Abstract
Ore bodies around the world are declining in grade, whilst increasing in complexity. The level of impurities relative to the valuable metal is steadily increasing, posing new challenges to existing operations. Ion exchange is widely used in the hydrometallurgical industry for both primary recovery of metals and the removal of impurities. The superior selectivity of ion exchange resins makes them exceptionally suitable for the removal of target impurities to very low levels, thereby saving operating costs, increasing the value of the final product and significantly improving revenue. The ion exchange process in these applications is very simple, using standard ion exchange equipment and acid regeneration. The process can be fully automated, requiring minimal operator interference and supervision Examples of impurities that are successfully removed via ion exchange include iron, antimony and bismuth from copper electrolyte. In the copper electrolysis process, antimony, bismuth and arsenic tend to form slimes which are dispersed in the electrolyte. These slimes contaminate the cathode and/or decrease the quality of the copper deposition. A special chelating resin was developed to remove antimony and bismuth, while at the same time ensuring minimal chloride leakage to the sulphate matrix. The work is described in more detail in this paper. Ion exchange is also used to remove copper, zinc and nickel from cobalt electrolyte. This paper addresses a few of these examples in more detail.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Ion exchange resins are used in a wide variety of industries, both to target specific metals of interest, and for the removal of impurities. Resins containing special chelating functional groups have superior selectivity for the target elements, allowing the removal of impurities to very low levels and ensuring final products of very high purity that fetch a premium price.
Examples of applications where ion exchange is used to remove impurities include:
-
Antimony and bismuth from copper electrolyte;
-
Copper, zinc and nickel from cobalt electrolyte;
-
Calcium and magnesium removal in the chlor-alkali process.
IX may also be instrumental in earning additional revenue by recovering valuable metals from waste or effluent streams that would otherwise have been discarded. Examples include:
-
Precious metals from reclaimed catalysts and electronics (urban mining);
-
Rhenium from copper smelters;
-
Scandium from titanium production.
The typical impurity contents for copper [1] and cobalt [2] metal are listed in Table 1. The list clearly shows that a wide range of elements are considered. The presence and/or concentration of these elements varies between ore bodies and the necessary impurity removal steps are not necessarily the same for every operation.
Copper Electrolyte Purification: Electrolysis
Method of Refining Copper
The industrial production of copper consists of two steps. The primary step is a pyrometallurgical process, producing blister copper with a purity of approximately 99% copper. This metal is cast into anodes. During the subsequent refining step, the anodes are subjected to electrolysis to produce high purity copper (>99.99%),
In the copper electrolysis process, the impure copper anodes and cathodes (made of stainless steel or high-purity copper) are immersed in an electrolysis tank filled with copper sulphate liquor. Direct current is applied, causing dissolution of Cu 2+ at the anodes with the release of two electrons. At the cathode, the opposite reaction takes place, i.e. the copper(II) ion receives two electrons and deposits as pure copper metal, as illustrated in Fig. 1.
Impurity Metals in Copper Electrolysis
According to K. Koyama et al., the copper concentrate used in pyrometallurgical processes contains copper, iron, sulphur, gold, silver, nickel, zinc, arsenic, and other impurities. These impurity metals report to the copper matte and slag during the smelting process or to the gas phase. A portion of the impurity metals contained in the copper matte reports to the anodes. During electrolysis, some of the impurity metals precipitate as slime at the bottom of the bath, while elements with a lower electrode potential than copper (0.337 V vs. SHE), are soluble under the prevailing conditions and report to the solution. The impurity metals can be categorized into three different groups, according to their solubility behaviour in copper electrolyte, as listed in Table 2.
When the concentration of impurities in the electrolyte increases above a certain level, it causes a reduction in electrical conductivity, leading to a decline of electrolytic efficiency which ultimately negatively affects the purity of the final copper product. To control the impurity level, a bleed of the copper electrolyte is treated De-copperization electrolysis (electrolysis using insoluble lead anodes to remove the impurity as copper removal slime) had been used conventionally. However, bismuth and antimony could not be removed efficiently by these conventional methods. This resulted in the study of using chelating resins for the purification of copper electrolyte [3].
