Abstract
This chapter is targeted to a broad scientific audience such as students and non-specialists who would like to explore and understand the diversity of the head, jaws and cranial muscles encountered within the large class of ray-finned fishes (Actinopterygii). Actinopterygians are a wide group of bony fishes including more than 30,000 species, which means that it is obviously not possible to carry out a case-by-case assessment or to condense the subject in a few pages. Therefore, we have described the role of the musculoskeletal elements of the head occurring during breathing and feeding in a ray-finned fish representative of the group, as well as we have demonstrated that the actinopterygian skull is truly distinctive among vertebrates. We have also tried to explain the main information concerning the diversification and evolution of the jaws and muscles of the five extant actinopterygian lineages with more specificities on teleostean fishes which are the most diverse and advanced clade.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
5.1.1 Osteichthyes
Next to the group of cartilaginous fishes (Chondrichthyes) comprising sharks, skates and rays (Elasmobranchii) and chimaeras (Holocephali), the clade Osteichthyes includes more than 50,000 living species with all bony fishes and tetrapods (Lecointre and Le Guyader 2001). The main feature of bony fishes is the presence of two types of bone in their skeleton: endochondral bones constitute the deep endoskeleton, whereas the dermal exoskeleton is made of dermal bones resulting from intramembranous ossification. The deep bones form from previously developed cartilage models which are then progressively replaced by the bone. The dermal bones involve the replacement of connective tissue membrane sheets with bone tissue (Lecointre and (Lecointre and Le Guyader 2001; Kardong 2012). Bony fishes also have other special features such as the body which is entirely covered by bony scales, the distal part of their fin membrane which is supported by lepidotrichia (i.e., double rows of small transformed scales) and the swim bladder which is an air sac connected to the digestive tract (i.e., oesophageal diverticulum) serving to regulate fish density relative to water density as well as many other species-specific characters.
Among these organisms with a bony endoskeleton, we generally distinguish two main subgroups: the ray-finned fishes ( Actinopterygii > 30,000 extant species) from the lobe-finned fishes and tetrapods ( Sarcopterygii > 24,000 living species) (Nelson 2006). The first subgroup of actinopterygians, also named “ray-finned fishes” because of the transformed scales on their fins forming their dermal rays, is the most diverse and extremely successful class of vertebrates. In terms of number, they group more than 30,000 species which provides an extraordinary basis for diversity.
A diversity which is equivalent to about a half of all living vertebrates and more than 95% of all living fish species which are gathered into 431 families and 42 orders (Nelson 2006; Helfman et al. 2009). However, the number of species in this taxon should be more impressive since it is expected many more species are still to be discovered and identified, including the strange species that inhabit the deep sea. Excluding the four-legged vertebrates (Tetrapoda), the subgroup of sarcopterygians (see Chap. 6) consists of a minority of lobe-finned fishes which are represented by only two extant species of coelacanths (Actinistia) and six living species of lungfishes (Dipnoi). Many different taxa of fossil actinopterygians (e.g., Palaeonisciformes, Pholidopleuriformes, Perleidiformes, Semionotidae, Pycnodontidae, Macrosemiidae) were also studied, but they are not discussed in this chapter. Readers can find information on these taxa in many different reviews (e.g., Blot 1966; Poplin 1984; Miller and McGovern 1996; Cloutier and Arratia 2004; Nelson et al. 2016).
5.1.2 Actinopterygii
Within the large class of actinopterygian fishes, five current separate lineages (Fig. 5.1) are encountered: the lineage of Polypteriformes with bichirs and reedfishes ( Cladistia ), the lineage of Acipenseriformes with sturgeons and paddlefishes ( Chondrostei ), the lineage of Lepisosteiformes with all living gars ( Ginglymodi ), the lineage of Amiiformes which contains the only species of bowfin Amia calva ( Halecomorphi ) and the lineage of teleostean fishes ( Teleostei ). The latter includes the amazing majority of living ray-finned fish species since it contains almost 99% of vertebrate species we can encounter in the aquatic environment (Nelson 2006). Polypteriformes are one of the earliest and basal clades of actinopterygians dating from the Devonian period but still have a debated phylogenetic position. This group is currently considered the sister group of the four other lineages (Venkatesh et al. 2001; Inoue et al. 2003; Nelson 2006; Diogo 2008) where Acipenseriformes, Lepisosteiformes and Amiiformes are also thought to be basal actinopterygians. Teleosteans are regarded as the most modern and advanced ray-finned fishes (e.g., Lauder and Liem 1983; Nelson 1994, 2006; Patterson 1994; Janvier 1996; Bemis et al. 1997). Teleostean fishes are the most rich species and diversified vertebrate lineage since there are more teleost species than all the other vertebrate species combined (Peng et al. 2009). According to molecular and morphological phylogenetic analyses, this large lineage is subdivided into four major teleostean subgroups (Fig. 5.1): (1) Elopomorpha (e.g., Elopiformes, Albuliformes, Notacanthiformes, Anguilliformes and Saccopharyngiformes), (2) Osteoglossomorpha (e.g., Hiodontiformes and Osteoglossiformes), (3) Otocephala (e.g., Clupeomorpha and Ostariophysi which includes Gonorynchiformes, Cypriniformes, Characiformes, Gymnotiformes and Siluriformes) and (4) Euteleostei (e.g., Argentiniformes, Esociformes, Osmeriformes, Salmoniformes and Neoteleostei) (Diogo 2008). Formerly, the Euteleostei subgroup was subdivided into three “superorders”: Protacanthopterygii (e.g., Esociformes, Osmeriformes and Salmoniformes), Paracanthopterygii (e.g., Batrachoidiformes, Gadiformes, Lophiiformes, Ophidiiformes and Percopsiformes) and Acanthopterygii (e.g., Atheriniformes, Beloniformes, Beryciformes, Cyprinodontiformes, Gasterosteiformes, Mugiliformes, Perciformes, Pleuronectiformes, Scorpaeniformes, Stephanoberyciformes, Synbranchiformes, Tetraodontiformes and Zeiformes) (Greenwood et al. 1966). Numerous studies have demonstrated that teleosts have extreme morphology and diversified heads, jaws and cranial muscles (e.g., Liem 1967; Osse 1969; Lauder and Liem 1981; Waltzek and Wainwright 2003; Hulsey and Garcia De Leon 2005; Geerinckx et al. 2007) which gives to the class a special position and a great importance for the study of evolutionary history.
Phylogenetic relationships among the major extant actinopterygian subgroups (modified from Diogo 2008) and illustrations of the head of some taxa. The five major lineages of actinopterygians are framed in red, and head schemata are those of Polypterus senegalus (Polypteriformes), Lepisosteus platyrhincus (Lepisosteiformes), Amia calva (Amiiformes), Megalops atlanticus (Elopiformes), Gymnothorax favagineus (Anguilliformes), Esox lucius (Esociformes), Hippocampus sp. (Syngnathiformes), Alburnus alburnus (Cypriniformes) and Serrasalmus sp. (Characiformes). In the skull illustrations, the black element highlights the lower jaw. The light and dark grey elements are, respectively, for the premaxilla and maxilla of the upper jaw. The eye and nares are circled in bold black. A note about the clade of Neoteleostei is that it includes many more orders than simply Aulopiformes and Stomiiformes, for example, Ateleopodiformes, Myctophiformes, Polymyxiiformes, Percopsiformes, Gadiformes, Zeiformes, Lampriformes, Perciformes, Beryciformes, etc.
In the framework of this chapter, it is therefore not possible to conduct an exhaustive description of all heads of bony fishes or actinopterygian species, their associated muscles and mechanisms. Although it is a truism, fishes are widespread worldwide and inhabit all aquatic biotopes as marine, brackish and freshwater systems. They can be encountered from the pelagic zone to the bottom of the ocean, as well as in lakes and rivers and in a variety of extreme environments including desert and thermal springs (e.g., pupfishes), sunless subterranean caves (e.g., cavefishes), torrential rivers (e.g., torrentfishes), hypersaline habitats (e.g., molly fishes), high-altitude lakes and streams (e.g., mountain carps), abyssal depths (e.g., anglerfishes), polar seas and arctic tundra (e.g., cods, flatfishes, salmons, trouts) (Helfman et al. 2009). Although this versatility of fishes to adapt to different environmental conditions is necessary to recall, they are all under the same basic constraint: in a dense and viscous aquatic medium , they all have to be able to ingest water at least to breathe and at best to feed. The respiration in bony fishes is mainly done by means of water flow entering through the mouth and flowing into the buccal cavity towards the pharyngeal cavity and the gills (i.e., pharyngeal arches and lamellae) in which respiratory gas exchanges occur. The water flow is created by the action of musculoskeletal pumps, which change the pressure and volume in the buccal and pharyngeal cavities. The existence of these successive suction-to-flowing pumps tends to streamline the water flow. In the same way, the most general way of feeding in actinopterygians corresponds to the ability to generate a strong pressure gradient inside the oral cavity by means of musculoskeletal pumps in order to draw a prey into the mouth (Lauder 1985; Wainwright et al. 2015). This mechanism has reached an important level of diversity (Westneat 1994; Liem 1978; Barel 1983; Ferry-Graham et al. 2001) because it is based on a high number of interconnected skeletal elements (e.g., up to 60 skeletal parts in adult teleosts) that are moved by an approximately equal number of muscles (Osse 1969; Aerts 1991). Although different innovations have been developed by ray-finned fishes and have been the subject of many different papers, the way to get food in an aquatic environment remains globally conserved. Its understanding requires however first the anatomical description of a generalized and simplified head musculoskeletal system.
