Abstract
Increasing numbers of trauma patients receive immediate and appropriate control of life-threatening injuries, but some of them eventually progress to brain death. Establishment of the best therapeutic strategies for patients with extensive and major trauma has led to significant improvement in donor conversion rates and increased utilization of deceased donors. Approximately 30% of all deceased organ donors are trauma patients who have suffered devastating neurologic injury and have progressed to brain death.
Donation rates have risen in the past decade, but many patients still die on the waiting list for organ transplants. Although most organs are from brain death donors, donation after cardiac death has come to be an additional resource in recent years. Optimizing selection and management of potential organ donors and exploring new strategies for maintenance of organ function have thus become a priority for expanding the transplantable organ pool. This chapter discusses organ donation from deceased donors, highlighting the margins of improvement in their management from declaration of death to organ recovery and outlining the possible applications of new perfusion technologies in this process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
-
Establishing the best therapeutic strategies for patients with major trauma has led to significant improvement in donor conversion rates and utilization of deceased donors.
-
Optimizing donor management is fundamental to increase the numbers of organs available for transplantation.
-
Declaration of death on the basis of neurologic criteria preserves transplantable organs from the detrimental warm ischemia that limits donation after cardiac death (DCD).
-
Brain death causes numerous physiological derangements, which make brain-dead donors fragile and essentially unstable.
-
Many countries worldwide have explored the option of DCD to expand the organ donor pool.
-
Normothermic regional perfusion (NRP) is a very effective method for preservation and functional assessment of DCD abdominal organs.
-
NRP permits unhurried warm organ dissection and cold flash directly through the femoral cannulae, minimizing the risk of graft injury.
-
The combination of NRP followed by machine perfusion can be considered a promising new frontier for organ recovery from DCD.
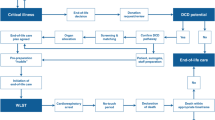
1 Introduction
Treatment of the trauma patient has improved enormously over the last few decades, achieving better results and survival rates. Although increasing numbers of trauma patients receive immediate and appropriate control of life-threatening injuries, some eventually progress to brain death. Establishment of the best therapeutic strategies for patients with extensive and major trauma, such as damage control surgery—a new emerging discipline with the aim of preventing patients progressing to an unsalvageable metabolic state—has led to significant improvements also in donor conversion rates and increased the utilization of deceased donors. Approximately 30% of all deceased organ donors come from trauma patients who have suffered devastating neurologic injury and have progressed to brain death.
Though donation rates have increased over the past decade, many patients still die on the waiting list for organ transplantation. Many individuals who satisfy the criteria for becoming organ donors eventually fail to donate because of lack of consent. But even when consent is given, organs may be deemed unsuitable for donation because of their condition, or the donor may develop cardiac arrest (CA) before organs can be recovered. Medical failures prior to organ procurement in brain-dead patients may be preventable in many cases, and optimizing donor selection and management has thus become a priority [1, 2]. Early recognition and physiological maintenance of the potential organ donor, and close coordination with the local organ procurement organization, are all important aspects of this process.
In the late 1990s, Jenkins et al. estimated that only 15–20% of potential donors actually become donors in the United States [1]. In 2012, brain deaths eligible for organ donation amounted to 8944 in the United States, with a donor conversion rate of 72.5 eligible donors per 100 eligible deaths [3]. In Italy, 2341 brain deaths were reported in 2015, and just under half were utilized as donors (1163, 49.8%) with lack of consent to donation 30.5% [4].
Since the definition of brain death diagnosis criteria in the 1960s, most organs have been procured from patients who have progressed to brain death. In recent years, however, donation after cardiac death (DCD) has grown substantially in the effort to expand the donor pool, and programs involving new perfusion methods have been developed to allow maintenance of organ function in spite of warm ischemia [2, 5,6,7].
This chapter discusses organ donation from deceased donors, highlighting the margins of improvement in their management from declaration of death to organ recovery and defining the possible applications of the new perfusion technologies. Living donation is another possibility to expand the donor poll, but it will be not analyzed here.
2 Donation After Brain Death (DBD)
The possibility of certifying death based on neurologic criteria in individuals who have lost all neurologic function, but continue to sustain a heartbeat and have their respiration supported, allows a flash of cold preservation solution simultaneously with CA during the donor operation, thus preserving the organs from detrimental warm ischemia [8, 9]. The most common causes of death in this group are cerebrovascular accident, traumatic brain injury, and anoxia. Traumatic brain injury, once the most common cause of brain death, has now been overtaken by cerebrovascular accident. Trauma donors are generally younger and healthier than stroke donors [10].