Bismuth, Antimony Removal Study with Purolite Chelation Resin
Testwork was performed on a synthetic liquor with a composition that is representative of typical electrolyte, with metals and concentrations as listed in Table 3.
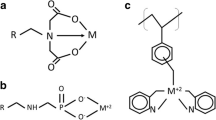
It was assumed that bismuth and antimony are present in trivalent cationic form in the copper electrolyte. The resin of choice had to have a good affinity for these impurities, whilst minimising the adsorption of divalent copper. Multiple studies on various chelating resins lead to the qualification of the Purolite® S950, with amino-phosphonic functional group (see Fig. 2).
The use of this resin for the removal of antimony and bismuth from copper electrolyte on large scale started in 2004. The system was a standard down-flow single bed with hydrochloric acid regeneration.
After this process had been in use for a number of years by several copper electrolysis sites, an additional requirement for the resin was stipulated by the end-users. It was requested that the Cl leakage after hydrochloric acid regeneration be reduced, as the presence of an excessive concentration of chloride in the electrolyte has a negative effect on the quality of the deposit.
In response, Purolite developed PurometTM MTS9510PF, with the goal to produce a resin that outperforms conventional amino-methyl-phosphonic resins in terms of Cl leakage, while retaining its good capacity to remove antimony and bismuth from copper electrolyte.
Laboratory tests simulated the different steps that the resin is subjected to in an actual operation, as listed in Table 4. The antimony and bismuth are first eluted with hydrochloric acid. The acid is displaced from the resin bed during a subsequent rinse with water. The resin is then contacted with sulphuric acid, to ensure displacement of chlorides that may still remain in the resin bed, thereby preventing the transfer of chlorides to the system when the resin is returned to the next adsorption cycle.
Two different resin samples were tested, a fresh resin sample and a “cycled” sample. The cycled sample was repeatedly contacted (100 cycles) with 15% w/v NaOH followed by 15% w/v H2SO4 to simulate the effect of osmotic shock. Osmotic shock is the successive swelling and shrinking of a resin bead when contacted with different types of solutions (alkaline, neutral, acidic). This weakens the resin over time and ultimately results in resin breakage and loss. The test was included to confirm that the resin not only had a good capacity for antimony and bismuth combined with low chloride leakage, but also provided sufficient osmotic stability to be economically viable.
Samples of the barren liquor during Step 1 (HCl regeneration) and Step 2 (water rinse) were taken in one bed volume (BV) fractions and analysed for chloride by titration with silver-nitrate. During Step 3 (H2SO4 displacement) samples were taken every 10 BV. A bed volume equals the volume of resin in the test.
A sample of the Purolite® S950, as well as a competitor’s equivalent resin, was included, for reference. The chloride leakage during Step 2 (water rinse) and Step 3 (H2SO4 displacement) are shown in Figs. 3 and 4, respectively.
Efficient displacement of the hydrochloric acid from both samples of the PurometTM MTS9510PF (fresh and cycled) was achieved within 3 bed volumes of the water rinse. Very low chloride leakage (<20 ppm) was detected in the barren during the subsequent sulphuric acid treatment step.
Tests by the end-user in their operation confirmed that the PurometTM MTS9510PF has a 10–20% higher capacity for bismuth and antimony when compared to conventional aminophosphonic chelating resins, such as Purolite® S950. Site tests also confirmed the lower Cl leakage that was obtained during laboratory tests. Due to its superior performance, the PurometTM MTS9510PF is the resin of choice for the removal of antimony and bismuth from copper electrolyte.
Iron from Copper Electrolyte
The FENIX Hydromet Iron control system [4] used at the Mount Gordon copper mine in Australia utilised a special sulphonic/phosponic resin to remove iron from the copper electrolyte. This resin was developed by Eichrom industries and is produced under licence by Purolite as Puromet MTS9570.
The iron control system was installed on a bleed stream of the Lean Electrolyte that is returned from the electrowinning cellhouse to the copper solvent extraction strip circuit, as shown in the simplified flow diagram in Fig. 5. The Lean Electrolyte contained 35 g/L Cu, 150–180 g/L H2SO4, 0.25 g/L Co and 3 g/L Fe.