5.2 Anatomy
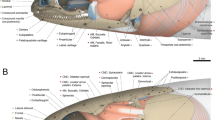
The anatomy of the head in primitive and modern actinopterygian fishes is an area of vertebrate morphology that has a long and distinguished history (Ferry-Graham et al. 2001) and has been the subject of numerous comparative studies. Moreover, it is a research field that has been and is still much studied because of the kinetics and incredible movements executed by the fish skull . The skull of most actinopterygians is actually distinctive among vertebrates due to the presence of a large number of independent and mobile cartilaginous and bony elements. This unique cranial composition (Fig. 5.2a, b) makes it more complex and kinetic (i.e., the skeletal elements that compose the skull can move with respect to each other) than the skull of chondrichthyans, for example (Motta and Huber 2004). These various skull elements result from compromises between different functions as breathing, feeding, hydrodynamic movements, protecting the brain and supporting the sensory organs. In addition, the cranial muscles of actinopterygian fishes play a major role in respiration and feeding by moving skull components to control the opening and closing of the buccal and pharyngeal cavities. In the scientific literature, there are many descriptions and illustrations that explain these anatomical aspects for simpler or more complex skulls of ray-finned fish species (suggestions for: Polypteriformes: Traquair 1870; Allis 1919, 1922; Lauder 1980; Acipenseriformes: Carroll and Wainwright 2003; Miller 2004; Lepisosteiformes: Allis 1922; Lauder 1980; Kammerer et al. 2006; Konstantinidis et al. 2015; Amiiformes: Allis 1897; Lauder 1980; Elopiformes: Vrba 1968; Anguilliformes: De Schepper et al. 2005, 2007; Eagderi and Adriaens 2010; Osteoglossiformes: Sanford and Lauder 1989; Camp et al. 2009; Gadiformes: Herbing et al. 1996; Salmoniformes: Wilson and Veilleux 1982; Perciformes: Deary and Hilton 2016; Gidmark et al. 2015; Cypriniformes: Gosline 1973). Thereafter, we have tried to present an anatomical description of the skull, jaws and cranial muscles for a representative actinopterygian model of the group by doing a simplified summary of different studies.
Schematic representations of a teleost (Carapus acus) neurocranium and splanchnocranium in (a) lateral view and (b) frontal view. (c) Representation of the different regions of neurocranium and the hyoid and branchial regions of the splanchnocranium. The neurocranium is represented as a cranial box (blue) that includes four regions: (1) the ethmoid region, (2) the orbital region, (3) the otic region and (4) the occipital region. The splanchnocranium comprises the upper and lower jaws, the suspensorium, the hyoid apparatus, the opercular series and the branchial arches. Schematic representations (a) and (c) are redrawn from Parmentier (2003) and the schema (b) from Lauder (1985)
5.2.1 Skull and Jaws
The skull (i.e., cranial skeleton) consists of two main parts: (1) the braincase called the neurocranium that protects the brain and sensory organs and (2) the splanchnocranium (i.e., visceral cranium) made of series of suspended skeletal elements supporting the jaws, cheeks and gills and offering attachment site for the respiratory and feeding muscles. Embryologically, the neurocranium is mainly formed from the cells having a mesodermal origin, whereas the splanchnocranium emerges from the cells of the neural crest (Kardong 2012). Neural crest cells migrate from the neural tube to the body wall where they contribute initially to pharyngeal arches and then give rise to a great variety of adult structures including the jaws and gill arches (e.g., in the zebrafish Danio rerio: Schilling and Kimmel 1994; Kimmel et al. 1995, 2001; Cubbage and Mabee 1996).
5.2.1.1 Neurocranium
Structurally, the neurocranium is divided into four regions (Fig. 5.2c): the olfactory region, the orbital region, the otic region and the occipital region (Helfman et al. 2009). The olfactory (or ethmoid) region is the most anterior region of the neurocranium that supports the nares related to smell (i.e., the ability to sense and detect odorous molecules) and consists mainly of the following bones: ethmoid, lateral ethmoids, vomer, preethmoids, mesethmoids, kinethmoid and nasals. The orbital region is the cavity of the skull in which the eye is located and is formed by several cartilaginous and bony elements: frontals, orbitosphenoid, pterosphenoids, sclerotic cartilage, suborbital series (i.e., lachrymal, jugal, postorbital, fourth orbital, fifth orbital and dermosphenoid) and the supraorbital series (i.e., supraorbital 1 and supraorbital 2). The otic region is the part of the skull delimited for the support of the hearing organs. It consists of numerous consolidated bones: sphenotics, pteroptics, prootics, epiotics, opisthotics, supratemporals, parietals, basisphenoid and parasphenoid. The occipital (or basicranial) region is at the back of the braincase and forms the cranial base. The region mainly consists of the following bones: exoccipital, basioccipital and supraoccipital (some reading suggestions: Liem 1967; Vandewalle et al. 1992; Diogo and Chardon 2000a, b; Bemis and Forey 2001; Parmentier et al. 2001).
5.2.1.2 Splanchnocranium
The splanchnocranium (Fig. 5.2a, c) is also divided into three regions or “functional units” in terms of feeding biomechanics: the oromandibular region, the hyoid region and the branchial region (Helfman et al. 2009; Kardong 2012). Each region is derived to a certain extent from an embryonic pharyngeal arch. The most anterior visceral arch gives rise to the oral jaws (i.e., mandibular arch), while the next arch becomes the hyoid apparatus and the main part of the suspensorium that support the jaws (i.e., hyoid arch). The other posterior pharyngeal arches contribute to the branchial basket which supports the gill arches and gill filaments (some reading suggestions: Vandewalle et al. 1997, 2000; Parmentier et al. 1998; Engeman et al. 2009; Carvalho and Vari 2015).
The oromandibular region is composed of the upper jaw (i.e., premaxilla, maxilla and supramaxilla) and lower jaw (i.e., dentary, anguloarticular, retroarticular, Meckel’s cartilage and coronomeckelian bone), the suspensorium (i.e., palatine, entopterygoid, metapterygoid, quadrate, hyomandibula and symplectic) and the opercular series (i.e., opercle, preopercle, interopercle, subopercle and subtemporal) corresponding to the gill cover elements.
The upper and lower jaws constitute the buccal jaws whose function is to grab food, whereas the prey processing is realized deeper in the buccal cavity, at the level of the pharyngeal cavity, where there is a second set of pharyngeal jaws (Vandewalle et al. 2000). The premaxilla and maxilla constituting the upper jaws articulate on the olfactory region of the neurocranium. Their morphology, length and shape are highly variable in actinopterygians just as it is also the case for teeth that can be found on both bones, on one of the bones or are absent.
Behind the upper and lower jaws, the suspensorium complex possesses at least five articulations (Fig. 5.3) which are important for the understanding of the mechanical principles of the respiration and feeding: (1) the autopalatine anteriorly articulates with the neurocranium in front of the orbit (i.e., neurocranial-autopalatine joint); (2) the hyomandibula posteriorly articulates with the neurocranium on the otic region (i.e., neurocranial-hyomandibula joint); (3) the posterior margin of the hyomandibula articulates with the opercular series which is related by a ligament to the caudal part of the lower jaw (i.e., hyomandibula-opercle joint); (4) the medial ventral margin of the hyomandibula articulates with the interhyal of the hyoid apparatus allowing back and forth movements of the branchial basket and (5) the quadrate of the suspensorium ventrally articulates with the anguloarticular bone of the lower jaw allowing the pivoting of the mandible (i.e., anguloarticular-quadrate joint). The two articulations of the suspensorium with the neurocranium can be compared to door hinges allowing lateral movements of the “cheeks” of the fish. In some species, a fifth articulation (6) can be found between the palatine and the maxilla (see later).
Illustration of the main articulations of the suspensorium in a teleost species (Carapus boraborensis): (1) between the palatine and the neurocranium, (2a), (2b) between the hyomandibula and the neurocranium, (3) between the hyomandibula and the opercle, (4) between the quadrate of the suspensorium and the lower jaw, (5) between the maxilla and the palatine, and (6) between the lower part of the hyomandibula and the interhyal of the hyoid bar (this articulation is in light blue because the articulation takes places on the medial side of the suspensorium). The dotted line represents an axis passing through the articulations between the suspensorium and neurocranium and allowing lateral movements of the “cheeks” of the fish. The schema is redrawn from Parmentier (2003)
The hyoid region includes the hyoid apparatus (i.e., hyoid bar ) which is the primary element of the mouth floor generally comprising (Fig. 5.4a, b) urohyal, basihyal, hypohyal, ceratohyal, epihyal, interhyal and branchiostegal rays (Aerts 1991; Faustino and Power 2001; Helfman et al. 2009). On the medial side of the hyomandibula, the hyoid apparatus articulates with the suspensorium to the branchial basket (Fig. 5.3) allowing the back-and-forth movements of the buccal roof (Liem 1967; Osse 1969).
Examples of (a) the hyoid apparatus in lateral external view and (b) the branchial basket in dorsal view in an actinopterygian (Carapus boraborensis). For the hyoid apparatus, urohyal is not shown (Parmentier 2003)
The branchial region corresponds to the region around the fish gills that includes the following skeletal elements (Fig. 5.4b): pharyngobranchials, pharyngeal plates, epibranchials, ceratobranchials, hypobranchials and basibranchials (e.g., Vandewalle et al. 2000; Faustino and Power 2001; Helfman et al. 2009). Pharyngeal jaws are located in the branchial region and are used to process food (Fraser et al. 2009).
5.2.2 Cranial Musculature
The cranial musculature (i.e., the muscles associated with the skull, jaws and other skeletal components) is essential for the understanding of mechanisms and linkages involved in breathing and feeding movements as these muscles are the primary contributors involved in the opening and closing of the buccal and pharyngeal cavities. Besides, most of the cranial muscles are formed before yolk exhaustion to allow exogenous respiration and feeding (Herbing et al. 1996). The cranial muscles are divided into four main groups: mandibular muscles, hyoid muscles, branchial muscles and hypobranchial muscles (e.g., Edgeworth 1935; Diogo and Abdala 2010).
5.2.2.1 Mandibular Muscles
The mandibular muscles are directly or indirectly involved in movements of the lower jaw and are innervated by the trigeminal nerve (i.e., cranial nerve V). The main mandibular muscles are four in number: adductor mandibulae, intermandibularis, levator arcus palatini and dilatator operculi. They originate from an embryonic mandibular muscle plate that progressively contributes to the development of three structures: (1) the premyogenic condensation constrictor dorsalis that dorsally develops, (2) the adductor mandibulae that medially develops, and (3) the intermandibularis that ventrally develops. The premyogenic condensation constrictor dorsalis then gives rise to the levator arcus palatini and the dilatator operculi (Edgeworth 1935; Diogo et al. 2008). The studies of Edgeworth (1935) are a fundamental source of information about the development of the cranial muscles in actinopterygians.
