Abstract
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to an infection. The definition of sepsis was updated in 2016 following publication of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). The 2016 consensus definitions recommend that the Sequential (Sepsis-related) Organ Failure Assessment (SOFA) criteria and “quick” (q)SOFA criteria be used to identify sepsis, in place of the currently used systemic inflammatory response syndrome (SIRS) criteria, which were the basis for the previous definition of sepsis. SOFA is an ICU-based mortality score, and qSOFA is a rapid, shortened version of SOFA designed for use outside the ICU. The SOFA scores are no clinical predictors of sepsis; they rely on clinical suspicion for the scores to be assessed. There are as many as 750,000–900,000 cases of sepsis per year, resulting in around 200,000 deaths per year. It is likely that there are as many as 30.5 million cases of sepsis annually worldwide, with an estimated 5.3 million deaths annually. Sepsis is a spectrum of disease, where there is a systemic and dysregulated host response to an infection. Risk of progression to fulminant disease is determined by various factors: magnitude and nature of the infective focus, timing and quality of interventions and genetic and acquired predisposition of the patient. Early recognition and diagnosis is essential because early treatment is associated with significant short- and long-term benefits in outcome. There is ongoing debate about the most appropriate criteria for diagnosing sepsis in clinical practice, with several different approaches suggested: SIRS criteria in the presence of infection, SOFA score, and use of risk stratification system as recommended by guideline groups. Strong risk factors are underlying malignancy, age > 65 years, immunocompromise, hemodialysis, alcoholism, diabetes mellitus, recent surgery or other invasive procedures, breached skin integrity, indwelling lines or catheters, intravenous drug misuse and pregnancy. Early recognition and treatment of sepsis is key to improving outcomes. Treatment guidelines have been produced by the Surviving Sepsis Campaign and remain the most widely accepted standards. Current best practice is based upon evidence for care bundles in sepsis. They include the following: obtain blood cultures prior to administration of antibiotics; administer broad-spectrum antibiotics that target the suspected pathogen(s); administer 30 mL/kg crystalloid for hypotension or lactate ≥36 mg/dL (≥4 mmol/L); obtain serial measurement of blood lactate; use vasopressors to maintain a mean arterial pressure (MAP) ≥65 mmHg in patients refractory to fluid therapy, in patients with an initial lactate ≥36 mg/dL (≥4 mmol/L), or who are persistently hypotensive (i.e. MAP < 65 mmHg); and assess volume status and perfusion using either a repeat focused exam or two of the following methods—measurement of central venous pressure, measurement of central venous oxygen saturation (ScvO2), bedside cardiovascular ultrasound and dynamic assessment of fluid responsiveness with passive leg raise or fluid challenge. One bundle dealing with basic therapies, the “Sepsis Six”, has been shown to improve outcomes in septic patients. If the six factors are completed within the first hour following recognition of sepsis, the associated mortality has been reported to reduce by as much as 50%. The six factors are the following: administer high-flow oxygen to maintain target oxygen saturations greater than 94% (or 88–92% in people at risk of hypercapnic respiratory failure), take blood cultures, give intravenous antibiotics, start intravenous fluid resuscitation, check lactate level and monitor hourly urine output. Patients who are refractory to initial treatments, in particular those with septic shock, may require invasive monitoring and consideration for organ support, so management on a High Dependency Unit or ICU may well be required. Patients who fail to respond to the rapid delivery of adequate volumes of intravenous fluids are in septic shock. The immediate priority in this group of patients is the restoration of the circulation and oxygen delivery. Monitoring of vital signs and response to fluid therapy is essential. Assessment of oxygenation via pulse oximetry and serial lactate measurements should be performed, along with monitoring of urinary output. A failure of lactate to improve with therapy is indicative of a poor outcome. Lactate clearance has been shown to correlate positively with survival. All patients receiving vasopressors should have an arterial catheter inserted as soon as it is practical to do so to aid more accurate monitoring of arterial blood pressure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sepsis definition
- Septic shock definition
- SIRS
- SOFA score
- qSOFA
- MODS
- Severe infection
- Sepsis bundle
- Sepsis guidelines
- Diagnostic stewardship
- Antibiotic stewardship
Sepsis is defined as a life-threatening organ dysfunction due to a dysregulated host response to the infection.
Septic shock is defined as a subset of sepsis with circulatory, cellular and metabolic dysfunction associated with a higher risk of mortality compared to sepsis alone.
In the previous 1992 and 2003 definitions, sepsis was defined as a suspected, probable or certain infection that was accompanied by at least two out of four SIRS (systemic inflammatory response syndrome) criteria [4, 5]. Sepsis was shown as a continuum that went from sepsis (infection plus SIRS) to severe sepsis (sepsis plus at least one organ failure) to septic shock (sepsis with arterial hypotension in spite of adequate fluid provision).
Currently the new definition abolishes this continuum and defines sepsis as an infection that becomes complicated due to the “dysregulated host response to the infection”, generating organ failure that is life-threatening. The new definitions remove the SIRS as they are too sensitive and lacking in clinical specificity and also remove severe sepsis as it is considered to be redundant.
Systemic inflammatory response syndrome was diagnosed when at least two of the criteria shown in Fig. 9.1 were found [the SIRS criteria did not include MAP—mean arterial pressure or SBP-systolic blood pressure—but did include fever, heart rate and the white blood cell count].
Yesterday, like today, there must be the suspicion or certainty of infection to make a sepsis diagnosis. Today, with the new definition (Fig. 9.2), to diagnose sepsis or organ dysfunction, all we need is the SOFA score, a score that tells us whether there is organ dysfunction, but in the context of which the body temperature is not taken, the heart rate and breathing rate do not appear, and the white blood cell count is not taken, criteria that, even if a specific, have always been considered useful, together with other clinical signs and symptoms, for making the diagnosis of infection (or at least for suspecting an infection).
The SOFA score therefore underlines the importance of organ failure contained in the new definition, but does not make one immediately reflect on the infection, as SIRS does instead.
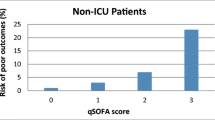
As the SOFA score requires some laboratory data, it was believed appropriate to run the quick SOFA (qSOFA) alongside the SOFA score, which provides for three simple, rapid factors (breathing rate >22 breaths/min, systolic pressure <100 mmHg and altered mental state—0 = mortality <1%, 1 = mortality 2–3%, >2 mortality >10%), but yet again does not include body temperature or heart rate or even the white blood cell count.
All the definitions must have a clinical suspicion of infection or microbiological certainty of the infectious pathogen(s) at the basis, but while useful indicators of infection were contained in the 1992 and 2003 definitions, they disappear in the new definition that focuses completely on infection complications, i.e. on organ failure.
Why was it decided it was necessary to change over from the SIRS criteria to the SOFA score?
The 1992 ACCP/SCCM Consensus Conference generated the old definition which was then taken up by the Surviving Sepsis Campaign guidelines published in 2003, 2008 and 2012. These definitions of sepsis and septic shock had shown good clinical resistance over the years (at least 20 years) and had proved useful for hospitals and research. However, over the past 20 years, their use showed that the SIRS definition could be overly sensitive and non-specific.
In 2006, a critical review of some European Intensive Care Units [6] showed that the SIRS criteria were 100% sensitive but only 18% specific. In 2012, a prospective observational study carried out in Holland [7] showed that even minor changes to timing and the SIRS criteria capture method, for example, manually rather than automatically capturing, significantly changed the measured incidences of sepsis. This study showed that from 6% to 17% (depending on the SIRS criteria capture system) of the infected patients in Intensive Care did not meet the SIRS criteria. SIRS criteria are so sensitive that more than 90% of patients admitted to Intensive Care meet these criteria [8].
More recently, a study of 14 years of work in New Zealand Intensive Care Units [9] highlighted significant potential problems with the definition of sepsis. This study found that the SIRS criteria were lost in one out of eight patients with serious infections and that these lost cases were associated with substantial morbidity and hospital mortality. More specifically, “SIRS-negative” septic patients still had high percentages of organ failure, with 42% affected by septic shock, 55% requiring mechanical ventilation and 12% with acute kidney failure. Also, when compared to “SIRS-positive” septic patients, the “SIRS-negative” septic patients had a lower but still substantial hospital mortality (16% versus 23%). These data have raised the problem of the use of the SIRS component of sepsis diagnostic criteria in helping with early recognition and with early treatment of severe infections that are complicated by organ failure. By defining sepsis as the presence of SIRS criteria plus an infection and emphasising what was said previously, i.e. that almost all critical patients satisfy the SIRS criteria, sepsis is the same as having an infection, but if it is true that all patients with sepsis have an infection, the contrary is not necessarily true, i.e. that all patients with an infection have sepsis. In a few words, SIRS criteria are extremely sensitive but not very specific [9, 10].
This is the context in which the task force from the third “International Consensus Conference” on the definitions of sepsis proposed new criteria that were then shared for the first time in the 2016 Surviving Sepsis Campaign guidelines [11].
However, many clinicians and the Surviving Sepsis Campaign still see the SIRS criteria as a useful tool for identifying the infection and for suspecting the infection, but no longer use them as criteria for defining sepsis.
The quick SOFA score and the SOFA score have been, respectively, introduced as screening tools (the former) and as clinical diagnostic criteria for sepsis (the latter). The term sepsis has been removed, and septic shock has been defined as persistent hypotension that requires the use of vasopressors with elevated serum lactate (>2 mmol/L) in spite of fluid resuscitation.
The authors of the new definition acknowledge that simplicity, outcome prediction, test weighting and pathobiology were all taken into consideration for the burdensome task, a true challenge, of deriving the clinical criteria for a syndrome with different aetiologies and without any diagnosis confirmation test, without a gold standard.
Initial performance assessment showed an improved prediction of by SOFA and qSOFA of intra-hospital mortality compared to the SIRS criteria, with criteria met in 67%, 70% and 55% of deaths with initial, clinically suspected infection in Intensive Care with SOFA and outside Intensive Care (e.g. in the emergency department) with qSOFA [12, 13]. While these new criteria have improved predictive ability, they are also used as a reminder for caution that the necessary trust in a definition, that is, syndromic rather than pathologically defined, will continue to introduce errors of misclassification in epidemiological surveillance. In fact, some researchers have studied [14] the incidence of SIRS and qSOFA, on a population of emergency department patients with suspected severe infection, basing their studies on the new sepsis definitions and the overlap with mortality. Of the patients who died, eight out of 276 (2.9%) were identified by qSOFA alone, 101 out of 276 (36.5%) by the SIRS criteria alone and 128 out of 276 (46.4%) by qSOFA and SIRS, and 39 out of 276 (14.2%) did not meet either one or the other criteria. Among the patients with infections in the emergency department who died during hospitalisation, the SIRS criteria showed greater sensitivity than among the patients who survived, the SIRS criteria showing greater ability in identifying the risk of death (higher specificity). The qSOFA in this study identified 21% fewer patients with infection who died while hospitalised.
