Abstract
Extant cyclostomes are jawless vertebrates and include hagfishes and lampreys. They are the closest extant relatives to jawed vertebrates (often called gnathostomes, which also include jawless extinct species) and share with them vertebrate-specific characters, like the presence of somites, neural crest cells, eyes (absent in hagfish), among others. There are also cyclostome-specific characteristics as the lingual apparatus, velum, and posterior hypophyseal process-derived cartilages. The comparison of development and anatomy between cyclostomes and gnathostomes provides insights into the evolution of the jaws and associated muscles. Furthermore, comparative anatomical and evolutionary developmental studies help to reconstruct the last common ancestor of vertebrates, cyclostomes, and gnathostomes. The evolutionary appearance of the jaw and associated muscles is of particular interest, as those are said to be one of the main reasons for the success of jawed vertebrates. Interestingly, the mechanisms to make an upper jaw are in place in cyclostomes and jawed vertebrates, but the lower jaw developmental program is only found in gnathostomes. The lower jaw is therefore a novelty that evolved in gnathostomes. Another example is the mandibular arch mesoderm and the associated neural crest cells which give rise to mandibular arch muscles and the jaws in gnathostomes, respectively. Cyclostomes have many muscles derived from the mandibular arch mesoderm, but only few can be homologized with those muscles from gnathostomes. However, those muscles that are homologous between those taxa must be present in their last common ancestor, i.e., vertebrates. Interestingly, some studies seem to indicate that mandibular arch (first arch) development was only secondarily included in the vertebrate branchial arch series, what would explain several differences in mandibular arch development between jawed and jawless vertebrates. However, this hypothesis is controversially discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
There are two major extant vertebrate groups: jawed and jawless vertebrates (“gnathostomes” and cyclostomes, respectively). The former includes jawless fossil taxa. The “ostracoderms” (i.e., arandaspids, heterostracans, thelodonts, galeaspids, osteostracans, pituriaspids; see Chap. 2) are currently regarded as a paraphyletic group which are characterized by having an array of bone- and dentine-producing tissues and are therefore viewed as jawless stem gnathostomes (e.g., Janvier 2008). Here, I continue to use the term gnathostome throughout the chapter as jawed vertebrates are almost identical with modern gnathostomes. Cyclostomes are animals that on first sight resemble giant worms (Fig. 3.1) and comprise hagfishes ( Myxiniformes ) and lampreys ( Petromyzontiformes ) (Heimberg et al. 2010). Their name indicates the presence of a round mouth (Fig. 3.1e, f), and they are often grouped with other jawless extinct vertebrates in the paraphyletic group agnathans (see Chap. 2), i.e., vertebrates without jaws, from which jawed vertebrates diverged 430–520 million years ago. The jawless vertebrates were diverse during the mid-Paleozoic, but only lampreys and hagfishes are still extant (Potter 1980). It is not the intention of this chapter to analyze the relationship between fossil and/or extant hagfishes and lampreys. Information about the fossil record can be found in diverse literature (e.g., Gess et al. 2006 and citations within).
Cyclostomes: (a) slime hag, Eptatretus sp. (formerly Bdellostoma); (b) hagfish, Myxine sp.; (c) lamprey, Petromyzon sp.; (a–c) from Romer (1950). (d) Ammocoete (larval lamprey), from Hardisty et al. (1989). (e) Mouthpart from Petromyzon marinus, from Potter (1980). (f) Ventral view of the mouthpart of a hagfish during maximum gape, from Clark et al. (2010)
There are currently 38 extant lamprey species known, which live in the sea but spawn in rivers (Gee 2018). Larval lampreys are commonly known as “ammocoetes” (Fig. 3.1d) because they were erroneously regarded as adult forms (Leach 1944). Lampreys are distributed antitropical and the distribution is dependent on the lethal temperature of the ammocoetes which lies between 28° C and 32° C (Potter 1980). As adults, lampreys are parasitic or nonparasitic, with the latter being marked by an extended larval live, reduced postmetamorphic time, and smaller adult size (Potter 1980). Furthermore, nonparasitic forms do not feed during postlarval life (Potter 1980). Lamprey larvae live burrowed in river mud with their front end exposed to the water from which they filter particles. Larval and adult lampreys are often characterized by their mouthparts (dentition, tentacles), length, coloration, and “tongue” precursor/lingual apparatus. The body proportions are also important to distinguish different life stages from larval to adult specimens (Potter 1980). Adult parasitic lampreys have a circular sucker with many teeth (Fig. 3.1e) and a tongue that also contains teeth, which can be protruded from the mouth to grab onto passing fishes to rib chunks out of them. They only have a single, medial nostril which is connected to the olfactory capsule. Seven gill slits are located behind the eye; in a historical description, the unpaired nostril, the lateral eye, and those seven gill slits together led to the misleading German name “Neunaugen” (nine eyes; Fig. 3.1c).
All extant hagfishes are benthic, opportunistic scavengers of marine invertebrates and vertebrates (Auster and Barber 2006). Their feeding apparatus has teeth (Fig. 3.1f) and cartilage but is dominated by muscles; the proportions of the feeding apparatus and the number of horny teeth are used to distinguish different species (Clark and Summers 2007). Hagfishes are able to forcefully remove tissue from carcasses and to ingest large pieces of food, despite having no jaws (Clark and Summers 2007). The use of gape cycles to grasp, ingest, and intraorally transport food was described by Clark and Summers (2007) and Clark et al. (2010).
It is often assumed that hagfishes are the more basal taxon in cyclostomes, partly because of the secondary loss of structures (Forey and Janvier 1993; Gess et al. 2006), which even included traits that are used to define vertebrates, as, for example, eyes and eye-related structures. However, developmental studies in hagfish have shown the presence of neural crest, somites, and even the appearance of putative vertebrae in the most caudal trunk region (Ota et al. 2007, 2011). There are also hagfish-specific traits such as the secondary opening of the nasohypophyseal duct into the pharynx (Oisi et al. 2013b) and the posterior shift of the caudal branchial arches (Holmgren 1946).
The monophyly of cyclostomes, i.e., that lampreys and hagfishes belong to the same taxon, was long questioned, but more recent molecular and developmental studies support this view (e.g., Kuraku et al. 1999; Delsuc et al. 2006; Heimberg et al. 2010). Their phylogenetic position as sister taxon to extant jawed vertebrates, Gnathostomata (Heimberg et al. 2010), makes them the most interesting group to study the origin and evolution of vertebrate structures (e.g., Janvier 1996, 2007; Kuratani et al. 2001; Kuratani 2004, 2005a, b, 2008a, b,). The comparative analysis of traits in those groups enables the uncovering of evolutionary patterns across early vertebrate lineages. In particular ammocoetes are often studied to understand vertebrate evolution as they resemble closer to the ancestral vertebrate (see below).
For example, cyclostomes are studied to understand the evolution of hypophyses and thyroid gland development (Leach 1944), thyroid hormone receptors (Holzer and Laudet 2017), adaptive immune system (Poole et al. 2017), heart physiology (Augustinsson et al. 1956), Tbx1/10 gene expression (Sauka-Spengler et al. 2002; Tiecke et al. 2007), oxygen transport with hemoglobin (Hoffmann et al. 2010), telencephalon (Sugahara et al. 2013), hindbrain segmentation (Parker et al. 2014), neural crest gene regulatory network (Ota et al. 2007; Green et al. 2015), vertebrate paired fins (Tulenko et al. 2013), and many other structures.
Comparing the two major taxa of living vertebrates, the cyclostomes and gnathostomes, revealed many shared traits that had to be present in their last common ancestor (LCA), the vertebrates (Oisi et al. 2013b). The LCA of vertebrates had a musculoskeletal body plan that only consisted of branchial and axial structures, including skeletal arches that supported the gills, segmental myotomes, vertebrae, and median fins (Janvier 1996; Ota et al. 2011). Recently, it was shown that gills in cyclostomes and gnathostomes are homologous (Gillis and Tidswell 2017), a subject discussed for several decades (Mallatt 1984). There are also cyclostome-specific developmental and morphological traits that cannot be identified in gnathostomes.
This book focuses on the evolution of heads, jaws, and associated muscles in vertebrates. Therefore, I focus in this chapter on characters shared between jawless and jawed vertebrates. Those characters include extraocular muscles (e.g., Suzuki et al. 2016), branchiomeric muscles (e.g., Ziermann et al. 2014), and neural crest cells (Horigome et al. 1999; Ota et al. 2007). Importantly, neural crest cells interact with other tissues and influence not only the craniofacial but also the cranial musculoskeletal development (Green et al. 2015).
3.2 Skull and Jaw Evolution
The importance to compare cyclostomes with other vertebrates and even cephalochordates to understand the evolution of the cranium was already recognized in the nineteenth century (Huxley 1876). The chondrocrania of gnathostomes and cyclostomes are very difficult to compare, even at the modular level, but the results of comparative studies shed light onto the evolution of vertebrate crania. Furthermore, the origin of the vertebrate jaw has fascinated scientists for centuries. Recent advances in the ability to study cyclostomes lead to an abundance of studies that try to shed light on the emergence of jaws (Kuratani et al. 2001; Shigetani et al. 2002, 2005; Kuratani 2004, 2012; Mallatt 2008; Cerny et al. 2010; Medeiros and Crump 2012; Gillis et al. 2013; Miyashita 2016).