The adductor mandibulae (i.e., jaw muscle, Fig. 5.5) is the more easily accessible cranial muscle and the largest superficial muscle complex of the fish cheek (Winterbottom 1974). It is specifically innervated by the ramus mandibularis nerve which is a motor branch of the trigeminal nerve. It is the more significant mandibular muscle in feeding biomechanics because it is responsible for the closing of the lower jaw and is consequently present in all actinopterygians. Structurally, the adductor mandibulae ranges from simple and undivided jaw muscle to a highly complex architecture incorporating up to ten discrete subdivisions. According to the new terminology of Datovo and Vari (2013), the adductor mandibulae muscle is composed of a large facial segment (i.e., segmentum facialis) and a smaller mandibular segment (i.e., segmentum mandibularis). The facial segment is positioned lateral to the suspensorium, whereas the mandibular segment is located medial to the lower jaw. These two muscle segments are usually connected by a tendinous complex (i.e., intersegmental aponeurosis) which is attached to the medial surface of the lower jaw. In many fishes, the facial segment can be also subdivided into three muscle sections, a ventrolateral section (i.e., pars rictalis), a dorsolateral section (i.e., pars malaris) and an anteromedial section (i.e., pars stegalis), and the mandibular segment can be separated into two muscle sections: a dorsal section (i.e., pars coronalis) and a ventral section (i.e., pars mentalis). Each muscle section can also be subdivided and differentiated into different subsections which are well explained and illustrated in the reference publications (Datovo and Vari 2013, 2014 for teleosteans).
Schematic representation of some cranial muscles in an actinopterygian (Carapus boraborensis). Mandibular muscles: adductor mandibulae (A1, A2, A3), levator arcus palatini and dilatator operculi. Hyoid muscles: adductor operculi, adductor arcus palatini and levator operculi. Protractor hyoideus (i.e., geniohyoideus) and sternohyoideus that can participate to the mouth opening are not shown. All the muscles and adductor arcus palatini and adductor operculi have at least one insertion on the lateral side of the suspensorium and opercle. In adductor arcus palatine, the insertion is on the medial side of the suspensorium, and in adductor operculi, the insertion is on the medial side of the opercle. The schema is redrawn from Parmentier (2003)
The intermandibularis is a muscle which ventrally connects the two mandibles (i.e., dentaries) and is present in virtually all actinopterygians. It is unsubdivided in basal actinopterygians such as Cladistia, Chondrostei and Ginglymodi, but it is subdivided into intermandibularis anterior and posterior in the Halecomorphi Amia calva and Teleostei. The intermandibularis posterior combines with the interhyoid muscle and is involved in the mouth opening (see below).
The levator arcus palatini (Fig. 5.5) is also found in all actinopterygians apart from Chondrostei where there is instead the protractor hyomandibulae that is responsible for the protraction of the hyomandibula. The levator arcus palatini originates from the neurocranium and has an attachment site often along the hyomandibula on the suspensorium to lift the palatal arch.
The dilatator operculi (Fig. 5.5) is found in all actinopterygians but is also absent in Chondrostei. This muscle originates from the neurocranium and inserts along the dorsolateral faces of the opercula to move them apart and expand the pharyngeal cavity.
5.2.2.2 Hyoid Muscles
The hyoid muscles are closely related to movements occurring in the mouth opening and motions of the hyoid apparatus . They are generally innervated by the facialis nerve (i.e., cranial nerve VII). The four main hyoid muscles are interhyoideus, hyohyoideus, adductor operculi and adductor arcus palatini. Embryologically, they arise from the premyogenic condensation constrictor hyoideus that gives rise ventrally to the interhyoideus and hyohyoideus and dorsomedially to the adductor operculi and the adductor arcus palatini (Edgeworth 1935).
The interhyoideus operates in the opening of the mouth by having a site of origin from the basihyal and ceratohyal of the hyoid apparatus and an attachment site on the lower jaw. This hyoid muscle is found in all actinopterygians but is specifically fused in Teleostei with the intermandibularis posterior of the mandibular muscles to constitute the protractor hyoideus (i.e., geniohyoideus ).
The hyohyoideus is a ventral muscle in contact with the hyoid apparatus, which is unsubdivided in basal actinopterygians such as Cladistia, Chondrostei and Ginglymodi but is subdivided into hyohyoideus inferior and superior in Amia calva and Teleostei. The hyohyoideus superior is also notably divided into one hyohyoideus abductor and two hyohyoidei adductors in Amia calva and Teleostei. The hyohyoideus abductor is responsible for the expansion of the branchiostegal membrane because of its origin from branchiostegal rays. The hyohyoidei adductors are in contrast responsible of the constriction of the branchiostegal membrane. This muscle originates from the opercle and subopercle and inserts on branchiostegal rays.
The adductor operculi (Fig. 5.5) is a dorsal hyoid muscle that has a site of origin from the neurocranium and an attachment site on the opercles causing their adduction. This muscle is present without exception in all actinopterygians.
The adductor arcus palatini (Fig. 5.5) is present in all actinopterygians (exclusive of Chondrostei where there is rather a retractor hyomandibulae ) where it originates from the neurocranium and inserts on the medial side of several elements of the suspensorium such as hyomandibula, metapterygoid and entopterygoid in order to raise the suspensorium (i.e., suspensorial adduction). In addition to these major hyoid muscles, a levator operculi and an adductor hyomandibulae are, respectively, is found in Halecomorphi Amia calva and Teleostei and more advanced Teleostei such as Euteleostei, Otocephala and Clupeomorpha.
The levator operculi (Fig. 5.5) originates from the neurocranium and inserts on the opercles which moves essentially to the opercular series, which may interfere in lower jaw depression through the interoperculo-mandibular ligament.
The adductor hyomandibulae is a dorsal hyoid muscle that originates from the neurocranium to attach on the dorsomedial faces of the hyomandibula. Its function is to adduct the hyomandibula.
5.2.2.3 Branchial Muscles
The branchial muscles include the branchial muscles sensu stricto that are innervated by the glossopharyngeus and vagus nerves (i.e., cranial nerves IX and X, respectively) and the other branchial muscles such as the cucullaris, laryngeal, coracobranchialis and epibranchial muscles that are normally innervated by the spinal accessory nerve (i.e., cranial nerve XI). The development, organization, nomenclature and function of branchial muscles are complex and are not discussed herein. Research works such as those of Winterbottom (1974), Vandewalle et al. (2000) and others could be consulted for specific examples as well as better representation and understanding.
5.2.2.4 Hypobranchial Muscles
The hypobranchial muscles are usually innervated by spinal nerves. There is a single hypobranchial muscle in teleosteans such as in the zebrafish, the sternohyoideus (Schilling and Kimmel 1994, 1997; Diogo et al. 2008), while there are two hypobranchial muscles in basal actinopterygians such as Cladistia (e.g., Polypterus senegalus): the coracomandibularis (i.e., branchiomandibularis ) and the sternohyoideus (Noda et al. 2017). The coracomandibularis connects the branchial arches to the lower jaw and is missing in living Lepisosteiformes and Teleosteans. The sternohyoideus is innervated by the anterior branches of the occipito-spinal nerves. It plays a major role in hyoid depression, and, through a series of mechanical linkages, in mouth opening and suspensorial abduction.
5.3 Breathing and Feeding Biomechanics
5.3.1 Breathing
Most actinopterygians breathe with gills that enable them to release carbon dioxide and to recover the oxygen that is dissolved in the aquatic environment (Brainerd and Ferry-Graham 2005).The respiratory cycle (Fig. 5.6) begins typically with the mouth opening that first implies the depression (i.e., ventral rotation) of the lower jaw. This mouth opening is directly followed by the depression of the hyoid apparatus and the lateral expansion of the suspensorium, which are caused by the contraction of the sternohyoideus and levator arcus palatini muscles, respectively. The volume increase allows moving water from the buccal to the pharyngeal cavity. The spreading of the opercular series allows creating a more important volume on the lateral parts of the branchial basket. As a result, the water flow is directed towards the gill opening. The mouth closing increases the pressure in the buccal cavity forcing again the water to move in the branchial basket. The rising of the hyoid apparatus (i.e., dorsal rotation) and the adduction of the suspensorium complete the pressure increase. Once the water is ejected from the opercular cavity, the opercular series returns against the fish body, and the passive part of the branchiostegal membranes moves away from it. Finally, the mouth begins to open again in order to start a new respiratory cycle. The increase and decrease in volume is related to the decrease and increase in pressure, respectively, which results in the displacement of water towards the gills (e.g., Hughes and Shelton 1958; Ballintijn and Hughes 1965; Herbing et al. 1996).
Schematic representation of the respiratory cycle in an actinopterygian. The mouth opening begins with the depression (i.e., ventral rotation) of the lower jaw which is followed by the depression of the hyoid apparatus and the lateral expansion of the suspensorium leading to the opercular enlargement. The mouth closing results from the inverse movements which are the elevation (i.e., dorsal rotation) of the lower jaw and then the hyoid apparatus induced the adduction of the suspensorium and the opercular series. Clei cleithrum of the pectoral girdle, Hy hyoid apparatus (hatched in black), Jj lower jaw (black), Mx maxillary (dark grey), Neuro neurocranium (black), Oper opercular series, Pmx premaxilla (light grey), Susp suspensorium. The black arrows indicate the direction of movements
5.3.2 Feeding
Feeding is actually more complex than simply opening the mouth and then closing it around a prey item (Shadwick and Lauder 2006). This action can be accomplished in two ways, depending of the fish movements. In the first case, the fish can swim with large gape allowing the water and potential prey to enter the mouth. The water leaves the fish through the gill openings, whereas food is directed towards the digestive tract. This mode of feeding is called the ram feeding . In the second option, the fish develops by means of its musculoskeletal system a large volume of the buccal cavity which in turn provokes a pressure decrease in the mouth cavity and results in entering of water (Lauder 1980). This mode of feeding is called the suction feeding and could be assimilated to an exaggeration of respiration movements. Powerful buccal expansion and rapid mouth opening are associated with extreme suction generation (Ferry-Graham et al. 2001). Ram and suction feeding were first considered as extremes of a continuum from pure ram to pure suction feeding, and it has been shown that many species of fish procure food using combinations of ram and suction feeding (Wainwright et al. 2001, 2007; Carroll 2004; Carroll et al. 2004; Day et al. 2005, 2007; Van Wassenbergh et al. 2005; Higham et al. 2006a, b; Staab et al. 2012). Even fish species that have abandoned capturing prey by suction feeding retain the mechanism during the processing and manipulation of prey (Wainwright et al. 2015). More recently, a third mode has been incorporated to create the ram-suction-biting domain, the action of biting being simply to close the jaws on the prey (Ferry et al. 2015). Adding this mode can provide more acute description of the feeding mechanism and give insight on the species ecology but does not change the basic fact that, after the biting, the fish has to find a way to move the prey into the mouth which requires suction and/or ram. In this way, there are three main feeding strategies that are encountered in fishes, ram feeding, suction feeding and feeding with manipulation (biting), but each mechanism relies on the use of the same musculoskeletal elements to capture prey.
