Abstract
The management of patients with nontuberculous mycobacterial (NTM) disease, especially pulmonary disease (PD), is neither intuitive nor simple. As is often lamented, treatment of NTM PD is typically more complicated and difficult than the treatment of tuberculosis (TB). Complexity begins with the requirement to select patients that meet established diagnostic criteria and who would benefit from therapy. Essentially all patients with NTM PD have respiratory comorbidities, typically bronchiectasis and/or chronic obstructive lung disease which also requires evaluation and treatment. Management of these comorbidities in the presence of NTM PD is critical but presents additional challenges such as symptomatic overlap with NTM PD that obfuscates NTM treatment responses. Likewise, the decision to begin a multidrug mycobacterial regimen must always involve careful risk-benefit assessment on an individual patient basis. In those instances that treatment for NTM PD is not started, continued monitoring and follow-up of patients are essential. For successful treatment outcomes, clinicians managing NTM PD patients must be familiar with the characteristics of individual NTM PD pathogens and especially the nuances and peculiarities of drug susceptibility patterns. For selected pathogens and antibiotics, the avoidance of acquired mutational drug resistance is imperative. Management requires close and ongoing patient monitoring to assess treatment response and drug toxicity. There are multiple impediments to successful NTM PD therapy so that even with optimal management strategies, treatment success can be elusive. In that context, however, adherence to basic management principles will maximize the chances for a successful treatment outcome. Specific recommendations for comprehensive NTM disease management are offered in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The reader will undoubtedly notice that throughout this volume, there are repeated caveats about the complexities, incongruities, paradoxes, and frustrations involved with nontuberculous mycobacterial (NTM) disease management, especially pulmonary disease (PD). This chapter is an attempt to address as many of the difficult management problems as possible in one section. Obviously, many areas in this discussion lack a firm evidence base and are still controversial and hotly debated. Where possible we offer corroborating evidence for recommendations but also frequently call upon almost three decades of experience managing NTM patients. It should also be noted that perspectives and recommendations made in this chapter are primarily made from a North American perspective and may or may not apply equally to other areas in the world, especially in developing countries. We fully recognize that the recommendations in this chapter will not be universally endorsed, but they will hopefully serve as a starting point for readers to explore difficult management decisions in more depth. While the initial admonition for this volume is not to use it as a quick “how-to” guide, this chapter is the exception. We hope the reader will find the recommendations in this chapter helpful in the practical day-to-day management of NTM disease patients.
Diagnosis
The first essential element in the management of NTM patients is adequate mycobacteriology laboratory support . All patient management decisions discussed below require accurate NTM identification and in vitro susceptibility testing results [1].
The management of patients with NTM PD begins with confidently establishing the diagnosis using published diagnostic criteria [1]. This topic is discussed in detail in chapter “Diagnosis of NTM Disease: Pulmonary and Extrapulmonary”. With very rare exception, we do not recommend or endorse empiric treatment for NTM infection, especially NTM PD. Meeting diagnostic criteria for NTM PD is the essential first step for confidently approaching subsequent management decisions such as whether to proceed with an often complicated and prolonged NTM PD treatment course. We also understand the limitations of the diagnostic criteria which are clearly not applicable to all NTM species isolated from respiratory specimens. It is absolutely essential for clinicians to be familiar with the virulence and disease-causing potential of NTM species to intelligently apply the NTM PD diagnostic criteria [2]. And while newer serologic tests are being developed to assist in the diagnosis of NTM PD (especially MAC), there is a large unmet need for additional meaningful biomarkers and diagnostic tools , representing an area under active investigation [3,4,5,6,7,8].
Diagnosis
-
Diagnosis of NTM PD requires clinical, microbiologic, and radiographic confirmation
-
Empiric treatment of NTM PD without confirmed diagnosis is not recommended
NTM Pulmonary Disease and Tuberculosis
Pulmonary tuberculosis (TB) is invariably mentioned as the first differential diagnostic consideration for NTM PD, so it seems reasonable to discuss the four important intersections between the two disease processes here.
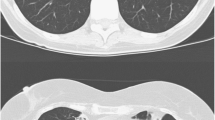
First, patients with cavitary NTM PD present with chest radiographs consistent with reactivation pulmonary TB disease and with sputum that is often acid-fast bacilli (AFB) smear positive. Problems expeditiously differentiating TB and NTM PD in this type of patient are greatly alleviated by the widespread availability of sputum nucleic acid amplification testing (NAAT) for TB. In the setting of sputum AFB smear positivity, a negative NAAT carries a high negative predictive value and can in most, but not all, instances exclude a diagnosis of TB [9]. However, there may be instances where patients are at high risk for having TB based on the clinical setting where NAAT is either not available or negative, and the initial organism identification by some other means such as high-performance liquid chromatography (HPLC) suggests an NTM pathogen. In that circumstance we believe it is prudent to treat for TB while waiting for microbiologic confirmation of the diagnosis (Fig. 1).
(a) 57-year-old male from Mexico found as part of a TB contact investigation to have a positive QuantiFERON test. Patient with chronic cough and abnormal chest radiograph. Initial sputum AFB smear positive but nucleic acid amplification test (NAAT) negative. Patient started on multidrug anti-tuberculosis therapy. (b): After 3 months of multidrug anti-tuberculosis therapy, no symptomatic improvement and progression of radiographic abnormalities. Multiple sputum specimens culture positive for MAC. (c) After 3 months of guideline-based MAC therapy including macrolide, patient symptomatically better with radiographic improvement and conversion of sputum to culture negative
A second more unusual occurrence is the isolation of MTB during the course of NTM PD therapy in a patient with established NTM disease. This circumstance represents a more difficult diagnostic challenge. Experience in the USA suggests that most M. tuberculosis (MTB) isolated during NTM PD therapy represent specimen laboratory contamination . We have, however, also rarely seen newly acquired TB in NTM PD patients who were close or household contacts to TB cases. In either case, genotyping of the MTB isolate is essential for determining its significance. The processing lab must be notified that specimen contamination is suspected which should trigger the appropriate investigation including genotyping of M tuberculosis isolates processed in proximity to the specimen in question. Robust communication with laboratory colleagues will facilitate efficient and quality decision-making on behalf of the patient. Unfortunately, redirecting therapy against TB, at least temporarily, may be unavoidable. This analysis is significantly more complicated in regions outside the USA with high TB incidence and high risk for TB transmission.
A third and more common occurrence is the isolation of NTM respiratory pathogens during the course of TB therapy [10,11,12]. There are limited data suggesting that most NTM isolated in this circumstance are not clinically significant; however, that determination must be made on an individual basis. We generally recommend that NTM isolated during TB therapy do not require a change in therapy and that TB therapy should be completed before addressing the clinical significance of the NTM isolate. Obviously, patients must have ongoing evaluation to determine if the isolated NTM is adversely affecting the patient’s course, thereby necessitating redirection of therapy to include coverage of the NTM pathogen. Most patients undergoing TB therapy in the USA are managed by public health entities, and evaluating the significance of NTM isolates is beyond their scope and resources which means that these patients will need evaluation in the community to determine the significance of NTM isolates. In that context, patients should be referred to appropriate specialists to address and possibly treat the NTM PD after TB treatment has been completed.
A fourth situation can occur when biopsies of an extrapulmonary organ unexpectedly yield an AFB smear-positive specimen. Too often cultures are not obtained during these procedures so that culture confirmation of the organism identity is not available. In this situation, patients are often assumed to have TB, especially if there is also a positive tuberculin skin test or interferon gamma release assay. Recently, molecular testing of the AFB smear-positive tissue has been employed through a service offered by the Centers for Disease Control and Prevention (CDC) that can identify MTB as well as NTM organisms from preserved tissue specimens [13]. The invaluable information obtained is not necessarily diagnostic of active mycobacterial disease and must be interpreted in the context of the patient’s overall clinical status.
