Abstract
Mesowear and microwear analyses use data from worn tooth surfaces as proxies for feeding ecology. Mesowear is based on gross dental wear and forms over months to years. The method was originally developed for ungulates but has recently been expanded to other groups, at least preliminarily. Dental microwear has been investigated for well over half a century and continues to be refined. It forms over days to weeks. Wide varieties of techniques are currently used for microwear analysis, all of which require attention to detail. Among these techniques, three-dimensional microwear texture analysis has the greatest potential for accurately reconstructing feeding ecology, yet the “recipe” for analyzing microwear data remains a work in progress. Combining mesowear and microwear with one another and other dietary proxies can permit robust inferences about the feeding ecology of extinct species.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Summary
Both dental mesowear and microwear analysis use data from the “damaged” wear surface of a tooth as a proxy for feeding ecology in extant and extinct vertebrates. Mesowear is a relatively new method of dietary interpretation in herbivores that is based on gross dental wear. It measures diet (and, to some extent, habitat) in individuals and populations over the span of months to years. Collecting data is relatively quick and easy. The method was originally developed for ungulates (mostly artiodactyls and perissodactyls) but has recently been expanded to other groups, at least in a preliminary fashion. Dental microwear analysis has been a rich area of investigation for well over half a century and continues to be refined today. It can discriminate not only large-scale dietary differences among taxa but also dietary variation within populations (among individuals). However, all steps of microwear data collection (from cleaning a tooth to analyzing data) require attention to detail and careful planning to ensure the collection of accurate, objective results. Investigators that analyze dental microwear are currently using five distinct techniques: (1) scanning electron microscopy (SEM); (2) stereoscopic low-magnification light microscopy (LM) ; (3) photomicrographic LM; (4) three-dimensional microwear texture analysis (MTA) with scale-sensitive fractal analysis (SSFA) ; and (5) MTA with International Organization for Standardization (ISO) 25178-2 parameters. Three-dimensional MTA has the greatest potential for accurately reconstructing feeding ecology, yet the “recipe” for analyzing microwear data remains a work in progress as technology advances and our understanding of the events that create microwear improves. Mesowear and microwear can each be a very powerful tool for paleoecological and environmental reconstruction, but both require strict protocols. Combining these techniques with one another and with other dietary proxies can permit robust inferences about the feeding ecology of extinct species.
Terms
Abrasion = tooth wear caused by interactions between a tooth and exogenous particles (e.g., food, grit).
Attrition = tooth wear that is caused by tooth-on-tooth interactions.
Browser = an herbivore that feeds primarily (>90%) on leaves, twigs, buds, flowers, and/or fruits.
Cast = an exact replica of a specimen that is produced by filling a mold with epoxy or another material.
Confocal Microscope = a microscope that uses point illumination and a spatial pinhole placed at the confocal plane of the lens to reduce out-of-focus light, creating a three-dimensional image.
Cusp Relief = the relative distance between the cusp tip and base.
Cusp Shape = a measure of the facet development in a cusp.
Erosion = tooth wear that is caused by chemical or acid dissolution.
Facet = a smooth, flat area of enamel on the occlusal surface of a tooth that is formed by wear.
Grazer = an herbivore that feeds primarily (>90%) on grasses.
Mesowear = gross (macroscopic) tooth wear (facet development).
Microwear = microscopic scars that form on a tooth surface from tooth use (use-wear) during life.
Mixed Feeder = an herbivore that feeds on a mixture of leaves, twigs, buds, and grasses.
Mold = a negative copy of a specimen that can be used to produce an exact replica (cast ).
Scanning Electron Microscope = a microscope that uses the interaction of a focused beam of electrons with a sample to produce a detailed image; designed for high-magnification use (typically ≥100×).
Stereomicroscope = a microscope in which light refracting through or reflecting off an object is viewed through two eye pieces, producing a three-dimensional visualization; designed for low-magnification use (typically ≤ 100×).
Ungulate = a hoof-bearing mammal; not a natural (monophyletic) group.
Theoretical and Historical Background
Mesowear
Mesowear refers to macroscopic wear on teeth: wear that is visible to the naked eye as a smooth, flat area of enamel on the occlusal surface. Such wear comprises what is known as a facet . Mesowear analysis seeks to describe the degree to which such facets are developed on the teeth of herbivorous mammals and to correlate such patterns with diet . Unlike microwear analysis, mesowear analysis has only been applied to herbivorous mammals. In herbivores that consume relatively soft foods, such as the leaves of dicotyledonous plants, twigs, buds, flowers, and fruits (i.e., browsers ), the teeth working against one another is the primary cause of tooth wear; this type of wear is attrition. Attrition creates enamel surfaces that have sharp edges and well-developed facets. In grazing herbivores, which mainly consume grasses and other low-growing vegetation, the food consumed causes more tooth wear than the teeth themselves. This type of wear is termed abrasion , and it tends to obscure facets and round off enamel edges. Mesowear analysis was originally developed for ungulates , particularly artiodactyls and perissodactyls, but it has recently been expanded to other groups including rodents , rabbits, elephants (proboscideans), extinct ungulate-like mammals from South America, and even marsupials (see below).
The term mesowear (meaning “intermediate wear”) was coined by Fortelius and Solounias (2000) because it highlighted the intermediate time scale at which mesowear forms: more slowly than microwear, which reflects an animal’s diet over days to weeks (see below), but more quickly than changes in the overall structure of a tooth, which only occur over evolutionary time (over many generations). Mesowear develops over an animal’s lifetime, or at least at a time scale long enough so that seasonal changes in diet cannot be detected (Rivals et al. 2013). Thus, it reflects “the average diet of a particular species from a particular location in space and time” (Fortelius and Solounias 2000: 2). Different scoring techniques for mesowear exist, but they differ from one another primarily in detail. The greatest advances in mesowear analysis have been in extending the technique to groups of herbivorous mammals beyond artiodactyls and perissodactyls. Collecting mesowear data is generally quick and easy, making the technique highly amenable to large samples (e.g., Mihlbachler et al. 2011).
Microwear
Microwear is the result of microscopic damage to dental tissues (usually enamel) that forms during mastication. This microscopic wear is not a cumulative (long-term) record of chewing (in contrast to mesowear), but rather is continuously recorded and erased with each subsequent feeding event (Teaford and Oyen 1989). As a result, it generally only records an animal’s last meal(s), a characteristic dubbed the “Last Supper” phenomenon by Grine (1986). High turnover rates in microwear have been recorded in most vertebrates, ranging from days to weeks (Teaford and Oyen 1989; Baines et al. 2014; Hoffmann et al. 2015). The relative orientations of striations on tooth surfaces is a proxy for direction of jaw movement during chewing (Gordon 1984a), although most microwear studies tend to focus on the relationship between microwear patterns and the texture of particles being consumed (Teaford 1991). Such analyses can target the proportions and densities of discrete features (scratches, pits, gouges) on a wear surface (Teaford and Walker 1984; Solounias and Semprebon 2002), or, increasingly commonly, the overall texture (roughness) of a chewing surface (Scott et al. 2005). Regardless of the approach, it has long been recognized that microwear patterns in animals with different diets can be qualitatively and quantitatively (even statistically) distinguished (Walker et al. 1978; Teaford and Walker 1984; Solounias and Semprebon 2002; Ungar et al. 2003). This correlation has led to the popularity of microwear as a proxy for reconstructing paleoecology in vertebrates.
Despite the many known correlations between diet and microwear, researchers are still trying to understand the details of these relationships (Ungar 2015). Indeed, a fundamental question that remains unanswered is whether the primary causative agent of tooth wear is endogenous (i.e., a property of the food itself, such as hardness; Erickson 2014; Rabenold and Pearson 2014; Xia et al. 2015) or exogenous (i.e., due to non-biological particles such as dust or sand; Lucas et al. 2013; Hoffmann et al. 2015; Spradley et al. 2016). Data support both interpretations, leading to the inescapable conclusion that a seemingly simple correlation is actually far more complex. The number of microwear studies published each year continues to increase, highlighting its importance in the field of paleoecological reconstruction.
Ungar et al. (2008) provided a thorough review of the deep history of microwear analysis including its origins, philosophical shifts, and methodological innovations. Here, we briefly review its foundations before focusing on the past decade, a time of rapid innovation in the field.
The first studies of microwear were qualitative in nature, using light microscopy to examine the orientations of scratches to determine directionality of chewing motion (Butler 1952; Mills 1955). Subsequent researchers abandoned the light microscope in favor of the high resolution and magnification of scanning electron microscopy (Rensberger 1978; Walker et al. 1978); the quantification of microwear patterns to discern dietary differences soon followed (Gordon 1982; Teaford and Walker 1984). This early research mainly focused on primates ; it demonstrated relatively consistent microwear within species (Gordon 1982) and distinct microwear among species of differing diets (Teaford and Walker 1984). Gordon (1984b) recognized the need for standardization of SEM instrumentation parameters, magnification, and tooth sampling loci to control for intra- and inter-individual variation in tooth wear. Quantifying scar features on teeth permitted statistical testing to discriminate between genuine and random microwear patterns, thus leading to a more advanced understanding of the relationship between microwear and feeding ecology (Teaford and Walker 1984; Strait 1993; Ungar and Spencer 1999). In the late 1980s and early 1990s, researchers sought to more accurately quantify microwear using a variety of approaches and variables (e.g., scratch:pit ratios, scratch length/breadth, feature orientation; Grine 1986; Maas 1991; Ungar 1995).
By the early 2000s, it was recognized that observer consistency in microwear feature recognition and measurement on SEM images limited interpretations (Grine et al. 2002) and that SEM analysis could be expensive and time-consuming (Solounias and Semprebon 2002). In this light, Solounias and Semprebon (2002) suggested returning to low-magnification light microscopy (LM) for dental microwear analysis as an effective yet inexpensive (in both time and cost) method. Interest in LM increased rapidly during the subsequent decade, with a concomitant increase in the diversity of non-primate mammals analyzed (e.g., ungulates : Solounias and Semprebon 2002; chalicotheres: Coombs and Semprebon 2005; proboscideans: Green et al. 2005; Rivals et al. 2012; xenarthrans: Green 2009a; rodents : Nelson et al. 2005; Townsend and Croft 2008b; notoungulates: Townsend and Croft 2008a). As with SEM methods, observer consistency was questioned (Ungar et al. 2008). Early studies suggested that intra- and inter-observer error were non-significant in LM (Semprebon et al. 2004b), but later studies have disagreed (Mihlbachler and Beatty 2012; Mihlbachler et al. 2012).
Concerns about observer error and lack of consistency among researchers using SEM and LM fueled the development of an automated three-dimensional technique called “microwear texture analysis” (MTA) (Ungar et al. 2003; Scott et al. 2005). This method differed from previous methods in using computer software to analyze the texture of a wear surface as a whole rather than to quantify discrete features (“scratches” vs. “pits”) on a tooth surface. Because this technique analyzes microwear in three dimensions rather than two (as in SEM and some variants of LM; Merceron et al. 2005), it can provide greater power for discriminating subtle differences in feeding ecology, such as seasonal variations in diet (DeSantis et al. 2013).
Microwear researchers presently use all three types of microscopy (listed above) and variations of each are still being proposed. For example, investigators in some recent LM studies have counted features on photomicrographs (under randomized, blind conditions) instead of directly through a microscope in an effort to create a more standardized approach (Fraser et al. 2009; Mihlbachler et al. 2012; Hoffmann et al. 2015); SEM researchers have used randomized, blind conditions for counting features (Green and Resar 2012; Green and Kalthoff 2015); and MTA now uses standardized ISO parameters to characterize textural patterns in addition to scale-sensitive fractal analysis (SSFA) (Purnell et al. 2012; Gill et al. 2014). In all of these cases, it is important to remember that each technique has its limitations and shortcomings . MTA is the most widely used technique today and has greatest promise for the future of microwear research (Calandra and Merceron 2016). Nevertheless, the other two methods can still provide significant insights when properly applied (Green and Resar 2012; Mihlbachler et al. 2012).
Collecting and Analyzing Data
Selecting Specimens
Accurate mesowear and microwear analyses require selecting appropriate comparative specimens. This means using teeth (either fossil or modern) that have well defined, undamaged wear facets . A tooth that has not yet come into wear (i.e., has no obvious wear facet) is unusable, as the processes that result in tooth wear have not yet been initiated. Additionally, overly worn teeth (from older individuals) should be excluded from mesowear analyses (Rivals et al. 2007) because facet development is obscured. Teeth that are in situ (in a skull) are ideal since they leave no ambiguity as to: (1) species identification; (2) tooth position and orientation; and (3) ontogenetic age. For extant species, specimens should preferably come from live or recently culled animals whose diet is known and controlled. However, since this is generally not possible, most studies have relied on museum collections, which offer large sample sizes but lack specific (individual) dietary information (Kay and Covert 1983). In such cases, specimens are assigned to the dietary category typical for the species. The lack of precise correspondence between specimens and their presumed diet can be a significant source of error or noise, particularly for species with diets that vary temporally (day to day or seasonally) or spatially (in different parts of their range). Targeting museum specimens associated with detailed collection information (including season of death and longitude-latitude, where available) can help mitigate this error, but parsing specimens into increasingly fine dietary and/or locality bins usually comes at the cost of decreasing sample sizes.
