Abstract
The incidence of neurodegenerative diseases is increasing progressively, and unfortunately the molecular mechanisms that lead to them are still unknown. Several studies are advancing in the comprehension of the processes involved in the establishment of the neurodegeneration. In this sense, the current approaches are focused on genes associated with the neurodegenerative diseases, reactional gliosis or microglial activation, pro-inflammatory cytokine production, IL-6 increase as a transcription factor, growth factor decrease, mitochondrial dysfunction, antioxidant defense system, cellular metabolism, protein degradation/aggregation, and oxidative stress triggering redox-dependent signals, among others. Parkinson’s and Alzheimer’s diseases show an increased diagnosis worldwide and are examples of deleterious effects associated with aging.
The present chapter is focused on the implication of the oxidative stress, aging, and inflammation processes on neurodegenerative alterations that lead to neuronal dysfunctions, tissue disturbances, and motor-cognitive disorders. In regard to this, the neurotrophic factors of clinical interest prevent the degeneration and enhance recovery of remaining neurons. Among them, insulin-like growth factor 1 (IGF-1), which is strongly induced by microglial cells after different insults such as ischemia, cortical injury, and inflammatory processes, is emerging as a powerful neuroprotective molecule. For this reason, we will close this chapter with the implications of IGF-1 on neurodegenerative diseases as a key neuroprotective and neuromodulator molecule.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Oxidative Stress and Neurodegeneration
Oxidative stress (OS) is defined as the imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense system . Living organisms produce ROS from molecular oxygen as a consequence of normal cellular metabolism . In order to prevent damage, cells have an antioxidant defense system constituted by an enzymatic component (including catalases, superoxide dismutases, etc.) and nonenzymatic antioxidants component (glutathione, α-tocopherol, ascorbic acid, etc.). When the levels of ROS exceed cell capacity, it can cause damage in cellular components such as carbohydrates, nucleic acids, lipids, and proteins, thus altering their function. Whenever this imbalance occurs within the central nervous system, it can lead to the development of the neurodegenerative disorders.
Neurodegenerative diseases are characterized by the loss of neuronal cells and, in most cases, by the aggregates of proteins that form intracytoplasmic and intranuclear inclusions in neurons and glial cells. Data on the literature show that there are two possible mechanisms involved in most of neurodegenerative diseases: (1) mutations and/or aggregation of characteristic proteins of each disease such as α-synuclein in Parkinson’s disease (PD) or beta-amyloid peptide in Alzheimer’s disease (AD) and (2) dysfunction of mitochondrial energy metabolism in neurons. In this section, we will focus on this last one.
In the CNS, mitochondria are in charge of the production of needed energy to drive most cellular reactions through oxidation of glucose under aerobic conditions, ending up in the electron transport chain (ETC) [1]. A loss of mitochondrial function at this level is associated with neurodegenerative processes. Consequently, in view of the susceptibility of the ETC to oxidative damage, loss of ETC activity, due to an increment of ROS levels, has been proposed to be a plausible mechanism for the neuronal cell death associated with PD, AD, and other neurodegenerative disorders [2]. Furthermore, the “beta-amyloid (Aβ) cascade hypothesis” that dominated the field of AD research for the past decades has been challenged, and a new “mitochondrial cascade hypothesis ” has been proposed. The mitochondrial cascade hypothesis states that in sporadic, late-onset AD, loss of mitochondrial function associated with age affects the expression and the processing of APP, initiating Aβ accumulation [3]. It is known that not only the partial reduction of O2 increases superoxide radical (O2 −) in normal conditions but also complex I participates [4, 5]. Complex I (CI or NADH:ubiquinone oxidoreductase) is the largest ETC enzyme, containing 44 subunits and the main contributor to ROS production. Regarding this, many authors have described that complex I is disturbed in AD and PD [6, 7], increasing even more ROS levels [8]. Moreover, Schapira et al. [9] have reported a decreased activity of complex I in the substantia nigra of nine postmortem patients with PD. Giachin et al. [5] reviewed that neuronal cells are particularly susceptible to ROS-induced damage because they rely mainly on oxidative metabolism for ATP generation, contrary to glial cells, which are highly glycolytic. This overproduction of ROS not only increases OS able to oxidize biomolecules [10,11,12] but also leads to the activation of poly(ADP-ribose) polymerase (PARP), responsible for the repair of the DNA using NADH, inhibiting the glycolytic pathway caused by a reduction in NAD+ content. This leads to the subsequent compromise of the respiration at complex I where NADH is a key cofactor [13, 14].
Mitochondrial complex IV (cytochrome c oxidase) is also associated with development of neurodegeneration, in particular AD. Some authors described decreased levels of cytochrome c oxidase in neuronal mitochondria of AD postmortem brain patients [10]. Moreover, it is also known that Aβ specifically inhibit cytochrome c oxidase. This decrease could also be able to rise ROS levels [8, 12], leading to neuronal death.
