Abstract
Restrictive blood transfusion strategies, with a hemoglobin transfusion threshold of 70 g/L, are safe for the majority of critically unwell patients in the ICU. However, patients with cardiovascular disease have a myocardium that is vulnerable to injury at rest. Critical illness may both decrease oxygen supply to and increase oxygen demand from the myocardium increasing the risk of myocardial infarction. Anemia may exacerbate this oxygen supply-demand imbalance. In the absence of high-quality evidence, guidelines for patients with acute coronary syndromes, and for patients with acute illness and coexisting chronic cardiovascular disease, currently recommend more liberal transfusion strategies although the optimum transfusion threshold and hemoglobin are uncertain. However, a restrictive hemoglobin transfusion threshold of 75 g/L appears safe for patients undergoing cardiac surgery.
This chapter will discuss the diagnosis of myocardial infarction in critical illness and the evidence for transfusion strategies for patients with acute coronary syndrome and chronic coexisting cardiovascular disease and for patients undergoing cardiac surgery.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cardiovascular disease (CVD) is the collective term for all diseases affecting the heart and blood vessels. Statistics from the British Heart Foundation (UK charity) show that approximately seven million people in the UK have coexisting CVD [1, 2]. In general non-cardiac critical care units, approximately 25–30% of all patients admitted will have coexisting cardiovascular disease, according to intensive care unit (ICU) casemix [3, 4]. CVD is the leading global cause of death, accounting for more than 17.3 million deaths in 2013 (31% of all global deaths), a number that is expected to grow to more than 23.6 million by 2030 [5]. Considerable geographical variation in mortality rates can occur within and between countries. For example, in 2014, in the UK, death rates from coronary heart disease were 45% higher in Scotland compared with the southeast of England. CVD accounted for 10% of all inpatient episodes in the UK National Health Service (NHS) in men and 6.2% in women. Unsurprisingly, the economic burden of CVD is high: in 2010, the estimated global cost of CVD was $863 billion and is estimated to rise to $1044 billion by 2030 [5].
Anemia in Cardiovascular Disease
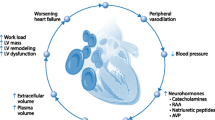
The myocardium has a limited anaerobic capacity and is dependent on a continuous supply of oxygen from the coronary circulation. At rest, the coronary blood flow is approximately 250 mL/min, representing 5% of cardiac output. The myocardium extracts 75% of the oxygen, which cannot increase significantly in response to increased oxygen demand, and the coronary sinus PO2 is subsequently very low (3 kPa) [6]. This oxygen extraction ratio (OER) is higher than for all other major organs, for which OERs of 25–30% are typical. In order to match the considerable increases in myocardial O2 consumption that occur during exercise (up to five times resting consumption), there must be substantial increases in coronary blood flow. Flow across the myocardium largely depends on the pressure gradient between the aortic root and the right atrium. The force from the contracting heart muscle is greatest in the left ventricular subendocardial layers where it approximates to intramyocardial pressure, and significant left ventricular coronary flow can occur only during diastole. The right coronary flow is less affected by systole because of the smaller right ventricular muscle mass and lower chamber pressures during the cardiac cycle. Any increase in heart rate will result in a reduction in diastolic time and will reduce perfusion time; the ratio of systolic to diastolic time becomes closer to one as heart rate increases. Coronary blood flow is also controlled by the diameter of the coronary arteries. This is under nervous and humoral control as well as local vasorestrictors and vasoconstrictors in the endothelium. Hypoxia causes coronary vasodilatation directly and also releases adenosine and opens ATP-sensitive potassium channels [7]. It follows from these features of normal coronary physiology that any reduction in coronary flow and/or coronary blood oxygen content will decrease myocardial oxygen delivery, which could cause myocardial ischemia.