The choice of tooth position and wear facet to sample depends on the question being addressed and the group being studied. It is optimal to have a standardized sampling protocol that targets the same wear facet on the same tooth across all individuals (Gordon 1982). This helps reduce variation in wear that may arise from differences in chewing mechanics across the tooth row (e.g., a posterior or distal tooth will experience less shearing and higher bite forces than a more anterior or mesial one; Gordon 1982). This is usually straightforward when comparing species within particular families or orders. Comparing members of different orders can be problematic if members of the groups under consideration have (or had) different chewing mechanics (Croft and Weinstein 2008; Fraser and Theodor 2010; Saarinen et al. 2015; Ulbricht et al. 2015; Mihlbachler et al. 2016). Studies that have examined intra- and inter-tooth variability have sampled multiple teeth and different surfaces within a single tooth to capture the range of variability that is present (e.g., Kaiser and Fortelius 2003; Krueger et al. 2008; Green 2009b; McAfee and Green 2015).
Extending mesowear and microwear analysis to extinct species poses additional challenges. First, sample sizes are generally smaller due to the rarity of fossil specimens. Second, fossil specimens may not be suitable if they cannot confidently be identified to species and/or if their position in the tooth row cannot be determined. In addition, the process of fossilization itself can significantly alter or erase the original microwear scars on the wear surface (Teaford 1988; King et al. 1999), though this can also be the case for seemingly well-preserved modern specimens (Teaford 1988). A simple comparison of the chewing and non-chewing surfaces of a tooth using a low-magnification microscope can be used to identify fossil teeth that should be discarded. The chewing surface should have visible microwear in regular patterns, whereas the non-occlusal surfaces should be devoid of similar features; if similar scar patterns are observed on chewing and non-chewing surfaces on the same tooth, the specimen has most likely been altered and should be removed from the sample (Teaford 1988). Fortunately, taphonomic processes tend to obliterate microwear patterns rather than mimic genuine scar features (King et al. 1999; Martínez and Pérez-Pérez 2004). Taphonomic breakage is generally obvious for mesowear, as it results in irregular surfaces that lack the smooth polish typical of well-preserved enamel.
Specimen Preparation
Since mesowear is scored on a gross level with the naked eye, no special cleaning of specimens is generally required. It is only necessary that the development of the tooth facets can be observed. This is not true of microwear, as debris (dirt, dust), preservatives, and/or other residues can completely obscure microscopic features (Teaford 1988). Whether to clean the entire tooth or simply a portion of it depends on the size of the tooth and the facet(s) targeted for analysis (described above); the teeth of small mammals (e.g., rodents, primates ) can usually be cleaned and molded entirely, whereas only a portion of the tooth of a large mammal (e.g., proboscideans) is usually cleaned. When only part of a tooth is cleaned, the anatomical position and orientation should be recorded so that it is readily available during the analysis phase. Cleaning via physical scrubbing or preparation, especially with metal tools, brushes, etc., should be avoided as this can remove and/or alter scar patterns on the tooth. For fossil specimens, chemical preservatives (e.g., Butvar, Glyptal) are usually dissolved with acetone but may require multiple applications, depending on the stubbornness of the preservative. Researchers should identify islands of pooled preservative on the tooth surface before conducting an analysis. Once glues are removed (or if none were ever applied), applying ethanol with a cotton swab is an excellent technique for physically washing the tooth surface. The tooth should dry completely before proceeding.
Both mesowear and microwear can be scored using replicas (casts ) rather than actual specimens. However, since scoring mesowear does not require specialized equipment, making casts is usually unnecessary; original specimens can be scored directly in museum collections. Microwear, by contrast, is frequently scored on casts because microscopes are not easily portable and borrowing large numbers of specimens from a museum for study in a laboratory usually is not possible. It is also much easier to manipulate a tooth cast under a microscope than a mandible or cranium, particularly for medium to large mammals, but original teeth can be analyzed where specimens are available for laboratory study.
The first step in making a cast is creating a mold , which is an impression of the tooth surface. A high quality dental molding compound should be used so that sub-micron level detail is preserved with little (if any) alteration. Polyvinylsiloxane compounds such as President Jet Light Body (low viscosity ) and President Jet Regular Body (medium viscosity) consistently produce molds with both high accuracy and precision (Goodall et al. 2015). Other products should be used with caution, and data from studies using different molding compounds should not be compared directly (Goodall et al. 2015). The molding compound should be mixed according to its label, applied to the clean tooth surface in a thin coat, and allowed to dry. A first mold serves as the final cleaning step and should be discarded, as it will be contaminated with any debris remaining on the tooth surface (Solounias and Semprebon 2002). A second mold should be created using a thicker layer that completely covers the cleaned area. The specimen number, species, and any other pertinent information should be kept with this second and final mold . Applying a small tag to the mold as it dries is one way to ensure that the specimen number remains with the mold. Plastic bags or plastic organizer boxes with subdivided compartments are affordable options for transporting and storing molds. Some researchers prefer to analyze microwear directly from molds rather than casts (e.g., Schulz et al. 2010), but this is not feasible for techniques that rely on light refraction or electron beam interaction.
Prior to casting, a dam made from two-part laboratory putty is constructed around each mold to constrain the casting compound. The casts themselves are usually made with a high quality two-part epoxy (resin + hardener) capable of replicating the sub-micron detail captured in the mold. Epo-Tek #301, EpoKwick, and Feropur PR-55 are common choices (Solounias and Semprebon 2002; Galbany et al. 2004). Adjust the ratio of resin to hardener and the mixing time according to manufacturer instructions. Before pouring the mold, excess air bubbles must be removed from the mixture via depressurization in a vacuum chamber and/or by using a centrifuge (poured molds should not be placed under vacuum). Surface bubbles present in the mixture after pouring are removed using a toothpick. Casts should polymerize for at least 48 hours. Up to four casts can be made from a single mold before the replicated microwear pattern is compromised (Galbany et al. 2006).
Mesowear Data Collection
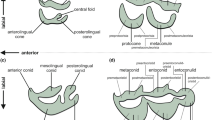
As originally conceived by Fortelius and Solounias (2000), two qualitative variables of a tooth are scored for a mesowear analysis of an ungulate or ungulate-like mammal: occlusal relief (cusp height; scored as high or low), and cusp shape (scored as sharp, rounded, or blunt) (Fig. 5.1A). Occlusal relief can be scored metrically instead of subjectively using digital photographs and pre-determined cutoffs for high versus low (see Croft and Weinstein 2008). In cases where the two buccal cusps differ in sharpness, the sharper of the two is scored. Mesowear is typically scored on an upper cheek tooth in buccal view (usually the second molar ), but other upper tooth positions can also be evaluated (Kaiser and Solounias 2003) as can lower teeth, at least in ruminant artiodactyls (Fraser et al. 2014). This is particularly useful for fossil populations, where sample size can be a limiting factor. For both extant and extinct species, at least 10 individuals (preferably 20) should be scored to adequately sample variation within the population. The percentages of specimens (individuals) of each species exhibiting high occlusal relief, blunt cusps, and sharp cusps are used for comparisons among species and for interpreting diets of extinct species. This two-variable method (i.e., using occlusal relief and cusp height) has recently been termed mesowear I (Solounias et al. 2014).
Examples of mesowear scoring. A, notoungulate right upper second molars in buccal view scored qualitatively for occlusal relief (low or high; asterisk indicates that relief varies depending on metric cutoff used) and cusp shape (blunt, rounded, or sharp) based on Croft and Weinstein (2008); from left to right, Archaeohyrax suniensis UF 172969, A. suniensis UF 172473, Trachytherus alloxus UF 149261; B, mesowear “ruler” of Mihlbachler et al. (2011), digitally reversed so scores read from 0–6; C, inner mesowear (=mesowear III) scoring method of Solounias et al. (2014) and Danowitz et al. (2016); left, clay models (courtesy of N. Solounias) illustrating the four wear stages of the inner enamel band and the junction (J) between its mesial (anterior) and distal (posterior) facets; right, upper molars of a typical browser (above; white-tailed deer, Odocoileus virginianus) and a typical grazer (below; domestic cow, Bos taurus); the asterisk in each photo indicates the inner enamel band of the paracone; D, left upper molars of the murid Apodemus flavicollis in anterior (mesial) view, scored qualitatively for mesowear; relief is scored for both valleys, and shape is scored for all three cusps ; modified from Ulbricht et al. (2015: Fig. 3); E, lower molar of an African savanna elephant (Loxodonta africana UM R1910) in oblique occlusal view (digitally reversed, anterior to right), illustrating the angle of the worn dentine valley measured by Saarinen et al. (2015) in their study of proboscideans. Abbreviations: UM, University of Michigan Museum of Paleontology; UF, Florida Museum of Natural History, University of Florida
Rivals and Semprebon (2006; see also Rivals et al. 2007) were the first to use a single-variable method for scoring mesowear that combined occlusal relief and cusp height into a single qualitative (categorical) score ranging from zero (high relief and sharp cusps ) to three (blunt cusps and essentially no relief). This modified version of mesowear analysis (later dubbed mesowear II; Solounias et al. 2014) was predicated on the observation that occlusal relief and cusp sharpness are correlated with one another and that some morphologies – such as high relief with blunt cusps – are rare or non-existent. Other researchers have used similar approaches, sometimes combined with more traditional scoring (e.g., Croft and Weinstein 2008; Kaiser et al. 2009). Mihlbachler and Solounias (2006) used the percentage of specimens exhibiting blunt or rounded cusps as a single mesowear score, but this approach has not been used in other studies. Mihlbachler et al. (2011) facilitated use of a single, combinatorial variable for mesowear by using a mesowear “ruler” of tooth cusp casts illustrating the two endpoints and five intermediate stages in this categorical variable (Fig. 5.1B). Although these authors used a different numerical scale than Rivals and Semprebon (2006) (0–6 versus 0–3, respectively), the number of categories was the same. The major conceptual difference between this strategy and that of Rivals and Semprebon (2006) is that mesowear is scored as a single variable rather than two separate variables that are later combined into a single value. Fraser et al. (2014) also used a single-variable scale for scoring mesowear but included only five stages (two endpoints and three intermediate stages). The “extended mesowear method” of Winkler and Kaiser (2011; see also Taylor et al. 2013) calculates a single mesowear score using cusp shape and a modified scoring scheme for occlusal relief with more than two categories .
Most recently, Solounias et al. (2014) and Danowitz et al. (2016) have developed a new mesowear method for selenodont artiodactyls known as mesowear III or inner mesowear. The latter term highlights the main difference between this mesowear scoring technique and traditional scoring methods (=mesowear I and II), which Danowitz et al. (2016) refer to as outer mesowear: inner mesowear evaluates wear on the inner (more lingual) enamel band of a tooth in occlusal view rather than the outer (external or buccal) enamel band in lateral view. Inner mesowear is evaluated qualitatively using a four-point scale that incorporates facet shape, distinctiveness of facets, and the presence of gouges, among other characteristics (Fig. 5.1C). Three parts of the inner enamel band of an upper tooth cusp (paracone or metacone) are scored separately: (1) the mesial (anterior) facet ; (2) the distal (posterior) facet; and (3) the junction between the two facets (j). The resulting inner mesowear data can be analyzed using the same visualization and statistical techniques as outer microwear data (discussed below), and the two can be combined with one another to provide a more nuanced picture of tooth wear (Danowitz et al. 2016).
Mesowear data collection for other groups of mammals varies depending on tooth morphology. Mesowear data for marsupials , murine rodents (mice; Fig. 5.1D), and rabbits (lagomorphs) can be collected using the same variables as for ungulates (Butler et al. 2014; Ulbricht et al. 2015). For proboscideans, which have a very different tooth structure, angular data are obtained from the occlusal surface using a digital angle meter (Saarinen et al. 2015; Fig. 5.1E).