In conclusion, although neurodegeneration is associated with multiple etiologies and pathophysiologic mechanisms, oxidative stress appears as a major part of the pathophysiologic process. Here, neuronal loss is caused by mitochondrial dysfunction and oxidative stress, among other factors. Mitochondrial function declines with age, with a concomitant increase of OS.
Neuroinflammation and Neurodegenerative Diseases
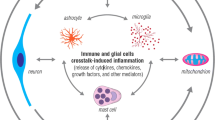
The central nervous system (CNS) presents a unique microenvironment given to the isolation due to the blood-brain barrier (BBB), which separates the brain from the periphery. BBB maintains chemical balance within the CNS to support neuronal function as well as limits the entrance of invading microorganisms [15]. Moreover, glial cells – microglia and astrocytes – constitute the neuroimmune system monitoring the CNS. These cells participate in various processes, such as phagocytosis, steroid and growth factor release, free radical reduction, and cellular repair, in both healthy and diseased brain [16,17,18,19]. Therefore, due to these peculiar characteristics, the CNS could be considered as an immunologically privileged organ.
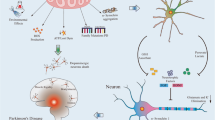
In the last decades, the average lifespan has increased due to the improvement of medical care and hygienic conditions. This fact comes together with an increment in the incidence of age-related diseases. Alzheimer’s disease is the most common neurodegenerative disorder, which is characterized by the presence of extracellular β-amyloid (Aβ) deposits and intracellular neurofibrillary tangles composed of phosphorylated protein Tau, resulting eventually in the characteristic memory loss [20]. The second most common neurodegenerative disease is PD, which is characterized by the aggregation of α-synuclein into Lewy bodies and Lewy neurites and the specific loss of dopaminergic neurons in the substantia nigra pars compacta, resulting in the exhibition of tremors, stooped posture, and dementia in some cases [21]. Regarding Huntington’s disease (HD), it is an autosomal dominant disease caused by a mutation in the huntingtin gene. Its manifestations include chorea and cognitive and behavioral decline [22].
Neurodegenerative diseases are accompanied by chronic inflammation, where the neuroimmune cells, mainly microglial cells, activate and adopt pro-inflammatory states (known as M1 phenotype). This state is characterized by the production of pro-inflammatory cytokines, which amplify the inflammatory response by recruiting and activating more microglial cells as well as promoting their proliferation. Many of these cytokines such as tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β) have been shown to lead to neuronal death both in vivo and in vitro [23, 24]. TNFα can induce cell death through (i) apoptosis via activation of caspase-8; (ii) necrosis via activation of receptor-interacting protein 1 (RIP1), receptor-interacting protein 3 (RIP3), and mixed lineage kinase domain-like protein (MLKL); and (iii) inflammation via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Moreover, M1 microglia characteristically expresses inducible NO synthase (iNOS), which produces high levels of NO continuously. These high levels of NO cause neuronal death by inhibition of mitochondrial cytochrome oxidase in neurons, leading to neuronal depolarization [25].
It is worth highlighting that, though inflammation contributes to the development and maintenance of degenerative diseases, it is not an initiation factor. In fact, an inflammatory response necessarily has to occur in order to guarantee the removal of harmful agents and repair of injuries. In this acute inflammation, microglial cells adopt an anti-inflammatory phenotype (termed M2), characterized by the release of anti-inflammatory cytokines such as tumor growth factor beta (TGF- β) and interleukin 10 (IL-10) and the expression of arginase-1, which inhibit the release of pro-inflammatory factors [26]. Consequently, we can conclude that it is the imbalance between the pro- and anti-inflammatory molecules secreted by microglial cells that defines whether the immune response will be beneficial or detrimental for the CNS.
Aging and Neurological Dysfunctions
The twentieth century was the century of population growth, and the twenty-first century will go into the history books as the century of aging. Global aging has been accelerating since 1969 when there were 550 million older adults, with a population of 1250 million in the year 2025 [27]. Only in the United States, the number of adults over the age of 64 will grow to 70 million by the year 2030, and they become the fastest-growing segment of the population [28]. With advanced age comes a decline in sensorimotor control and functioning and cognitive and learning impairment. As a consequence, aging is the major risk factor for the development of human neurodegenerative process such as Alzheimer’s, Huntington’s, and Parkinson’s diseases [29].