The most common mode of prey capture in actinopterygian fishes is suction feeding, in particular, among teleosteans (Liem 1980; Lauder 1985) and could be understood as an exaggeration of the respiration movements, where some musculoskeletal elements can be modulated to modify the mouth gape or increase the feeding performance. The underlying mechanisms of suction feeding are complex and have been extensively studied. They could be divided into four phases: a preparatory phase, an expansive phase , a compressive phase and a recovery phase (Lauder 1980, 1985). The preparatory phase, which consists in buccal cavity compression and buccal volume decreasing, is absent in basal actinopterygians such as Polypteriformes, Lepisosteiformes and Amiiformes and can be observed only in acanthopterygian teleosteans. The most important phase of suction feeding is the expansive phase, which is defined by Lauder (1980, p. 294) as “the time from the start of the mouth opening to peak gape”. During this phase, the mouth opens quickly with a rapid expansion of the buccal cavity, which occurs as a result of cranial elevation (i.e., dorsal rotation of the neurocranium) generated by epaxial muscles (Westneat and Olsen 2015), jaw opening (i.e., ventral rotation of lower jaws) that can be caused in different ways such as the contraction of either geniohyoideus, levator operculi, sternohyoideus (and related hyoid depression), hypaxial or epaxial muscles and at the same time lateral expansion of the suspensorium (Schaeffer and Rosen 1961; Lauder 1982; Grubich 2001) (Fig. 5.7). The rapid expansion creates a drop in pressure into the buccal cavity, which generates a flow of water directed towards the mouth (Higham et al. 2006b). The resulting water flow exerts a hydrodynamic force on the prey item and draws it towards the beginning of the digestive tract. The compressive phase, defined by Lauder (1980, p. 294) as “the time from the peak gape to complete closure of the jaws”, involves the compression of buccal and pharyngeal cavities via hyoid protraction and suspensorium adduction and at the same time of the lower jaw closure via the adductor mandibulae (Grubich 2001). The last recovery phase results in the return in their original position of all the skeletal elements of the feeding system (Wainwright et al. 2001, 2007; Carroll 2004; Carroll et al. 2004; Day et al. 2005, 2007; Van Wassenbergh et al. 2005; Higham et al. 2006a, b).
Schematic representation of one of the different mechanisms that can be used for the expansive phase during suction feeding (a) and schematic representation of the lateral expansion (b). The mouth can open following the isolated or combined contraction of different muscles: (1) the contraction of the epaxial muscles causes the cranial elevation; (2) the contraction of the geniohyoideus (i.e., protractor hyoidei) and levator operculi involves the lower jaw depression; (3) the hyoid depression can be due to the isolated contraction of the sternohyoideus or to a combination with the contraction of the hypaxial muscles that lead to the pectoral girdle retraction; and (4) the contraction of the levator arcus palatini conducts to the lateral expansion of the suspensorium
The mechanical principles of suction feeding were mainly based on studies using high-speed camera and electromyography (e.g., Osse 1969). Actually three mechanisms can allow the mouth opening and correspond to the so-called expansive phase. According to the high amount of actinopterygian species and their related specificities, it is not possible to describe accurately all the different mechanisms encountered. They are voluntary simplified, and the reader has to keep in mind that they are not necessarily found in all species (Lauder 1982; Westneat 2005). Whatever the mechanism, the aim is basically to depress the lower jaw and to elevate the skull. The power required for suction expansion would be mainly generated by the epaxial swimming muscles , in which the body muscles just behind the head cause the skull to rotate upward during feeding (Camp et al. 2015).
-
1.
The first basic mechanism implies from the back to the front the coupling of the ventral hypaxial musculature , the pectoral girdle, the sternohyoideus muscle, the hyoid bar, the geniohyoideus muscle and the lower jaw. Fundamentally, the contraction of the hypaxial musculature stabilizes at least the pectoral girdle and at best pulls it backward. Then, the contraction of the sternohyoideus muscle pulls the hyoid bar posteroventrally. This action is transferred to the geniohyoid muscle that depresses the lower jaw because it pivots around the articulation with the quadrate. Isolated or different combinations of contraction of the three muscles can modify the movement amplitude. In basal actinopterygians (Cladistia, Chondrostei, Ginglymodi and Halecomorphi), the geniohyoideus muscle is not found. In Teleostei, the hyoid apparatus can be related to the mandible by the mandibulo-hyoid ligament, while in other primitive species, this ligament is changed into interoperculo-hyoid ligament. This ligament connects the hyoid apparatus and the interoperculum which is connected by the interoperculo-mandibular ligament to the lower jaw (Lauder 1982).
-
2.
The second mechanism consists in the elevation or dorsal rotation of the neurocranium. It has been modeled on the coupling between the skull, the epaxial musculature, the pectoral girdle, the urohyal from the hyoid apparatus and the lower jaw (Muller 1987). The contraction of the epaxial musculature inserting on the posterior part of the skull causes the neurocranium elevation because it pivots clockwise around the rostral end of the vertebral column (Schaeffer and Rosen 1961; Lauder 1982; Carroll et al. 2004). This skull movement induces the backward displacement of the pectoral girdle. This movement is transferred to urohyal from the hyoid apparatus and by the first coupling explained above transmitted to the lower jaw.
-
3.
The third mechanism implies the opercular series (i.e., operculum, suboperculum and interoperculum), the levator operculi muscle and the lower jaw. The contraction of the levator operculi muscle that connects the dorsal margin of the opercle to the neurocranium causes the elevation of the operculum that pivots around its articulation with the hyomandibula of the suspensorium. This motion pulls posteriorly the interoperculum which possesses on its anterior edge an interoperculo-mandibular ligament passing under the articulation between the quadrate and the lower jaw and inserting on the posterior part of the mandible. Consequently, the posterior movement of the interoperculum starts the depression of the lower jaw.
On another note while the mobility of the jaws and the shape of the opening of the mouth are modified in some species that have departed from a primary reliance on suction feeding, the anterior-to-posterior wave of expansion persists. The suction would be more efficient when the buccal cavity is shaped like a large cone with a small circular mouth opening (Liem 1990). The rate of expansion of the cone can change the shape of the cone, determining the water flow velocity and the resulting suction efficiency. Therefore, the buccal cavity may be modeled as an expanding cylinder with surrounding buccal pressure distributed across its internal surface (Muller et al. 1982). In addition, the action of the mouth opening, or the lower jaw depression, tends to pull on the upper jaw (maxilla and/or premaxilla) and protrude it due to linkages in most teleostean fishes between the upper and lower jaws (Westneat 2004). When the upper jaw protrudes, the descending arm of the premaxilla and the maxilla typically rotate forward and occlude the sides of the open mouth (Gibb 1996). Indeed, this helps create the round or planar opening of the mouth thought to be a key component of effective suction feeding.
5.4 Evolution Trends in Actinopterygians
The success of actinopterygians and mainly teleosteans has been associated with different evolutionary trends , but it remains to be shown. It would concern the repositioning and specialization of the dorsal fin, the change in placement and function of pectoral and pelvic fins, the elaboration of homocercal tail and the improvement of the swim-bladder function (Rosen 1982). At the skull level, there is fusion and reduction in a number of bony elements, such as dermal bones that originally constituted the exoskeleton of the braincase (Helfman et al. 2009). Dermal bones (i.e., exoskeleton) seem to have merged with deep bones (i.e., endoskeleton) to contribute to the development of a more laterally kinetic skull.
Nonetheless, it is rather difficult to generalize evolutionary trends within the skull, jaws and cranial muscles of actinopterygians because of the plethora of species from different taxa that were able to take advantage of different habitats and types of prey. The results are that jaw mechanics show numerous patterns of both diversification and convergence. A common large gap can be found, for example, in distant-phylogenetic species such as the Northern pike Esox lucius (Esociformes) and the grouper (Perciformes), but they are not phylogenetically related to meaning features which result from evolutionary convergence. Comparable observations concern herrings (Clupeiformes), minnows (Cypriniformes) or damselfishes (Perciformes) that have circular mouth to feed on plankton but use different mechanisms to do it. Although having different anatomy, all species are able to drop the lower jaw and then abduct the hyoid bar and the suspensorium before abducting the opercular series. This is because ray-finned fishes are characterized by an extremely large number of mobile bony elements in the skull allowing various mouth opening mechanisms.
Moreover, in relation to their actinopterygian Bauplan, the mandibular lever system of the mandible is present in virtually all ray-finned fishes. The lower jaw possesses however different shapes that directly impact both the force and velocity of the lower jaw closing abilities. Species vary from having high force transmission to those specialized for speed of jaw motion (Barel 1983; Alfaro et al. 2001; Westneat 2004; Wainwright et al. 2015). In parallel, there are important patterns concerning the number and shape of teeth on the jaws. Biters show rows of large conical teeth directed towards the buccal cavity, whereas many suction feeders can have minute teeth or are simply toothless (Schaeffer and Rosen 1961; Motta 1984).
Throughout actinopterygian phylogeny the increasing mobility of upper jaws from basal to more derived taxa is a subject of much interest. In the bichir Polypterus (Polypteriformes) and the gars Lepisosteus (Lepisosteiformes), both premaxilla and maxilla are firmly attached to the neurocranium and do not contribute to mouth opening. A first innovation is found in Amia calva (Amiiformes) where the maxilla is free from the cheek and is able to pivot anteriorly because it has gained a rotational joint with the neurocranium (Lauder 1980). The maxilla is attached by connective tissue to the palatine bone and is connected to the mandible via the maxillo-mandibular ligament. At the jaw opening, the lower jaw is dropped and pulls the posterior end of the maxilla that swings forward. As a result, maxilla and associated connective tissue form the lateral walls of the gape. This novelty has enhanced the control of fluid flow and has increased the velocity of water movement, both of which can improve suction feeding abilities (Lauder 1980).