The definition of sepsis has changed, but the suspected or certain infection remains the crux of the problem. The fundamental enigma in the field of infectious diseases is the huge clinical variability among individuals during the course of the same infection [15].
An infectious (or contagious) illness is defined as an illness caused by a specific infectious agent or by its toxic products that result from the transmission of that agent or its products from another infected person, animal or other reservoir to a susceptible host, either directly or indirectly, via vegetable or animal host and a vector or even an inanimate environment. An infection is on the other hand the dynamic term that defines the entry and development of an infectious agent into a human being or animal body, whether the illness develops of not. When we are faced with infections, we must try to define them from where they manifest themselves as community or nosocomial infections, defining their paths and the risks connected with their deterioration.
An infection may only be suspected at home or at the entrance to hospital (triage in the emergency department). The suspected infection is a clinical case in which the signs and symptoms that a person is showing are consistent and compatible with a particular infectious illness. The patient may be severe or become more severe during observation; therefore the SIRS criteria, or their systemic involvement, become important at two or more of the following: heart rate >90 beats/min, breathing rate >20 breaths/min, body temperature >38 °C or <36 °C (hypothermia), white blood cells >12/mm3 or <4/mm3, or >10% band form, presence of pus or inflamed tissue and presence of skin marbling. The qSOFA is fast and frugally indicates organ impairment breathing rate, hypotension and altered mental status that cause us to go immediately to the hospital.
An improbable infection combined with a positive microbial culture constitutes a colonisation (today important to know due to multiresistant bacteria).
An infection can only be considered not probably or probable and then be confirmed in the emergency department, or on the ward, or in Intensive Care via the microbiological path.
This category of probable infections may include the expert opinion accompanied by positive biomarkers (e.g. PCT) but sometimes also by non-pathogenic germ cultures. Therefore, a suspected or probable infection in hospital must always be confirmed microbiologically and become a possible certain infection as soon as possible.
However, in all this complexity and variability, there is a new exceptionally important problem: time passing.
The real problem: an infection that deteriorates to the point of sepsis and septic shock happens in the space of a few hours. Sepsis and septic shock are real medical emergencies.
Different doctors and different healthcare organisations are working on this “Sepsis Path” but it is the same patient experiencing it. Care and assistance must be integrated to respect time, to avoid wasting time and to avoid allowing wasted time to further complicate the infection.
Physiological changes, location and confirmation of the infection, investigations, determination of the gravity, target treatment and revision and personalisation of the treatment are all steps in a row, that is, the classic Bayesian approach to diagnosis and treatment of the infection.
The infection is the crux and TIME is the fundamental problem for diagnosis and treatment.
Infection is a dynamic process. It can be resolved, it can stabilise, or it can deteriorate to sepsis. Without cultures or with sterile cultures when a doctor strongly suspects the infection, the day of infection will be defined as the day on which suspicion of the infection starts. With positive cultures, on the other hand, the day of infection is the day on which the sample is found positive in microbiology.
But why does an infection deteriorate? What is an infection that deteriorates and when does an infection deteriorate? Is a particular infection that deteriorates easier than another?
Four complementary theories contribute to creating the infectious phenotype and consequently the septic phenotype: germ theory (microbiology allows us to know microbiological variability in quantitative and qualitative terms); ecological theory that allows us to know environmental variability; immunological theory that allows us to learn the host’s response, deficiencies in somatic, adaptive (both genetic and epigenetic) acquired immunity; and genetic theory with inherent errors of germ-line encoder immunity (both inherent and adaptive) (Fig. 9.3).
Always think of sepsis when suspecting an infection. When should you think it? When a person shows signs and symptoms that indicate a possible infection that is deteriorating with organ failure or shock. Always take into consideration those people with sepsis who may show non-specific, non-located signs, for example, feeling extremely ill and not having a fever and having normal white blood cells. Pay special attention to the problems raised by one person or by the family or carers, for example, changes in usual behaviour and altered mental state. Assess people who may have sepsis with greater attention if they can’t communicate easily (e.g. Italian is their second language or people with communication problems). Assess people with any suspected infection to identify the possible source or infectious factors that increase the risk of sepsis, every clinically important indication such as sudden changes in behaviour, circulation and breathing. Use a structured observation set to assess people and to stratify risk if sepsis is suspected. Consider using an “early warning score” to assess people with suspected sepsis in acute hospital settings (medical or surgical wards, mother-infant setting). Suspect neutropenic sepsis in patients who are having cancer treatment and suddenly don’t feel well. Take these patients immediately to the emergency department for a hospital assessment.
What can the sepsis risk factors be?
Here are the main ones: very young infants (under 1 year of age) or the elderly (over 75 years of age) or extremely fragile subjects. People with a damaged immune system due to illness or drugs taken also include people treated with chemotherapy for cancer in this group. People who have damaged immune functions; people who have taken steroids for a long time; people who take immunosuppressor drugs for non-tumoural diseases such as rheumatoid arthritis; people who have undergone surgery or other invasive procedure in the last 6 months; people who have skin lesions, for example, cuts, burns, blisters or skin infections; people who use intravenous drugs; and people who wear intravenous catheters or other types of catheter (Fig. 9.4).
Sepsis and septic shock must be recognised and treated rapidly otherwise mortality and morbidity increase. Sepsis is a systemic infection that goes bad to organ failure (Fig. 9.5).
The host’s responses to severe infection are complex and multiple. Recent studies show that activation of both pro-inflammatory and anti-inflammatory immune responses occurs immediately after the onset of sepsis. The inherent immune system cells, including monocytes and neutrophils, release large amounts of pro-inflammatory cytokines that activate inflammation. The intensity of the initial inflammatory response can vary in each patient, depending on several factors including pathogen load and virulence, co-morbidity in patients and the host’s genetic factors. Early deaths in sepsis are usually due to a hyperinflammatory cytochemical storm, with fever, refractory shock, acidosis and hypercatabolism. One classic example of this could be that of a young patient who dies from toxic shock syndrome or from meningococcemia. Most patients have an inherent, adaptive immunity recovery and survive infection usually between the fifth and sixth day. If sepsis persists, there is an insufficiency of critical elements in both the inherent immune system and the adaptive system, so that the patient enters a deep immunosuppressive state. Deaths are due to the patient’s inability to eliminate the infections and to the development of secondary infections. The most accredited pathophysiology is the one that even if both pro-inflammatory and anti-inflammatory responses begin together rapidly after sepsis, the initial response in previously health patients is usually an important hyperinflammatory phase with fever, hyperdynamic circulation and shock. Deaths in this early phase are usually due to cardiovascular collapse, metabolic alterations and multiple organ dysfunctions. Although no anti-inflammatory therapy has been found to improve survival in large clinical trials, short-acting anti-inflammatory or anti-cytochemical therapies could still offer a theoretical benefit [16,17,18].
Many patients that develop sepsis are however elderly with several comorbidities that contribute to altering the already altered—by their very age—immune response. When these individuals develop sepsis, it is common to see that the hyperinflammatory stage is of reduced intensity or even absent, and the patients rapidly develop altered immunity, an actual anti-inflammatory state.
In these situations, which nowadays are increasingly more frequent, an immune adjuvant therapy that can strengthen their immune response is rather promising.
Another immunological response to sepsis may be cycling between hyperinflammatory and hypoinflammatory states. According to this theory, patients that develop sepsis have an initial hyperinflammatory response followed by a hypoinflammatory state. When patients develop a new secondary infection, they have a repeated hyperinflammatory response and can either recover or return to the hypoinflammatory phase. Patients can die in either of the two states. There is not much scientific evidence for this theory, and the longer sepsis continues, the more likely it is that the patient develops a severe immunosuppression.
Due to their advance aged (immuno-senescence) and due to their comorbidities and the specific treatments for said comorbidities, and also due to highly pathogenic, antibiotic-resistant bacteria, many patients are immunodepressed or even immunoparalysed [19].
Sepsis has several, deep effects on all cells that make up the inherent immune system. Sepsis rapidly gives rise to the onset of widespread apoptosis of dendritic cells, monocytes and immature macrophages and natural killer cells and “myeloid-derived suppressor cells” (MDSC). The reduced expression of HLA-DR on cells with the antigen that include monocytes/macrophages and dendritic cells is a true distinguishing sign of sepsis that can harm the optimal presentation of microbial antigens to T cells. Sepsis also causes massive losses of CD4+ e CD8+ cells and B cells. T-regulatory (Treg) cells are more resistant to apoptosis induced by sepsis, and there is therefore an increased percentage of Treg cells in circulation compared to the other lymphocyte subsets. This contributes to a more immunosuppressive phenotype. The CD4+ e CD8+ cells that survive have either a shift from one pro-inflammatory Th1 cell phenotype to an anti-inflammatory Th2 cell phenotype or develop a thorough phenotype characterised by an increased expression of “programmed cell death 1” and reduced cytokine secretion. CD4+ T cells have a reduced expression of CD28 and a reduced receptor diversity for the T cell (TCR) that likely both contribute to the damaged microbial response to invading pathogens.
It has recently been shown that patients with sepsis can develop—if they survive the acute phase but do not recover from organ failure—a so-called persistent critical illness (PCI) [19,20,21] with clear organ failure that lasts for weeks and months. In the past, these patients would have died, but today they survive due to organ support and therapies provided to them. Death from persistent critical illness is 20–40%, and survivors are frequently handicapped in their cognitive functions, with neuropathies and myopathies, immune dysfunction and other serious complications.
Sepsis is an immunopathology; very early administration of antibiotics, fluids and oxygen (SEPSIS Bundles and SEPSIS SIX) (Figs. 9.6, 9.7, and 9.8) to patients with sepsis has lowered the percentage of mortality, even if this has not prevented or overturned the development of persistent critical illness that is often associated with immunosuppression. Sepsis and persistent critical illness may also not be immunopathologies but be the failure to recover homeostasis that results in dysfunction of the immune and neuroendocrine system: the two major systems that maintain healthy intercommunication between the organs. The components pertaining to efferent to both the systems are damaged in sepsis and in persistent critical illness via molecular and cellular mechanisms that are currently the focus of active research.