For instance, dysfunctions in fine motor control, locomotion, and balance affect the ability of older adults to perform activities of daily living and maintain their independence. The causes of these motor deficits are multifactorial, with central nervous declines and changes in sensory receptors, muscles, and peripheral nerves playing a role [30]. As a result, aging drives changes in brain structural regions: an age-related degeneration of the cerebellum [31], which may contribute to deficits in multi-joint coordination and postural stability in older adults [30] and a reduced volume of gray matter, often present with greater ventricular and cerebrospinal fluid volume [32, 33]. Furthermore, the prefrontal cortex is particularly susceptible to gray matter atrophy [34], and the differential thickness in prefrontal and parietal cortices may be relevant to motor age performance deficits in old age because motor control is more dependent on these brain regions in older adults. In 2004, the group of Salat found significant age effects in both the primary motor cortex, contributes to the movement slowing seen with age, and the somatosensory cortex, increased falls, poor balance, and increased reliance on visual feedback for motor performance in older adults, and they marked a potential vulnerability of these regions of the brain to age-related atrophy [35].
Aging also drives changes in neuronal and cognitive function. The hippocampus, a brain region subserving roles of spatial and episodic memory and learning, is sensitive to the detrimental effects of aging at morphological and molecular levels. With advancing age, synapses in various hippocampal subfields exhibit impaired long-term potentiation [36], an electrophysiological correlate of learning and memory. At the molecular level, immediate early genes are among the synaptic plasticity genes that are both induced by long-term potentiation and downregulated in the aging brain [37]. Besides, age-related cognitive impairment is associated with a specific set of synaptic plasticity proteins with roles in structural and functional synaptic systems [38]. Deak and Sonntag observed that an evolving area of research in brain aging is focused on the balance between excitatory and inhibitory synaptic systems [39, 40], especially in a reduction of synaptic markers [41].
Despite brain structural effects, there are also prominent differences in brain neurochemistry of older adults. For example, dopaminergic system has been most widely studied and appears to have dramatic effects. There is a significant decline in dopamine transmission levels – reduction of neurotransmitters, receptors, and transporters – and due to this general decrease in dopamine levels, the aging brain is often considered to be located in the preclinical continuum of Parkinson’s disease. Dopamine also plays a significant role in higher cognitive functions such as working memory [42], which is very important for motor skill acquisition [43]. So, these cognitive impairments associated with dopaminergic degeneration may indirectly contribute to age-related motor dysfunction.
In addition to a dopaminergic decline, a cholinergic reduction with age has been found in the medial forebrain and hippocampus [44]. Several studies have associated cholinergic decline in hippocampus with Alzheimer dementia, which involves severe deficits in learning and memory. Also, there is a serotonergic decline in the cingulate cortex and putamen [45] which has been related to cognitive deficits ([46] and motor dysfunctions [47]. There is a significant reduction associated with age of norepinephrine levels due to a loss of neurons in the locus coeruleus [48].
Aging and age-related disease are a mounting challenge for individuals, for families, and for social, economic, and healthcare systems. Implementation of preventive health strategies to decrease, delay, or prevent these affections may increase health expectancy and allow people to age gracefully and mantain an independent life style, without disability, for as long as possible [49].
Estrogen as Neuromodulators and Neuroprotectors
Estrogen is a potent steroid of both gonadal and neuronal origin implicated in reproductive functions as well as in neuromodulatory and neuroprotective actions on the central nervous system [50, 51]. Local synthesis of this molecule in the central nervous system may prevent or reduce neurodegeneration. Steroids modulate the expression of neuropeptides such as neuropeptide Y (NPY), galanin, and β-endorphin. NPY regulates the reproductive cycle, and estradiol administration has been shown to decrease NPY expression in the arcuate nucleus [52, 53], whereas the absence of this hormone results in an increase of NPY mRNA levels [54]. β-Endorphin is another peptide involved in energy and reproductive homeostasis. Estradiol stimulates the activity of pro-opiomelanocortin (POMC) cells, increases the levels of POMC mRNA, and even increases the number of these cells [53, 55, 56].
Moreover, estrogens participate in a series of protective function processes. One example is the promotion of cell survival and synaptic plasticity. In vitro studies have shown that the addition of 17β-estradiol to primary cultures of various neuronal populations (hypothalamic, neocortical, hippocampal, and amygdala) increased viability, survival, and differentiation [42, 57,58,59]. This molecule also regulates the expression and the action of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) and their receptors (trk, p74) [60]. Neurotrophins are a family of proteins that favor the survival, development, and function of neurons, and estrogens can interact with them by convergence (e.g., sharing the same signaling cascade, acting synergistically), by inducing their expression through estrogen response elements, or by dependence, where the two components are needed to obtain a complete effect [61, 62]. Estrogens exert their functions via the classical nuclear receptors, regulating transcription factors that control the expression of estrogen-sensitive genes and membrane receptors (independent of transcriptional activation), which elicit faster responses than genomic effects. The most well-characterized nuclear receptors are estrogen receptor α (ERα) and estrogen receptor β (ERβ) [61], and among the membrane receptors is G-protein-coupled receptor 30 (GPR30) which, according to in vitro studies in cortical neurons, is involved in neuroprotection against oxidative stress [63].
