Abstract
The cardiac natriuretic peptides (NPs) family comprises three main peptides (ANP, BNP and CNP) which are provided with properties relevant for cardiovascular and fluid homeostasis. They produce their cardiovascular and renal effects through the binding with guanylyl cyclase (GC)-coupled receptors, namely NPR-A and NPR-B. The NPR-C and neutral endopeptidase (NEP) contribute to NPs clearance. NPs levels progressively increase in heart failure (HF), in parallel with deterioration of left ventricular function and increased cardiac wall stress. The marked involvement of NPs in HF makes them valuable diagnostic and prognostic biomarkers. In view of their therapeutic implications in HF, inhibition of NPs catabolism through NEP has been obtained with the development of ARNi which comprises Angiotensin II-AT1 receptor blocker (valsartan) and a NEP inhibitor (sacubitril). ARNi has been recently introduced for the treatment of HF with reduced ejection fraction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The natriuretic peptide (NP) family includes three well-characterized hormones, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP), which play a key role in the maintenance of cardiorenal and body fluid homeostasis [1].
ANP is largely produced from cardiac atria [1], whereas BNP is predominantly secreted from the heart ventricles [1]. NPs are produced to a lesser extent in other organs, including the brain, kidney, and vessels [2]. Within the heart, they are mostly synthetized in response to increased volume overload and myocyte stress [1]. In addition, a neuroendocrine regulation of cardiac NPs involves angiotensin II, endothelin-1, and phenylephrine that, by signaling through receptors coupled to Gq proteins, increase ANP and BNP in a more gradual manner than stretch [3]. On the other hand, CNP is mainly produced by endothelial cells and is considered a noncirculating hormone [1]. In addition, urodilatin , an amino-terminal 4-amino acid extended form of ANP, is considered a renal ANP [4]. Additional components of the family, dendroaspis natriuretic peptide (DNP) and vasonatrin peptide (VNP), have been identified in the green mamba snake and in the eel [5].
ANP, BNP, and CNP derive from separate genes. ANP and BNP genes are located in the distal arm of chromosome 1 (1p36.2). Their structure is similar and includes three exons and two introns. The signal sequences are located in exon 1, whereas the coding sequences are located in exon 2; exon 3 encodes the terminal tyrosine and the 3’ untranslated region [1]. The CNP gene is located on chromosome 5 (5p13.3) and includes 13 exons [1].
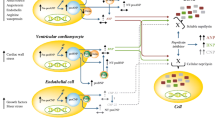
NPs are synthetized as pre-prohormones and are subsequently cleaved to obtain a biologically active α-carboxy-terminal peptide along with the amino-terminal end (Fig. 6.1). Human ANP is released as a 152-amino acid pre-prohormone. After removal of the signal peptide, the proANP1–126 is released and stored into granules within the atrial cardiomyocytes. Before secretion, proANP1-126 is processed by corin, a type II transmembrane serine protease, into the circulating forms of ANP(1–98) and of ANP(99–126). Of note, the active corin protease is obtained through the cleavage of procorin by proprotein convertase subtilisin/kexin type 6 (PCSK6) [6]. The major form of biologically active ANP is the 28-amino acid carboxy-terminal peptide, ANP(99–126). More recently, a biological functional relevance has been proven also for ANP(1–98) [7]. In addition, three peptides are cleaved from the ANP(1–98): the long-acting natriuretic peptide, LANP (1–30), the vessel dilator (31–67), and the kaliuretic peptide (79–98) [8]. All of them appear to exert some diuretic and natriuretic effects.
BNP is synthetized as a 134-amino acid pre-prohormone. After processing by furin, a subtilisin/Kex2p-like endoprotease, the biologically active peptide consisting of a 32-amino acid peptide, BNP(77–108), is released.
CNP is synthetized as a 103-amino acid prohormone which is processed by furin. The active CNP form is a 22-amino acid peptide [1].
