Abstract
The eye is an immune-privileged site and is more amenable to genetic therapy. A number of diseases in the posterior segment of the eye have genetic origin. The posterior segment of the eye is difficult to access. But routes to deliver drugs and genes have been worked upon. The genetic therapy ensures long-term treatment of the disease in the eye. The genes that are to be delivered to their proper site can be given through viral and non-viral vectors. Viral vectors offer a number of advantages when it comes to transfection efficiency and gene expression but are associated with immunity issues and cannot be loaded with genes which are more than 5 kb. Over the years non-viral gene vectors are becoming increasingly popular because of their higher gene loading capacity and number of distinct advantages over the viral vectors. But the genes that are to be delivered to the nucleus have to escape a number of degradative mechanisms outside and inside the cell to reach the nucleus. Lipoplexes and polyplexes have been successfully used in the treatment in nonclinical studies, but the clinical applications have still to see the light of the day.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Introduction
The expected number of people with visual disability in the world is 285 million, 39 million blind and 246 million having low vision ; 65% of people visually impaired and 82% of all blind are 50 years and older. Posterior segment diseases are a foremost cause of visual impairment globally and likely to become more prominent, with the rapid growth of the aging population. The fraction of the total visual impairment and blindness from age-related macular degeneration, glaucoma, and diabetic retinopathy is at present greater than from infective causes such as trachoma and corneal opacities (http://www.who.int/blindness/causes/priority/en/index8.html).
Gene therapy deals with the modification of individual’s genes so as to correct their expression or correction of abnormal gene. This involves the administration of a specific DNA (or RNA). Inherited eye diseases are attractive targets for gene therapy for several reasons. Inherited eye retinal degenerations have been studied intensively, and the mutations leading to death of photoreceptor cells have been described in over 200 genes (https://sph.uth.edu/retnet/home.htm), and so the targets are known. The retina is accessible from outside the body, and injections may be viewed through the lens. The thickness of the retinal layers and integrity of photoreceptors can be measured through clear cornea and lens in the living eye. The genetic disorders in the eye like diabetic retinopathy, glaucoma, and age-related macular degeneration require long-term treatment that may be approachable by gene therapy. [6].
Vectors that encapsulate and deliver foreign genetic materials into specific cell types need to be efficient and nontoxic. These carriers may be viral in origin or may be non-viral, i.e., lipid-based or polymer-based. Viral vectors like adeno-associated virus (AAV) have high gene transduction capability, but the issues with their toxicity still need to be addressed. The size of viral vectors, which restricts the inclusion of genes to <5 kb, is another constraint [5]. Non-viral vectors offer promising platform for delivering plasmid DNA for gene therapy because of their safety and higher payload capacity [8].
The Eye as a Therapeutic Target [9]
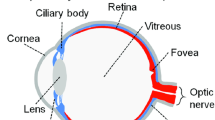
The eye can be separated in two anatomical segments : the anterior segment, where the cornea, lens, and conjunctiva are the most significant structures, and the posterior segment, where the retina plays an important role in image attainment (Fig. 16.1). The anterior segment concentrates light onto the photoreceptor cells in the retina, while photoreceptors translate the light image to electrical signals and transmit it to the brain through the optic nerve to enable vision.
The posterior segment of the eye is made up of three layers, the sclera, choroid, and retina, which surround the vitreous cavity filled by the vitreous, a transparent gel made of water, collagen, hyaluronic acid (HA), and proteoglycans. The vitreous supports the contour of the eye by keeping the retina flat aligned with Bruch’s membrane. The sclera is a strong external layer and is largely composed of connective tissue. The choroid is a vascular layer and endows oxygen and sustenance to the outer layers of the retina. The retinal pigment epithelium (RPE ) is a monolayer of pigmented epithelial cells that outlines the back of the retina and is responsible for the protection of the neural retina and vision. Further the tight junctions between RPE cells comprise the blood-retinal barrier (BRB ) which grants the eye its immune-privileged status.
