement
-
Type 2: steatosis with portal inflammation and/or fibrosis, in the absence of ballooning degeneration or perisinusoidal involvement
-
Overlap type: coexisting elements from both type 1 and 2
9.1 Steatosis
In children, steatosis is most prominent in the zone 1 (periportal zone) of the hepatic acinus, with a decreasing trend of fatty hepatocyte presence from zone 1 to zone 3. In adolescents and adults, the steatosis pattern is opposite. In NAFLD hepatocellular steatosis is mainly macrovescicular (single fat lipid droplet in the cytoplasm shifting the nucleus to periphery) rather than microvescicular (multiple small drops not-displacing nucleus), but mixed macro-microvescicular steatosis is often observed [35].
9.2 Ballooning
Ballooning degeneration of the hepatocyte is the histological lesion indicatives of cell damage due to fat droplet accumulation, cytoskeletal injury, and intracellular fluid retention. Cells lose their original polygonal shape and become enlarged; cytoplasm is vacuolated and Mallory-Denk bodies (MDB) could be found. MDB are intracellular protein aggregates (mainly intermediate filaments) which are a typical feature of NASH even if they may be present in other chronic liver diseases (e.g., alcoholic steatohepatitis, Wilson disease, chronic cholestasis, and metabolic disorders) [36].
9.3 Inflammation
Inflammation in NAFLD, when present, is usually mild. Evidence of severe, confluent inflammation should raise suspicion for other liver diseases. The major type of inflammation highlighted in NASH is lobular inflammation; portal inflammation may be present and represents the stigma of type 2 NASH. Cells mainly involved in the inflammatory process are lymphocytes, histiocytes, and PMN leukocytes. These last can surround ballooned hepatocytes containing MDB, forming a complex known as satellitosis. Histiocytes instead are responsible for the formation of lipogranulomas [35].
9.4 Fibrosis
Fibrosis is the result of collagen and other extracellular matrix fiber depositions due to hepatic stellate cell activation. Fibrosis distribution pattern varies with age: in adults and older adolescents, onset of fibrosis is typically observed in acinar zone 3 (perisinusoidal/pericellular fibrosis), and as NASH progresses, portal areas are involved. In advanced diseases, bridging fibrosis and cirrhosis may develop [37]. On the other hand, in children and younger adolescents, fibrosis may begin from the acinar zone 1 with bridging fibrosis connecting portal areas [35]. Cirrhosis is rarely observed in pediatric subset, and when it’s present, it is most commonly macronodular or mixed. At the cirrhotic stage, the whole hepatic architecture may be subverted.
Other microscopic findings can be found in NAFLD as megamitochondria, acidophilic bodies, and vacuolated glycogen-filled nuclei, but they are nonspecific elements with little diagnostic significance.
9.5 Scoring Systems
In recent years, in parallel with the rise of pediatric obesity, an exponential increase in NAFLD frequency was observed in children, and consequently a greater number of LBs have been performed. This has imposed the need to design a standardized approach for assessing the severity of the disease; three principal scoring systems are used in clinical and research activities: the Brunt system, the NASH Clinical Research Network (CRN) system, and the Pediatric NAFLD Histological Score (PNHS). The Brunt system was described for the first time in 1999 and takes account of steatosis, ballooning, lobular and portal inflammation, and cell infiltrates to classify NAFLD in three categories (mild or grade 1, moderate or grade 2, severe or grade 3) [38].
The NASH CRN validated a histological score (NAFLD Activity Score or NAS) based on the evaluation of three parameters each of which is assigned a score (steatosis, 0–3; lobular inflammation, 0–3; and ballooning, 0–2). NAS results from the unweighted sum of these parameters for a total score ranging from 0 to 8. A NAS ≥5 is suggestive of NASH, while a NAS ≤2 excludes NASH [39].
PNHS was designed with the aim of developing a new scoring system that included portal inflammation. PNHS results from the weighted sum of steatosis, ballooning, and lobular and portal inflammation scores; a PNHS value of 85 has a sensitivity of 77% and a specificity of 98% for the prediction of NASH [40] (Fig. 17.2).