NTM PD and Tuberculosis
-
Differentiation of TB disease from NTM PD may be challenging and requires careful clinical assessment, collaboration with laboratory colleagues, and use of available molecular diagnostic tools.
-
NTM PD during treatment of TB pulmonary disease and, to lesser extent, TB pulmonary disease during NTM PD treatment can occur.
-
Treatment of TB is the highest priority with need for referral (from public health) of coinfected NTM PD patients after completing TB treatment to appropriate pulmonary or infectious disease specialists.
Respiratory Comorbidities
The most important initial NTM lung disease management effort is typically how best to address underlying and often complex comorbidities. The most common of these comorbidities are bronchiectasis , chronic obstructive lung disease (COPD) , sinus disease , and gastroesophageal reflux disease (GERD) . These diagnoses are covered in more detail in chapter “Management of Lung Diseases Associated with NTM Infection”, but some key points are worth emphasizing.
It was observed more than four decades ago that some patients with non-cavitary Mycobacterium avium complex (MAC) disease experienced conversion of sputum AFB cultures to negative with “pulmonary toilet” measures, usually chest percussion and postural drainage [14]. At the time of that observation, patients with bronchiectasis and nodular bronchiectatic (NB) MAC lung disease were not recognized as such. More recent studies have confirmed this phenomenon [15,16,17]. It is our experience that treatment of bronchiectasis can be symptomatically transformative for some patients. The management of bronchiectasis symptoms, which frequently mimic and are indistinguishable from the symptoms of NTM PD, can also help clinicians decide whether or not to start NTM therapy. Patients with mild and/or indolent NTM PD may experience sufficient symptomatic improvement with airway clearance measures and treatment of other comorbidities that NTM therapy may not be necessary even though NTM PD is still present.
Controversy exists about the utility of efforts to determine the etiology of bronchiectasis in patients without an obvious explanation. A careful and detailed history is unquestionably the most important aspect of any effort to determine the etiology of bronchiectasis. Recent guidelines have recommended core laboratory evaluation for all patients with additional evaluations based on clinical suspicion of specific contributing etiologies [18, 19]. The one consensus indication for a more extensive etiologic search is young age at onset of bronchiectasis. In our opinion this indication includes patients diagnosed with bronchiectasis at a young age but also older bronchiectasis patient with symptoms that began at a young age (Fig. 2). Severe and/or extensive bronchiectasis would be other indications for considering an etiologic evaluation (Figs. 3 and 4). The extent of the evaluation is influenced by cost for the testing and the potential impact of a positive test including the availability of interventions for a specific diagnosis and genetic counselling for family members.
(a) PA chest radiograph from 32-year-old female with history of recurrent pneumonia since childhood. Undergoing infertility evaluation . Sputum culture positive for MAC, M. abscessus, three strains of Pseudomonas. Chest radiograph with abnormalities consistent with diffuse bronchiectasis. (b) Chest CT slice from the same patient showing extensive upper lobe bronchiectasis consistent with cystic fibrosis. Sweat chloride level > 90 mmol/L
(a) Chest CT slice from 69-year-old patient, never smoker, with bronchiectasis in 2006 showing bilateral bronchiectasis. Patient with history of recurrent respiratory infections including pneumonia since early adulthood. (b) Chest CT slice at comparable level from the same patient in 2012 showing progression of bronchiectasis. Patient’s sputum culture positive for MAC, M. abscessus, Nocardia, Pseudomonas, Stenotrophomonas, Burkholderia, and Aspergillus. Alpha-one-antitrypsin level < 30 mg/dl, Zz phenotype
(a) Chest radiograph from 80-year-old patient with history of recurrent respiratory infections including pneumonia since childhood. Mother and maternal grandmother with chronic cough without diagnosis. Patient diagnosed with MAC disease 6 years prior with multiple subsequent treatment efforts including left lower lobe lobectomy. (b) Chest CT slice from the same patient showing extensive cavitary consolidation in the remaining left lung. Sputum cultures positive for MAC, M. abscessus, Pseudomonas, and Aspergillus. IgG level < 400 mg/dl
The management of COPD may also provide significant symptomatic benefit to the patient; however, it is less likely to allow NTM disease treatment avoidance because COPD patients are more likely to have cavitary NTM PD which requires aggressive treatment [17, 20,21,22]. Regardless, the potential symptomatic benefit from COPD treatment is a worthwhile goal of its own. It should be noted that appropriate treatment of advanced COPD may involve use of inhaled corticosteroids (ICS), but that ICS may increase the risk of NTM lung disease [23, 24] Our approach is to avoid ICS in the management of NTM PD in the absence of a COPD phenotype that specifically benefits from ICS administration.
A second important consideration for cavitary NTM PD patients is the potential for surgical intervention, which can dramatically improve NTM disease outcomes. Surgery for NTM disease is discussed in detail in chapter “Surgical Management of NTM Diseases”. Medical therapy alone for NTM PD has a low likelihood of sterilizing large cavities and converting associated positive sputum cultures when present. Best outcomes, defined as culture conversion, frequently require a combination of medical therapy and surgical intervention. It should be further emphasized that medical therapy for NTM PD should be given pre- and postoperatively when adjunctive lung resection for cavitary NTM PD is considered. Optimizing pulmonary function is critical for a successful surgical outcome and requires comprehensive and coordinated care by a multidisciplinary perioperative program including an experienced mycobacterial disease thoracic surgeon [25]. In our opinion, surgery is sufficiently effective that it should be considered, if only briefly, for all NTM PD patients and especially those with severe or treatment refractory disease.
Respiratory Comorbidities
-
Symptoms of NTM PD are not specific and similar to other comorbidities such as bronchiectasis or COPD.
-
Treatment of comorbidities is of paramount importance to facilitate etiologic discernment of nonspecific symptoms.
-
Consider etiologic evaluation for young bronchiectasis patients and those with long-standing symptoms or severe disease.
-
Bronchial hygiene is a core component of treatment programs for NTM PD patients understanding that most patients with NTM PD also have bronchiectasis.
-
Minimize exposure to ICS unless justified by comorbidities.
-
Select NTM PD patients may benefit from adjunctive surgical therapy.
Starting NTM PD Therapy
After optimizing management of associated comorbidities, the next decision for the NTM PD patient is whether to begin therapy. For cavitary NTM PD patients, that decision is not difficult due to the inarguable risk for disease progression with attendant morbidity and mortality [20, 21]. Thus, the risk/benefit assessment overwhelmingly favors initiating therapy at the time of NTM cavitary PD diagnosis.
For patients with NTM NB PD, especially NB MAC PD, that decision is frequently complicated and requires a more deliberate approach. It is evident that some, perhaps many patients with NB MAC PD have indolent disease so that MAC isolation from a respiratory specimen, whether or not diagnostic criteria are met, does not reflexively or automatically require initiation of therapy. While many questions remain about the natural history of MAC PD, it is clear that not all patients with MAC isolated from respiratory specimens subsequently or inevitably have progressive MAC lung disease that requires treatment [26]. There is even less data regarding the natural history for other NTM PD. Nonetheless, the general concept of a variable natural history likely holds true for NTM PD other than MAC as well as MAC PD.
