Abstract
The incidence of new thoracoabdominal aortic aneurysms is increasing. Enhanced awareness combined with improved diagnostic imaging modalities has led to an increased diagnosis of thoracoabdominal aneurysms. With the current trend in population aging, more and more elderly patients will be diagnosed, and proper clinical management will be required. Patients with TAAA have a high prevalence of significant comorbid conditions which may decrease their long-term survival and increase their perioperative mortality and morbidity. Thus, it is important to know the natural history of TAAA in order to make the proper decision on operative indications. Further problems come from the complications of endovascular treatment in dissected patients. Very often it happens that patients with type B dissection are treated with thoracic endografting. The late expansion of the aorta can lead to an urgent situation for the onset of thoracic pain in the reperfused false lumen of the aorta is difficult to manage.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
-
Under anesthesiological point of view, in emergency always monitor right radial artery (in endovascular repair) and left femoral artery (in open repair) and position a single endotracheal tube in endo-repair or double-lumen in open repair. Always CSF is mandatory, in open repair, while in endo repair only if extensive coverage is expected.
-
In emergency, open technique always prepare the patient for extracorporeal cannulation and cooling especially in dissecting aneurysm.
-
In emergency, if possible, an endovascular approach is preferable.
-
The 24-h availability of various sizes of thoracic graft and cover graft, in your hospital, allows you to treat with an endovascular technique almost all the patients.
-
The patient must be treated in centers with experiences in both techniques open and endovascular.
1 Epidemiology
Improvement in imaging modalities has led to increased diagnosis and awareness of thoracoabdominal aortic diseases and their potentially lethal outcome. Despite progress in anesthetic and surgical management, open and endovascular repair of the thoracoabdominal aorta has remained a challenging operation with potential catastrophic complications as paraplegia and renal failure. Sound judgment is required to decide whether or not an elective operation is justified. This should be based on an understanding of the natural history of thoracic aortic pathology. Etiology of thoracoabdominal pathology has been divided into two groups, dissecting and nondissecting. Atherosclerotic or degenerative etiology is the main nondissecting etiology for approximately 80% of these aneurysms. Dissecting thoracoabdominal aneurysms have been associated with an increased production of symptoms and an increased risk of death from rupture. The presence of dissection decreased the 5-year survival in patients with TAAA from 71% without dissection to 46% if the aneurysm was associated with dissection. The male-female ratio was established to average 2:1 for nondissecting pathology. This gender ratio increased to an average of 3:1 for dissecting pathology. Gender difference was found to have no impact on the risk of rupture and the expansion rate of aneurysms, while the female sex was found to be associated with an increased risk of postoperative death [1]. Patients often have a high prevalence of comorbidities like hypertension (83%), coronary artery disease (35%), peripheral artery disease, and visceral occlusive disease (25%). Furthermore, chronic renal failure and chronic obstructive pulmonary disease were found to be predictive variables of early postoperative death.
2 Classification
Thoracoabdominal aneurysms can be classified according to the Crawford classification into four types with the adjunct of the fifth type by Safi (Fig. 35.1). Type I aneurysm extends from the level of the left subclavian artery to renal arteries. Type II involves the entire thoracic descending aorta at the level of the left subclavian artery, all the visceral branches, and finishes to the iliac arteries. Type III usually begins distal to T6 level extending to the iliac bifurcation. Type IV involves the supra-celiac aorta, at or below the diaphragm, to the iliac bifurcation, Safi’s type V starts from middle descending to the renal arteries.
This classification still has an importance since it concerns the extent of the pathology, and it reflects the operative approach. The extent of involvement in the thoracoabdominal aorta is not associated with an increased risk of rupture.