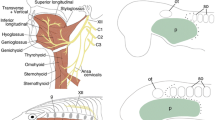
In order to enable a comparison of cyclostomes and gnathostomes, it is important to understand the homology of cranial elements between the taxa. Most studies compared each skeletal element, including the relation to cranial muscles and cranial nerves, in order to establish homology (e.g., Holmgren 1946; Yalden 1985). However, even the comparison between hagfish and lamprey crania is difficult because their anatomical pattern differ substantially (Fig. 3.2; Fürbringer 1875; Oisi et al. 2013a). Therefore, the evaluation of the development of the crania is essential for the homologization of the skeletal elements in cyclostomes (e.g., Johnels 1948).
Cranial skeleton in cyclostomes. (a) Adult hagfish, (b) larval lamprey (ammocoetes), (c) adult lamprey, (d) hypothetical pan-cyclostome embryonic pattern. (a) Asterisk (*)—this cartilage is indicated as PHP derivative by Oisi et al. (2013a) but should be part of the nasal duct cartilages and was recolored as ANP derivative; (c) Oisi et al. (2013a) mark the branchial cartilages in lampreys with the same abbreviation as the internal branchial arch in the adult hagfish. However, in their Table 1, they correctly homologize the extrabranchiale of hagfishes with the branchiale of lampreys. The styliform cartilage (stc) is listed as PHP-derivative in their Table 1, but their Fig. 1 and Fig. 10 compared would conclude it is hyoid derivative as also shown here. (a–c) Recolored and modified from Oisi et al. (2013a), terminology follows Oisi et al. (2013a) if not otherwise mentioned; (d) recolored from Oisi et al. (2013b). adp anterior dorsal plate, alac anterior lateral apical cartilage, alp anterior lateral plate, anc annular cartilage, ANP anterior nasal process, avnb anterior vertical nasal bar, br 3 branchiale 3 (extrabranchiale sensu Marinelli and Strenger 1954; intrabranchiale sensu Oisi et al. 2013a), cc cornual cartilage, da dorsal arcualia, dc dental cartilage (after Marinelli and Strenger 1956b), exbr1/2 extrabranchiale 1/2, exhy extrahyal, exqp extra palatoquadrate, hcom hypophyseal commissure, hy hyoid, ibr1 internal branchial arch 1, jcv joint caput for velum, lc labial cartilage, lmp lateral mouth plate, MA Mandibular arch mp medial part of basal plate, mrp medio-rostral part of basal plate, mvc medioventral cartilage, nc nasal capsule, ndc nasal duct cartilages, NHP nasohypophyseal plate, otc otic capsule, pc piston cartilage, pdp posterior dorsal plate, ph pharynx, PHP posthypophyseal process, plp posterior lateral plate, pvnb posterior vertical nasal bar, rdp rostrodorsal plate, snc subnasal cartilage, soca subocular arch, stc styliform cartilage, styc stylet cartilage, T1–T4 tentacles with supporting cartilages, tc tongue cartilage, trab trabecula, vb velar bar, vlp ventrolateral plate, vmlb ventromedial longitudinal bar
Lampreys are the better accessible extant jawless vertebrates, and therefore more studies are published about them than about hagfishes. The larval and adult crania in lampreys are well studied (Fig. 3.2b, c; e.g., Huxley 1876; Marinelli and Strenger 1954; Oisi et al. 2013a and citations within). The embryonic development and metamorphosis of the lamprey cranium was described by Johnels (1948). In hagfish the adult cranium was described by several researchers (e.g., Fig. 3.2a; Marinelli and Strenger 1956b; Miyashita 2012; Oisi et al. 2013a and citations within), but only few developmental descriptions exist (Holmgren 1946; Ota et al. 2007; Ota and Kuratani 2008; Oisi et al. 2013a and references within; Oisi et al. 2013b; Miyashita and Coates 2015 and citations within). The most detailed description up to today of the development of the chondrocranium in hagfishes (Eptatretus burgeri, E. atami) is by Oisi et al. (2013a).
Hagfish embryos and lamprey larvae share similar ontogenetic skeletal features (Fig. 3.2; Oisi et al. 2013a, b), but lamprey adults have structures they share with hagfish but that are underdeveloped in larvae. The lingual apparatus, for example, is present in both adult taxa but only appears after metamorphosis in lampreys (Yalden 1985). Larval lampreys (ammocoetes) are filter feeders, which are found usually in the soft sediment of streams (Moore and Mallatt 1980); they possess some apparently plesiomorphic (“primitive,” ancestral) characters like an endostyle. Hagfish do not have an endostyle, and the homology of the lamprey’s endostyle with that of amphioxus or ascidians is also still questioned by some, as is the homology of thyroid gland and endostyle (e.g., Holland and Chen 2001). The early embryonic pattern of lampreys is similar to that of hagfishes but their oral apparatus, including the lips, resembles those of some adult fossil heterostracans or osteostracans better than does the lips of adult lampreys (Kuratani et al. 2002).
3.2.1 The Cyclostome Chondrocranium
The hagfish chondrocranium includes the nasal capsule cartilages, otic capsule , neurocranial base (mesodermal neurocranium), lingual cartilages , other branchial arch cartilages, and premandibular cartilages (Fig. 3.2a; Oisi et al. 2013a). The latter includes also the cartilages that support the tentacles. The otic capsule, the trabecula, and the dorsal longitudinal bar present likely the entire mesodermal-derived neurocranial elements (Oisi et al. 2013a). Elements derived from the anterior nasal process (ANP in Fig. 3.2) are in hagfishes the cartilages of the supranasal region (nasal duct cartilages and cartilaginous elements of the nasal capsule) and in lampreys the dorsal wall posterior to the nostril. Elements derived from the posterior hypophyseal process (PHP in Fig. 3.2) include also cartilages derived from both the premandibular crest and mandibular arch cells: tentacular (T1–4) cartilages (perhaps with the exception of the T4 cartilage) and the subnasal cartilage of hagfishes, along with the palatine bar and the hypophyseal commissure, and perhaps the dorsal longitudinal bar and the trabecula (Oisi et al. 2013a). The mucocartilage in the upper lip of lampreys and possibly the rostral trabeculae parts appear to develop from the equivalent anlage (Fig. 3.2c). Therefore, all posterior hypophyseal process derived cartilages of hagfishes should be homologous to the rostral dorsal plate and lateral wall of the upper lip, the trabecula, and part of the nasal capsule of lampreys (Oisi et al. 2013a). Based on the innervation pattern in hagfishes and lampreys, the lateral wall in lampreys may correspond to the tentacular cartilages T1, T3, and T4 in hagfishes, while T2 seems to be more similar to the dorsal roof (Oisi et al. 2013a).
Based on development, innervation, and gene expression pattern, Oisi et al. (2013a) summarized the homologous relationship of cyclostome crania (Fig. 3.2); but see Kuratani et al. (2016) for an updated interpretation of a cartilaginous element at the level of the hyoid arch. Several cyclostome-specific characters were identified (Oisi et al. 2013a): differentiation of the lingual apparatus and the velum in the ventral and middle mandibular arch region, respectively, and lateral and posterior hypophyseal process-derived cartilages. The (external) branchial arch skeleton is also thought to be cyclostome specific (Mallatt 1984), but a recent cell lineage tracing study demonstrated an endodermal origin of gills in both gnathostomes and cyclostomes which supports the homology of the gills in both taxa (Gillis and Tidswell 2017). Compared to gnathostomes, cyclostomes lack homologues to the intertrabecula and have no occipital vertebrae (Oisi et al. 2013a). It is currently not clear which of those characters are plesiomorphic (retained from the LCA of vertebrates and lost in gnathostomes) or synapomorph (newly developed in the LCA of cyclostomes).
3.2.2 Development of the Chondrocranium
Oisi et al. (2013a, b) compared the development of the chondrocranium in Eptatretus with the development in a lamprey (Lethenteron reissneri). They not only showed that there is a conserved embryonic pattern of head development in cyclostomes but also that chondrocrania of lampreys and hagfishes can be compared at least at the module level (Fig. 3.2); the latter corresponds to the craniofacial primordia that build up the cyclostome morphotype (Oisi et al. 2013a). The most conserved stage during cyclostome development is the pharyngula stage, which is before the chondrification of the cranium. However, as adults, the crania are very different from each other (Fig. 3.2), and homology establishment is difficult because of the adaptations in both taxa and because of the highly apomorphic nature of the hagfish cranium.
Both hagfishes and lampreys have a neural crest development comparable to that of gnathostomes, but the nasohypophyseal process/plate is unique to cyclostomes (Fig. 3.2d; Ota et al. 2007; Oisi et al. 2013b; Kuratani et al. 2016), but it was suggested that this process might even be plesiomorphic present for all vertebrates. However, the similarity between the nasohypophyseal complex in lampreys and osteostracans is likely due to convergent evolution (Gai et al. 2011). Neural crest cells give rise to numerous skeletal elements of the head, connective tissue, tendons, etc., but they do not from head muscles (Noden 1983; Noden and Francis-West 2006). However, muscle fibers form within cranial neural crest-derived connective tissue in a coordinated manner (Ziermann et al. 2018). That leads to the association of muscles with the proper skeletal region; i.e., muscles of a certain branchial arch are associated with connective tissue and through this with skeletal elements from the same arch (Köntges and Lumsden 1996).