While some iron (2–3 g/L) is required in the electrolyte stream, iron builds up over time and excess iron lowers the current efficiency in the cellhouse and must be removed via a bleed. This bleed causes the loss of acid and cobalt (50–150 mg/L cobalt which is added to improve surface morphology), resulting in additional cost for neutralisation of the acid and replacement of the cobalt.
The high affinity of the resin for iron allows efficient extraction of iron from the copper electrolyte liquor. Due to this high affinity, a very specific eluant, cuprous sulphate, is required, to elute the resin. The cuprous sulphate is formed by contacting a small volume of lean electrolyte with copper metal, such as copper wire scrap.
Cobalt Electrolyte Purification
The specifications for cobalt metal vary, but are always very strict, requiring the removal of impurities prior to the production of metal (refer to Table 1). IX can be used to remove nickel, copper and zinc. A typical flowsheet for the production of cobalt metal for a copper/cobalt operation in the African Copperbelt is shown in Fig. 6.
Copper and Zinc
Cobalt advance electrolyte (AE) typically contains around 50–60 g/L Co and between 50 and 200 mg/L Cu and Zn. The cobalt concentration is thus orders of magnitude higher than that of the impurities. This necessitates the use of a resin with a very high selectivity for Cu and Zn over Co such as an amino methylphosphonic acid (AMP) resin. The selectivity order of this resin is:
Laboratory and pilot plant testwork was done at Mintek for the Kakanda Tailings treatment project [5] to obtain operating conditions for the removal of Cu and Zn from Co Advanced Electrolyte (AE). The feed to the IX unit operation contained 65 g/L Co, 370 mg/L Cu and 5 mg/L Zn, with a pH of 4–5. A copper loading of 10–12 g/L was achieved when the removal of copper alone was targeted. The copper loading on the resin was lower, at 7 g/L, when both zinc and copper removal was targeted. Some (<0.5%) cobalt co-loading occurred. Copper, zinc and cobalt were easily eluted with sulphuric acid. The capacity of the AMP resin for the target metals is sensitive to pH. As such conversion of the resin to the sodium form is required, prior to returning the resin to the adsorption circuit, to prevent the pH inside the resin bed from dropping too low, thereby ensuring maximum impurity metal loading.
A drawback of this resin is its strong affinity for ferric ion, to the extent that any Fe3+ loaded onto the resin is not eluted during the standard sulphuric acid elution and a reductive strip is required. Efficient removal of iron from the AE liquor prior to contact with the AMP resin is thus important.
Nickel
The chemical properties of nickel and cobalt are quite similar, making it difficult to remove small amounts of nickel from cobalt sulphate liquor. Various methods exist, with varying degrees of success. Precipitation typically result in high levels of co-precipitated cobalt and subsequent recycling of the cobalt within the process. Solvent extraction introduces the risk of fire and organic contamination, as well as the introduction of undesirable sodium via the pH control with sodium hydroxide. IX has several advantages over both precipitation and solvent extraction. As such IX, using the Dow M4195 chelating resin with bis-picolylamine functionality, has been used with success at Vale’s nickel refinery in Port Colborne, Canada and Chambishi Metals in Zambia. The selectivity order of the resin is:
Testwork done for the Cosac project at Chambishi [6] showed that the resin has good selectivity for nickel over cobalt, in spite of the high ratio of cobalt: nickel in the feed. The cobalt electrolyte contained 60 g/L Co and ~350 mg/L Ni. A resin loading of 29 g/L cobalt and 6 g/L nickel was obtained. The resin’s selectivity for nickel over cobalt was improved at increased Ni concentration in the feed and also by decreasing the relative flowrate. Both factors allowed nickel to displace cobalt from the resin, minimising the loss or unnecessary in-process recycling of cobalt. Additional separation of cobalt and nickel could be achieved by a split elution, during which the cobalt was eluted first using 10 g/L H2SO4, followed by nickel elution with 150 g/L H2SO4.
