Abstract
Prevention and treatment of the complications of immobility play an important role on the care of hospitalized patients with traumatic injuries. Effects of immobility involve several body functions and may lead to future morbidity, disability, and even mortality, with significant socioeconomic impact. In addition to the most evident complications, such as pressure ulcers, deformities, joint pain, loss of muscle and bone mass, deep vein thrombosis and pulmonary embolism, atelectasis and pneumonia, and also injuries to the cardiovascular, endocrine, immune, gastrointestinal, excretory, vestibular, cognitive, and psychological systems have been reported. Regarding traumatic brain injury patients, disorders resulting from prolonged bed rest periods are combined with injury-related morbidities and complications. Early mobilization strategies have proven to be a feasible and safe approach that may promote improved physical function, higher independence levels, and an accelerated process of the return to premorbidity condition and activities. Moreover, early rehabilitation has also been associated with the reduction of the length of hospital stay and costs.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Bed rest
- Immobilization
- Immobility complications
- Traumatic brain injury
- Disuse
- Bone loss
- Muscle loss
- Pressure ulcers
- Deformity
- Postural hypotension
- Deep vein thrombosis
- Pulmonary embolism
- Early mobilization
- Early rehabilitation
The conception, among many others, that the human being was created to work [1] is one of the oldest and most debated principles that guide humanity. However, without addressing the merit of the anthropological discussion of this concept, what is increasingly proved by science is that the body was made to function. Vital function is intrinsically associated with the production of energy, and an organ that is not stimulated degenerates, atrophies, and may even irreversibly lose its function [2].
Indicated as a “medical treatment” since the nineteenth century [3], most of the scientific community ignored the deleterious effects of bed rest until the mid-1950s [4]. Bed rest, in principle harmless, was seen as something that allowed body restoration by reducing metabolic demand [5], and so it was of the utmost importance for one’s recovery. Thus, prescriptions recommended excessive bed resting periods or even contained incorrect indications [6]. Vestiges of this conduct exist to this day, not because of the persistence of the bed rest culture in the popular scope but because of its persistence also in the medical environment [7].
Many studies published in subsequent years analyzed the effects of immobilization from the organic and functional points of view and its impact on the quality of life of bedridden patients [8].
A meta-analysis consisting of 39 randomized studies about the effect of bed rest on 15 different diseases and after 24 medical procedures showed that immobilization was not beneficial; on the contrary, it could be harmful [6].
The advancement of aerospace science and the development of studies submitting normal individuals to experimental models of forced bed rest (“elevated limb,” “casted limb,” and “bed rest and microgravity”) provided better physiological understanding of immobilization [9].
In addition to the most evident complications , such as deformities , joint pain, loss of muscle mass, deep vein thrombosis, and atelectasis [10], injuries to the cardiovascular [11], endocrine [12], immune [13], gastrointestinal [14], excretory [15], vestibular [16], cognitive [17], and psychological [18] systems have also been reported.
With the growing survival of individuals in critical conditions [19], this fact became even more evident. This occurred because scientific and technological knowledge increased the number of so-called intensive care unit survivors, which resulted in a threefold increase in the number of patients referred to rehabilitation centers to treat hospital-acquired disabilities [20].
Roughly, 60–70% of individuals who are released from intensive care units present some degree of motor disability [21], and 50–70% have some type of neurocognitive impairment, both acquired during hospital stay [22].
According to the World Health Organization [23], quality of life is directly related to the degree of independence and physical, psychological, social, and spiritual statuses of individuals. Hence, as bed rest can have a negative impact on most of these domains, many studies have correlated immobilization with worse quality of life in hospitalized patients submitted to prolonged bed rest [24,25,26].
Given above, medical services around the world have been working on the development and implementation of early intervention protocols aiming at fast mobilization of bedridden patients in detriment of harmful bed rest [27,28,29].
Until now, early mobilization provided by an effective multidisciplinary approach has proven a positive, viable, and low-risk strategy [10, 30].
Older patients or patients with chronic diseases or disabilities are especially more susceptible to the adverse effects of immobilization [31].
However, studies in the literature correlating immobilization in patients with traumatic brain injury are scarce. The few studies on the topic reported that bed rest did not provide a positive, but possibly a negative, contribution to the recovery of these patients [32,33,34].
Thus, debating and further studying this topic are of the utmost importance, not only because of the long hospital stays that these individuals experience but also because of the harm caused by the incorrect management of orthosis and the disabling nature of this type of injury. The earlier an injured nervous system is stimulated, the better the prognosis [35].
The objective of this chapter is to review the negative effects of bed rest on many systems, correlating them to the peculiarities of patients with traumatic brain injury. The available interventional strategies in the rehabilitation scope for early, effective, and safe mobilization will also be approached.
Musculoskeletal, Nervous, and Integumentary Systems
The integumentary, nervous, and musculoskeletal systems have an intrinsic relationship with one’s degree of functionality and independence.
Given the neuromuscular impairment inherent to traumatic brain injury, patients who suffer this type of injury are more susceptible to the deleterious effects of immobilization of these systems as a result of many factors, which will be discussed.