Ocular Disorders [1]
Retinitis Pigmentosa
Retinitis pigmentosa (RP) is a cluster of clinically and genetically diverse disorders. RP has a worldwide prevalence of 1 in 3000 people. This is characterized by night blindness (tunnel vision) at the onset because of the loss of rod cells in the periphery of the retina. As the disease advances, cone cells begin to degenerate, which leads to complete blindness of the patient. The reason behind the loss of cone cells in RP cases is less understood, although rod cells possess defective genes. In this context, it is hypothesized that these cone cell dystrophies might be the result of loss of rod cell-based supporting factors. Numerous genetic inheritance patterns have been found in RP patients. These genetic inheritance patterns can be autosomal recessive (arRP), autosomal dominant (adRP), or x-linked. In general, RP is divided into two categories: one is nonsyndromic, which is limited to the eye, and the other one is syndromic, which also affects other organs and tissues in the body. RP is linked to more than 100 mutations in the different regions of the rhodopsin gene that accounts for close to 30–40% of adRP. Mutation in the rhodopsin gene can affect the rod cell functions at variable severity levels. The mutation in the Phosphodiesterase 6b gene is responsible for the arRP in humans. This earliest and most harsh form of the disease contributes to 5% of all arRP cases.
Usher Syndrome
Usher syndrome (USH) is a genetically diverse group of autosomal recessive genes that influence both hearing and vision, along with occasional loss of balance. Clinically, there are three types of Usher syndrome (USH1, USH2, and USH3). Regarding the onset of hearing loss, USH1 is the most severe followed by USH2 in terms of severity. In USH3 cases, the child is born with usual hearing and balance, but there is loss of hearing by adolescence. There are five USH1 genes that codify known products, and these are myosin VIIA, cadherin 23, protocadherin 15, scaffold protein containing ankyrin repeats and sam domain, and calcium- and integrin-binding protein 2. The USH2 (usherin, VLGR1, and whirlin) and USH3 (clarin-1) proteins are also found in the same sections of photoreceptor cells in the mouse retina. Mutations in these genes can result in the loss of protein functions that finally lead to loss in hearing and defects in the photoreceptor cells of retina.
Stargardt’s Disease
Stargardt’s is an autosomal recessive juvenile disorder which mainly occurs in children between the ages of 6 and 16 years, with a prevalence of 1 in 8000–10,000 patients. This common genetic macular disorder presents throughout the world, because of the mutation in a gene that encodes a PR ATP-binding cassette (ABC) lipid transporter protein (more commonly known as ABCA4).
Leber Congenital Amaurosis
Leber congenital amaurosis (LCA) is an autosomal recessive inheritance pattern. So far, 14 genes have been evaluated that are associated with this severe form of congenital blindness that presents in early childhood. The worldwide incidence of this disease is 1 of 30,000 cases, 20% of all inborn blindness and 5% of all innate retinal dystrophies. One of the most important mutations is the RPE65 gene that encodes RPE65 (RPE-specific 65 KDa) protein in RPE cells. This has led investigators to hypothesize that restoration of function of RPE65, which is involved in photoreceptor cell cycling, might prevent progression of degeneration and may allow restoration of visual function.
Diabetic Retinopathy
Diabetic retinopathy (DR) affects 93 million people worldwide. Diabetes might cause damage to the retinal blood vessels that nourish the retina. Blood and other fluids that leak from these vessels can cause thickening and inflammation of the retina. Fluid that builds up because of chronic high blood sugar levels causes blurred vision. DR can sometimes be restricted if the blood glucose level is stabilized. DR associated with gene alteration, however, may not always be controlled by the long-term management of blood sugar by using insulin therapy.
Age-Related Macular Degeneration
Symptoms of age-related macular degeneration (AMD) usually present around age 60 and are caused by deterioration of the macula. AMD manifests in two forms: one is “dry” (non-neovascular) and the other is “wet” (neovascular) AMD. The dry form of the disease is more common than the wet form. Dry AMD is related with the deposition of yellowish lipid proteins known as drusen beneath the retina that build up slowly and cause detachment of the retina. All these events lead to steady loss in central vision. Dry AMD can evolve to geographic atrophy or the more destructive wet form. In wet AMD, an atypical angiogenesis rapidly leads to the choroidal neovascularization (CNV) within the retina and deteriorates PR cells in the macula. Ninety percent of AMD-related blindness have been attributed to this angiogenesis. Though the pathophysiology of AMD is complicated and multifactorial, the core of treatment for the neovascular form is inhibition of vascular endothelial growth factor (VEGF), which requires monthly assessment and repeat intravitreal administrations. Additionally, pigment epithelium-derived factor and placental growth factor and new molecules such as endostatin and angiostatin modify the permeability of the retinal and choroidal vasculature and represent interesting targets for gene therapy.
Gene Therapy in the Eye
Gene therapy in the eye can be divided into three categories [2]:
-
1.
Gene replacement.
-
2.
Knockdown technology.
-
3.