All mature bioactive peptides contain a 17-amino acid ring structure that is essential for their biological activities [1]. Eleven of the 17 amino acids are identical in ANP, BNP, and CNP. On the other hand, the amino- and carboxy-terminal sequences vary in length and composition among the three peptides. The primary structure of NPs is conserved across species apart few variations. With regard to the ANP sequence, an isoleucine is present at position 10 in rats, mice, and rabbits, whereas humans, dogs, and bovines have a methionine at this position.
The half-life of ANP ranges from 2 to 2.5 min in humans. BNP has both a short half-life (3–4 min) and a long half-life (20–23 min). Finally, CNP has a half-life of 2–3 min.
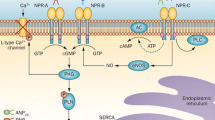
ANP, BNP, and CNP bind to specific cell membrane receptors which mediate the biological functions [1]. In particular, soluble guanylyl cyclase (GC) receptors mediate NP effects in target tissues [1]. GC-A receptor (or type A natriuretic peptide receptor, NPR-A) is the main effector of both ANP and BNP actions, whereas GC-B (or NPR-B) mediates CNP actions. GC-A and GC-B receptors contain three domains: an extracellular ligand-binding domain that binds to NPs, a short transmembrane domain, and an intracellular domain that acts as a docking site for other proteins. An increase in cyclic guanylate monophosphate (cGMP) levels reflects the activation of both GC-A and B receptors (Fig. 6.2).
Type A and type B natriuretic peptide receptors mediate the biological effects of the NPs through the activation of guanylyl cyclase and release of cGMP, with consequent increase of protein kinase G (PKG) and of phosphodiesterase (PDE). On the other hand, type C natriuretic peptide receptor is mainly responsible of NP clearance. In addition, NPR-C inhibits, through the Gi proteins, the adenylate cyclase with consequent decrease of protein kinase A and production of biological effects
An additional natriuretic peptide receptor (type C natriuretic peptide receptor, NPR-C) plays a fundamental role in NP clearance [9]. This receptor is mostly expressed in the glomerular and vascular structures of the kidney and also in the adrenals, lungs, brain, heart, and vascular wall. It recognizes an eight-amino acid linear fragment of ANP molecule to perform the peptide clearance. This process requires the ANP-NPR-C internalization and is followed by the ANP hydrolysis by lysosomes. Of interest, different from GC-A and B receptors, NPR-C contains a 37-amino acid cytoplasmic domain with a Gα inhibitory protein-activating sequence, and it is devoid of kinase and GC activities [9]. By activating NPR-C a decrease in cyclic adenylate monophosphate levels follows (Fig. 6.3). It has been shown that NPR-C mediates vasoprotective properties of CNP, and it has been also involved in cellular signaling pathways leading to antiproliferative and pro-apoptotic effects in specific circumstances [10]. In fact, a molecular variant of ANP (T2238C) acts through NPR-C to exert vascular effects opposing those of the regular peptide [11].
In addition to NPR-C, the circulating NPs are cleared by proteolytic cleavage by neutral endopeptidase (NEP) [1] (Fig. 6.3). NEP is a zinc-dependent type II integral membrane metallopeptidase with ubiquitous distribution [12]. Among the NPs, αANP is the main known target of NEP. Additional target peptides of NEP are angiotensins I and II, endothelin 1, bradykinin, and substance P.
Through the activation of GC-A receptor , ANP and BNP produce natriuretic and diuretic effects [13]. These effects are achieved mostly through an increase of glomerular filtration rate and filtration fraction by dilating afferent arterioles and constricting efferent arterioles with an increase of glomerular capillary hydrostatic pressure. They reduce sodium reabsorption at the level of collecting ducts, and this effect is achieved also by a decrease of vasopressin secretion from the pituitary gland. Moreover, by limiting renin production and release from the juxtaglomerular cells and aldosterone secretion at the zona glomerulosa level [14], ANP interferes with sodium reabsorption at the proximal tubular level and sodium transport at the distal tubule.
In addition, ANP and BNP contribute to modulate systemic vascular resistance mainly by inhibiting the contraction of vascular smooth muscle cells through cGMP-dependent kinases [1]. At the heart level, ANP inhibits cardiac and pulmonary chemo- and baroreceptor activity causing a suppression of sympathetic outflow to the heart [15]. The inhibition of sympathetic activity associated with an increase of vagal afferent activity and the interference with SNS leads to reduction of heart rate and of cardiac output [16]. Moreover, ANP and BNP reduce salt and water appetite [1].