The critical element in the decision to start or withhold therapy is a careful risk/benefit determination for an individual patient. For mild NB MAC PD, the risks of treatment with uncertain benefit that potentially exposes patients to medication toxicity and side effects must be balanced against possible undertreatment of progressive disease, which exposes patients to disease morbidity . A common scenario is the patient with persistently positive sputum AFB cultures for MAC who has minimal symptoms and stable NB radiographic abnormalities. There is a consensus that the benefit of therapy for this type of patient would not likely outweigh the risks of MAC therapy. Fortunately, NB MAC lung disease is sufficiently indolent that careful longitudinal appraisal without therapy is safe and presents little risk for rapid progression of MAC PD or later hindrance to favorable therapeutic response. Should MAC therapy be held, it is imperative that macrolides are not used for exacerbations of bronchiectasis or other indications so as to mitigate the risk of developing macrolide-resistant MAC .
The next critical element is persistence in longitudinal follow-up. Those patients not started on therapy must be followed indefinitely as there is increasing evidence for significant risk of irreversible radiographic progression and pulmonary function decline in some patients not on therapy even if guideline-based treatment is started at a later date [27]. Our approach to initiating MAC lung disease therapy has rested on three essential factors, patient symptoms, microbiologic results, and radiographic findings. The most important factor is the radiographic appearance, especially the development of cavitation, which would strongly favor initiation of therapy regardless of symptomatic or microbiologic stability.
There are few objective markers of NTM PD disease progression. A recent study compared the clinical characteristics of MAC PD patients who had a progressive course resulting in treatment initiation within 3 years of diagnosis with patients who exhibited a stable course for at least 3 years [26]. Compared to stable MAC PD, patients with progressive MAC PD had lower body mass index (BMI) and more systemic symptoms, positive sputum AFB smears, and fibrocavitary radiographic findings. Hopefully, other biomarkers of disease progression will emerge to supplement or even supplant the current dependence on these clinical, microbiologic, and radiographic criteria.
The intensity and frequency of clinical scrutiny is also function of the specific NTM causing the patient’s lung disease. It has been recognized for many years that there is a spectrum of virulence among nontuberculous mycobacterial lung pathogens (chapters “ Mycobacterium avium Complex Disease”, “NTM Disease Caused by M. kansasii, M. xenopi, M. malmoense and Other Slowly Growing NTM”, and “Disease Caused by Mycobacterium abscessus and Other Rapidly Growing Mycobacteria (RGM)”) [2]. It is not as clear if other NTM PD pathogens in the setting of NB disease such as M. abscessus, M. kansasii, or M. xenopi behave as benignly as MAC. It is incumbent upon the treating physician to be familiar with the relative virulence of common NTM pathogens in general as well as locally isolated NTM species [28, 29]. For instance, a clinician in Central Texas would need to be familiar with the virulence and natural history of M. simiae disease, whereas physicians in the Northern United States must be familiar with the disease-causing potential and natural history of M. xenopi.
No single or simple algorithm is adequate for determining the intensity of follow-up for all patients. The physician must be familiar with the virulence of the NTM pathogen in question and the pattern of disease stability and/or progression for each patient rather than depending on arbitrary recommendations for the frequency of clinical, microbiologic, and radiographic follow-up. For patients who meet NTM PD diagnostic criteria but who do not start therapy, we recommend 3–6-month (or sooner if symptoms worsen) follow-up pulmonary visits with sputum collection and radiographic assessment over at least a 24-month period. A 24-month period is generally adequate for determining which ostensibly stable patients will need therapeutic intervention. If therapy is not started in that time, we recommend at least yearly follow-up thereafter. We again stress that individualized patient assessment schedules are required. It is quite possible that management of other comorbid conditions such as bronchiectasis will dictate more frequent physician visits. We strongly urge indefinite follow-up for these patients as there is no recognized or consensus statute of limitations for when progressive NTM PD might develop after isolation of NTM from a respiratory specimen.
Patient participation is mandatory and an essential aspect of these considerations. A frank discussion with the patient should not be limited to a list of medication toxicities but also weighing the possibility of progressive lung disease if therapy is held. Too often patients are simply told that “the treatment is worse than the disease” to prejudice the patient against treatment. Our experience is that most patients are quite willing to tolerate some diagnostic uncertainties while knowing that they are part of a careful and deliberate long-term evaluation. Patients must trust that the process will not push them into unnecessary therapy nor abandon them to untreated disease progression. In our experience a major advantage of this deliberate approach is that by the time it is clear to the physician that treatment initiation is necessary, it is also usually clear to the patient as well. Attaining confidence in the need for therapy is absolutely essential for patient adherence with extended anti-mycobacterial treatment regimens. In our experience the sequential and incremental introduction of (oral) NTM medications has generally enhanced tolerance at the beginning of treatment in contrast to starting all NTM medications at full doses all at once. Buildup to full dosing and starts of new medication are frequently recommended at 2–3-day intervals so as to reach full dosing of all medications in an average of 2–3 weeks. Use of probiotics has been associated with a reduction of antibiotic-associated diarrhea and, in our experience, may improve gastrointestinal tolerance of medications [30].
Starting NTM PD Therapy
-
The decision to start treatment or observe for NTM PD is complex and unique to each patient, comorbidities, and overall risk-benefit assessment.
-
The sequential and incremental introduction of NTM medications may improve tolerance.
-
If treatment for NTM PD is not started, regular and longitudinal follow-up is essential.
Choosing Anti-mycobacterial Treatment Regimens
Two of the greatest challenges for choosing NTM treatment regimens are understanding NTM drug resistance mechanisms and recognizing the limitations of in vitro susceptibility testing for guiding NTM anti-mycobacterial therapy . The latter consideration is neither intuitive nor facile and is perhaps the greatest source of frustration among clinicians treating patients with NTM disease. This topic is so important that it is covered in detail in two chapters in this volume (chapters “Laboratory Diagnosis and Antimicrobial Susceptibility Testing of Nontuberculous Mycobacteria” and “Drug Susceptibility Testing of Nontuberculous Mycobacteria”). The reader is strongly urged to read both of these discussions as they have somewhat different perspectives and emphasis. Even so it is worth reiterating that in vitro susceptibility testing for many NTM pathogens is frequently not a reliable guide to effective anti-mycobacterial drug choices and clinical responses (Table 1). The most important and common example is MAC where only macrolide and amikacin in vitro susceptibilities predict in vivo treatment response. This observation is so important and so frequently ignored or misunderstood that it bears reinforcing. Awareness of the potential for acquired macrolide resistance for MAC means inclusion of adequate companion medications (usually ethambutol) to prevent acquired macrolide resistance which is associated with significantly worse clinical outcomes compared with macrolide-susceptible MAC isolates (Table 2; Fig. 5) [31].
68-year-old patient diagnosed with MAC lung disease . Patient started on guideline-based therapy including macrolide, ethambutol, and rifampin. Ethambutol discontinued after “resistant” MIC for ethambutol reported by reference laboratory. Moxifloxacin substituted for ethambutol because of “susceptible” MIC reported by the reference lab. Rifampin stopped due to patient intolerance. After 12 months of therapy with macrolide and moxifloxacin, the patient had progressive cavitary destruction of the right upper lobe and a macrolide-resistant MAC isolate
Formulating an adequate treatment regimen for a specific NTM pathogen and achieving therapeutic success require familiarity with both innate and acquired resistance mechanisms for that pathogen [32]. Conversely, a lack of familiarity with these mechanisms is not likely to be associated with treatment success and may in fact exacerbate or worsen the patient’s status. While specific NTM PD regimens including oral, inhaled, and/or parenteral antimicrobial agents vary considerably across different NTM species, the concept of using multidrug regimens for avoidance of acquired drug resistance is universally applicable.