Treatments for neurodegenerative disorders (glaucoma, age-related macular degeneration) that do not have a monogenic cause.
Gene delivery for LCA arising from mutations in the RPE65 gene is the most successful example of gene therapy in the eye. RPE65 encodes the 65-kDa RPE-specific isomerase which is important for recycling 11-cis retinal, the chromophore of rod and cone opsins. rAAV vector-mediated gene replacement has led to the rescue of vision in the Swedish Briard dog, a spontaneous RPE65-null model, and stable vision improvement has continued for over 8 years after a single rAAV vector administration. These results, in addition to the absence of side effects after rAAV vector subretinal delivery in nonhuman primates, have cemented the way to three ongoing clinical trials using rAAV2/2 vectors for RPE65 gene replacement in patients affected by LCA due to mutations in RPE65.
Many of the common disease-causing mutations in the retina are dominant, gain-of-function mutations. In these cases, gene replacement alone is not a viable treatment option. Researchers have confirmed the ability of small interfering RNAs contained in AAV-2 vectors to knock down cotransduced reporter gene expression in retinal ganglion cells39. Researchers were able to specifically knockdown mouse rhodopsin expression (in cultured retinal explants) using short hairpin RNAs and concomitantly express (at ~90% of wild-type levels) a co-transfected mouse rhodopsin with silent mutations in the shRNA recognition sequence.
There are diseases which are not monogenetic, i.e., they do not involve single genetic mutation. Diseases like glaucoma and age-related macular degeneration cannot be related to a single genetic component. Efforts are being directed to evaluate the expression of neurotrophic or antiapoptotic factors in the eye for the suppression of angiogenic factors.
Gene Delivery Routes [4]
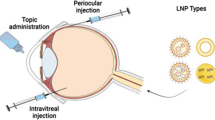
The posterior segment of the eye can be targeted through topical, systemic, intravitreal, and periocular routes. Some of the limitations of the topical route to target posterior segment include rapid drainage through the nasolacrimal ducts, poor permeability of the corneal epithelium, blood aqueous barrier, and systemic absorption. Diffusion of the drugs which are administered through systemic route is hindered by blood-retinal barrier (BRB) .
Intravitreal injections have gained considerable impetus during the last two decades. In this method drug solution is injected directly into vitreous through pars plana using a 30 G needle. Intravitreal injection gives higher drug concentrations in vitreous and retina than the other routes of administration. Intravitreal injection offers high concentrations of drugs in the retina, but it is associated with various short-term complications such as retinal detachment, endophthalmitis, and intravitreal hemorrhages. This mandates that the patients be carefully monitored following intravitreal injections. The inner limiting membrane (ILM) and the BRB are the main biological barriers that hamper drug transport from the vitreous to the retina. The ILM forms a frame between the vitreous humor and the retina and is the major barrier to drug diffusion to the retina . The endothelial cells of retinal blood vessels and retinal pigmented epithelium (RPE) form the BRB which acts as a secondary barrier hindering the transport of the drugs to the inner retinal cells.
Drugs from the vitreous humor are eliminated through the anterior chamber or across the retinal surface . Passive diffusion through the BRB leads to rapid clearance of lipophilic drugs administered into the vitreous. Further, the molecules that are substrates for the innate active transport mechanisms are rapidly eliminated by the efflux transporters in the endothelium.
Periocular Routes
The periocular route has an equal merit for administering drugs to posterior eye segment. Periocular refers to the region surrounding the eye. Periocular pathways used for the delivery of drugs to the posterior tissues of the eye include the retrobulbar, peribulbar, subtenon, and subconjunctival routes. Drug solutions are placed close to the sclera which results in high retinal and vitreal concentrations. Sclera is made up of fibrous tissue and offers less resistance to permeability of drugs. The periocular route of drug delivery enables the deposition of molecules against the external surface of the sclera, thereby minimizing the risk of endophthalmitis and retinal damage associated with the intravitreal route of administration. It is considered to be the least painful and the most efficient route of drug delivery to the posterior eye.
The direct penetration pathway is the main route in achieving high concentrations of a drug in the vitreous following subtenon injections. The sclera, with its large surface area (16.3 cm2), is less resistant to permeation of molecules and has lower protease activity compared to the cornea. Nevertheless, only minute concentrations of a drug administered via the transscleral route end up in the vitreous. This low bioavailability can be attributed to the loss of the drug from the periocular space, BRB , choroidal circulation, and the binding of drugs to tissue proteins as well as efflux transporters.