The indication to proceed with operative repair of a TAAA must be based on the available information on the natural history of TAAA and the results of surgical repair. Data on the expansion rate and the risk of rupture of abdominal aortic aneurysms has led to clear recommended indications to proceed with operative repair for a 5 cm and greater or with rapid expansion. The information that a large aneurysm has a higher risk of rupture than a smaller one has been known for decades. The size of the aneurysm plays a role in surgical decision-making only when many other factors must be weighed in the scales of judgment. In a young good-risk patient, it is reasonable to proceed with AAA repair even for an aneurysm smaller than 5 cm although we do know the risk of rupture to be minimal. This rationale does not apply for small thoracoabdominal aneurysm because of the higher operative risk. The main criteria have remained the size. Patients with symptomatic aneurysms greater than 6 cm have a decreased 5-year survival of 61.1% versus 38.2% (size less than 6 cm). The presence of symptoms, of course, increases the risk of rupture. Knowledge of the expansion rate of the aneurysm is essential to properly manage small aneurysms which might be observed with serial CT scan. The estimated change in maximal diameter was found to be 0.43 cm per year. The presence of chronic obstructive pulmonary disease is the only factor associated with an increased expansion rate.
3 Diagnosis
Transesophageal echocardiography (TEE) has been the first-choice technique for a long time for the advantage to be performed in emergency and OR. The diagnostic sensibility is very high (98%) similar to NMR and spiral CT; however the specificity is inferior (77%) due to false positive secondary to artifacts in the dilated aorta. Limits are the availability of an expert operator and the reduced visualization of the aortic arch for the presence of the left main bronchus. CT scan represents the diagnostic gold standard for the thoracoabdominal aorta with a sensibility of 94% and a specificity of 87%. The possibility of 3D reconstruction allows evaluating the coronary involvement and the exact morphology of the dissected flap.
4 Treatment in Emergency
4.1 Patient Preoperative Assessment in Emergency
The preoperative assessment is crucial for the anesthesiologist to evaluate the real physiopathological status, evaluating the standard blood tests, stratifying the correlated risk, and, when possible, optimizing the medical therapy to improve the outcome [2].
In an urgent-emergent setting, this could not be possible because of the short period available. So what is the “ideal bundle care” in this situation? The anesthesiologist work must be focalized on feasible and efficient, but not time-consuming, protective organ strategies. Cardiac assessment should be used as an important criterion to evaluate the real functional capacity, because a poor one, measured as metabolic equivalents (METs), is associated with an increased incidence of postoperative cardiac events. METs evaluation can be useful to understand if the patient could fit for open repair (with very aggressive surgical approaches such as thoracotomy) or for an endovascular procedure (suitable anatomy), which represents a therapeutic solution for the high-risk patient. Preoperative plasma levels of biomarkers like cardiac troponins T and I, measured even in the postoperative days, are useful due to their sensibility and specificity. Other biomarkers such as pro-BNP and BNP, corrected for factors like renal dysfunction or obesity, should be considered. Pulmonary assessment is important too: chest X-ray and arterial blood gas analysis can be performed easily and quickly. The anesthesiologist must know if there is any evidence of tracheal or bronchial compression by the aneurysm. During open repair (OR), one-lung ventilation with a left-side double-lumen endotracheal tube (DLT) is necessary to proceed to left thoracotomy (during AATA type I, II, and III repair). Renal assessment is another important issue because of the high risk of renal injury following both the surgical and the endovascular approach. A protocol to prevent contrast-induced nephropathy (including hydration, administration of N-acetylcysteine, withdrawal of potential nephrotoxic drugs) must be undertaken as soon as possible. Arterial blood gas analysis can demonstrate a status of metabolic acidosis related to previous renal impairment. The hemostatic status is often altered, due to the vascular pathology and to patients’ therapy. Most of the vascular patients take antiplatelet agents. The functional platelet assay could help to identify the therapeutic window to reduce the bleeding risk and to perform one of the most invasive anesthesiological procedures more safely, the cerebrospinal fluid (CFS) drainage [3]. According to the literature, there is a wide range, from 4 to 30%, of patients taking clopidogrel that are not responders to the drug: in this subgroup, with high on-treatment platelet reactivity, we could consider to perform CFS drainage