The observation of the growth and transformation of the posthypophyseal process in larval lampreys showed that the nostril ( nasohypophyseal opening ) is moved to the dorsal side of the larval head (Damas 1944; Kuratani et al. 2016). In hagfishes the process enlarges anteriorly and forms a septum that divides the oronasal cavity dorsoventrally, as well as the ventral margin of the nostril rostrally; the posterior root of this process disappears during further development which leads to the formation of a continuous connection between the nasohypophyseal duct and the pharynx (Oisi et al. 2013b). Due to those developmental processes, the hagfish and lamprey heads are less comparable during later developmental stages, while during early development, the set of craniofacial primordia are identical in cyclostomes (Kuratani et al. 2016). This is just one example of how embryological studies can help to identify similarities and even homologies based on the assumption that all included taxa share the same ancestral developmental plan. However, when studying the emergence of the jaw it is important to keep in mind that the jaw elements (derivatives from the mandibular arch) evolved likely after the divergence of cyclostomes and gnathostomes. Therefore, Oisi et al. (2013a, b) used deeper levels of homology to establish their homology hypotheses. In their first study (here “2013b” because of sorting in alphabetical order), they established a pan-cyclostome embryonic pattern (Fig. 3.2d; see below), which is shared by cyclostomes but not by crown gnathostomes. They did not only compare the embryological development of lampreys and hagfishes but also gene expression patterns in different tissues. In later studies it was suggested that this embryonic pattern might even represent the ancestral vertebrate embryonic pattern.
The pan-cyclostome embryonic pattern includes the presence of a nasohypophyseal plate (a single median placode that yields the nasal epithelium and adenohypophysis), which is bordered by an anterior nasal process and a posthypophyseal process (Fig. 3.2d). Both of those processes and the ventral part of the mandibular arch serve as craniofacial primordia in cyclostomes—similar to the nasal prominences and maxillomandibular processes in jawed vertebrates (Oisi et al. 2013a, b). The anterior nasal process of cyclostomes differentiates into the posterodorsal margin of the nasohypophyseal duct, and the posthypophyseal process differentiates into the upper lip of lamprey larva (or the oral funnel in adult lampreys) and the oronasohypophyseal septum in hagfishes (Oisi et al. 2013b).
The mandibular arch mesoderm gives rise to three parts: the dorsal one shifts rostrally to reside in the posthypophyseal process and its derivatives (Kuratani et al. 2004), the mid-part transforms into the velum, and the ventral part differentiates into the tongue apparatus (Kuratani 2012). The described pattern is not present in gnathostomes (Oisi et al. 2013b). Even with this knowledge, the comparison with the gnathostome pattern is still difficult, e.g., comparing the undifferentiated mandibular arch mesoderm before the taxon-specific compartmentalization. It was furthermore shown that the trigeminal nerve divisions and pattern of innervation is comparable within cyclostomes but not between them and gnathostomes (Oisi et al. 2013b; Higashiyama and Kuratani 2014) (see below: trigeminal innervation).
Cephalic neural crest -derived ectomesenchyme contributes to craniofacial components in cyclostome embryos and to craniofacial primordia in gnathostomes (Horigome et al. 1999; Kuratani et al. 1999; Shigetani et al. 2002; Oisi et al. 2013b). Furthermore, the initial migration pattern and anteroposterior specification of neural crests as well as the expression patterns of regulatory genes are similar between those taxa (Horigome et al. 1999; McCauley and Bronner-Fraser 2003; Green et al. 2015). However, the otocyst is slightly more rostral in cyclostome embryos than in gnathostome embryos with respect to the hyoid arch, and the hyoid neural crest stream is found medial to the otocyst in cyclostomes (Horigome et al. 1999; Oisi et al. 2013a, b).
Besides some cyclostome-specific traits, it is likely that basic ectomesenchymal (neural crest) distribution and skeletogenic properties are very similar in cyclostomes and gnathostomes. This in turn suggests that a craniofacial skeleton with pharyngeal arch components and prechordal neurocranial elements can also be identified in cyclostomes. In fact, the mesodermal cranial elements in hagfishes and lampreys (Fig. 3.2) are similar to gnathostomes, and the head mesoderm distribution in early lamprey embryos resembles that of gnathostome embryos (Kuratani et al. 1999; Adachi and Kuratani 2012).
The so-called trabeculae are described in hagfishes, lampreys, and gnathostomes. However, as detailed in (Oisi et al. 2013a), they are likely not homologous, because they develop differently in all three taxa. Trabeculae in gnathostomes are neural crest-derived prechordal cranial elements (Couly et al. 1993; Wada et al. 2011), in lampreys they are mesodermal elements (Kuratani et al. 2004), and in hagfishes they seem to be composites of the trabecula and the dorsal longitudinal bar (Oisi et al. 2013a) (Fig. 3.2). However, the anterior portion of the trabeculae of hagfishes might be homologous to the gnathostome trabeculae, which appears to be supported by its position within the posthypophyseal process (Oisi et al. 2013a).
3.2.3 The Evolution of Jaws
As the name indicates, extant gnathostomes possess an upper and lower jaw that derives from the mandibular arch (see also Chap. 2 for discussion on the origin of the jaw). The cartilaginous primordia are usually called palatoquadrate (which is the main part of the upper jaw; the latter also includes premandibular components, e.g., trabecula) and Meckel’s cartilage (lower jaw) (e.g., Goodrich 1930). The mandibular arch is characterized by the absence of Hox gene expression, while all posterior arches have a specific Hox gene patterning (Rijli et al. 1993, 1998). This is also shared by lampreys (Takio et al. 2004, 2007). The homologizations of caudally located branchial arch skeletons between hagfishes and lampreys are usually done by branchial muscle distribution (Marinelli and Strenger 1954, 1956b; Oisi et al. 2013b) and their cranial nerve innervation patterns (e.g., Song and Boord 1993; Oisi et al. 2013b), because each cranial nerve can be associated with a specific branchial arch (1st arch = mandibular arch = trigeminal nerve, cranial nerve V; 2nd arch= hyoid arch = facial nerve, cranial nerve VII; 3rd and following arches = caudal branchial arches = cranial nerves IX, X; Edgeworth 1935).
However, the cyclostome mandibular arch cannot easily be divided into upper and lower jaw elements. The dorsoventral patterning of branchial arches is regulated by Dlx gene expression in the ectomesenchyme (Depew et al. 2002, 2005; Minoux and Rijli 2010; Gillis et al. 2013). In mouse, Dlx5 and Dlx6 are specifically expressed in the ventral (lower) half of the mandibular arch (Depew et al. 2002). The simultaneous disruption of those genes leads to an upper jaw morphology instead of a lower jaw morphology. If in turn their upstream regulator Ednra is activated in the upper jaw domain, lower jaw morphology develops (Sato et al. 2008).
Lampreys have at least six Dlx genes (A–F); five of them are expressed in the branchial arch ectomesenchyme including the mandibular arch (Kuraku et al. 2010). However, there seems to be no dorsoventrally nested expression (see Chap. 2); but, a dorsoventrally symmetrical nested expression pattern around the gill pores was suggested (Cerny et al. 2010). Bapx1 specifies the jaw joint in gnathostomes (Miller et al. 2003), but its lamprey homologue is not expressed in the lamprey’s mandibular arch (Cerny et al. 2010; Kuraku et al. 2010). Yet, dHand cognate, a ventral pole specifier, is expressed in a way supporting a dorsoventral patterning in lampreys, while the unpolarized Dlx expression is consistent with the dorsoventrally symmetrical morphology of its posterior branchial arches. Hagfishes also do not have an apparent dorsoventral polarity in the preliminary analyses of Oisi et al. (2013a). The lingual apparatus in hagfishes derives from the ventral portion of the mandibular arch, and the homology of this structure to the lingual apparatus in lampreys is well established (Yalden 1985). The musculoskeletal structure, however, seems to develop through a different mechanism as it is independent of Dlx expression, and it is therefore not homologous to Meckel’s cartilage (lower jaw) of gnathostomes (Oisi et al. 2013a).
The dorsal half of the crown gnathostome mandibular arch is developmentally patterned as the default state of the Dlx code (Depew et al. 2002; Gillis et al. 2013; see also Chap. 2). As those genes are ubiquitously expressed in the branchial arch ectomesenchyme (neural crest), it is assumed that the “upper jaw” is the default state of the Dlx code in gnathostomes (Kuratani et al. 2013; Oisi et al. 2013a). Therefore, Dlx expression in ectomesenchyme of branchial arches seems to be a common vertebrate character. However, the patterning in those arches changes during the evolution of gnathostomes, which may play an important role in the establishment of the lower jaw (for a detailed discussion, see Miyashita 2016) but also leads to questioning of the homology between palatoquadrate (upper jaw) and dorsal mandibular arch skeletal derivates in gnathostomes (Oisi et al. 2013a).
3.3 Muscle Evolution
Cephalic muscles , that is, muscles associated with the head, can be grouped based on their developmental origin into eye (extraocular), mandibular, hyoid, branchial (including epibranchial and laryngeal muscles), and hypobranchial muscles (Edgeworth 1935; Diogo and Abdala 2010). The muscles, except the extraocular and hypobranchial muscles, originate from mesodermal anlagen associated with the same named branchial arches (aka pharyngeal arches) and are innervated by nerves associated with their respective region of origin (Edgeworth 1935; Diogo and Abdala 2010; Harel and Tzahor 2013). For example, the first branchial arch is called the mandibular arch , and the tissues associated with this arch give rise to the upper and lower jaw elements, the associated mandibular muscles (e.g., muscles of mastication: adductor mandibulae), and the connective tissue. The nerve innervating the muscles and receiving sensory information from the mandibular region is the trigeminal nerve (cranial nerve V). It is not the aim of this chapter to review the complete development of all the cephalic muscles; for more detailed information, see, for example, Diogo and Abdala (2010), Harel and Tzahor (2013), and references within.