As a consequence of their multiple functions, the biological signature of NPs is to reduce body fluid and maintain blood pressure and cardiovascular homeostasis. On this basis, NPs become the physiological antagonist of both renin and angiotensin II.
They also modulate endothelial function [17]. Consistently with the latter effect, ANP level showed the best correlation with endothelial function among several tested biomarkers in the Framingham population [18].
Apart from the well-known hemodynamic functions, NPs are known to preserve vascular health in both endothelial and vascular smooth muscle cells by interfering with the key mechanisms of atherosclerosis, i.e., proliferation, angiogenesis, apoptosis, and inflammation [19]. In this regard, a low dose of ANP induced an increase in endothelial cell numbers, DNA synthesis, and cell migration through an increased expression of cGMP-regulated protein kinase (cGK) and, consequently, of protein kinase B (Akt) and extracellular signal-regulated kinase (ERK)1/2 [(p44/42 mitogen-activated protein kinases (MAPK)] pathways [20]. Thus, physiological concentrations of ANP promote endothelial regeneration and could be useful in the regeneration of endothelial cells after injury in atherosclerosis. On the other hand, supraphysiological levels of ANP exert an opposite effect leading to reduced endothelial cell regeneration, DNA synthesis, and cell migration [20]. In addition, NPs exert anti-hypertrophic and anti-fibrotic effects within the heart [21] by activating several signaling pathways including the calcineurin/nuclear factor of activated T cells (NFAT), the sodium-hydrogen exchanger (NHE)-1, and the transforming growth factor (TGF)β1/Smad [22]. Interestingly, a negative inotropic effect of ANP has been described in normal left ventricular myocytes [19].
Finally, a role of NPs in the control of lipid metabolism [19], in the promotion of mitochondria biogenesis in adipocytes, and in the process of “browning” of white adipocytes to increase energy expenditure has been described [23]. The cardiac cachexia of HF patients has been partially attributed to the increased levels of NPs that are present in HF.
As a proof of their fundamental cardiovascular properties, several dramatic effects can be observed in the absence of either NPs or of their receptors. In fact, lack of ANP leads to salt-sensitive hypertension [24]. Disruption of NPR-A causes hypertension, cardiac hypertrophy, interstitial fibrosis, and sudden death [25]. Moreover, lack of BNP leads to cardiac fibrosis [26]. Finally, lack of CNP causes bone deformation with skeletal overgrowth [27].
NPs are currently viewed as key players in the process of cardiovascular function and remodeling as well as in the natural history of cardiovascular diseases including heart failure (HF) [28].
2 Natriuretic Peptides in HF
ANP and BNP levels increase in HF, and their properties could at least partially balance the overactivation of the RAAS and of the SNS, hence contributing to cardiovascular and fluid-electrolyte homeostasis [29, 30]. However, their increase is not sufficient to preserve the cardiovascular hemodynamics, particularly with the progression of the disease. Thus, the physiological responses to NPs are blunted in HF patients. As a consequence, HF is recognized as a disease state characterized by defective NP processing and synthesis and by a resistance to NPs. Consistently with these observations, a decreased expression of corin gene and protein, with consequent reduced conversion of proANP or proBNP into ANP and BNP, was reported in the left atrium of experimental HF [31]. Moreover, serum corin levels decrease over time during progression of HF [32]. Consistently, corin overexpression improves cardiac function and survival in a mouse model of dilated cardiomyopathy [33]. Moreover, low levels of BNP(1–32) were detected in HF patients by the use of mass spectrometry immunoassay technology [34], raising the issue that commonly used BNP assays are unable to distinguish between different peptide fragments and that relatively greater abundance of immature BNP forms, that are less active biologically, are present in HF. These experimental and clinical observations may contribute to explain the paradoxical compromised natriuretic response in HF. The latter condition can also be explained by a reduced responsiveness due to natriuretic peptide receptor downregulation, with reduced guanylyl cyclase activity, in certain tissues including the heart [35], by increased local degradation [36], and by increased degradation of cGMP [37].