in hemodynamically stable patients. The presence of an anticoagulant therapy with vitamin K antagonists (VKAs) should be checked with laboratory standard tests and, if available, with the point-of-care (POC) viscoelastic tests such as thromboelastography (TEG) or rotational thromboelastogram (ROTEM). The effect of the anticoagulant therapy must be antagonized with the administration of prothrombin concentrate complex (PCC) at the recommended dose of 25 up to 50 UI/kg, related to patient body weight and the INR value, and the administration of vitamin K 10 mg IV. The exact reversal of the drug can be monitored by laboratory standard tests or using POCs. The goal is to achieve an INR value below 1.5 as early as possible. Recent guidelines suggest the use of four-factor PCCs because it is a balanced drug, thanks to the presence of anticoagulant factor proteins C and S. The reversal of direct oral anticoagulants (DOAC) is more complex because the antidote for thrombin inhibitor (dabigatran), idarucizumab, is not widely available and the antidote for direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) is in experimental phase. As first-line therapy, four-factor PCCs can be used. We can monitor the efficacy of the reversal therapy with POC. The PCCs available on market available as three (II, IX, and X) or four factors (II, VII, IX, and X), allow for a rapid re-coagulation of the patient in order to avoid fresh frozen plasma and its transfusional collateral effects, such as hemodilution, allergic reaction, transfusion-related acute lung injury (TRALI), and transfusion acute cardiac overload (TACO) [4]. Once the patient is “recoagulated,” we can proceed with the invasive anesthesiological procedures, in stable patients. All these procedures require a short time (up to 1 h) to be performed, and they can be crucial for end-organ protective strategies.
4.2 Intraoperative Management
Five-lead electrocardiogram is used to detect early signs of myocardial ischemia through the continuous monitoring of D2-V5 derivations. Right radial (or brachial) and right femoral artery lines are fundamental to measure, respectively, arterial pressure in the upper part of the body and the distal aortic perfusion pressure during partial cardiopulmonary bypass (CPB). Temperature control is important too: bladder and nasopharyngeal probes are required, in order to control a mild permissive hypothermia (core temperature of 32–34 °C) to prevent neurological injuries (paraparesis, paraplegia). The use of intraoperative monitoring with transesophageal echocardiography can help to guide the placement of the venous cannula of the CBP ant to detect any cardiac dysfunction. Because of the possibility of severe bleeding during OR, a large-bore central catheter must be available (best 12.5 Fr high-flow) and additional central venous line may be disposable (the “double-stick” technique is simple and feasible using echo-guide, providing two large-bore catheters in the same central vein, usually right internal jugular vein). A rapid high-flow infusion device is fundamental to treat blood losses immediately and effectively. A goal-directed hemostatic therapy is essential to optimize the coagulation status and to reduce transfusions. TAAA repair is associated with a major risk of intraoperative bleeding and coagulopathy. This is related to several factors: hypothermia, acidosis, hemodilution, surgical bleeding, heparin administration, and continuous activation of the coagulation with consumption of clotting factors and fibrinogen. Heparin reversal could represent a major challenge for the anesthesiologist, and it could be guided by POCs; TEG and ROTEM can show residual heparin comparing reaction time R-kaolin TEG or clotting time CT-INTEM, respectively, with and without adding heparinase to the cuvette. Other pro-hemorrhagic factors are thrombocytopenia, platelet dysfunction, and hyperfibrinolysis. All these alterations may be monitored and corrected using POC coagulation tests [5]. Thrombocytopenia and platelet dysfunction can be treated with platelet concentrate transfusion; hyperfibrinolysis, monitored by D-dimers, FDP, and viscoelastic tests, can be treated with antifibrinolytic drugs such as tranexamic acid (TXA) at a dose of 12.5 mg/kg. In case of a severe bleeding, fibrinogen is the first factor to decrease critically, and its rapid restoration must be considered. The value of fibrinogen can be measured either by laboratory test (conventional Clauss method) or with POC. The viscoelastic tests measure clot firmness (TEG-MA maximum amplitude, ROTEM-MCF maximum clot firmness), which is determined by fibrinogen and platelets. Fibrinogen levels can be evaluated by using tests (TEG-FF and ROTEM-FIBTEM), which eliminate platelet contribution to clot strength [6]. The trigger value to fibrinogen administration is debated: Ranucci et al. propose a value of 115 mg/dL as a trigger value for fibrinogen supplementation in cardiac surgery [7]. Fibrinogen can be supplemented with different strategies. Fresh frozen plasma (FFP) is widely used to achieve this