Anatomical descriptions of cyclostome musculature were performed several times during the past 150 years, but without a comparison to gnathostomes or between cyclostomes (Fürbringer 1875; Cole 1907; Tretjakoff 1926; Marinelli and Strenger 1954, 1956b). More recent publications, however, compare the morphology and development of the head muscles between cyclostomes and gnathostomes (Miyashita 2012; Ziermann et al. 2014; Diogo and Ziermann 2015). Functional analyses of feeding in hagfishes and lampreys reveal the underlying kinematics (Moore and Mallatt 1980; Rovainen 1996; Clark and Summers 2007; Clark et al. 2010).
In addition to the above-mentioned differences in the head skeleton between cyclostomes and extant gnathostomes, the associated musculature seems also to be quite different. Yet, comparing the morphology (attachments, number of bellies), innervation, overall position, and development of muscles associated with the head in cyclostomes and jawed fishes can provide insights into the homology and evolution of those muscles. Such a comparative study was performed, for example, by Ziermann et al. (2014) and Diogo and Ziermann (2015). The most intriguing observation, besides the obvious different morphology of the head muscles, is the difference in number of cranial muscles (Fig. 3.3). While cyclostomes have over 20 mandibular arch muscles , gnathostome fishes possess less than 10. The adult hagfish has four hyoid arch muscles which is similar to most of the gnathostome fishes (2–3), but the larval lamprey has only one hyoid muscle that is absent in adult lampreys. The branchial arch muscles are largely reduced in hagfishes (3); the numbers are increased in adult lampreys (76) but similar in larval lampreys (32) and cartilaginous fishes (16–28).
Number of cephalic muscles in vertebrates (based on results from: Ziermann et al. 2014; Diogo and Ziermann 2015; Diogo et al. 2015b). Cephalic muscles are colored according to their developmental origin: mandibular arch muscles (red); hyoid arch muscles (green); branchial arch muscles (blue); hypobranchial muscles (yellow); laryngeal muscles (purple); pharyngeal muscles (black). Comparing and homologizing the muscles of diverse vertebrates, it is possible to infer the muscles in the last common ancestor (LCA) of extant taxon
Based on the dissection and comparison of cyclostomes with chondrichthyans (cartilaginous fishes like sharks, skates, chimera), Ziermann et al. (2014) inferred the ancestral condition of cephalic muscles in cyclostomes, gnathostomes, and even vertebrates (Fig. 3.3). In order to study the ancestral condition in cyclostomes, they studied and reviewed the literature of embryonic, larval, and adult hagfishes (Fig. 3.4) and lampreys (Fig. 3.5). The last common ancestor (LCA) of vertebrates had a single intermandibularis (i.e., a ventral muscle sheet) and other mandibular muscles (e.g., labial muscles), some constrictores hyoidei and branchiales, and epibranchial and hypobranchial muscle sheets (Ziermann et al. 2014). From this condition, the number of mandibular arch muscles increased toward the LCA of cyclostomes (synapomorphy) and then further in the different lineages of hagfishes and lampreys with 24 and 26 mandibular muscles in adult hagfishes and lampreys, respectively (red in Figs. 3.3, 3.4, and 3.5). Alternatively, the increase in mandibular muscles could have evolved independently from each other as the amount of branchial muscles also differs significantly in both taxa (see below).
Muscles of the Atlantic hagfish, Myxine glutinosa. Specimens not to scale. Not all muscles are shown on both sides in ventral view. Cephalic muscles are colored according to their developmental origin: mandibular arch muscles (red, orange, pink); hyoid arch muscles (green); true branchial arch muscles (blue); epibranchial muscles (brown); hypobranchial muscles (yellow). (a) Embryo, left lateral view, redrawn from Miyashita (2012); somites (not shown) extend to just behind the otic capsule. (b) Adult, left lateral view; (c) Adult, ventral view. (b) Parietalis and decussatus cut to enable view of deeper layers; white box—velar muscles in window on the right side of the animal. (c) Basitentacularis (basitent.) cut on right side. (b, c) Modified from Ziermann et al. (2014) and Diogo and Ziermann (2015). basitent. basitentacularis, co.ph constrictor pharynges, co.subna cornuosubnasalis, cran.bas craniobasalis, cran.hy craniohyoideus, cran.li carniolingualis, lev.cart.bas levator cartilagines basalis, lo.li longitudinalis lingua, cran.vel.a. d & v craniovelaris anterior dorsalis & ventralis, cran.vel.p. craniovelaris posterior, pal.co palatocoronarius, pal.lat palatinalis lateralis, pal.subn palatosubnasalis, perpen. perpendicularis, pr.cart.bas.a + p protractor cartilagines basalis anterior and posterior, pr.dent.prf protractor dentium profundus, pr.dent.spf protractor dentium superficialis, retr.muc.o retractor mucosae oris, subna.ba subnasobasalis, subn.sa subnasonasalis, te.po tentacularis posterior, te.subn tentaculosubnasalis, trans.o transversus oris, tub. tubuatus
Muscles of the sea lamprey, Petromyzon marinus. Specimens not to scale. Cephalic muscles are colored according to their developmental origin: mandibular arch muscles (red, orange, pink); hyoid arch muscles (green); true branchial arch muscles (blue); epibranchial muscles (brown); hypobranchial muscles (yellow). (a) Ammocoete larva, left lateral view; redrawn from Tulenko et al. (2013). (b) Ammocoete larva, left lateral view; redrawn from Miyashita (2012). (c) Adult, left lateral view; all branchial muscles (blue) are present in each segment but not shown in each segment. (d) Adult, ventral view; not all muscles shown on both sides; the hypobranchialis (hypobr) extends backward but was cut to show the branchial basket; the subocularis (suboc) on the left of the animals was cut and reflected. Modified from Ziermann et al. (2014) and Diogo and Ziermann (2015). add.br.d + v adductores branchiales dorsales + ventrales, ann annularis, ann.gl annuloglossus, bas basilaris, bas.gl basilariglossus, bu.a buccalis anterior, bu.spf buccalis superficialis, cgl cornuoglossus, co.br.ext constrictores branchiales externi, co.bu constrictor buccalis, co.cor.s constrictor cornualis superficialis, co.gl.p.e constrictor glossae profundus externus, co.pre constrictor prebranchialis, com.b.br.cir compressores bursae branchiales circulares, cop.r copuloglossus rectus, cop.o copuloglossus obliquus, cor.t cornuotaenalis, corn cornealis, epibr epibranchialis, hypobr hypobranchialis, ibr interbranchiales, lev.lab.v levator labialis ventralis, ph.a pharyngicus anterior, ph.p pharyngicus posterior, probr probranchialis, sp.br.ext.a + p sphincters branchiales anteriores + posteriores, pro.veli protractor veli, re.lab.d retractor labialis dorsalis, re.lab.v retractor labialis ventralis, re.pap retractor papillaris, sp.co spinosocopularis, sta styloapicalis, st.t stylotectalis, sub.oc subocularis, sup.oc supraocularis, tcl tectolateralis, tsa tectospinosus anterior, tsp tectospinosus posterior, ve.hy velohyoideus, ve.thy velothyroideus
The number of hyoid muscles stays almost constant throughout vertebrates until the diversification of amniotes (reptiles, birds, mammals; Fig. 3.3; see also Table 11.2 in Chap. 11). Interestingly, adult lampreys do not have any hyoid muscles, but the associated nerve (facial nerve, cranial nerve VII) can clearly be identified (Figs. 3.3 and 3.5). However, hagfishes have four hyoid muscles and larval lampreys also have one (constrictor prebranchialis, Fig. 3.5b). Therefore, the LCA of cyclostomes had at least one hyoid arch muscle. The number of branchial muscles in hagfishes and lampreys are quite different from each other, while adult lampreys have 76, larval lampreys possess 32, and adult hagfishes only 3. Cartilaginous fishes have on average about 19 branchial arch muscles. Ziermann et al. (2014) inferred from those numbers and their studies of vertebrate muscles that the LCA of cyclostomes had at least four branchial arch muscles, and with the evolution and adaptation to their specific lifestyles, lampreys increased and hagfishes reduced the number of branchial arch muscles. The number of hypobranchial muscles is almost constant throughout vertebrates and only slightly increases in tetrapods (Fig. 3.3).
Based on the comparison of morphology, innervation, overall position, and development, Ziermann et al. (2014) suggested to group the mandibular muscles of cyclostomes into five groups (red, orange, pink in Figs. 3.4 and 3.5): derivatives of the intermandibularis muscle sheet, labial muscles, nasal muscles, lingual and dental muscles, and velar muscles. Importantly, it is argued that because there are significant differences in the developmental patterning of mandibular arch derivatives (muscles, cartilages, nerves), no homologies can be established between mandibular muscles of cyclostomes and gnathostomes; this is because if there are homologies, then it would have to be concluded that there are upper and lower jaws in cyclostomes (Kuratani pers. com.). As also pointed out with respect to the velar muscles (see below), gene expression does not always support homology. However, mandibular arch muscles were present before the split of the cyclostomes from the stem gnathostomes. Therefore, even if there is no one-to-one homology and if there is an increase of mandibular muscles in cyclostomes (addition), this does not exclude that at least some muscle groups are (as group) homologous to gnathostome mandibular muscles, as are the mammalian sternocleidomastoid and the trapezius together homologous to the protractor pectoralis of reptiles and adult amphibians (see Chap. 7).