The limits of this new definition of sepsis emerge in all current scientific evidence, together with deep uncertainty (Figs. 9.9 and 9.10).
Doctors and nurses need a less controversial and less confused definition. They need it to be made operational like a set of clinical criteria that can be used to make clinical decisions and even guide them in testing and using therapies that can modulate the host’s response. The first task requires identification of specific diagnostic criteria, others a further refinement of these criteria via true therapeutic stratification.
We don’t currently screen patients for sepsis; instead we search for diagnostic tests that can establish or exclude diagnosis in a patient with early and non-specific signs of acute illness. The critical question is: what are we trying to diagnose?
Sepsis is a complex structure that incorporates both causes, infection and the consequent complications: development of a life-threatening organ dysfunction and thus the diagnostic criteria for sepsis could address four different needs:
-
(a)
DIAGNOSING THE INFECTION AT AN EARLY STAGE, so that we can treat it suitably and thus prevent organ dysfunction.
-
(b)
IN PATIENTS WITH SUSPECTED OR KNOWN INFECTION, IDENTIFY THE ONES THAT HAVE AN INCREASED RISK OF DEVELOPING LIFE-THREATENING ORGAN DYSFUNCTION, so that we can monitor them better and intervene earlier.
-
(c)
IDENTIFY THOSE PATIENTS WITH EARLY ORGAN DYSFUNCTION THAT ARE AT A HIGH RISK OF DEATH, so that we can intervene to alter their trajectory.
-
(d)
EXCLUDE THOSE PATIENTS THAT RISK ORGAN DYSFUNCTION BUT WHERE THE INFECTION IS NOT THE CAUSE.
The infection and sepsis must integrate care and assistance in time because sepsis can appear or develop from the territory to the hospital or inside the hospital, in Intensive Care and/or on the wards or in the maternity wards. If it develops in the territory, diagnosis must be carried out by the family GP if the patient calls him. Therefore, we can consider a delay caused by the patient. If first contact is with a family GP, he can diagnose or not diagnose sepsis and delay causing the emergency services. If he does call emergency services, there may be a delay in transport either due to delay in replying or a delay in the transport itself. On arriving at the emergency department, there may be a delay in the department itself due to an undertriage of the sepsis. Only an integrated system can diagnose and treat sepsis in the right timescales. In the hospital, it is necessary to know what the correct methods are for diagnosing sepsis in the different settings but also to know where sepsis and septic shock must be treated: sepsis and septic shock must always be treated either in Intensive Care or High Dependency Units (HDU).
Sepsis at home can be diagnosed with simple, rapid approaches such as the qSOFA may be, perhaps expanded to temperature and heart rate and with strong empowerment of the families. There is need for a new family GP and to teach the patient signs of infection and sepsis.
Sepsis and septic shock can be addressed in emergency medical systems also in these settings, via simple adaptive tools. When we analyse these simple tools, it is possible to see that they all start from MEWS (Fig. 9.11).
With the introduction of the more recent rapid diagnosis microbiological techniques in clinical practice for identification of the pathogen and for its antibiotic resistance (Figs. 9.12 and 9.13), severe infections in high intensity areas of hospital care can be addressed by the clinician in a totally new way. With this new diagnostic approach, the information obtained using the molecular microbiological technique and phenotype microbiological technique can be used rapidly by the doctor, as required by sepsis, thus overcoming one of the main problems of traditional microbiology, i.e. the prolonged response time. Finding negative cultures in patients that have already received antibiotics occurs frequently [22]. This problem can also be resolved in part using molecular biology techniques. Delay in diagnosis due to the microbiological laboratory is accompanied by a delay in administering targeted antibiotic therapy, with deterioration in the patients’ outcome, especially if they are in septic shock. There is much solid scientific evidence that shows a mortality increase if the empiric antibiotic therapy proves to be inadequate. At the end of the 1990s, in a prospective surveillance study, it was proven that 8.5% of infected patients admitted to Intensive Care received inadequate empiric therapy, and this was an important independent factor of hospital mortality: 42% versus 17.7% in the infected group treated adequately from the start [23].
Some authors, using a broad range of cases, assessed the appropriateness of antibiotic therapy in bacteraemia and the related clinical outcome and found a clear increase in hospital mortality in the critical group of patients who were given an inappropriate empiric therapy [24].
In an article often cited in literature, the concept that the duration of hypotension—expressed in hours—in septic shock before an effective empiric antibiotic is commenced is a critical decisive factor closely related to mortality is emphasised. This work reports a 7.6% increase in mortality for each hour of delay in administering effective therapy, in the first 6 h from diagnosis of septic shock [25]. This concept has been misinterpreted by many, extending the need to establish a rapid, prompt antibiotic therapy in all infectious conditions far removed from septic shock, the only one where such evidence is solid.
More recently, on analysing an enormous global case study of patients in septic shock/with severe sepsis, other authors have again shown the close relationship between delay in commencing appropriate antibiotic therapy in the early hours since diagnosis of septic shock/severe sepsis and hospital mortality. Mortality increased in line in the early hours, as in the previously cited work, but in lower percentages [26]. Some of the independently associated factors to inappropriate antibiotic therapy include a previous colonisation by multiresistant germs and previous antibiotic therapy, especially one with broad-spectrum molecules such as carbapenems [27]. More than 2/3 of the bacteraemias acquired in Intensive Care are caused by MDR or XDR (multidrug-resistant, extensively drug-resistant) germs, with a clear prevalence of isolated gram-negatives, characterised [28] by more complex problems in treatment, mainly due to the limited number of effective antibiotic molecules. The problem of the negative impact (in terms of mortality) of inappropriate empiric antibiotic therapy on outcome has once again been confirmed by a large recently published meta-analysis [29]. All this clearly imposes rapid identification of the pathogen causing the infection, especially in septic patients admitted to Intensive Care, together with its pattern of resistance and susceptibility to currently used antimicrobial molecules. Access to rapid diagnosis is currently possible for only a few clinicians as such methods are extremely expensive. It is therefore essential to design clinical care paths with patient complexity at the centre, able to direct a certain “setting” of septic patients to rapid diagnosis. On this matter, the microbiology laboratory should travel at two different speeds, clearly differentiating the routine path of a sample from that of the critical septic patient who requires much different times. A well-prepared clinician should stratify patients who need a preferential diagnostic path based on several factors. The combined, integrated use of validated scores helps the clinician in this task which is often not easy. Above all, the SOFA score was introduced by Vincent in 1996 [30]. Originally, the SOFA score was designed not to predict the outcome but to describe a developing sequence of organ damage. On admission, the SOFA score is independently associated with the possibility of developing bacteraemia in critical patients; in itself, it does not predict the outcome for those patients who will then develop this complication, but if we analyse its value on the first day of bacteraemia, then it is a powerful, independent prognostic factor. A high SOFA score value is associated with a higher probability of death [31].
With the introduction of the new sepsis and septic shock definitions, this score takes on an even more important clinical role in accurately defining a septic patient [1,2,3]. Today, sepsis involves organ dysfunction, focusing attention on a much more complex pathology than a simple infection plus SIRS criteria. This new way of thinking underlines the supremacy of the non-homeostatic host response to the infection, with the connected greater potential fatality compared to a simple infection, and this forces the clinician to rapidly recognise these patients in order to reduce said risk. Current clinical criteria for sepsis are the presence of an infection that has brought about a dysregulated host response together with a 2-point increase on the SOFA score, considering 0 as the initial state when the starting value is unknown. A patient in these conditions has a 10% higher hospital mortality risk. Septic shock, as has already been mentioned, means sepsis, as defined above, associated with the needs to vasopressors for maintaining a MAP > 65 mmHg and with lactacidemia >2 mg/L with no hypovolaemia. However, it is necessary to remember that the concept of SIRS has been removed from the definition of sepsis, but the clinician must be well aware that its use is most certainly useful when making a presumptive diagnosis of infection. The infection to be verified is still the crux of the sepsis problem, in fact.
Alongside the SOFA score, there are other scores of great and renewed interest for stratifying which patients are worthy of rapid microbiological diagnosis, including the CPIS score (clinical pulmonary infection score) when a lung infection is suspected and the PITT score when bacteraemia is suspected. Recently, the CPIS score was included in more complex diagnostic strategies, including both quali-/quantitative microbiological analyses and biomarkers [32], or due to its non-elevated diagnostic performance highlighted by various studies published in literature [33, 34] integrated with procalcitonin levels and a chest echography [35] (CEPPIS Chest Echography and Procalcitonin Pulmonary Infection Score). A CEPPIS score >5 analysed retrospectively on 221 patients, 108 of which with microbiological VAP confirmation was found to be higher-performing in predicting VAP compared to a CPIS score >6 (OR 23.78; sensitivity 80.5%, specificity 85.2% versus OR 3309; sensitivity 39.8%, specificity 83.3%). PITT bacteraemia score is associated with mortality in patients with bacteraemia [36]. Another essential important aspect in risk stratification for septic patients is assessing the probability that the infection is caused by multiresistant germs. It is possible in this case too to use clinical scores to quantify this risk. Some authors [37] have validated a specific score for hospital-acquired infections from multiresistant gram-negative pathogens in critical patients, based on the following factors:
-
1.
A stay of more than 5 days in Intensive Care
-
2.
Use of carbapenems in the previous 6 months
-
3.
Presence of a gram-negative infection in the previous 6 months
-
4.
Dialysis with end-stage kidney disease
-
5.
Surgery that precedes identification of gram-negative MDR
-
6.
Carbapenem therapy in patients in Intensive Care for more than 5 days
-
7.
Presence of CoNS
A coefficient is attributed to each of these factors, and the sum total allows the patients to be stratified into three classes of MDR risk: low risk, medium risk and high risk.
Another advantage of correct risk stratification is that within the MDR low-risk group of patients with less organ impact, the abuse/misuse of broader-spectrum molecules can be considerably reduced, thus contributing to interrupting the vicious cycle that leads to resistance. There are also predictive infection models for KPC KlebsiellaKPC, which are also based on several factors that are easy to analyse at the patient’s bedside [38].
Some authors [39] have carried out retrospective studies on the risk factors of pneumonia caused by MDR in microbiologically confirmed cases and have constructed a new predictive score: the DRIP score (Drug Resistance in Pneumonia Score) which can identify these patients better than the less precise criteria of the CDC’s HCAP (healthcare-associated pneumonia). At a threshold value >4, the DRIP score shows a sensitivity of 0.82 (95% CI, 0.67 at 0.88), a specificity of 0 (95% CI, 0.73 at 0.87), a positive predictive value (PPV) of 0.68.81 (95% CI, 0.56 at 0.78) and a negative predictive value (NPV) of 0.90 (95% CI 0.81 at 0.93).