The main musculoskeletal and neurological complications related to immobilization cited in the literature, regarding these systems, are muscle atrophy, fatigue, changes in bone density, heterotopic ossification, contracture deformities , peripheral and central nervous system involvement, behavioral and cognitive changes, pressure ulcers, and pain.
Muscle Atrophy and Fatigue
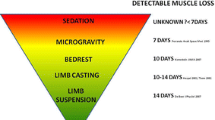
A person in complete bed rest loses from 1% to 3% of their muscle strength per day of immobilization [36]. In 4 weeks, immobilized individuals are estimated to lose 69% of their net muscle weight [37], the loss being more significant between the second and tenth day of bed rest due to edema and tissue fatty replacement [8, 31, 38].
The lower limbs of a healthy individual in complete bed rest are more affected than the upper limbs [39], and among muscles, the most affected are those composed predominantly of type II fibers and related to movement instead of joint stabilization, such as the rectus femoris muscle compared with the vastus intermedius muscle [8], and especially antigravitational movement.
The mechanism responsible for muscle atrophy and weakness involves a complex group of interrelated processes [10]. Lack of use and inflammation seems to be the main factors related to the deterioration of the muscular system, promoting mass loss, reduction of the number of contraction fibers, and reduction of contraction strength, especially of type II fibers [40].
Muscle immobilization seems to change the balance between muscle protein synthesis and lysis, affecting the former more intensely [41]. Low protein synthesis would stem from 4E-BP-1 mRNA inhibition of protein synthesis initiation factors. Excessive protein lysis would be triggered by three distinct pathways: the calcium-dependent protease calpain, lysosomal cathepsins, and the ubiquitin-proteasome system [10].
Meanwhile, the inflammatory pathway would be triggered in later phases of prolonged bed rest by increasing pro-inflammatory cytokines (IL-1 beta, IL-2, and interferon gamma) [42] and producing reactive oxygen species (ROS), which promotes a negative protein balance [43]. This same pathway includes the relationship between the extra cytokines and higher insulin resistance, since hyperglycemia has been demonstrably associated with higher neuromuscular involvement. Simultaneously, low oxidative enzyme capacity, which decreases the contraction of type II fibers, associated with low vascular flow in the immobilized muscles and changes in the size of the plaque of terminal acetylcholine receptors, induces low fatigue resistance.
The majority of patients with traumatic brain injury in the first stages of recovery have low protein intake, which, when associated with the motor and cognitive impairments caused by the trauma, further increase the abovementioned muscle degradation. On the other hand, studies of spastic patients have found an increase in the muscle mass of the most spastic limbs compared with those with mild or without spasticity [44].
Changes in Bone Density
Numerous are the factors that affect bone quality, such as age and genetic factors, pharmaceuticals, and diseases, among others. Nevertheless, one of the main factors associated with inactivity that reduce bone mineral density is the absence or decreased level of gravity-induced mechanic compression and traction stimuli and/or muscle contraction stimuli [45].
Animals submitted to different gravities present changes in the trabecular and cortical bone structures, mineralization pathways, collagen metabolism, and calcium excretion [46].
These losses begin quickly, occurring a few weeks after immobilization. They are self-limited and reach a peak after 20–30 weeks. In more severe cases, they are partly irreversible, even years after resuming mobility [47]. The immobilization of forearms and wrists in males and females for almost 5 weeks resulted in significant bone loss, which was not recovered after 5 weeks of hand remobilization and therapy [48]. Individuals submitted to microgravity had bone deficiency in the calcaneus bone as long as 5 years after exposure [49].
In the first months of immobilization, bone mineral loss is mainly attributed to higher bone resorption [50]. Although the pathophysiology has not been well established, osteoblasts in vitro in microgravity environments responded differently to systemic hormones and growth factor [51].
Given that the fluid movement inside bone gap junctions and canaliculi provides a significant collaboration to the preservation of the skeletal matrix, studies in vivo have found that bone movements change the pressure of this liquid in the skeletal cells, which in turn stimulate the release of signaling molecules that mediate bone remodeling [52, 53].
Another bone remodeling mechanism, also impaired by the lack of mechanic bone stressors and increasingly accepted by the scientific community, is promoted by the sympathetic nervous system, which stimulates remodeling via beta-adrenergic receptors. This pathway could also be influenced by lesions in the central nervous system, which, in turn, would cause metabolic, neurovascular, and molecular disorders, generating the so-called neurogenic osteoporosis.
Hence, one can speculate that individuals with traumatic brain injury are more susceptible to bone involvement because they have more than one causal trigger of bone mass reduction stimuli.
Age and injury location aside, this fact could also be reinforced by bone density changes, which indicate the different bone qualities of a healthy and a compromised limb of patients with stroke or spinal cord injury [54, 55].
Additionally, in the initial critical stage of traumatic brain injury, patients often present nutritional changes, severe weight loss, inflammatory processes and hormonal changes secondary to thyroid and gonadal disorders, and changes related to the growth hormone and insulin-like growth factor 1 [56]. All these factors may have a negative impact on bone integrity, along with immobilization and nervous system injury.