Microwear Data Collection
Light Microscopy (LM) Data Collection: LM analysis uses a stereomicroscope and manipulated light to illuminate microwear features on a clear epoxy cast (Solounias and Semprebon 2002; Semprebon et al. 2004b; Fig. 5.2A). These analyses occur either by counting features directly through the lens (stereoscopic LM; Solounias and Semprebon 2002) or via digital images (photomicrographic LM: Merceron et al. 2005; Fraser et al. 2009; Mihlbachler et al. 2012). Solounias and Semprebon (2002) developed the popular observer-based stereoscopic LM method using a standardized magnification of 35× and a reticle with a 0.4 × 0.4 mm counting square placed in the ocular. Some later studies that focused on smaller mammals have used higher magnifications and/or smaller counting areas (e.g., 70×; Nelson et al. 2005; Townsend and Croft 2008b; Christensen 2014). Directing a light source across the occlusal surface at a shallow angle (“external oblique illumination” sensu Semprebon et al. 2004b) highlights microwear features in the target area.
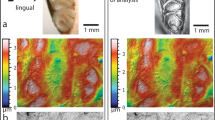
Examples of enamel microwear on the second lower molar of Mammut americanum (late Pleistocene, Indiana, USA; INSM 71.3.226.1), imaged via three different microscopes. Images were all captured in the same general region on the central mesial facet of the metalophid. A, light microscope image using external oblique illumination, captured at a resolution of 1199 pixels/mm, scale bar = 400 µm; B, SEM image, captured at 500× magnification, 20 kV, secondary electron image, surface oriented normal to the electron beam, scale bar = 50 µm; C, three-dimensional white-light scanning confocal microscope , image x-y axes equal 276 × 204 µm2 respectively, colors represent scale of z-axis. INSM = Indiana Museum of Natural History, Indianapolis, IN
The original LM variables of Solounias and Semprebon (2002) include: (1) number of scratches; (2) number of pits; (3) presence of >4 large pits; (4) texture of scratches (fine, coarse, or mixed); (5) presence of hypercoarse scratches; (6) presence of >4 cross-scratches; and (7) presence of gouges. Scratches and pits are counted inside the 0.4 × 0.4 mm reticle in two separate (non-overlapping) areas on the target region per individual (specimen), and the values for all individuals of a particular species are averaged to generate a species-specific value. Identifying scratch texture (predominately fine, predominately coarse, or a mixture of fine and coarse), pit size (small vs. large), hypercoarse scratches, and gouges is done qualitatively through the differential refraction of light (Solounias and Semprebon 2002). Deeper features (hypercoarse scratches, large pits) have low refractivity and thus appear darker on a light background, whereas shallower features are highly refractive and shiny on a dark background (Semprebon et al. 2004b; Christensen 2014). The angle of the light source is manipulated in a standardized fashion (e.g., Christensen 2014) to create both bright and dark fields, which helps illuminate the full range of features. Changes in the angle of the light source can directly change the types of features that are visible (Solounias and Semprebon 2002); thus, it is important that an experienced user teach new individuals interested in stereoscopic LM the technique. For these qualitative variables, the microwear of a particular species is defined as the percentage of individuals showing a particular feature (e.g., hypercoarse scratches). Subsequent studies have introduced additional variables, such as the percent of individuals with a low scratch percentage (Semprebon and Rivals 2007; Rivals et al. 2012). Collectively, these variables provide a quantitative mean microwear signature for each species that can be analyzed statistically.
One limitation of stereoscopic LM is that the full range of features visible through the lens using external oblique lighting cannot be captured as a digital image without loss of resolution (Semprebon et al. 2004b). Alternative photomicrographic LM techniques have been developed to compensate for this problem (Merceron et al. 2005; Fraser et al. 2009; Mihlbachler et al. 2012). These techniques all use a digital camera mounted on a light stereomicroscope but vary in their placement of the light source and other aspects. Merceron et al. (2005) and Mihlbachler et al. (2012) placed the specimen on a glass stage and transmitted light from beneath, whereas Fraser et al. (2009) continued to use external oblique illumination but specified an angle of ~45° relative to the surface of the cast . In all photomicrographic LM studies, the surface of the cast is subhorizontal to ensure equal illumination and good focus. Contrast and other variables can be manipulated to produce the best image either before the image is captured (Merceron et al. 2005) or afterwards (using software; Fraser et al. 2009; Mihlbachler et al. 2012). All of these decisions should follow the specifications of the chosen methodology.
In photomicrographic LM, images are analyzed on a computer display, so it is necessary to report digital resolution of both the image (resulting from the combination of pixel density and digital magnification) and the monitor display. It is critical to bear in mind that optical magnification is not the same as digital magnification; since light passing into the camera does not pass through the eyepiece, the magnification of the eyepiece is irrelevant. Confusion with regard to these specifications can lead to a lack of repeatability and inappropriate comparisons among independent microwear analyses (Mihlbachler and Beatty 2012). Digital resolution is reported as pixel density (sensu Mihlbachler and Beatty 2012), and an optimal resolution for photomicrographic LM is chosen on a case-by-case basis, depending on the size of the scars being analyzed (Mihlbachler and Beatty 2012). Highest resolutions can lead to overestimates of feature densities that are difficult to reproduce, as researchers will be tempted to count and analyze the finest scars visible (Mihlbachler and Beatty 2012).
Data collection on digital micrographs is user-dependent yet more controlled than counting directly through the lens (Mihlbachler et al. 2012). Counts should still be restricted to a standardized area, delineated as a digital box or by cropping the image. Users can save images used for analysis and even upload them to open-access online databases (e.g., Dental Microwear Image Library, maintained by NYIT College of Osteopathic Medicine) so that independent researchers can perform counts on the same images to improve long-term repeatability. In addition to counting feature density, photomicrographic analysis allows sizes of scars to be measured either directly (Merceron et al. 2005) or estimated using predefined shapes (Mihlbachler et al. 2012). The lowest levels of observer error and bias in LM (see Microwear Limitations) occur when users have prior experience with microwear analysis and conduct their analyses in a randomized, blind (to taxonomic identity and dietary category) fashion (Mihlbachler et al. 2012). Dimensionless variables (e.g., relative proportions of scratches and pits) may be more robust to repeatability than absolute ones (Mihlbachler and Beatty 2012; Mihlbachler et al. 2012; DeSantis et al. 2013).
Scanning Electron Microscopy (SEM) Data Collection: As mentioned previously, the bulk of dental microwear research prior to the re-introduction of LM or texture analysis was SEM-based, as high resolution micrographs could be captured at high magnification to measure the density and proportions of even the smallest features on a tooth (Gordon 1988; Teaford 1991; Ungar et al. 2008; Fig. 5.2B). This technique requires a conductive specimen to prevent electrostatic charging with the electron beam during imaging (unless an environmental SEM is used; Rose 1983; Galbany et al. 2004). This process is non-reversible, so casts of teeth are usually analyzed rather than actual specimens. The cast is first mounted on a brass disc or aluminum stub (using an adhesive such as term fusible gum, carbon tabs, or PVA) and then a colloidal argent belt (e.g., silver, graphite) is usually applied to the base of the cast and between the cast and stub. The mounted cast is then sputter-coated with a colloidal metal, typically silver or gold. The thickness of the coating can vary (100–400 Å; Gordon 1982; Galbany et al. 2004) but should only be as thick as necessary to produce a quality image with the microscope being used. The specimen is kept in a clean, dust-free container and gently cleaned with compressed air prior to imaging, as dust interferes with electron dispersal during analysis (Galbany et al. 2004).
A mounted specimen is inserted in the SEM and placed under vacuum, following the specified procedure for the instrument being used. A litany of imaging settings can be adjusted, including magnification level, operating voltage, working distance, specimen orientation/tilt, and the type of electrons used. The values used will depend on operator knowledge, the quality of the instrument, and the questions being addressed. However, it cannot be emphasized enough that all of these parameters should be standardized during imaging, as even subtle changes can affect the number, size, and type of scar features that are visible (Gordon 1988; Galbany et al. 2004). Magnification level varies depending on the analysis, with an obvious tradeoff between area sampled and accuracy and precision of measurements (Teaford 1991). Lower magnification levels (100–150×) cover a wider surface area (e.g., 0.4 mm2 at 150×); as a result, the largest and most representative scars are visible (up to 400 individual features per tooth; Teaford 1991) but finer features are either invisible or too small to permit accurate measurements (Gordon 1988; Fig. 5.3A). Higher magnification levels (300–500×) cover a much smaller area (e.g., 0.03 mm2 at 500×; Fig. 5.3B), which can be advantageous when working with small teeth and measuring the proportions of very small features. However, larger scars extend beyond the field of view (i.e., their size cannot be accurately measured), and variation in the overall scar pattern is underestimated (Gordon 1988; Fig. 5.3B–C). Thus, data collected from images taken at different magnifications are not directly comparable (e.g., Fig. 5.3), so standardizing this variable in a study is critical (Gordon 1988).
Examples of microwear visible at different SEM magnification scales on the same specimen (Mammut americanum, m2, late Pleistocene, Indiana, USA; INSM 71.3.226.1). Images captured on central mesial facet of metalophid, using secondary electrons at 20 kV with surface oriented normal to the electron beam. A, 100× magnification, scale bar = 100 µm, box represents region visible in B; B, 500× magnification, scale bar = 50 µm, box represents region visible in C; C, 1000× magnification, scale bar = 10 µm. INSM = Indiana Museum of Natural History, Indianapolis, IN
Operating (electron accelerating) voltage and working distance can vary depending on the size of the cast and capability of the instrument. Reported accelerating voltages usually range from 10–25 kV (Galbany et al. 2004; Grine et al. 2006; Green and Resar 2012), while working distances usually range from 10–30 mm (Galbany et al. 2004; Green and Resar 2012). Specimen tilt within the chamber should be consistent, as visual distortion occurs with varying levels of tilt relative to the electron detector, leading to errors in feature measurement (Gordon 1982, 1988). SEM analyses usually employ either back-scattered (e.g., Strait 1993) or secondary electrons (e.g., Ungar and Spencer 1999). The former provides excellent surface relief but may not illuminate scratches aligned with the electron detector (Galbany et al. 2004). Secondary electron imaging is recommended where categories of feature orientation are being considered (Galbany et al. 2004). The final SEM image should be adjusted for brightness and contrast using standardized procedures (e.g., using the “levels” feature in Adobe Photoshop such that the lightest pixel is white and darkest pixel is black; Mihlbachler et al. 2012; McAfee and Green 2015) to maximize feature visibility in a controlled fashion. Digital resolution (in pixel density) of the image and the computer display should be reported. Images are usually either cropped to a standardized size for analysis (with every feature in the cropped image being counted; e.g., Patnaik 2015), or a standardized digital box is constructed around the area to be counted (e.g., Green and Resar 2012).
Quantification of scar patterns on SEM micrographs has its roots in the early days of microwear research (Gordon 1982), with different techniques varying in magnification, field of view, measurement method, and feature category designation (scratches vs. pits) (Teaford and Walker 1984; Grine 1986; Ungar 1995). Grine et al. (2002) showed that different methods are all prone to inter-observer error (an observer has to identify individual features, an inherently subjective process) and recommended using the semi-automated software package Microware developed by Ungar (1995) to maximize ease of use and consistency. Subsequent SEM studies (Grine et al. 2006; Purnell et al. 2006; Green and Resar 2012; Resar et al. 2013; Green and Kalthoff 2015; Patnaik 2015) have used the latest version of the program (Microware 4.02; Ungar 2002). This software requires that the user identify genuine scars on the image and use mouse-clicks to mark the four endpoints (long axis and short axis) of each feature. Even features with edges that extend past the image are counted and measured. The software automatically generates summary data for each micrograph, including feature density, mean length, mean width, and mean orientation (relative and standard). Scratches are automatically distinguished from pits based on a length:width ratio that can be set by the user (and therefore should be reported); the default setting is a ratio of >4:1 for scratches, which is based on the findings of Grine (1986).
MTA Microscopy Data Collection: The ability to measure microwear in three dimensions (quantifying not only length and width of scars but also depth) adds a level of detail that can discriminate subtle dietary distinctions not obvious using LM or SEM methods (Purnell et al. 2012; DeSantis et al. 2013; Purnell et al. 2013). The advent of MTA (Ungar et al. 2003; Scott et al. 2005, 2006) achieved this result by using a white-light scanning confocal microscope with 100× objective to scan tooth casts . Some authors have used interferometry to acquire three-dimensional surface data (Estebaranz et al. 2007; Merceron et al. 2014), yet confocal microscopy remains the most widely applied platform in MTA (Calandra and Merceron 2016). The user chooses the wear facet to analyze and is responsible for positioning the tooth cast such that the appropriate target surface (i.e., enamel vs. dentine; Haupt et al. 2013) is scanned. Four adjacent scans per tooth (a total field of view of 204 × 276 µm2; Fig. 5.2C) are then digitally leveled, and identifiable surface defects and artifacts (dust particles) are manually removed from the image (Scott et al. 2006). Postmortem alteration, either anthropogenic or sedimentary, is easily recognizable under MTA (see Calandra and Merceron 2016 for examples). A data point cloud is generated and analyzed in Toothfrax (Surfact) and SFrax programs using scale-sensitive fractal analysis (SSFA), which characterizes changes in surface texture (length-scale, area-scale, and volume-filling versus scale analyses) at differing scales (Scott et al. 2006). Since the entire pattern on the surface of the tooth is characterized automatically rather than by an individual user, problems associated with observer error are greatly reduced (Ungar et al. 2003). However, selection of the wear facet to be scanned still requires user competency, which can be a source of error for untrained individuals (Calandra and Merceron 2016). Likewise, differences in settings on the confocal imaging system (e.g., resolution, lateral point spacing, source light type) can lead to incomparable data if such factors are not taken into account by researchers using different instruments (Arman et al. 2016). Manually deleting dust particles and casting artifacts from confocal scans (prior to analysis) is another potential source of error, as both excessive and conservative editing can reduce data quality. Some authors choose to discard scans that include dust particles and casting artifacts, rather than digitally edit each scan (Calandra et al. 2012).
