Abstract
Diabetic retinopathy is a complication of diabetes. Majority of diabetic patients with high blood glucose face the challenge of dealing with retinopathy and macular edema as the disease progresses. Although treatment choices and care are available to manage and symptomatically treat Diabetic retinopathy the current understanding is limited and lacks options for treatment and rescue strategies. Pharmacological options include anti-VEGF treatment strategies and surgical procedures. The present review provides insights in to type of diabetic retinopathy, along with different stages of the disease. In addition, the roles for health care providers, importance of pharmacists for treatment and management of diabetic retinopathy patient care are discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Diabetic retinopathy (DR) is a complication of diabetes that affects the eyes. Diabetes affects insulin production and sensitivity and therefore the ability to absorb glucose, leading to high blood sugar levels. When blood sugar levels are high, damage can occur to the blood vessels of the light-sensitive retina, which allows for vision. For instance, high blood sugar levels can cause a narrowing or blockage of the retinal arteries and lead to reduced or no blood flow to the retina. As a result, endogenous processes trigger angiogenesis allowing growth of new blood vessels, but this leads to further complications. These changes to the retina affect vision and can cause blindness in diabetics.
Significance
DR holds medical, social, and economic significance. The disease affects up to 80% of people who have had diabetes for more than 20 years and accounts for 12% of all new cases of blindness. Globally, it is the leading cause of vision loss, affecting an estimated 285 million people worldwide. Recent studies have determined that about one in three people with diabetes have DR. Since it is such a prevalent health issue, the economic significance of DR is also high. In the United States alone, it is estimated that $500 million is spent on diabetes-related blindness costs. Worldwide, it is estimated that $232 billion is spent on diabetes and its complications. Given the enormous cost and financial burden that DR brings, it is essential to take measures to prevent DR, possibly by keeping good control of blood sugar levels and by early detection of eye diseases. Given the increasing prevalence of diabetes, the worldwide costs associated with it are projected to rise even more.
Studies have also been conducted to find correlations between DR and socioeconomic status . However, clear, strong relations could not be found. This weak or absent correlation can be attributed to a number of competing influences, including lifestyle, health behaviors, attitude, mortality rate, and health-care systems. For instance, a higher socioeconomic group, which receives benefits of good diabetes care and treatment, may counter those effects with a sedentary lifestyle and the consumption of western foods. This lack of correlation does not negate the importance.
There are different classifications of DR , including nonproliferative diabetic retinopathy (NPDR), proliferative DR, diabetic maculopathy, and advanced diabetic eye disease.
Patient Care Overview
Although with recent studies diabetic retinopathy (DR) and diabetes macular edema (DME) are two common ophthalmic complications for the diabetic patients, it can be kept under control through patient awareness and by adopting simple changes in lifestyle. Early detection, treatment, and improved glycemic control can limit the onset or progression of DR and DME.
The primary management for DR and DME includes three main therapies:
-
Laser photocoagulation
-
Intravitreal vascular endothelial growth factor (VEGF) inhibitors
-
Intravitreal corticosteroid implants
DR and DME have multifactorial etiology due to the fact that combination therapy is gaining more popularity. Even though the change in lifestyle and regular screening can prevent and/or reduce the effect of DR and DME, health-care providers and patient’s adherence is poor and needs to be regulated with proper management. Screening and prevention goes hand in hand, and it is observed that 40% of the patients with DM and DME can prevent further ocular complications presented with their routine ocular screening. Diabetic eye exam compliance in a US Medicaid population increased from 46% to 64% between 2010 and 2012. The economic cost for treating vision complication due to diabetes mellitus is estimated to be about $490 million each year indicating the burden for patients and managed care system imposed by DR and DME.
Though treatments are available to manage complications due to DR and DME, the length of the treatment causes additional burden for patients and managed care system due to necessity of longer duration of the treatment. Health plans, accountable care organizations, and other providers have more interest in investing time and proper education in ensuring their patients with diabetes (DM) receive proper vision screening and maintain adequate disease control to avoid complication due to DM which includes DR and DME . It is crucial to manage cost-effectiveness of currently available treatments of DR or DME as well as identify opportunities to improve patient adherence to treatment.
Health-Care Providers and Pharmacist’s Role
Encourage adherence to eye exam visits in patients with diabetes and for managing DR or DME.