Interestingly, ventricles express ANP in the developing embryo and fetus with a rapid decline of gene expression during the prenatal period [38]. Ventricular ANP expression is reinduced in adult life in cardiac disease states [39].
2.1 Diagnostic Implications
The diagnostic role of NPs in both acute and chronic HF is well established [28,29,30]. In fact, both ANP and BNP plasma levels increase in parallel with the degree of left ventricular dysfunction and hemodynamic stress, although they are not useful to discriminate between reduced and preserved ejection fraction (EF) [28]. Both ANP and BNP reduce preload and afterload of the heart. The increase of BNP is 10- to 100-fold greater than that of ANP, and it appears to perform better than ANP. Furthermore, since the N-terminal prohormones are more stable and their half-lives in the plasma are longer than that of the carboxy-terminal peptides, the NT-proBNP and NT-proANP (particularly, the mid-regional NT-proANP, MR-proANP) are currently considered suitable and informative biomarkers in cardiovascular diseases [28].
The highest levels of NPs are expected in HF with reduced EF (HFrEF) compared to HF with preserved EF (HFpEF). However, when considering the levels of circulating NPs in both HFrEF and HFpEF, other factors, apart from the hemodynamic stress and left ventricular function, should be taken into account. In fact, age, sex, NP gene variants, BMI, renal function, sodium levels, and comorbidities such as atrial fibrillation exert a significant impact on circulating BNP and NT-proBNP levels. In particular, obesity is associated with reduced NP levels, whereas atrial fibrillation and renal failure are associated with increased NP levels [28]. Carrier status for the rs5068 ANP gene variant is associated with higher NP levels [19, 28].
At present, BNP and NT-proBNP are the established diagnostic biomarkers for HF, as well as for ventricular remodeling after myocardial infarction, cardiomyopathies, left ventricular hypertrophy, and pulmonary hypertension [28]. BNP and MR-proANP provide similar and useful diagnostic information in HF, particularly in the context of acutely destabilized HF (ADHF) [28]. This evidence suggests that the combination of either BNP or NT-proBNP and MR-proANP can provide superior diagnostic accuracy than either NP alone, and they greatly contribute to exclude noncardiac causes of acute dyspnea [40]. In fact, the current ESC guidelines recommend measuring levels of BNP, NT-proBNP, or MR-proANP [41].
The important clinical diagnostic implications of NPs in HF indicate that NP level measurement may represent a useful marker to monitor the course of the disease in relation to the benefits of therapeutic strategies [28]. In ADHF, BNP measurement led to better accuracy in diagnosis and reduced rate of hospitalizations and of admissions in intensive care units and had favorable effects on treatment costs and mortality rates. In fact, a useful algorithm for BNP-guided treatment of ADHF has been developed [28]. In patients with chronic HF due to systolic dysfunction, a meta-analysis performed in the attempt to overcome existing controversies and including 12 randomized clinical trials has shown that NP-guided therapy reduced all-cause mortality and HF-related hospitalizations. It was observed that individuals older than 75 years are those who benefit less, as a possible result of increased rate of comorbidities in this age range [28]. However, the recently published GUIDE-IT study has reported that a strategy of NT-proBNP-guided therapy was not more effective than a usual care strategy in improving outcomes in high-risk patients with HFrEF [42]. As a consequence of the uncertainties produced by the different studies, a low level of recommendation for NP-guided therapy in chronic HF has been assigned by AHA-ACC-HF guidelines [43]. Future studies will add important insights on this topic.
2.2 Prognostic Implications
The value of NPs as reliable markers for the long-term prognostic stratification both in acute and chronic HF conditions is well established. The prognostic value of NPs has been shown in both HF with reduced and preserved EF [44].