Ecologically significant SSFA variables include: (1) complexity; (2) anisotropy; (3) textural fill volume; (4) scale of maximum complexity; and (5) heterogeneity of complexity. Each of these is briefly summarized below (see Scott et al. 2006 for additional details). Complexity measures change of roughness across different scales, with high complexity usually correlated with a surface that has scratches and pits of different sizes that overlie one other. Anisotropy measures the orientation of roughness on a surface, with high anisotropy corresponding to a surface with long, consistently oriented scratches. Textural fill volume describes how much material has been removed from a surface, with high values correlating with the presence of deep features. Scale of maximum complexity records fine-scale limits of complexity, with higher values corresponding to a greater proportion of small features on a surface. Finally, variation in complexity across a surface (heterogeneity) is measured using subdivisions of the analyzed surface (either a 3 × 3 or 9 × 9 grid), with low heterogeneity representing surfaces of more uniform texture.
Three-dimensional MTA using SSFA is applied today as it was originally developed by Ungar et al. (2003; see Withnell and Ungar 2014; Hua et al. 2015; Shearer et al. 2015), although alternative techniques employing standardized areal texture parameter data (e.g., ISO 21578-2) are also in use (see Schulz et al. 2010; Calandra et al. 2012; Purnell et al. 2012, 2013; Schulz et al. 2013; Gill et al. 2014). Extracting ISO parameters requires similar pre-processing as SSFA, but the final scan must also be spatially filtered and adjusted for gross tooth form alterations such as curvature (Calandra et al. 2012; Purnell et al. 2013). Generated parameters include up to 30 variables that define basic geometric properties of surface texture (e.g., height, hybrid, volume, spatial), each quantifying a specific attribute. The ability of particular variables to discriminate diet depends on the taxonomic group; diets of primates and bats are discriminated by a unique combination of height, spatial, and volume variables (Calandra et al. 2012; Purnell et al. 2013), whereas fish require height and hybrid combinations (Purnell et al. 2012). Recently, an analytical technique that quantifies three-dimensional microwear on virtual reality models has been developed and tested (Tausch et al. 2015). Preliminary results suggest that this alternative to MTA may be useful for mapping directionality of chewing in molar facets in extinct taxa and may also have clinical applications in humans (Tausch et al. 2015).
Mesowear Data Analysis
In the case of analyses using mesowear I, the variables analyzed are percentages of individuals in a population or species with a particular trait (e.g., % with sharp cusps ). Since percentage data are not normally distributed, they should be transformed using the arcsine transformation if standard (parametric) significance tests follow. Alternatively, non-parametric tests could be employed. Mesowear profiles using these variables can be compared using multivariate techniques such as principal components analysis (PCA) , discriminant function analysis (DFA), and hierarchical cluster analysis (HCA) (Fortelius and Solounias 2000; Franz-Odendaal et al. 2003; Semprebon et al. 2004a; Croft and Weinstein 2008; Fraser and Theodor 2011; Louys et al. 2011). Mesowear II analyses use univariate statistical tests to compare means (e.g., ANOVA; Rivals et al. 2013) and can examine correlations using regression (Kaiser et al. 2013). Mesowear distributions can be visualized using histograms and box and whisker diagrams.
Statistical tests should only be used if their a priori assumptions are not violated. Many such tests assume that data points are statistically independent, and this is not necessarily the case for biological data; a trait common to two species can be the result of phylogeny (inheritance from a common ancestor) as well as ecology (adaptation to a particular lifestyle). Thus, strong phylogenetic signal in the species and traits being analyzed can confound a paleoecological study and lead to type I errors (“false positives”). The degree to which this impacts mesowear analyses is not entirely clear and likely depends on the particular data set being analyzed and the specific question(s) being asked. Nevertheless, assuming phylogeny is a potentially confounding factor is a conservative approach, and several studies have controlled for non-independence of data using techniques such as phylogenetic generalized least squares (PGLS; Kaiser et al. 2013; Fraser et al. 2014) and phylogenetically independent contrasts (PIC; Mihlbachler and Solounias 2006). Münkemiller et al. (2012) provide a useful review of other statistical techniques that can be used to test for phylogenetic signal in ecological data.
Microwear Data Analysis
Plots of mean scratch vs. mean pit counts among sampled taxa reveal broad dietary differences (grazer vs. leaf browser vs. fruit browser) using both LM and SEM techniques. Grazers tend to have high scratch but low pit counts, leaf browsers have low scratch and low or high pit counts (i.e., “dirty” browsers; Semprebon and Rivals 2007), and fruit browsers have moderate scratch and high pit counts. This relative difference in scratch vs. pit values (i.e., the “trophic triangle”) is consistently reproduced in stereoscopic and photomicrographic LM studies (e.g., Semprebon et al. 2004b; Mihlbachler et al. 2012; Christensen 2014). Although absolute values of scratch and pit counts vary among observers (see Microwear Limitations), relative differences will be consistent across observers (Mihlbachler et al. 2012). Thus, it may be more useful to look for statistically significant relative trends across a dataset rather than absolute “cut-offs” between dietary morphospaces (e.g., differentiating browsing from grazing ungulates based on number of scratches; Solounias and Semprebon 2002). Dimensionless variables (e.g., Microwear Index = number of scratches/number of pits; MacFadden et al. 1999) that rely on proportional rather than absolute similarities may also be useful (DeSantis et al. 2013). For SSFA variables in MTA, a significant negative relationship exists between complexity and anisotropy among mammalian herbivores (DeSantis et al. 2013), and these two variables are commonly plotted against one another to delineate dietary ecomorphospaces (Ungar et al. 2007; Donohue et al. 2013; Merceron et al. 2014; Shearer et al. 2015). For a more detailed review of how MTA can be used to study mammalian ecology, see Calandra and Merceron (2016) and DeSantis (2016).
Regardless of how data are collected, statistical tests are necessary to distinguish random noise from significant variation among species (Gordon 1988). Tests should be chosen based on: (1) the nature of the sample population; (2) the assumptions of the test; and (3) the hypotheses being tested. Univariate parametric tests (e.g., student’s t-test, ANOVA) can be used to compare species means, but should only be used if the assumptions of normality and equal variance are met. Otherwise, (as is common for microwear data; Scott et al. 2006), data should be transformed beforehand (Conover and Iman 1981), or non-parametric tests (e.g., Mann-Whitney U, Kruskal-Wallis test; Kruskal and Wallis 1952) can be applied (e.g., Calandra et al. 2012; Donohue et al. 2013; Purnell et al. 2013; Withnell and Ungar 2014). Multivariate analyses (PCA, DFA, and HCA) amenable to both continuous and discrete variables can provide a robust view of how LM, SEM, or MTA variables change statistically among taxa (e.g., Solounias and Semprebon 2002; Godfrey et al. 2004; Green et al. 2005; Townsend and Croft 2008b; Purnell et al. 2013; Gill et al. 2014; Green and Kalthoff 2015). As discussed above for mesowear, the presence of a strong phylogenetic signal in microwear data can be tested and controlled for using statistical techniques (e.g., Fraser and Theodor 2011). Independent researchers should be well-versed with statistical techniques or collaborate with statistically-knowledgeable colleagues to avoid potentially catastrophic mistakes (Vaux 2012).
Strengths
General Strengths
A major strength of mesowear and microwear as paleodietary proxies is that only well-preserved teeth are required. The need for relatively complete skeletal remains (e.g., skulls, articulated limbs, etc.) is often a major limiting factor for studies of extinct species using functional morphology (e.g., finite element analysis). Teeth are frequently preserved in the fossil record due to the extreme durability of enamel, and in the case of mammals, even isolated teeth usually preserve sufficient information for a generic or specific identification. This means that studies can be based on relatively large sample sizes (at least from a paleontological perspective), engendering greater confidence in interpretations and providing opportunities to examine patterns within and between populations. Both mesowear and microwear are independent of one another, and statistical power for reconstructing diet is higher when they are used in conjunction (Fraser and Theodor 2011). In contrast to isotopic studies (Higgins 2018), mesowear and microwear are non-destructive techniques.
A second major strength of mesowear and microwear analyses is that they appear to be relatively taxon-independent (see Andrews and Hixson 2014). In other words, since the wear being evaluated is due in large part to the particles consumed, similar ingesta are thought to produce similar features in different groups. Nevertheless, differences in enamel microstructure can influence the size and distribution of microwear features (Maas 1991), and a recent study of artiodactyls and perissodactyls concluded that similar diet does not necessarily result in similar microwear (Mihlbachler et al. 2016). Likewise, since mesowear was developed for ungulates with selenodont or lophodont teeth, extending the method to groups with other types of teeth and/or mastication styles has required developing new scoring methods (see below). Thus, scores are not directly comparable among all groups. The degree to which mesowear and microwear data are taxon-independent (i.e., affected by phylogeny) will undoubtedly be a topic of additional research in coming years. If such data are found to be highly correlated with clade membership, it may limit their utility for inferring diets in families and orders of mammals that have no extant representatives and/or that differ significantly in craniodental morphology from their modern relatives (e.g., Schulz et al. 2007; Croft and Weinstein 2008; Townsend and Croft 2008a; Green 2009a, b; Semprebon et al. 2011; Resar et al. 2013; Green and Kalthoff 2015).
Mesowear Strengths
In addition to the general strengths noted above, mesowear has the advantage of being relatively quick and easy to record on original fossil material. The ease of scoring varies to some degree with the specific method employed, but even more complex schemes only require a pair of calipers or a digital camera and laptop computer. As a result, specimens can be scored directly in museum collections, permitting large samples to be obtained for minimal expense (essentially only the cost of visiting the museum). Mesowear scores tend to be more consistent among different observers than microwear scores, and the technique can be learned relatively quickly (Loffredo and DeSantis 2014).
Microwear Strengths
Dental microwear data provide direct evidence of food and chewing mechanics on tooth surfaces if care is taken with specimen selection and preparation (Gordon 1982; Teaford 1988; Ungar et al. 2007). Microwear thus provides direct evidence of how teeth were actually used by an individual animal, not just what they are evolutionarily capable of doing (Teaford 2007). This proxy can provide detailed information on individual feeding behavior based on physical properties (abrasiveness) of ingesta, which can be used to determine variation in food preference within and among populations of a species (e.g., Ungar and Spencer 1999; Grine et al. 2006; Rivals and Solounias 2007; Merceron et al. 2010; Henton et al. 2014), as well as seasonal and chronologic (evolutionary) changes in feeding ecology at the species, clade, or paleocommunity-level (e.g., Merceron et al. 2010; Rivals et al. 2013; Merceron et al. 2014; Patnaik 2015). Hypotheses of the cause-effect relationship between food texture (size, shape, hardness) and dental biomechanics (tooth shape , tissue hardness, bite force, chewing mechanics) can be experimentally tested under controlled laboratory conditions (e.g., Gordon 1984; Teaford and Oyen 1989; Maas 1991; Lucas et al. 2013; Borrero-Lopex et al. 2015; Constantino et al. 2015; Hua et al. 2015; Xia et al. 2015), and ideally, in vivo in wild or captive animals (e.g., Teaford and Oyen 1989; Organ et al. 2006; Schulz et al. 2013; Baines et al. 2014; Hoffmann et al. 2015).
Traditionally, microwear analyses were limited to enamel surfaces, as it was assumed that orthodentine was too complex and heterogeneous to preserve regular, measureable microwear (Lucas 2005). However, multiple recent studies into orthodentine microwear (focused on xenarthrans, which lack enamel on adult tooth surfaces) reveal that scar patterns on this tissue can be statistically quantified (Oliveira 2001; Green 2009a, b; Green and Resar 2012; Haupt et al. 2013; Resar et al. 2013; Green and Kalthoff 2015; McAfee and Green 2015). Although orthodentine has less power to discriminate diet than enamel (Haupt et al. 2013), microwear still varies predictably among xenarthrans with distinct diets regardless of the technique applied (Green and Kalthoff 2015). Thus, microwear can now be used as an ecological proxy for species with enameless teeth (i.e., a presumed limitation is now a strength).