Focus on Preventative Strategies
-
Glycemic control
-
Blood pressure control
-
Lipid control
-
Proteinuria and BUN/creatinine ratio
DR and DME Patient Education
-
Increase awareness, describe risk of vision loss, explain how to prevent by addressing barriers to effective diabetes care, and use motivational interviewing.
-
Lack of education – speak in layperson terms and provide reminders for routine eye exams.
-
Explain therapy requirements (frequent visits), cost, and possible adverse effects.
-
Monitor therapy safety and efficacy; describe what to expect with therapy, stopping vision loss, vision improvement expectations, etc.
The following simple chart can help the patient to manage DR and DME during different disease stages
Disease stage | Presents with one of the following | Management |
|---|---|---|
Early | Multiple small drusen Few medium-sized drusen Mild retinal pigment epithelial (RPE) abnormalities | Quite smoking Control body mass index (BM) and blood pressure Increase dietary intake of antioxidants |
Intermediate | Numerous medium-sized drusen At least one large druse Geographic atrophy | Keep lifestyle the same as the early disease stage Antioxidant supplements like AREDS and AREDS2 |
Advanced “dry” stage | Drusen with atrophy in the center of the macula | Keep lifestyle the same as the early disease stage Antioxidant supplements like AREDS and AREDS2 |
Advanced “wet “stage | Neovascularization with hemorrhage Lipid deposits Swelling and damage to the macula capillaries | Keep lifestyle the same as the intermediate disease stage Vascular endothelial growth factor(VEGF) inhibitors Anti-angiogenic therapy Laser therapy |
Study on Different VEGF Inhibitor Treatment Costs
In a previously published report [1], the researchers calculated the incremental cost-effectiveness ratios (ICERs) of the three drugs. One-year trial data were used to calculate cost-effectiveness for 1 year for the three anti-VEGF drugs. In addition, the researchers used mathematical modeling to project 10-year cost-effectiveness. In the patients with worse vision, 20/50 or more, aflibercept improves vision to a greater extent than bevacizumab or ranibizumab . At vision levels better than this, all drugs perform equally well.
The current study found that the more expensive agent (aflibercept) performs better in patients with worse vision. The study results also underscore the possibility of effective as-needed treatment [2]. Fixed-interval dosing would be superior to as-needed dosing. They randomly assigned participants to receive intravitreous aflibercept (2.0 mg), bevacizumab (1.25 mg), or ranibizumab (0.3 mg). Patients were treated on a needed basis as often as every 4 weeks, as opposed to on fixed dose intervals. From baseline to 1 year, the mean visual acuity letter score improved by 13.3 with aflibercept, 9.7 with bevacizumab, and 11.2 with ranibizumab. A closer examination of the data, however, revealed that the difference between the drugs was driven by improvements in eyes with worse visual acuity at baseline.
Laser photocoagulation therapy reduces risk of vision loss in patients with high-risk proliferative diabetic retinopathy and, in some cases, severe nonproliferative diabetic retinopathy.
Per Year Cost Comparison

Gaps in DR and DME Care
-
Studies involving anti-VEGF therapies need to better translate to clinical practice and results be clinically significant.
-
Nine injections during the first year of treatment are impractical and lead to noncompliance.
-
Ability to read one additional line on an eye chart may not have meaningful functional value.
-
Lack of evidence for treatment non-responders.
-
Necessary DME-related services such as screening, diagnosis, treatment, and ongoing care may not be covered by insurance providers.
-
Precise data on DME financial impact to individual and society are needed to justify costs advocating for improved treatment and outcomes for diabetic macular edema.
References: The American Journal of Managed Care
Pharmacy Times Continuing Education
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3627413/
Medscape Medical News from the Source: U.S. Census Bureau, Current Population Survey, 1968 through 2016 Annual Social and Economic Supplements.