Although many studies have found a negative correlation between spasticity and bone mineral density, some studies have reported a positive correlation between high spasticity and worse bone mass quality [57, 58].
Heterotopic Ossification
Still on bone involvement, heterotopic ossification [HO] could not be omitted. This disease develops in situations of immobilization and is usually associated with central nervous system lesions [59].
HO is defined as the presence of bone tissue in places where bone normally does not exist. This abnormality stems from a metaplastic process with bone neoformation in soft tissues, usually adjacent to large joints (hip, elbows, knees, and shoulders) [60]. Its etiology is still unknown, but many factors responsible for osteoblastic activation through bone-forming proteins have been studied, such as the stimulus caused by the central neurological lesion itself [61].
Potential HO-related complications are limited joint amplitude with functional impairment, pain, nerve compression, and worsening of spasticity [62].
Contracture Deformities
As mentioned earlier, significant changes in the muscle tissues of previously healthy limbs occur after some weeks of immobilization. These, in addition to changes of the other structures that involve the joints, such as ligaments, capsules, disks, and menisci, may cause structural and disabling deformities .
Experimental animal studies have found roughly a 12.5° loss of amplitude of joint movement after 2 weeks of immobilization, which may increase to 51.4° after 32 weeks of bed rest [63].
The pathophysiology of this joint phenomenon has not been well defined, but replacement of type III collagen fibers by type I collagen fibers after 1 week of immobilization of a healthy joint has been described, with reduction of total elasticity [64]. Yet, low production of collagen fibrils in the ligaments reduces their long-term resistance and increases osteoclastic activity in the ligament-bone interface [65].
An inflamed joint deserves special attention given that short-term immobilization is indicated to reduce the concentration of type I interleukin and increase proteoglycans, necessary for cartilage protection. However, the presence of inflammation and pain increases the risk of long-term contracture deformities , so the former cannot be excessive [31].
Given the traumatic nature of the lesion, patients with traumatic brain injury may have three factors that promote the development of contracture deformities in addition to immobilization: motor changes, spasticity, and/or other joint inflammation processes stemming directly from the polytrauma, such as fractures, ligament lesions, etc.
Since paresis or paralysis in traumatic brain injury patients in the initial stages of the trauma can even progress to complete functional recovery, segmental immobilization, with the introduction and maintenance of orthosis, should be done carefully and under supervision.
Pressure Ulcers
In addition to the collagen changes mentioned earlier, which increases contracture deformities , another frequent and disabling change in patients that remain immobilized for prolonged periods is pressure ulcers.
A 3-month study of 530 patients hospitalized for clinical or surgical reasons found that 11.3% of these patients had pressure ulcers, and immobilization was the main risk factor for pressure ulcer development [66].
Likewise, a study proposing an early mobilization program over a period of 12 months in 3233 intensive care unit patients found a 3% reduction in the incidence of pressure ulcers [67].
In addition to motricity changes stemming from the central nervous system lesion, traumatic brain injury patients may also present other risk factors for skin lesions, such as sensory changes, nutritional deficiencies, metabolic changes, spasticity, neurotrophic changes, and long intensive care unit stays. The strategies proposed to lower the incidence of pressure wounds in patients with traumatic central nervous system lesions include not only early mobilization, with assistive position change when active movement is impaired, and assistive devices that reduce friction and shear, but also rigorous surveillance and, if necessary, correction of plasma hemoglobin level and introduction of oral feeding, as soon as possible, and skin hydration [68].
Neuronal Involvement and Cognitive and Behavioral Changes
Neuroplasticity occurs in adulthood in response to natural cell maturation associated with neuronal use or lack of use [69]. As the field of diagnostic tests involving functional analyses of the various levels of the nervous system advances, more pieces of evidence of the negative impacts of immobilization on this system are frequently being discovered.
The changes seen in bedridden patients or patients in microgravity environments for prolonged periods include sensory disorders, altered vestibular reflex responses, lack of coordination, balance changes, reaction speed, attention, planning, memory, spatial-temporal orientation, body perception, level of anxiety, depression, and insomnia [9, 16, 17, 64, 70, 71].
The pathophysiology of these changes has not been well defined, but some studies have suggested a correlation between stimulus to the vagal tone and growth of white and gray masses in the prefrontal cortex after observing the higher brain volume of individuals submitted to frequent aerobic exercises [17]. Regarding the peripheral nervous system, animal nerve diameter changes proportionally to immobilization duration [72].
Two entities often mentioned in the current literature, which have immobilization and sensory deprivation as their greatest risk factors, must also be cited: delirium and post-intensive care syndrome. The latter is proof that the sequelae of immobilization may continue after the patient is released from the hospital and may even become permanent.
In addition to the damages to central nervous system and motor system secondary to trauma, traumatic brain injury patients may also develop post-traumatic stress disorder, further aggravating the cognitive-behavioral domain [73].
Strategies must be addressed toward the provision of information to promote better orientation of the patient, and stimulus must be done during the day, in respect to the physiological sleep-wake cycle.
Pain
Pain may be related to immobilization in many domains, but little is known about its pathophysiology. It may result from involvement of the nociceptive, neuropathic, and mixed pathways.