aim, but it contains fibrinogen at variable concentrations, and, consequently, large volumes are needed (about 30 mL/kg FFP increase fibrinogen concentration by 1 g/L). Hence, fibrinogen concentrate could be an effective and safer alternative, thanks to its high concentration (20 g/L) [8]. However, the concentrate is still not available to treat acquired bleeding in the UK and USA. Thus, we can affirm that the need of transfusion of allogenic blood components may be reduced and optimized targeting “all the prohemostatic strategies” guided by POC viscoelastic test (real-time, bedside, and time-sparing) or standard laboratory test. After the surgical or endovascular planning (aneurysm extension, coverage or reimplantation of left subclavian artery, coverage or reimplantation of the intercostal arteries, status of pelvic circulation, and previous abdominal aortic surgery), we would have to proceed with the insertion of CBF drainage (usually a 16-g needle), just before the final positioning of the patient. It must be inserted at the level of L3–L4, and the cerebrospinal fluid pressure (CBFP) must be monitored in continuous in order to measure the cerebrospinal fluid perfusion pressure.
Ischemic spinal cord injury (SCI) remains the most devastating complication after TAAA repair by any modality. Main risk factors for SCI after TAAA repair are listed in Table 35.1. The risk of SCI may also be described according to the “Crawford classification,” [9] even with the recent advances in neuroprotective strategies, and CSF drainage types I and type II remain the categories associated with the highest percentages of SCI (Fig. 35.1). TEVAR offers a less invasive approach, but it is still associated with a significant risk of SCI. Crawford et al. reported a significant increase in the incidence of permanent SCI for ruptured thoracic aneurysm compared to elective repair [10]. In a more recent case series, Gaudino and colleagues reported that the risk of SCI was not significantly higher in the ruptured group [11] (Table 35.2, Fig. 35.2).
Monitoring spinal cord viability is crucial to prevent SCI. The use of motor-evoked potentials (MEPs) and somatosensory-evoked potentials (SSEPs) has been reported to guide therapeutic maneuverers (CSF drainage, MAP augmentation, and CVP lowering) during TAAA repairs. Even though evoked potentials are widely accepted, they are challenging, invasive, and not available for postoperative surveillance. The use of NIRS has been proposed for monitoring SCI. According to the “collateral network” concept [21], blood supply to the spinal cord is provided by a rich network of intra- and paraspinous arterial collaterals that enables sufficient blood flow when segmental arteries are occluded. NIRS optodes used to detect paraspinous muscle oxygenation may provide an indirect, noninvasive, real-time monitoring of spinal cord blood flow. In particular, monitoring paraspinous vasculature at lumbar level seems to identify spinal malperfusion during TAAA repair. NIRS measurements can be easily performed in the postoperative period for delayed SCI detection [22, 23]. Performing cerebrospinal fluid drainage (CSFD) is suggested as a class I recommendation, level of evidence B in high-risk patients undergoing both open repair and endovascular thoracic aortic repair, by current American Heart Association guidelines [24]. Several studies also support the effects of CSFD in preventing SCI [16, 19, 25,26,27]. On the other hand, important complications, such as subdural hematoma, have been reported [28, 29]. Complications of CSFD include those related to lumbar puncture, the presence of an indwelling catheter, and those connected to CSF drainage. In the setting of an urgent TAAA repair, CSFD should be used as protective measure only as the emergent situation may allow [30]. In fact, in a situation of hemodynamic instability, CSFD can be impracticable [31] like other organ-protective strategies. Furthermore, in the urgent TAAA repair, the risk associated with CSFD can be even higher because of concomitant coagulopathy or ongoing antiplatelet/anticoagulant therapy. Again, the use of point-of-care coagulation tests and a goal-directed, selective hemostatic approach might guide a safe CSF drain insertion. In literature, there is only a case report of such use of thromboelastometry to guide hemostatic therapy before CSFD placement and extraction in a patient with severe coagulopathy undergoing TEVAR [32]. Insertion of the lumbar CSF drain should ideally be performed in the awake patients, at L2–L4 level. CSF should be drained to maintain a pressure less than 10–15 mmHg. If signs of SCI develop, lowering goal pressure should be considered. In order to minimize the risk of intracranial hypotension and subdural hematoma, no more than 10–15 mL/h should be drained [12, 33]. Initial results with an automated, pressure-controlled system for CSF drainage have been reported. The spinal drain is generally maintained for 48–72 h if no symptoms of SCI develop. In case of delayed SCI, repeated drain placement has to be taken into account.