The problem of antibiotic resistance and therefore of acquiring an MDR germ responsible for the infection is priority, but we must not forget that it is the gravity of the septic syndrome that has the greatest influence on the outcome and likewise the condition in which an appropriate antibiotic therapy was established. In a retrospective study on 510 patients affected by bacteraemia in sepsis, severe sepsis and septic shock, all with appropriate empiric therapy, it was proven that it is the gravity of the septic syndrome rather than the acquired MDR state that is an important predictor of death [40]. Therefore, it is only a careful initial assessment, layering the clinical gravity risk related to the outcome with the help of scores, the severity of the septic syndrome and the risk of having MDR pathogen infections that can guide microbiological diagnostics in septic patients, as part of a rapid path shared at every stage with the clinical microbiology colleague, who is today a vital figure in the “sepsis path”.
As a part of this decision-making path (diagnostic-therapeutic), a role of primary importance is played by the use of biomarkers, such as procalcitonin (PCT) and pro-adrenomedullin (proADM). Evidence supporting the use of PCT is truly important. In 2004, Liliana Simon published her first review and comparative meta-analysis between PCT and PCR as bacterial infection markers, concluding that PCT was more sensitive (88% versus 75%) and more specific (81% versus 67%) than PCR in differentiating a bacterial infection from non-infectious causes [41]. A few years later, the proCAP study began to lay the foundations for the use of PCT in deciding to interrupt antibiotic therapy earlier, showing that the 151 patients guided by PCT on average interrupted antibiotic therapy in CAPs after 5 days compared to the 12 days of the control group, with a reduction of prescription on entry from 99% to 85% [42].
The proHOSP study published in 2009 extended the analysis to all lower airway infections and on analysing 1359 patients in a multicentre, randomised, controlled trial, confirmed what had already been stated that the PCT-guided group had a lower exposure to antibiotics and a smaller percentage of drug-related adverse events related to a similar clinical outcome than the control group [43]. Once the PRORATA trial appeared in a basic, reference article supporting current clinical use of PCT, it was finally confirmed that the 307 patients in the PCT group (algorithm for commencing and/or discontinuing the PCT-guided antibiotic therapy) had a mortality rate at 28 and at 60 days that was fully comparable with that of the 314 patients in the control but with a significantly lower daily exposure to antibiotics [44]. In 2012, the Cochrane collaboration published a review of 14 randomised, controlled clinical trials, concluding that use of PCT in commencing or discontinuing antibiotic therapy did not lead to a higher frequency of mortality and/or therapeutic failure but did, on the other hand, lead to an important reduction of days of exposure to the antibiotic and a lower drug-related effect and lower antibiotic-resistance rate [45]. Another meta-analysis from 2013 introduced the concept that the PCT value should be contextualised and interpreted within the clinical context [46]: this is vitally important, when PCT is used in clinical practice, as it is not the single value alone that must be considered but the meaning that that value has in that clinical context and above all the kinetics in the first 72 h of PCT. It is the dynamic change of PCT in the first 48–72 h that expresses the predictive value of survival and of antibiotic therapy efficacy [47, 48]. In addition to this placement of PCT, which has now been consolidated for some time and is based on the fact that no marker has ever been assessed with such a high level of methodological rigour in randomised clinical trials, the marker has already been paired for a few years now with new microbiological diagnostic and rapid identification technologies, which is a new and promising possibility for treating serious infections in the critical patient [49]. PCT plays a primary role today, alongside a careful stratification of patient risk, in guiding rapid diagnosis. In clinical practice based on close daily collaboration with his clinical microbiology colleagues, the clinician’s request, shared with the microbiologist after reasoning on each clinical case, for rapid diagnosis, therefore for a dedicated path from the laboratory, starts after identifying the patient deserving of such a customised diagnostic path, based on suitable stratification of risk, including scores (adaptive, heuristic tools) and biomarkers, the first of which is PCT, and more recently proADM that strengthens the former for some aspects. PCT has been included in all the most advanced antimicrobial stewardship programmes [50] especially for critical patients, at which point it is now truly difficult not to consider this marker as part of the clinical path of this particular patient setting. Mortality data in terms of reduction in the PCT-guided group that was missing in all the trials published thus far has recently come from the results in a large-scale Dutch open-label, randomised, controlled trial including 1575 patients divided into two groups (PCT-guided and standard-guided) where mortality at 28 days in the PCT group was 19.6% versus 25% of the control group [51].
One ideal biomarker to be included in daily clinical practice should not only have a high positive predictive value (PPV) but also a high negative predictive value (NPV). Anyone who currently uses PCT in an advanced and innovative manner focuses their attention on the NPV of this marker, taking the rest for granted and consolidated. It is this very particular characteristic that differentiates PCT from other biomarkers used in clinical practice in the context of an initial structured diagnostic path for a markedly septic patient, without however known or common infection sites. The use of this marker as part of an integrated, complex clinical path stimulates the clinician’s diagnostic ability, refining his accuracy. In a patient with sepsis/septic shock with negative or extremely low PCT for the severity of the clinical picture, it is automatically mandatory to guide diagnosis with the exclusion of syndrome situations such as deep and/or compartmentalised abscesses, meningitis/ventriculitis, endocarditis without embolism, specific atypical pneumonia and “bloodstream infections” from CoNS or from fungus.
In clinical practice in these groups of patients, instrumental diagnosis using advanced total body imaging with contrast media, in both CT and MRI scans, are rapidly carried out, the latter at the same time as the CT scan, in the cases where the site can be studied better or characterised using this method—see particular deep muscle or CNS locations. Alongside the CT and/or MRI test, another important diagnostic test is a trans-thoracic or trans-oesophageal ultrasound with the intention to exclude valve vegetations.
Alongside PCT, another marker of current interest is the MR-proADM amino acid fragmentation of the adrenomedullin peptide of 52 amino acids secreted by endothelial cells and vascular smooth muscles. ADM is involved in the systemic control of circulation and has a probable autocrine/paracrine vasoactive action. The molecule also has a diuretic and natriuretic action, increasing glomerular filtration rate and reducing the distal absorption of sodium. ADM also has a bactericide action, further increased by being regulated and by modulation of the complementary activity. It is not therefore surprising that high blood levels of ADM have been found raised in septic patients, making it one of the parameters to evaluate in both the diagnostic and the prognostic and monitoring paths. Unfortunately, measuring ADM is often unreliable due to its rapid clearance in blood circulation. Furthermore, circulating ADM is associated with a binding protein that makes it inaccessible for any direct immunometric dosage. However, the problem has recently been resolved by identifying its mean regional fragment, named pro-adrenomedullin (MR-proADM), in the plasma of septic patients, as a stable, reliable surrogate marker for release of ADM. Secretion of proADM increases during the immune response to viruses, fungi and bacteria in relation to stimulation intensity, and its presence is also found therefore during serious infections [52]. The MR-proADM amino acid fragment also provides more prognostic information and is an expression of greater endothelial damage. It is closely related to the gravity of the disease and would appear to play an important role in defending the organism from the host and is in fact an antibacterial peptide [53]. ProADM is therefore a strong prognostic biomarker that can be used together with the clinical parameters of gravity (APACHE II score, SOFA score) to stratify the risk of septic patients. Therefore, having a more specific marker for prognosis on entry can help the clinician to formulate both diagnostic and most of all clinical situation evolution hypotheses, by modulating several therapeutic strategies on several levels of care intensity. Pairing both the PCT and proADM markers with TNF alpha in one compound score has proven that identification performance for a sepsis diagnosis is notably refined [54]. It has recently been proven that MR-proADM differentiates sepsis from non-infection-based SIRS situations with very high specificity and that if paired with PCT in septic patients, the post-test diagnosis probability increases noticeably if compared to that of the two markers taken individually [55,56,57]. The following hypotheses can be validated by assessing daily blood levels of proADM together with those of PCT, as part of a multi-parametric analysis of sepsis using existing scores (SOFA score, PITT score, CPIS score, MDR score) and extremely advanced rapid microbiological diagnostic techniques:
-
(a)
Identifying diagnostic cut-offs for infectious situations
-
(b)
Testing the association between associated patterns compared to each proADM and PCT text
-
(c)
Diagnostic accuracy of the infection and in response to therapy
-
(d)
Testing whether the diagnostic values of proADM and PCT, their maximum values or their cumulative values or quantification of their trends add predictiveness to classical prediction models in critical patients
From an initial analysis, it is possible to see an existing close correlation between proADM and SOFA score and sometimes even the proADM rises before the SOFA score, allowing the clinician to anticipate his suspicion of a probable deterioration in organ failure. The proADM also correlates with the PITT score, more than the PCT. Another characteristic aspect to validate during the analysis phase is the fact that in several cases of initial PCT values that can also be high, the proADM remains low if the organ is not affected. It is as if the marker, not seeing the gravity of the patient, differs itself from the PCT positive result. The matter is more complex for the cut-off of proADM as it is not clear in literature what the most reliable cut-off may be. Cut-off < 1 indicates the absence of an infectious pathology, with organ dysfunction ongoing. Cut-off between 1 and 2, even without clear clinical signs and with low PCT, may indicate that the organs are affected with a latent infectious picture or one that is not yet fully resolved. This latter data is extremely important and innovative for a biomarker, as it encourages the clinician to reason and to set up a more accurate diagnostic-therapeutic plan. Cut-off between 2 and 4 indicates a severe infection associated with organ dysfunction and is usually associated in line with the SOFA score.
All patients who are admitted in septic shock with a proADM value >6 are all dead, as if this was a cut-off of no return in this group of patients. By pairing up the two markers, the clinician can refine his diagnostic capacity in the initial phase even further.
As everyone knows, PCT does not move during a viral or fungal infection, although with fungal infections, things are slightly different, in fact. A cut-off of 2 ng/mL separates sepsis from Candida from bacterial ones, with a 92% sensitivity and a 93% specificity and a NPV of 94% [58]. Therefore, when evaluating the NPV in PCT, also in fungal infections, the information it provides is very important for a well-prepared clinician. From data found in literature, a septic patient with PCT > 3 on the seventh day plus a Candida score >3 is associated with the high probability of candidemia to the point that an empiric antifungal therapy will be recommended [55, 59].
With the new definition of sepsis and with the important role attributed to the SOFA score, the possibility of using a prognostic marker, related to the SOFA score as an expression of organ damage, such as proADM most definitely increases leaning towards more advanced diagnostic-therapeutic strategies during the decision-making stage.