Anemia is associated with worse outcomes in patients with CVD, both in terms of severity of illness and mortality. Anemia is associated with poor outcome in ischemic heart disease [8], chronic heart failure [9], rhythm disturbance, and mortality and major adverse cardiovascular events in acute coronary syndrome [10, 11]. This may be due to increased myocardial workload and adverse left ventricular and large artery remodeling. Anemia is also associated with worse postoperative mortality in patients with CVD [12], suggesting that patients with coexisting CVD are less tolerant of anemia than patients without CVD. This is consistent with animal studies which showed that dogs with experimentally created coronary stenoses developed ischemic ECG changes at higher Hb concentrations compared to those with normal coronary arteries (stenoses 70–100 g/L vs normal 30–50 g/L) [13]. However, the evidence in humans is mainly from observational studies, and it is difficult to tease out whether the anemia is exacerbating the underlying condition or is a reflection of the severity of the underlying disease. It follows, therefore, that reversing anemia with RBC transfusion may not improve patient prognosis. These questions can only be answered with certainty by well-designed randomized trials in relevant patient populations.
There are additional reasons why patients with acute coronary syndrome (ACS) may have poorer outcomes if they are anemic. Reduction in the oxygen delivery to infarcted or ischemic myocardium may promote arrhythmias, worsen hypotension, and increase infarct size [14]. Patients with anemia may also have antiplatelet therapy withheld, due to concerns of the associated bleeding risks. For example, in the CADILLAC trial, a trial investigating the effectiveness of Abciximab (a platelet glycoprotein IIb/IIIa inhibitor), 18% of patients with anemia at the time of their ACS were no longer receiving aspirin at 1 year follow-up [15]. The prevalence of anemia among patients presenting with ACS is increasing, in part because of the aging population and the presence of coexisting morbidities. Anemia at presentation with ACS is associated with poorer short- and long-term outcomes [16], and bleeding events during treatment for ACS are associated with greater mortality and other cardiovascular events [17]. Some studies have found that hemoglobin decrement during hospitalization for ACS is an independent predictor of subsequent death or hospitalization with myocardial infarction (MI) [18, 19]. These associations highlight the importance of establishing whether blood transfusions to correct anemia can modify risk of adverse outcomes among anemic patients with ACS.
Anemia in Critically Ill Patients with CVD
Anemia causes an increase in cardiac output, achieved by an increase in heart rate and stroke volume and the reduction in vascular resistance associated with reduced blood viscosity (lower hematocrit) . In acute and critical illness, tachycardia reduces diastolic filling time and hypotension reduces the pressure gradient across the left ventricle. Both of these reduce blood flow through the coronary arteries. The use of catecholamines increases myocardial O2 demand, and global O2 demand is also increased. The myocardial oxygen supply is reduced in patients with anemia, and patients with coexisting cardiovascular disease with potentially atheroma-related flow-limiting disease have limited ability to compensate.
Myocardial Infarction
Myocardial infarction (MI) is defined according to the Third Universal Definition as evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. This requires the presence of a rise and/or a fall pattern of cardiac biomarkers (usually Troponin I (TnI) or Troponin T (TnT)), with at least one value above the 99th percentile of the upper reference limit. This should occur with at least one of the following [20]:
-
Symptoms of ischemia
-
Electrocardiographic (ECG) evidence of myocardial ischemia (new or presumed new significant ST-segment-T-wave changes or new left bundle branch block or pathological Q wave changes in the ECG)
-
Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality or identification of an intracoronary thrombus by angiography
MI can be further categorized according to its likely cause [20] (Table 8.1).
Patients with myocardial necrosis (elevated troponin concentrations) in the absence of symptoms or signs of myocardial ischemia are classified as having myocardial injury. Acute myocardial injury occurs where troponin concentrations display a dynamic rise and fall pattern, compared with chronic myocardial injury, where troponin concentrations remain elevated but unchanged on serial testing. Chronic myocardial injury may be found in patients with chronic heart failure, renal failure, and coronary artery disease in the community [21, 22]. Acute myocardial injury is common in critically ill patients with CVD and may be secondary to sepsis [23, 24], acute exacerbation of COPD [25], acute intracerebral pathology [26], or pulmonary embolism [27, 28].