The intermandibularis muscle sheet in gnathostomes derives from the ventral part of the mandibular muscle plate (Edgeworth 1935). The tubulatus muscle in hagfishes and the constrictor cornualis superficialis and constrictor glossae profundus internus of lampreys are suggested to belong to the intermandibularis group. Therefore, the LCA of vertebrates likely also possessed an intermandibularis muscle sheet.
The labial muscles were suggested to be conserved across vertebrates (Mallatt 1996, 1997b, 2008). However, the “upper lips” of cyclostomes and gnathostomes seem not to be homologous as they differ fundamentally in their developmental process (Horigome et al. 1999; Noden and Francis-West 2006; Kuratani 2012; Kuratani et al. 2013; Oisi et al. 2013b). Based on their anatomical comparisons, Ziermann et al. (2014) tentatively suggested that the constrictor buccalis in larval lampreys (Fig. 3.5b), which seems to develop from the “mandibular branchiomere” (Mallatt 1996), is homologous to the labial muscles in gnathostomes. The labial muscles in holocephalans (e.g., ratfish) are innervated by cranial nerve V2 (CNV2; maxillary branch of trigeminal nerve) (Song and Boord 1993; Mallatt 1996). The hagfish Myxine glutinosa has six muscles that are innervated by CNV2 (retractor mucosae oris, longitudinalis linguae, protractor dentium profundus, protractor dentium superficialis, tubulatus, and perpendicularis; Fig. 3.4). Corresponding muscles in the lamprey Petromyzon marinus are all but one innervated by CNV2 ramus mandibularis (sensu Marinelli and Strenger 1954) according to the analyzes of Miyashita (2012) and (Ziermann et al. 2014) (CNV2 ramus velaris: pharyngicus posterior; CNV2 ramus mandibularis: levator valvulae velaris, cardioapicalis, annuloglossus, copuloglossus rectus, constrictor cornualis superficialis, constrictor glossae profundus internus). Those observations can be interpreted in two ways: (1) holocephalans retained a cyclostome-like innervation of labial muscles, which was lost in other gnathostomes and (2) cyclostomes and holocephalans independently developed an innervation of labial muscles by CNV2. Currently, the latter hypothesis is supported as it is more parsimonious (two steps of independent gain of innervation, as compared to three steps: one gain and two losses of innervation by CNV2). Furthermore, it is questionable if the branches of the trigeminal nerve are homologues as currently assumed (Miyashita 2012; Higashiyama and Kuratani 2014; Modrell et al. 2014).
Velar muscles are suggested to derive from the same anlage that also gives rise to the gnathostome levator arcus palatini. The levator arcus palatini and spiracularis are gnathostome muscles that derive from the dorsal part (aka constrictor dorsalis) of the mandibular muscle plate (Edgeworth 1935). The expression of engrailed in the velothyroideus muscle of lamprey larvae (Fig. 3.5b) and in the gnathostome levator arcus palatini was used to infer homology between these muscles (Holland et al. 1993). However, similar gene expression does not support unambiguously homology, and it is not clear if the larval lamprey muscles degenerate during metamorphosis entirely or if they give rise to adult muscles; NB: the levator arcus palatini would corresponds to an “adult” muscle in gnathostomes (Miyashita 2012; Ziermann et al. 2014). With respect to the velothyroideus, two hypotheses exist: (1) the muscle is reduced at metamorphosis together with the larval velum and (2) the muscle becomes incorporated in adult velar muscles (e.g., depressor veli). Ziermann et al. (2014) favored the latter hypothesis and suggested further that velar muscles of hagfishes and lampreys derive from the same anlage as the dorsal mandibular (“constrictor dorsalis”) muscles of gnathostomes such as the levator arcus palatini. The description that in both hagfishes and lampreys, the velum arises from the middle portion of the mandibular arch, between the rostral endodermal wall of the first branchial pouch and oral ectoderm, supports the homology of the velum in cyclostomes (Oisi et al. 2013b).
The adductor mandibulae of gnathostomes is related to “biting” of the jaw and derives from the transversely medial and dorsoventrally intermediate part of the mandibular muscle plate (Edgeworth 1935). However, it is currently not possible to identify a clear homologue to the adductor mandibulae in cyclostomes. Furthermore, it was suggested that the mandibular muscle development in gnathostomes is tightly linked to the patterning of the jaw skeleton (Noden 1983; Rinon et al. 2007; Medeiros and Crump 2012), which is supported by developmental defects observed in knockdown mutants (Schilling et al. 1996; Heude et al. 2010; Hinits et al. 2011).
Hagfishes have four hyoid muscles (green in Fig. 3.4), from which only two (craniolingualis, craniohyoideus) are likely homologous to the constrictor hyoideus dorsalis of gnathostome fishes (Ziermann et al. 2014). Adult lampreys have no hyoid muscle, but larval lampreys possess the constrictor prebranchialis (Miyashita 2012; Diogo and Ziermann 2015).
True branchial muscles include branchial muscles sensu stricto (sensu Diogo and Abdala 2010) and the cucullaris muscle and its derivatives. Laryngeal and epibranchial muscles are included as “other” branchial muscles, but laryngeal muscles do not evolve until gnathostomes (Ziermann et al. 2014). Interestingly, larval lampreys and sharks share two functions of their branchial muscles sensu stricto, as in both (1) the expiration is due to peristaltic action of superficial branchial constrictors and interbranchiales, and (2) the inspiration is caused by a passive recoil of the branchial arches (Mallatt 1996). This might indicate that this type of ventilation could be ancestral for the LCA of vertebrates. However, while the superficial branchial constrictors seem to be homologous throughout vertebrates, the interbranchiales could not be identified in cyclostomes and osteichthyans (bony fishes and tetrapods), which weakens this idea as both or one of those muscles could have been present in the LCA of vertebrates or gnathostomes (Ziermann et al. 2014).
The cucullaris muscle was discussed intensively in recent literature (e.g., Diogo and Abdala 2010; Diogo and Ziermann 2015) and basically two hypothesis regarding its developmental origin are most common: (1) the cucullaris is a true branchial muscle (Diogo and Abdala 2010; Ziermann et al. 2014), and (2) it is from somitic origin (Kusakabe and Kuratani 2005; Kusakabe et al. 2011; Sambasivan et al. 2011) (see also Chaps. 2 and 7 for discussion on the evolution of the cucullaris and its derivatives). Due to topographic similarities of the “infraoptic” muscles in lampreys (Fig. 3.5a) and the gnathostome muscle cucullaris, and based on the observation that the infraoptic muscles derive from anterior somites, it was suggested that those muscles are homologous and that they resemble epibranchial muscles (Kusakabe and Kuratani 2005; Kusakabe et al. 2011; Sambasivan et al. 2011). However, there are three infraoptic muscles in lampreys (subocularis, cornealis, and probranchialis) (Kusakabe et al. 2011), and developmentally the cucullaris resembles closer the hypobranchial migratory muscles of lampreys (Matsuoka et al. 2005). The cucullaris and its derivatives are in most gnathostomes innervated by cranial nerve XI (CNXI; accessory nerve; spinal accessory nerve) but might also be innervated by spinal nerves (Edgeworth 1935). Based on ontogenetic studies and comparative anatomical studies, it was suggested that the cucullaris derives from the same anlage as true branchial muscles do, followed by an extension toward the pectoral region (Diogo and Abdala 2010). This is furthermore supported by genetic studies in mice, where branchiomeric muscle differentiation is regulated by Pitx2 and Tbx1, while trunk muscles (somitic origin) are regulated by Pax3. Pax3 mutant mice lack somitic-derived muscles, but the cucullaris derivatives trapezius and sternocleidomastoid are still present (Tajbakhsh et al. 1997; Ericsson et al. 2013; Minchin et al. 2013). Further supporting the branchial identity of cucullaris and derivates is that Tbx1 mutant mice lack branchial muscles, including trapezius and sternocleidomastoid (Theis et al. 2010). As the presence of a cucullaris homologous muscles in cyclostomes is not proven yet, I infer that the muscle evolved in the LCA of gnathostomes and was not present in the LCA in vertebrates (Ziermann et al. 2014).
Epibranchial and hypobranchial muscles arise from anterior myotomes, migrate into the head below the pharynx, and retain spinal innervation (Edgeworth 1935) (brown, yellow in Figs. 3.4 and 3.5). Epibranchial muscles derive from anterior parts of somites, whereas hypobranchial muscles derive from ventral parts of somites (Edgeworth 1935; Lours-Calet et al. 2014). Cyclostomes have one (hagfish) or more (lamprey) epibranchial muscles and three (hagfish) or one (lamprey) hypobranchial muscles . As those muscles are present in cyclostomes and gnathostomes, the LCA of vertebrates also possessed an undifferentiated epibranchial muscle sheet and an undifferentiated hypobranchial muscle sheet (Ziermann et al. 2014).