2.1 Death Declaration Based on Neurologic Criteria
Death was defined by the ad hoc committee of the Harvard Medical School in 1968 as the irreversible cessation of whole brain function [8, 11]. The diagnosis of death by neurologic criteria requires clinical and instrumental evidence of complete and irreversible absence of brain function, and the exclusion of confounding factors [8]. Confounding factors that must be ruled out include hypothermia, circulatory shock, drug intoxication, endogenous metabolic intoxication states such as renal or hepatic failure, and the prolonged effects of neuromuscular blocking agents and sedatives [11].
Different brain death criteria have been adopted in different countries. In the United States and in Italy, declaring death on the basis of neurologic criteria uses the concept of whole brain death, according to which loss of cortical function and brainstem reflexes has to be demonstrated. In the United Kingdom, only the loss of brainstem function is required [8]. According to Italian law, brain death is diagnosed according to the following criteria: (1) deep coma, (2) absence of brainstem reflexes and positive apnea testing (no breathing effort is observed at a partial arterial pressure of carbon dioxide more than 60 mmHg and pH more than 7.4 after discontinuation of mechanical ventilation), (3) flat electroencephalogram, and (4) absence of cerebral blood flow at imaging (e.g., cerebral angiography) for children younger than 1 year of age and when there are factors affecting clinical evaluation or electroencephalography. There must be an observation period lasting at least 6 h [12].
2.2 Authorization for Organ Donation
From a legal point of view, organ donation is an anatomical gifting and not a healthcare decision for the donor. For this reason it does not involve standard informed consent [13, 14]. The approach to authorization for organ donation internationally adheres to a model of either explicit or presumed consent. An explicit consent system requires the consent of the individual or family. Mechanisms for designating oneself as an organ donor include signing a donor card or registering through a donor state registry. In a presumed consent system, consent is instead presumed unless the individual has exercised the right to refuse donation. The presumed consent model still follows voluntary gift law principles [14].
An interview study in the United States has shown that, among families that deny donation, 53% did not receive an adequate explanation of brain death, and those who decide against donation generally have less understanding of brain death than those who agree to it [15]. Several recent studies have shown that in-house transplant coordinators at various trauma centers, in the form of a dedicated physician or other specially trained healthcare providers, were able to significantly increase family consent rates [10, 16]. The discussion with the potential donor’s family needs to be caring but unambiguous and direct in stating that the individual is dead, and the distinction should always be clear between the event of death and organ donation [8].
2.3 Physiological Maintenance of the Brain-Dead Donor
Once the established neurologic criteria have been fulfilled, the patient is declared dead. Physicians are under no moral or legal obligation to continue medical support for a cadaver, except when they are dealing with an organ donor [8]. In this case, every effort must be made to maintain homeostasis of the brain-dead donor until the procurement, thus preserving the organs to be transplanted.
2.3.1 Brain Death Physiopathology
Brain death causes numerous physiological derangements, producing profound hemodynamic and metabolic abnormalities (Table 55.1). The two main mechanisms responsible for these changes are the loss of integrated neurologic regulation and the sustained release of pro-inflammatory cytokines, which have negative effects on the function of numerous systems [11]. This makes the brain-dead donor physiologically fragile and unstable. Thus, organ recovery must proceed promptly to avoid potential donor loss.
Cardiovascular effects of brain death result first from the so-called autonomic storm and later from profound reductions in sympathetic outflow [11]. When ischemia reaches the vagal nuclei in the lower medulla, unopposed sympathetic stimulation occurs, and this autonomic storm causes a significant increase in vascular resistance. Subsequently, loss of spinal sympathetic pathways causes total sympathectomy, with consequent systemic vasodilatation, bradycardia, and hemodynamic instability. Restoration of blood pressure typically requires volume replacement and vasopressors. High doses of vasopressors may lead to organ ischemia and worsen the transplant outcome, but clinical data are conflicting. Nevertheless, combination therapy may avoid excessively high doses of a single agent with strong vasoconstrictor effects [2].
The pulmonary capillaries may be subjected to excessive hydrostatic pressure when left atrial pressure exceeds pulmonary artery pressure because of vasoconstriction. This results in capillary wall disruption and leakage of protein-rich fluid into the pulmonary interstitium, explaining what is termed neurogenic pulmonary edema [11]. Other potential donor respiratory complications include ventilator-associated and aspiration pneumonia [10].
The hematologic system is adversely affected as the high catecholamine and cortisol levels create a temporary hypercoagulable state. The passage of cerebral gangliosides into the systemic circulation and the consequent release of fibrinolytic factors can lead to significant coagulopathy and spontaneous hemorrhage [2, 11].