Patients exposed to stress or excessive sensory deprivation present low tolerance to painful stimuli because of neuroplasticity changes [74, 75]. Likewise, changes in integumentary and muscle tissues (atrophy, contracture deformities , skin lesions) may trigger constant unpleasant stimuli, which generate even greater suffering in underweight, restrained, and/or functionally dependent patients, who often have impaired communication skills.
Traumatic brain injury patients are no exception, and to make matters worse, they may have various inflammatory processes due to the etiology of the disease and to nervous system lesions that may evolve to sensory changes (allodynia/ dysesthesia) and/or presence of pain of central nervous system origin.
About 51.5% of traumatic brain injury patients will develop significant chronic pain after the accident, including patients with mild lesions. This pain does not appear to be associated with a history of depression or the development of post-traumatic stress disorder [76].
Analgesic measures must watch out for sedative effects and may include physical modalities.
Cardiovascular and Pulmonary Systems
The cardiovascular and pulmonary systems are two other systems that may suffer significantly with immobilization. The first one suffers from the rapid adaptation of the blood vessels and the cardiac pump to the decubitus position, and the slow and often risky recovery of their function as orthostatism is regained. The second one suffers because of adaptations to the lower oxygen demand during bed rest, adaptations associated with numerous complications, such as pneumonia and atelectasis.
Cardiovascular Adaptation
When a healthy person stands up from a lying position, the heart rate increases by 32%, 62%, and 89% after 3 days, 1 week, and 6 weeks, respectively, of complete bed rest. Systolic volume may reduce by 15% after 2 weeks of bed rest [77]. After 20 days of bed rest, VO2 max can decrease by roughly 27% [78]. Three weeks of immobilization may decrease cardiac performance by 25% [79]. In addition to the physiological cardiovascular adaptations to the horizontal position, time will promote further pathological changes to the structures responsible for the adaptive cardiac response to postural changes, impairing cardiovascular function recovery as patients exposed to immobilization stand up [9].
Deep Vein Thrombosis
Another known complication of immobilization is deep vein thrombosis, promoted mainly by venous blood stagnation and increased blood coagulability [80]. Stagnation may lead to an increase of thrombin, which promotes platelet aggregation and thrombosis [81]. If left untreated, venous thrombosis may evolve to potentially fatal pulmonary embolism. In traumatic brain injury patients, the presence of lower-limb paralysis and the trauma itself may increase the risk of developing this complication [82].
Trauma patients with prolonged bed rest, frequently combined with segmental paralysis that might result from brain injury, are at high risk for deep vein thrombosis and demand mechanical and pharmacological interventions for its prevention.
Postural Hypotension
As mentioned earlier, in addition to cardiovascular changes, immobilization also causes changes to the sympathetic-adrenergic system [83]. Hence, the response to baroreceptor stimulation in individuals not exposed to bed rest is different in individuals exposed to approximately 3 weeks of bed rest [84]. This process may take from 20 to 72 days to recover once mobilization is resumed [31]. Increased beta-adrenergic activity caused by immobilization may be responsible for this intolerance [85], associated with the more recent finding of intolerance associated with changes in the vestibulosympathetic reflex [16].
Progressive early mobilization strategies, care of fluid balance and systemic hydration, and surveillance toward the possible adverse effects of the medication in use must be applied.
Atelectasis and Pneumonia
Immobilization causes changes in the respiratory system, namely, changes in pulmonary blood flow, tissue structure, and ciliary movement, and decreased diaphragmatic excursion, with repercussions on functional residual capacity and effectiveness of coughing [86, 87]. In turn, these changes increase the risk of atelectasis and risk of airway infection.
Traumatic brain injury patients are four times more susceptible to Pneumonia than the general population [88]. Among other reasons, this higher susceptibility occurs as a result of dysphagia and the frequent use of invasive mechanical ventilation [89].
Hence, preventive measures to the deleterious effects of immobilization on the respiratory system are critical in this type of patient to reduce their morbidity and mortality rates. Respiratory exercises, with or without the use of support and assistive devices, must be part of the rehabilitation interventions, as adequate positioning and early mobilization.
Endocrine and Metabolic Disorders
The greatest and best known changes in the endocrine system associated with immobilization are increased peripheral insulin resistance, which is related to higher morbidity [90]; high parathormone associated with low growth hormone, which changes bone density [91]; and high adrenocorticotrophic hormone, possibly triggered by the stress experienced by critical care patients [31].
With respect to metabolic changes, potassium-, sodium-, nitrogen-, magnesium-, and calcium-related disorders have been associated with immobilization. Immobilization rarely causes severe electrolyte imbalance, but it is important to bear in mind patients with renal failure [31] and the association between high serum potassium, sodium, and calcium and low cerebral blood flow in traumatic brain injury patients [92].
Digestive and Excretory Systems
Immobilization repercussions on absorptive functions are commonly found in these two systems, such as atrophy of the intestinal mucosa and glands; intestinal motility disorder, which causes constipation and reflux; and sphincter dysfunction, which generates urinary stasis with the formation of calculi and higher incidence of infections [31].