Extraocular muscles (EOMs) are highly conserved throughout vertebrates, and all vertebrates have six, with the exception of (some) placoderms that have seven (Burrow et al. 2005). While cell lineage studies in mice suggest that EOMs are not branchiomeric muscles (Harel and Tzahor 2013), clonal studies also performed in mice suggest that they are branchiomeric muscles that are related to mandibular arch and right ventricle (heart) musculature (Lescroart et al. 2010). The latter scenario is supported by cell-labeling studies in lampreys, which showed that mandibular mesodermal cells migrate near the eye; however, it is unclear if those cells differentiate to EOMs (Kuratani et al. 2004). Interestingly, disruption of the Pitx2 gene during mesoderm differentiation disrupts the morphogenesis of all EOMs and some mandibular arch muscles, but also the myogenesis of the body wall and appendicular muscles (Shih et al. 2007; Sambasivan et al. 2011).
Overall it seems that the adult basal gnathostomes share more similarities with hagfish embryos and larval lampreys than with adult cyclostomes due to peramorphic events that occurred in the evolutionary history of cyclostomes (Diogo and Ziermann 2015). Peramorphic events describe the appearance of ancestral adult characters in descendant juveniles due to additions to terminal somatic developmental stages (McNamara 1990). As described above, Ziermann et al. (2014) and Diogo and Ziermann (2015) presented hypotheses about the homology and evolution of adult and larval muscles in cyclostomes and gnathostomes. According to them, the LCA of extant vertebrates had an undifferentiated intermandibularis muscle sheet, labial muscles, and some other mandibular muscles, at least one hyoid muscle (constrictor hyoideus = constrictor prebranchialis), at least some constrictores branchiales (branchial muscles), and undifferentiated epibranchial and hypobranchial muscle sheets. The adductores branchiales (branchial muscles) were likely independently acquired in lampreys and chondrichthyans. Furthermore, lamprey larvae seem to be a better model for cranial muscles of adults of the LCA of gnathostomes and LCA of vertebrates, than is the adult lamprey, because the inferred adult muscles in LCA of vertebrates is amazingly similar to lamprey larva. The absence of a hyoid muscle in adult lampreys as compared to the presence of one larval hyoid muscle is the most striking supporting this hypothesis. Another example is the presence of two muscles within the labial and intermandibularis group in lamprey larvae and LCA of vertebrates, while the adult lamprey has many more labial muscles. At least one velar and/or dorsal mandibular muscle was inferred for the LCA of vertebrates, and lamprey larvae have two of these muscles, while adult lampreys seem to have no muscles that can be easily put into this group. Also, branchial arch muscles are more similar in larval lampreys to the LCA of vertebrates, because the adult branchial muscles are far more complex. This is also true for epibranchial muscles. Therefore, larval lampreys are more similar to adult members of the LCA of extant gnathostomes, supporting the idea that peramorphic events occurred in the history of cyclostomes.
Metamorphosis is a process in which larval structures are remodeled into an adult form; the adult form differs from the larval form in morphology and ecology (see Chap. 7). In lampreys, the mouth, eyes, gut epithelium, larval kidney, and endostyle (thyroid gland in adults) are remodeled during metamorphosis (Youson 1980, 1997). Hagfishes have direct development. The adult cephalic muscles in lampreys develop from blastema as larval cephalic muscles degenerate during metamorphosis. Currently, it is not clear if the larval muscles are direct precursors of adult muscles. The rebuilding process during metamorphosis (Marinelli and Strenger 1954) is so dramatic that it is hard to make any assumptions about correspondence of larval and adult muscles. However, if one would accept the homology as proposed by Holland et al. (1993) that the larval velothyroideus in lampreys is homologous to the levator arcus palatini and dilatator operculi of embryonic and adult teleosts, then the velum of lampreys could be homologous to the palatoquadrate (upper jaw) of gnathostomes, or at least homogenic (Miyashita 2012). The velum is reduced during metamorphosis of lampreys, and instead a lingual apparatus develops; this would be another example where larval lampreys represent better adult gnathostomes than adult lampreys do.
3.3.1 Evolution of the Gnathostome Jaw and Mandibular Arch Muscles
Some species develop their mandibular (first) arch muscles before or simultaneously with hyoid (second) arch muscles; however, others develop first hyoid arch muscles (see Table 2 in Ziermann et al. 2017). Furthermore, it appears that the most anterior (first) arch in basal chordates (cephalochordates and fossils like Haikouella) corresponds to the hyoid (second) arch of vertebrates (Mallatt and Chen 2003; Mallatt 2008). This in turn would imply that the mandibular arch of vertebrates was secondarily incorporated into the branchial arch series in derived chordates; the similarity between the jaws and the patterning of branchial arches are suggested to have evolved due to functional reasons (Janvier 1996). Supported is this view by the expression of Hox genes in all branchial arches, except the mandibular arch (see above). Miyashita (2016) followed up on this idea, and based on his studies and an extensive literature review, he suggested the “ mandibular confinement theory ” (see Chap. 2 for more details). Specifically, this theory proposes that the jaw in gnathostomes evolved through a developmental spatial confinement of an ancestral oral (anterior) chordate structure. This confinement lead to a co-option of genetics and patterning that is normally found in more posterior arches which lead to the evolution of the mandibular arch and its derivatives (in particular the gnathostome jaw).
A problem with the mandibular confinement theory is that it depends on the assumption that the common ancestor of cyclostomes and gnathostomes would be similar to modern cyclostomes, which has yet to be proven. For example, Miyashita (2016) assumes that LCA of vertebrates would have possessed a lingual apparatus and a velum (anteroposteriorly elongated mandibular arch) like cyclostomes; however, an equally likely hypothesis is that this is a cyclostome synapomorphy (Oisi et al. 2013a). Furthermore, the mandibular confinement theory is dependent on refuting several previous “branchial arch theories” for the origin of the jaw, including those of Goodrich (1930), Mallatt (1996, 1997a, 2008), Kuratani (2004, 2005b, 2012), and many others. Therefore, carefully designed experiments (e.g., gene manipulation), detailed comparative developmental and anatomical studies, etc., will be necessary in the upcoming years to test the proposed hypotheses regarding the evolution of the vertebrate jaw.
The findings that the cardiopharyngeal field (Diogo et al. 2015a; see Chap. 1 for more details) gives rise to branchiomeric (head) muscles and to the myocardium (heart musculature) partially supports the hypothesis from Miyashita (2016), because the oral siphon muscles in ascidians, which are urochordates, derive not from the cardiopharyngeal field, while the atrial siphon muscles do. However, the oral siphon is said to correspond to the mandibular region of gnathostomes, where all branchiomeric muscles including the mandibular muscles derive from the cardiopharyngeal field (Diogo and Ziermann 2015). Therefore, the mandibular arch was integrated secondarily into this field. As mentioned above Tbx1 is a branchiomeric muscle marker, and in lamprey development, Tbx1/10 is expressed first in the mesodermal core of the branchial and pharyngeal region below the otic vesicle and only later in the labial/oral and velar muscle-deriving mesoderm that corresponds to the mandibular mesoderm (Sauka-Spengler et al. 2002). This would furthermore explain why it is so difficult to homologize the mandibular arch muscles of cyclostomes with those of gnathostomes (see above: adductor mandibulae).
3.4 Summary
Cyclostomes are very peculiar and fascinating animals. Both the larval chondrocrania (Oisi et al. 2013a, b) and the larval muscles of cyclostomes (Diogo and Ziermann 2015) resemble the adult plesiomorphic vertebrate and gnathostome condition better than adult cyclostome structures do. This is currently best explained by peramorphic events during the evolution of cyclostomes. The upper jaw development is the default developmental mode in vertebrates. The lower jaw, however, is a novelty that evolved in the LCA of gnathostomes. The musculature associated with the different branchial arches can be homologized in vertebrates based on gene expression patterns, attachments, innervation, and overall position.