In the next structural change that evolved in distantly related groups (e.g., Salmoniformes, Esociformes, Aulopiformes, Stomiiformes, Elopiformes, Clupeiformes), the proportionally small premaxilla acquires some mobility and can articulate with the maxilla (Gosline 1980; Wainwright et al. 1989; Grubich 2001). Both bones are joined on a butt joint meaning that an anterior swing of the maxilla causes (small) movements of the premaxilla (Rosen 1982). Although the fine structural organization between Elops and Clupea appears to be different, the result of the maxillary rotation is to rock the dental surface of the premaxilla forward and outward in both taxa (Gosline 1980). The maxilla has thus a propulsive function. These short movements of both bones (maxilla and premaxilla) could result in the protrusion of the premaxilla of higher teleosts (Alexander 1967; Motta 1984). Moreover, the maxillary articulation with the palatine is modified in these taxa since the ligamentous joint found in Amia is now replaced by a ball-and-socket joint articulation. As it was the case in Amia, the maxillary rotation forms a tubular mouth for suction feeding.
The next major structural specialization is encountered in teleosteans and is related to the increasing mobility of premaxilla and maxilla that are loosely connected by ligaments. It allowed many species to develop the upper jaw protrusion which is the ability to extend the premaxilla and maxilla towards the prey during feeding. Functional advantages of jaw protrusion include at least (1) the increase in the rate of approach of the predator to the prey (Westneat and Wainwright 1989; Ferry-Graham et al. 2001; Waltzek and Wainwright 2003), (2) the increase of the distance from which a prey may be sucked, (3) the decrease of lower jaw movements to close the mouth, (4) the reduction of energy expenditure during suction feeding (Osse 1985) and (5) the increase of the hydrodynamic force exerted on prey (Holzman et al. 2008; Staab et al. 2012). Morphologically, optimized anterior mouth opening for suction feeding also reduces the length of the toothed jaw edge to grasp, retain or bite a prey (Osse 1985). This mechanism would have evolved at least five times in distantly related phylogenetic groups and may help to explain the extraordinary diversity seen in ray-finned fish skulls (Westneat 2004, 2005; Wainwright et al. 2015) (Fig. 5.8). The upper jaw protrusion ability is found in taxa showing the fastest rates of speciation (Alfaro et al. 2009), and interestingly, three of these independent origins have occurred within Ostariophysi (e.g., Gonorynchiformes, Cypriniformes, Characiformes). Although the morphological and kinematical details of jaw protrusion appear to be quite variable (Liem 1980; Motta 1984), a particular system of premaxilla projection appears to be basic to modern teleosts (Osse 1985). Whatever the detailed mechanism, the upper jaw protrusion is always related to the rotation of the maxillary. The shift from the single premaxilla rotation to protrusion seems due to different features.
Schematic representations in left lateral view of the different protraction mechanisms in Actinopterygii: (1) Acipenseriformes redrawn from Carroll and Wainwright (2003), (2) Perciformes redrawn from Motta (1984), (3) Cypriniformes redrawn from Staab et al. (2012), (4) Characiformes Bivibranchia sp. redrawn from Géry (1963) and (5) Gonorynchiformes Phractolaemus sp. redrawn from Géry (1963). Lower jaws are in orange, and the upper jaws are in blue. The kinethmoid bone of Cypriniformes is illustrated in green. The red circles localize the main articulations that are involved in the mechanism of the mouth opening. The dotted circles indicate the mouth opening, and the arrows show the direction of movements
5.4.1 Acipenseriformes
Sturgeons and paddlefishes ( Acipenseriformes or Chondrostei ) constitute a basal group in Actinopterygii. They are notably characterized by reduced ossification of the endoskeleton, but they have numerous dermal bones that are associated with the head and the body. They also have a hyostylic jaw suspension, meaning the upper jaw (or palatoquadrate in sturgeons) is not directly connected to the cranium but it is suspended through loose connective tissue between the upper jaw and ventral surface of the neurocranium (Carroll and Wainwright 2003). The palatoquadrate articulates with the lower jaw (i.e., Meckel’s cartilage), both parts being supported caudally by the hyoid bar. This organization is similar to the jaw anatomy of sharks (Wilga and Motta 1998; Huber et al. 2005). The protrusion mechanism could be summarized as follows (Carroll and Wainwright 2003); (Fig. 5.8). (1) the retraction of the hyoid bar is associated with lower jaw depression; and (2) the contraction of the protractor hyomandibularis, connecting the anterior margin of the hyomandibula to the neurocranium, would provoke forward dorsal rotation of the hyomandibula. This forward displacement would push the symplectic bone rostrally resulting in the jaws being placed outside (i.e., moving anteriorly) of the oral cavity and thus protruded.
5.4.2 Acanthopterygii (e.g., Perciformes)
In acanthopterygian protrusion, the proximal part of the premaxilla moves forward relative to the skull; this kinesis involves different modifications at the level of the skull, the ligaments, the shapes of the maxilla and premaxilla (Fig. 5.8). There is, for example, the development of a sliding articulation between the premaxilla and the skull that corresponds to the development of an ascending process extending over the anterior part of the neurocranium. Another modification corresponds also to the elongation of the toothed process of the premaxilla, excluding the maxilla from the gape (Alexander 1967; Gosline 1980). It would prevent the formation of an angle between maxilla and premaxilla, favouring the development of a rounded mouth gape. The cylindrical shape of the mouth is due to many connective tissues between both bones of the upper and lower jaws. In this system, the twisting of the maxilla during mouth opening does no more have propulsive function because the membranous attachment that previously concerned only the maxilla and the lower jaw is now also found at the level of the premaxilla: the lowering of the mandible directly pulls the premaxilla downward (Schaeffer and Rosen 1961). The maxilla, connective tissue and ligaments (between the premaxilla and the skull) determine the premaxilla protrusion distance. According to the species, this basic system can have numerous adaptations at the level of the morphology (of the upper jaw, anterior part of the skull, etc.) and on the moving mechanism (Motta 1984). The system of levers formed by the lower jaw, maxilla and premaxilla has been modeled as a four-bar linkage (Westneat 2004).
5.4.3 Cypriniformes
In Cypriniformes (carps, minnows, loaches and relatives), an additional sesamoid and synapomorphic bone, called the kinethmoid , is involved in the jaw protrusion mechanism (Fig. 5.8). This bone is located at the rostral neurocranium and is entirely suspended by ligaments which names provide information about their attachments premaxilla-kinethmoid ligament, mesethmoid-kinethmoid ligament, palatine-kinethmoid ligament and maxilla-kinethmoid ligament (Hernandez et al. 2007; Staab and Hernandez 2010). During mouth opening, the kinethmoid makes an anterior 90°–180° rotation that protrudes the premaxilla. The amplitude of the displacement is the function of the kinethmoid size and shape of the ligaments. In this case, the cypriniformes does not have a long ascending process of the premaxilla. The fine mechanism still is not fully understood because its complexity is more important than in the previous group. It implies more components, and there are more connections between the elements (Staab and Hernandez 2010). It was first thought the lower jaw depression drives the premaxilla protrusion as it is the case in Acanthopterygii (Alexander 1967; Motta 1984). However, a recent study on five different species of Cypriniformes has shown it was not the case since the timing of peak gape is not correlated with the timing of peak protrusion (Staab et al. 2012). It shows at least lower jaw movement is not the only force acting on upper jaws. In Cypriniformes, the adductor mandibulae A1 complex (see hereafter) inserts on the maxilla. The A1 bundle organization is more complex than in Acanthopterygii and seems to be implicated in jaw protrusion. Moreover, its high diversity in terms of insertion sites combined with diversity in jaw and kinethmoid shapes highlight specialization in different kinds of movements, increasing the ability of the fish to interact with its environment (Hernandez et al. 2007; Hernandez and Staab 2015). Electromyographic-based studies support the contraction of the A1 bundles and can lower the maxilla (Ballintijn et al. 1972). As a result, the ventral displacement of the maxillae produces tension in the paired maxilla-kinethmoid ligament and the anterior rotation of the kinethmoid. The main functional difference between Cypriniformes and Acanthopterygii (e.g., Perciformes) would be in the flexibility of the movements relative to jaw protrusion (Hernandez and Staab 2015). In acanthopterygians, jaw protrusion takes place simultaneously with full mandible lowering. In cypriniform, the full lower jaw depression is not required to have jaw protrusion. Upper jaw protrusion is decoupled from lower jaw depression, meaning the production can take place with closed or open mouth. According to Gidmark et al. (2012), this functional difference could be related to the ability to feed (lowered mandible + protrusion) or to sort food (raised mandible + protrusion). Additional studies showed movements are more flexible in the relative timing of jaw protrusion and suction flows (Staab et al. 2012). These differences could be related to the feeding niches. Acanthopterygians are found in different feeding niche (Wainwright et al. 2007) but are preferentially feeding on elusive prey in the water column: correlated movements between upper and lower jaw are required to provoke powerful water flow. The Cypriniformes are mostly benthic feeders (López-Fernández et al. 2012; Hernandez and Staab 2015) and could be compared to a vacuum cleaner: the higher kinesis of the jaw allows positioning of a rounded mouth on the substrate that prolongs the sucking action.
5.4.4 Characiformes (Bivibranchia protractila)
The characiform Bivibranchia protractila (junior synonym of Bivibranchia fowleri) is so named due to its protrusible upper jaw (Vari 1985; Vari and Goulding 1985). This feature can be found in different species of Hemiodontidae, but some differences can be found among species (Alexander 1964; Roberts 1974; Vari 1985). In this clade, the small premaxilla is fused to the maxilla, both structures being S-shaped (Fig. 5.8). The upper jaw has lost is ligamentous attachment to the ethmoid and is not articulated to the palatine. However, a ligament can be found between the palatine and the premaxilla, and there is also a maxilla-mandibular ligament between the maxilla and the dentary (Géry 1962). At the level of the rostral part of the suspensorium, the palatine, ectopterygoid and entopterygoid appear to be firmly connected, supporting the neurocranium and most probably articulating with the quadrate (Regan 1911; Alexander 1964). During the mouth opening, the ligament between the dentary and the maxilla pulls the upper jaw downwards and forwards. The upper jaw then pulls the anterior margin of the palatine and rotates the rostral complex of the suspensorium downwards. This movement is feasible thanks to the loose connection between the complex and the quadrate. Therefore, the upper jaw is protracted (Géry 1962; Vari 1985; Vari and Goulding 1985).