Outcomes of Type II Myocardial Infarction and Myocardial Injury
Outcomes for patients with both type II MI and myocardial injury are poor, especially in the critical care setting. There is currently no consensus on the optimal cardiac investigation, management, or treatment strategy for these conditions. For consecutive unselected hospital inpatients out with the critical care setting, patients with type II MI or myocardial injury have worse outcomes than patients who present with type I MI, with a third of patients dead at 1 year [29] and 60% of patients with type II and 75% of patients with myocardial injury dead at 5 years [30]. However, this reflects all-cause mortality, and it is not known whether therapeutic intervention can improve outcomes. Patients with type II MI were twice as likely as those with myocardial injury to be admitted with a type I MI during the subsequent year, suggesting that a proportion of patients with type II MI may benefit from further investigation and treatment for coronary artery disease.
Diagnosis of MI in Critical Illness
Diagnosis of MI in patients with critical illness is not straightforward. Many patients are unable to communicate any symptoms due to sedation and ventilation, strong analgesia, distracting injuries, and delirium. TnI elevation is common in critical illness and in addition to cardiac causes has multiple non-cardiac etiologies as described above. ECGs are typically not performed routinely, and ECG interpretation is difficult due to tachycardia, arrhythmias, and non-specific changes. Two groups have looked at ECGs taken routinely in heterogeneous critically ill patients [31,32,33]. They found that ECG interpretation by clinicians had poor agreement for the presence of myocardial ischemia or infarction. This was improved to moderate agreement once the ECG was interpreted alongside the patient’s troponin values. Specific ECG changes such as bundle branch block had high reliability, compared to non-specific T-wave flattening.
Bedside imaging is limited to transthoracic echocardiography, which may miss small but important regional wall motion abnormalities. In critical care settings, echocardiography is frequently technically difficult, and an injury involving >20% of myocardial wall thickness may be required to detect a wall motion abnormality [34].
Diagnosis of MI in Critically Ill Patients with Coexisting CVD
Patients with coexisting CVD are at high risk of further myocardial injury during critical illness. Surgery and trauma induce an inflammatory state, with increase in the concentrations of cytokines such as TNF-alpha, interleukin-1, interleukin-6, and CRP. Patients are hypercoagulable due to increases in PAI-1, factor VIII, and platelet reactivity and decreases in antithrombin III concentrations. Furthermore, patients have increased catecholamine and cortisol levels as a result of physiological stress. All of these may lead to coronary artery shear stress, plaque fissuring, and subsequent acute coronary thrombosis, or type I MI [35]. Even in the absence of plaque rupture, increased oxygen demand and reduced oxygen delivery in the presence of stable atherosclerotic stenosis may result in type II myocardial infarction.
It is important to attempt to delineate the mechanism of raised TnI in critically ill patients with CVD, in order to identify patients where cardiac or coronary investigations or therapies may be indicated. For the patient who presents with sub-massive pulmonary embolism, TnI elevation may be secondary to right ventricular strain or hypoxia, and coronary angiography is both unwarranted and an unnecessary risk. For the patient who presents with community acquired pneumonia, chest pain, and ECG changes, TnI elevation may be due to hypoxia, tachycardia, or hypotension, with the acute illness representing a physiological stress test. In this context, it is appropriate to diagnose acute MI. These examples illustrate how the complexity of cases presenting to critical care, especially the interplay between pre-existing and complex acute disease, make the diagnosis of MI difficult in individual patients.