4.3 Open Surgical Technique
The surgical approach in emergency does not differ from one of the elective cases. The approach depends on the history of the patient (previous operations) and from the extension of the aneurysm. In case of previous arch surgery with a median sternotomy, it is important to guarantee a proximal control which can be reached through a fourth space left thoracotomy extended with a transversal sternotomy which can give the possibility to proceed to cannulation of supra-aortic trunks. In this case, the surgical isolation of the left axillary artery can give support for perfusion. The thoracotomy must be extended, depending on the level of the aneurysm in a radial phrenotomy and pararectal laparotomy. A second thoracotomy in the seventh space may be required. Visceral vessels and renal vessels must be accurately isolated at their origin (apart from the right renal artery) with vessel loops to prepare it for cannulation (Fig. 35.3). The aortic bifurcation can be easily reached, and also the origin of the right common iliac artery can be obtained without problems through extra- or transperitoneal space.
The surgical exposure of femoral vessels can allow the cannulation (Fig. 35.4) to start with femoro-femoral extracorporeal circulation.
Once the aorta is clamped proximally, a second clamp can be positioned just 10–15 cm below to guarantee, during the proximal anastomosis, the perfusion of visceral and intercostal arteries. The proximal anastomosis (Fig. 35.5) can be done with a 4/0 Prolene and must be reinforced with Teflon pledgets and with a glue.
The proximal anastomosis can be done with a 4/0 Prolene and must be reinforced with Teflon pledgets and with a glue.
In the thoracoabdominal aorta, a multibranched graft is used and no segment of the native aorta left (Fig. 35.7). This is true especially for genetic disease in which a late dilatation of the aorta can be observed. After the completion of the proximal anastomosis, the clamp is moved down along the aorta above the origin of the celiac axis, and a meticulous reimplantation of the intercostal arteries is achieved (Fig. 35.6).
Once the intercostal anastomosis is completed, a dedicated line of the arterial line is used for blood perfusion of spinal cord (Fig. 35.6).
The anastomosis on the intercostal arteries can be performed variously and reattached on the aortic graft in different shapes: C-shape (Fig. 35.7) or Y-shape (Fig. 35.8). Then the clamp is moved to the aortic bifurcation, the extracorporeal perfusion through the femoral artery is lowered for perfusion of the left hypogastric artery, and a complete separated cannulation of visceral vessels is achieved (Fig. 35.9).
Each visceral vessel is sutured with an end-to-end anastomosis to each branch of the graft and reinforced with a Teflon pledget (Fig. 35.10). After the completion of each anastomosis, the vessel is perfused with antegrade flow from the aortic graft (Fig. 35.11).
Then the diaphragm is reconstructed completely together with the integrity of thoracic and abdominal wall. A double drainage tube is positioned into the pleural space in paravertebral and supradiaphragmatic position and an additional drainage tube into the retroperitoneal space. No need for coverage of the graft.
4.4 Endovascular Techniques in Emergency
In emergency, it is difficult to obtain a fenestrated graft because of the need for customization. So the possibilities of endovascular treatment in emergency are (1) the chimney-sandwich technique for atherosclerotic aneurysm and (2) the exclusion of the false lumen expansion in a patient with previous TEVAR in a dissected aorta.
4.4.1 Sandwich Techniques for Atherosclerotic Aneurysms
This technique, first described by Lobato [34], consists in the exclusion of the aneurysm with a double endovascular graft covering all the diseased aorta with revascularization of visceral vessels in the space between the grafts (Fig. 35.12).