Neuroendocrine changes occur in relation to hypothalamic-pituitary axis dysfunction. The most commonly reported manifestation is diabetes insipidus due to antidiuretic hormone deficiency, which can further exacerbate low vascular resistance hypovolemia. It can be treated with desmopressin or vasopressin, if vasopressors are otherwise required. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) may also be observed. Anterior pituitary deregulation frequently translates into significant reduction of thyroid hormone and cortisol levels [11].
Hormone therapy to the donor has been a topic of controversy. There are data suggesting that thyroid hormone may relieve hemodynamic instability in brain-dead donors. Its use has been associated with complete reversal of anaerobic metabolism, reducing the need for vasopressor support, preventing cardiovascular collapse, and stabilizing the hemodynamically unstable donor [10, 11, 16].
Different electrolyte derangements can occur in the brain-dead donor, secondary to the altered cellular membrane permeability and diabetes insipidus. Wide variations in body temperature and hypothermia are the result of central dysregulation, thyroid dysfunction, and exposure and infusion of large volumes of crystalloids and blood products and can exacerbate coagulopathy or precipitate arrhythmia and CA.
2.3.2 Optimizing the Management of the Brain-Dead Donor
Even with aggressive treatment, more than 25% of potential donors may be lost due to hemodynamic instability [11]. The American Society of Transplantation and American Society of Transplant Surgeons advocate the use of standardized donor management protocols [16]. Early admission to the intensive care unit with an aggressive donor management protocol has reduced the incidence of cardiovascular collapse in donors and improved organ recovery and function in recipients [10]. A protocol of aggressive donor management should include the following: (1) pulmonary artery catheterization to monitor hemodynamic status and tissue perfusion; (2) maintenance of an adequate systemic perfusion pressure with aggressive infusion of fluids and vasopressors; (3) hormonal therapy, consisting of thyroid hormone; and (4) identification of brain death-related complications and prompt intervention [11, 16].
2.4 Donor Evaluation and Risk Assessment
Exclusion criteria for a potential organ donor are essentially based on the history, clinical examination, and blood tests; specific tests and imaging may serve to complete the evaluation of individual organs (e.g., coronary angiography). Donors are consequently stratified according to specific classes of risk, as shown in Table 55.2. If the evaluation does not allow a clear definition of the class of risk, the opinion of some expert consultants (second opinion) is needed.
Age is not an absolute exclusion criterion for organ donation, and in fact the percentage of liver transplants from octogenarian donors has risen steadily in Western countries in the last few years [17]. Absolute exclusion criteria are human immunodeficiency virus (HIV) infection; active malignant neoplasms with risk of metastases; untreatable, generalized infections; and documented prion disease. However, some HIV-positive to HIV-positive transplants have been recently reported in the literature [18]. Relative and organ-specific contraindications may vary from center to center and in relation to the recipient’s conditions and urgency. A specific discussion of these aspects is beyond the scope of this chapter [19].
2.5 General Principles of Surgical Technique for DBD Organ Recovery
The guiding principle of organ procurement in DBD is the avoidance of warm ischemia. This can be achieved during procurement with in situ organ cooling by intravascular infusion of the cold preservation solution at the time of circulatory arrest. For abdominal organ recovery in stable donors, we recommend first gaining access to the retroperitoneal space and checking the abdominal aorta, with subsequent accurate dissection of the hepatic hilum, splenic artery, and left gastric artery and recognition of any vascular abnormalities before in situ cooling. A rapid en bloc retrieval technique should be used only if the donor becomes unstable. This procedure minimizes warm ischemia times during hemodynamic instability and can be safely applied in critical situations even by non-expert surgeons, but the risk of vascular and graft injury is significantly higher [9].
3 Donation After Cardiac Death (DCD)
Many countries worldwide have explored the option of donation after circulatory death (DCD) to expand the organ donor pool [20]. The DCD procedure seeks to obtain transplantable organs from patients previously declared dead following cessation of their circulatory and respiratory functions. DCD are classified, in accordance with the Maastricht criteria [21], in the following four categories (Table 55.3): donors who are declared dead outside the hospital and are brought into hospital without any attempt at resuscitation (Type 1); donors in whom CA occurs unexpectedly, and for whom resuscitation attempts are unsuccessful (Type 2); donors for whom CA is expected after withdrawal of treatment (Type 3); and donors in whom CA occurs during or after brain death diagnostic procedures (Type 4).