The preoptic area, the hypothalamus, and the amygdala are the regions with the highest expression of ERs, although expression of these is also observed in regions of the cortex, hippocampus, and midbrain. Although ERα is the subtype with the highest expression in most regions, there are areas such as the mesencephalon, where ERβ predominates [64].
Estrogens can also prevent neuronal dysfunction by alterations in the levels of neurotransmitters, their receptors, and second messengers, through estrogen-binding sites in the plasma membrane [65]. For example, estradiol could regulate the release and uptake of dopamine by decreasing the affinity of dopamine receptors in order to protect the nigrostriatal system from excitotoxicity [66,67,68].
Several studies evidence the neuroprotective effects of estrogens in neurodegenerative diseases and traumatic damage. In Alzheimer’s disease, estradiol could affect β-amyloid aggregation by regulating its metabolism and apolipoprotein E (ApoE) expression [69, 70]. In addition, this hormone could be involved in the regulation of cholinergic and serotonergic neurotransmissions, thus reducing the cognitive and affective symptoms of the disease.
Estradiol has anti-inflammatory effects in the brain that are mediated, at least in part, by a reduction of reactive gliosis [60, 71] and contributing to neuronal regeneration [72,73,74].
As it has already discussed, there are numerous neuroprotective actions of estrogens that have direct relevance to neurodegenerative diseases. Despite these actions, the promise of estrogen-based therapies for reducing the risk for neurodegeneration remains to be fulfilled. The application of estradiol as a neuroprotectant in humans presents numerous limitations, mainly due to the endocrine actions of the molecule on peripheral tissues, including estrogen dependent tumors. Therefore, as ongoing research continues to address these crucial and immediate concerns, an emerging area of investigation is the development of natural and synthetic hormone mimetics that will preferentially activate estrogen neuroprotective mechanisms while minimizing adverse effects in other tissues.
Neurological Dysfunctions and Brain’s Growth Factors
Neurotrophic factors (NTFs) are diffusible peptides secreted from neurons and neuron-supporting cells. They serve as growth factors for the development, maintenance, repair , and survival of specific neuronal populations, and they act via retrograde signaling from target neurons by paracrine and autocrine mechanisms [75, 76]. NTFs have different functions depending on the stage of life. During development they contribute to the formation of synaptic network [77, 78], neuronal survival, and axonal growth [79, 81]. In adulthood they are associated with neuronal survival and maintenance of different neural phenotypes [80].
An additional basic activity of many growth factors in the nervous system is influencing on the proliferation, migration, and differentiation of stem cells in the developing and adult nervous system [81, 82]. These basic properties are also employed by the nervous system in the case of disease and injury and thus constitute a powerful endogenous repair and maintenance system. NTFs act together in a coordination mode, so that any alteration on their local synthesis and/or transport caused by traumas, pathologies, or even aging could lead to neuronal death. Loss or dysregulation of endogenous trophic factors could contribute to the development of neurodegenerative diseases.
Neurotrophic factors are classified in superfamilies, according to their structure or function, as follows: (i) nerve growth factor (NGF) superfamily; (ii) transforming growth factor (TGF)-beta superfamily, consisting of the glial cell line-derived neurotrophic factor (GDNF) family, the TGF-beta family, and the bone morphogenetic protein (BMP) family; (iii) neurokine or neuropoietin superfamily; and (iv) non-neuronal growth factors that include IGF-1 and acidic and basic fibroblast growth factors (aFGF and bFGF, respectively). In the last decade, there have been described two new NTFs that cannot be included in the previous classification. They are cerebral dopamine neurotrophic factor (CDNF) and mesencephalic astrocyte-derived neurotrophic factor MANF [83]. Both are structurally and functionally clearly distinct from the classical, target-derived neurotrophic factors (NTFs) that are solely secreted proteins [82].
The potential for a therapeutic application of these factors has been realized early on, and the last two decades have seen several approaches to exploit this potential in different neurological disorders. The next section summarizes the current data on NTFs and their potentiality as therapeutic agents for neurodegenerative diseases.
NTFs and Neurodegenerative Diseases: Evidences and Perspectives
There is clear evidence that neurodegenerative diseases course with impairment levels of neurotrophic factors or their receptors. For example, in PD there is a small decrease in staining density for BDNF and dopaminergic projection areas; GDNF levels are reduced (about 20%) in the substantia nigra pars compacta (SNpc) from PD patients compared with age-matched controls. The researchers also found that ciliary neurotrophic factor (CNTF) decreased as compared with age-matched controls, 11.1%/neuron and 9%/neuropil, and markedly increased levels of tumor necrosis factor and interleukin 6 (IL-6) in the nigrostriatal DA regions of PD patient [84].
Respect to the other major neurodegenerative disease, Alzheimer’s disease (AD), it had been described increases in NGF and decreases in BDNF in hippocampal and neocortical regions. It was also observed decreases in tropomyosin receptor kinase A (TrkA) in the cortex and nucleus basalis in advanced AD [85, 86].