Management of MI in Critically Ill Patients with Coexisting CVD
If type I MI is suspected , then invasive coronary angiography should be considered. However, if TnI elevation is in the context of oxygen supply-demand imbalance, then the need for further investigation and treatment is uncertain [36]. A survey of 310 intensivists regarding treatment strategies for critically ill patients with elevated troponin and without typical symptoms of MI or ECG changes found that 76% would start aspirin or clopidogrel, 47.4% would start heparin, 48.9% would start high-dose statins, 68.7% would start beta-blockers, and 37.6% would use an ACE-inhibitor. 72.7% would request a cardiology consultation, and 51.3% would refer for an angiogram once the patient was stable [37]. These responses indicate substantial clinical variation and uncertainty regarding best practice. In addition, patients with pre-existing CVD are frequently already on secondary prevention therapies such as antiplatelet agents and statins. The risk to benefit ratio of continuing agents during a critical illness episode, which agents should be prioritized, and the optimum timing of restarting therapies during recovery are all areas of clinical uncertainty. Based on lack of clear evidence, the risks and benefits of primary and secondary treatment have to be assessed on an individual basis. For example, critically ill patients often have significant coagulation abnormalities. Approximately 25–34% of critically ill medical patients are thrombocytopenic [38], and patients with platelet counts of <50 × 109/L have a four- to fivefold increased risk of bleeding compared with patients with higher platelet counts [39]. In these patients, the risk of bleeding from heparin and antiplatelet medication may outweigh potential benefits to myocardial function.
Transfusion in Critically Ill Patients with CVD
Red blood cell transfusion may increase oxygen delivery to the myocardium, thereby reducing the risk of myocardial ischemia and necrosis. Current guidelines advocate a restrictive use of blood transfusions for general hospital inpatients including those who are critically unwell (Table 8.2) [41, 42, 44, 45]. These have highlighted the lack of evidence and uncertainty regarding best practice for patients with acute or chronic cardiovascular disease [41, 42, 44, 45]. The National Institute for Health and Care Excellence (NICE) blood transfusion guideline, published in November 2015, stated that the optimal transfusion threshold for patients with ongoing acute coronary syndrome was 80–100 g/L but made no specific recommendation for patients with chronic cardiovascular disease and highlighted the need for further research in this specific population [43].
Transfusion in Acute Coronary Syndrome
Until recently, the only data available exploring the association between anemia, transfusion practice, and clinical outcomes in ACS were observational cohort studies. These provide contrasting and contradictory data but in meta-analysis suggest an association between blood transfusions and higher risk of mortality and reinfarction [46]. However, as with all observational research exploring the association between anemia, transfusion, and clinical outcomes, these studies are potential subjects to confounding by indication [47, 48]. At best, these studies are hypothesis generating but indicate the need for randomized trials in the setting of ACS.
There are no completed large RCTs, but two pilot RCTs of RBC transfusion in acute coronary syndrome have been published. The CRIT pilot trial randomized 45 patients with ACS to either a liberal transfusion threshold (transfusion at hematocrit <30%) or a restrictive transfusion threshold (hematocrit <24%). Baseline hematocrit was similar (liberal 26.9% vs restrictive 27.5%, p = 0.4). More patients in the liberal arm were transfused (100% vs 54%, p < 0.001), and the average number of units transfused per patient was higher in the liberal arm (2.5 vs 1.6, p = 0.07). The primary composite endpoint of in-hospital death, recurrent myocardial infarction, or congestive heart failure occurred in eight patients in the liberal arm and three in the conservative arm (38% vs 13%, p = 0.046) [49]. However, most of the excess events in the liberal group (eight versus two patients) were accounted for new or worsening heart failure, which could have been related to transfusion. There was no difference in deaths.
The MINT pilot trial (liberal Hb 100 g/L vs restrictive 80 g/L or symptoms of ischemia) found a trend for fewer major cardiac events and deaths in patients randomized to the liberal arm [50]. Baseline characteristics were similar between groups except age (liberal, 67.3; restrictive, 74.3). The mean number of units transfused was 1.6 in the liberal group and 0.6 in the restrictive group. The primary outcome (composite of death, myocardial infarction, or unscheduled revascularization up to 30 days) occurred in 6 patients (10.9%) in the liberal group and 14 (25.5%) in the restrictive group (risk difference = 15.0%; 95% confidence interval of difference 0.7–29.3%; p = 0.054 and adjusted for age p = 0.076). Death at 30 days was less frequent in liberal group (n = 1, 1.8%) compared to restrictive group (n = 7, 13.0%; p = 0.032).