References
Adachi N, Kuratani S (2012) Development of head and trunk mesoderm in the dogfish, Scyliorhinus torazame: I. Embryology and morphology of the head cavities and related structures. Evol Dev 14:234–258
Augustinsson K-B, Fänge R, Johnels A, Östlund E (1956) Histological, physiological and biochemical studies on the heart of two cyclostomes, hagfish (Myxine) and lamprey (Lampetra). J Physiol 131:257–276
Auster P, Barber K (2006) Atlantic hagfish exploit prey captured by other taxa. J Fish Biol 68:618–621
Burrow CJ, Jones AS, Young GC (2005) X-ray microtomography of 410 million-year-old optic capsules from placoderm fishes. Micron 36:551–557
Cerny R, Cattell M, Sauka-Spengler T, Bronner-Fraser M, Yu F, Medeiros DM (2010) Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc Nat Acad Sci 107:17262–17267
Clark AJ, Maravilla EJ, Summers AP (2010) A soft origin for a forceful bite: motorpatterns of the feeding musculature in Atlantic hagfish, Myxine glutinosa. Zoology 113:259–268
Clark AJ, Summers AP (2007) Morphology and kinematics of feeding in hagfish: possible functional advantages of jaws. J Exp Biol 210:3897–3909
Cole FJ (1907) XXVI.—a monograph on the general morphology of the Myxinoid fishes, based on a study of Myxine. Part II. The anatomy of the muscles. Earth Env Sci Trans R Soc Edinb 45:683–757
Couly GF, Coltey PM, Douarin NML (1993) The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117:409–429
Damas H (1944) Recherches sur la developpement de Lampetra fluviatilis L. Contribution a l’etude de la cephalogenese des vertebres. Arch Biol Paris 55:1–289
Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439:965–968
Depew MJ, Lufkin T, Rubenstein JL (2002) Specification of jaw subdivisions by Dlx genes. Science 298:381–385
Depew MJ, Simpson CA, Morasso M, Rubenstein JLR (2005) Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat 207:501–561
Diogo R, Abdala V (2010) Muscles of vertebrates—comparative anatomy, evolution, homologies and development. CRC Press; Science Publisher, Enfield, New Hampshire
Diogo R, Ziermann JM (2015) Development, metamorphosis, morphology, and diversity: the evolution of chordate muscles and the origin of vertebrates. Dev Dyn 244:1046–1057
Diogo R, Kelly RG, Christiaen L, Levine M, Ziermann JM, Molnar JL, Noden DM, Tzahor E (2015a) A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520:466–473
Diogo R, Ziermann JM, Linde-Medina M (2015b) Specialize or risk disappearance - empirical evidence of anisomerism based on comparative and developmental studies of gnathostome head and limb musculature. Biol Rev 90:964–978
Edgeworth FH (1935) The cranial muscles of vertebrates. Cambridge at the University Press, London
Ericsson R, Knight R, Johanson Z (2013) Evolution and development of the vertebrate neck. J Anat 222:67–78
Forey P, Janvier P (1993) Agnathans and the origin of jawed vertebrates. Nature 361:129–134
Fürbringer P (1875) Untersuchungen zur vergleichenden Anatomie der Muskulatur des Kopfskelets der Cyclostomen. Jenaer Zeitschriften
Gai Z, Donoghue PC, Zhu M, Janvier P, Stampanoni M (2011) Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature 476:324
Gee H (2018) Across the bridge: understanding the origin of the vertebrates. University of Chicago Press, Chicago
Gess RW, Coates MI, Rubidge BS (2006) A lamprey from the Devonian period of South Africa. Nature 443:981–984
Gillis JA, Modrell MS, Baker CV (2013) Developmental evidence for serial homology of the vertebrate jaw and gill arch skeleton. Nat Commun 4:1436
Gillis JA, Tidswell OR (2017) The origin of vertebrate gills. Curr Biol 27:729–732
Goodrich ES (1930) Studies on the structure and development of vertebrates. Dover Publications Inc. P, USA
Green SA, Simoes-Costa M, Bronner ME (2015) Evolution of vertebrates as viewed from the crest. Nature 520:474–482
Hardisty M, Potter I, Hilliard R (1989) Physiological adaptations of the living agnathans. Earth Env Sci Trans R Soc Edinb 80:241–254
Harel I, Tzahor E (2013) Head muscle development. In: McLoon LK, Andrade FH (eds) Craniofacial muscles. Springer, New York, pp 11–28
Heimberg AM, Cowper-Sal lari R, Sémon M, Donoghue PCJ, Peterson KJ (2010) microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Nat Acad Sci 107:19379–19383
Heude É, Bouhali K, Kurihara Y, Kurihara H, Couly G, Janvier P, Levi G (2010) Jaw muscularization requires Dlx expression by cranial neural crest cells. Proc Nat Acad Sci 107:11441–11446
Higashiyama H, Kuratani S (2014) On the maxillary nerve. J Morphol 275:17–38
Hinits Y, Williams VC, Sweetman D, Donn TM, Ma TP, Moens CB, Hughes SM (2011) Defective cranial skeletal development, larval lethality and haploinsufficiency in Myod mutant zebrafish. Dev Biol 358:102–112
Hoffmann FG, Opazo JC, Storz JF (2010) Gene cooption and convergent evolution of oxygen transport hemoglobins in jawed and jawless vertebrates. Proc Nat Acad Sci 107:14274–14279
Holland ND, Chen J (2001) Origin and early evolution of the vertebrates: new insights from advances in molecular biology, anatomy, and palaeontology. BioEssays 23:142–151
Holland ND, Holland LZ, Honma Y, Fujii T (1993) Engrailed expression during development of a lamprey, Lampetra japonica: a possible clue to homologies between agnathan and gnathostome muscles of the mandibular arch. Develop Growth Differ 35:153–160
Holmgren N (1946) On two embryos of Myxine glutinosa. Acta Zooligica XXVII:1–91
Holzer G, Laudet V (2017) New insights into vertebrate thyroid hormone receptor evolution. Nucl Recept Res 4
Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, Kuratani S (1999) Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol 207:287–308
Huxley TH (1876) The nature of the craniofacial apparatus of Petromyzon. J Anat Physiol 10:412–429
Janvier P (1996) Early vertebrates. Oxford University Press, Clarendon
Janvier P (2007) Homologies and evolutionary transitions in early vertebrate history. In: Anderson JS, Sues HD (eds) Major transitions in vertebrate evolution. Indiana University Press, Bloomington, pp 57–121
Janvier P (2008) Early jawless vertebrates and cyclostome origins. Zool Sci 25:1045–1056
Johnels AG (1948) On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool 29:139–279
Köntges G, Lumsden A (1996) Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122:3229–3242
Kuraku S, Hoshiyama D, Katoh K, Suga H, Miyata T (1999) Monophyly of lampreys and hagfishes supported by nuclear DNA–coded genes. J Mol Evol 49:729–735
Kuraku S, Takio Y, Sugahara F, Takechi M, Kuratani S (2010) Evolution of oropharyngeal patterning mechanisms involving Dlx and endothelins in vertebrates. Dev Biol 341:315–323
Kuratani S (2004) Evolution of the vertebrate jaw: comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. J Anat 205:335–347
Kuratani S (2005a) Craniofacial development and the evolution of the vertebrates: the old problems on a new background. Zool Sci 22:1–19
Kuratani S (2005b) Developmental studies of the lamprey and hierarchical evolutionary steps towards the acquisition of the jaw. J Anat 207:489–499
Kuratani S (2008a) Evolutionary developmental studies of cyclostomes and the origin of the vertebrate neck. Develop Growth Differ 50:S189–S194
Kuratani S (2008b) Is the vertebrate head segmented?—evolutionary and developmental considerations. Integr Comp Biol 48:647–657
Kuratani S (2012) Evolution of the vertebrate jaw from developmental perspectives. Evol Dev 14:76–92
Kuratani S, Adachi N, Wada N, Oisi Y, Sugahara F (2013) Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: revising the heterotopy scenario of gnathostome jaw evolution. J Anat 222:41–55
Kuratani S, Horigome N, Hirano S (1999) Developmental morphology of the head mesoderm and reevaluation of segmental theories of the vertebrate head: evidence from embryos of an agnathan vertebrate, Lampetra japonica. Dev Biol 210:381–400
Kuratani S, Kuraku S, Murakami Y (2002) Lamprey as an evo-devo model: lessons from comparative embryology and molecular phylogenetics. Genesis 34:175–183
Kuratani S, Murakami Y, Nobusada Y, Kusakabe R, Hirona S (2004) Developmental fate of the mandibular mesoderm in the lamprey, Lethenteron japonicum: comparative morphology and development of the Gnathostome jaw with special reference to the nature of the trabecula cranii. J Exp Zool (Mol Dev Evol) 302B:458–468
Kuratani S, Nobusada Y, Horigome N, Shigetani Y (2001) Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Philos Trans R Soc Lond Ser B Biol Sci 356:1615–1632
Kuratani S, Oisi Y, Ota KG (2016) Evolution of the vertebrate cranium: viewed from hagfish developmental studies. Zool Sci 33:229–238
Kusakabe R, Kuraku S, Kuratani S (2011) Expression and interaction of muscle-related genes in the lamprey imply the evolutionary scenario for vertebrate skeletal muscle, in association with the acquisition of the neck and fins. Dev Biol 350:217–227
Kusakabe R, Kuratani S (2005) Evolution and developmental patterning of the vertebrate skeletal muscles: perspectives from the lamprey. Dev Dyn 234:824–834
Leach WJ (1944) The archetypal position of amphioxus and ammocoetes and the role of endocrines in chordate evolution. American Naturalist, pp 341–357
Lescroart F, Kelly RG, Le Garrec J-F, Nicolas J-F, Meilhac SM, Buckingham M (2010) Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137:3269–3279
Lours-Calet C, Alvares LE, El-Hanfy AS, Gandesha S, Walters EH, Sobreira DR, Wotton KR, Jorge EC, Lawson JA, Lewis AK (2014) Evolutionarily conserved morphogenetic movements at the vertebrate head–trunk interface coordinate the transport and assembly of hypopharyngeal structures. Dev Biol 390:231–246