The well-documented role for neurotrophic factors to prevent cell death and to maintain cellular function has led scientists to investigate their use as therapeutic drugs and benefits. In the last years, there have been numerous preclinical and clinical studies involving treatment of neurodegenerative diseases with NTFs (for review see [84, 87,88,89]).
Despite the evident heterogeneity, the results from the reviewed studies can aid in conducting human trials applying NTFs. The reviews mentioned highlight that targeted local deliveries of NTFs led to favorable safety and efficacy outcomes when administration regimens successfully target a degenerated neuronal population. The previous works suggest that the application of NTFs is generally safe and well tolerated when administered locally.
In conclusion the strong potential of NTFs to exert pro-survival and pro-functional responses in neurons of the peripheral and central nervous system makes them good target candidates for treatment of a multitude of neurodegenerative disorders. However significant problems need to be overcome before translating the potential of neurotrophic factors into the therapeutic area.
Insulin-Like Growth Factor-1 as a Therapeutic Factor
As we previously mentioned, neurotrophic factors that prevent degeneration and restore the function of the remaining neuronal populations are currently of increasing clinical interest as targets of therapeutic possibility in the treatment of neurodegenerative diseases. One of these neurotrophic factors is IGF-1. It is known, in rats, that tissue level of IGF-1 and its receptor decrease significantly in the hippocampus and cortical layers II/III and V/VI throughout life [90]. It is also known that in situations of cytotoxic hippocampal damage, the microglia of this region substantially increases the production of IGF-1 and IGF-binding protein 2, suggesting a neuroprotective role of these molecules in the central nervous system [91].
It has also been documented that IGF-1 protects hippocampal neurons from the toxic effects of amyloid peptides [92] and that treatment with IGF-1 in mice overexpressing a mutant Aβ-amyloid peptide markedly reduces the peptide levels and improves their cognitive ability [93]. In vitro studies have shown that IGF-1 increases the cell survival of primary cultures and hypothalamic neurons [94] and stimulates the differentiation of rat dopaminergic mesencephalic neurons [95]. Also, IGF-1 modulates the inflammatory response of astrocyte cultures treated with lipopolysaccharide (LPS) [96].
There are mechanisms that suggest neuroinflammation is harmful, at least partially, because it avoids the neuroprotective action of IGF-1 [97]. Moreover, a protective effect of IGF-1 on hypothalamic cells exposed to glutathione-depleting agents has also been described [98], as well as in human, dopaminergic culture cells exposed to the salsolinol toxin and in human and murine neuronal cultures exposed to high doses of dopamine [99]. Recently, Rodriguez-Perez et al. [100] demonstrated that IGF-1 participates together with the local renin-angiotensin system to inhibit or activate neuroinflammation (M1-M2 phenotype transition), oxidative stress, and dopaminergic degeneration induced by MPP + neurotoxin.
Short-term studies of our group revealed that gene therapy with IGF-1 in the senile female rats with DA neurodegeneration is highly effective to restore hypothalamic DA neuronal function, thus correcting the chronic hyperprolactinemia associated with the dysfunction of tuberoinfundibular dopaminergic neurons (TIDA) in senile rats [101]. Also, ICV gene therapy with IGF-1 partially but significantly restores motor performance in this model of spontaneous TIDA neurodegeneration in senile animals [102, 103].
Among the possible effector fields of IGF-1 on synaptic plasticity and hippocampus, it is known that within a glutamatergic synapse, there is a positive effect of IGF-1 on synaptic transmission and consequently a prevention of cognitive deterioration. IGF-1 (as well as ghrelin, GLP-1, and insulin) is reported to be one of the molecules that stimulate glucose metabolism, providing energy for the biosynthesis of neurotransmitters. In this way, it would facilitate hippocampal circuits in terms of plasticity and synaptic structure, as well as participating in neurogenic aspects modifying learning, memory, and cognitive functionality [104]. This indirect mechanism of action would be mediated by the IGF-1 signal receptor that leads to voltage-dependent calcium channel phosphorylation, causing increased calcium and release of neurotransmitters that would facilitate synaptic conduction [105].
Currently, it is unknown whether IGF-1 exerts a direct effect on the mobilization of synaptic vesicles and the core complex of soluble NSF attachment protein (SNARE) proteins during neurotransmitter exocytosis. Interestingly, at the postsynaptic site, IGF-1 could inhibit the activity of glycogen synthase kinase 3 beta (GSK3β), a key factor in the hyperphosphorylation of the microtubule-associated protein (Tau). By reducing the activity of GSK3, IGF-1 could potentially prevent the formation of neurofibrillary agglomerates (an important pathological marker of AD disease). In addition, through phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) activation, IGF-1 may be involved in the incorporation and transport of glutamatergic receptors in dendrites [41].