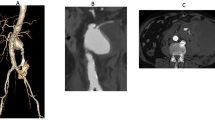
With this technique, it is necessary to proceed to a surgical isolation of both the femoral and omeral arteries. In Figs. 35.13 and 35.14 is shown the CT scan of a huge 10 cm of diameter Type III thoracoabdominal aneurysm who arrived in emergency with thoracic pain.
In this case, a 12 Fr introducer sheet 30 cm long is bilaterally introduced into the omeral arteries. From each side, it is possible to cannulate the renal arteries and the SMA and celiac trunk (Figs. 35.16, 35.17, and 35.18). Once the visceral vessels are cannulated, the first thoracic graft is advanced through one femoral artery. The graft is released from the proximal neck down to the origin of the celiac trunk (in the case described, the celiac trunk is occluded as you can see in Fig. 35.12).
A first thoracic graft has been positioned with the distal end just above the origin of the celiac trunk. The visceral vessel all cannulated from above through the omeral accesses. A second thoracic graft is positioned inside the first one according to Fig. 35.12
After the opening of the first graft and the cannulation of all vessels, the second graft is delivered inside the first one at the level of the origin of the stent grafts for visceral arteries. After the release of the visceral stent graft, a rapid angiographic check is performed to control the patency of the vessels (Figs. 35.17 and 35.18).
Then the introducer is removed and the arteries closed.
In the early postoperative period, it is necessary to perform a CT scan to check the complete thrombosis of the aneurysmal sac because there is still the risk of blood perfusion through the space between the various grafts (gutter). In the CT scan performed after three months, there is the complete exclusion of the aneurysm (Figs. 35.17, 35.18, and 35.19). In the following Figs. 35.19, 35.20, and 35.21, a complete exclusion of the aneurysm is shown with patency of the visceral vessels.
4.4.2 Candy-Plug Technique
This technique is used to exclude the dilated false lumen in a dissected aorta already treated with a frozen elephant trunk technique or a descending aorta endografting (TEVAR) (Figs. 35.22 and 35.23).
It consists in the positioning of a parallel graft or plug into the false lumen. The graft must be filled up with spiral coils to occlude completely the communication and the reperfusion from the entry tear below the distal end of the previous endovascular graft.
In this case from the femoral artery, it is possible to cannulate the false lumen (Fig. 35.24).
After the cannulation of the false lumen, it is possible to close the false lumen with a plug or spiral coils to close the flow in the false lumen. In the case shown, the false lumen was too large. So we have decided to position a parallel graft and to fill it with spiral coils (Figs. 35.25, 35.26, and 35.27).
In Fig. 35.28 the complete exclusion of the false lumen sac is shown.
Case Scenario
A 47-year-old woman is admitted in emergency ward for thoracic interscapular pain. The patient had a previous diagnosis of type B aortic dissection but with stability of the diameter of the aorta in previous CT scan (Fig. 35.29).
The patient underwent a CT scan.
-
1.
What do you expect?
-
A.
Aortic rupture.
-
B.
Aortic re-dissection.
-
C.
Retrograde dissection.
-
D.
All the precedent answers are correct.
-
A.
-
2.
How many aortic lumens can you recognize in the CT scan (Figs. 35.29, 35.30, 35.31, and 35.32)?
-
A.
1
-
B.
2
-
C.
3
-
D.
More than 3
-
A.
-
3.
Which therapy do you suggest?
-
A.
Medical
-
B.
Open surgery
-
C.
Endovascular
-
D.
Hybrid
-
A.
Self-Evaluation Questions
-
1.
Should the anesthesiologist perform the CSF drainage in an urgent-emergent setting of TAAA repair?
-
A.
It can be considered only in endovascular repair.
-
B.
Only for surgical repair.
-
C.
Always in setting of TAAA repair.
-
D.
YES, in hemodynamically stable patients. Rescue use of CSF drainage should be reserved for patients with postoperative signs of spinal cord injury and in those taking antiplatelet agents without the possibility to perform a preprocedural platelet functional test.