The full MINT trial of RBC transfusion in ACS started recruiting early in 2017 and is aiming to recruit 3500 patients by 2021. The liberal arm will be transfused at a hemoglobin concentration of 100 g/L compared to 80 g/L (or symptoms of angina) for the restrictive arm. The primary outcome will be a composite outcome of all-cause mortality or nonfatal myocardial reinfarction. Until this trial reports, and despite the paucity of evidence, guidelines currently recommend a higher transfusion threshold of >80 g/L for patients with ACS. This threshold is higher than the value of 70 g/L suggested in guidelines for most other groups but is still close to the restrictive threshold used in these inconclusive pilot trials. Clinicians currently need to make decisions based on individual patient status, for example, whether patients are tachycardic and hypotensive or have evidence of ischemia .
Chronic Cardiac Disease
We conducted a systematic review and meta-analysis assessing the effect of restrictive vs liberal red cell transfusion strategies on patient outcomes restricted to adult patients with coexisting cardiovascular disease, excluding patients undergoing cardiac surgery [51]. We were able to extract data on patients with CVD from 11 RCTs that compared restrictive and liberal strategies for 30-day mortality and 9 RCTs for new events of ACS.
We found no evidence of a difference in 30-day mortality between restrictive and liberal transfusion threshold groups. However, we found that a restrictive transfusion threshold was associated with a 78% increased risk of ACS in patients with cardiovascular disease with low heterogeneity between trials suggesting this increased risk is a consistent finding (RR 1.78, 95% CI 1.18–2.70, moderate quality of evidence as assessed by GRADE). There was no difference in the incidence of pulmonary edema between restrictive and liberal transfusion strategies, but heterogeneity existed between trials, and the GRADE quality of evidence was judged as very low. It is possible that this outcome includes cases of transfusion associated circulatory overload (TACO), which is more likely with liberal strategies. There was no difference in hospital length of stay between restrictive and liberal transfusion strategies, and other outcomes were rare, with inadequate data for meta-analysis. There were limitations in the systematic review, notably the definition and diagnosis of ACS . In some trials ACS was diagnosed by the clinicians, who were not blinded to the transfusion arm, which increases the chance of ascertainment bias. Another limitation was the heterogeneity of the clinical setting of the trials, varying between orthopedics, critical care, and GI bleeding. The extent and duration of physiological stress and the duration of exposure to anemia also varied between settings, and this could have an impact on the risk-benefit balance for transfusion.
This review suggested that for anemic patients with CVD, the use of restrictive hemoglobin thresholds (Hb concentration 70–80 g/L) was associated with higher rates of ACS than liberal thresholds (90–100 g/L). No effects on mortality or other important outcomes were demonstrated. The currently available quality of evidence for all outcomes was low. These data support the use of a more liberal transfusion threshold (greater than 80 g/L) for patients with both acute and chronic cardiovascular disease, until adequately powered high-quality randomized trials have been undertaken in this patient population.
Cardiac Surgery
Cardiac surgery represents a major consumer of red blood cells in all healthcare systems. There is a physiological rationale that anemia may be harmful during cardiac surgery, when coronary blood flow may be compromised, the myocardium acutely injured by surgery, and global oxygen demands increased by the stress response to surgery. However, there are theoretical reasons for harmful effects from transfusion of allogeneic blood, including immune suppression and infection and the pro-inflammatory and procoagulant effects of stored red blood cells [52].
Observational cohort studies mostly show associations between anemia and a range of adverse outcomes following cardiac surgery, especially infection and mortality. However, associations also exist between blood transfusions and these outcomes, even after attempts to adjust for hemoglobin concentrations [53]. This observational research is unable to delineate the relative risk to benefit ratio of anemia and blood transfusion, especially as other issues such as patient case mix and the red cell product could modify the association.