Limitations
General Limitations
The temporal scale of any dietary proxy must be taken into consideration when reconstructing feeding ecology. As noted previously, mesowear reflects an animal’s diet over months to years (but see Solounias et al. 2014 for a demonstration of more rapid changes in experimental animals). As a consequence, it cannot detect transitions in diet on shorter time scales, such as with the seasons (e.g., Rivals et al. 2013). However, mesowear can detect dietary transitions before such shifts are reflected in skeletal morphology (tooth shape , chewing mechanics, bite force potential), since changes in mesowear take place more rapidly than evolutionary changes in morphology (e.g., Mihlbachler et al. 2011). Microwear reflects diet on an even shorter time scale than mesowear, generally days to weeks. This can be useful for detecting seasonal changes in diet or dietary differences among extinct and extant populations (e.g., Rivals and Solounias 2007; Rivals et al. 2015), but it can also result in misrepresentations of typical diet if the population sampled is not sufficiently representative (Kay and Covert 1983). When comparing mesowear and microwear across major clades, statistical techniques that account for non-independence of data due to phylogeny should be used to limit the potential for type I errors. Carbon (δ13C ) and nitrogen (δ15N) isotope ratios preserved in fossilized bones and/or teeth can also provide dietary information (Higgins 2018). This geochemical information is recorded over an interval similar to mesowear (months to years) but reflects ecological conditions when the tissue was mineralizing (see Higgins 2018) rather than the last few months or years of life. Apparently conflicting dietary information from different proxies can often be resolved by considering the specific intervals that each reflects.
Mesowear Limitations
Perhaps the greatest limitation of mesowear analysis is that it only reflects the relative contributions of attrition (tooth-on-tooth wear) and abrasion (food-on-tooth wear) to tooth facet development. In other words, it gauges the toughness or abrasiveness of the food being consumed. The method was originally developed for relatively large-bodied herbivores (primarily folivores or leaf-eaters) with the primary goal of discriminating abrasion-dominated grazers and/or open habitat feeders from attrition-dominated browsers in forested habitats (as well as identifying varying types of mixed feeders ; Fortelius and Solounias 2000). This same grazing-browsing/open habitat-closed habitat axis has formed the basis for all subsequent mesowear analyses, even those focusing on non-ungulates (e.g., Butler et al. 2014; Ulbricht et al. 2015). Unlike microwear, it is unclear whether mesowear (relative attrition vs. abrasion) can be used to distinguish among other types of foods. The relative contributions of food (particularly grass) and habitat to mesowear development continue to be debated (e.g., Kaiser and Schulz 2006; Kaiser et al. 2013; Damuth and Janis 2014; Kubo and Yamada 2014).
Another primary limitation of mesowear is that it is scale-dependent; the cusps on the tooth of a large, browsing rhinoceros do not look the same as those of a small, browsing deer, even though each has teeth with high, pointed cusps compared to close relatives that have more abrasive diets . Thus, although the mesowear ruler of Mihlbachler et al. (2011) functions well for members of the horse family (Equidae), the focus of that study, the scale is less useful for species with much smaller or much larger teeth. This limitation can be overcome relatively easily for species that have modern relatives of similar size but is an impediment for extinct herbivores such as South American notoungulates that span four orders of magnitude but have no living representatives (Croft 1999; Croft and Weinstein 2008).
Until recently, it seemed that overall tooth structure and mastication style were a significant limitation for mesowear investigations. Several recent studies have demonstrated that the basic principles of paleodietary reconstruction based on attrition and abrasion can also be applied to groups as diverse as rodents , rabbits, marsupials , and elephants , and that it is possible to develop methods for mesowear analysis in these groups (Fraser and Theodor 2010; Butler et al. 2014; Saarinen et al. 2015; Ulbricht et al. 2015). Thus, tooth structure and mastication style may be a less significant limitation than previously thought.
Microwear Limitations
Teaford (2007) provides a thorough review of the theoretical limits of dental microwear; the highlights of Teaford’s discussion are outlined below. As mentioned previously, microwear is only as good as the specimen on which it is preserved. Once scars are altered or destroyed via post-mortem processes, they are lost forever. If all known specimens of a fossil species have been altered, then that animal’s diet must be determined using dietary proxies other than microwear. Even in well-preserved teeth, information about an animal’s feeding behavior can be invisible to microwear analysis if it does not scar the tooth surface, as can occur with very soft foods. Microwear in fossil populations is also limited by the fact that not all past environments and habitats still exist today, and that such environments may have had unknowable and untestable impacts on microwear. However, these limits will continue to be pushed as technologies used to image and analyze wear surfaces improve along with the ability to experimentally test the direct impacts of particles on tooth surfaces (e.g., Hua et al. 2015).
Dental microwear relies on the calibration of microwear patterns among extant animals (for which diet is known) to test hypotheses of paleodiet in fossil organisms (Teaford 1991). Unless in vivo experimental analyses are applied, microwear studies must rely on specimens in museum collections and generalized dietary information for the species (which may itself be scarce, such as for two-toed sloths ; Green and Resar 2012) rather than specific information about the foods consumed by a particular individual (Kay and Covert 1983). Lack of associated climate and environmental data can exacerbate this problem (Teaford 2007). Many mammals are opportunistic and change their diet seasonally and/or regionally (e.g., Bowyer et al. 1983; Heiduck 1997; Sidorovich 2000; Stokke and du Toit 2000). As a result, simply pooling individuals of a single species from different regions could produce a skewed microwear signal (Rivals et al. 2010). In both extant and paleontological studies, this can be minimized by only pooling individuals from limited spatial and temporal intervals (e.g., from the same geographic regions or geological formations).
A large sample of individuals is necessary to accurately estimate microwear signatures and identify normal departures from the mean at either the population or specific level (Gordon 1988). Acquiring a sufficient sample depends on not only the researcher’s ability to visit museum collections, but also the time required to analyze each tooth. The latter variable is directly correlated with the technique being applied. Stereoscopic LM is the most amenable to analyzing a large number of samples (in some cases, hundreds of teeth; Solounias and Semprebon 2002; Green 2009a) in the shortest amount of time (hours to days). Confocal MTA also permits analysis of large samples over short time frames, although not as quick as stereoscopic LM, as each specimen takes approximately 20–30 minutes to scan. Photomicrographic LM and SEM require the most time per specimen, as the position and instrumentation settings have to be adjusted manually to capture an appropriate image, and then each image has to be analyzed independently by the user. Additionally, SEM and MTA labs may charge for instrumentation use, whereas this is generally not the case for LM setups. The pros and cons of time required to conduct an analysis versus the ability of a technique to address the study’s goals should be considered when designing an analysis.
Arguably, the single largest source of error in microwear studies is consistent identification and measurement of discrete features, either through the lens or on images (LM and SEM; Grine et al. 2002; Semprebon et al. 2004b; Galbany et al. 2005; Mihlbachler et al. 2012; DeSantis et al. 2013). Error rates among users examining the same specimens can be high and statistically significant for all user-based techniques (1–21% [9% average] for SEM; 8–45% for light microscopy), with variation depending on user experience, the specific variable being identified, sample size, and number of iterations (Grine et al. 2002; Galbany et al. 2005; Mihlbachler et al. 2012). In general, a significant negative relationship exists between user experience with microwear feature identification and the magnitude of error (Galbany et al. 2005; Mihlbachler et al. 2012). Even though inter-observer error clearly impacts LM and SEM studies (Grine et al. 2002; Galbany et al. 2005; Mihlbachler et al. 2012; DeSantis et al. 2013), a wide variety of user-based microwear analyses have made and continue to make important contributions toward elucidating feeding ecology (e.g., Walker et al. 1978; Gordon 1982; Teaford and Walker 1984; Teaford and Oyen 1989; Strait 1993; Solounias and Semprebon 2002; Purnell et al. 2006; Fraser et al. 2009; Green 2009a; Green and Resar 2012; Resar et al. 2013; Christensen 2014; Strait 2014; Hoffmann et al. 2015). Microwear studies using MTA provide the solution to the problem of significant user error but are limited by the availability of confocal microscopes , which are available to fewer researchers than pre-existing SEMs and stereomicroscopes . Until more researchers have access to such technologies, studies using user-based techniques should follow strict protocols to minimize noise from observer error. For example, researchers should count their own features on each image or cast that is analyzed (Galbany et al. 2005; Mihlbachler et al. 2012) rather than comparing their values to those of others, such as those compiled for comparative microwear databases (e.g., Green et al. 2005; Rivals et al. 2010, 2012). Such practices are becoming more common among user-based studies (e.g., Green 2009a, b; Green and Resar 2012; Christensen 2014; Hoffmann et al. 2015). Although is not possible to retroactively test for the effects of inter-observer error in past LM studies (Mihlbachler et al. 2012), there is no direct evidence that the fundamental conclusions of such studies were invalid. Indeed, most independent studies found similar, predictable microwear trends among species with distinct diets (Green et al. 2005; Townsend and Croft 2008b; Rivals et al. 2010, 2012).
Observers should also always count under blind conditions, with specimen, taxonomic, and dietary information invisible, in one sitting (see Green and Resar 2012 and Mihlbachler et al. 2012 for examples of how to set up such analyses). This ensures that a microwear analysis is not a “self-fulfilling prophecy” (Mihlbachler et al. 2012).
Examples of Applications
Mesowear and microwear have been used in a wide variety of applications, and no single study can stand as an exemplar of their many uses in paleoecology . Therefore, we briefly summarize below recent representative publications that together provide examples of the techniques and approaches discussed in this chapter.
Mesowear
-
1.
Mesowear and hypsodonty over time: Mihlbachler, M. C., Rivals, F., Solounias, N., & Semprebon, G. M. (2011). Dietary change and evolution of horses in North America. Science, 331, 1178–1181. Mihlbachler et al. integrate mesowear data (mesowear II) with crown height (hypsodonty) and body size data (as inferred from tooth size) to examine how dietary abrasiveness in horses (family Equidae) changed over a period of some 55 million years. They find a strong correlation between hypsodonty and dietary abrasiveness and were able to correlate both with changes in climate and vegetation. They use the amount of variation in mesowear scores as an indicator of the relative strength of selective pressure, with high variation corresponding to low selective pressure, and note several instances during which particular horse populations were apparently under greatest selective pressure for increased hypsodonty. This study is an example of how mesowear can be used to examine ecological changes in a single group over an extended interval.
-
2.
Combined mesowear and stereoscopic LM: Sánchez-Hernández, C., Rivals, F., Blasco, R., & Rosell, J. (2016). Tale of two timescales: combining tooth wear methods with different temporal resolutions to detect seasonality of Paleolithic hominin occupational patterns. Journal of Archaeological Research: Reports, https://doi.org/10.1016/j.jasrep.2015.09.0114. Sánchez-Hernández et al. collect mesowear (mesowear II) and microwear data from archaeological cave site samples of red deer (Cervus elaphus; a mixed feeder ) and horse (Equus ferus; a grazer). They find E. ferus mesowear and microwear to be consistent with one another and invariant among caves and stratigraphic intervals, as would be expected for a committed grazer . By contrast, they find discrepancies between microwear and mesowear data for C. elaphus, as well as stratigraphic variation in microwear (but not mesowear) scores. They interprete these differences as reflecting the differing time scales over which mesowear and microwear form, with mesowear scores reflecting a year-long average diet and microwear scores recording diet during a particular season. This paper is an example of how combining microwear and mesowear data can provide greater insight than analyzing either one separately.
-
3.
Combined mesowear and MTA: Stynder, D. D. (2011). Fossil bovid diets indicate a scarcity of grass in the Langebaanweg E Quarry (South Africa) late Miocene/early Pliocene environment. Paleobiology, 37, 126–139. Stynder collects mesowear data (mesowear I) from fossil horses (family Equidae) and compares these data with microwear data from two previous studies (one using traditional microwear, the other using MTA). He interprets most species as browsers based on mesowear scores and infers that previous interpretations of some of these species as grazers based on microwear (using traditional techniques but not MTA) could be due to their feeding on less abrasive C3 (as opposed to C4) grasses during a specific time of year. Based on the diets of these horses, he reconstructs South Africa’s southwest coast as being heavily wooded about 5 million years ago. This study provides an example how mesowear and microwear data can be combined to infer vegetational structure.
In Vivo Microwear in Laboratory Animals
-
1.