5.4.5 Gonorynchiformes (Phractolaemus ansorgii) (Grande and Poyato-Ariza 1999)
To the best of our knowledge, the mechanism of the jaw protrusion in the gonorynchiform Phractolaemus ansorgii is not known but inferred from dissection and handly manipulations. At rest, the mouth is unusually positioned being dorsally directed (Fig. 5.8). In this situation, the raised lower jaw forms a semicircle with the upper jaw (Géry 1963). The upper jaw is located under the mesethmoid. It connects both the suspensorium (through the palatine) and neurocranium (through the prevomer) by a ligament (Grande et al. 2010), meaning the upper jaw is extremely movable. During the lowering of the mandible, the lower jaw rotates anteriorly around the quadrate to gain a horizontal position. In this situation, the maxilla-mandibular ligaments pull the upper jaws anteriorly, what results in the loss of connection of the jaw with the skull and facilitates the protrusion. When the mouth is totally protracted, the oral cavity is completely directed anteriorly or anteroventrally (Thys van den Audenaerde 1961).
It is worth mentioning that some species can also show protrusible lower jaws (Westneat and Wainwright 1989). In the sling-jaw wrasse, Epibulus insidiator, this unusual ability is mainly related to deep modifications at the level of the suspensorium and opercle. In Perciformes, the quadrate found at the lower part of the suspensorium has usually the role of a stationary support for the lower jaw because it is firmly attached to other bones (symplectic, metapterygoid, etc.) of the jaw. In Epibulus, the quadrate can articulate with the metapterygoid and make rotations that push the lower jaw rostrally (Delsman 1925; Westneat and Wainwright 1989). However, it is also important to bear in mind that most of the skeletal pieces of the skull and jaws are able to perform these incredible movements because of the contraction of cranial muscles .
During the evolution of actinopterygians and more generally those of vertebrates, the cranial muscles underwent enormous diversification that was crucial to the success of each clade (Goodrich 1958). Within the large class of Actinopterygii, it is important to understand that virtually all species have the cranial musculature described in the “anatomy” part of this chapter. Some of these cranial muscles have however differentiated by subdividing into several muscle sections to probably respond to the increasing complexity and kinetics of the teleostean skull. Nevertheless, the role of each muscle element remains fundamentally conserved in all ray-finned fishes, except from species which have early diverged such as sturgeons or paddlefishes (Acipenseriformes) which are having deeply anatomical and functional differences (Carroll and Wainwright 2003; Miller 2004).
In the cranial musculature of actinopterygians, the most studied and differentiated muscle is undoubtedly the adductor mandibulae muscle complex since it participates both in breathing and feeding movements by raising the lower jaw and closing the mouth. However, the evolution and nomenclature of the different muscle bundles of the adductor mandibulae has been the subject of many discussions and predominantly for teleostean fishes. There are many hypotheses about the early differentiation of the adductor mandibulae muscle in the course of evolution of actinopterygians and mainly in the teleostean lineage (Lauder 1980; Gosline 1989) and numerous publications are devoted to the terminology (e.g., Owen 1846, 1866; Winterbottom 1974; Diogo and Chardon 2000a, b; Wu and Shen 2004; Diogo et al. 2008; Datovo and Bockmann 2010; Datovo and Castro 2012). In the framework of this chapter, we have decided to bring to the fore the new terminology of Datovo and Vari (2013) instead of that proposed by Vetter in 1878 and subsequently used by Winterbottom (1974) and other authors with some misinterpretations. Table 5.1 highlights the main differences with regard to the way of naming and understanding the subdivisions of the adductor mandibulae muscle. The terminology of Datovo and Vari (2013) concerns only teleostean fishes and is more intuitive since it is possible to designate the different muscle subdivisions based on their position. Besides, it is easy to understand the instances of evolutionary subdivision and/or coalescence of muscle subdivisions (McCord and Westneat 2016). For further discussion, the following reference books should be consulted (Winterbottom 1974; Datovo and Vari 2013, 2014).
In basal actinopterygians (Polypteriformes, Lepisosteiformes and Amiiformes), the adductor mandibulae muscle is generally subdivided into three main portions: anterior, medial and posterolateral portions (Lauder 1980) that do not have the same nomenclature as teleostean fishes (see hereafter). In Polypterus, the anterior portion is absent, whereas it is subdivided in Lepisosteus and Amia calva. In any basal actinopterygian lineage, the medial portion is also separated into two subdivisions, but they have different pathways of differentiation and muscle terminology. The posterolateral portion is not subdivided, and the entire muscle has a unique attachment site on the medial face of the lower jaw (Lauder 1980).
In the teleostean lineage, the complex configuration of the adductor mandibulae muscle, coupled with the fact that its different muscle sections are found in diverse groups, has suggested several pathways of differentiation (Gosline 1989). It must be imagined that at the beginning, there was only a single muscle adductor mandibulae mass and that the first differentiation of the jaw muscle would have been in the segregation between the facial and mandibular adductor mandibulae segment where the latter would have separated as a distinct entity (Edgeworth 1935; Winterbottom 1974; Gosline 1989). In addition, this first differentiation would be an actinopterygian plesiomorphy (Lauder 1980) which means that all ray-finned fishes have this first subdivision for the adductor mandibulae. Secondly, the facial segment would have begun to differentiate even though an unsubdivided facial segment is observable, for example, in Elopiformes (Elops), Osteoglossiformes (Hiodon), Salmoniformes (Salvelinus) and Clupeiformes (Clupea) (Lauder and Liem 1980; Gosline 1989; Datovo and Vari 2014). According to Gosline (1989), two pathways of second differentiation can be observed in teleosteans. In the first pathway of differentiation found in acanthopterygians (e.g., Atheriniformes, Cyprinodontiformes, Gasterosteiformes, Perciformes, Scorpaeniformes, Tetraodontiformes), an antero-dorso-lateral part of the facial segment is differentiated and develops an attachment site on the upper jaw at the level of the maxilla [that can be the A1 section for Winterbottom (1974) and the pars malaris section or the pars promalaris subsection for Datovo and Vari (2013)]. Then, another part of the facial segment separates more medially, which is observed in most of teleosteans [that can be the A2/A3 section for Winterbottom (1974) and the pars malaris and pars stegalis sections for Datovo and Vari (2013)]. The second pathway of differentiation is encountered in most Ostariophysi (i.e., Gonorynchiformes, Cypriniformes, Characiformes, Gymnotiformes, Siluriformes) where an antero-ventro-lateral part of the facial segment appears and attaches on the medial face of the lower jaw [that can be the A2 section for Winterbottom (1974) and the pars rictalis section for Datovo and Vari (2013)]. In that case, this division of the adductor mandibulae seems to have developed as a “supplementary system for raising the mandible”(Diogo and Chardon 2000a, b). There is also another part of the facial segment that differentiates more medially, but it is followed by a more external differentiation of the first ventral section. This new subdivision develops, via the primordial ligament, an attachment site on the upper jaw at the level of the maxilla [that can be the A1-OST section for Winterbottom (1974) and the pars ectorictalis subsection for Datovo and Vari (2013)]. Among some Siluriformes, another special differentiation would appear externally with an attachment site on the maxilla and could be termed the retractor tentaculi muscle (Diogo and Chardon 2000a, b; Datovo and Vari 2013, 2014).
In this way, the evolution and differentiation of the adductor mandibulae muscle is one of the most notable within the cranial musculature because, in our opinion, this development may be mainly related to the parallel specialization of the buccal jaws which become able to protrude for suction feeding in more advanced ray-finned fishes (Westneat 2004, 2005; Wainwright et al. 2015).