9.6 Genetic Tests
Genetic screening tests are now available: they are easy to perform and have a low cost and can assess the risk of the subject of developing severe forms of NAFLD. Currently, a simple oral swab that searches for mutations in a combination of four genes (KLF6, PNPLA3, SOD2, LPIN1), each of which is related to NAFLD, is able to estimate the risk of severe hepatopathy [41].
10 Therapy
The main objective of the NAFLD therapy is to halt the progression of liver damage and possibly restore the hepatic original histology, with the ultimate goal of improving the patient’s quality of life and reducing NAFLD-related morbidity and mortality. The cornerstone of therapy is represented by lifestyle changes, with hypocaloric diet and regular physical exercise, aimed at weight loss although this approach is burdened with a high failure rate due to poor adherence often associated with a low perception of disease. Pharmacological approaches for treating NAFLD are limited by the small number of randomized, controlled trials conducted in pediatric population, but recent studies have shown promising results.
11 Non-pharmacological Treatment
The first step in the treatment of pediatric NAFLD is lifestyle intervention, based on a healthy and balanced diet and physical activity.
In literature, no specific dietary regimens are described for children with NAFLD; however intervention must always take into account the state of health, comorbidity, and degree of activity of the child. As a general rule, caloric intake should be around 25–30 kcal/kg/day for overweight/obese patients and 40–45 kcal/kg/day for normal weight patients with macroelements respecting the following proportions: 50–60% carbohydrates, 25–30% fat, and 15–20% protein. Unsaturated fatty acids (ideally 2/3 of total lipid intake) should be preferred than saturated fatty acids (1/3 of total lipid intake), with a ω3–ω6 ratio equal to 1:4. The dietary program should encourage consumption of food with low glycemic index, in order to improve insulin sensitivity; it is therefore necessary to reduce the quantity of simple sugars, white rice, and white bread in favor of fruit, vegetables, and legumes.
As previously stated, fructose may play a central role in NAFLD pathogenesis, so it is crucial to avoid foods and beverages with high fructose content including soft drinks and energy drinks.
The dietary regimen should be accompanied with an active lifestyle, with regular physical activity preferring aerobic exercise, and restriction of time spent in front of the screens (TV, PC, smartphone, and tablet).
In patients with NAFLD, a gradual loss of weight is desirable since a rapid weight loss may be associated with a paradoxical effect resulting in worsening of the liver damage and increase in metabolic comorbidities. For these reasons, a weight reduction not exceeding 10% in 6 months is recommended.
LS intervention can improve liver histology, mainly steatosis, and other metabolic features as insulin resistance and dyslipidemia, but it is limited by the high failure rate, due to the poor and non-lasting compliance of patients and their families with a success rate estimated <10% 2 years after the onset of treatment [42].
Bariatric surgery (BS) and nonsurgical obesity treatments based on minimally invasive intragastric balloons are emerging as therapeutic alternatives to be carefully considered in obese children with NAFLD, mostly in patients with numerous, unsuccessful weight loss attempts. In 2015 a position paper of ESPGHAN has established eligibility criteria for BS in pediatrics: selected obese patients with BMI >40 kg/m2 and severe comorbidities (including NASH with advanced fibrosis) or with BMI more than 50 kg/m2 and mild comorbidities (including NASH) [43].
In a recent pediatric trial, laparoscopic sleeve gastrectomy (a restrictive intervention consisting in the removal of the gastric fundus) has proved to be more effective than lifestyle approach, even when combined with intragastric devices, in reducing NASH and fibrosis in obese patients after 1 year of treatment and in improving dyslipidemia, sleep apnea, and hypertension [44]. Despite encouraging results, BS should not be considered as a first-line therapy, and a careful evaluation of the patient, considering emotional, psychological, and clinical features, should be performed before performing surgery. Moreover, further studies are needed in order to evaluate long-term efficacy and safety of these procedures.
11.1 Pharmacological Treatment
In recent years, numerous studies have contributed to elucidate new aspects of the pathogenesis of NAFLD, which remains partially unknown. Understanding the pathogenic mechanisms is crucial to identify molecular targets in order to change the natural history of the disease. Limited knowledge of the pathogenesis and the low number of uniform RCTs available in pediatrics currently limit the use of pharmacological approaches in NAFLD.