The diagnostic/therapeutic path proposed for sepsis and septic shock can be tracked as follows in different hospital settings (Figs. 9.14, 9.15, and 9.16):
-
1.
Stratification of risk
-
Scores: SOFA score/PITT score/CPIS score/MDR score + BIOMARKERS: PCT/proADM + lactate and grading of gravity of septic syndrome
-
-
2.
Rapid microbiological diagnostics in patients selected based on their gravity, with the criteria stated above and its correct interpretation
-
Rapid identification/resistance pattern/rapid antibiogram
-
-
3.
Reasoned empiric therapy on the infection site possible, on the patient’s history and on the department’s epidemiology
-
4.
Therapeutic upgrading/downgrading once the information from point 2 has been obtained
-
Narrowing of action spectrum of molecules used, reduction in the number of molecules and changeover from combination therapy to monotherapy
-
-
5.
Synergism test (customised therapy)
-
6.
Reduction in the duration of antibiotic therapies based on the clinic and the PCT kinetics
-
7.
Close sharing in all parts of the process described above between clinician and clinical microbiologist
-
8.
Possibility of a positive return in terms of both local epidemiology and infection control, with a considerable impact on patients’ outcome
-
Shorter stays in hospital, lower mortality, lower costs for related drug, etc.
-
1 The Surviving Sepsis Campaign 2016 Guidelines
The international SSC guidelines for sepsis and septic shock management reached their fourth edition in 2016. The recommendations contained therein have been evaluated using the GRADE system and clearly show us that there is still a high rate of uncertainty that the clinician must still embrace. However, they contain a new section: Good Practice. Eighteen best practice statements appear in the guidelines, 13 of which concern the first 3 h of intervention, i.e. initial resuscitation, diagnosis, antibiotic therapy, source control and fluid therapy (Figs. 9.17, 9.18, and 9.19).
One good practice defines sepsis and septic shock as medical emergencies and recommends that treatment and resuscitation commence immediately. This means that healthcare organisations must consider sepsis and septic shock on the same basis as trauma, IMA and strokes, where times for treatments must be measured and considered as indicators. The good practices that concern fluid therapy recommend that after the start of early fluid resuscitation, additional fluids must be guided by frequent reassessment of the hemodynamic state and that further hemodynamic assessments are carried out if a clinical examination of the patient does not lead to a clear diagnosis; the “fluid challenge” technique is also recommended, when fluids must continue to be administered for the entire time that the hemodynamic factors continue to improve. On diagnosis, not much is added to the recommendation that the appropriate routine microbiological cultures (including blood) must be taken before starting antimicrobial therapy in patients with suspected sepsis or septic shock, if these procedures do not delay excessively the start of antibiotic therapy. Most of the good practices concern antibiotic therapy and “source control”. With regard to antibiotic therapy, empiric antibiotic therapy should be narrowed down once the pathogen and the antibiogram have been identified and/or a suitable clinical improvement has been noted. Sustained systemic prophylaxis is also not recommended for patients with severe inflammatory states of a non-infectious origin, such as severe pancreatitis and burn injuries. Antibiotic dosing must be optimised based on the accepted principles of pharmacokinetics and pharmacodynamics and the specific properties of the drug. It is also recommended that if a combination therapy is initially used for septic shock, de-escalation should be carried out with discontinuation of the combination therapy within the first few days in response to clinical improvement and/or evidence of infection resolution. This is applied to both targeted combination therapy (for culture-positive infections) and empiric combination therapy (for culture-negative infections). Lastly, therapy should be assessed daily for de-escalation of antimicrobial therapy in patients with sepsis and septic shock.
Other good practices concern controlling the site of infection. The first good practice recommends a specific anatomic diagnosis of infection that requires emergency source control is identified or excluded as early as possible and that each requested source control intervention be implemented as soon as it is medically and logistically possible after diagnosis. A recommendation is also made for prompt removal of intravascular access devices that may be the source of sepsis and septic shock, after other vascular access sites have been established. As we can see, the best practices regard the entire initial phase. One important best practice states that hospitals and hospital systems must have a performance improvement programme that includes sepsis screening for high-risk, acutely ill patients.
On comparing the 2012 recommendations with the ones from 2016, with regard to initial resuscitation, we can note that some important trials have also influenced this change. In 2012, initial resuscitation of patients with sepsis and tissue hypoperfusion (defined as hypotension persisting after initial fluid challenge or blood lactate concentration ≥4 mmol/L) was protocolised under the influence of the EGDT. The goals to be achieved in the first 6 h of resuscitation were:
-
(a)
CVP 8–12 mmHg
-
(b)
MAP ≥65 mmHg
-
(c)
Urine output ≥0.5 mL/kg/h
-
(d)
Central or mixed venous saturation of = 70% or 65%, respectively (grade 1C)
-
(e)
With elevated lactate levels, resuscitation must tend towards normalising the lactate (grade 2C)
In 2016, sepsis and septic shock are considered to be a medical emergency, and it is recommended that treatment and resuscitation begin immediately. A minimum of 30 mL/kg of intravenous crystalloids are recommended in resuscitation in sepsis-induced hypoperfusion, to be administered in the first 3 h (strong recommendation with low quality of evidence). A frequent reassessment of the hemodynamic state is recommended after initial fluid resuscitation, which must include a full clinical examination and an assessment of the physiological variables available: heart rate, blood pressure, oxygen saturation, breathing rate, temperature, urinary output and others if available, such as other invasive and non-invasive forms of monitoring. A further hemodynamic assessment is recommended (such as the one to assess heart function) to determine the type of shock if the clinical examination does not lead to a clear diagnosis. Dynamic variables should be used rather than static ones to predict “fluid responsiveness”, when they are available, of course (weak recommendation, low quality of evidence). An initial target for mean arterial pressure of 65 mmHg in patients with septic shock requiring vasopressors is recommended (strong recommendation with moderate quality of evidence). Resuscitation should be guided to normalising lactate in patients with high levels of lactate as a marker for tissue hypoperfusion (weak recommendation, low quality of evidence)
With regard to diagnosis, the use of the 1.3-sD glucan and of the antibody mannan and the antibody anti-mannan for the different diagnoses with fungus infections disappear, and the use of imaging carried out to promptly confirm a potential source of infection also disappears.
For antibiotic therapy, the recommendation of administering antibiotics within 1 h for both sepsis and septic shock remains (strong recommendation with moderate quality of evidence). Not much changes for all the rest apart from the increase in this treatment in the best practices as already stated. The same for source control. Follow the EGDT protocol or monitor the patient and administer the treatment based on the clinical signs [60,61,62,63,64].
In a recent Canadian study [65], an association was shown between time to treatment and the outcome for patients with sepsis or septic shock treated in the emergency department. In this important study, it was found that a longer time in completing the “bundle of care” of 3 h for septic patients and the administration of broad-spectrum antibiotics were associated with a higher hospital mortality, adjusted for risk. No association was found in the same study between completion of the initial bolus of fluids and hospital mortality. Time to treatment varied widely between hospitals. The results of the study were in agreement with other smaller, observational-type studies [66,67,68,69].
However, a recent meta-analysis of 11 observational studies did not show a significant benefit of administering antibiotics within 3 h, compared to administration after 3 h, after triage in the emergency department (odds ratio 1.16; 95% CI, 0.92 at 1.46) or within 1 h of recognising shock (odds ratio 1.46; 95% CI, 0.89 at 2.40) [26].
The odds ratios cited in the study are similar, but the confidence intervals are narrower, given the larger sample included in the Canadian study. This study complements a meta-analysis of goal-directed therapy in sepsis and septic shock (PRISM trial) [60]. More than three out of four patients in the PRISM trial received elements of the 3-h bundle before randomisation, after which the various trials making up the PRISM trial tested to see whether the protocolised resuscitation strategies improved the outcome. However, the Canadian study asked another question: is timing important for these earlier, more basic elements? This population data also places the relatively high compliance with these steps in the control groups of the various trials making up the PRISM trial before randomisation into the context. Only half the hospitals conducted themselves at this level. There are several biological explanations for the association between time to completion of the 3-h bundle and the outcome. First of all, a faster administration of antibiotics reduces the pathogenic load, modifies the host’s response and may reduce the incidence of subsequent organ failure. Secondly, doctors who decide more rapidly to measure lactate levels in the blood can identify non-recognised shock better and are better prepared for facing lactate-guided resuscitation than clinicians who are slower in measuring lactate levels, a strategy that may improve the outcome of the randomised trials [72]. Thirdly, doctors vary vastly in how they identify sepsis, even when presented with similar cases [73].
Early administration of treatment in sepsis, even within the framework of rigid protocols, requires a prompt clinical suspicion of both the infection and of the organ dysfunction that is deteriorating. [70–71] Even if we do not find any association between the completion time of the initial bolus of intravenous fluids and the outcome, this data should not be interpreted as evidence in favour of abandoning early fluid resuscitation. Analysis of the completion time of the initial bolus of fluids is more likely to be confused by indication (e.g. sicker patients will receive fluids earlier and are also more likely to die) [74].
A greater volume of fluids administered rapidly may contribute to adverse effects such as pulmonary edema, fluid overload and a longer duration of organ support in selected patients [6]. A variation of 1–2 times was found in the study between hospitals regarding the speed in which the 3-h bundle was completed, antibiotics were administered, and the bolus of intravenous fluids were completed in the emergency departments. Generally speaking, adherence ranged from 60% to 90% and was higher than the comparable one in programmes to improve the quality of treatments for strokes in New York [75]. This performance may derive from the public’s growing attention towards sepsis [76]. Adherence was greater in emergency departments in smaller, non-university hospitals, a result that differs from a previous study [77]. These hospitals may have fewer doctors to train, a lower census in their emergency departments and a different case mix compared with the larger reference centres that perhaps helps with the faster implementation of sepsis protocols.