In chronic HF, subsequent measurements of either BNP or NT-proBNP levels provide independent information regarding the risk for disease progression across a wide spectrum of adverse outcomes: ventricular remodeling, malignant ventricular arrhythmias, hospitalization for HF, need for transplantation, and death. Due to its longer half-life and higher circulating concentrations, NT-proBNP may behave as a more accurate marker than BNP in disease prognosis. In fact, in the Valsartan in Heart Failure Trial (Val-HeFT), NT-proBNP, compared to BNP, had a greater predictive value for mortality, morbidity, and hospitalization for HF [45]. In one of the longest available follow-up study of patients with chronic HF, evaluating the prognostic power of multiple biomarkers (not including BNP), plasma ANP levels turned out to be the strongest long-term predictor of death in all disease stages [28].
In a study of patients presenting with acute HF, measurement of BNP, NT-proBNP, and MR-proANP levels showed significant diagnostic performance, although only MR-proANP had long-term prognostic value [46]. In a large collection of patients hospitalized for acutely destabilized HF (ADHF), the prognostic performance of both NT-proBNP and MR-proANP levels was confirmed by the evidence of an incremental prognostic value with respect to clinical risk factors for predicting mortality at 1 year [47].
2.3 NPs as Marker of Transition from Cardiac Diseases to HF in the General Population
Based on the knowledge that NPs increase in the attempt to maintain cardiorenal homeostasis, it has been postulated that higher circulating NP levels may anticipate the development of a cardiovascular disease. Over the last decade, several efforts have been performed in the attempt to establish the potential predictive role of NP levels toward the risk of developing cardiovascular diseases including HF in the general population.
A preventive role of NP-based screening toward HF development has been documented in different populations, being confirmed in both sexes, in older men and even among individuals with obesity [28]. Among patients with CV risk factors, at risk of HF, BNP assessment, associated with collaborative care, reduced the combined rates of LV systolic dysfunction, diastolic dysfunction, and HF [48].
More recently, the Natriuretic Peptides Studies Collaboration demonstrated the ability of NT-proBNP level to predict future HF development, as well as ischemic heart disease and stroke occurrence, in subjects without baseline cardiovascular disease from a large cohort of the general population, obtained through the analysis of 40 different populations collected worldwide [49].
Therefore, it appears that NP measurement predicts the risk of HF development beyond current routine assessment, and it may be a useful, cost-effective screening test for the identification of individuals at high risk of HF.
2.4 NPs for the Treatment of HF
It would be ideal to use oral NP in HF. However, this has not become a feasible approach in the clinical setting. Moreover, the subcutaneous administration of NPs did not reveal a complete absorption, and it was substantially ineffective [50].
Synthetic peptides for intravenous administration have been made available in the recent past to overcome the abovementioned difficulties. Among them, anaritide and carperitide are synthetic forms of ANP. Nesiritide is a synthetic form of BNP. Ularitide and cenderitide are the synthetic forms of urodilatin and of CNP, respectively. These synthetic peptides have shown some positive effects in the treatment of HF, particularly in acute HF (AHF) [51,52,53]. Carperitide led to a satisfactory recovery from AHF [53] and also to renal protection following infusion of contrast medium. Only carperitide was approved for the treatment of AHF in Japan in 1995, whereas there is not enough evidence to support the clinical use of the other peptides. Among others, CD-NP is a chimeric natriuretic peptide in which the 15-amino acid C-terminal tail of DNP is coupled to the 22-amino acid human C-type natriuretic peptide. CD-NP is able to bind to all three natriuretic peptide receptors (NPR-A, NPR-B, and NPR-C). Animal and human studies demonstrated that CD-NP improves cardiovascular and renal function without inducing significant levels of hypotension [54]. Although preliminary data suggested improved renal function in human HF patients, no indication has ever been obtained for the use of CD-NP in the treatment of HF.
Notably, the ANP gene delivery was also tested as an alternative way of administration. However, its use in experimental models of hypertension did not lead to conclusive results [50].
Interestingly, either stimulation of corin expression or of its activity should favor the proANP processing to ANP. In fact, by increasing corin expression, a significant improvement of cardiac function was observed in a mouse model of dilated cardiomyopathy [33].
Degradation of ANP takes place by NPR-C and by NEP. Since NPR-C plays pleiotropic functions other than NP clearance, its inhibition in order to increase ANP levels does not appear feasible.