Conclusion
It is well known that age is the major risk factor for neurodegenerative diseases, and the endogenous level of neurotrophic factors decreases with age, suggesting that trophic factor loss may contribute to the etiology of neurodiseases. Neurodegenerative diseases such as Parkinson’s disease or Alzheimer’s disease and even the natural process of aging generate, as we detailed, several cognitive disorders and motor dysfunctions associated with excessive ROS from cellular metabolism, alterations in growth factor production, in pro-inflammatory cytokines and inflammatory markers, and in neurotransmitter receptors expression, and structural changes in dendritic morphology and electrophysiological properties.
In this context, insulin-like growth factor-1 is an important neurotrophic factor/hormone with receptors widely expressed in the brain. It is affected in a range of neurodegenerative disorders and may play a role in brain dysfunction. It is well known that IGF-1 promotes neurogenesis and synaptogenesis in the postnatal dentate gyrus and acts as fast modulator of brain activity, specifically, in cognitive processes. Also, the chronic administration of IGF-1 or administration of Gly-Pro-Glu, an N-terminal peptide of IGF-1, attenuated loss of TH-immunoreactive cells, terminals, and behavioral deficits in response to 6-hydroxydopamine (6-OHDA) infusion into the dopaminergic axons within the nigrostriatal pathway. Moreover, IGF-1 therapy improved glucose and lipid metabolism, increasing testosterone levels and antioxidant ability and reducing oxidative damage, and it was also associated with a normalization of antioxidant enzyme activities.
Taken together, the IGF-1 action as neuroprotective molecule, the interaction with estrogen as neuromodulators, its crucial role in oxidative stress, and its important function in synaptic plasticity are valuable aspects to study and evaluate in depth of this growth factor considering that it may be a potential therapeutic molecule inside neurodegenerative diseases.
References
Pope S, Land JM, Heales SJ. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim Biophys Acta. 2008;1767(7–8):782–9.
Spano M, Signorelli M, Vitaliani R, Aguglia E, Giometto B. The possible involvement of mitochondrial dysfunctions in Lewy body dementia: a systematic review. Funct Neurol. 2015;30(3):151–8.
Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1105–21.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13.
Giachin G, Bouverot R, Acajjaoui S, Pantalone S, Soler-López M. Dynamics of human mitochondrial complex I assembly: implications for neurodegenerative diseases. Front Mol Biosci. 2016;3:43.
Berndt N, Holzhütter HG, Bulik S. Implications of enzyme deficiencies on mitochondrial energy metabolism and reactive oxygen species formation of neurons involved in rotenone-induced Parkinson’s disease: a model-based analysis. FEBS J. 2013;279(20):5079–92.
Zhang L, Zhang S, Maezawa I, Trushin S, Minhas P, Pinto M, Jin LW, Prasain K, Nguyen TD, Yamazaki Y, Kanekiyo T, Bu G, Gateno B, Chang KO, Nath KA, Nemutlu E, Dzeja P, Pang YP, Hua DH, Trushina E. Modulation of mitochondrial complex I activity averts cognitive decline in multiple animal models of familial Alzheimer’s disease. EBioMedicine. 2015;2(4):293–305.
Sipos I, Tretter L, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem. 2003;82(1):112–8.
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1978;1(8539):1268.
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21(9):3017–23.
Cristalli DO, Arnal N, Marra FA, de Alaniz MJ, Marra CA. Peripheral markers in neurodegenerative patients and their first-degree relatives. J Neurol Sci. 2012;314(1–2):48–56.
Avetisyan AV, Samokhin AN, Alexandrova IY, Zinovkin RA, Simonyan RA, Bobkova NV. Mitochondrial dysfunction in neocortex and hippocampus of olfactory bulbectomized mice, a model of Alzheimer’s disease. Biochemistry (Mosc). 2016;80(6):605–23.
Abeti R, Abramov AY, Duchen MR. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain. 2011;134(Pt 6):1648–71.
Delgado-Camprubi M, Esteras N, Soutar MP, Plun-Favreau H, Abramov AY. Deficiency of Parkinson’s disease-related gene Fbxo7 is associated with impaired mitochondrial metabolism by PARP activation. Cell Death Differ. 2017;24(1):120–31.
Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1163–7.
Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:482–94.
Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–51.
Xia CY, Zhang S, Gao Y, Wang ZZ, Chen NH. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int Immunopharmacol. 2015;25(2):376–81.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(7):35.
O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:184–204.
Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:S207–9.
Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord. 2012;27:1082–90.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:908–24.
McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflamm. 2008;5:45.
Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1618:287–96.
Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:64–85.
Lunenfeld B. An aging world- demographics and challenges. Gynecol Endocrinol. 2008;24:1.
He W, Sengupta M, Velkoff VA, et al. 641 in the United States: 2005. Washington, DC: National Institute on Aging; U.S. Census Bureau; 2005.