Choosing Anti-mycobacterial Treatment Regimens
-
Discordance between in vitro susceptibilities and in vivo treatment responses for NTM is common.
-
Understanding the mechanisms of drug resistance in NTM is important for successful treatment of NTM.
Patient Evaluation During NTM PD Therapy Including Response to Therapy
Once the patient has been started on an appropriate anti-mycobacterial treatment regimen, there are multiple potential impediments to the completion of adequate therapy. Most of these impediments are related to the long duration of treatment and the need for multiple potentially toxic anti-mycobacterial medications. Other impediments are generally related to the poor overall physical status of NTM patients due to other comorbidities, usually bronchiectasis or chronic obstructive lung disease. These comorbidities can obfuscate or confuse symptoms of mycobacterial disease. In one recent study, it was found that even for patients who responded well to anti-mycobacterial therapy, there was invariably at least one bronchiectasis exacerbation while on anti-mycobacterial therapy [33]. Some of the potential obstacles to successful anti-mycobacterial therapy are listed in Table 3.
It is noteworthy that a major impediment to successful therapy is a lack of adherence by treating physicians to recommended NTM PD guideline-based treatment [34, 35]. We readily concede that current treatment guidelines are suboptimal and frequently do not result in treatment success. We just as readily suggest that nonadherence to the treatment guidelines does not improve the chances for treatment success and may adversely affect a patient’s disease course and prognosis [34, 35].
Given the combination of coexisting pulmonary comorbidities and multiple potentially toxic medications, it is not surprising that NTM PD patients frequently experience problems with anti-mycobacterial therapy requiring therapeutic adjustments. In our experience, the majority of adjustments are needed at the front end of the NTM PD regimen. One example of an effective adjustment is the improved tolerance of intermittent (three times weekly) medication compared with daily medication administration for NB MAC PD [36, 37]. Changes in mycobacterial treatment required due to gastrointestinal drug intolerances generally become less likely once patients are well into the treatment course.
Exceptions for late intolerances include hearing loss with the longer-term use of amikacin, and to lesser extent macrolide, and optic nerve toxicity with ethambutol. It is essential for the clinician to manage these patients in such a way as to maintain as many “first-line” drugs as possible in the patient’s treatment regimen as there are few effective alternatives. We recommend audiograms for patients on parenteral amikacin and visual and color vision testing for patients on ethambutol in accordance with monitoring recommendations from recent TB guidelines [38].
Macrolides are the most important anti-mycobacterial component in MAC and macrolide-susceptible M. abscessus treatment regimens. Simply stated, there is not a comparably effective replacement in either circumstance. It is clear that maintaining a macrolide in the treatment regimen in these situations has the highest priority. Patients who are intolerant of one macrolide (clarithromycin or azithromycin) because of drug toxicity can frequently tolerate the other. Even for mild hypersensitivity responses, there is not complete cross-reaction between the two drugs so that patients with a rash on one macrolide should be challenged (under appropriate observation and monitoring) with the other macrolide. Although not as critical for successful therapy, patients with intolerance to one rifamycin (rifampin or rifabutin), including mild hypersensitivity reactions, may tolerate the other rifamycin. Rifampin-related hypersensitivity reactions can also be addressed through established rifampin desensitization protocols [39]. This step would be especially important for maintaining rifampin in M. kansasii (rifampin susceptible) treatment regimens.
Patient Evaluation During NTM PD Therapy and Assessment of Response to Therapy – 1
-
Established guidelines should be followed to optimize chances of successful NTM PD treatment outcomes.
-
Monitoring while on NTM PD treatment with blood work, visual assessments, and hearing/vestibular testing is required without exception and should be tailored individually to patients and associated comorbidities as well as specific anti-mycobacterial regimen (see text).
In MAC disease, the most important of the macrolide companion drugs is ethambutol. While ethambutol is not a potent anti-MAC drug per se , it has been shown to protect against the emergence of acquired mutational macrolide resistance [40]. If ethambutol is lost in the treatment regimen, it cannot be easily replaced. Intermittent ethambutol administration appears to be associated with less ocular toxicity than daily ethambutol. It was also shown that older patients taking ethambutol who develop ocular symptoms frequently have explanations for those symptoms other than ethambutol. Even though many, perhaps most, ophthalmologists currently in practice are unfamiliar with ethambutol ocular toxicity, it is our practice to heed the advice of an ophthalmology consultant about discontinuation of ethambutol in a patient with new or worsening ocular symptoms. Patients with ethambutol hypersensitivity reactions can be successfully desensitized via published protocols so that ethambutol can remain in the treatment regimen [39].
Two particularly poor ethambutol replacement strategies for MAC disease are the substitution of fluoroquinolone for ethambutol (macrolide + fluoroquinolone ± rifamycin) or the use of macrolide with only a rifamycin (Fig. 5). The rifamycins decrease macrolide serum levels, and the fluoroquinolones do not protect against the emergence of acquired mutational macrolide resistance. Possible substitutions include parenteral amikacin, inhaled amikacin, and clofazimine although there is little data to support this recommendation.
Patient Evaluation During NTM PD Therapy and Assessment of Response to Therapy – 2
-
The use of fluoroquinolones for the treatment of MAC, either alone or in combination with macrolide, is not recommended.
-
Ethambutol as a companion drug in MAC treatment regimens protects against development of macrolide resistance.
-
Rifamycins should not be a single companion drug to macrolide in MAC PD treatment regimens.
-
Alternative MAC companion medications to protect macrolide include amikacin and clofazimine.
One trend in MAC PD therapy deserves special mention, that is, the use of inhaled generic amikacin [41,42,43,44]. Our impression is that inhalation of a parenteral amikacin preparation is widely prescribed in the USA, although there is little published clinical experience with inhaled amikacin. There is not an FDA-approved inhalation form of amikacin, so there is no standardization in dosing, delivery, or administration. We believe that amikacin is effective against MAC when given parenterally, but its effectiveness by inhalation is less certain. Variable and heterogenous lung deposition and concentrations conceivably could promote acquired amikacin resistance or even acquired macrolide resistance if it is the only companion drug for macrolide-susceptible isolates. It is understandable that patients and clinicians prefer amikacin inhalation to parenteral amikacin administration, but both should be aware that there is no proof that they are comparably effective.
In contrast to inhaled generic amikacin, results from a phase II study using liposomal amikacin for inhalation for refractory MAC PD were promising [45]. A second Phase III study has been published and confirms the effectiveness of inhaled liposomal amikacin for producing sputum conversion in refractory MAC lung disease [46]. These studies with provide sufficient safety and efficacy information for establishing appropriate placement of inhaled liposomal amikacin in treatment regimens for MAC.
Treatment of other NTM PD pathogens such as M. abscessus, M. kansasii, and M. xenopi is discussed in detail in chapters “NTM Disease Caused by M. kansasii, M. xenopi, M. malmoense and Other Slowly Growing NTM” and “Disease Caused by Mycobacterium abscessus and Other Rapidly Growing Mycobacteria (RGM)”. Some of the concepts discussed for MAC PD are pertinent to these pathogens although each NTM pathogen presents its own challenges and obstacles to successful therapy, such as the need for parenteral therapy for M. abscessus. Our impression is that for most non-MAC NTM pathogens, expert consultation is sought more frequently than for MAC PD so that familiarity with each one may not be as critically important as with MAC for the general pulmonary or infectious disease specialist.