SEM: Baines, D. C., Purnell, M. A., & Hart, P. J. B. (2014). Tooth microwear formation rate in Gasterosteus aculeatus. Journal of Fish Biology, 84, 1582–1589. Baines and colleagues undertake a controlled feeding study in a population of tank-reared three-spined stickleback. Prior to the analysis, all fish had an exclusive limnetic feeding regime and were fed bloodworms in the water column in a tank with no abrasive sediment on the bottom. At the start of the study, a group of fish were transferred to a benthic feeding regime, where they fed exclusively on bloodworms on the tank bottom with quartz sediment substrate. On four consecutive days, four fish were sacrificed per day for study. Microwear feature density (scratch and pit counts) increased significantly from day 0 (control) to day 4 within the study group. These results reveal that: (1) microwear turnover rate is high even in aquatic vertebrates with non-occluding teeth; and (2) an abrasive substrate has a significant influence on microwear formation.
-
2.
Photomicrographic LM: Hoffman, J. M., Fraser, D., & Clementz, M. T. (2015). Controlled feeding trials with ungulates : a new application of in vivo dental molding to assess the abrasive factors of microwear. The Journal of Experimental Biology, 218, 1538–1547. Hoffmann and colleagues use a novel live-animal sampling technique to test the influence of ingested abrasives on ungulate tooth microwear. Over a one-month period, four pen-raised sheep were fed three dietary regimes: (1) a control diet of clean hay; (2) hay with fine sand; and (3) hay with coarse sand. Sheep were anesthetized and microwear molds taken between feeding treatments. Under blind, randomized conditions, two independent observers consistently found that pit counts were positively correlated with an increase in abrasive size. This study was the first to use ungulates in a repeatable, controlled feeding experiment and to study the effect of different-sized abrasive particles on microwear formation.
-
3.
MTA: Schulz, E., Piotrowski, V., Clauss, M., Mau, M., Merceron, G., & Kaiser, T. M. (2013). Dietary abrasiveness is associated with variability of microwear and dental surface texture in rabbits. PLoS ONE, 8, e56167. Schulz and colleagues measure the effect of silica content on microwear formation in four groups of laboratory rabbits. For 25 weeks, each group was fed a different controlled diet , including lucerne (alfalfa), lucerne plus crushed oats, grass meal with crushed oats, and grass meal. The lucerne group had the lowest silica content and was correlated with higher variability in microwear texture parameters, whereas abrasive diets (grass) had more uniform wear patterns. This suggests that variation in a species’ microwear texture signature likely reflect changes in abrasive content of consumed food.
Reconstructing Diet Using Microwear
-
1.
SEM: Resar, N. A., Green, J. L., & McAfee, R. K. (2013). Reconstructing paleodiet in ground sloths (Mammalia, Xenarthra) using dental microwear analysis. Kirtlandia, 58, 61–72. Resar and colleagues compare orthodentine microwear patterns in three extinct ground sloths with those of extant two-toed and three-toed sloths. Each fossil species was sampled from a single locality, representing one population. Under blind, randomized conditions, two independent observers found that living and extinct forest-dwelling sloths had a low number of predominately coarse scratches, whereas the more open-habitat sloth, Thinobadistes, had unusually high numbers of fine scratches. This result supports the efficacy of orthodentine microwear as a proxy for paleoecology and suggests a possible environmental influence in sloth microwear.
-
2.
Stereoscopic LM: Christensen, H. B. (2014). Similar associations of tooth microwear and morphology indicate similar diet across marsupial and placental mammals. PLoS ONE, 9, e102789. Using a large sample of 153 extant mammal species, Christensen combines LM with tooth morphology and body mass to produce an accurate method to predict diet in a species with unknown ecology. The author collected all LM data independently rather than relying on comparative data collected by others. The “trophic triangle” (where grazers, browsers , and hard object feeders vary in scratch and pit counts) was recovered, consistent with independent LM studies. This study demonstrates that microwear data used in conjunction with other variables will more accurately predict ecology than microwear data alone.
-
3.
Photomicrographic LM: Fraser, D., Mallon, J. C., Furr, R., & Theodor, J. M. (2009). Improving the repeatability of low magnification microwear methods using high dynamic range imaging. PALAIOS, 24, 818–825. Fraser and colleagues introduce a robust method of LM using high dynamic range imaging to produce high-resolution photomicrographs that can be scored manually. All ranges of scratch and pit categories are visible on a single image, which eliminates the need to manually adjust the light angle, as in stereoscopic LM. Using these images, two independent researchers were able to consistently and correctly classify exant species by diet . This method improves the repeatability of LM studies.
-
4.
MTA: Donohue, S. L., DeSantis, L. R. G., Schubert, B. W., & Ungar, P. S. (2013). Was the Giant short-faced bear a hyper-scavenger? A new approach to the dietary study of ursids using dental microwear textures. PLoS ONE, 8, e77531. Donohue and colleagues compare the microwear textures of the extinct Arctodus simus (whose diet has been strongly debated) with that of five extant bear species. They found that high anisotropy is characteristic of leaf-eating (folivory) in living bears, whereas high complexity is more correlated with feeding on hard objects, such as bone or fruit. Arctodus simus had a microwear texture inconsistent with hard-object feeding and therefore did not consume large amounts of bone (i.e., it was not a hyper-scavenger), falsifying at least one paleodietary hypothesis for this extinct species. This study highlights the ability of MTA to reconstruct paleodiet in extinct species via comparisons with living relatives.
Future Prospects
Several recently published studies have highlighted areas in which mesowear research will likely advance over the next decade. These include extending mesowear analysis to non-ungulate groups of mammals (Butler et al. 2014; Saarinen et al. 2015; Ulbricht et al. 2015) and examining more detailed aspects of tooth morphology to provide additional data on abrasion and attrition (Solounias et al. 2014; Danowitz et al. 2016). Additionally, controlled studies of the relationship between diet and mesowear (Solounias et al. 2014) are vital for understanding the processes underlying observed patterns. Since mesowear is still a relatively new method, it is likely to be used with increasing frequency to study the diets of extinct species.
One area of mesowear analysis that seems ripe for advancement is automated scoring of teeth. Since mesowear I and II (“outer mesowear”) analyses focus on the buccal profile of a tooth, it should be possible to create an automated computer program for extracting a simple shape from a photograph and calculating relevant parameters (heights, angles, etc.). This could eliminate all subjectivity in scoring mesowear and permit more refined statistical tests (and perhaps shape-based analyses; see Curran 2018). On the other hand, since ease of data collection is a major strength of mesowear analysis, the benefits of a more sophisticated approach over traditional methods would have to be clearly demonstrated before justifying wider adoption.
In response to the issue of subjectivity in data collection of LM and SEM, MTA was developed as a fully automated system that was free from observer error (Ungar et al. 2003; Scott et al. 2005). However, 10 years later, revisions to the automated procedure (e.g., incorporating ISO data; Purnell et al. 2012) have now been applied, and improvements to instrumentation parameters are still being made (Arman et al. 2016). The more traditional LM and SEM techniques are still used, but their methodological limits are better defined, and this has improved the objectivity of their conclusions. There is every reason to believe that further advances in LM, SEM, and texture analyses will be forthcoming, although more changes are likely for the latter than the two former methods. The greatest promise involves improvements to three-dimensional modeling and automated reconstruction of tooth surfaces, which will directly improve the level of precision and accuracy of microwear and mesowear data.
One major element that has been traditionally lacking is in vivo studies of microwear formation. These studies are truly the source of the majority of our knowledge of how microwear forms as a result of food texture. Fortunately, recent attempts to record microwear in laboratory animals (Schulz et al. 2013; Baines et al. 2014; Hoffmann et al. 2015) have significantly improved our knowledge base. As protocols for such experimental research become better defined, it will be increasingly possible to acquire permits and permissions to analyze microwear in vivo in both captive and wild animals. This, in turn, will rapidly refine our interpretations of fossil microwear data (Ungar 2015). Combined with technological advances, future investigators will be better equipped to answer important questions that drive current research. How does the abrasiveness of ingested particles directly influence microwear formation (Hoffmann et al. 2015)? Can microwear features of specific sizes and/or shapes be directly correlated with specific particles (Constantino et al. 2015)? How much of an animal’s microwear signature is influenced by its ingested diet and how much by its external surroundings (Xia et al. 2015)? Future studies that apply a combined approach of mesowear and microwear analyses to the same samples will provide robust data on dental wear as it relates to ecology (Sánchez-Hernández et al. 2016). True understanding of the complexity of tooth wear as a natural process is still a speck on the scientific horizon, but every study inches us closer to the destination.
References
Andrews, P., & Hixson, S. (2014). Taxon-free methods of palaeoecology. Annales Zoologici Fennici, 51, 269–284.
Arman, S. D., Ungar, P. S., Brown, C. A., DeSantis, L. R. G., Schmidt, C., & Prideaux, G. J. (2016). Minimizing inter-microscope variability in dental microwear texture analysis. Surface Topography: Metrology and Properties, 4, 024007.
Baines, D. C., Purnell, M. A., & Hart, P. J. B. (2014). Tooth microwear formation rate in Gaterosteus aculeatus. Journal of Fish Biology, 84, 1582–1589.
Borrero-Lopez, O., Pajares, A., Constantino, P. J., & Lawn, B. R. (2015). Mechanics of microwear traces in tooth enamel. Acta Biomaterialia, 14, 146–153.
Bowyer, R. T., McKenna, S. A., & Shea, M. E. (1983). Seasonal changes in coyote food habits as determined by fecal analysis. The American Midland Naturalist, 109, 266–273.
Butler, P. M. (1952). The milk molars of Perissodactyla, with remarks on molar occlusion. Proceedings of the Zoological Society of London, 121, 777–817.
Butler, K., Louys, J., & Travouillon, K. (2014). Extending dental mesowear analyses to Australian marsupials, with applications to six Plio-Pleistocene kangaroos from southeast Queensland. Palaeogeography, Palaeoclimatology, Palaeoecology, 408, 11–25.
Calandra, I., & Merceron, G. (2016). Dental microwear texture analysis in mammalian ecology. Mammal Review, 46, 215–228.
Calandra, I., Schulz, E., Pinnow, M., Krohn, S., & Kaiser, T. M. (2012). Teasing apart the contributions of hard dietary items on 3D dental microtextures in primates. Journal of Human Evolution, 63, 85–98.
Christensen, H. B. (2014). Similar associations of tooth microwear and morphology indicate similar diet across marsupial and placental mammals. PLoS ONE, 9, e102789.
Conover, W. J., & Iman, R. L. (1981). Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician, 35, 124–129.
Constantino, P. J., Borrero-Lopez, O., Pajares, A., & Lawn, B. R. (2015). Simulation of enamel wear for reconstruction of diet and feeding behavior in fossil mammals: a micromechanics approach. BioEssays, 38, 89–99.
Coombs, M., & Semprebon, G. (2005). The diet of chalicotheres (Mammalia, Perissodactyla) as indicated by low magnification stereoscopic microwear analysis. Journal of Vertebrate Paleontology, 25, 47A.
Croft, D. A. (1999). Placentals: South American ungulates. In R. Singer (Ed.), Encyclopedia of Paleontology (pp. 890–906). Chicago: Fitzroy-Dearborn Publishers.
Croft, D. A., & Weinstein, D. (2008). The first application of the mesowear method to endemic South American ungulates (Notoungulata). Palaeogeography, Palaeoclimatology, Palaeoecology, 269, 103–114.
Curran, S. C. (2018). Three-dimensional geometric morphometrics in paleoecology. In D. A. Croft, D. F. Su & S. W. Simpson (Eds.), Methods in paleoecology: Reconstructing Cenozoic terrestrial environments and ecological communities (pp. 317–335). Cham: Springer.
Damuth, J., & Janis, C. M. (2014). A comparison of observed molar wear rates in extant herbivorous mammals. Annales Zoologici Fennici, 51, 188–200.
Danowitz, M., Hou, S., Mihlbachler, M., Hastings, V., & Solounias, N. (2016). A combined-mesowear analysis of late Miocene giraffids from North Chinese and Greek localities of the Pikermian Biome. Palaeogeography, Palaeoclimatology, Palaeoecology, 449, 194–204.
DeSantis, L. R. G. (2016). Dental microwear textures: reconstructing diets of fossil mammals. Surface Topography: Metrology and Properties, 4, 023002.
DeSantis, L. R. G., Scott, J. R., Schubert, B. W., Donohue, S. L., McCray, B. M., Van Stolk, C. A., et al. (2013). Direct comparisons of 2D and 3D dental microwear proxies in extant herbivorous and carnivorous mammals. PLoS ONE, 8, e71428.
Donohue, S. L., DeSantis, L. R. G., Schubert, B. W., & Ungar, P. S. (2013). Was the Giant short-faced bear a hyper-scavenger? A new approach to the dietary study of ursids using dental microwear textures. PLoS ONE, 8, e77531.