Drugs may be conveyed to the posterior ocular segment through the systemic blood circulation. Drugs that have high permeability can cross the blood-retina barrier to reach the retina and vitreous. The choroid is easily accessible owing to the extensive blood flow and leaky vasculature in this tissue. High doses are required, and systemic undesirable effects are common (e.g., systemic treatment of glaucoma with carbonic anhydrase inhibitors) through this route since only a very small fraction of the blood circulates through the posterior ocular segment. Such approach is not feasible for drugs that have small dose and narrow therapeutic index.
Viral Vectors
Viral vectors include adenovirus (Ad), AAV , retrovirus (RV), and lentivirus (LV). These viral vectors are disabled genetically so that they do not cause disease in the infected target cell. AAV and LV are the more commonly used. AAV is a member of the parvovirus family and is non-enveloped, replication-defective virus having a size of 18 to 26 nm. LV is a member of Retroviridae family and tends to be larger in size (80–120 nm) which can infect nondividing cells. AAV has a smaller size than LV, so less genetic material can be packaged into AAV. AAV can incorporate up to 4.7 kb of genetic material, while LV can accommodate up to 10 kb. However, the smaller size of AAV makes it a more adaptable option for delivery to the outer retina and photoreceptors. Further AAV does not integrate its genome into the host cell genome. Transgenic material exists as an episome in the case of AAV, while LV vectors integrate genetic material into host chromosomes which may cause mutagenesis. Table 16.1 lists clinical trials that are in progress or have been completed. Viral vectors seem to be the most used gene delivery systems for ocular diseases as shown by these clinical trials.
Improvement of Cellular and Nuclear Uptake of Non-viral Vectors [11]
Well-organized uptake of non-viral vectors in the target cell is imperative for the success of the gene therapy . The uptake of DNA plasmids is a Herculean task because, when they reach the cell membrane, there are several barriers the particle has to overcome to reach the nucleus and attain expression. The overall effectiveness of a gene delivery system is dependent on three key factors: (1) cellular uptake of NPs, (2) escape of NPs from endosomal vesicles into the cytosol, and (3) transfer of the plasmid DNA to the nucleus (Fig. 16.2).
Cellular Uptake
The plasma membrane is the first barrier that is to be overcome to get into the cell. RPE cells have a high rate of phagocytosis. Particles in their environment are therefore readily taken up. Photoreceptors do not exhibit phagocytosis and so are difficult to transfect. Non-phagocytic cells, however, require a number of proteins to maintain their function, and these pathways can be exploited for the delivery of transgene.
Clathrin-Coated Pits
Clathrin-coated pits are formed on the surface of plasma membrane when a ligand binds to its specific receptor forming a complex. This complex is internalized by endocytosis where it fuses with cytoplasmic organelles for further processing. Small receptor ligands like transferin and hyaluronic acid have been used to increase the uptake of nanoparticles. Hyaluronic acid targets the CD44 receptor that is overexpressed in RPE cell layer and retinal glia cells. When uptake occurs using CD44 pathway, it decreases the intracellular degradation of nanoparticle as well.
Cell-Penetrating Peptides (CPPs)
Proteins such as Tat (human immunodeficiency virus) and VP22 (herpes simplex virus) can pass through intact biological membranes and are called cell-penetrating peptides. These CPPs can be coated on the surface of nanoparticles to affect their internalization. Once inside the cell, the delivery system has to survive the endosomal uptake so that the plasmid remains intact and enters the nucleus. The mechanism of survival depends on the type of non-viral vector and will be discussed at relevant place.
For the transgenic DNA to be transcribed, it is imperative that it enters the nucleus. It is easy for a plasmid DNA to enter into the nucleus when the cell is in a state of active division as nuclear envelope splits down during mitosis. But for cells like photoreceptors, postmitotic cells, the entry into the nucleus is possible only through the nuclear pores of 9 nm in diameter, and entry of molecules with molecular weight greater than 50 kDa is thus restricted. Nuclear localization sequence from simian virus 40 (SV40), a single seven-amino stretch (PKKKKRKV) [141], has been found to increase the transfection efficiency. These sequences combine with different proteins like nuclear pore transport proteins in the cell and lead to the active translocation of proteins into the nucleus. These NLS sequences can be attached to the surface of non-viral vectors to facilitate their nuclear entry.