Several RCTs have explored the effectiveness of liberal versus restrictive transfusion practice among anemic patients undergoing cardiac surgery. A systematic review and meta-analysis by Patel and colleagues reviewed all studies published to May 2015, identifying six RCTs involving 3352 patients [54]. Meta-analysis found a pooled fixed effects mortality odds ratio (liberal versus restrictive transfusion threshold) of 0.70 (95% CI 0.49–1.02; p = 0.060), indicating a trend toward better outcomes with more liberal practice. This contrasted with the direction of effect in RCTs in the non-cardiac surgery setting supporting the hypothesis that patients undergoing cardiac surgery may benefit from a more liberal practice; however, data were inconclusive. Important differences between the trials included the timing of intervention (during cardiopulmonary bypass (CPB), on ICU post-CPB, or throughout the perioperative period) and the hemoglobin thresholds used. These issues may be important, especially in relation to the acute hemodilution that occurs during CPB.
The two largest trials involved 502 [55] and 2007 [56] patients. Hajjar and colleagues randomized all consecutive patients undergoing CPB surgery in a single Brazilian center to a transfusion threshold of hematocrit 30% or 24% throughout the perioperative period. No difference in the 30-day composite of mortality and major morbidity was observed (10% vs 11%), or mortality alone (5% vs 6%). Murphy and colleagues randomized patients in 17 UK hospitals to a restrictive (Hb 75 g/L) or liberal (Hb 90 g/L) transfusion strategy post-surgery in the ICU if their hemoglobin concentration was 90 g/L or less. They found no difference in the composite outcome of serious infection or ischemic events within 3 months post-randomization (35.1% vs 33.0%), with no heterogeneity across pre-defined patient subgroups. However, more deaths occurred in the restrictive threshold group (4.2% vs 2.6%; hazard ratio, 1.64; 95% CI, 1.00–2.67; p = 0.045). As this was a secondary outcome, its significance was uncertain, but the finding made a major contribution to the effect observed in the meta-analysis [54].
Two further studies have been published since 2015. Koch and colleagues randomized 722 patients undergoing cardiac surgery in two US hospitals to a transfusion threshold of hematocrit 24% or 28% throughout hospitalization. This trial was stopped at the second interim analysis because a pre-defined futility boundary was crossed. There was no difference in the composite outcome of mortality and morbidity (16% vs 19%). These data supported the safety of a restrictive transfusion practice. Most recently, Mazer and colleagues randomized 5243 adults undergoing cardiac surgery with a European System for Cardiac Operative Risk Evaluation (EuroSCORE) I of six or more (on a scale from 0 to 47) to a restrictive red cell transfusion threshold (Hb < 75 g/L) or a liberal red cell transfusion threshold (Hb < 95 g/L) [57].
The intervention lasted from induction of anesthesia throughout hospitalization. They found no difference in the primary composite outcome of death from any cause, myocardial infarction, stroke, or new-onset renal failure with dialysis (11.4% vs 12.5%); mortality was also similar (3.0% vs 3.6%). There were no differences between the groups in any of the secondary outcomes or for any pre-defined subgroups including patients with worse preoperative left ventricular function, renal dysfunction, or diabetes mellitus. The non-inferiority design of this trial provides high-quality evidence for the safety of this restrictive strategy in cardiac surgery.
Clinical trial evidence therefore provides clarity about current best practice for patients undergoing cardiac surgery. Despite the association between anemia and adverse outcomes in this population, red blood cell transfusions are only indicated when the hemoglobin concentration is 75 g/L or less during the perioperative period and subsequent hospitalization. More liberal transfusion practices confer no clinical benefit to the patient and increases red cell use.