Erickson, K. L. (2014). Prairie grass phytolith hardness and the evolution of ungulate hypsodonty. Historical Biology: An International Journal of Paleobiology, 26, 737–744.
Estebaranz, F., Galbany, J., Martínez, L. M., & Pérez-Pérez, A. (2007). 3-D interferometric microscopy applied to the study of buccal enamel microwear. In S. E. Bailey & J.-J. Hublin (Eds.), Dental Perspectives on Human Evolution (pp. 391–403). New York: Springer.
Fortelius, M., & Solounias, N. (2000). Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. American Museum Novitates, 3301, 1–36.
Franz-Odendaal, T. A., Kaiser, T. M., & Bernor, R. L. (2003). Systematics and dietary evaluation of a fossil equid from South Africa. South African Journal of Science, 99, 453–459.
Fraser, D., & Theodor, J. M. (2010). The use of gross dental wear in dietary studies of extinct lagomorphs. Journal of Paleontology, 84, 720–729.
Fraser, D., & Theodor, J. M. (2011). Comparing ungulate dietary proxies using discriminant function analysis. Journal of Morphology, 272, 1513–1526.
Fraser, D., Mallon, J. C., Furr, R., & Theodor, J. M. (2009). Improving the repeatability of low magnification microwear methods using high dynamic range imaging. PALAIOS, 24, 818–825.
Fraser, D., Zybutz, T., Lightner, E., & Theodor, J. M. (2014). Ruminant mandibular tooth mesowear: a new scheme for increasing paleoecological sample sizes. Journal of Zoology, 294, 41–48.
Galbany, J., Martinez, L. M., & Perez-Perez, A. (2004). Tooth replication techniques, SEM imaging and microwear analysis in primates: methodological obstacles. Anthropologie, 42, 5–12.
Galbany, J., Martínez, L. M., López-Amor, H. M., Espurz, V., Hiraldo, O., Romero, A., et al. (2005). Error rates in buccal-dental microwear quantification using scanning electron microscopy. Scanning, 27, 23–29.
Galbany, J., Estebaranz, F., Martínez, L. M., Romera, A., De Juan, J., Turbón, D., et al. (2006). Comparative analysis of dental enamel polyvinylsiloxane impression and polyurethane casting methods for SEM research. Microscopy Research and Technique, 69, 246–252.
Gill, P. G., Purnell, M. A., Crumpton, N., Brown, K. R., Gostling, N. J., Stampanoni, M., et al. (2014). Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature, 512, 303–305.
Godfrey, L. R., Semprebon, G. M., Jungers, W. L., Sutherland, M. L., Simons, E. L., & Solounias, N. (2004). Dental use wear in extinct lemurs: evidence of diet and niche differentiation. Journal of Human Evolution, 47, 145–169.
Goodall, R. H., Darras, L. P., & Purnell, M. A. (2015). Accuracy and precision of silicon based impression media for quantitative areal texture analysis. Scientific Reports, 5, 10800.
Gordon, K. D. (1982). A study of microwear on chimpanzee molars: implications for dental microwear analysis. American Journal of Physical Anthropology, 59, 195–215.
Gordon, K. D. (1984a). The assessment of jaw movement direction from dental microwear. American Journal of Physical Anthropology, 63, 77–84.
Gordon, K. D. (1984b). Hominoid dental microwear: complications in the use of microwear analysis to detect diet. Journal of Dental Research, 63, 1043–1046.
Gordon, K. D. (1988). A review of methodology and quantification in dental microwear analysis. Scanning Microscopy, 2, 1139–1147.
Green, J. L. (2009a). Dental microwear in the orthodentine of the Xenarthra (Mammalia) and its use in reconstructing the palaeodiet of extinct taxa: the case study of Nothrotheriops shastensis (Xenarthra, Tardigrada, Nothrotheriidae). Zoological Journal of the Linnean Society, 156, 201–222.
Green, J. L. (2009b). Intertooth variation of orthodentine microwear in armadillos (Cingulata) and tree sloths (Pilosa). Journal of Mammalogy, 90, 768–778.
Green, J. L., & Kalthoff, D. C. (2015). Xenarthran dental microstructure and dental microwear analyses, with new data for Megatherium americanum (Megatheriidae). Journal of Mammalogy, 96, 645–657.
Green, J. L., & Resar, N. A. (2012). The link between dental microwear and feeding ecology in tree sloths and armadillos (Mammalia: Xenarthra). Biological Journal of the Linnean Society, 107, 277–294.
Green, J. L., Semprebon, G. M., & Solounias, N. (2005). Reconstructing the palaeodiet of Florida Mammut americanum via low-magnification stereomicroscopy. Palaeogeography, Palaeoclimatology, Palaeoecology, 223, 34–48.
Grine, F. E. (1986). Dental evidence for dietary differences in Australopithecus and Paranthropus: a quantitative analysis of permanent molar microwear. Journal of Human Evolution, 15, 783–822.
Grine, F. E., Ungar, P. S., & Teaford, M. F. (2002). Error rates in dental microwear quantification using scanning electron microscopy. Scanning, 24, 144–153.
Grine, F. E., Ungar, P. S., Teaford, M. F., & El-Zaatari, S. (2006). Molar microwear in Praeanthropus afarensis: evidence for dietary stasis through time and under diverse paleoecological conditions. Journal of Human Evolution, 51, 297–319.
Haupt, R. J., DeSantis, L. R. G., Green, J. L., & Ungar, P. S. (2013). Dental microwear texture as a proxy for diet in xenarthrans. Journal of Mammalogy, 94, 856–866.
Heiduck, S. (1997). Food choice in Masked titi monkeys (Callicebus personatus melanochir): selectivity or opportunism? International Journal of Primatology, 18, 487–502.
Henton, E., MCorriston, J., Martin, L., & Oches, E.A. (2014). Seasonal aggregation and ritual slaughter: isotopic and dental microwear for cattle herder mobility in the Arabian Neolithic. Journal of Anthropological Archaeology, 33, 119–131.
Higgins, P. (2018). Isotope ecology from biominerals. In D. A. Croft, D. F. Su & S. W. Simpson (Eds.), Methods in paleoecology: Reconstructing Cenozoic terrestrial environments and ecological communities (pp. 99–120). Cham: Springer.
Hoffman, J. M., Fraser, D., & Clementz, M. T. (2015). Controlled feeding trials with ungulates: a new application of in vivo dental molding to assess the abrasive factors of microwear. The Journal of Experimental Biology, 218, 1538–1547.
Hua, L., Brandt, E. T., Meullenet, J., Zhou, Z., & Ungar, P. S. (2015). Technical note: an in vitro study of dental microwear formation using the BITE Master II chewing machine. American Journal of Physical Anthropology, 158, 769–775.
Kaiser, T. M., & Fortelius, M. (2003). Differential mesowear in occluding upper and lower molars: opening mesowear analysis for lower molars and premolars in hypsodont horses. Journal of Morphology, 258, 67–83.
Kaiser, T. M., & Schulz, E. (2006). Tooth wear gradients in zebras as an environmental proxy – a pilot study. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 103, 187–210.
Kaiser, T. M., & Solounias, N. (2003). Extending the tooth mesowear method to extinct and extant equids. Geodiversitas, 25, 321–345.
Kaiser, T. M., Brasch, J., Castell, J. C., Schulz, E., & Clauss, M. (2009). Tooth wear in captive wild ruminant species differs from that of free-ranging conspecifics. Mammalian Biology – Zeitschrift für Säugetierkunde, 74, 425–437.
Kaiser, T. M., Müller, D. W. H., Fortelius, M., Schulz, E., Codron, D., & Clauss, M. (2013). Hypsodonty and tooth facet development in relation to diet and habitat in herbivorous ungulates: implications for understanding tooth wear. Mammal Review, 43, 34–46.
Kay, R. F., & Covert, H. H. (1983). True grit: a microwear experiment. American Journal of Physical Anthropology, 61, 33–38.
King, T., Andrews, P., & Boz, B. (1999). Effect of taphonomic processes on dental microwear. American Journal of Physical Anthropology, 108, 359–373.
Krueger, K. L., Scott, J. R., Kay, R. F., & Ungar, P. S. (2008). Technical note: dental microwear textures of “Phase I” and “Phase II” facets. American Journal of Physical Anthropology, 137, 485–490.
Kruskal, W. H., & Wallis, W. A. (1952). Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association, 47, 583–621.
Kubo, M. O., & Yamada, E. (2014). The inter-relationship between dietary and environmental properties and tooth wear: comparisons of mesowear, molar wear rate, and hypsodonty index of extant sika deer populations. PLoS ONE, 9, e90745.
Loffredo, L. F., & DeSantis, L. R. G. (2014). Cautionary lessons from assessing dental mesowear observer variability and integrating paleoecological proxies of an extreme generalist Cormohipparion emsliei. Palaeogeography, Palaeoclimatology, Palaeoecology, 395, 42–52.
Lucas, P. W. (2005). Dental functional morphology: How teeth work. New York: Cambridge University Press.
Lucas, P. W., Omar, R., Al-Fadhalah, K., Almusallam, A. S., Henry, A. G., Michael, S., et al. (2013). Mechanisms and causes of wear in tooth enamel: implications for hominin diets. Journal of the Royal Society Interface, 10, 20120923.
Maas, M. C. (1991). Enamel structure and microwear: an experimental study of the response of enamel to shearing force. American Journal of Physical Anthropology, 85, 31–49.
MacFadden, B. J., Solounias, N., & Cerling, T. E. (1999). Ancient diets, ecology, and extinction in 5-million-year-old horses from Florida. Science, 283, 824–827.
Martínez, L. M., & Pérez-Pérez, A. (2004). Post-mortem wear as an indicator of taphonomic processes affecting enamel surfaces on hominin teeth from Laetoli and Olduvai (Tanzania): implications to dietary interpretations. Anthropologie, 42, 37–42.
McAfee, R. K., & Green, J. L. (2015). The role of bite force in the formation of orthodentine microwear in tree sloths (Mammalia: Xenarthra: Folivora): implications for feeding ecology. Archives of Oral Biology, 60, 181–192.
Merceron, G., Blondel, C., De Bonis, L., Koufos, G. D., & Viriot, L. (2005). A new method of dental microwear analysis: application to extant primates and Ouranopithecus macedoniensis (Late Miocene of Greece). PALAIOS, 20, 551–561.
Merceron, G., Escarguel, G., Angibault, J., & Verheyden-Tixier, H. (2010). Can dental microwear textures record inter-individual dietary variations? PLoS ONE, 5, e9542.
Merceron, G., Hofman-Kamińska, E., & Kowalczyk. (2014). 3D dental microwear texture analysis of feeding habits of sympatric ruminants in the Bialowiża Primeval Forest, Poland. Forest Ecology and Management, 328, 262–269.
Mihlbachler, M. C., & Beatty, B. L. (2012). Magnification and resolution in dental microwear analysis using light microscopy. Palaeontologia Electronica, 15, 25A.
Mihlbachler, M. C., & Solounias, N. (2006). Coevolution of tooth crown height and diet in Oreodonts (Merycoidodontidae, Artiodactyla) examined with phylogenetically independent contrasts. Journal of Mammalian Evolution, 13, 11–36.
Mihlbachler, M. C., Rivals, F., Solounias, N., & Semprebon, G. M. (2011). Dietary change and evolution of horses in North America. Science, 331, 1178–1181.
Mihlbachler, M. C., Beatty, B. L., Caldera-Siu, A., Chan, D., & Lee, R. (2012). Error rates and observer bias in dental microwear analysis using light microscopy. Palaeontologia Electronica, 15, 12A.
Mihlbachler, M. C., Campbell, D., Ayoub, M., Chen, C., & Ghani, I. (2016). Comparative dental microwear of ruminant and perissodactyl molars: implications for paleodietary analysis of rare and extinct ungulate clades. Paleobiology, 42, 98–116.
Mills, J. R. E. (1955). Ideal dental occlusion in primates. Dental Practice, 6, 47–61.
Münkemüller, T., Lavergne, S., Bzeznik, B., Dray, S., Jombart, T., Schiffers, K., et al. (2012). How to measure and test phylogenetic signal. Methods in Ecology and Evolution, 3, 743–756.
Nelson, S., Badgley, C., & Zakem, E. (2005). Microwear in modern squirrels in relation to diet. Palaeontologia Electronica, 8, 14A.
Oliveira, E. V. (2001). Micro-desgaste dentario em alguns Dasypodidae (Mammalia, Xenarthra). Acta Biologica Leopoldensia, 23, 83–91.