2 Controlling the Infection Site (Source Control) [78,79,80,81]
In the German MEDUSA trial, surgical (84.8%) or intervention (15.9%) source control was carried out in 422 patients: the overall average time was 3 h (−0.1 to 13.7 h) and 6 h, respectively (2–20 h), in those patients where source control was begun after development of organ dysfunction (314 patients). Of these 314 patients, 158 (50.3%) received source control within 6 h of the onset of infection-related organ dysfunction. Time to source control was significantly longer in non-surviving patients than in surviving ones. In 55 patients (13.3%), source control was evaluated as inadequate. Mortality at 28 days was 65.5% in patients with inadequate source control, compared to a 26.7% mortality rate in patients with adequate source control (P < 0.01). There was no relation between time to source control and risk of death at 28 days (odds ratio per hour of increase in time to source control, 1.0 (95% CI: 1–1.0 P = 0.725)). Patients who suffered a delay in source control for more than 6 h had a significantly higher mortality (42.9% versus 26.7% P < 0.001). This delay was independently associated with an increased risk of death. There was no statistically significant or linear interaction between time to antibiotic treatment and time to source control. Other studies found that a cut-off of 12 h is a better indicator between early and late times, with better outcomes observed in the groups with early intervention. In a Spanish prospective observational study by the EDUSEPSIS Study Group, a third of the patients with sepsis admitted to ICU required source control, especially the ones with abdominal infections and soft tissue infections. Although the patients going to source control were more serious and received worse initial resuscitation, their outcome was better than those that did not go to source control. The study, however, failed to prove a lower mortality with early source control compared to later source control in specific infection sites. Educational and quality control programmes are required to identify and control infection sites in patients with sepsis and septic shock.
All these results from different studies must not be an excuse for doctors to use a slow approach with critically ill patients, in a population of vulnerable patients. These studies support the notion that personalised medicine and personalised surgery are the optimal approach for timing to source control in septic patients. Some patients require early intervention, while other can tolerate a delayed source control certainly until their clinical condition has improved. It is difficult to imagine a situation where a prospective, randomised clinical trial can be designed to strictly determine the optimal time for commencing source control in septic patients. This trial may be possible, but it would be difficult to justify it on an ethical level. Evidence to date shows that careful supervision of a drainable infection site is mandatory in septic patients. Timing to source control interventions should raise attention to the fact that source control is a challenge and a difficult, individual decision that needs to be based on all the evidence available for each patient.
3 Some Critical Questions
3.1 Does the New Definition of Sepsis Improve Early Diagnosis of Sepsis and Its Treatment?
The question of how it is better to define sepsis is one doctors have asked for the last 20 years. Previous efforts, in 1991 and in 2001 [82, 83], have continued to cause much controversy; one of the observations has been that there was not much agreement between the definitions agreed at the table and the one that the doctors at the patient’s bedside thought at the time [5]. Many believed that the huge heterogeneity of the population found in existing definition was partly responsible for the difficulties found by phase three trials that had overall failed to identify new treatments for sepsis. Several authors [84, 85] had pointed out that there was an urgent need for reviewing these definitions. This was then enacted by an international group of experts which led to the SEPSIS-3 proposal. As soon as this new definition was published, it started up a real torrent of comments and criticisms [1, 86,87,88]. What are the strengths and weaknesses of the new definitions?
Strengths
-
(a)
Proposals with solid foundations based on widely validated cohorts
-
(b)
Pragmatic proposals that reduce complexity: they remove redundant terms such as severe sepsis and septicaemia
-
(c)
qSOFA that is extremely easy to apply without the need for using a laboratory
-
(d)
qSOFA that can be used for both early recognition in the emergency department and in clinical and epidemiological trials
Weaknesses
-
(a)
It is still a syndrome diagnosis based on the probability of hospital mortality.
-
(b)
There are still no clinical tests that are easy to measure and that can reflect the concept of “dysregulated immune response”.
-
(c)
The criteria for recognising the infection have not been defined; microbiology is totally ignored.
-
(d)
Paediatric patients are currently excluded.
-
(e)
The definition is developed for the first world and cannot be generalised for use in countries with low or medium levels of income.
The definition is based on an “injury severity score”, the SOFA score that actually establishes whether you have sepsis or not or whether you have a very serious infection that is complicating and becoming organ failure. The fact that the work derives from data from North America and to a lesser extent from Europe means that the conclusions need to be reviewed for doctors who work, for example, in third world countries. As others have debated [89], there is confusion between having sepsis and what doctors recognise as a septic patient. SEPSIS-3 is actually focused on bacterial disease in Intensive Care Units in Europe and North America, but does not say so and does not clarify that fact; in other parts of the world, malaria may respond well to such requests. Perhaps what has been lost in all these discussions is a real understanding of what these definitions have been drawn up for. There is inevitable tension between a meaning whose primary function is to accurately and without ambiguity identify a homogeneous population that is available for inclusion in a clinical trial and a pragmatic definition that is easy to apply in the clinic that can allow a rapid diagnosis and immediate treatment.
The experts who drew up SEPSIS-3 attempted to address this problem by developing the idea of the quick SOFA, a shorter version of the more detailed SOFA score [90]. The qSOFA has three physiological variables that are easy to measure, every two of these is a positive result and an indication that the patient is at risk of sepsis. A positive qSOFA is not a replacement definition of sepsis but an indication that a patient may be at increased risk of sepsis. The difficulty here lies in the concern that such a low threshold (all that is necessary is rapid breathing and blood pressure slightly lower than normal) will result in a super-sensitive signal, similar to the problems found with SIRS [9, 91].
It is helpful to compare the high simplicity of the qSOFA with the recent guide published by NICE, where the algorithms used are much more complex [92].
The application of the new definition of sepsis is actually more interesting: a life-threatening organ dysfunction due to a dysregulated host response to the infection. This definition has caused a lot of debate: Are all cases of sepsis really life-threatening? What does dysregulated mean? How do we measure a dysregulated host response in a patient? This new definition helps on each of the two key requests: improved definition of each case or improved treatment of each case? In Great Britain, the NHS has published an action plan to support hospitals in improving the outcome of sepsis patients. The so-called SEPSIS SIX, based on early recognition with the NEWS has been widely adopted, and evidence suggests that this method has been effective [93, 94]. It is not clear how the new SEPSIS-3 definition, based on a 2-point change in the SOFA score can improve these outcomes. A fundamental criticism of this new definition is that it perpetuates the notion of sepsis as a single entity with a common pathophysiological bias, i.e. that of being susceptible to a single therapeutic intervention (if only it is possible to identify what that might be).
Moving increasingly towards the world of customised medicine, the idea of being able to separate patients with sepsis into subgroups becomes more attractive than leaving them all grouped together in a single category [95]. These subtypes could be characterised by an entire spectrum of phenotypic or biological characteristics: We could identify adult patients with pneumococcus pneumonia or patients with a particular combination of biomarkers or a genotype at risk plus a specific clinical risk factor. This approach faces significant challenges. The first is to establish plausible hypotheses, although this is becoming more probable with the use of big data and bio-information technology. The second is that these populations are by definition subgroups and therefore smaller in number than we were used to working with, and there are both scientific and commercial pressures to try and avoid narrow focuses. However, the introduction of rapid microbiological diagnostic processes, such as MALDI TOF [96] or molecular ones, means that real-time bacterial diagnosis is now a reality, and there are examples of drugs developed for the treatment of specific types of bacterial sepsis [97].
3.2 Should Combined Antibiotic Therapy Be Used Routinely in the Empiric Treatment of Septic Patients?
The main principles for using antibiotics in sepsis are now free of controversy:
-
(a)
They should be commenced as soon as a clinical diagnosis of sepsis is made: speed is essential.
-
(b)
It is important to use a regime with adequately broad spectrum of activity that will be active against the most likely pathogen agents.
-
(c)
The dose should be optimised using a load dose if necessary and taking some variables into consideration (e.g. the use of hemoperfusion) that may alter pharmacokinetics in septic patients.
-
(d)
Ideally, the regime should be quickly de-escalated to reduce the spectrum.
There is a further consideration that is sometimes discussed that the use of bactericide drugs is preferable to bacteriostatics. Although this may seem intuitively correct, there is not much clinical data to support it. The problem that clinicians encounter is whether an adequately broad spectrum of activity suggests the routine use of a combination of antibiotics in these critically ill patients. The problem is that selecting an antibiotic regime that then proves to be inactive against the organism that is subsequently isolated in blood cultures is associated with a significantly higher mortality [98]. This is a strong incentive to choose a cautious, defensive approach in prescribing multiple antibiotics. There are advantages and disadvantages in using combination therapy; although it is likely that if it were possible to prove a measurable benefit for survival from the routine use of two or more drugs, then this would act as a counterweight to potential disadvantages.
Advantages of combined antibiotics
-
(a)
They will guarantee a broader spectrum of activity than the one obtained by a single drug.
-
(b)
They can produce an additive or even a synergic effect.
-
(c)
They can reduce the risk of the emergency of resistance during treatment.
-
(d)
They can produce non-antimicrobial, beneficial pharmacological effects.
Disadvantages
-
(a)
A generally wider use of antibiotics would probably bring about the problem of resistance to antimicrobials.
-
(b)
It would probably increase the risk of toxicity.
-
(c)
It can increase the risk of superinfection (e.g. with fungi).
-
(d)
It can increase the probability of undesired and unexpected interactions between drugs.
-
(e)
It can increase costs.
The current recommendations of the Surviving Sepsis Campaigns are that combination therapy is indicated for:
-
(a)
Neutropenic patients with severe sepsis
-
(b)
Patients that have or are likely to have infections with organisms that are resistant to most drugs such as Acinetobacter or Pseudomonas or Klebsiella
-
(c)
Selected patients with severe infections associated with respiratory failure and/or septic shock associated with bacteraemia from Pseudomonas
-
(d)
Shock from infection from Streptococcus pneumoniae
The evidence that supports these recommendations varies in quality, but putting to one side these special cases, the question remains of whether there can really be a benefit from using a combination routine in routine treatment of most septic patients. A widely cited work [99] that is often reported [100] shows that there is actually a benefit for survival from using combination therapy. However, this work is a retrospective analysis and not a controlled prospective, randomised study and even here the apparent benefit is limited to some subgroups of patients. The more powerful beta-lactams (e.g. carbapenems and third and fourth generation anti-pseudomonal cephalosporins) have failed to prove any benefits in combinations, probably because they already act with a virtually 100% bactericide activity against the most common pathogens, so that there is little room for other improvements [22]. Some authors have carried out a meta-regression analysis of about 50 trials and have proven that there are no benefits from multiple agents, although there was a statistically significant effect in the more critical patients [101]. The most convincing evidence comes from a well-conducted, controlled, randomised trial on patients in Intensive Care with varying levels of sepsis that compared meropenem alone with a combination of meropenem and moxifloxacin [102] in which there was no evidence of benefits from combining the two antibiotics. It is difficult to interpret the information in this area as the populations of patients studied in the various trials and the regimes of antibiotics used vary greatly. In spite of this, taken as a whole, this data shows that there is no evidence to support the routine use of combined antibiotic therapy for most septic patients.