The inhibition of NEP, aimed at increasing ANP levels, was first attempted several years ago through the introduction of the first inhibitor, omapatrilat. Its use in HFrEF led to a greater improvement compared to enalapril [55]. However, omapatrilat was stopped because of the higher occurrence of angioedema due to the coincident action on the substrates of angiotensin-converting enzyme (ACE) and NEP (bradykinin and substance P). More recently, the combined inhibition of NEP and AT1R (ARNi) was realized with the target to prevent a rise of Ang II and its effects during NEP inhibition that may offset the advantages of the increased action of NPs. This was accomplished in a compound (LCZ696) formed by the NEP inhibitor sacubitril and the AT1 receptor blocker valsartan. In the first proof-of-concept trial, the PARADIGM-HF, the ARNi LCZ696, today commercialized as Entresto, reduced the risk of death and of rehospitalization in HFrEF compared to enalapril, and it did not promote angioedema [56]. The use of Entresto, the first drug of this new class, has been indicated in patients affected by HFrEF, and it promises to be a valid tool in the treatment of HF [57].
The relevance of NEP inhibition in order to reinforce the natriuretic peptide actions led to the design of M-ANP, a synthetic peptide which consists of the 28 amino acids of native ANP and of a 12-amino acid terminus extension, which is highly resistant to NEP degradation [58]. M-ANP lowers blood pressure, induces natriuresis and glomerular filtration rate (GFR), and inhibits aldosterone [50]. In a model of hypertensive HF, M-ANP exhibited significant renal enhancing and left ventricular unloading properties that were greater than those of a conventional vasodilator (nitroglycerin) [50] and make M-ANP an attractive candidate for the treatment of HF.
As a key interesting issue, the knowledge that miR425 binds to a region of the 3′ untranslated region (UTR) of the ANP gene to stimulate its expression [59] underscores the potential usefulness of a miR425-based therapeutics to modulate ANP expression in HF. Additional miRNAs, able to interfere with ANP expression, may also provide future potential targets for the treatment of HF [60].
3 Conclusions
Thirty-six years after the discovery of the first component of the family, we keep reinforcing our knowledge on NPs as a class of cardiovascular hormones with fundamental regulatory functions on hemodynamics, as well as on atrial, ventricular, and vascular remodeling processes. Following the initial pioneering physiological studies, the subsequent application of several molecular biology and genetic approaches has allowed a full characterization of the multiple roles of NPs in physiology and pathology within the cardiovascular system. In the context of HF, NPs emerge as useful biomarkers with important diagnostic and prognostic implications. More importantly, based on the notion that HF is a disease state characterized by a deficient natriuretic peptide response, NPs merit full consideration as a rational therapeutic target for the treatment of this disease. From the latter point of view, several attempts have been made to define efficacious therapeutic strategies that, either by increasing NP levels through reduced degradation or by mimicking the physiological peptides actions through synthetic molecules, can restore the cardiovascular hemodynamics in both acute and chronic HF conditions. Many other approaches deserve to be tested in the near future to establish novel NP-based therapeutic drugs that can help to fight a dreadful common disease such as HF.
References
Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–66.
Gardner DG, Deschepper CF, Ganong WF, Hane S, Fiddes J, Baxter JD, Lewicki J. Extra-atrial expression of the gene for atrial natriuretic factor. Proc Natl Acad Sci U S A. 1986;83:6697–701.
de Bold AJ, Bruneau BG, Kuroski de Bold ML. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res. 1996;31:7–18.
Saxenhofer H, Raselli A, Weidmann P, Forssmann WG, Bub A, Ferrari P, Shaw SG. Urodilatin, a natriuretic factor from kidneys, can modify renal and cardiovascular function in men. Am J Phys. 1990;259(5 Pt 2):F832–8.
Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem. 1992;267:13928–32.
Chen S, Cao P, Dong N, Peng J, Zhang C, Wang H, Zhou T, Yang J, Zhang Y, Martelli EE, Naga Prasad SV, Miller RE, Malfait AM, Zhou Y, Wu Q. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med. 2015;21:1048–53.