Organ, J. M., Ruff, C. B., Teaford, M. F., & Nisbett, R. A. (2006). Do mandibular cross-sectional properties and dental microwear give similar dietary signals? American Journal of Physical Anthropology, 130, 501–507.
Patnaik, R. (2015). Diet and habitat changes among Siwalik herbivorous mammals in response to Neogene and Quaternary climate changes: an appraisal in the light of new data. Quaternary International, 371, 232–243.
Purnell, M. A., Crumpton, N., Gill, P. G., Jones, G., & Rayfield, E. J. (2013). Within-guild dietary discrimination from 3-D textural analysis of tooth microwear in insectivorous mammals. Journal of Zoology, 291, 249–257.
Purnell, M. A., Hart, P. J. B., Baines, D. C., & Bell, M. A. (2006). Quantitative analysis of dental microwear in threespine stickleback: a new approach to analysis of trophic ecology in aquatic vertebrates. Journal of Animal Ecology, 75, 967–977.
Purnell, M., Seehausen, O., & Galis, F. (2012). Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. Journal of the Royal Society Interface, 9, 2225–2233.
Rabenold, D., & Pearson, O. M. (2014). Scratching the surface: a critique of Lucas et al. (2013)’s conclusion that phytoliths don’t abrade enamel. Journal of Human Evolution, 74, 130–133.
Rensberger, J. M. (1978). Scanning electron microscopy, of wear and occlusal event in some small herbivores. In P. M. Butler & K. A. Joysey (Eds.), Development, function, and evolution of teeth (pp. 415–438). New York: Academic Press.
Resar, N. A., Green, J. L., & McAfee, R. K. (2013). Reconstructing paleodiet in ground sloths (Mammalia, Xenarthra) using dental microwear analysis. Kirtlandia, 58, 61–72.
Rivals, F., & Semprebon, G. M. (2006). A comparison of the dietary habits of a large sample of the Pleistocene pronghorn Stockoceros onusrosagris from the Papago Springs Cave in Arizona to the modern Antilocapra americana. Journal of Vertebrate Paleontology, 26, 495–500.
Rivals, F., & Solounias, N. (2007). Differences in tooth microwear in populations of caribou (Rangifer tarandus, Ruminatia, Mammalia) and implications to ecology, migration, glaciations and dental evolution. Journal of Mammalian Evolution, 14, 182–192.
Rivals, F., Mihlbachler, M. C., & Solounias, N. (2007). Effect of ontogenetic-age distribution in fossil and modern samples on the interpretation of ungulate paleodiets using the mesowear method. Journal of Vertebrate Paleontology, 27, 763–767.
Rivals, F., Semprebon, G., & Lister, A. (2012). An examination of dietary diversity patterns in Pleistocene proboscideans (Mammuthus, Palaeoloxodon, and Mammut) from Europe and North America as revealed by dental microwear. Quaternary International, 255, 188–195.
Rivals, F., Rindel, D., & Belardi, J. B. (2013). Dietary ecology of extant guanaco (Lama guanicoe) from southern Patagonia: seasonal leaf browsing and its archaeological implications. Journal of Archaeological Science, 40, 2971–2980.
Rivals, F., Prignano, L., Semprebon, G. M., & Lozano, S. (2015). A tool for determining duration of mortality events in archaeological assemblages using extant ungulate microwear. Scientific Reports, 5, 17330.
Rivals, F., Mihlbachler, M. C., Solounias, N., Mol, D., Semprebon, G. M., de Vos, J., et al. (2010). Palaeoecology of the Mammoth Steppe fauna from the late Pleistocene of the North Sea and Alaska: separating species preferences from geographic influence in paleoecological dental wear analysis. Palaeogeography, Palaeoclimatology, Palaeoecology, 286, 42–54.
Rose, J. J. (1983). A replication technique for scanning electron microscopy: applications for anthropologists. American Journal of Physical Anthropology, 62, 255–261.
Saarinen, J., Karme, A., Cerling, T., Uno, K., Säilä, L., Kasiki, S. et al. (2015). A new tooth wear-based dietary analysis method for Proboscidea (Mammalia). Journal of Vertebrate Paleontology, e918546.
Sánchez-Hernández, C., Rivals, F., Blasco, R., & Rosell, J. (2016). Tale of two timescales: combining tooth wear methods with different temporal resolutions to detect seasonality of Paleolithic hominin occupational patterns. Journal of Archaeological Research: Reports, 6, 790–797.
Schulz, E., Calandra, I., & Kaiser, T. M. (2010). Applying tribology to teeth of hoofed mammals. Scanning, 32, 162–182.
Schulz, E., Fahlke, J. M., Merceron, G., & Kaiser, T. M. (2007). Feeding ecology of the Chalicotheriidae (Mammalia, Perissodactyla, Ancylopoda). Results from dental micro- and mesowear analyses. Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg, 43, 5–31.
Schulz, E., Piotrowski, V., Clauss, M., Mau, M., Merceron, G., & Kaiser, T. M. (2013). Dietary abrasiveness is associated with variability of microwear and dental surface texture in rabbits. PLoS ONE, 8, e56167.
Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Grine, F. E., Teaford, M. F., et al. (2005). Dental microwear texture analysis reflects diets of living primates and fossil hominins. Nature, 436, 693–695.
Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Childs, B. E., Teaford, M. F., et al. (2006). Dental microwear texture analysis: technical considerations. Journal of Human Evolution, 51, 339–349.
Semprebon, G., & Rivals, F. (2007). Was grass more prevalent in the pronghorn past? An assessment of the dietary adaptations of Miocene and Recent Antilocapridae (Mammalia: Artiodactyla). Palaeogeography, Palaeoclimatology, Palaeoecology, 253, 332–347.
Semprebon, G., Janis, C., & Solounias, N. (2004a). The diets of the Dromomerycidae (Mammalia: Artiodactyla) and their response to Miocene vegetational change. Journal of Vertebrate Paleontology, 24, 427–444.
Semprebon, G., Godfrey, L. R., Solounias, N., Sutherland, M., & Jungers, W. L. (2004b). Can low-magnification stereomicroscopy reveal diet? Journal of Human Evolution, 47, 115–144.
Semprebon, G., Sise, P., & Coombs, M. (2011). Potential bark and fruit browsing as revealed by stereomicrowear analysis of the peculiar clawed herbivores known as chalicotheres (Perissodactyla, Chalicotherioidea). Journal of Mammalian Evolution, 18, 33–55.
Shearer, B. M., Ungar, P. S., McNulty, K. P., Harcourt-Smith, W. E. H., Dunsworth, H. M., & Teaford, M. F. (2015). Dental microwear profilometry of African non-cercopithecoid catarrhines of the Early Miocene. Journal of Human Evolution, 78, 33–43.
Sidorovich, V. E. (2000). Seasonal variation in the feeding habits of riparian mustelids in river valleys of ME Belarus. Acta Theriologica, 45, 233–242.
Solounias, N., & Semprebon, G. (2002). Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. American Museum Novitates, 3366, 1–49.
Solounias, N., Tariq, M., Hou, S., Danowitz, M., & Harrison, M. (2014). A new method of tooth mesowear and a test of it on domestic goats. Annales Zoologici Fennici, 51, 111–118.
Spradley, J. P., Glander, K. E., & Kay, R. F. (2016). Dust in the wind: how climate variables and volcanic dust affect rates of tooth wear in Central American howling monkeys. American Journal of Physical Anthropology, 159, 210–222.
Stokke, S., & du Toit, J. T. (2000). Sex and size related differences in the dry season feeding patterns of elephants in Chobe National Park, Botswana. Ecography, 23, 70–80.
Strait, S. G. (1993). Molar microwear in extant small-bodied faunivorous mammals: an analysis of feature density and pit frequency. American Journal of Physical Anthropology, 92, 63–79.
Strait, S. G. (2014). Myrmecophagous microwear: implications for diet in the homin fossil record. Journal of Human Evolution, 71, 87–93.
Stynder, D. D. (2011). Fossil bovid diets indicate a scarcity of grass in the Langebaanweg E Quarry (South Africa) late Miocene/early Pliocene environment. Paleobiology, 37, 126–139.
Tausch, J., Kullmer, O., & Bromage, T. G. (2015). A new method for determining the 3D spatial orientation of molar microwear. Scanning, 37, 446–457.
Taylor, L. A., Kaiser, T. M., Schwitzer, C., Müller, D. W. H., Codron, D., Clauss, M., et al. (2013). Detecting inter-cusp and inter-tooth wear patterns in rhinocerotids. PLoS ONE, 8, e80921.
Teaford, M. F. (1988). Scanning electron microscope diagnosis of wear patterns versus artifacts on fossil teeth. Scanning Microscopy, 2, 1167–1175.
Teaford, M. F. (1991). Dental microwear: what can it tell us about diet and dental function? In M. A. Kelley & C. S. Larsen (Eds.), Advances in Dental Anthropology (pp. 341–356). New York: Wiley-Liss.
Teaford, M. F. (2007). What do we know and not know about dental microwear and diet? In P. S. Ungar (Ed.), Evolution of the Human Diet: The known, the unknown, and the unknowable (pp. 106–131). Oxford: Oxford University Press.
Teaford, M. F., & Oyen, O. J. (1989). In vivo and in vitro turnover in dental microwear. American Journal of Physical Anthropology, 80, 447–460.
Teaford, M. F., & Walker, A. (1984). Quantitative differences in dental microwear between primate species with different diets and a comment on the presumed diet of Sivapithecus. American Journal of Physical Anthropology, 64, 191–200.
Townsend, K. E., & Croft, D. A. (2008a). Diets of notoungulates from the Santa Cruz Formation, Argentina: new evidence from enamel microwear. Journal of Vertebrate Paleontology, 28, 217–230.
Townsend, K. E., & Croft, D. A. (2008b). Enamel microwear in caviomorph rodents. Journal of Mammalogy, 89, 728–742.
Ulbricht, A., Maul, L. C., & Schulz, E. (2015). Can mesowear analysis be applied to small mammals? A pilot-study on leporines and murines. Mammalian Biology – Zeitschrift für Säugetierkunde, 80, 14–20.
Ungar, P. S. (1995). A semiautomated image analysis procedure for the quantification of dental microwear II. Scanning, 17, 57–59.
Ungar, P. S. (2002). Microware software. Version 4.02. A semiautomated image analysis system for the quantification of dental microwear. Unpublished. Fayetteville.
Ungar, P. S. (2015). Mammalian dental function and wear: a review. Biosurface and Biotribology, 1, 25–41.
Ungar, P. S., & Spencer, M. A. (1999). Incisor microwear, diet, and tooth use in three Amerindian populations. American Journal of Physical Anthropology, 109, 387–396.
Ungar, P. S., Merceron, G., & Scott, R. S. (2007). Dental microwear texture analysis of Varswater bovids and Early Pliocene palaeoenvironments of Langebaanweg, Western Cape Province, South Africa. Journal of Mammalian Evolution, 14, 163–181.
Ungar, P. S., Brown, C. A., Bergstrom, T. S., & Walker, A. (2003). Quantification of dental microwear by tandem scanning confocal microscopy, and scale sensitive fractal analysis. Scanning, 25, 185–193.
Ungar, P. S., Scott, R. S., Scott, J. R., & Teaford, M. (2008). Dental microwear analysis: historical perspectives and new approaches. In J. D. Irish & G. C. Nelson (Eds.), Technique and application in dental anthropology (pp. 389–425). New York: Cambridge University Press.
Vaux, D. (2012). Research methods: know when your numbers are significant. Nature, 492, 180–181.
Walker, A., Hoeck, H. N., & Perez, L. (1978). Microwear of mammalian teeth as an indicator of diet. Science, 201, 908–910.
Winkler, D. A., & Kaiser, T. M. (2011). A case study of seasonal, sexual and ontogenetic divergence in the feeding behaviour of the moose (Alces alces LINNÉ, 1758). Verhandlungen des Naturwissenschaftlichen Vereins Hamburg, 46, 331–348.
Withnell, C. B., & Ungar, P. S. (2014). A preliminary analysis of dental microwear as a proxy for diet and habitat in shrews. Mammalia, 78, 409–415.
Xia, J., Zheng, J., Huang, D., Tian, Z. R., Chen, L., Zhou, Z., et al. (2015). New model to explain tooth wear with implications for microwear formation and diet reconstruction. Proceedings of the National Academy of Sciences, USA, 112, 10669–10672.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Green, J.L., Croft, D.A. (2018). Using Dental Mesowear and Microwear for Dietary Inference: A Review of Current Techniques and Applications. In: Croft, D., Su, D., Simpson, S. (eds) Methods in Paleoecology. Vertebrate Paleobiology and Paleoanthropology. Springer, Cham. https://doi.org/10.1007/978-3-319-94265-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-94265-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94264-3
Online ISBN: 978-3-319-94265-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)