The original Maastricht classification was modified during the International DCD Conference in Paris in 2013, to define the exact circumstances of the circulatory arrest and consequent warm ischemic organ damage better [22]. The two main discriminant factors are maintained in the modified Maastricht classification: the circumstances of CA (sudden or planned) and the initial therapeutic procedure (resuscitation or not). The “location” has been added: where the sudden CA occurs (outside or inside the hospital).
DCD donors can be considered either uncontrolled (uDCD) or controlled (cDCD). Types 1 and 2 are defined as uncontrolled donors on the basis of unexpected CA and unsuccessful resuscitation. uDCD are considered to be very marginal donors because of the deleterious effect of warm ischemia. There is also the risk of being unable to obtain all necessary medical data within the short time frame provided by uDCD procedures. Donation in this setting should be designed to minimize the duration of warm ischemia and its impact on organ viability, to ensure the greatest possible safety for the donated organ.
Type 3 is defined as controlled donors, when death occurs in an ICU patient who is deemed to have a catastrophic and non-recoverable brain injury. In these patients, after the decision that any additional care is futile, life-sustaining treatment can be withdrawn. In cDCD the CA is expected, allowing the organ recovery to be planned [23].
DCD remains an activity restricted to a limited number of countries, and there are also considerable differences in its practice between countries. In Australia, Belgium, the Netherlands, the United Kingdom, and the United States, DCD donors are predominantly controlled, whereas in Spain, France, and Italy, DCD donors are most commonly uncontrolled (although these countries too have recently embarked on cDCD programs). The different approaches may be related to different legislations, ethical concerns, practices at the end of life, and organizational approaches to the treatment of out-of-hospital CA [24]. In Belgium and the Netherlands, cDCD is also possible after euthanasia.
The main impediment to DCD is the higher incidence of complications or of impaired graft function as a result of extended periods of warm ischemia. Organs from these donors sustain appreciable warm ischemic injury, and graft function must be carefully assessed for their potential use for transplantation. The unpredictable consequences of warm ischemic injury during low-flow or no-flow prior to death, together with the no-touch period required for death declaration according to national laws, result in extensive ischemia-reperfusion injury (IRI). IRI is the main factor causing graft dysfunction after transplantation [23, 25].
During ischemia transport mechanisms involving Na+/K+ and Ca2+/Mg2+ ATPase are inhibited, resulting in entry of water and cell swelling. Ischemia also causes rapid accumulation of toxic products such as lactate and hypoxanthine produced by anaerobic metabolism. These processes are exacerbated by reperfusion, resulting in an inflammatory response with subsequent release of free radicals.
There is still no univocal definition of warm ischemia time (WIT) [26]. In controlled DCD, WIT can be defined as the interval between support withdrawal and the start of cold perfusion [20]. Research has shown that agonal periods of more than 2 h duration are not acceptable for transplantation purposes. Currently there is a tendency to define and record the warm ischemia at the onset of hemodynamic instability (referred to as “functional warm ischemia”). It involves sustained hypotension and may have a substantial impact on warm ischemic damage to DCD organs.
In uncontrolled DCD, WIT can have an even more significant impact, since the exact period of circulatory arrest is usually not known [27]. Furthermore, between CA and the start of organ perfusion, there is a period of cardiopulmonary resuscitation (CPR), varying in duration and efficacy.
The time required to define the irreversibility of death varies widely between different countries. The no-touch period, defined as the time between the cessation of circulation and respiration and the determination of death, ranges from 5 to 20 min [28]. In Italy, the interval to diagnosis of death is 20 min of CA, demonstrated by a continuous electrocardiography recording. This is clearly longer than the interval adopted in the United States and other European countries and may negatively affect organ viability, prolonging the WIT.
3.1 Warm Ischemia Time and Organ Preservation in DCD
The key for the recovery of most DCD grafts has been the implementation of cytoprotective mechanisms in preservation techniques in order to stop or even reverse cellular injury [29]. By the end of 2000, there was already a fair amount of data, showing that the use of normothermic recirculation, namely, abdominal regional perfusion (ARP), could avoid and reverse warm ischemia injury. ARP can restore a warm, oxygenated blood flow through the organs and thus ensure adequate tissue perfusion between the donor’s death and organ procurement [30]. ARP has proved to be the most effective method for preservation and functional assessment of abdominal organs. It reverses ischemic injury and at the same time allows an evaluation of the quality of potential grafts.