Cobalt electrolyte in operations in the African Copperbelt often contain appreciable concentrations of copper. Any copper present in the cobalt electrolyte loads strongly on the bis-picolylamine resin and is not eluted with sulphuric acid. An alkaline ammonia elution is needed to remove the copper. This introduces an undesirable additional step and reagent to the process. An alternative resin, the Dow XUS-43605 utilising N-(2-hydroxypropyl) picolylamine (HPPA) functionality [7], achieves nickel loadings from cobalt electrolyte that are comparable with that of the bis-picolylamine resin, and, while it also loads copper, the affinity for copper is significantly less and copper is eluted during the standard sulphuric acid elution. An additional split between cobalt, nickel and copper is achieved by eluting cobalt first, using 20 g/L H2SO4, followed by nickel and copper elution using 60 g/L H2SO4.
Waste Treatment
Environmental regulations are becoming increasingly more stringent and the disposal of any effluents is heavily regulated. Exceeding the prescribed concentration of specific components in effluents, either knowingly or due to unplanned spillages, can result in hefty fines. IX may be used, either as a stand-alone operation, or as part of a bigger effluent treatment operation, to remove specific contaminants to an acceptable level, thereby avoiding such fines. Regulated components may include nitrates (by-product of blasting activities) and arsenic (present in the ore). Treatment may also result in improved revenues by the recovery of otherwise ‘lost’ valuable metal, such as copper. Additional treatment also often makes it possible to recycle the stream back to the process, reducing the need to spend money on fresh water.
Conclusions
The use of ion exchange resins in a large variety of industries is well-established. The examples in this paper clearly show how the removal of impurities can improve revenue, by
-
Allowing the production of a high-purity product that fetches a premium price, as in the case of cobalt metal;
-
Improving operating conditions, such as improved current efficiency in a copper electrowinning cellhouse which leads to lower operating costs.
Relatively small adjustments to the composition of IX resins can result in a substantial improvement to the end-user’s process, such as the reduced chloride leakage obtained with the new PurometTM MTS9510PF. Such tweaking of existing products does not necessarily require lengthy and expensive research programmes and most manufacturers of IX resins are willing to work with their customers to improve the bottom line.
References
Moriya M, Imachi T (1993) Method for removing impure metal ion in copper electrolyte, Japanese Patent, 05-214576, A, (filed in 1985), Description of the Prior Art
Marston CR, Rodgers ML (2010) New ion exchange technique for removal of copper and nickel from cobalt electrolyte. In: Alta metallurgical conference 2010. ALTA Metallurgical Services; Melbourne, Australia
Jurrius Y, Sole KC, Hardwick E, (2014) Removal of copper and zinc from a cobalt electrolyte by ion exchange at Kamoto Copper Company’s Luilu plant. In: Hydrometallurgy 2014, vol II. Canadian Institute of Mining, Metallurgy and Petroleum. Vancouver, Canada
Baily C, Harris GB, Kuyvenhoven R, Du Plessis J (2003) Removal of Nickel from cobalt sulphate electrolyte using ISEP continuous ion exchange. In: Ian MR (ed) Hydrometallurgy 2003: 5th international symposium honouring, Vancouver, Canada
Wyethe JP, Kotze MH (2000) Impurity removal by fixed bed ion exchange from cobalt electrolyte derived from the Kakanda Tailings Dump. In: ALTA SX/IX 2000; ALTA Metallurgical Services: Melbourne, Australia
Case Study—Puromet™ MTS9510PF P-000129-NPOLD-0817-R3 Purolite Confidential Communication
Shaw RD, Dreisinger DB, Lancaster T, Richmond GD, Tomlinson M (2004) The commercialisation of the FENIX iron control system for purifying copper electrowinning electrolytes. JOM J Miner Metals Mater Soc 56(7):38–41
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
van Deventer, J., Mori, Y. (2018). The Use of Ion Exchange to Improve Revenue via the Removal of Impurities. In: Davis, B., et al. Extraction 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95022-8_180
Download citation
DOI: https://doi.org/10.1007/978-3-319-95022-8_180
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95021-1
Online ISBN: 978-3-319-95022-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)