When the patient and the clinician embark on a treatment course for NTM PD, there must be a clear understanding that treatment difficulties and medication intolerances will inevitably arise but can generally be managed with modifications of drug doses or dosing intervals. In our experience, modification of treatment regimens during the course of NTM PD therapy is the rule rather than the exception. Given the paucity of effective drugs for treating NTM PD pathogens, premature abandonment of “first-line” therapy or specific components of that therapy will usually not result in a successful treatment outcome and adversely impact long-term prognosis. Some frequently encountered medication-related problems are outlined in Table 5.
Just as there are three components (clinical, radiographic, and microbiologic) for establishing MAC PD diagnosis and for deciding when to begin NTM PD therapy, there are also three major components for evaluating treatment response. Clinically it is expected that patients would have symptomatic improvement, with symptoms such as cough, sputum production, fatigue, and weight loss. The use and role of a recently developed quality of life (QOL) instrument developed for NTM in clinical practice remains to be fully clarified [47]. In our experience, improvements in microbiologic status (i.e., culture conversion) parallel improvements in clinical symptoms and stabilization of radiographic abnormalities [33]. The universal coexistence of pulmonary comorbidities such as bronchiectasis and chronic airflow obstruction frequently make this assessment difficult and unsatisfying for the patient. As discussed above the overlapping symptoms between comorbid pulmonary conditions and NTM PD make optimal management of those comorbid conditions absolutely critical for adequate NTM PD assessment. Clinicians should be particularly mindful to recognize undertreated comorbidities if NTM microbiologic improvements occur in the setting of progressive clinical symptoms.
We recommend clinic visits at least every 2–3 months while on therapy to evaluate patient medication tolerance , treatment response, and toxicity monitoring understanding that the frequency may need to be more often for some patients on parenteral-based regimens and/or with substantial intolerances. Patients should be regularly and systematically questioned at each visit as to any symptoms including common drug toxicity symptoms (Table 4). As noted above, we also recommend visual acuity and color vision testing for all patients who are on ethambutol on a regular 2- to 3-month interval basis or sooner if symptoms develop. For patients on a rifamycin and/or macrolide, we also recommend a complete blood count and chemistry panel including liver enzymes at each visit although the utility of this approach has not been rigorously evaluated. Patients who are receiving an intravenous aminoglycoside should have baseline audiometry and vestibular function with follow-up monitoring studies guided by patient’s symptoms and published guidelines. Initial clinical assessment and follow-up of hearing and vestibular function are also required for those patients receiving inhaled aminoglycoside [48]. Baseline EKG is generally warranted in all patients starting macrolides to assess for significant baseline EKG abnormalities. A role for the ongoing monitoring of patients on macrolide with EKG’s is not established nor, in our opinion, justified at this point unless there are abnormalities at baseline or if cardiac rhythm risk factors are present. The presence of comorbid pulmonary conditions especially bronchiectasis can also complicate the interpretation of radiographic response to therapy. Patients with bronchiectasis with or without NTM lung disease frequently have waxing and waning densities associated with secretion retention in the airways as well as acute inflammatory responses that can be associated with bronchiectasis exacerbations. It is our experience that acute symptomatic and radiographic changes with the development of purulent sputum production most often reflect a bronchiectasis exacerbation rather than failure of a NTM PD regimen. These varying radiographic features of bronchiectasis often cloud the radiographic assessment of response to NTM PD therapy. Once again the aggressive management of the underlying pulmonary comorbidity can greatly facilitate interpretation of the patient’s response to anti-mycobacterial therapy. Radiographic abnormalities are not expected to resolve completely even after successful completion of a full course of NTM PD therapy. In most instances improved, albeit not resolved, radiographic abnormalities parallel improvements in clinical symptoms and microbiologic responses while on NTM PD therapy.
We recognize that there is not a universally endorsed approach to the radiographic follow-up for NTM patients on therapy. Patient’s generally have both plain chest radiography and chest CT scans at the initiation of therapy. For those patients with obvious abnormalities on plain chest radiograph, it may be adequate to obtain serial follow-up radiographs for detecting significant radiographic changes. For other patients the abnormalities are more subtle and may require serial (low dose) chest CT scans to detect changes. Patients are very cognizant about radiation exposure with chest CT scans, and our general approach is to limit the number of chest CT scans as much as possible. Ideally after starting therapy, patients would only require one follow-up chest CT scan at the termination of therapy.
The microbiologic analysis is ostensibly the most straightforward, but even that analysis has important caveats and pitfalls. Arguably the most important single metric for evaluating mycobacterial treatment response is conversion of the patient’s sputum to AFB culture negative. There is ongoing debate about the relative importance of patient symptomatic and radiographic responses and for determining the efficacy of therapy. However, it would be difficult to argue that an anti-mycobacterial intervention was effective without AFB sputum culture conversion to negative. We recommend collection of sputum for AFB analysis on a monthly basis so as to establish sustained sputum culture conversion as early as possible while patients are undergoing treatment but no less than every 2–3 months. We recommend expectorated sputum collection if possible or sputum induction during clinic visits if expectorated sputum is not available. Monitoring of patients with additional sputum culture collections is also recommended during NTM therapy (e.g., MAC) understanding that a second NTM species (e.g., M. abscessus) may appear [49]. We do not recommend routine bronchoscopy for specimen collection while patients are undergoing therapy or at the end of therapy even if the original diagnosis was made based on a bronchoscopic specimen. The legitimacy of serial bronchoscopic cultures for evaluating treatment response including the impact on the treatment success criterion of 12 months sputum culture negativity has not been rigorously tested.
Patient Evaluation During NTM PD Therapy and Assessment of Response to Therapy – 3
-
The frequency of regular clinic visits and sputum collections during NTM PD therapy are dependent on patient factors and specific medications of the NTM PD regimen.
-
A second NTM species, sometimes needing treatment, may occur during NTM PD therapy.
-
Radiographic abnormalities are not expected to resolve completely even when successful NTM PD therapy is completed.
Treatment Endpoints
The current primary NTM PD treatment endpoint most often is microbiologic . Patients should achieve 12 months of negative sputum AFB cultures while on therapy. This treatment duration was chosen because it was observed in the past to be associated with sustained sputum culture negativity after discontinuation of MAC therapy [1]. A major confounding factor for this treatment success criterion is the observation that some patients with NB MAC lung disease will have microbiologic recurrence either while still receiving therapy (after sputum conversion) or after completing adequate therapy due to new or unique MAC genotypes that are different from the initial MAC genotype at the time of diagnosis. The source of these new MAC genotypes is not clear. They may represent reinfection or it is possible that the original MAC infection was polyclonal and the new genotypes were preferentially selected to grow during therapy. It is noteworthy however that when these new genotypes occur during therapy, they are invariably also macrolide susceptible in vitro although the patient was invariably receiving as part of the original guideline-based MAC therapy [36].
Likewise, the goal of 12 months of treatment after sputum conversion is often not practically possible when using NTM PD regimens that incorporate one or more parenteral agents when alternative transition oral or inhaled medications are not available. This practical limitation adds to the risk-benefit assessment complexities not only about starting treatment but also about determining the duration of therapy. In some instances treatment holidays from parenterally based regimens should be considered, even if sputum culture conversion has not occurred. It has been our experience that patients and treating physicians are best served by articulating expectations and endpoints a priori before the start of a course of NTM therapy.