Conclusions
Coexisting cardiovascular disease is prevalent among patients admitted to ICU. Critical illness places significant strain on the vulnerable myocardium, and both atheromatous plaque rupture and supply-demand oxygen imbalance may result in myocardial infarction. Clinical trials have shown that red blood cell transfusions are only indicated when the hemoglobin concentration is ≤75 g/L for patients undergoing cardiac surgery. However, there is biological plausibility, supported by work from pilot trials and systematic reviews, that more liberal transfusion strategies may be beneficial in patients with both acute and chronic cardiovascular disease. We would recommend a more liberal transfusion strategy of ≥80 g/L until data from high-quality RCTs in these populations are available.
References
British Heart Foundation. Cardiovascular disease statistics 2015. Oxford: British Heart Foundation; 2015.
Townsend N, Bhatnagar P, Wilkins E, Wickramasinghe K, Raynor M. Cardiovascular disease statistics, 2015. London: British Heart Foundation; 2015.
Ostermann M, Lo J, Toolan M, Tuddenham E, Sanderson B, Lei K, et al. A prospective study of the impact of serial troponin measurements on the diagnosis of myocardial infarction and hospital and six-month mortality in patients admitted to ICU with non-cardiac diagnoses. Crit Care. 2014;18(2):R62.
Walsh TS, McClelland DB, Lee RJ, Garrioch M, Maciver CR, McArdle F, et al. Prevalence of ischaemic heart disease at admission to intensive care and its influence on red cell transfusion thresholds: multicentre Scottish Study. Br J Anaesth. 2005;94(4):445–52.
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985). 2004;97(1):404–15.
Ramanathan T, Skinner H. Coronary blood flow. Contin Educ Anaesth Crit Care Pain. 2005;5(2):61–4.
Zeidman A, Fradin Z, Blecher A, Oster HS, Avrahami Y, Mittelman M. Anemia as a risk factor for ischemic heart disease. Isr Med Assoc J. 2004;6(1):16–8.
Szachniewicz J, Petruk-Kowalczyk J, Majda J, Kaczmarek A, Reczuch K, Kalra PR, et al. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003;90(2–3):303–8.
Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111(16):2042–9.
Mamas MA, Kwok CS, Kontopantelis E, Fryer AA, Buchan I, Bachmann MO, et al. Relationship between anemia and mortality outcomes in a National Acute Coronary Syndrome Cohort: insights from the UK Myocardial Ischemia National Audit Project Registry. J Am Heart Assoc. 2016;5(11):e003348.
Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055–60.
Hagl S, Heimisch W, Meisner H, Erben R, Baum M, Mendler N. The effect of hemodilution on regional myocardial function in the presence of coronary stenosis. Basic Res Cardiol. 1977;72(4):344–64.
Nikolsky E, Aymong ED, Halkin A, Grines CL, Cox DA, Garcia E, et al. Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;44(3):547–53.
Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346(13):957–66.
Turner SJ, Ketch TR, Gandhi SK, Sane DC. Routine hematologic clinical tests as prognostic markers in patients with acute coronary syndromes. Am Heart J. 2008;155(5):806–16.
Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774–82.
Nabais S, Gaspar A, Costa J, Azevedo P, Rocha S, Torres M, et al. Prognostic impact of hemoglobin drop during hospital stay in patients with acute coronary syndromes. Rev Port Cardiol. 2009;28(4):383–95.
Marechaux S, Barrailler S, Pincon C, Decourcelle V, Guidez T, Braun S, et al. Prognostic value of hemoglobin decline over the GRACE score in patients hospitalized for an acute coronary syndrome. Heart Vessel. 2012;27(2):119–27.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–67.
Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–47.
Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;125(2):280–8.
Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39(7):1181–9.
Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: a meta-analysis. Heart Lung. 2015;44(1):75–81.
Soyseth V, Bhatnagar R, Holmedahl NH, Neukamm A, Hoiseth AD, Hagve TA, et al. Acute exacerbation of COPD is associated with fourfold elevation of cardiac troponin T. Heart. 2013;99(2):122–6.
Bruder N, Rabinstein A, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Cardiovascular and pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):257–69.