ARP has been applied clinically using both hypo- and normothermic strategies. With hypothermic perfusion, metabolic processes are suppressed; with normothermic regional perfusion (NRP), cell metabolism is fully restored [31]. A period of postischemic NRP in DCD donors is useful to restore cellular energy substrates, lower the levels of nucleotide degradation products, and improve the concentrations of endogenous antioxidants prior to graft recovery. Like ARP, NRP has proved to be the most effective method for preservation and functional assessment of abdominal DCD organs. It reverses ischemic injury and at the same time allows evaluation of the quality of potential grafts. NRP allows organs to recover in situ from warm ischemic damage [32] providing oxygen and nutrients to restore metabolic processes, which in turn enable repair of damaged cells, correct the acidosis, restore the depleted adenosine triphosphate (ATP), regulate calcium homeostasis, and remove free radicals. Hypothermic perfusion has been mainly limited to DCD kidney transplantation, and NRP has been used to improve and assess the quality of DCD kidneys, livers, and even pancreas for transplantation.
NRP has marked a significant advance in organ recovery and has the potential to increase organ recovery rates, with its applicability in both controlled and uncontrolled DCD donors. Furthermore, its ability to restore ATP supplies and permit dynamic assessment of organ function prior to transplantation may allow more precise graft selection and achieve better long-term outcomes in comparison with kidneys or livers that were flushed in situ directly after the declaration of death [33].
3.2 Uncontrolled Donation After Circulatory Death
Although this type of donation can substantially boost the potential donor pool, it is still restricted to a few countries. Spain and France have the most experience with uDCD. It has also been developed in other countries such as Italy and Belgium and recently in Portugal, Russia, and Poland [7, 32, 33, 34].
Patients with witnessed in- or out-of-hospital refractory cardiac arrest (CA) are considered eligible. In the out-of-hospital scenario, the emergency medical service is mobilized to the scene of witnessed CA, where it starts advanced life support in accordance with international standard guidelines, using an automated chest compression system. If the CA is refractory, the patient is maintained on an automated chest compression system and transferred to a hospital. In the hospital, if the asystolic period persists and no reversible cause is identified, the CA is considered irreversible and further attempts at resuscitation futile. The declaration of death is formalized in the hospital, based on the absence of ECG and spontaneous respiratory activity, in accordance with local law. Heparin may be administered before death is declared (in countries where this is allowed by law).
After declaration of death, external cardiac massage is restarted with automated chest compression; the femoral artery and veins are cannulated, and ARP or NRP is set in motion, with the aim of preserving organ function while the family may express non-opposition to donation [34]. A Fogarty balloon catheter is inserted through the contralateral femoral artery and inflated in the supraceliac aorta, to reduce the total perfused volume and prevent cardiac and brain perfusion. Cannulae are connected to an extracorporeal circuit incorporating a membrane oxygenator, a centrifugal pump, and a heat exchanger (Fig. 55.1). The circuit is primed with 100–800 mL of Ringer’s acetate solution, sometimes buffered with sodium bicarbonate.
Pump flow is maintained in the 1.7–3.0 L/min range, temperature at 35.5–37.5 °C, and pH 7.0–7.4. The circuit sweep gas flow and FiO2 are adjusted to keep PaCO2 in the 30–45 mmHg range and SaO2 98% or more. Full heparinization is warranted, and autologous blood perfusate from the donor cadaver is used. During the period of NRP, blood samples are collected for biochemical and gasometric parameters, and the donor is assessed for possible contraindications to donation [35]. Indicative criteria for donation include witnessed arrest, age less than 65 years for kidneys and livers, 50–55 for lungs, cause of death known or presumed, and non-bleeding abdominal injuries. Total WIT (time from CA to start of NRP) should be less than 150 min.
3.3 Controlled Donation After Circulatory Death
In the case of cDCD, CA occurs following planned withdrawal of life-sustaining support (WLST) after it has been demonstrated that further intensive treatment is “futile” and inappropriate, in the best interest of a critically ill patient, according to the patient’s personal rights. DCD donation accounts for over 40% of deceased donation in the United Kingdom, and similar percentages are seen in other Northern European countries. Unlike other types of DCD, in the controlled scenario, the CA is expected [36]. Although the majority of actual cDCD donors die from catastrophic non-recoverable brain injury, data from the Netherlands, Spain, the United Kingdom, and the United States suggest that up to 15% of cDCD die from other conditions such as end-stage respiratory failure or neuromuscular disease [37]. When the planned WLST is discussed with the relatives of patients who have a nonreversible disease, organ donation can be an appropriate consideration. Currently, in countries where such programs are implemented, there are useful guidelines to assist physicians in therapy withdrawal.
After WLST in cDCD, a variable period of progressive hypoxia and hypotension develops until the onset of CA and determination of death, known as the agonal phase. Most transplant programs will limit this phase to 60–90 min to exclude potential harmful effects. However, data are still very limited.