Pathogenesis
The pathogenesis or development of DR can occur in various ways. Due to the high blood sugar levels, arterial walls of diabetics can thicken and become narrow. In the eyes, this leads to less blood flow, therefore causing DR [3]. The vascular and hematological changes of diabetic patients also lead to thickening of the capillary basement membrane, causing capillary endothelial cell damage. Red blood cells become deformed, leading to increased stickiness of platelets and increase plasma viscosity [3]. All these symptoms can in turn lead to microvascular occlusions, in which the artery leading to the eyes, the ophthalmic artery, is clamped off. This prevents bleeding and rupture. In order to bypass this occluded artery, new arteries could grow and branch off as seen in proliferative DR. Occlusion then leads to retinal ischemia, which is the state in which blood supply to the eye is cut off [4]. The blood in the artery before the occlusion pools up, causing the artery to enlarge. This ballooning and weakened area in the artery, referred to as a microaneurysm, could rupture, leading to a hemorrhage and retinal edema . This can lead to neovascularization [4].
Factors Affecting DR
Many factors influence the likelihood and onset of DR. For instance, age and puberty significantly affect DR because of the hormonal factors responsible for growth that are involved. High levels of IGF1 and IGF2, smoking, anemia, obesity, and hyperlipidemia can all lead to the progression of DR [5]. Poor metabolic control, specifically hyperglycemia, can accentuate the progress of DR, along with ocular factors such as glaucoma, hypertension, and pregnancy [5]. DR can also be genetic, leading to increased risk of proliferative retinopathy in people with HLA DR4 and DR3 genes [5]. Whereas all of these factors increase the prevalence and likelihood of DR, myopia can decrease the prevalence and severity of retinopathy.
Different Types of DR
Nonproliferative Diabetic Retinopathy
There are two main types of DR, namely, nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy. NPDR is an early stage of DR in which tiny blood vessels within the retina leak blood or fluid, causing the retina to swell. NPDR can be characterized by microaneurysms, retinal hemorrhages, edema, hard exudates (yellowish waxy patches arranged in circinate pattern), and venous abnormalities such as beading, looping, and dilation [6]. Apart from these, NPDR can also be characterized by cotton wool spots that are small, white, superficial areas which represent areas of nerve fiber infarcts. These are a sign that the eye is not getting enough oxygen. Intraretinal microvascular abnormalities (IRMA), which are fine, irregular red lines connecting arterioles with venues representing AV shunts, are also characteristic of NPDR. NPDR can be classified into different stages , namely, mild, moderate, severe, and very severe [6].
Proliferative Diabetic Retinopathy
As the conditions of NPDR approach the severe stage, the eye may be forced to form new arteries through a process called neovascularization. This then becomes known as proliferative DR, in which new arteries form in order to bring oxygen to the hypoxic retina. Proliferative DR affects around 5–10% of the total population and develops in more than 50% of DR cases 25 years after the onset of diabetes [7]. The primary feature of PDR is neovascularization, which is caused by the angiogenic factors elaborated by the retinal tissue in an attempt to neovascularize the hypoxic retina. The angiogenic factors are most commonly the vascular endothelial growth factors(VEGF) with isoforms like VEGF-A, VEGF-B, VEGF-C, and VEGF-D, placental growth factors, pigment epithelium-derived factors, etc. Similarly, there are several endogenous inhibitors of angiogenesis such as endostatin, platelet factor 4, and angiostatin [7]. It is hypothesized that the net balance between the VEGF and endostatin is associated with retinopathy. About one quarter of the retina has to be non-perfused before PDR develops [7].
There are two main types of proliferative DR, including PDR without high-risk characteristics and PDR with high-risk characteristics, which is also known as advanced PDR. The high-risk characteristics include neovascularization of the disc (NVD) to one fourth of the disc area, less than one fourth of the disc area , or more than half of the disc area with vitreous hemorrhage (VH) or preretinal hemorrhage (PRH) [7].
Further Side Effects
Diabetic Maculopathy
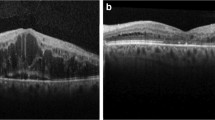
DR can lead to further complications of the patient, such as diabetic maculopathy and advanced diabetic eye disease. Diabetic maculopathy is a condition that arises from retinopathy [8]. It is concerned with damage to a specific part of the retina, the macula. When this swelling occurs in the central part of the retina (the macula), it is known as macular edema. Since the macula is the region of keenest vision, swelling of the macula could lead to reduced or blurred vision, whereas leakage or swelling elsewhere in the retina will usually not have too severe of an effect on vision. This swelling, or edema, occurs due to increased permeability of the retinal capillaries. Symptoms for diabetic maculopathy include trouble reading and recognizing faces in the center of your vision. There are also four different classifications of maculopathy, namely, focal exudative maculopathy , diffuse exudative maculopathy, ischemic maculopathy, and mixed maculopathy [8].