Moll L, El-Ami T, Cohen E. Selective manipulation of aging: a novel strategy for the treatment of neurodegenerative disorders. Swiss Med Wkly. 2014;144:w13907.
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon M, Gwin J, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional and biomechanical effects. Neurosci Biobehav Rev. 2011;34(5):711–23.
Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1666–88.
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 464 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36.
Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):662–81.
Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3294–301.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2014;14(7):711–30.
Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40.
Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, Shen JC, Zou B, Xie XS, Tingle M, Hinkson IV, Angst MS, Wyss-Coray T. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544(7541):487–91.
Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;23:8.
Lu T, Pan Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:872–81.
Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;463:529–35.
Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;66(6):601–25.
Arimatsu Y, Hatanaka H. Estrogen treatment enhances survival of cultured fetal rat amygdala neurons in a defined medium. Brain Res. 1975;390(1):151–9.
Seidler RD, Bo J, Anguera JA. Neurocognitive contributions to motor skill learning: the role of working memory. J Mot Behav. 2012;44(6):445–53.
Bañuelos C, LaSarge CL, McQuail JA, Hartman JJ, Gilbert RJ, Ormerod BK, Bizon JL. Age-related changes in rostral basal forebrain cholinergic and GABAergic projection neurons: relationship with spatial impairment. Neurobiol Aging. 2013;34(3):845–62.
Gottfries CG. Human brain levels of monoamines and their metabolites. Postmortem investigations. Acta Psychiatr Scand. 1980;61(S280):49–61.
Cowen P, Sherwood AC. The role of serotonin in cognitive function: evidence from recent studies and implications for understanding depression. J Psychopharmacol. 2013;27(7):575–83.
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lippset DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–33.
Mann JJ, Stanley M, Kaplan RD, Sweeney J, Neophytides A. Central catecholamine metabolism in vivo and the cognitive and motor deficits in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46:905–10.
Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventive strategies. Best Pract Res Clin Obstet Gynaecol. 2014;27(5):633–49.
Do Rego JL, Seon JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon M, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol (Elsevier Inc). 2009;30(3):259–301.
Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56.
Crowley WR, Tessel RE, O’Donohue TL, Adler BA, Kalra SP. Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: correlation with serum luteinizing hormone (LH) and median eminence LH-releasing hormone. Endocrinology (Oxford University Press). 1974;117(3):1151–5.
Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol (Blackwell Publishing Ltd). 2007;19(6):426–31.
Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1985;204(1):80–2.
Thornton JE, Loose MD, Kelly MJ, Rönnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol (Wiley Subscription Services, Inc., A Wiley Company). 1983;341(1):67–76.
Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13(1):88–93.
Chowen JA, Torres-Alemán I, García-Segura LM. Trophic effects of estradiol on fetal rat hypothalamic neurons. Neuroendocrinology (Karger Publishers). 1981;56(6):885–90.
Brinto RD, Tran J, Proffitt P, Montoya M. 17β-estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem Res (Kluwer Academic Publishers-Plenum Publishers). 1986;22(11):1339–51.
Sudo S, Wen TC, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M. β-Estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res. 1986;29(4):345–54.
Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;62(1):29–60.
Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–35.
Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front Neuroendocrinol. 2006;27(4):390–403.
Liu SB, Han J, Zhang N, Tian Z, Li XB, Zhao MG. Neuroprotective effects of oestrogen against oxidative toxicity through activation of G-protein-coupled receptor 30 receptor’. Clin Exp Pharmacol Physiol. 2011;38(9):576–84.
Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1986;387(4):507–25.
Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front Neuroendocrinol. 1985;17(4):402–39.
Disshon KA, Dluzen DE. Estrogen as a neuromodulator of MPTP-induced neurotoxicity: effects upon striatal dopamine release. Brain Res. 1986;753(1–2):9–16.
Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1987;345(2):207–11.
Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol (Kluwer Academic Publishers). 2000;29(5–6):386–98.
Bales KKR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APPV707F transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci. 1989;95(26):15233–8.
Xu H, Gouras GK, Greenfield JP, Vincen B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer bold beta-amyloid peptides. Nat Med. 1987;4(4):447–51.
Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29(4):507–19.
Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A (National Academy of Sciences). 2003;99(16):9504–9.
Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology (Oxford University Press). 2006;147(5):2262–71.
Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1982;618(1–2):270–7.
Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1983;14(1):14 LP–28.
Valdés JJ, Weeks OI. Estradiol and Lithium chloride specifically Alter NMDA receptor subunit NR1 mRNA and excitotoxicity in primary cultures. Brain Res. 2009;1267(May):1–12.
Lo DC. Chapter 7 instructive roles of neurotrophins in synaptic plasticity. Prog Brain Res. 1987;117:64–9.
Vicario-Abejon C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. 2002;3(12):954–74.