11.2 Insulin Sensitizers
Insulin resistance is a common feature in obese patients with NAFLD. Metformin is a biguanide that acts both on lipid metabolism (inhibition of lipogenesis) and on glucose metabolism (reduction of gluconeogenesis and improvement of insulin sensitivity). The TONIC trial, conducted in 173 children with biopsy-proven NAFLD, showed that metformin (500 mg twice daily) was not superior to placebo in decreasing ALT level and/or in improving any histological lesion but ballooning degeneration [45]. To date metformin is not recommended for treating NAFLD in nondiabetic children.
Thiazolidinediones are agonists of peroxisome proliferator-activated receptors-gamma (PPAR-G), a group of nuclear receptors that improves insulin sensitivity and reduce hepatic fat content. Although promising results in adult studies, their applicability in pediatrics is limited by the risk of cardiotoxicity associated with the use of these drugs.
11.3 Antioxidants
Oxidative stress is implicated in NAFLD pathogenesis and acts a key role in the progression from steatosis to steatohepatitis mainly through the release of ROS (reactive oxygen species) from mitochondria. Vitamin E is the most studied antioxidant agent, but its real therapeutic value has not been fully clarified. In the TONIC trial, an arm of patients was treated with vitamin E (400 UI/day), but this group did not benefit from the treatment. Recently, other antioxidants, such as polyphenols (e.g., resveratrol, silymarin, epigallocatechin gallate, anthocyanin, curcumin, and quercetin), have been studied, but despite encouraging findings observed on murine models, human studies have not provided solid results and therefore require further investigations.
11.4 Ursodeoxycholic Acid
Ursodeoxycholic acid (UDCA) is a secondary bile acid, endogenously produced by the gut microbiota, which regulates cholesterol absorption, and it is largely used in biliary diseases in order to prevent formation of cholesterol gallstones. Recently UDCA was speculated to be involved in several other mechanisms, as glutathione synthesis and activation of glucocorticoid receptor, contributing to the antioxidant and anti-inflammatory pathways. Despite these effects, administration of UDCA does not result in concrete benefits in treating NAFLD [46].
11.5 Omega-3 Fatty Acids
Omega-3 fatty acids are a variety PUFAs. The most studied omega-3 in humans are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which can be found in fish, algae, and fish oil. Omega-3 are modulators of the transcription of genes regulating lipid metabolism and provide anti-inflammatory and insulin-sensitizing systemic activity.
Several trials evaluated the efficacy of omega-3 supplementation, alone or in combination with other compounds (e.g., vitamin D, Colin, and vitamin E), in improving the biochemical and histological parameters of NAFLD. DHA is effective in reducing liver steatosis, and, when combined with vitamin D, it ameliorates insulin resistance and lipid profile [47].
11.6 Probiotics
Considering the recent findings regarding the central role of intestinal dysbiosis in the pathogenesis of NAFLD through the so-called gut-liver axis, probiotics have been suggested as a possible therapeutic option for the treatment of the disease. In 2016, a triple-blind trial, conducted among 64 obese children with sonographic NAFLD, who were randomly allocated to receive probiotic capsule (containing Lactobacillus acidophilus, Bifidobacterium lactis, B. bifidum, and L. rhamnosus) or placebo for 12 weeks, showed that the probiotic group improved their lipid profile and waist circumference, decreased levels of AST and ALT, and had a higher percentage of subjects with remission of steatosis to US evaluation compared to placebo group [48].
11.7 Vitamin D
Vitamin D deficiency is frequently reported in obese patients with NAFLD: it is estimated that about 80% of children with steatosis present with Vitamin D insufficiency (≤20 ng/mL) or deficiency (≤10 ng/mL). Vitamin D is not only responsible for the calcium-phosphorus metabolism but is involved in different immunoregulatory processes (pleiotropic action). The combination of DHA and Vitamin D has proved to be effective in reducing the activation of hepatic stellate cells (involved in fibrotic accumulation) and fibrillary collagen deposit with the prospect of being able to revert the already existing fibrotic lesions [47].