The diagnoses for clinically significant macular edema (CSME) can be made if one of the three criteria is present on slit-lamp examination within a 90D lens: thickening of hard exudates at or within 500 microns of the center of the fovea associated with adjacent retinal thickening, the retina at or within 500 microns of the center of the fovea , and development of zone of retinal thickening 1 disc diameter or larger in size at least a part of which is within 1 disc diameter of foveal center.
Treatment for diabetic maculopathy is most commonly done using laser photocoagulation [9]. One specific type of laser treatment is the focal treatment, in which burns are applied to microaneurysms and microvascular lesions located 500–3000 μm from the center of the macula. The spot size is 50–100 μm, and the exposure time is 0.1 s, with sufficient power to obtain a gentle whitening or darkening of the lesion. In another laser treatment, known as the grid treatment , burns are applied to areas of diffuse retinal thickening of more than 500 μm from the macula. The spot size is again 100 μm, and the exposure time is 0.1 s, resulting in a high-intensity burn [9].
Another treatment option apart from the laser photocoagulation is a pars plana vitrectomy [10]. Vitrectomy is a surgery to remove the vitreous gel containing retinal detachment or blood. This procedure can give better access to the retina of the eye and can get rid of the edema. The pars plana vitrectomy is named as such since the instruments used to do the procedure go through the pars plana, or the flat portion of the ciliary body located near the point where the iris and sclera touch. This procedure is done only if severe persistent vitreous hemorrhage is present or if there is a premacular subhyaloid hemorrhage [10].
Advanced Diabetic Eye Disease
Another side effect of DR is advanced diabetic eye disease. This is characterized by vision-threatening complications in patients whose laser photocoagulation treatments have been unsuccessful or inadequate. There are many methods of diagnosis for this disease. Some characteristics used for diagnosis are persistent vitreous hemorrhage, tractional retinal detachment (caused by progressive contraction of fibrovascular membranes over areas of vitreoretinal attachment), tractional retinoschisis, and rubeosis iridis (caused by retinal ischemia) [11]. This disease can be preretinal, intragel, or both. Intragel hemorrhages take longer to clear than preretinal hemorrhages because the former result in more extensive bleeding. Patients should be warned that bleeding might be precipitated by severe exertion or straining , hypoglycemia, or direct ocular trauma. The treatment for this disease again is usually pars plana vitrectomy [10].
Treatment
Screening
Patients with DR should be screened frequently to monitor the condition of their disease. Typically, diabetics should be screened every year for symptoms of NPDR. Patients who already have moderate NPDR should be screened every 6 months to ensure that it is under control and not worsening. Patients with severe NPDR should be screened every 3 months, and patients with PDR should be screened every 2 months. Frequent screenings can be advantageous for the prevention and control of DR in diabetic patients [12].
Drug Delivery
Medical drugs can be taken to help treat DR. Delivery of drugs to the posterior eye is challenging, owing to anatomical and physiological constrains of the eye [13]. There is an increasing need for managing rapidly progressing posterior eye diseases, such as age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa. Drug delivery to the posterior segment of the eye is therefore compounded by the increasing number of new therapeutic entities (e.g., oligonucleotides, aptamers, and antibodies) and the need for chronic therapy. Currently, the intravitreal route is widely used to deliver therapeutic entities to the retina. However, frequent administration of drugs via this route can lead to endophthalmitis, increased intraocular pressure, and retinal detachment. Various controlled delivery systems, such as biodegradable and non-biodegradable implants, liposomes, and nanoparticles, have been developed to overcome such adverse effects, with some success [13]. The periocular route is a promising alternative, owing to the large surface area and the relatively high permeability of the sclera. Yet, the blood–retinal barrier and efflux transporters hamper the transport of therapeutic entities to the retina . As such, the efficient delivery of drugs to the posterior eye remains a major challenge facing the pharmaceutical scientist [13].