Following the Spanish experience with uDCD, several countries have explored the feasibility of NRP in cDCD using similar technology (heat exchanger, oxygenator, and pump). Few studies have described the use of regional perfusion in controlled DCD donation in normothermic as well as hypothermic conditions [5]. These studies reported an increase in organ recovery rates compared with standard preservation procedures. However, the essential prerequisite in these studies was the possibility of using heparin and vascular cannulation prior to the patient’s death. Interestingly, in different countries, different interventions are allowed antemortem: heparin and vessel cannulation are allowed in Spanish and French guidelines, and specific informed consent must be obtained.
3.4 General Principles of Surgical Technique for DCD Organ Recovery
For controlled DCD donors, a super-rapid recovery technique is generally adopted. Prior to withdrawal of support, the donor is draped, and the surgical instruments, preservation solution, and tubing are prepared. Once death has been formally declared, the surgeons return to the operating room. Speedy access to the abdomen is gained through a midline laparotomy, the distal aorta is cannulated, and perfusion with cold preservation solution is started. Then the sternum is split, the thoracic aorta is cross-clamped, and the inferior vena cava is vented into the right chest. The inferior mesenteric vein may be cannulated to perfuse the portal system [9, 38, 39]. Because visceral dissection is performed in the cold without the possibility of taking pulses, the risk of graft and vessel injury is higher than in DBD donors [9, 40].
Conversely, NRP in DCD donors permits an unhurried donor operation, which can be conducted in the same way as for DBD donors, drastically minimizing the risk of graft injury. Cannulation of the aorta is generally not necessary, and cold preservation solution can be flushed directly through the previously inserted femoral arterial cannula [5,6,7, 34]. Simultaneous portal perfusion is always advisable.
3.5 Ex Situ Machine Perfusion of the DCD Grafts
Static cold storage (SCS) is a suboptimal means of maintaining the viability of grafts from DCD. Increasing importance has been paid in recent years to machine perfusion (MP) for the ex situ phase of DCD organ preservation. MP preserves the organs dynamically, providing continuous circulation of preservation solution or blood at various temperatures. Experimental data suggest either hypothermic or normothermic MP as superior to SCS [41]. Vascular resistance during kidney MP correlates with delayed graft function and survival. Although the predictive value is low, this information could be used as an extra parameter for pretransplant evaluation of DCD kidneys [42, 43].
Hypothermic oxygenated perfusion (HOPE) has shown a protective effect in DCD livers against hepatocyte injury and Kupffer cell activation, but we still lack reliable markers and cutoffs predicting liver function posttransplant [44]. The Zurich group first reported that HOPE offered important benefits in preserving high-risk DCD livers and increasing graft survival [45, 46]. The combination of NRP with subsequent MP could offer the best way to improve the viability and transplantability of livers and kidneys from DCD, drawing on the recognized advantages of these two technologies [6].
3.6 The Italian Scenario in DCD
In Italy, the main, limiting obstacle to the development of DCD programs so far has been the legal framework for the determination of death: using the cardiocirculatory criteria necessary for DCDs, death can be legally declared only after a 20-min period in which no cardiac electrical activity and a flat ECG have been recorded.
The first program on DCD donor management in Italy, the Alba Program, started in 2007 at the Policlinico San Matteo in Pavia [47]. Three different scenarios are now under consideration for enrollment in this DCD protocol:
-
1.
Subjects who undergo witnessed CA, without restoration of spontaneous circulation with advanced life support in accordance with international guidelines (refractory CA), and are transferred to the hospital under automated chest compression. If there is no indication for extracorporeal life support (ECLS), these subjects are enrolled in the DCD program. The declaration of “cardiac” death is formalized in the hospital, based on the absence of ECG for 20 min, according to Italian law.
-
2.
Subjects under ECLS, in whom the extracorporeal support is withdrawn because of futility according to current criteria. The death declaration will be formalized according to recent Italian National Transplant Center guidelines.
-
3.
Subjects with catastrophic non-recoverable brain injury, who undergo CA and no attempt at resuscitation is made.
Organ preservation was based on NRP and started immediately after death had been declared using the circulatory criteria. For the first experimental years, the program focused only on kidney DCDs, considered more resilient to ischemic damage. The results so far are encouraging on many accounts such as organ function and recovery rates.
More recently, the procedure has been redefined to include liver recovery, which was first done in September 2015 by the transplant team of Niguarda Hospital (Milan) [5]. The results of the DCD liver transplant series originating from this experience, which subsequently included cases from a few other regional and extra-regional donor hospitals, show that DCD liver transplantation is possible in Italy with good results despite the exceptionally long WIT [7, 48, 49]. The use of NRP followed by MP – first introduced by the group of Niguarda – can be considered a new, promising frontier for liver recovery from DCD in clinical practice [6, 7].