References
Aerts P (1991) Hyoid morphology and movements relative to abducting forces during feeding in Astatotilapia elegans (Teleostei: Cichlidae). J Morphol 208(3):323–345
Alexander RM (1964) Adaptation in the skulls and cranial muscles of south American characinoid fish. Zool J Linnean Soc 45(305):169–190
Alexander RM (1967) The functions and mechanisms of the protrusible upper jaws of some acanthopterygian fish. J Zool 151(1):43–64
Alfaro ME, Janovetz J, Westneat MW (2001) Motor control across trophic strategies: muscle activity of biting and suction feeding fishes. Am Zool 41(6):1266–1279
Alfaro ME et al (2009) Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc Natl Acad Sci U S A 106(32):13410–13414
Allis EP (1897) The cranial muscles and cranial and first spinal nerves in Amia calva. Ginn
Allis EP (1919) The homologies of the maxillary and vomer bones of Polypterus. Dev Dyn 25(4):348–394
Allis EP (1922) The cranial anatomy of Polypterus, with special reference to Polypterus bichir. J Anat 56(3-4):189–294
Ballintijn CM, Hughes GM (1965) The muscular basis of the respiratory pumps in the trout. J Exp Biol 43(2):349–362
Ballintijn CM, Van Den Burg A, Egberink BP (1972) An electromyographic study of the adductor mandibulae complex of a free-swimming carp (Cyprinus carpio L.) during feeding. J Exp Biol 57(1):261–283
Barel CDN (1983) Towards a constructional morphology of cichlid fishes (Teleostei, Perciformes). Neth J Zool 33(4):357–424
Bemis WE, Forey PL (2001) Occipital structure and the posterior limit of the skull in actinopterygians. In: Major events in early vertebrate evolution: palaeontology, phylogeny, genetics and development. Taylor & Francis, London, pp 41–62
Bemis WE, Findeis EK, Grande L (1997) An overview of Acipenseriformes. Environ Biol Fish 48(1-4):25–71
Blot J (1966) Étude des Palaeonisciformes du bassin houiller de Commentry. Allier, Paris
Brainerd EL, Ferry-Graham LA (2005) Mechanics of respiratory pumps. Fish Physiol 23:1–28
Camp AL, Konow N, Sanford CPJ (2009) Functional morphology and biomechanics of the tongue-bite apparatus in salmonid and osteoglossomorph fishes. J Anat 214(5):717–728
Camp AL, Roberts TJ, Brainerd EL (2015) Swimming muscles power suction feeding in largemouth bass. Proc Natl Acad Sci U S A 112(28):8690–8695
Carroll AM (2004) Muscle activation and strain during suction feeding in the largemouth bass Micropterus salmoides. J Exp Biol 207(6):983–991
Carroll AM, Wainwright PC (2003) Functional morphology of prey capture in the sturgeon, Scaphirhynchus albus. J Morphol 256(3):270–284
Carroll AM et al (2004) Morphology predicts suction feeding performance in centrarchid fishes. J Exp Biol 207(22):3873–3881
Carvalho M, Vari RP (2015) Development of the splanchnocranium in Prochilodus argenteus (Teleostei: Characiformes) with a discussion of the basal developmental patterns in the Otophysi. Zoology 118(1):34–50
Cloutier R, Arratia G (2004) Early diversification of actinopterygians. In: Recent advances in the origin and early radiation of vertebrates. Pfeil, Munich, pp 217–270
Cubbage CC, Mabee PM (1996) Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae). J Morphol 229(2):121–160
Datovo A, Bockmann FA (2010) Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol 8(2):193–246
Datovo A, Castro RMC (2012) Anatomy and evolution of the mandibular, hyopalatine, and opercular muscles in characiform fishes (Teleostei: Ostariophysi). Zoology 115(2):84–116
Datovo A, Vari RP (2013) The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (osteichthyes: actinopterygii). PLoS One 8(4):e60846
Datovo A, Vari RP (2014) The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linnean Soc 171(3):552–622
Day SW et al (2005) Sucking while swimming: evaluating the effects of ram speed on suction generation in bluegill sunfish Lepomis macrochirus using digital particle image velocimetry. J Exp Biol 208(14):2653–2660
Day SW, Higham TE, Wainwright PC (2007) Time resolved measurements of the flow generated by suction feeding fish. Exp Fluids 43(5):713–724
De Schepper N, Adriaens D, De Kegel B (2005) Moringua edwardsi (Moringuidae: Anguilliformes): cranial specialization for head-first burrowing. J Morphol 266(3):356–368
De Schepper N, De Kegel B, Adriaens D (2007) Pisodonophis boro (Ophichthidae: Anguilliformes): specialization for head-first and tail-first burrowing. J Morphol 268(2):112–126
Deary AL, Hilton EJ (2016) Comparative ontogeny of the feeding apparatus of sympatric drums (Perciformes: Sciaenidae) in the Chesapeake Bay. J Morphol 277(2):183–195
Delsman HC (1925) Fishes with protrusile mouths. Treubia 6:98–106
Diogo R (2008) The origin of higher clades: osteology, myology, phylogeny and evolution of bony fishes and the rise of tetrapods. Science, New York
Diogo R, Abdala V (2010) Muscles of vertebrates: comparative anatomy, evolution, homologies and development. CRC Press, Boca Raton
Diogo R, Chardon M (2000a) Anatomie et fonction des structures céphaliques associées à la prise de nourriture chez le genre Chrysichthys (Teleostei: Siluriformes). Belg J Zool 130(1):21–37
Diogo R, Chardon M (2000b) Homologies among different adductor mandibuale sections of teleostan fishes, with special regard to catfishes (Teleostei: Siluriformes). J Morphol 243(2):193–208
Diogo R, Hinits Y, Hughes SM (2008) Development of mandibular, hyoid and hypobranchial muscles in the zebrafish: homologies and evolution of these muscles within bony fishes and tetrapods. BMC Dev Biol 8(1):24
Eagderi S, Adriaens D (2010) Cephalic morphology of Pythonichthys macrurus (Heterenchelyidae: Anguilliformes): specializations for head-first burrowing. J Morphol 271(9):1053–1065
Edgeworth FH (1935) The cranial muscles of vertebrates. Cambridge University Press, Cambridge
Engeman JM, Aspinwall N, Mabee PM (2009) Development of the pharyngeal arch skeleton in Catostomus commersonii (Teleostei: Cypriniformes). J Morphol 270(3):291–305
Faustino M, Power DM (2001) Osteologic development of the viscerocranial skeleton in sea bream: alternative ossification strategies in teleost fish. J Fish Biol 58(2):537–572
Ferry LA, Paig-Tran EM, Gibb AC (2015) Suction, ram, and biting: deviations and limitations to the capture of aquatic prey. Integr Comp Biol 55(1):97–109
Ferry-Graham LA, Lauder GV, Hulsey CD (2001) Aquatic prey capture in ray-finned fishes: a century of progress and new directions. J Morphol 248(2):99–119
Fraser GJ et al (2009) An ancient gene network is co-opted for teeth on old and new jaws. PLoS Biol 7(2):e1000031
Geerinckx T et al (2007) A head with a suckermouth: a functional-morphological study of the head of the suckermouth armoured catfish Ancistrus cf. triradiatus (Loricariidae, Siluriformes). Belg J Zool 137(1):47–66
Géry J (1962) Pterohemiodus luelingi sp. nov., un curieux poisson characoïde à nageoire dorsale filamenteuse, avec une clé des genres d’Hemiodontinae (Ostariophysi-Erythrinidae). Bonner zoologische Beiträge 59(12):332–342
Géry J (1963) L’appareil protracteur buccal de Bivibranchia (Characoidei) avec une note sur Phractolaemus (Chanoidei) (Pisces). Vie et Milieu 13(4):729–740
Gibb A (1996) The kinematics of prey capture in Xystreurys liolepis: do all flatfish feed asymmetrically? J Exp Biol 199(10):2269–2283
Gidmark NJ et al (2012) Flexibility in starting posture drives flexibility in kinematic behavior of the kinethmoid-mediated premaxillary protrusion mechanism in a cyprinid fish, Cyprinus carpio. J Exp Biol 215(13):2262–2272
Gidmark NJ et al (2015) Functional morphology of durophagy in black carp, Mylopharyngodon piceus. J Morphol 276(12):1422–1432
Goodrich ES (1958) Studies on the structure and development of vertebrates, vol II. Macmillan, London
Gosline WA (1973) Considerations regarding the phylogeny of cypriniform fishes, with special reference to structures associated with feeding. Copeia 1973(4):761–776
Gosline WA (1980) The evolution of some structural systems with reference to the interrelationships of modern lower teleostean fish groups. Japan J Ichthyol 27(1):1–28
Gosline WA (1989) Two patterns of differentiation in the jaw musculature of teleostean fishes. J Zool 218(4):649–661
Grande T, Poyato-Ariza FJ (1999) Phylogenetic relationships of fossil and recent gonorynchiform fishes (Teleostei: Ostariophysi). Zool J Linnean Soc 125(2):197–238
Grande T, Poyato-Ariza FJ, Diogo R (2010) Gonorynchiformes and Ostariophysan relationships: a comprehensive review. Science, New York
Greenwood PH et al (1966) Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bulletin of the AMNH 131:4
Grubich JR (2001) Prey capture in actinopterygian fishes: a review of suction feeding motor patterns with new evidence from an elopomorph fish, Megalops atlanticus. Am Zool 41(6):1258–1265
Helfman GS et al (2009) The diversity of fishes: biology, evolution, and ecology. Wiley, Hoboken, NJ
Herbing IHV et al (1996) Ontogeny of feeding and respiration in larval Atlantic cod Gadus morhua (Teleostei, Gadiformes): I. Morphology. J Morphol 227(1):15–35
Hernandez LP, Staab KL (2015) Bottom feeding and beyond: how the premaxillary protrusion of cypriniforms allowed for a novel kind of suction feeding. Integr Comp Biol 55(1):74–84
Hernandez PL, Bird NC, Staab KL (2007) Using zebrafish to investigate cypriniform evolutionary novelties: functional development and evolutionary diversification of the kinethmoid. J Exp Zool B Mol Dev Evol 308(5):625–641
Higham TE, Day SW, Wainwright PC (2006a) Multidimensional analysis of suction feeding performance in fishes: fluid speed, acceleration, strike accuracy and the ingested volume of water. J Exp Biol 209(14):2713–2725
Higham TE, Day SW, Wainwright PC (2006b) The pressures of suction feeding: the relation between buccal pressure and induced fluid speed in centrarchid fishes. J Exp Biol 209(17):3281–3287
Holzman R et al (2008) Jaw protrusion enhances forces exerted on prey by suction feeding fishes. J R Soc Interface 5(29):1445–1457
Huber DR et al (2005) Analysis of the bite force and mechanical design of the feeding mechanism of the durophagous horn shark Heterodontus francisci. J Exp Biol 208(18):3553–3571
Hughes GM, Shelton G (1958) The mechanism of gill ventilation in three freshwater teleosts. J Exp Biol 35(4):807–823
Hulsey CD, Garcia De Leon FJ (2005) Cichlid jaw mechanics: linking morphology to feeding specialization. Funct Ecol 19(3):487–494
Inoue JG et al (2003) Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the “ancient fish”. Mol Phylogenet Evol 26(1):110–120
Janvier P (1996) Early vertebrates. Oxford University Press, New York, NY