-
A.
-
2.
Could POC viscoelastic tests guide a goal-directed hemostatic therapy?
-
A.
Only the standard laboratory test can be used.
-
B.
POCs can measure only some features of coagulation setting.
-
C.
POCs cannot guide the transfusional blood requirements.
-
D.
POC can monitor real-time during all phases of surgery.
-
A.
-
3.
Could NIRS be useful to monitor indirectly spinal cord perfusion in an urgent-emergent setting of TAAA repair?
-
A.
It’s difficult to perform.
-
B.
In an urgent-emergent setting, MEP and SSEP are recommended because they can detect early signs of spinal cord hypoperfusion.
-
C.
Spinal NIRS can be used only in intraoperative setting.
-
D.
Spinal NIRS is a potential, real-time, noninvasive, and always available technique to monitor the spinal cord perfusion indirectly during and after endovascular or open TAAA repair.
-
A.
Please see Chap. 58 for the correct answer.
References
Schepens MAAM, Deafauw JJAM, Hamerlijinck RPHM. Surgical treatment of TAAA by simple cross clamping. Risk factors and late results. J Thorac Cardiovasc Surg. 1994;107:134–42.
Anton JM, Herald K. Anesthetic management of open thoracoabdominal aortic aneurysm repair. J Int Anesthesiol Clin. 2016;54(2):76–101.
Tantry US, Bonello L, Aradi D, et al. Working group on on-treatment platelet reactivity. J Am Coll Cardiol. 2013;62(24):2261–73.
Ranucci M, Sim P. Point-of-care tests for severe hemorrhage. A manual for diagnosis and treatment. 1st ed. Berlin: Springer; 2016.
Ortmann E, Rubino A, Altemimi B, Collier T, Besser MW, Klein AA. Validation of viscoelastic coagulation tests during cardiopulmonary bypass. J Thromb Haemost. 2015;13:1207–16.
Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, Pichlmaier M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138(3):694–702. https://doi.org/10.1016/j.jtcvs.2008.11.065. Epub 2009 May 17.
Ranucci M, Pistuddi V, Baryshnikova E, Colella D, Bianchi P. Fibrinogen levels after cardiac surgical procedures: association with postoperative bleeding, trigger values, and target values. Ann Thorac Surg. 2016;102(1):78–85.
Rahe-Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. J Thorac Cardiovasc Surg. 2013;145(Suppl 3):S178–85.
Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17(2):357–70.
Crawford ES, Hess KR, Cohen ES, Coselli JS, Safi HJ. Ruptured aneurysm of the descending thoracic and thoracoabdominal aorta. Analysis according to size and treatment. Ann Surg. 1991;213(5):417.
Gaudino M, Lau C, Munjal M, Girardi LN. Open repair of ruptured descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2015;150(4):814–23.
Etz CD, Weigang E, Hartert M, Lonn L, Mestres CA, Di Bartolomeo R, Bachet JE, Carrel TP, Grabenwöger M, Schepens MA, Czerny M. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery. Eur J Cardiothorac Surg. 2015;47:943–57.
Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118:808–17.
Conrad MF, Ye JY, Chung TK, Davison JK, Cambria RP. Spinal cord complications after thoracic aortic surgery: long-term survival and functional status varies with deficit severity. J Vasc Surg. 2008;48:47–53.
Fehrenbacher JW, Siderys H, Terry C, Kuhn J, Corvera JS. Early and late results of descending thoracic and thoracoabdominal aortic aneurysm open repair with deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg. 2010;140:S154–60; discussion S85–S90.
Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: result of a randomized clinical trial. J Vasc Surg. 2002;35:631–9.
Zoli S, Roder F, Etz CD, Brenner RM, Bodian CA, Lin HM, et al. Predicting the risk of paraplegia after thoracic an thoracoabdominal aneurysm repair. Ann Thorac Surg. 2010;90:1237–44; discussion 45.
Sundt TM, Flemming MD, Oderich GS, Torres NE, Li Z, Lenoch J, et al. Spinal cord protection during open repair of thoracic and thoracoabdominal aortic aneurysms using profound hypothermia and circulatory arrest. J Am Coll Surg. 2011;212:678–83; discussion 84–5.