In spite of this, there is legitimate concern that in some clinical situations and actually in some countries, the risk of sepsis from gram-negative MDR is so high that combination therapy is essential. In particular, it has been suggested that in a somewhat counterintuitive way, organisms that produce carbapenemases such as strains of Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter may benefit from treatments with combinations of carbapenems [103, 104]. The logic is that the two drugs together can reach the MIC concentration threshold in spite of in vitro resistance. A more recent work [105] however presents data that contradicts all this, suggesting that combination therapy was only efficient if the drugs were effective in vitro and administered together with colistin or tigecycline. None of these studies is free of methodological problems, however, and this is still an unresolved problem.
3.3 Should Beta-Lactam Antibiotics Be Administered by Continuous Infusion in Septic Patients?
Antimicrobial broad-spectrum beta-lactams, with their good safety profile, are a good choice as empiric agents for septic patients. Different antibiotics have different modes of action, and in the case of beta-lactams, the time over the MIC (T > MIC) (which is the amount of time during which the antibiotic concentration in the relevant tissue space exceeds the minimum inhibitor concentration required to kill the bacteria) is the critical pharmacokinetic characteristic that determines efficacy. These are the reasons why many have believed that it is much more sensible to administer beta-lactams by continuous infusion as this will optimise the T > MIC. The situation is complicated even further because the profound shift of fluids that can occur in septic patients, and intervention such as hemofiltration, can considerably alter the pharmacokinetics of several drugs, including antibiotics [106]. Sepsis studies have actually shown marked variability in serum levels of beta-lactams during treatment and have also shown that the administration of these drugs by continuous infusion truly improves the pharmacokinetic parameters [107].
The answer is by far not a clear one. In a 2013 preliminary study, some Australian authors from Brisbane proved that even though infusion did improve the pharmacokinetics, there was no overall effect on survival in Intensive Care, but there was a statistically significant improvement in clinical [108–109]. More recently, the BLISS trial reached practically the same conclusions [110]. In the meantime, the Brisbane group had published another large-scale, controlled, randomised trial that failed to prove a statistically significant benefit from continuous infusion [111]. Lastly, a meta-analysis of 632 patients studied three large-scale controlled trials and concluded that there was a statistically significant benefit from continuous infusion for both clinical care and for survival, but when the multi-variate analysis was carried out, the independent effect of continuous infusion was lost [112]. Those who defend this approach could argue that it is unreasonable to require that survival in Intensive Care or hospital outcome are the two primary end points of these trials, as the purpose of the intervention is to improve treatment of the infection, and there are several other reasons why a patient may not survive in Intensive Care or on admission to hospital, even if the infection is treated effectively. Other hypotheses include the possibility that it is only a subgroup of septic patients (usually the most ill ones) that will benefit or that it is necessary to monitor the drug to ensure that optimal levels of the drug are reached even with continuous infusion [113]. Increasing the beta-lactams dosage to bring them into the optimal range would however bring with some risks with it: extremely high doses are associated with neurotoxicity although this generally unusual. Lastly, it has been suggested that rather than the seric level of antibiotics, the critical measurement for efficacy is that actual bactericide effect in vivo, in the appropriate tissue space, against the specific infecting organism. A method has been described for measuring all this based on the time required for a culture to become positive in the presence of an antibiotic (time to positivity: Tpos), and it has proven that there is a correlation between Tpos and the length of stay in Intensive Care [114]. It is a rather arduous method that measures seric levels, and it must still be decided whether it may have some clinical use.
To sum up, continuous infusion of beta-lactams is a logical indication with no significant risk; however if we take survival in Intensive Care as the final test of a new intervention, this strategy is not supported by current data.
3.4 Should We Use Biomarkers to Limit the Duration of Antibiotic Treatment?
Most clinicians agree that generally speaking, it is right for the duration of antibiotic treatment to be as short as possible, commensurate with obtaining a satisfactory clinical outcome. This applies to all clinical contexts and not just to sepsis, but it is interesting that the matter of therapy duration is not frequently studied. There are some well-known examples—infections of the urinary tract or tuberculosis—but most antibiotic prescriptions are based on habits and practice rather than on solid data obtained from comparative clinical trials. Again, the potential advantages of shorter courses of antibiotics are relatively non-controversial, and as Intensive Care is an area where antibiotics are highly used, there is much pressure to see how the use of antibiotics may be subject to limitations (antibiotic stewardship).
Biomarkers are a potentially attractive way of controlling the use of antibiotics. There is a large amount of literature regarding the use of biomarkers in sepsis, but in terms of their role in controlling antibiotics, recent works have concentrated on procalcitonin. There are two approaches that could be taken into consideration for the use of biomarkers (and one does not exclude the other): the first question—can I use a biomarker that tells me that the patient does not have an infection and I do not need to commence antibiotics or if I have already begun them I am now quite sure that there is no infection and can I suspend the antibiotics? Interestingly, the Surviving Sepsis Campaign takes this approach and recommends, but only with a moderate level of evidence, that procalcitonin or other similar biomarkers can be used to help the doctor in discontinuing empiric antibiotics in patients who initially appear to have sepsis, but who do not have subsequent evidence of infection. The alternative strategy is try to use a biomarker to answer the question: has the infection been cured and can I stop antibiotics?
There is still a large amount of evidence that suggests that the use of procalcitonin provides safe, credible information about when to discontinue antibiotics in patients with sepsis in Intensive Care [115]. Some studies have shown that sequential measures (normally every day) could identify positive- or negative-culture patients and also the ones that are destined to survive or to die [116] and several trials have shown that the use of antibiotics is less in patients who are followed with an active protocol based on procalcitonin measurements [117, 118]. Even more surprisingly a recent large trial also showed a benefit for survival [119]. A recent HTA [119] concluded that in spite of limited data, procalcitonin can be efficient and cost-effective when used to guide discontinuation of antibiotics in adults treated for suspected or confirmed sepsis in Intensive Care.
Still today, sepsis is a common, difficult problem. There is no magic bullet on the horizon, so in the meantime we need to optimise those aspects of the treatment that we can control by using the best quality data that we have. Faced with the problem of antibiotic resistance, we must use the antibiotics available with increasing shrewdness and while saving as much as possible. This is why it is necessary to run a microbiological path alongside the sepsis path, based on the modern development of molecular microbiological diagnostics and to run diagnostic stewardship alongside antibiotic stewardship [120, 121].
Sepsis stewardship, antibiotic stewardship and diagnostic stewardship are today both a moral and scientific obligation.
Change history
21 June 2019
This book was inadvertently published with incorrect figure captions for figures 9.3, 9.4, 9.5, 9.6, 9.7, 9.8, 9.9 and 9.10.
References
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10.
Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:775–87.
Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762–74.
Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55.
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6.
Vincent J-L, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53.
Klein Klouwenberg PM, Ong DS, Bonten MJ, et al. Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive Care Med. 2012;38:811–9.
Vincent JL, Opal SM, Marshal JC. Sepsis definitions: time for change. Lancet. 2013;381:774–5.
Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–38.
Churpek MM, Zadravecz FJ, Winslow C, et al. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015;192:958–64.
Rhodes A, et al. Surviving Sepsis Campaign International Guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43:304–77. Crit Care Med 2017; 45: 486–552.
Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317:301–8.
Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the sofa score, sirs criteria, and qsofa score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:290–300.
Henning DJ, et al. An emergency department validation of the SEP 3 Sepsis and Septic Shock definitions and comparison with 1992 consensus definitions. Am Emerg Med. 2017;70:544.
Casanova JL, Abel L. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu Rev Genomics Hum Genet. 2013;14:215–43.
Hotchkiss RS, Monneret G, Payen D. Sepsis induced immunosuppression from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74.
Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260.
Deutschmann CS, Tracey KJ. Sepsis: current dogma and new perspective. Immunity. 2014;40:463–75.
Mira J, et al. Sepsis pathophysiology, chronic critical illness and persistent inflammation Immunosuppression and catabolism syndrome. Crit Care Med. 2017;45:253–62.
Shankar-Hari M, Rubenfeld GD. Understanding long term outcomes following sepsis: implication and challenges. Curr Infect Dis Rep. 2016;18:31.
Monneret G, Venet F. Sepsis induced immune alterations monitoring by flow cytometry as a promising tool for individualized therapy. Cytometry B Clin Cytom. 2016;90:376–86.
Vincent JL, Bassetti M, Francois B, et al. Advances in antibiotic therapy in the critically ill. Crit Care. 2016;20:133.
Kollef MH, Sherman G, Ward S. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74.
Ibrahim EH, Sherman G, Ward S. Treatment of bloodstream infections on patients outcomes in the ICU setting. Chest. 2000;118:146–55.
Kumar A, Daniel R, Kenneth W. Duration of hypotension before initiation effective antimicrobial therapy is the critical determinant of serviva in human septic shock. Crit Care Med. 2006;34:1589–96.
Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline based performance improvement program. Crit Care Med. 2014;42:1749–55.
Vazquez-Guillamet C. Using the number needed to treat to assess appropriate antimicrobial therapy as a determinant of out come in severe sepsis and septic shock. Crit Care Med. 2014;42:2342–9.
Tabah A, Koulenti D, Lapland K. Characteristics and determinants of out come of hospital acquired bloodstream infections in intensive care units: the EUROBACT International color study. Intensive Care Med. 2012;38:1930–45.
Raman G, Avendano E, Berger S. Appropriate initial antibiotic therapy in hospitalized patients with gram negative infections: systematic review and meta analysis. BMC Infect Dis. 2015;15:395.
Vincent JL, Moreno R, Takala J. The SOFA (Sepsis related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10.
Vincent JL. Dear SIRS, I am sorry to say that I do not like you. Crit Care Med. 1997;25:372–4.
Vincent JL, de Mendonca A, Cantraine F, Working group on Sepsis Related Problems of the European Society of intensive Care Medicine. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter prospective study. Crit Care Med. 1998;26:1793–800.
Nair GB, Niederman MS. Ventilator associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015;41:34–48.
Fabregas N, Ewing S, Torres A. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post mortem lung biopsies. Thorax. 1999;54:867–73.
Lauzier F, Ruest A, Cook Canadian critical care trials group. The value of pretest probability and modified clinical pulmonary infection score to diagnose ventilator associated pneumonia. J Crit Care. 2008;23:50–7.
Zagli G, Cozzolino M, Terreni A. Diagnosis of ventilator associated pneumonia: a pilot, exploratory analysis of a new score based on procalcitonin and chest echography. Chest. 2014;146:1578–85.
Rhee JY, Kwon KT, Ki HK. Scoring systems for prediction of mortality in patients with intensive care unit acquired sepsis: a comparison of the PITT bacteremia score and the APACHE II scoring systems. Shock. 2009;31:146–50.