Kammerer CF, Grande L, Westneat MW (2006) Comparative and developmental functional morphology of the jaws of living and fossil gars (Actinopterygii: Lepisosteidae). J Morphol 267(9):1017–1031
Kardong KV (2012) Vertebrates: comparative anatomy, function, evolution. McGraw-Hill Higher Education, New York
Kimmel CB et al (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310
Kimmel CB et al (2001) Neural crest patterning and the evolution of the jaw. J Anat 199(1–2):105–119
Konstantinidis P et al (2015) The developmental pattern of the musculature associated with the mandibular and hyoid arches in the longnose gar, Lepisosteus osseus (Actinopterygii, Ginglymodi, Lepisosteiformes). Copeia 103(4):920–932
Lauder GV (1980) Evolution of the feeding mechanism in primitive actinopterygian fishes: a functional anatomical analysis of Polypterus, Lepisosteus, and Amia. J Morphol 163(3):283–317
Lauder GV (1982) Patterns of evolution in the feeding mechanism of actinopterygian fishes. Am Zool 22(2):275–285
Lauder GV (1985) Aquatic feeding in lower vertebrates. In: Hildebrand M, Bramble DM, Liem KF, Wake DB (eds) Functional vertebrate morphology. Harvard University Press, Cambridge, pp 210–229
Lauder GV, Liem KF (1980) The feeding mechanism and cephalic myology of Salvelinus fontinalis: form, function, and evolutionary significance. In: Charrs: Salomnids of the genus Salvelinus, pp 365–390
Lauder GV, Liem KF (1981) Prey capture by Luciocephalus pulcher: implications for models of jaw protrusion in teleost fishes. Environ Biol Fish 6(3):257–268
Lauder GV, Liem KF (1983) Patterns of diversity and evolution in ray-finned fishes. Fish Neurobiol 1:1–24
Lecointre G, Le Guyader H (2001) Classification phylogénétique du vivant, vol Vol. 2. Belin, Paris
Liem KF (1967) Functional morphology of the head of the anabantoid teleost fish Helostoma temmincki. J Morphol 121(2):135–157
Liem KF (1978) Modulatory multiplicity in the functional repertoire of the feeding mechanism in cichlids fishes. Part I. Piscivores. J Morphol 158(3):323–360
Liem KF (1980) Adaptive significance of intra-and interspecific differences in the feeding repertoires of cichlid fishes. Am Zool 20(1):295–314
Liem KF (1990) Aquatic versus terrestrial feeding modes: possible impacts on the trophic ecology of vertebrates. Am Zool 30(1):209–221
López-Fernández H et al (2012) Diet-morphology correlations in the radiation of South American geophagine cichlids (Perciformes: Cichlidae: Cichlinae). PLoS One 7(4):e33997
McCord CL, Westneat MW (2016) Evolutionary patterns of shape and functional diversification in the skull and jaw musculature of triggerfishes (Teleostei: Balistidae). J Morphol 277(6):737–752
Miller MJ (2004) The ecology and functional morphology of feeding of North American sturgeon and paddlefish. In: Sturgeons and paddlefish of North America. Springer, Dordrecht, pp 87–102
Miller RF, McGovern JH (1996) Preliminary report of fossil fish (Actinopterygii: Palaeonisciformes) from the Lower Carboniferous Albert Formation at Norton, New Brunswick (NTS 21 H/12). Current research, pp 97–104
Motta PJ (1984) Mechanics and functions of jaw protrusion in teleost fishes: a review. Copeia 1984(1):1–18
Motta PJ, Huber DR (2004) Prey capture behavior and feeding mechanics of elasmobranchs. In: Biology of sharks and their relatives, 2nd edn. Taylor & Francis, London, pp 153–197
Muller M (1987) Optimization principles applied to the mechanism of neurocranium levation and mouth bottom depression in bony fishes (Halecostomi). J Theor Biol 126(3):343–368
Muller M, Osse JWM, Verhagen JHG (1982) A quantitative hydrodynamical model of suction feeding in fish. J Theor Biol 95(1):49–79
Nelson JS (1994) Fishes of the world. Wiley, New York
Nelson JS (2006) Fishes of the world. Wiley, Hoboken
Nelson JS, Grande T, Wilson MVH (2016) Fishes of the world. Wiley, New York
Noda M, Miyake T, Okabe M (2017) Development of cranial muscles in the actinopterygian fish Senegal bichir, Polypterus senegalus Cuvier, 1829. J Morphol 278(4):450–463
Osse JWM (1969) Functional morphology of the head of the perch (Perca Fluviatilis L.): an electromyographic study. Neth J Zool 19(3):289–392
Osse JWM (1985) Jaw protrusion, an optimization of the feeding apparatus of teleosts? Acta Biotheor 34(2):219–232
Owen R (1846) Lectures on the comparative anatomy and physiology of the vertebrate animals: Delivered at the Royal College of Surgeons of England, in 1844 and 1846. Volume 2. Part I - Fishes. Longman, Brown, Green, and Longmans
Owen R (1866) Comparative anatomy and physiology of vertebrates: fishes and reptiles. Longman, Harlow
Parmentier E (2003) Contribution à l’étude des relations entre des poissons de la famille des Carapidae et leurs hôtes invertébrés: une approche mutidisciplinaire. University of Liège, Liège
Parmentier E et al (1998) Morphology of the buccal apparatus and related structures in four species of Carapidae. Aust J Zool 46(4):391–404
Parmentier E, Vandewalle P, Lagardere F (2001) Morpho-anatomy of the otic region in carapid fishes: eco-morphological study of their otoliths. J Fish Biol 58(4):1046–1061
Patterson C (1994) Bony fishes. In: Prothero DR, Schoch RM (eds) Major features of vertebrate evolution, Short courses in paleontology, vol 7. Paleontological Society, University of Tennessee, Knoxville, pp 57–84
Peng Z et al (2009) Teleost fishes (Teleostei). The timetree of life. Oxford University Press, Oxford, pp 335–338
Poplin CM (1984) Lawrenciella schaefferi n.g., n.sp. (Pisces: Actinopterygii) and the use of endocranial characters in the classification of the Palaeonisciformes. J Vertebr Paleontol 4(3):413–421
Regan CT (1911) LXV. The classification of the teleostean fishes of the order Ostariophysi—2. Siluroidea. Ann Mag Nat Hist 8(47):553–577
Roberts TR (1974) Dental polymorphism and systematics in Saccodon, a neotropical genus of freshwater fishes (Parodontidae, Characoidei). J Zool 173(3):303–321
Rosen DE (1982) Teleostean interrelationships, morphological function and evolutionary inference. Am Zool 22(2):261–273
Sanford CP, Lauder GV (1989) Functional morphology of the tongue-bite in the osteoglossomorph fish Notopterus. J Morphol 202(3):379–408
Schaeffer B, Rosen BE (1961) Major adaptive levels in the evolution of the actinopterygian feeding mechanism. Am Zool 1(2):187–204
Schilling TF, Kimmel CB (1994) Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120(3):483–494
Schilling TF, Kimmel CB (1997) Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development 124(15):2945–2960
Shadwick RE, Lauder GV (2006) Fish physiology: fish biomechanics, vol 23. Academic Press, Cambridge
Staab KL, Hernandez LP (2010) Development of the cypriniform protrusible jaw complex in Danio rerio: constructional insights for evolution. J Morphol 271(7):814–825
Staab KL et al (2012) Independently evolved upper jaw protrusion mechanisms show convergent hydrodynamic function in teleost fishes. J Exp Biol 215(9):1456–1463
Thys van den Audenaerde DFE (1961) L’anatomie de Phractolaemus ansorgei Blgr. et la position systématique des Phractolaemidae. Annales du Musée Royal de l’Afrique Centrale, Sciences Zoologiques, série 8(103):101–167
Traquair RH (1870) The cranial osteology of Polypterus. J Anat Physiol 5(Pt 1):166–184
Van Wassenbergh S, Aerts P, Herrel A (2005) Scaling of suction-feeding kinematics and dynamics in the African catfish, Clarias gariepinus. J Exp Biol 208(11):2103–2114
Vandewalle P et al (1992) Early development of the cephalic skeleton of Barbus barbus (Teleostei, Cyprinidae). J Fish Biol 41(1):43–62
Vandewalle P et al (1997) Postembryonic development of the cephalic region in Heterobranchus longifilis. J Fish Biol 50(2):227–253
Vandewalle P, Parmentier E, Chardon M (2000) The branchial basket in teleost feeding. Cybium 24(4):319–342
Vari RP (1985) A new species of Bivibranchia (Pisces: Characiformes) from Surinam, with comments on the genus. Proc Biol Soc Wash 98(2):511–522
Vari RP, Goulding M (1985) A new species of Bivibranchia (Pisces: Characiformes) from the Amazon River basin. Proc Biol Soc Wash 98(4):1054–1061
Venkatesh B, Erdmann MV, Brenner S (2001) Molecular synapomorphies resolve evolutionary relationships of extant jawed vertebrates. Proc Natl Acad Sci U S A 98(20):11382–11387
Vrba ES (1968) Contributions to the functional morphology of fishes. Part V The feeding mechanism of Elops taurus Linnaeus. Afr Zool 3(2):211–236
Wainwright PC et al (1989) Evolution of motor patterns: aquatic feeding in salamanders and ray-finned fishes. Brain Behav Evol 34(6):329–341
Wainwright PC et al (2001) Evaluating the use of ram and suction during prey capture by cichlid fishes. J Exp Biol 204(17):3039–3051
Wainwright P et al (2007) Suction feeding mechanics, performance, and diversity in fishes. Integr Comp Biol 47(1):96–106
Wainwright PC et al (2015) Origins, innovations, and diversification of suction feeding in vertebrates. Integr Comp Biol 55(1):134–145
Waltzek TB, Wainwright PC (2003) Functional morphology of extreme jaw protrusion in Neotropical cichlids. J Morphol 257(1):96–106
Westneat MW (1994) Transmission of force and velocity in the feeding mechanisms of labrid fishes (Teleostei, Perciformes). Zoomorphology 114(2):103–118
Westneat MW (2004) Evolution of levers and linkages in the feeding mechanisms of fishes. Integr Comp Biol 44(5):378–389
Westneat MW (2005) Skull biomechanics and suction feeding in fishes. Fish Physiol 23:29–75
Westneat MW, Olsen AM (2015) How fish power suction feeding. Proc Natl Acad Sci 112(28):8525–8526
Westneat MW, Wainwright PC (1989) Feeding mechanism of Epibulus insidiator (Labridae; Teleostei): evolution of a novel functional system. J Morphol 202(2):129–150
Wilga C, Motta P (1998) Conservation and variation in the feeding mechanism of the spiny dogfish Squalus acanthias. J Exp Biol 201(9):1345–1358
Wilson MVH, Veilleux P (1982) Comparative osteology and relationships of the Umbridae (Pisces: Salmoniformes). Zool J Linnean Soc 76(4):321–352
Winterbottom R (1974) A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Natl Sci Phila 125(125):225–317
Wu KY, Shen SC (2004) Review of the teleostean adductor mandibulae and its significance to the systematic positions of the Polymixiiformes, Lampridiformes, and Triacanthoidei. Zool Stud 43(4):712–736
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Huby, A., Parmentier, E. (2019). Actinopterygians: Head, Jaws and Muscles. In: Ziermann, J., Diaz Jr, R., Diogo, R. (eds) Heads, Jaws, and Muscles. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-93560-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-93560-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93559-1
Online ISBN: 978-3-319-93560-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)