11.8 Novel Treatments
The increase in the prevalence of NAFLD, associated with the absence of effective pharmacological treatments, has led to the study of new molecules to counteract NAFLD.
The farnesoid X receptor (FXR) is a nuclear receptor, expressed primarily in the liver and intestine, which binds to bile acids. When activated, FXR migrates into the cell nucleus and modulates the transcription of specific genes involved in the regulation of inflammation and glucose and lipid metabolism. Numerous studies, mostly based on animal models, have shown that the use of FXR-agonists (e.g., obeticholic acid) could improve hepatic steatosis and steatohepatitis [49].
Liraglutide is an analogue of glucagone-like peptide 1 (GLP-1), a gut-derived incretin hormone that induces weight loss and insulin sensitivity. In 2016, the LEAN study, conducted on 52 adult patients with NASH, showed that liraglutide led to histological resolution of steatohepatitis. The treatment was safe and well tolerated by the patients [50].
Among the various molecules under investigation, cysteamine bitartrate (an antioxidant) and pentoxifylline (PTX, a xanthine derivative with anti-inflammatory effects) are showing promising results, but further studies are needed to assess their efficacy and safety in pediatrics.
12 Implication for Liver Transplantation
NAFLD is often perceived by the patients as a “minor” disease compared to other liver conditions, but recent studies stated that fibrotic potential of NAFLD is as severe as that of chronic hepatitis C, with an average interval time of transition from NASH to cirrhosis estimated around 8–10 years [51]. Prevalence data have decreed NAFLD as the most widespread liver disease in Western countries. 30–40% and 3–5% of adult US population are affected by NAFLD and NASH, respectively: this reflects the risk for millions of people to develop, over the years, end-stage liver disease potentially requiring liver transplantation (LT). Over the past 25 years, in the USA, the number of LTs performed for NASH cirrhosis has doubled from 5.5 to 11% of all reported LTs, and to date NAFLD is the third cause of LT preceded only by alcoholic liver disease and hepatitis C virus. Considering the prevalence trend of NAFLD, the delay of diagnosis due to the absence of noninvasive diagnostic tool, the absence of effective treatments, and new antiviral drugs for HCV infections, NASH cirrhosis is expected to become the main indication for LT by 2030 [52].
Because of its natural history, LT for NASH cirrhosis is a rare occurrence in the pediatric context; in fact the average age of transplantation is around 58.5 ± 8 years old [53].
According to data extracted from the Scientific Registry of Transplant Recipients (SRTR) and United Network for Organ Sharing (UNOS) databases, the survival outcomes of NASH recipients at 1, 3, and 5 years after transplant were similar to LTs performed for other causes and were 87.6%, 82.2%, and 76.7%, respectively [54]. The principal cause of death after LT for NASH cirrhosis is attributable to sepsis and cardiovascular events. The latter account for 11% of death at 1 year in LT recipients, and it’s important to highlight that NAFLD patients have, per se, a higher cardiovascular risk because they often suffer from comorbidities such as insulin resistance, dyslipidemia, and high blood pressure. In fact, a BMI ≥ 40 kg/m2 and/or the diagnosis of diabetes mellitus type 2 are considered the principal risk factors for increased mortality after LT. In this perspective, a careful stratification of patients’ risk before transplantation is essential to predict the possibility of cardiovascular complications and to implement specific precautions.
A pathological entity that may occur in the follow-up of LT is posttransplant NAFLD, which may be a recurrence of disease or a first manifestation in patients previously not affected by NAFLD (de novo NAFLD). The posttransplant NAFLD is caused by the coexistence of both host and graft risk factors. In patients with NASH-related LT, the risk of recurrence is also due to the fact that transplantation does not improve the metabolic alterations (e.g., insulin resistance, dyslipidemia, intestinal dysbiosis) which predispose to NAFLD and can act on transplanted organ. Moreover, there are genetic factors that increase the odds of recurrence (e.g., the presence of specific polymorphisms of the PNPLA3 gene) which is why these patients should be identified before transplantation and subjected to more stringent controls over time.