Patients also, as noted above, sometimes have co-isolation of more than one NTM species. A common scenario is the isolation of M. abscessus during the course of treatment for MAC. Most of these patients have a limited number of M. abscessus isolates without an indication of progressive M. abscessus lung disease . No alteration in MAC therapy is necessary for these patients. Some patients however will have repeated M. abscessus isolation associated with progressive radiographic abnormalities including cavitation and worsening symptoms (Fig. 6). For these patients therapy may need to be altered to treat M. abscessus as well as MAC. This therapeutic shift is difficult because there are few agents with activity against both MAC and M. abscessus. Determining the significance of M. abscessus isolates during the treatment of MAC requires very close clinical, microbiologic, and radiographic assessment to dissect confidently the role of M. abscessus as the cause of clinical deterioration or progressive disease. Unfortunately there are no surrogate markers to aid in that determination so that the clinician must depend on clinical indicators in conjunction with the radiographic as well as microbiologic findings.
(a) 58-year-old patient with bronchiectasis and MAC lung disease on guideline-based therapy including macrolide with initial symptomatic, radiographic, and microbiologic improvement. (b) 58-year-old MAC lung disease patient improving on guideline-based therapy who subsequently had symptomatic regression associated with enlarging cavitary lesion on chest imaging and repeated isolation of M. abscessus from sputum
A second and equally difficult scenario is the isolation of other known respiratory pathogens such as fungi and Nocardia species during the course of NTM PD therapy. The isolation of fungi, especially Aspergillus species , is common in patients with cavitary disease presumably due to colonization of cavities with the fungus as well as the use of chronic antimicrobials. In most patients it has been our experience that the isolation of Aspergillus was not clinically significant. It is also evident, however, that there are patients with extensive cavitary disease who do not respond well to anti-mycobacterial therapy and are felt to have progressive cavitary fungal disease (Fig. 7) [50, 51]. Unfortunately, we are not aware of a simple diagnostic approach for determining the significance of Aspergillus in this circumstance, although measuring serum Aspergillus precipitins or other specific biomarkers has been touted in this role [52]. Because of the usually severe nature of the cavitary disease in these patients, this diagnostic uncertainty means that by default, patients will receive antifungal therapy. The assessment of positive fungal cultures is all the more important because antifungal and anti-mycobacterial therapies are often incompatible due to drug-drug interactions especially with combinations of rifamycins and macrolides. This is clearly an area that urgently needs more research. For now, clinicians must rely on a multidisciplinary approach to the patients and careful assessment of the effects of all interventions.
(a) 35-year-old patient with history of immunoglobulin G deficiency and steroid-dependent asthma diagnosed with cavitary MAC lung disease. (b) Patient treated with guideline-based therapy including macrolide and parenteral amikacin with symptomatic, radiographic, and microbiologic improvement. (c) After initial radiographic improvement on guideline-based MAC therapy including macrolide and amikacin, patient developed rapidly enlarging bilateral cavities associated with repeated isolation of Aspergillus from sputum and bronchoscopy
Similarly, the isolation of Norcadia species is not rare in NB NTM PD patients [53]. Prior to the recent expansion in bronchiectasis interest, it was assumed that the isolation of Nocardia from respiratory specimens was an ominous and always clinically significant finding and indicative of immune deficiency. More recently, it has become clear that bronchiectasis patients can sometimes have Nocardia isolated from sputum without evidence of immune deficiency or progressive Nocardia lung disease . The current situation of Nocardia isolated in sputum or other respiratory specimens is reminiscent of the situation 30 years ago with bronchiectasis and NTM. As with Aspergillus, this is also an area that urgently needs more research.
In contrast to the utility of the microbiologic endpoint, the role of clinical and radiographic changes as endpoints for treatment of NTM PD is even less clear. Certainly both symptomatic and radiographic endpoints are important, but they are simply more difficult to evaluate given their nonspecific character in relation to comorbid medical conditions and lack of validated instruments and endpoints. The culprit again is the common coexistence of underlying lung disease especially bronchiectasis. Bronchiectasis is associated with permanent radiographic abnormalities and shifting patterns of secretion retention with waxing and waning infiltrates. Chest radiographs are certain to remain abnormal throughout therapy and at the completion of MAC lung disease treatment. This is highlighted further with the understanding that all tree-in-bud infiltrates are not NTM PD related [54]. Without a clear-cut or unequivocal indication of mycobacterial disease progression , it would be difficult to justify treatment extension after the patient meets the microbiologic endpoint (culture conversion) for treatment success. Similarly while it is hoped that patients have overall symptomatic improvement, it would also be difficult to justify treatment extension without clear-cut or unequivocal indicators of symptomatic progression due to MAC disease. These areas exemplify the importance of close familiarity by the clinician with the MAC lung disease patient. As importantly in our experience, robust laboratory support and interactions between clinician and laboratorians will further optimize successful treatment outcomes. Assessing treatment response for MAC lung disease is clearly not as simple as the assessment of tuberculosis lung disease treatment response. Pulmonary function testing including forced expiratory volume at 1 s (FEV1) and forced vital capacity (FVC) is not considered to be sensitive or specific enough to use routinely as an endpoint in the assessment of response to NTM treatment. Often, abnormalities of pulmonary function are more reflective of the status of comorbidities rather than NTM PD.
It is worth emphasizing that treatment endpoints of NTM PD are not static universal endpoints and may vary as well through the initial evaluation period and the treatment course or in the follow-up period. For example, it may be decided based on status of disease severity, drug tolerance (or lack thereof), or other contributing factors that the goal of therapy is to control symptoms but not to achieve sputum culture conversion and or radiographic improvement. Goals of treatment and treatment endpoints are particularly important to discuss with patients prior to the start of treatment as well as during treatment. In this instance, modifications in the treatment regimen in intensity and duration may be appropriate. Alternatively, escalation of treatment may also be warranted if anticipated endpoints at the beginning of treatment are not being met with initial treatment regimens. One such escalation is adjunctive surgical resection of involved lung. This strategy is clearly effective for selected patients with localized disease and adequate cardiopulmonary reserve in the hands of experienced surgeons. Surgical intervention should be considered for all NTM PD patients who do not respond adequately to first-line anti-mycobacterial therapy and have surgically favorable lung disease involvement with the understanding that many such patients will still not be appropriate surgical candidates. Specific modifications of de-escalation or escalation in NTM PD therapy regimens are covered in other chapters addressing specific pathogens or interventions.
Treatment Endpoints
-
Goals of treatment and treatment endpoints should be discussed with patients prior to the start of therapy as well as during therapy.
-
Findings of a second NTM species or other pathogens (e.g.. Aspergillus or Nocardia) during NTM PD are not uncommon.
-
The significance of co-pathogens and need to treat requires complex assessments of clinical, microbiologic, and radiographic factors.
Post-therapy Evaluation
After successful treatment of MAC lung disease is completed, we recommend continued surveillance of sputum with AFB cultures obtained once in 2–3 months and then periodically (up to every 2–3 months) for at least 2–3 years along with longitudinal assessment of clinical symptoms and radiographic abnormalities. Even if the patient is successfully treated for MAC microbiologically, there is an approximate 50% chance of microbiologic recurrence after completion of therapy [36]. Most of these microbiologic recurrences are due to new or unique genotypes compared with the original genotype isolated from the patient [36]. When microbiologic recurrences occur that are due to new or unique genotypes, our approach is the same as for any patient with a new isolation of MAC. As previously mentioned above, macrolide susceptibility is generally expected to be preserved for recurrent MAC lung disease although recheck of macrolide susceptibility on the recurrent isolates is warranted. If the microbiologic recurrence is due to a MAC genotype identical to the originally isolated genotype, we consider those patients as having true disease relapse and generally reinstitute therapy at that point. Subsequent rates of microbiologic responses to repeat treatment courses have been observed to be less relative to initial treatment responses even with preserved macrolide susceptibility [1]. These considerations underscore the critical importance of having adequate laboratory support to make the appropriate determinations about the significance of microbiologic recurrence isolates. If, however, genotyping of microbiologic recurrence isolates is not available, we recommend the same systematic assessment (symptomatic, microbiologic, and radiographic) as would be applicable after an initial isolation of MAC in a respiratory specimen.