Traumatic brain injury patients already have a high incidence of gastrointestinal tract changes due to the nature of the lesion. These changes can be related to deglutition disorders, fecal incontinence, and intestinal constipation [93]. The excretory system may also be affected, resulting in urinary incontinence, which may affect up to 62% of these patients during the acute phase [94].
Given the negative repercussion of these problems on the patient’s social and clinical domains, and the high susceptibility of traumatic brain injury patients to these problems, the implementation of early mobilization strategies is vital to avoid the deleterious effects of immobilization. They include pharmacological and non-pharmacological interventions, which include dietetic measures, adequate positioning, abdominal maneuvers, and the use of the gastrocolic reflex, apart from the early mobilization strategies.
Early Mobilization
The mainstay of treatment lies on early mobilization strategies, since the acute phase, and that include critical ill patients. The early rehabilitation of critically ill patients has proven a feasible and safe approach that may promote improved physical function, greater independence in activities of daily living [ADL], and an accelerated process of the return to premorbidity activities, with reduced symptoms of fatigue and dyspnea [24, 28, 95,96,97,98,99]. In addition to those benefits, early rehabilitation has also been associated with other relevant clinical outcomes, including preventing the incidence of ICU-acquired muscle weakness and reducing the time of weaning from mechanical ventilation [MV], the length of hospital stay, and costs [27, 100,101,102,103,104].
Bedridden and comatose patients unable to cooperate with the therapy should receive passive mobilization and multisensory stimulation. Stimulation and activities must be applied in an organized, planned, and isolated manner during daytime. Family and caregivers should be involved. Passive mobilization should include progressive passive orthostatic training with the monitoring of vital signs. Orthostatic training contributes to sensory stimulation toward arousal, cardiovascular response, and the prevention of orthostatic hypotension [104], total lung capacity [105], gastrointestinal regulation and the prevention of contracture deformities and also to the alleviation of the pressure of some skin areas. As the patient improves collaboration and mobility, the use of active exercises and neuromuscular stimulation may be introduced. Contracture deformity prevention might demand the introduction of orthosis, and pharmacologic interventions might be needed if spasticity is present.
Regarding bone loss prevention, pharmacologic interventions must be added to mobilization strategies especially in the presence of paralysis to minimize bone reabsorption. Vitamin D must also be maintained or replaced, and minimum calcium intake must be provided [54, 58].
References
The Holy Bible. The new American Bible. Genesis. 1970;2:15.
Bortz WM. The disuse syndrome. West J Med. 1984;141(5):691–4.
Stiles A. The rest cure, 1873–1925. In: Felluga DF, editor. BRANCH: Britain, representation and nineteenth-century history; 2012. p. 1–11.
Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–90.
Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to earth: advances in human physiology from 20 years of bed rest studies (1986-2006). Eur J Appl Physiol. 2007;101:143–94.
Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet. 1999;354(9186):1229–33.
Corcoran PJ. Use it or lose it--the hazards of bed rest and inactivity. West J Med. 1991;154(5):536–8.
Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30(5):1151.e9–1151.e14.
Liu Q, Zhou R, Zhao X, Oei TPS. Effects of prolonged head-down bed rest on working memory. Neuropsychiatr Dis Treat. 2015;11:835–42.
Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit--from pathophysiology to clinical trials. Crit Care. 2009;13(4):216.
Bringard A, Pogliaghi S, Adami A, De Roia G, Lador F, Lucini D, et al. Cardiovascular determinants of maximal oxygen consumption in upright and supine posture at the end of prolonged bed rest in humans. Respir Physiol Neurobiol. 2010;172(1–2):53–62.
Belavy DL, Seibel MJ, Roth HJ, Armbrecht G, Rittweger J, Felsenberg D. The effects of bed rest and counter measure exercise on the endocrine system in male adults - evidence for immobilization induced reduction in SHBG levels. J Endocrinol Investig. 2011;35(1):54–62.
Hoff P, Belavy DL, Huscher D, Lang A, Hahne M, Kuhlmey AK, et al. Effects of 60-day bed rest with and without exercise on cellular and humoral immunological parameters. Cell Mol Immunol. 2015;12(4):483–92.
Iovino P, Chiarioni G, Bilancio G, Cirillo M, Mekjavic IB, Pisot R, et al. New onset of constipation during long-term physical inactivity: a proof-of-concept study on the immobility-induced bowel changes. PLoS One. 2013;8(8):e72608.
Okada A, Ohshima H, Itoh Y, Yasui T, Tozawa K, Kohri K. Risk of renal stone formation induced by long-term bed rest could be decreased by premedication with bisphosphonate and increased by resistive exercise. Int J Urol. 2008;15(7):630–5.
Dyckman DJ, Sauder CL, Ray CA. Effects of short-term and prolonged bed rest on the vestibulosympathetic reflex. Am J Physiol Heart Circ Physiol. 2012;302:H368–74.
Lipnicki DM, Gunga HC, Belavỳ DL, Felsenberg D. Bed rest and cognition: effects on executive functioning and reaction time. Aviat Space Environ Med. 2009;80(12):1018–24.
Ishizaki Y, Fukuoka H, Katsura T, Nishimura Y, Kiriyama M, Higurashi M, et al. Psychological effects of bed rest in young healthy subjects. Acta Physiol Scand Suppl. 1994;616:83–7.
Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–9.
Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. 2015;19(Suppl 3):S6.
Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–41.
Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–20.
Division of Mental Health and Prevention of Substance Abuse WHO. WHOQOL: measuring quality of life (Internet). Geneva: World Health Organization; 1997. Available from: http://apps.who.int/iris/bitstream/10665/63482/1/WHO_MSA_MNH_PSF_97.4.pdf.
Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. 2012;23(1):5–13.
Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175–83.
Parker A, Sricharoenchai T, Needham DM. Early rehabilitation in the intensive care unit: preventing physical and mental health impairments. Curr Phys Med Rehabil Rep. 2013;1(4):307–14.
Murakami FM, Yamaguti WP, Onoue MA, Mendes JM, Pedrosa RS, Maida ALV, et al. Functional evolution of critically ill patients undergoing an early rehabilitation protocol. Rev Bras Ter Intensiva. 2015;27(2):161–9.
Gosselink J, Bott J, Johnson M, Dean E, Nava S, Norrenberg M, Schonhofer B, Stiller K, van de Leur H, Vincent JLRB. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med. 2008;34(7):1188–99.
Stiller K. Safety issues that should be considered when mobilizing critically ill patients. Crit Care Clin. 2007;23:35–53.
Clark DE, Lowman JD, Griffin RL, Matthews HM, Reiff DA. Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther. 2012;93(2):186–96.
Halar EM, Bell KR. Imobilidade. In: DeLisa JA, Gans BM, editors. Tratado de Medicina de Reabilitação. 3rd ed. São Paulo: Editora Manole Ltda; 2002. p. 1067–89.
de Kruijk JR, Leffers P, Meerhoff S, Rutten J, Twijnstra A. Effectiveness of bed rest after mild traumatic brain injury: a randomised trial of no versus six days of bed rest. J Neurol Neurosurg Psychiatry. 2002;73(2):167–72.
Singer BJ, Jegasothy GM, Singer KP, Allison GT, Dunne JW. Incidence of ankle contracture after moderate to severe acquired brain injury. Arch Phys Med Rehabil. 2004;85(9):1465–9.
Carlile M, Nicewander D, Yablon SA, Brown A, Brunner R, Burke D, et al. Prophylaxis for venous thromboembolism during rehabilitation for traumatic brain injury: a multicenter observational study. J Trauma. 2010;68(4):916–23.
Grüner ML, Terhaag D. Multimodal early onset stimulation (MEOS) in rehabilitation after brain injury. Brain Inj. 2000;14(6):585–94.
Nicks DK, Beneke WM, Key RM, Timson BF. Muscle fibre size and number following immobilisation atrophy. J Anat. 1989;163:1–5.
Haggmark T, Eriksson E, Jansson E. Muscle fiber type changes in human skeletal muscle after injuries and immobilization. Orthopedics. 1986;9(0147-7447 (Print)):181–5.
Puthucheary ZA, Phadke R, Rawal J, McPhail MJW, Sidhu PS, Rowlerson A, et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43(8):1603–11.
LeBlanc D, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol. 1992;73:2172–8.
Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol. 2003;551(Pt 1):33–48.
Ashmore CR, Summers PJ. Stretch-induced growth in chicken wing muscles: myofibrillar proliferation. Am J Phys. 1981;241(3):C93–7.
Schmitt DA, Schwarzenberg M, Tkaczuk J, Hebrard S, Brandenberger G, Mauco G, et al. Head-down tilt bed rest and immune responses. Pflügers Arch Eur J Physiol. 2000;441(2–3 Suppl):R79–84.
Pawlak W, Kedziora J, Zolynski K, Kedziora-Kornatowska K, Blaszczyk J, Witkowski P. Free radicals generation by granulocytes from men during bed rest. J Gravit Physiol. 1998;5(1):P131–2.
Lofvenmark I, Werhagen L, Norrbrink C. Spasticity and bone density after a spinal cord injury. J Rehabil Med. 2009;41(13):1080–4.
Giannotti S, Bottai V, Dell’osso G, De Paola G, Bugelli G, Pini E, et al. Disuse osteoporosis of the upper limb: assessment of thirty patients. Clin Cases Miner Bone Metab (Internet). 2013 (cited 2016 Apr 30);10(2):129–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24133531.
Zerath E. Effects of microgravity on bone and calcium homeostasis. Adv Space Res Off J Comm Space Res (COSPAR). 1998;21:1049–58.
Steinberg FU. The immobilized patient. Boston: Springer US; 1980. p. 34–43.
Houde JP, Schulz LA, Morgan WJ, Breen T, Warhold L, Crane GK, et al. Bone mineral density changes in the forearm after immobilization. Clin Orthop Relat Res. 1995;(317):199–205.
Tilton FE, Degioanni JJ, Schneider VS. Long-term follow-up of Skylab bone demineralization. Aviat Space Environ Med. 1980;51(11):1209–13.
Gross TS, Rubin CT. Uniformity of resorptive bone loss induced by disuse. J Orthop Res. 1995;13(5):708–14.