Vector Engineering
Bacterial plasmid DNA has been the building block for non-viral gene therapy. Although plasmids have been very useful for non-viral gene transfer (especially when coupled with efficient compaction), there exist problems like gene silencing and transient expression because of the episomal nature of the plasmid and the bacterial elements required for propagation. Recent work has therefore focused on incorporating additional DNA elements. Enchancers, insulators, S/MARs, inverted terminal repeats (ITRs), and CpG depletion are some of the various techniques used to engineer vectors to increase the transgene expression and its longevity. Such complex vectors containing additional DNA elements require delivery tools that can compact them. Luckily, the availability of non-viral packaging methods which can deliver larger DNA payloads has made this possible.
Cationic Lipid/DNA Complexes (or Lipoplexes) [10]
Plasmid DNA can be coated with lipids in an organized structure of a liposome. When the organized structure is complexed with DNA, it is termed as lipoplex. The liposomes contain two components: a cationic lipid and a neutral lipid, also called helper lipid. Cationic lipids are amphiphilic molecules that contain a positively charged polar head group that is linked via an anchor to a hydrophobic domain comprising of two alkyl chains. The length and degree of nonsaturation of the alkyl chains contribute to the structural variations in the hydrophobic domain of cationic lipids. Amine group with different degrees of substitution, amidine, guanidium, and pyridinium form the positive charge of the cationic lipids. The size and charge of the cationic head group plays a more decisive role in transfection than that of alkyl chains. Synthetic lipids that are commercially available include DOTAP and DC-Chol with monovalent head groups and DOSPA with a multivalent head group. Lipopolyamines not only bind to DNA but also compact it. DOPE and cholesterol are used as neutral lipids.
Lipoplexes may be prepared by methods that are used of liposomes like hydration of a lipid film, dehydration-rehydration, ethanolic injection, reverse-phase evaporation, or the detergent analysis technique. The cationic liposomes are then complexed with DNA, forming lipoplex particles by spontaneous self-assembly. The concentration, temperature, environment, and kinetics of mixing are important for transfection efficiency and should be considered when forming lipoplexes. Lipoplexes with a net positive charge interact more efficiently with the negatively charged cell surface and therefore have higher transfection efficiency. At high positive- or negative-charge ratios, homogeneous, relatively small aggregates of lipoplexes are formed which may also contribute to better transfection efficiency.
After endocytosis by cells, DNA needs to be released from lipoplexes . Spermidine3+ and spermidine4+, the biogenic polycations, are known to be present in the cytosol during the cell cycle. These cations remove DNA from the lipoplexes to form hexagonally packed DNA-polyamine particles in cytosol. The positively charged lipids confer protection to the DNA against degradation by cellular nucleases, and therefore lipoplexes show better transfection.
Polymeric Nanoparticles: Polyplexes
Cationic polymers can form particulate complex with DNA, and the complexes so formed are smaller than that of cationic liposomes. Small particle size favors the transfection. The most potent polyplex formulations have accomplished efficiencies of viral vectors, although more number of particles per cell are required for successful transfection.
Cationic polymers include natural DNA-binding proteins like histones, synthetic polypeptides, cationic dendrimers, or carbohydrate-based polymers. Since most of these are synthetic in nature, the molecular weight can be tailored, and ligands can be attached to their surface. Poly(l-lysine) (PLL) and PEI are among the most widely studied polymers for gene delivery.
Cationic polymers do not contain a hydrophobic domain and so cannot destabilize the endosome by direct interaction with the endosomal membrane as cationic lipids. Actually the first-generation cationic polymers, such as polylysine or polyarginine, were quite ineffective in terms of endosomal escape and transfection abilities. Second-generation cationic polymers, such as polyethylenimine and polyamidoamine dendrimers (PAMAM), act as proton sponge and so can mediate endosome disruption.
Pollard et al. [7] projected the “proton sponge” hypothesis to clarify the high transfection efficiency of PEI-based polyplexes. The hypothesis suggests that at physiological pH only 1–6 nitrogen atoms of PEI-based polyplexes gets protonated. Upon lowering the pH, e.g., in endosomes, the proportion of protonated nitrogen increases and generates a charge gradient that causes a Cl − influx. The increase in Cl − concentration aggravates the water influx and, ultimately, osmotic swelling and rupture of endosome.
PEI-based gene delivery has been studied in the eye. Intravitreal delivery was not very successful; however, results from topical treatment (for some corneal conditions) have been more positive. Its capability to transfect RPE cells has been mostly tested in vitro, and the capacity of these polymers to generate panretinal transfection and treat RPE disease models has not been established.