Keefe MK, Sheikh SI, Smith MG. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18(3):548; doi: https://doi.org/10.3390/ijms18030548.
Mitre M, Mariga A, Chao MV. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin Sci. 2016;131(1):13–23.
Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1979;347(6194):751–64.
Kuiper SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9(5):579–85.
Lindahl M, Saarma M, Lindholm P. Unconventional neurotrophic factors CDNF and MANF: structure, physiological functions and therapeutic potential. Neurobiol Dis (Elsevier Inc.). 2016;96:89–101.
Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs: Clin Immunotherapeutics, Biopharmaceuticals Gene Ther. 2005;19(2):96–127.
Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Brain Res Rev. 1987;27(1):1–39.
Hock C, Heese K, Müller-Spahn F, Hulette C, Rosenberg C, Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1987;241(2–3):151–4.
Bartus RT, Johnson EM. Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: where have we been and what have we learned? Neurobiol Dis (Elsevier Inc.). 2015;96:156–67.
Bartus RT, Johnson EM. Clinical tests of neurotrophic factors for human neurodegenerative diseases, Part 2: where do we stand and where must we go next? Neurobiol Dis (Elsevier Inc.). 2016;96:168–77.
Bezdjian A, Kraaijenga VJC, Ramekers D, Versnel H, Thomeer HGXM, Klis SFL, Grolman W. Towards clinical application of neurotrophic factors to the auditory nerve; assessment of safety and efficacy by a systematic review of neurotrophic treatments in humans. Int J Mol Sci. 2016;17(12):1981. doi: https://doi.org/10.3390/ijms17121981.
Sonntag WE et al. Alterations in IGF-I gene and protein expression and type 1 IGF I receptors in the brains of ageing rats. Neuroscience. 1989;87.
Breese CR, D’Costa A, Rollins YD, Adams C, Booze RM, Sonntag WE, Leonard S. Expression of insulin‐like growth factor‐1 (IGF‐1) and IGF‐binding protein 2 (IGF‐BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J Comp Neurol. 369(3):388–404.
Doré S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against betaamyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1986;93:4761–7.
Carró E, Torres-Aleman I. Serum insulin-like growth factor I in brain function. Keio J Med. 2006;55:59–62.
Knusel B, Michel PP, Schwaber JS, Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. Journal of Neuroscience. 1990;10(2):558–70.
Carró E, Busiguina, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2916–23.
Bellini MJ, Hereñú CB, Goya RG, Garcia-Segura LM. Insulin-like growth factor-I gene delivery to astrocytes reduces their inflammatory response to lipopolysaccharide. J Neuroinflammation. 2011;8:21.
Trejo, Carro, Garcia-Galloway, Torres-Aleman. Role of IGF-I signaling in neurodegenerative diseases. J Mol Med. 2004;81:156–61.
Sortino, Canonico. Neuroprotective effect of insulin-like growth factor I in immortalized hypothalamic cells. Endocrinology. 1985;137:1418–22.
Shavali R, Ebadi. IGF-I protects human dopaminergic SH-SY5Y cells from salsolinol induced toxicity. Neurosci Lett. 2003;340:78–81.
Rodriguez-Perez AI, Borrajo A, Diaz-Ruiz C, Garrido-Gil P, Labandeira-Garcia JL. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. Oncotarget. 2016;7:30049–67.
Hereñú CB, et al. Restorative effect of insulin-like growth factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Ther. 2007;14:237–45.
Hereñú C, Sonntag W, Morel, Portiansky, Goya R. The ependymal route for IGF1 gene therapy in the brain. Neuroscience. 2009;162(1):442–7.
Nishida F, Morel GR, Hereñú CB, Schwerdt JI, Goya RG, Portiansky EL. Restorative effect of intracerebroventricular insulin-like growth factor-I gene therapy on motor performance in aging rats. Neuroscience. 2011;176:194–206.
Mainardi M, Fusco S, Grassi C. Modulation of hippocampal neural plasticity by glucose-related signaling. Neural Plast. 2015;2015:657928.
Liquitaya-Montiel A, Aguilar-Arredondo A, Arias C, Zepeda A. Insulin growth factor-I promotes functional recovery after a focal lesion in the dentate gyrus. CNS Neurol Disord Drug Targets. 2012;11(7):808–28.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Herrera, M.L., Falomir-Lockhart, E., Dolcetti, F.J.C., Arnal, N., Bellini, M.J., Hereñú, C.B. (2019). Implication of Oxidative Stress, Aging, and Inflammatory Processes in Neurodegenerative Diseases: Growth Factors as Therapeutic Approach. In: Gargiulo, P., Mesones Arroyo, H. (eds) Psychiatry and Neuroscience Update . Springer, Cham. https://doi.org/10.1007/978-3-319-95360-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-95360-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95359-5
Online ISBN: 978-3-319-95360-1
eBook Packages: MedicineMedicine (R0)