Carmeliet G, Nys G, Bouillon R. Microgravity reduces the differentiation of human osteoblastic MG-63 cells. J Bone Miner Res. 1997;12(5):786–94.
Mullender MG, Dijcks SJ, Bacabac RG, Semeins CM, Van Loon J, Klein-Nulend J. Release of nitric oxide, but not prostaglandin E2, by bone cells depends on fluid flow frequency. J Orthop Res. 2006;24(6):1170–7.
Knothe Tate ML, Steck R, Forwood MR, Niederer P. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol. 2000;203(Pt 18):2737–45.
Brito CMM, Battistella LR, Sakamoto H, Saito ET. Densidade Mineral Óssea após Lesão Medular. Acta Fisiátrica. 2002;9:127–33.
Sato Y, Kuno H, Kaji M, Ohshima Y, Asoh T, Oizumi K. Increased bone resorption during the first year after stroke. Stroke. 1998;29(7):1373–7.
Daci E, Van Cromphaut S, Bouillon R. Mechanisms influencing bone metabolism in chronic illness. Horm Res. 2002;58:44–51.
Pang MYC, Ashe MC, Eng JJ. Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporos Int. 2007;18(9):1243–52.
De Brito CMM, Garcia ACF, Takayama L, Fregni F, Battistella LR, Pereira RMR. Bone loss in chronic hemiplegia: a longitudinal cohort study. J Clin Densitom. 2013;16(2):160–7.
Hudson SJ, Brett SJ. Heterotopic ossification – a long-term consequence of prolonged immobility. Crit Care (Internet). 2006 (cited 2016 Apr 30);10(6):174. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17129365.
Hartmann APB, Ximenes AR, Hartmann LG, Fernandes AR, Natour J, D’Ippolito G. Diagnóstico por imagem na avaliação da ossificação heterotópica. Rev Bras Reumatol. Sociedade Brasileira de Reumatologia. 2004;44(4):291–3.
Argyropoulou MI, Kostandi E, Kosta P, Zikou AK, Kastani D, Galiatsou E, et al. Heterotopic ossification of the knee joint in intensive care unit patients: early diagnosis with magnetic resonance imaging. Crit Care (Internet). 2006 (cited 2016 Apr 30);10(5):R152. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17074077.
Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. (Review) (80 refs). J Rehabil Med. 2005;37(Sweden PT - Journal Article PT - Review LG - English DC - 20050725):129–36.
Trudel G, Uhthoff HK. Contractures secondary to immobility: is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil. 2000;81(1):6–13.
Oliveira RCC, Haddad MSCM, Koyama RCC. Síndrome da imobilização In: Greve JMGG, Amatuzzi MM, editors. Medicina de reabilitação aplicada à ortopedia e traumatologia. 1st ed. São Paulo: Rocca; 1999. p. 381–98.
Newton PO, Woo SL, Kitabayashi LR, Lyon RM, Anderson DR, Akeson WH. Ultrastructural changes in knee ligaments following immobilization. Matrix. 1990;10(5):314–9.
Lindgren M, Unosson M, Fredrikson M, Ek AC. Immobility - a major risk factor for development of pressure ulcers among adult hospitalized patients: a prospective study. Scand J Caring Sci. 2004;18(1):57–64.
Azuh O, Gammon H, Burmeister C, Frega D, Nerenz D, DiGiovine B, et al. Benefits of early active mobility in the medical intensive care unit - a pilot study. Am J Med. 2016;129:866.
Dhandapani M, Dhandapani S, Agarwal M, Mahapatra AK. Pressure ulcer in patients with severe traumatic brain injury: significant factors and association with neurological outcome. J Clin Nurs. 2014;23(7–8):1114–9.
Lundbye-Jensen J, Nielsen JB. Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans. J Physiol. 2008;586(17):4121–35.
Dupui P, Montoya R, Costes-Salon MC, Séverac A, Güell A. Balance and gait analysis after 30 days −6 degrees bed rest: influence of lower-body negative-pressure sessions. Aviat Space Environ Med. 1992;63(11):1004–10.
De la Torre G. Cognitive neuroscience in space. Life. 2014;4(3):281–94.
Malathi S, Batmanabane M. Effects of varying periods of immobilization of a limb on the morphology of a peripheral nerve. Acta Morphol Neerl Scand. 1983;21:185–98.
Bryant R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):251–62.
Dufton LM, Konik B, Colletti R, Stanger C, Boyer M, Morrow S, et al. Effects of stress on pain threshold and tolerance in children with recurrent abdominal pain. Pain. 2008;136(1–2):38–43.
Petrie A, Collins W, Solomon P. The tolerance for pain and for sensory deprivation. Am J Psychol. 1960;73(1):80.
Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711–9.
Taylor HL, Henschel A. Effects of bed rest on cardiovascular function and work performance. J Appl Physiol. 1949;2(5):223–39.
Chobanian AV, Lille RD, Tercyak A, Blevins P. The metabolic and hemodynamic effects of prolonged bed rest in normal subjects. Circulation. 1974;49(3):551–9.
Demida BF, Machinskiĭ GV. Use of rehabilitation measures for restoration of human physical work capacity after the prolonged limitation of motor activity. Kosm Biol Aviakosm Med. 1979;13(1):74–5.