Another issue regarding LT and NAFLD is the impact of NAFLD on liver donors. The decreasing liver quality could compromise LT volume; in fact a recent analysis has hypothesized that liver utilization could fall from 78 to 44% by 2030 because of NAFLD [55].
To date, there are no standardized protocols for the assessment of donor liver. A liver with a fat content <30% can be safely used for transplantation, while if the percentage is >60%, the organ cannot be used because of high risk of primary nonfunctioning due to the intrinsic reduced tolerance to ischemic stress and the greater possibility of reperfusion injury. To date, there are no univocal approaches when the steatosis is between 30 and 60% [56].
NAFLD is a pathology of public interest with health and economic implications. Actually the main obstacles in clinical practice are represented by the absence of a noninvasive method capable of diagnosing and staging the disease and the need to identify an effective treatment in reversing the natural history of the disease. Epidemiological data and projections on the future are not reassuring, so much so that NAFLD is expected to become the first cause of liver transplantation in the next 15 years.
Considering all these elements, it is essential to undertake national and international prevention policies in order to halt the “NAFLD epidemic” and to protect the health of children and adolescents.
References
Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–13.
Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218–29.
Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–10.
Sayin O, Tokgoz Y, Arslan N. Investigation of adropin and leptin levels in pediatric obesity-related nonalcoholic fatty liver disease. J Pediatr Endocrinol Metab. 2014;27:479–84.
Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:195–203.
Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–44.
Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080.
Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–20.
Nobili V, Putignani L, Mosca A, et al. Bifidobacteria and lactobacilli in the gut microbiome of children with non-alcoholic fatty liver disease: which strains act as health players? Arch Med Sci. 2018;14(1):81–7.
Li ZZ, Berk M, McIntyre TM, et al. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284(9):5637–44.
Lin YC, Chang PF, Chang MH, et al. Genetic variants in GCKR and PNPLA3 confer susceptibility to nonalcoholic fatty liver disease in obese individuals. Am J Clin Nutr. 2014;99:869–74.
Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20(5):379–85.
Pappachan JM, Babu S, Krishnan B, et al. Non-alcoholic fatty liver disease: a clinical update. J Clin Transl Hepatol. 2017;5(4):384–93.
Selvakumar PKC, Kabbany MN, Nobili V, et al. Nonalcoholic fatty liver disease in children: hepatic and Extrahepatic complications. Pediatr Clin N Am. 2017;64(3):659–75.
Mann JP, Raponi M, Nobili V. Clinical implications of understanding the association between oxidative stress and pediatric NAFLD. Expert Rev Gastroenterol Hepatol. 2017;11(4):371–82.
Doulberis M, Kotronis G, Gialamprinou D, et al. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism. 2017;71:182–97.
Poeta M, Pierri L, Vajro P. Gut-liver axis derangement in non-alcoholic fatty liver disease. Children (Basel). 2017;4(8).
Silva Figueiredo P, Carla Inada A, Marcelino G, et al. Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients 2017;9(10).
Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65(2):451–64.
Della Corte C, Vajro P, Socha P, Nobili V. Pediatric non-alcoholic fatty liver disease: recent advances. Clin Res Hepatol Gastroenterol. 2014;38(4):419–22.
Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2017;16(Suppl. 1: s3-105):s15–20. https://doi.org/10.5604/01.3001.0010.5494.
Molinaro A, Wahlström A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab. 2018;29(1):31–41.
Nobili V, Alisi A, Mosca A, et al. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int. 2018;38(2):342–9.
Carpino G, Pastori D, Baratta F, et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: a possible role for oxidative stress. Sci Rep. 2017;7(1):15756.
Nobili V, Bedogni G, Donati B, et al. The I148M variant of PNPLA3 reduces the response to docosahexaenoic acid in children with non-alcoholic fatty liver disease. J Med Food. 2013;16:957–60.
Mangge H, Baumgartner BG, Zelzer S, et al. Patatin-like phospholipase 3 (rs738409) gene polymorphism is associated with increased liver enzymes in obese adolescents and metabolic syndrome in all ages. Aliment Pharmacol Ther. 2015;42:99–105.
Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–34.
Glen J, Floros L, Day C, et al. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. 2016;354:i4428.
Mandelia C, Collyer E, Mansoor S, et al. Plasma cytokeratin-18 level as a novel biomarker for liver fibrosis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2016;63(2):181–7.
Walenbergh SM, Houben T, Hendrikx T, et al. Plasma cathepsin D levels: a novel tool to predict pediatric hepatic inflammation. Am J Gastroenterol. 2015;110(3):462–70.
Di Martino M, Koryukova K, Bezzi M. Imaging features of non-alcoholic fatty liver disease in children and adolescents. Children (Basel). 2017;4(8).
Di Martino M, Pacifico L, Bezzi M, et al. Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver steatosis in children and adolescents. World J Gastroenterol. 2016;22(39):8812–9.
Dezsőfi A, Baumann U, Dhawan A, et al. Liver biopsy in children: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2015;60(3):408–20.
Mann JP, De Vito R, Mosca A, et al. Portal inflammation is independently associated with fibrosis and metabolic syndrome in pediatric nonalcoholic fatty liver disease. Hepatology. 2016;63(3):745–53.
Kleiner DE, Makhlouf HR. Histology of NAFLD and NASH in adults and children. Clin Liver Dis. 2016;20(2):293–312.
Strnad P, Zatloukal K, Stumptner C, et al. Mallory-Denk-bodies: lessons from keratin-containing hepatic inclusion bodies. Biochim Biophys Acta. 2008;1782(12):764–74.
Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16(42):5286–96.
Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–74.
Kleiner DE, Brunt EM, Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Alkhouri N, De Vito R, Alisi A, et al. Development and validation of a new histological score for pediatric non-alcoholic fatty liver disease. J Hepatol. 2012;57(6):1312–8.
Nobili V, Donati B, Panera N, et al. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2014;58(5):632–6.
Nobili V, Marcellini M, Devito R, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44(2):458–65.
Nobili V, Vajro P, Dezsofi A, et al. Indications and limitations of bariatric intervention in severely obese children and adolescents with and without nonalcoholic steatohepatitis: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2015;60(4):550–61.
Manco M, Mosca A, De Peppo F, et al. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. J Pediatr. 2017;180:31–37.e2.
Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68.
Vajro P, Franzese A, Valerio G, et al. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–43.
Della Corte C, Carpino G, De Vito R, et al. Docosahexanoic acid plus vitamin D treatment improves features of NAFLD in children with serum vitamin D deficiency: results from a single centre trial. PLoS One. 2016;11(12):e0168216.
Famouri F, Shariat Z, Hashemipour M, et al. Effects of probiotics on non-alcoholic fatty liver disease in obese children and adolescents: a randomized clinical trial. J Pediatr Gastroenterol Nutr. 2017;64:413–7.
Kim S-G, Kim B-K, Kim K, et al. Bile acid nuclear receptor Farnesoid X receptor: therapeutic target for nonalcoholic fatty liver disease. Endocrinol Metab (Seoul). 2016;31(4):500–4.
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90.
Charlotte F, Le Naour G, Bernhardt C, et al. A comparison of the fibrotic potential of nonalcoholic fatty liver disease and chronic hepatitis C. Hum Pathol. 2010;41(8):1178–85.
Shaker M, Tabbaa A, Albeldawi M, et al. Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol. 2014;20(18):5320–30.
Alkhouri N, Hanouneh IA, Zein NN, et al. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl Int. 2016;29(4):418–24.
Zezos P, Renner EL. Liver transplantation and non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(42):15532–8.
Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21(8):1040–50.
Pais R, Barritt AS, Calmus Y, et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65(6):1245–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mosca, A., Veraldi, S., Dellostrologo, A., Sanseviero, M., Nobili, V. (2019). Nonalcoholic Fatty Liver Disease and Steatohepatitis in Children. In: D'Antiga, L. (eds) Pediatric Hepatology and Liver Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-96400-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-96400-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96399-0
Online ISBN: 978-3-319-96400-3
eBook Packages: MedicineMedicine (R0)