Chitosan particles have been studied for RPE transfection in vitro, and experiments have demonstrated efficient transfection, biocompatibility, and cell viability after treatment signifying positive outcomes in future in vivo studies if toxicity concerns can be answered.
Positively charged polylysine peptides (CK30) have been used to compact negatively charged DNA or RNA into nanoparticles, but these tend to aggregate and become unstable. However, conjugation of the CK30 to PEG (CK30PEG) has proved to facilitate improved gene expression. These particles have a diameter of <25 nm with the smallest ones having diameters in the range of 8–11 nm. Particle size and homogeneity are important advantages of the CK30PEG compacted DNA nanoparticles as compared to those of PEI and chitosan polyplexes. As opposed to AAVs , CK30PEG nanoparticles have a huge capacity, tested up to 20 kbp in the lung and 14 kbp in the eye and so can deliver large genes. These nanoparticles have been shown to bind to lipid raft-associated cell surface nucleolins, although it is not clear if the same pathway is responsible for RPE uptake. The nucleolin-NP complex is internalized by the nucleus, although the small size of these nanoparticles may allow transport through nuclear pores. Transfecting of both mitotic and postmitotic cells thus becomes possible, a benefit for RPE therapy. Additionally these nanoparticles have been shown to be nontoxic and non-immunogenic.
CK30PEG nanoparticles have been used in vivo to drive gene expression and mediate therapeutic rescue in the lung epithelium and nasal mucosa, brain, retina, and retinal pigment epithelium. Retinal expression was several folds higher than that achieved with naked DNA and on the same scale as that observed with AAVs . In adult mice at 2 days posttreatment, authors demonstrated that a single subretinal injection of CK30PEG nanoparticles yielded reporter gene expression in 55% percent of RPE cells. Further this expression and therapeutic rescue was sustained in the RPE for 2 years or more when the reporter gene plasmid contained a scaffold/matrix attachment region. Subretinal delivery of these particles was demonstrated as safe and nontoxic after delivery to both normal and diseased eyes. Combined, these features make CK30PEG nanoparticles exciting tools for RPE-based gene therapy.
Conclusion
The main driving force for translational research is the commercial ophthalmic market. The ultimate marker of success is whether the treatment makes it to the clinic and thereby becomes available to treat illness. There is a lot of growth potential in this largely untapped market; 55% of all debilitating ocular diseases are posterior segment diseases, yet only 5% of ophthalmic pharmaceutical sales in 2007 were for treating posterior segment diseases. The predictions of the future prevalence rates of posterior segment diseases issued in epidemiologic studies should be considered; increasing aging of populations throughout the world, paired with other factors, including an increase in the prevalence of age-related diseases and sedentary lifestyles, will ultimately lead to a far greater worldwide prevalence of many diverse ophthalmic diseases, in addition to those related to only aging and obesity [3]. Gene therapy holds lot of promise for the treatment of many genetic retinal diseases. This approach directly attacks the root of the disease, rather than treating symptoms, and is therefore supposedly the closest approach to a cure.
References
Adijanto J, Naash MI. Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm. 2015;95:353–67.
Conley SM, Cai X, Naash MI. Non-viral ocular gene therapy: assessment and future directions. Curr Opin Mol Ther. 2008;10(5):456–63.
Edelhauser HF, et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51(11):5403–20.
Kaur IP, Kakkar S. Nanotherapy for posterior eye diseases. J Control Release. 2014;193:100–12.
Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27.
Petit L, Khanna H, Punzo C. Advances in gene therapy for diseases of the eye. Hum Gene Ther. 2016;27(8):563–79.
Pollard H, Remy JS, Loussouarn G, Demolombe S, Behr JP, Escande D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J Biol Chem. 1998;273:7507–11.
Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9(1):GE01–6.
Thakur A, et al. Strategies for ocular siRNA delivery: potential and limitations of non-viral nanocarriers. J Biol Eng. 2012;6:7.
Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–70.
Zulliger R, Conley SM, Naash MI. Non-viral therapeutic approaches to ocular diseases: an overview and future directions. J Control Release. 2015;219:471–87.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Lalwani, A., Shelat, P., Patel, J.K. (2018). Nanomedicine-Based Gene Delivery for the Retina and Posterior Segment Diseases. In: Patel, J., Sutariya, V., Kanwar, J., Pathak, Y. (eds) Drug Delivery for the Retina and Posterior Segment Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95807-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-95807-1_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95806-4
Online ISBN: 978-3-319-95807-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)