The frequency of radiographic follow-up is less certain. Our approach is to obtain plain chest radiographs as often as possible reserving (low dose) chest CT scans for specific questions that might arise. Patients are frequently and understandably quite interested in limiting radiation exposure but also usually quite willing to cooperate with appropriately justified requests for CT scans. Clearly the frequency and type of radiographic follow-up will depend on the patient’s clinical status.
We view these patients as lifelong patients not only because of the need to manage their bronchiectasis and comorbidities but because there is no endpoint for exposure to ubiquitous NTM pathogens and presumably to acquisition and reacquisition of these pathogens in the lungs of patients. Moreover, the impact of residential environmental NTM mitigation including but not limited to increasing water heater temperature, the design of showerheads, and showering habits is unknown as to risk of recurrent NTM lung disease infection rates (see chapter “Environmental Niches for NTM and Their Impact on NTM Disease”) [55].
Certainly there are patients that were not started on anti-mycobacterial therapy but have persistently positive sputum AFB cultures and stable clinical and radiographic status who require long-term monitoring for evidence of progressive mycobacterial disease. We feel strongly that close familiarity with the course of an individual’s bronchiectasis and mycobacterial disease facilitates the evaluation of mycobacterial disease activity during the waxing and waning course of underlying lung disease, especially bronchiectasis. An overview of NTM lung disease management recommendations is provided in Table 5.
Post-therapy Evaluation
-
Microbiologic recurrence of NTM PD after successful therapy is not uncommon.
-
Patients with NTM PD need indefinite follow-up and should be considered lifelong patients.
Extrapulmonary NTM Disease
Early suspicion of NTM infection is the critical element for timely diagnosis of extrapulmonary NTM disease. It is typical for patients with NTM wound infections to have several courses of unsuccessful antibacterial antibiotic therapy before undergoing diagnostic evaluation for NTM pathogens. In some circumstances the typical features of NTM infection are present, such as the purplish nodules of cutaneous and disseminated NTM disease (Fig. 8). In that circumstance there should be an immediate diagnostic effort to culture for NTM. In other less clear or nonspecific circumstances, it is understandable that bacterial pathogens would be therapeutically addressed first, but hopefully, AFB cultures would also be sent to expedite the diagnosis and facilitate initiation of anti-mycobacterial therapy. Findings of granulomatous changes on histopathology, with or without the presence of AFB organisms on AFB stain, should promptly raise suspicion for NTM (or TB) infection.
The general principles of NTM therapy as outlined for NTM PD also generally apply for extrapulmonary NTM disease as well albeit frequently with shorter duration of therapy than as recommended for NTM PD [1].
Following the patient with extrapulmonary NTM disease is also challenging. Patients with cutaneous/disseminated disease often have the appearance of new nodular lesions even while undergoing appropriate therapy for the mycobacterial pathogen. For these patients it is critical to aspirate new lesions to determine if there is active infection in the lesions, possibly due to the emergence of resistance to the antibiotic regimen, or if they are sterile and immunologically mediated as part of the paradoxical inflammatory response to therapy. Similarly patients with mycobacterial lymphadenopathy due to either TB or NTM may show lymph node enlargement or the appearance of new lymph nodes even while on appropriate treatment. Again it is critical that new or enlarging lesions are aspirated for AFB culture to determine if there is ongoing infection versus an immunologically mediated paradoxical response (e.g., IRIS – immune reconstitution inflammatory syndrome).
For patients who have disseminated NTM infection, it is critically important to correct any modifiable underlying immune suppression. The antibiotics used for treating NTM disease are not as potent as the antibiotics used for treating TB so that without reversal or correction of severe underlying immunosuppression, the chances for long-term treatment success are low. The finding of disseminated NTM should prompt the clinician to assess the patient and exclude an immunodeficiency. Patients with NTM PD at large are not expected to have other immunocompromised infections or substantial immune defects although this is an area under active investigation [56, 57].
For patients who have NTM infection associated with a foreign body, it is also critically important to remove the foreign body for adequate treatment of the mycobacterial infection. Sometimes removal of these foreign bodies is relatively simple such as removal of an indwelling venous catheter, a breast augmentation device, or a peritoneal dialysis catheter. For other foreign bodies such as joint prostheses, removal of the foreign body is more problematic but no less necessary. While there may be instances of successful treatment of joint space mycobacterial infections without removal of a prosthetic joint, there are also instances where failure to remove the joint initially significantly prolongs the duration of antibiotic therapy and does not prevent the eventual removal of the joint prosthesis. We feel the evidence strongly supports prosthetic joint removal at the initiation of the anti-mycobacterial therapy. Iatrogenic or nosocomial extrapulmonary NTM infections often can be traced back to contamination from (tap) water supplies.
Following patients with extrapulmonary NTM disease is often more difficult than following patients with NTM lung disease. Aside from the paradoxical therapeutic responses that may occur with cutaneous and disseminated disease noted above, there are also limited methods of treatment evaluation for other sites of infection. One particularly troubling area is joint space infection. These areas are especially difficult to visualize radiographically and are problematic for obtaining serial specimens for culture. We find objective assessment of treatment response with these infections challenging and all too often frustrating. These types of infections are perhaps even more desperately in need of surrogate disease activity biomarkers than NTM PD.
Summary
In summary, management of pulmonary and extrapulmonary NTM disease requires the clinician to be experienced and familiar with the protean and complex clinical, microbiologic, and radiographic manifestations of NTM disease. Input from patients as stakeholders a priori in the planning of and articulation of the treatment goals of prolonged multidrug anti-mycobacterial treatment regimens is of paramount importance. Close relationships between the treating clinicians and their respective colleagues in the microbiology laboratory and surgery consultation services are equally critical for optimizing treatment success. We are reminded daily of the shortcomings in our treatment options for NTM infections [58]. Currently, success rates for treating macrolide-susceptible MAC are not as good as our success with multidrug-resistant TB, while the success rate for treating macrolide-resistant MAC is closer to the success rate of extensively drug-resistant TB (XDR-TB). We remain optimistic that this situation will improve in the near future given the current international interest and research momentum in NTM diseases.
Bibliography
Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
van Ingen J, Griffith DE, Aksamit TR, Wagner D. Chapter 3. Pulmonary diseases caused by non-tuberculous mycobacteria. Eur Respir Monogr. 2012;58:25–37.
Jeong BH, Kim SY, Jeon K, Lee SY, Shin SJ, Koh WJ. Serodiagnosis of Mycobacterium avium complex and Mycobacterium abscessus complex pulmonary disease by use of IgA antibodies to glycopeptidolipid core antigen. J Clin Microbiol. 2013;51(8):2747–9.
Shu CC, Ato M, Wang JT, et al. Sero-diagnosis of Mycobacterium avium complex lung disease using serum immunoglobulin a antibody against glycopeptidolipid antigen in Taiwan. PLoS One. 2013;8(11):e80473.
Kitada S, Kobayashi K, Ichiyama S, et al. Serodiagnosis of Mycobacterium avium-complex pulmonary disease using an enzyme immunoassay kit. Am J Respir Crit Care Med. 2008;177(7):793–7.
Kitada S, Maekura R, Toyoshima N, et al. Use of glycopeptidolipid core antigen for serodiagnosis of mycobacterium avium complex pulmonary disease in immunocompetent patients. Clin Diagn Lab Immunol. 2005;12(1):44–51.