Safi HJ, Estrera AL, Miller CC, Huynh TT, Porat EE, Azizzadeh A, et al. Evolution of risk for neurologic deficit after descending and thoracoabdominal aortic repair. Ann Thorac Surg. 2005;80:2173–9; discussion 9.
Stone DH, Brewster DC, Kwolek CJ, Lamuraglia GM, Conrad MF, Chung TK, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;44:1188–97.
Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg. 2007;83(2):S865–9; discussion S890–S892.
Etz CD, von Aspern K, Gudehus S, et al. Near-infrared spectroscopy monitoring of the collateral network prior to, during, and after thoracoabdominal aortic repair: a pilot study. Eur J Vasc Endovasc Surg. 2013;46(6):651–6.
Luehr M, Mohr FW, Etz CD. Indirect neuromonitoring of the spinal cord by near-infrared spectroscopy of the paraspinous thoracic and lumbar muscles in aortic surgery. Thorac Cardiovasc Surg. 2016;64(04):333–5.
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/ACA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369.
Svensson LG, Hess KR, D’Agostino RS, Entrup MH, Hreib K, Kimmel WA, et al. Reduction of neurologic injury after high-risk thoracoabdominal aortic operation. Ann Thorac Surg. 1998;66:132–8.
Acher CW, Wynn MM, Hoch JR, Kranner PW. Cardiac function is a risk factor for paralysis in thoracoabdominal aortic replacement. J Vasc Surg. 1998;27:821–30.
Sugiura J, Oshima H, Abe T, Narita Y, Araki Y, Fujimoto K, Mutsuga M, Usui A. The efficacy and risk of cerebrospinal fluid drainage for thoracoabdominal aortic aneurysm repair: a retrospective observational comparison between drainage and non-drainage. Interact Cardiovasc Thorac Surg. 2017;24(4):609–14.
Wynn MM, Mell MW, Tefera G, Hoch JR, Acher CW. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg. 2009;49:29–35.
Murakami H, Yoshida K, Hino Y, Matsuda H, Tsukube T, Okita Y. Complications of cerebrospinal fluid drainage in thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2004;39:243–5.
Loddo P, Degiudici A, Maxia A, Pibiri L, Pisu F, Ruiu G, Zanetti PP. Emergency surgery in thoracoabdominal aneurysms repair. Clinical outcome. G Ital Chir Vasc. 2003;10:255–67.
Zanetti PP, Krasoń M, Walas R, Cebotaru T, Popa C, Vintila B, Steiu F. “Open” repair of ruptured thoracoabdominal aortic aneurysm (experience of 51 cases). Pol J Cardiothorac Surg. 2015;12(2):119.
Bevilacqua S, Casini A, Galeotti I, Corsoni V, Romagnoli S. Rotational thromboelastometry–guided hemostatic therapy for management of cerebrospinal fluid catheter in patients undergoing endovascular aortic repair. Reg Anesth Pain Med. 2015;40(5):631–4.
Fedorow CA, Moon MC, Mutch WA, et al. Lumbar cerebrospinal fluid drainage for thoracoabdominal aortic surgery: rationale and practical considerations for management. Anesth Analg. 2010;111:46–58.
Lobato AC, Camacho-Lobato L. Enovascular treatment of complex aortic aneurysms using the sandwich technique. J Endovasc Ther. 2012;19:691–706.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Pirrelli, S., Mazzola, A., Ticozzelli, G., Bianchi, I.M., di Matteo, M., Quaretti, P. (2019). Update in the Management of Non-traumatic Thoracoabdominal Vascular Emergencies. In: Aseni, P., De Carlis, L., Mazzola, A., Grande, A.M. (eds) Operative Techniques and Recent Advances in Acute Care and Emergency Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-95114-0_35
Download citation
DOI: https://doi.org/10.1007/978-3-319-95114-0_35
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95113-3
Online ISBN: 978-3-319-95114-0
eBook Packages: MedicineMedicine (R0)