References
Jenkins DH, Reilly PM, McMahon DJ, Hawthorne RV. Minimizing charges associated with the determination of brain death. Crit Care. 1997;1:65–70.
Keegan MT, Wood KE, Coursin DB. An update on ICU management of the potential organ donor. In: Vincent JL, editor. Intensive care medicine. Annual update 2010. Heidelberg: Springer; 2011. p. 547–59.
Israni AK, Zaun D, Bolch C, Rosendale JD, Snyder JJ, Kasiske BL. Deceased organ donation. Am J Transplant. 2016;16:195–215.
Trapianti—Statistiche. http://www.trapianti.salute.gov.it/cnt/cntStatistiche.jsp. Accessed 26 Mar 2017.
De Carlis L, Lauterio A, De Carlis R, Ferla F, Di Sandro S. Donation after cardiac death liver transplantation after more than 20 minutes of circulatory arrest and Normothermic regional perfusion. Transplantation. 2016;100:e21–2.
De Carlis L, De Carlis R, Lauterio A, Di Sandro S, Ferla F, Zanierato M. Sequential use of normothermic regional perfusion and hypothermic machine perfusion in donation after cardiac death liver transplantation with extended warm ischemia time. Transplantation. 2016;100:e101–2.
De Carlis R, Di Sandro S, Lauterio A, Ferla F, Dell’Acqua A, Zanierato M, De Carlis L. Successful donation after cardiac death liver transplants with prolonged warm ischemia time using normothermic regional perfusion. Liver Transpl. 2017;23:166–73.
Manno EM, Wijdicks EFM. The declaration of death and the withdrawal of care in the neurologic patient. Neurol Clin. 2006;24:159–69.
De Carlis R, Sguinzi R, Grande AM, Aseni P. Multiple organ retrieval: general principles, organ preservation, and new strategies. In: Aseni P, Grande AM, De Carlis L, editors. Multiorgan procurement for transplantation. A guide to surgical technique and management. Heidelberg: Springer; 2016. p. 79–90.
Ley EJ, Salim A. Organ donation. In: Velmahos GC, Degiannis E, Doll D, editors. Penetrating trauma: a practical guide on operative technique and peri-operative management. 2nd ed. Heidelberg: Springer; 2017. p. 623–8.
Jenkins DH, Reilly PM, Schwab CW. Improving the approach to organ donation: a review. World J Surg. 1999;23:644–9.
Gazzetta Ufficiale—Decreto 11 aprile. 2008. http://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2008-06-12&atto.codiceRedazionale=08A04067&elenco30giorni=false. Accessed 26 Mar 2017.
Abt PL, Marsh CL, Dunn TB, Hewitt WR, Rodrigue JR, Ham JM, Feng S. Challenges to research and innovation to optimize deceased donor organ quality and quantity. Am J Transplant. 2013;13:1400–4.
Glazier AK. The principles of gift law and the regulation of organ donation. Transpl Int. 2011;24:368–72.
Franz HG, DeJong W, Wolfe SM, Nathan H, Payne D, Reitsma W, Beasley C. Explaining brain death: a critical feature of the donation process. J Transpl Coord. 1997;7:14–21.
Salim A, Velmahos GC, Brown C, Belzberg H, Demetriades D. Aggressive organ donor management significantly increases the number of organs available for transplantation. J Trauma. 2005;58:991–4.
Ferla F, De Carlis R, Mariani A, De Carlis L. Liver transplant using octogenarian donors. Liver Transpl. 2016;22:1040–1.
Lauterio A, Moioli MC, Di Sandro S, Travi G, De Carlis R, Merli M, Ferla F, Puoti M, De Carlis L. HIV-positive to HIV-positive liver transplantation: To be continued. J Hepatol. 2018;S0168-8278:32180–9.
Grande AM, Aseni P. Preoperative evaluation and arrangements for multiorgan donation: general principles and contraindications. In: Aseni P, Grande AM, De Carlis L, editors. Multiorgan procurement for transplantation: a guide to surgical technique and management. Heidelberg: Springer; 2016. p. 19–34.
Neyrinck A, Raemdonck D, Van Monbaliud D. Donation after circulatory death: current status. Curr Opin Anaesthesiol. 2013;26:382–90.
Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893–4.
Thuong M, Ruiz A, Evrard P, Kuiper M, Boffa C, Akhtar MZ, Neuberger J, Ploeg R. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29:749–59.