Qvist T, Pressler T, Taylor-Robinson D, Katzenstein TL, Hoiby N. Serodiagnosis of Mycobacterium abscessus complex infection in cystic fibrosis. Eur Respir J. 2015;46(3):707–16.
Kitada S, Levin A, Hiserote M, et al. Serodiagnosis of Mycobacterium avium complex pulmonary disease in the USA. Eur Respir J. 2013;42(2):454–60.
Peralta G, Barry P, Pascopella L. Use of Nucleic Acid Amplification Tests in Tuberculosis Patients in California, 2010-2013. Open Forum Infect Dis. 2016;3(4):ofw230.
Kendall BA, Varley CD, Hedberg K, Cassidy PM, Winthrop KL. Isolation of non-tuberculous mycobacteria from the sputum of patients with active tuberculosis. Int J Tuberc Lung Dis. 2010;14(5):654–6.
Huang CT, Tsai YJ, Shu CC, et al. Clinical significance of isolation of nontuberculous mycobacteria in pulmonary tuberculosis patients. Respir Med. 2009;103(10):1484–91.
Chung MJ, Lee KS, Koh WJ, et al. Drug-sensitive tuberculosis, multidrug-resistant tuberculosis, and nontuberculous mycobacterial pulmonary disease in nonAIDS adults: comparisons of thin-section CT findings. Eur Radiol. 2006;16(9):1934–41.
Yakrus MA, Metchock B, Starks AM. Evaluation of a u.S. public health laboratory service for the molecular detection of drug resistant tuberculosis. Tuberc Res Treat. 2015;2015:701786.
Ahn CH, Lowell JR, Onstad GD, Ahn SS, Hurst GA. Elimination of Mycobacterium intracellulare from sputum after bronchial hygiene. Chest. 1979;76(4):480–2.
Prince D, Peterson D, Steiner R, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–8.
Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129(2):341–8.
Lee G, Lee KS, Moon JW, et al. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Natural course on serial computed tomographic scans. Ann Am Thorac Soc. 2013;10(4):299–306.
Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629.
Martinez-Garcia MA, Maiz L, Olveira C, et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol. 2018;54:79.
Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;185(5):575–83.
Lee MR, Yang CY, Chang KP, et al. Factors associated with lung function decline in patients with non-tuberculous mycobacterial pulmonary disease. PLoS One. 2013;8(3):e58214.
Aksamit TR. Mycobacterium avium complex pulmonary disease in patients with pre-existing lung disease. Clin Chest Med. 2002;23(3):643–53.
Andrejak C, Nielsen R, Thomsen VO, Duhaut P, Sorensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–62.
Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50(3):1700037.
Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008;85(6):1887–92. discussion 1892-1883
Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537.
Kim SJ, Park J, Lee H, et al. Risk factors for deterioration of nodular bronchiectatic Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2014;18(6):730–6.
Ford ES, Horne DJ, Shah JA, Wallis CK, Fang FC, Hawn TR. Species-specific risk factors, treatment decisions, and clinical outcomes for laboratory isolates of less common Nontuberculous mycobacteria in Washington state. Ann Am Thorac Soc. 2017;14(7):1129–38.
Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604–13.
Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959–69.
Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174(8):928–34.
van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 2012;15(3):149–61.
Griffith DE, Adjemian J, Brown-Elliott BA, et al. Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;192(6):754–60.
Adjemian J, Prevots DR, Gallagher J, Heap K, Gupta R, Griffith D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc. 2014;11(1):9–16.
van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017;49(2).
Wallace RJ Jr, Brown-Elliott BA, McNulty S, et al. Macrolide/Azalide therapy for nodular/Bronchiectatic: Mycobacterium avium complex lung disease. Chest. 2014;146:276.
Jeong BH, Jeon K, Park HY, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;191(1):96–103.
Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147-e195.
Matz J, Borish LC, Routes JM, Rosenwasser LJ. Oral desensitization to rifampin and ethambutol in mycobacterial disease. Am J Respir Crit Care Med. 1994;149(3 Pt 1):815–7.
Bermudez LE, Nash KA, Petrofsky M, Young LS, Inderlied CB. Effect of ethambutol on emergence of clarithromycin-resistant Mycobacterium avium complex in the beige mouse model. J Infect Dis. 1996;174(6):1218–22.
Olivier KN, Shaw PA, Glaser TS, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014;11(1):30–5.
Yagi K, Ishii M, Namkoong H, et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis. 2017;17(1):558.
Davis KK, Kao PN, Jacobs SS, Ruoss SJ. Aerosolized amikacin for treatment of pulmonary Mycobacterium avium infections: an observational case series. BMC Pulm Med. 2007;7:2.
Safdar A. Aerosolized amikacin in patients with difficult-to-treat pulmonary nontuberculous mycobacteriosis. Eur J Clin Microbiol Infect Dis. 2012;31(8):1883–7.
Olivier KN, Griffith DE, Eagle G, et al. Randomized trial of liposomal Amikacin for inhalation in Nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2017;195(6):814–23.
Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, Addrizzo-Harris DJ, O’Donnell AE, Marras TK, Flume PA, Loebinger MR, Morgan L, Codecasa LR, Hill AT, Ruoss SJ, Yim JJ, Ringshausen FC, Field SK, Philley JV, Wallace RJ Jr, van Ingen J, Coulter C, Nezamis J, Winthrop KL; CONVERT Study Group. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT): a Prospective, Open-Label, Randomized Study. Am J Respir Crit Care Med. 2018.
Henkle E, Aksamit T, Barker A, et al. Patient-centered research priorities for pulmonary Nontuberculous mycobacteria (NTM) infection. An NTM research consortium workshop report. Ann Am Thorac Soc. 2016;13(9):S379–84.
Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–62.
Griffith DE, Philley JV, Brown-Elliott BA, et al. The Significance of Mycobacterium abscessus Subspecies abscessus Isolation During Mycobacterium avium Complex Lung Disease Therapy. Chest. 2015;147(5):1369–75.
Kunst H, Wickremasinghe M, Wells A, Wilson R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur Respir J. 2006;28(2):352–7.
Zoumot Z, Boutou AK, Gill SS, et al. Mycobacterium avium complex infection in non-cystic fibrosis bronchiectasis. Respirology. 2014;19(5):714–22.
Pinel C, Fricker-Hidalgo H, Lebeau B, et al. Detection of circulating Aspergillus fumigatus galactomannan: value and limits of the Platelia test for diagnosing invasive aspergillosis. J Clin Microbiol. 2003;41(5):2184–6.
Woodworth MH, Saullo JL, Lantos PM, Cox GM, Stout JE. Increasing Nocardia incidence associated with bronchiectasis at a tertiary care center. Ann Am Thorac Soc. 2017;14(3):347–54.
Miller WT Jr, Panosian JS. Causes and imaging patterns of tree-in-bud opacities. Chest. 2013;144(6):1883–92.
Falkinham JO 3rd. Reducing human exposure to Mycobacterium avium. Ann Am Thorac Soc. 2013;10(4):378–82.
Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. American journal of respiratory and critical care medicine. 2008;178(10):1066–74.
Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med. 2013;34(1):110–23.
Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119:107–59.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Aksamit, T.R., Griffith, D.E. (2019). Nontuberculous Mycobacterial Disease Management Principles. In: Griffith, D. (eds) Nontuberculous Mycobacterial Disease. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-93473-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-93473-0_10
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-93472-3
Online ISBN: 978-3-319-93473-0
eBook Packages: MedicineMedicine (R0)