Mallatt J (1984) Early vertebrate evolution: pharyngeal structure and the origin of gnathostomes. J Zool 204:169–183
Mallatt J (1996) Ventilation and the origin of jawed vertebrates: a new mouth. Zool J Linnean Soc 117:329–404
Mallatt J (1997a) Crossing a major morphological boundary: the origin of jaws in vertebrates. Zoology 100:128–140
Mallatt J (1997b) Shark pharyngeal muscles and early vertebrate evolution. Acta Zool 78:279–294
Mallatt J (2008) The origin of the vertebrate jaw: neoclassical ideas versus newer, development-based ideas. Zool Sci 25:990–998
Mallatt J, Chen J (2003) Fossil sister group of craniates: predicted and found. J Morphol 258:1–31
Marinelli W, Strenger A (1956a) Vergleichende Anatomie und Morphologie der Wirbeltiere: von W. Marinelli und A. Strenger. Myxine glutinosa (L.). Franz Deuticke:80
Marinelli W, Strenger A (1956b) Vergleichende Anatomie und Morphologie der Wirbeltiere: von W. Marinelli und A. Strenger. Myxine glutinosa (L.). Franz Deuticke:80
Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G (2005) Neural crest origins of the neck and shoulder. Nature 436:347–355
McCauley DW, Bronner-Fraser M (2003) Neural crest contributions to the lamprey head. Development 130:2317–2327
McNamara KJ (1990) The role of heterochrony in evolutionary trends. Evolutionary Trends. K. J. McNamara, London
Medeiros DM, Crump JG (2012) New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev Biol 371:121–135
Miller CT, Yelon D, Stainier DYR, Kimmel CB (2003) Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development 130:1353–1365
Minchin JE, Williams VC, Hinits Y, Low S, Tandon P, Fan C-M, Rawls JF, Hughes SM (2013) Oesophageal and sternohyal muscle fibres are novel Pax3-dependent migratory somite derivatives essential for ingestion. Development 140:2972–2984
Minoux M, Rijli FM (2010) Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137:2605–2621
Miyashita T (2012) Comparative analysis of the anatomy of the Myxinoidea and the ancestry of early vertebrate lineages. M.Sc. In: University of Alberta
Miyashita T (2016) Fishing for jaws in early vertebrate evolution: a new hypothesis of mandibular confinement. Biol Rev 91:611–657
Miyashita T, Coates MI (2015) Hagfish embryology. Hagfish Biol 95
Modrell MS, Hockman D, Uy B, Buckley D, Sauka-Spengler T, Bronner ME, Baker CV (2014) A fate-map for cranial sensory ganglia in the sea lamprey. Dev Biol 385:405–416
Moore JW, Mallatt JM (1980) Feeding of larval lamprey. Can J Fish Aquat Sci 37:1658–1664
Noden DM (1983) The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 96:144–165
Noden DM, Francis-West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235:1194–1218
Oisi Y, Ota KG, Fujimoto S, Kuratani S (2013a) Development of the chondrocranium in hagfishes, with special reference to the early evolution of vertebrates. Zool Sci 30:944–961
Oisi Y, Ota KG, Kuraku S, Fujimoto S, Kuratani S (2013b) Craniofacial development of hagfishes and the evolution of vertebrates. Nature 493:175–180
Ota KG, Fujimoto S, Oisi Y, Kuratani S (2011) Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish. Nat Commun 2:373
Ota KG, Kuraku S, Kuratani S (2007) Hagfish embryology with reference to the evolution of the neural crest. Nature 446:672–675
Ota KG, Kuratani S (2008) Developmental biology of hagfishes, with a report on newly obtained embryos of the Japanese inshore hagfish, Eptatretus burgeri. Zool Sci 25:999–1011
Parker HJ, Bronner ME, Krumlauf R (2014) A Hox regulatory network of hindbrain segmentation is conserved to the base of vertebrates. Nature 514:490
Poole JRM, Paganini J, Pontarotti P (2017) Convergent evolution of the adaptive immune response in jawed vertebrates and cyclostomes: an evolutionary biology approach based study. Dev Com Immunol 75:120–126
Potter I (1980) The Petromyzoniformes with particular reference to paired species. Can J Fish Aquat Sci 37:1595–1615
Rijli FM, Gavalas A, Chambon P (1998) Segmentation and specification in the branchial region of the head: the role of the Hox selector genes. Int J Dev Biol 42:393–401
Rijli FM, Mark M, Lakkaraju S, Dierich A, Dollé P, Chambon P (1993) A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75:1333–1349
Rinon A, Lazar S, Marshall H, Büchmann-Møller S, Neufeld A, Elhanany-Tamir H, Taketo MM, Sommer L, Krumlauf R, Tzahor E (2007) Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 134:3065–3075
Romer AS (1950) The vertebrate body. WB Saunders, London
Rovainen CM (1996) Feeding and breathing in lampreys. Brain Behav Evol 48:297–305
Sambasivan R, Kuratani S, Tajbakhsh S (2011) An eye on the head: the development and evolution of craniofacial muscles. Development 138:2401–2415
Sato T, Kurihara Y, Asai R, Kawamura Y, Tonami K, Uchijima Y, Heude E, Ekker M, Levi G, Kurihara H (2008) An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci 105:18806–18811
Sauka-Spengler T, Le Mentec C, Lepage M, Mazan S (2002) Embryonic expression of Tbx1, a DiGeorge syndrome candidate gene, in the lamprey Lampetra fluviatilis. Gene Expr Patterns 2:99–103
Schilling TF, Piotrowski T, Grandel H, Brand M, Heisenberg C-P, Jiang Y-J, Beuchle D, Hammerschmidt M, Kane DA, Mullins MC, Eeden FJM, Kelsh RN, Furutani-Seiki M, Granato M, Haffter P, Odenthal J, Warga RM, Trowe T, Nüsslein-Volhard C (1996) Jaw and branchial arch mutants in zebrafish I: branchial arches. Development 123:329–344
Shigetani Y, Sugahara F, Kawakami Y, Murakami Y, Hirano S, Kuratani S (2002) Heterotopic shift of epithelial-Mesenchymal interactions in vertebrate jaw evolution. Science 296:1316–1319
Shigetani Y, Sugahara F, Kuratani S (2005) A new evolutionary scenario for the vertebrate jaw. BioEssays 27:331–338
Shih HP, Gross MK, Kioussi C (2007) Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc Natl Acad Sci 104:5907–5912
Song J, Boord R (1993) Motor components of the trigeminal nerve and organization of the mandibular arch muscles in vertebrates. Cells Tissues Organs 148:139–149
Sugahara F, Murakami Y, Adachi N, Kuratani S (2013) Evolution of the regionalization and patterning of the vertebrate telencephalon: what can we learn from cyclostomes? Curr Opin Genet Dev 23:475–483
Suzuki DG, Fukumoto Y, Yoshimura M, Yamazaki Y, Kosaka J, Kuratani S, Wada H (2016) Comparative morphology and development of extra-ocular muscles in the lamprey and gnathostomes reveal the ancestral state and developmental patterns of the vertebrate head. Zool Lett 2:1
Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89:127–138
Takio Y, Kuraku S, Murakami Y, Pasqualetti M, Rijli FM, Narita Y, Kuratani S, Kusakabe R (2007) Hox gene expression patterns in Lethenteron japonicum embryos—insights into the evolution of the vertebrate Hox code. Dev Biol 308:606–620
Takio Y, Pasqualetti M, Kuraku S, Hirano S, Rijli FM, Kuratani S (2004) Lamprey Hox genes and the evolution of jaws. Nature:1–2
Theis S, Patel K, Valasek P, Otto A, Pu Q, Harel I, Tzahor E, Tajbakhsh S, Christ B, Huang R (2010) The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137:2961–2971
Tiecke E, Matsuura M, Kokubo N, Kuraku S, Kusakabe R, Kuratani S, Tanaka M (2007) Identification and developmental expression of two Tbx1/10-related genes in the agnathan Lethenteron japonicum. Dev Genes Evol 217:691–697
Tretjakoff D (1926) Das Skelett und die Muskulatur im Kopfe des Flussneunauges. Z Wiss Zool 128:267–304
Tulenko FJ, McCauley DW, MacKenzie EL, Mazan S, Kuratani S, Sugahara F, Kusakabe R, Burke AC (2013) Body wall development in lamprey and a new perspective on the origin of vertebrate paired fins. Proc Natl Acad Sci 110:11899–11904
Wada N, Nohno T, Kuratani S (2011) Dual origins of the prechordal cranium in the chicken embryo. Dev Biol 356:529–540
Yalden D (1985) Feeding mechanisms as evidence for cyclostome monophyly. Zool J Linnean Soc 84:291–300
Youson J (1980) Morphology and physiology of lamprey metamorphosis. Can J Fish Aquat Sci 37:1687–1710
Youson JH (1997) Is lamprey metamorphosis regulated by thyroid hormones? Am Zool 37:441–460
Ziermann JM, Diogo R, Noden DM (2018) Neural crest and the patterning of vertebrate craniofacial muscles. Genesis:e23097
Ziermann JM, Freitas R, Diogo R (2017) Muscle development in the shark Scyliorhinus canicula: implications for the evolution of the gnathostome head and paired appendage musculature. Front Zool 14:1–17. https://doi.org/10.1186/s12983-12017-10216-y
Ziermann JM, Miyashita T, Diogo R (2014) Cephalic muscles of cyclostomes (hagfishes and lampreys) and Chondrichthyes (sharks, rays and holocephalans): comparative anatomy and early evolution of the vertebrate head muscles. Zool J Linnean Soc 172:771–802
Acknowledgments
I would like to thank Shigeru Kuratani and Philippe Janvier for their constructive reviews which improved the chapter.
Further Reading
From the extensive literature list in this chapter, I suggest for further reading on the relevance of cyclostomes to understand vertebrate and gnathostome evolution: Janvier (2008), Kuratani (2008a), and Miyashita (2016).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ziermann, J.M. (2019). Cranium, Cephalic Muscles, and Homologies in Cyclostomes. In: Ziermann, J., Diaz Jr, R., Diogo, R. (eds) Heads, Jaws, and Muscles. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-93560-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-93560-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93559-1
Online ISBN: 978-3-319-93560-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)