Balibrea JM, Núñez-Peña JR, García-Martín MC, Olmedilla Y, Martín-Antona E, Berthuin J, Rancan L, Vara E, Balibrea JL. The differential tissue expression of inflammatory, oxidative stress, and apoptosis markers in human uncontrolled non-heart-beating donors. Transplantation. 2013;95:1346–53.
Monbaliu D, Pirenne J, Talbot D. Liver transplantation using donation after cardiac death donors. J Hepatol. 2012;56:474–85.
Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24:676–86.
Manara AR, Murphy PG, O’Callaghan G. Donation after circulatory death. Br J Anaesth. 2012;108:i108–21.
Rodríguez-Arias D, Deballon IO. Protocols for uncontrolled donation after circulatory death. Lancet. 2012;379:1275–6.
Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 2014;97:258–64.
Net M, Valero R, Almenara R, et al. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am J Transplant. 2005;5:2385–92.
Valero R, Cabrer C, Oppenheimer F, et al. Normothermic recirculation reduces primary graft dysfunction of kidneys obtained from non-heart-beating donors. Transpl Int. 2000;13:303–10.
Hessheimer AJ, Billault C, Barrou B, Fondevila C. Hypothermic or normothermic abdominal regional perfusion in high-risk donors with extended warm ischemia times: impact on outcomes? Transpl Int. 2015;28:700–7.
Hessheimer AJ, García-Valdecasas JC, Fondevila C. Abdominal regional in-situ perfusion in donation after circulatory determination of death donors. Curr Opin Organ Transplant. 2016;21:322–8.
Fondevila C, Hessheimer AJ, Flores E, et al. Applicability and results of Maastricht type 2 donation after cardiac death liver transplantation. Am J Transplant. 2012;12:162–70.
Fondevila C, Hessheimer AJ, Ruiz A, et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant. 2007;7:1849–55.
Savier E, Dondero F, Vibert E, et al. First experience of liver transplantation with type 2 donation after cardiac death in France. Liver Transpl. 2015;21:631–43.
Oniscu GC, Randle LV, Muiesan P, Butler AJ, Currie IS, Perera MTPR, Forsythe JL, Watson CJE. In situ normothermic regional perfusion for controlled donation after circulatory death—the United Kingdom experience. Am J Transplant. 2014;14:2846–54.
Magliocca JF, Magee JC, Rowe SA, Gravel MT, Chenault RH, Merion RM, Punch JD, Bartlett RH, Hemmila MR. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58:1095–101.
Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Bronsther O, Randhawa P, Broznick B, Fung JJ, Starzl T. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation. 1995;59:197–203.
Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004–11.
Ausania F, White SA, Pocock P, Manas DM. Kidney damage during organ recovery in donation after circulatory death donors: data from UK National Transplant Database. Am J Transplant. 2012;12:932–6.
Shapey IM, Muiesan P. Regional perfusion by extracorporeal membrane oxygenation of abdominal organs from donors after circulatory death: a systematic review. Liver Transpl. 2013;19:1292–303.
Moers C, Smits JM, M-HJ M, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19.
Jochmans I, Moers C, Smits JM, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant. 2011;11:2214–20.
Schlegel A, de Rougemont O, Graf R, Clavien P-A, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58:278–86.
Dutkowski P, Schlegel A, De Oliveira M, Müllhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765–72.
Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, DeOliveira ML, Kron P, Clavien P-A. First comparison of hypothermic oxygenated PErfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg. 2015;262:764–71.
Geraci P, Sepe V. Non-heart-beating organ donation in Italy. Minerva Anestesiol. 2011;77:613–23.
Vergano M, Magavern E, Baroncelli F, et al. Making a case for controlled organ donation after cardiac death: the story of Italy’s first experience. J Crit Care. 2017;38:129–31.
De Carlis R, Di Sandro S, Lauterio A, Botta F, Ferla F, Andorno E, Bagnardi V, De Carlis L. Liver grafts from donors after cardiac death on regional perfusion with extended warm ischemia compared with donors after brain death. Liver Transpl. 2018 [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
De Carlis, R., Zanierato, M., Iotti, G.A., Aseni, P., De Carlis, L. (2019). The Potential Organ Donor: Current Trends and Management. In: Aseni, P., De Carlis, L., Mazzola, A., Grande, A.M. (eds) Operative Techniques and Recent Advances in Acute Care and Emergency Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-95114-0_55
Download citation
DOI: https://doi.org/10.1007/978-3-319-95114-0_55
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95113-3
Online ISBN: 978-3-319-95114-0
eBook Packages: MedicineMedicine (R0)