Gibbs NM. Venous thrombosis of the lower limbs with particular reference to bed-rest. Br J Surg. 1957;45(191):209–36.
Okoye GC, Evans JH, Beattie J, Lowe GD, Lorimer AR, Forbes CD. Response of femoral venous oxygen tension to graduated pressure stockings--possible relationship to deep vein thrombosis. Thromb Haemost. 1984;51(1):103–4.
Reiff DA, Haricharan RN, Bullington NM, Griffin RL, McGwin G, Rue LW. Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacological prophylaxis. J Trauma. 2009;66(5):1436–40.
Melada GA, Goldman RH, Luetscher JA, Zager PG. Hemodynamics, renal function, plasma renin, and aldosterone in man after 5 to 14 days of bedrest. Aviat Space Environ Med. 1975;46(8):1049–55.
Lamb LE, Stevens PM, Johnson RL. Hypokinesia secondary to chair rest from 4 to 10 days. Aerosp Med (Internet). 1965 (cited 2016 Apr 30);36:755–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14334709.
Pequignot JM, Guell A, Gauquelin G, Jarsaillon E, Annat G, Bes A, et al. Epinephrine, norepinephrine, and dopamine during a 4-day head-down bed rest. J Appl Physiol. 1985;58(1):157–63.
Schulz H, Hillebrecht A, Karemaker JM, ten Harkel AD, Beck L, Baisch F, et al. Cardiopulmonary function during 10 days of head-down tilt bedrest. Acta Physiol Scand Suppl. 1992;604:23–32.
Svanberg L. Influence of posture on the lung volumes, ventilation and circulation in normals; a spirometric-bronchospirometric investigation. Scand J Clin Lab Invest. 1957;9(Suppl 25):1–195.
Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: a retrospective cohort study. Arch Phys Med Rehabil. 2009;90(9):1506–13.
Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620–5.
Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239(4):553–60.
Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29(2):197–206.
Weber JT. Altered calcium signaling following traumatic brain injury. Front Pharmacol. 2012;3:60.
Lim YH, Kim DH, Lee MY, Joo MC. Bowel dysfunction and colon transit time in brain-injured patients. Ann Rehabil Med. 2012;36(3):371–8.
Chua K, Chuo A, Kong KH. Urinary incontinence after traumatic brain injury: incidence, outcomes and correlates. Brain Inj. 2003;17(6):469–78.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik A, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82.
Feliciano V, Albuquerque CG, Andrade FM, Dantas CM, Lopez A, Ramos FF, et al. A influência da mobilização precoce no tempo de internamento em unidade de terapia intensiva. ASSOBRAFIR Ciênc. 2012;3(2):31–42.
Dantas CM, Silva PF, Siqueira FH, Pinto RM, Matias S, Maciel C, et al. Influence of early mobilization on respiratory and peripheral muscle strength in critically ill patients. Rev Bras Ter Intensiva. 2012;24(2):173–8.
Soares TR, Avena KM, Olivieri FM, Feijó LF, Mendes KM, Souza Filho SA, et al. Retirada do leito após a descontinuação da ventilação mecânica: há repercussão na mortalidade e no tempo de permanência na unidade de terapia intensiva? Rev Bras Ter Intensiva. 2010;22(1):27–32.
Pires-Neto RC, Pereira AL, Parente C, Sant’anna GN, Esposito DD, Kimura A, et al. Characterization of the use of a cycle ergometer to assist in the physical therapy treatment of critically ill patients. Rev Bras Ter Intensiva. 2013;25(1):39–43.
Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–43.
Pinheiro AR, Christofoletti G. Motor physical therapy in hospitalized patients in an intensive care unit: a systematic review. Rev Bras Ter Intensiva. 2012;24(2):188–96.
McWilliams D, Weblin J, Atkins G, Bion J, Williams J, Elliott C, et al. Enhancing rehabilitation of mechanically ventilated patients in the intensive care unit: a quality improvement project. J Crit Care. 2015;30(1):13–8.
Lord RK, Mayhew CR, Korupolu R, Mantheiy EC, Friedman MA, Palmer JB, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717–24.
Sibinelli M, Maioral DC, Falcão ALE, Kosour C, Dragosavac D, Lima NMFV. Efeito imediato do ortostatismo em pacientes internados na unidade de terapia intensiva de adultos. Rev Bras Ter Intensiva. 2012;24(1):64–71.
Smelt WL, de Lange JJ, Booij LH. Cardiorespiratory effects of the sitting position in neurosurgery. Acta Anaesthesiol Belg. 1988;39(4):223–31.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
de Brito, C.M.M., Battistella, L.R., Guarita, M.L.C. (2018). Challenges and Complications of Immobility. In: Anghinah, R., Paiva, W., Battistella, L., Amorim, R. (eds) Topics in Cognitive Rehabilitation in the TBI Post-Hospital Phase. Springer, Cham. https://doi.org/10.1007/978-3-319-95376-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-95376-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95374-8
Online ISBN: 978-3-319-95376-2
eBook Packages: MedicineMedicine (R0)