Vasudevan A, Mukhopadhyay A, Li J. A prediction tool for nosocomial multi drug resistant gram negative bacilli infections in critically ill patients: prospective observational study. BMC Infect Dis. 2014;14:615.
Tumbarello M, Trecarichi EM, Tumietto F. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2014;58:3514–20.
Webb A, Dascomb K, Stenehjem E. Derivation and multicenter validation of the drug resistance in pneumoniae clinical prediction score. Antimicrob Agents Chemother. 2016;60:2652–63.
Burham JP, Lane MA, Kollef MH. Impact of sepsis classification and multidrug resistance status on outcome among patients treated with appropriate therapy. Crit Care Med. 2015;43:1580–6.
Simon L. Serum procalcitonin and c reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17.
Christ-Crain M, Stolz D, Bingisser R. Procalcitonin guidance of antibiotic therapy in community acquired pneumonia. Am J Respir Crit Care Med. 2006;174:84–93.
Schuetz P, Christ-Crain M, Thomann R. Effect of procalcitonin based guidelines versus standard guidelines on antibiotic use in lower respiratory tract infections. JAMA. 2009;302:1059–66.
Bouadma L, Luyt CE, Tubach F. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial) a multicenter randomized controlled trial. Lancet. 2013;375:463–74.
Schuetz P, Muller B, Christ-Crain M. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections (review). Cochrane Database Syst Rev. 2012;(9):CD007498.
Wacker C, Prkno A, Brunkhorst FM. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:426–35.
Guan J, Zhaofen L, Hong L. Dynamic change of procalcitonin rather than concentration itself, is predictive of serviva in septic shock patients when beyond 10ng/ml. J Shock. 2011;36:570–4.
Georgopoulou AP, Savva A, Giamarellos-Bourboullis EJ. Early changes of procalcitonin may advise about prognosis and appropriateness of antimicrobial therapy in sepsis. J Crit Care. 2011;26:331.e1–7.
Mitsuma SF, Mansour MK, Dekker JP. Promising new assays and technologies for the diagnosis and management of infectious diseases. Clin Infect Dis. 2013;56:996–1002.
Bassetti M, De Waele JJ, Eiggmann P. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med. 2015;41:776–95.
De Jong E, Van Oers JA, Beishuizen A. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomized, controller, open label trial. Lancet Infect Dis. 2016;16:819–27.
Linscheid P, Seboek D, Zulewski H. Autocrine/paracrine role of inflammation mediated calcitonin gene related and adremedullin expression in human adipose tissue. Endocrinology. 2005;146:2699–707.
Christ-Crain M, Morgenthaler NG, Strunck J. Mid regional pro adrenomedullin as a prognostic marker in sepsis; an observation study. Crit Care. 2005;9:R816–24.
Angeletti S, Dicuonzo G, Fioravanti M, Procalcito N. Procalcitonin levels in surgical patients at risk of candidemia. J Infect. 2010;60:425–30.
Angeletti S, et al. Procalcitonin, MR proadrenomedullin and cytokines measurement in sepsis diagnosis: advantages from test combination. Dis Markers. 2015;2015:951532.
Angeletti S, Battistoni F, Fioravanti M. Procalcitonin and mid regional pro-adrenomedullin test combination in sepsis diagnosis. Clin Chem Lab Med. 2013;51:1059–67.
Suberviola B, Castellanos-Ortega A, Ruiz A. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and pro ADM in a single determination on ICU admission. Intensive Care Med. 2013;39:1945–52.
Charles PE, Castro C, Ruiz-Santana S. Serum procalcitonin levels in critically ill patients colonized with Candida Spp:new clues for the early recognition of invasive candidiasis. Intensive Care Med. 2009;35:2146–50.
The PRISM Investigators. Early, goal-directed therapy for septic shock — a patient-level meta-analysis. N Engl J Med. 2017;376:2223–34.
The ProCESS Investigators. A randomized trial of protocol based care for early septic shock. N Engl J Med. 2014;370:1683–93.
Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–11.
The ARISE Investigators and the ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496.
Berger RE. Management of septic shock: a woman with septic shock. N Engl J Med. 2017;376:2282–5.
Seymour CW. Time to tretment and Mortality during mandate emergency care for sepsis. N Engl J Med. 2017;376:2235–44.
Liu VX, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:934.
Lee CC, et al. Timing of appropriate empirical antimicrobial administration and outcome of adults with community onset bacteremia. Crit Care. 2017;21:119.
Peltan ID, et al. Physician variation in time to antimicrobial treatment for septic patients presenting to emergency department. Crit Care Med. 2017;45:1011–8.
Leisman DA. Delayed second dose antibiotics for patients admitted from the emergency department with sepsis: prevalence, risk factors and outcomes. Crit Care Med. 2017;45:956–65.
Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med. 2015;43:1907–15.
Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39:2066–71.
Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61.
Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care. 2016;20:89.
Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–54.
Gropen TI, Gagliano PJ, Blake CA, et al. Quality improvement in acute stroke: the New York State Stroke Center Designation Project. Neurology. 2006;67:88–93.
Cooke CR, Iwashyna TJ. Sepsis mandates: improving inpatient care while advancing quality improvement. JAMA. 2014;312:1397–8.
Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189:548–55.
Bloos F, et al., for the Medusa Study group. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multicenter study. Crit Care. 2014;18:R42.
Boyer A, Vargas F, Coste F, et al. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med. 2009;35:847–53.
Kobayashi L, Konstantinidis A, Shackelford S, et al. Necrotizing soft tissue infections: delayed surgical treatment is associated with increased number of surgical debridements and morbidity. J Trauma. 2011;71:1400–5.
Martinez ML, et al., for the Edusepsis Study group. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017;45:11–9.
Opal SM. Source control in sepsis urgent or not so fast. Crit Care Med. 2017;45:130–2.
Bone RC. Sepsis, the sepsis syndrome, multi-organ failure: a plea for comparable definitions. Ann Intern Med. 1991;114:332–3.
Brown T, Ghelani-Allen A, Yeung D, et al. Comparative effectiveness of physician diagnosis and guideline definitions in identifying sepsis patients in the emergency department. J Crit Care. 2015;30:71.
Cohen J, Opal S, Calandra T. Sepsis studies need new direction. Lancet Infect Dis. 2012;12:503–5.
Deutschman CS. Imprecise medicine: the limitations of sepsis-3. Crit Care Med. 2016;44:857–8.
Whittle J, Walker D. The new international sepsis guidelines (sepsis-3): the central message remains. Br J Hosp Med. 2016;77:208–11.
Marshall JC. Sepsis-3: what is the meaning of a definition? Crit Care Med. 2016;44:1459–60.
Petersen E, Zumla A. To have sepsis or to be septic – is the difference between these clinical conditions important? Int J Infect Dis. 2016;48:118–9.
Vincent J-L, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Klein Klouwenberg PM, Ong DS, Bonten MJ, et al. Classification of sepsis, severe sepsis and septic shock: the impact of minorvariations in data capture and definition of SIRS criteria. Intensive Care Med. 2012;38:811–9.
National Institute of Health and Care Excellence. Sepsis: recognition, diagnosis and early management. 2016. https://www.nice.org.uk/guidance/ng51.
NHS England. Improving outcomes for patients with sepsis. A cross system action plan. 2015. https://www.england.nhs.uk/wp-content/uploads/2015/08/Sepsis-Action-Plan-23.12.15-v1.pdf.
Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011;28:507–12.
Prescott HC, Calfee CS, Thompson BT, et al. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194:147–55.
Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614.
Bulger EM, Maier RV, Sperry J. A novel drug for treatment of necrotizing soft-tissue infections: a randomized clinical trial. JAMA Surg. 2014;149:528–36.
Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. https://doi.org/10.1007/s00134-012-2769-8.
Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38:1773–85.
Kumar A, Safdar N, Kethireddy S, et al. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38:1651–64.
Brunkhorst FM, Oppert M, Marx G, et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390–9.
Cprek JB, Gallagher JC. Ertapenem-containing double-carbapenem therapy for treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2016;60:669–73.
Oliva A, D’Abramo A, D’Agostino C, et al. Synergistic activity and effectiveness of a double-carbapenem regimen in pan drug resistant Klebsiella pneumoniae bloodstream infections. J Antimicrob Chemother. 2014;69:1718–20.
Bass SN, Bauer SR, Neuner EA, et al. Impact of combination antimicrobial therapy on mortality risk for critically ill patients with carbapenem-resistant bacteremia. Antimicrob Agents Chemother. 2015;59:3748–53.
Cotta MO, Roberts JA, Lipman J. Antibiotic dose optimization in critically ill patients. Med Intensiva. 2015;39:563–72.
Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double blind, randomized controlled trial. Clin Infect Dis. 2013;56:236–44.
Bates A, Joffe AR. Is there a role for continuous infusion of betalactam antibiotics in severe sepsis? J Thorac Dis. 2016;8:E437–9.
Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, openlabelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42:1535–45.
Dulhunty JM, Roberts JA, Davis JS, et al. A multicenter randomized trial of continuous versus intermittent β-lactam infusion in severe sepsis. Am J Respir Crit Care Med. 2015;192:1298–305.
Roberts JA, Abdul-Aziz MH, Davis JS, et al. Continuous versus Intermittent β-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194:681–91.
Jager NG, van Hest RM, Lipman J, et al. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol. 2016;9:961–79.
Jerwood S, Hankins M, Cohen J. A pilot clinical trial to evaluate a novel time-to-positivity assay to measure the effectiveness of antibiotic therapy for septic patients in intensive care. J Crit Care. 2012;27:320–5.
Hohn A, Heising B, Schutte JK, et al. Procalcitonin-guided antibiotic treatment in critically ill patients. Langenbecks Arch Surg. 2017;402:1.
Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190:1102–10.
Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–66.
de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomized controlled open label trial. Lancet Infect Dis. 2016;16:819–27.
Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in Intensive Care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost effectiveness analysis. Health Technol Assess. 2015;19:v.
Arena F, et al. Molecular antibiogram in diagnostic clinical microbiology: advantages and challenges. Future Microbiol. 2017;12:361–4.
Messacor K, et al. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017;55:715–83.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tulli, G. (2019). Diagnosis and Management of Sepsis and Septic Shock: An Evidence-Based Review. In: Chiumello, D. (eds) Practical Trends in Anesthesia and Intensive Care 2018. Springer, Cham. https://doi.org/10.1007/978-3-319-94189-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-94189-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94188-2
Online ISBN: 978-3-319-94189-9
eBook Packages: MedicineMedicine (R0)