The first line of drugs in the treatment of DR are anti-VEGF (anti-vascular endothelial growth factors). These drugs work by stopping a protein called vascular endothelial growth factor (VEGF), which is produced by the cells in the retina, from working. The overproduction of VEGFs has been connected to hypoxia, growth of new blood vessels, and consequently blindness. The two most widely used anti-VEGF drugs to counter this problem are bevacizumab (Avastin) and ranibizumab (Lucentis) [14, 15].
Specific Anti-VEGF Drugs
Bevacizumab, commercially known as Avastin, is a full-length, recombinant, humanized monoclonal antibody that works against all VEGF isoforms. It is used to treat eye diseases as well as a number of different cancers. It works by binding to all isoforms of VEGF-A and inhibiting their activity. The typical dose taken is 1–1.25 mg or 0.05 mL [14].
Ranibizumab, commercially known as Lucentis, is a genetically manipulated version of bevacizumab. It is a monoclonal antibody fragment (Fab) that is anti-angiogenic and has been approved to treat macular diseases and vision loss. Because it is genetically manipulated from the same parent mouse antibody as bevacizumab, its effectiveness is also similar to that of bevacizumab. The antibody works by inhibiting VEGF-A. All isoforms are also bound, including VEGF-110, a plasmin-cleaved form of VEGF165. The normal dosage of ranibizumab is 0.3–0.5 mg. Although ranibizumab has been proven to be relatively safe, some side effects may include conjunctival hemorrhage, eye pain, or intraocular inflammation [16].
Another anti-VEGF drug that is used is pegaptanib sodium, commercially known as Macugen . This is another anti-angiogenic drug, used to treat neovascular macular degeneration. It acts by binding specifically to the pathological 165 isoform of VEGF, which is the most important in angiogenesis, and blocking its actions, therefore reducing the growth of blood vessels and working to control leakage and swelling. An advantage of this drug is that it spares the normal vasculature, therefore giving it a dual mechanism of anti-angiogenesis and anti-permeability. The normal dosage is 0.3 mg or 90 μL [17, 18].
Anecortave acetate, commercially known as Retaane, is also known as an angiogenic steroid because of its functions. It inhibits the remodeling of basement membranes and extracellular matrix components in angiogenesis, as well as the expression of VEGF in smooth muscles. It can also be used to treat age-related macular degeneration and to reduce intraocular pressure. Anecortave acetate is delivered via the posterior juxtascleral depot (PJD) that delivers the drug onto the sclera near the macula. This method allows for decreased intraocular infection and retinal detachment. Retaane is typically delivered once every 6 months. Possible complications of this drug include endophthalmitis, vitreous hemorrhage, persistent floaters, rise in IOP, retinal pigment epithelial tear, and retinal detachment [19].
Other Methods of Treatment
Although anti-VEGF drugs are the most common method of treatment for DR, there are other options as well. For example, protein kinase C is an intracellular signaling molecule, whose activation plays an important role in the development of ocular complications. PKC inhibitors can diminish blood flow related to hyperglycemia and therefore has potential use as a therapy for DR. Other methods can include the use of aldose reductase and ACE inhibitors, antioxidants such as vitamin E, and intravitreal steroids such as fluocinolone acetonide implants and intravitreal injections of triamcinolone at a 2–4 mg dosage [17].
Routes of Drug Delivery
Systemic, topical, periocular, and intravitreal routes are used to deliver pharmaceuticals to the posterior segment of the eye. The topical route has a lower bioavailability due to rapid drainage through the nasolacrimal ducts, a hydrophobic corneal epithelium, the blood–aqueous barrier, and the systemic absorption. Conversely the blood–retinal barrier (BRB) hinders the diffusion of systemically administered drugs to the posterior segment of the eye. Thus, the ideal routes of drug delivery are the periocular and the intravitreal routes.
Intravitreous injection of anti-VEGF, antibiotics, and steroids is the currently accepted route of administration to treat posterior segment diseases, such as diabetic retinopathy, age-related macular degeneration (AMD), vascular occlusions, cystoid macular edema, uveitis, viral retinitis, endophthalmitis, and retinal detachment. This enables direct application of the drug eliminating the barriers which are common with topical and systemic administration. A higher intraocular bioavailability yields more efficacious treatment of posterior segment diseases. Intravitreal injections are typically given at pars plana 3.5–4 mm posterior to the limbs. The availability of infusion devices such as insulin pumps (Fornia, Zhuhai, Guangdong, China) has also added to improve treatment modalities [17].