Douketis JD, Leeuwenkamp O, Grobara P, Johnston M, Sohne M, Ten Wolde M, et al. The incidence and prognostic significance of elevated cardiac troponins in patients with submassive pulmonary embolism. J Thromb Haemost. 2005;3(3):508–13.
Giannitsis E, Muller-Bardorff M, Kurowski V, Weidtmann B, Wiegand U, Kampmann M, et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation. 2000;102(2):211–7.
Shah AS, McAllister DA, Mills R, Lee KK, Churchhouse AM, Fleming KM, et al. Sensitive troponin assay and the classification of myocardial infarction. Am J Med. 2015;128(5):493–501.e3.
Chapman A, Shah A, Anand A, Strachan F, McAllister D, Newby D, et al. Long term outcomes of patients with type 2 myocardial infarction or injury. Heart. 2016;102(Suppl 6):A80.
Lim W, Qushmaq I, Cook DJ, Devereaux PJ, Heels-Ansdell D, Crowther MA, et al. Reliability of electrocardiogram interpretation in critically ill patients. Crit Care Med. 2006;34(5):1338–43.
Lim W, Tkaczyk A, Holinski P, Qushmaq I, Jacka M, Khera V, et al. The diagnosis of myocardial infarction in critically ill patients: an agreement study. J Crit Care. 2009;24(3):447–52.
Mehta S, Granton J, Lapinsky SE, Newton G, Bandayrel K, Little A, et al. Agreement in electrocardiogram interpretation in patients with septic shock. Crit Care Med. 2011;39(9):2080–6.
Devereaux PJ, Goldman L, Yusuf S, Gilbert K, Leslie K, Guyatt GH. Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: a review. CMAJ. 2005;173(7):779–88.
Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627–34.
Chapman AR, Adamson PD, Mills NL. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart. 2017;103(1):10–8.
Gundre P, Kleyn M, Kulbak G, Kupfer Y, Tessler S. Elevated troponin Cs in intensive care units – a nationwide survey of critical care physicians. Chest. 2011;140(4 (MeetingAbstracts)):1013A.
Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10(4):222.
Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30(8):1765–71.
American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208.
Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157(1):49–58.
Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, Stanworth S, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160(4):445–64.
National Institute for Health and Clinical Excellence, 2015. Transfusion. Department of Health. Available http://www.nice.org.uk/guidance/ng24/evidence/full-guidance-2177160733.
Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R, et al. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia. 2016;71:829–42.
Norfolk D. Handbook of transfusion medicine. Norwich: TSO; 2013.
Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis. JAMA Intern Med. 2013;173(2):132–9.
Middelburg RA, van de Watering LM, van der Bom JG. Blood transfusions: good or bad? Confounding by indication, an underestimated problem in clinical transfusion research. Transfusion. 2010;50(6):1181–3.
Carson JL, Hebert PC. Here we go again--blood transfusion kills patients?: comment on “Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis”. JAMA Intern Med. 2013;173(2):139–41.
Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol. 2011;108(8):1108–11.
Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165(6):964–71.e1.
Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst L, et al. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis. BMJ. 2016;352:i1351.
Chen D, Serrano K, Devine D. Introducing the red cell storage lesion. ISBT Sci Ser. 2016;11(S1):26–33.
Loor G, Rajeswaran J, Li L, Sabik JF 3rd, Blackstone EH, McCrae KR, et al. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146(6):1480–7.e6.
Patel NN, Avlonitis VS, Jones HE, Reeves BC, Sterne JA, Murphy GJ. Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. Lancet Haematol. 2015;2(12):e543–53.
Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67.
Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372(11):997–1008.
Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. 2017;377(22):2133–44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Docherty, A.B., Walsh, T.S. (2018). Hematologic Challenges in ICU Patients with Cardiovascular Disease. In: Shander, A., Corwin, H. (eds) Hematologic Challenges in the Critically Ill. Springer, Cham. https://doi.org/10.1007/978-3-319-93572-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-93572-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93571-3
Online ISBN: 978-3-319-93572-0
eBook Packages: MedicineMedicine (R0)