Periocular routes comprising of the retrobulbar, peribulbar, subtenon, and subconjunctival administration of drugs enable the molecules to be deposited on the external surface of the sclera, thus minimizing the risk of endophthalmitis and toxic retinal reactions. Of these, the subtenon route is considered to be the most effective method to treat posterior segment diseases of the eye [17].
Conclusion
DR is a widely prevalent disease and a common cause of visual loss. It can progress in the absence of symptoms, producing irreversible damage to the retina. The key to managing this ailment is realizing that prevention is better than treatment and a repeated follow-up of these patients to detect the earliest sign of diabetic retinopathy. Interventions are most efficacious when started early in the disease, when retinal damage is minimal and clinical findings are few or absent. Periodic ophthalmoscopic examinations are essential in detecting the progression of retinopathy and development of disease characteristics which indicate a need for treatment. Regular screening examinations along with intensive control of hyperglycemia, serum lipid levels, and blood pressure not only retard the progression of DR but also contribute to reducing cardiovascular mortality.
References
Colquitt J, Jones J, Tan S, Takeda A, Clegg A, Price A. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess. 2008;12(16):iii.
Spooner K, Hong T, Wijeyakumar W, Chang AA. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017;Volume 11:161–77.
Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38(10):1203–6.
Bresnick GH, Venecia GD, Myers FL, Harris JA, Davis MD. Retinal ischemia in diabetic retinopathy. Arch Ophthalmol. 1975;93(12):1300–10.
Marshall G, Garg SK, Jackson WE, Holmes DL, Chase HP. Factors influencing the onset and progression of diabetic retinopathy in subjects with insulin-dependent diabetes mellitus. Ophthalmology. 1993;100(8):1133–9.
Bandello F, Lattanzio R, Zucchiatti I, Petruzzi G. Non-proliferative diabetic retinopathy. In: Clinical strategies in the management of diabetic retinopathy. Springer-Verlag: Berlin Heidelberg; 2014. p. 19–63.
Shah KB, Han DP. Proliferative diabetic retinopathy. Int Ophthalmol Clin. 2004;44(4):69–84.
Ivanisevic M. Stage M1: Maculopathy. A practical manual of diabetic retinopathy management; 2009. p. 70–98.
Dowler JGF. Laser management of diabetic retinopathy. J R Soc Med. 2003;96(6):277–9.
Schwartz SG, Flynn HW. Pars plana vitrectomy for primary rhegmatogenous retinal detachment. Clin Ophthalmol. 2008;2:57.
Cleary PE. The treatment of advanced diabetic eye disease. Ir J Med Sci. 1979;148(S2):38–44.
Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283(7):889.
Macha S, Hughes P, Mitra A. Overview of ocular drug delivery. In: Ophthalmic drug delivery systems, vol. 25. 2nd ed. New York: Dekker; 2003. p. 1–12.
Grisanti S, Ziemssen F. Bevacizumab: off-label use in ophthalmology. Indian J Ophthalmol. 2007;55(6):417.
Waisbourd M, Goldstein M, Loewenstein A. Treatment of diabetic retinopathy with anti-VEGF drugs. Acta Ophthalmol. 2010;89(3):203–7.
Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M, Loke YK. A safety review and meta-analyses of bevacizumab and Ranibizumab: off-label versus Goldstandard. PLoS One. 2012;7(8):e42701.
Abraham A, Senthil S. Clinical ophthalmology: made easy. New Delhi: Jaypee Brothers Medical Publishers; 2013.
Galvez MIL. Protein kinase C inhibitors in the treatment of diabetic retinopathy. Review. Curr Pharm Biotechnol. 2011;12(3):386–91.
Augustin A. Anecortave acetate in the treatment of age-related macular degeneration. Clin Interv Aging. 2006;1(3):237–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Gudla, S., Tenneti, D., Pande, M., Tipparaju, S.M. (2018). Diabetic Retinopathy: Pathogenesis, Treatment, and Complications. In: Patel, J., Sutariya, V., Kanwar, J., Pathak, Y. (eds) Drug Delivery for the Retina and Posterior Segment Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95807-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-95807-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95806-4
Online ISBN: 978-3-319-95807-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)