Ichiki T, Huntley BK, Sangaralingham SJ, Burnett JC Jr. Pro-atrial natriuretic peptide: a novel guanylyl cyclase-A receptor activator that goes beyond atrial and B-type natriuretic peptides. JACC Heart Fail. 2015;3:715–23.
Vesely DL. Atrial natriuretic hormones originating from the N-terminus of the atrial natriureticfactor prohormone. Clin Exp Pharmacol Physiol. 1995;22:108–14.
Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–59.
Rubattu S, Sciarretta S, Morriello A, Calvieri C, Battistoni A, Volpe M. NPR-C: a component of the natriuretic peptide family with implications in human diseases. J Mol Med. 2010;88:889–97.
Sciarretta S, Marchitti S, Bianchi F, Moyes A, Barbato E, Di Castro S, Stanzione R, Cotugno M, Castello L, Calvieri C, Eberini I, Sadoshima J, Hobbs AJ, Volpe M, Rubattu S. The C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res. 2013;112:1355–64.
Turner AJ, Brown CD, Carson JA, Barnes K. The neprilysin family in health and disease. Adv Exp Med Biol. 2000;477:229–40.
Melo LG, Steinhelper ME, Pang SC, Tse Y, Ackermann U. ANP in regulation of arterial pressure and fluid-electrolyte balance: lessons from genetic mouse models. Physiol Genomics. 2000;3:45–58.
Laragh JH. Atrial natriuretic hormone, the renin-aldosterone axis, and blood pressure-electrolyte homeostasis. N Engl J Med. 1985;313:1330–40.
Volpe M. Atrial natriuretic peptide and the baroreflex control of circulation. Am J Hypertens. 1992;5:488–93.
Volpe M, Cuocolo A, Vecchione F, Mele AF, Condorelli M, Trimarco B. Vagal mediation of the effects of atrial natriuretic factor on blood pressure and arterial baroreflexes in the rabbit. Circ Res. 1987;60:747–55.
Kuhn M. Endothelial actions of atrial and B-type natriuretic peptides. Br J Pharmacol. 2012;166:522–31.
Kathiresan S, Gona P, Larson MG, Vita JA, Mitchell GF, Tofler GH, Levy D, Newton-Cheh C, Wang TJ, Benjamin EJ, Vasan RS. Cross-sectional relations of multiple biomarkers from distinct biological pathways to brachial artery endothelial function. Circulation. 2006;113:938–45.
Rubattu S, Sciarretta S, Valenti V, Stanzione R, Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733–41.
Kook H, Itoh H, Choi BS, Sawada N, Doi K, Hwang TJ, Kim KK, Arai H, Baik YH, Nakao K. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am J Physiol Heart Circ Physiol. 2003;284:H1388–97.
Molkentin JD. A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest. 2003;111:1275–7.
Calvieri C, Rubattu S, Volpe M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J Mol Med. 2012;90:5–13.
Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–36.
John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–81.
Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–5.
Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–44.
Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–21.
Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–25.
Burnett JC Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–7.
Mukoyama M, Nakao K, Saito Y, et al. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;323:757–8.
Ichiki T, Boerrigter G, Huntley BK, Sangaralingham SJ, McKie PM, Harty GJ, Harders GE, Burnett JC Jr. Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R102–9.
Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–20.
Gladysheva IP, Wang D, McNamee RA, Houng AK, Mohamad AA, Fan TM, Reed GL. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension. 2013;61:327–32.
Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, Jaffe AS. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355–60.
Dickey DM, Dries DL, Margulies KB, Potter LR. Guanylyl cyclase (GC)-A and GC-B activities in ventricles and cardiomyocytes from failed and non-failed human hearts: GC-A is inactive in the failed cardiomyocyte. J Mol Cell Cardiol. 2012;52:727–32.
Knecht M, Pagel I, Langenickel T, Philipp S, Scheuermann-Freestone M, Willnow T, Bruemmer D, Graf K, Dietz R, Willenbrock R. Increased expression of renal neutral endopeptidase in severe heart failure. Life Sci. 2002;71:2701–12.
Margulies KB, Burnett JC Jr. Inhibition of cyclic GMP phosphodiesterases augments renal responses to atrial natriuretic factor in congestive heart failure. J Card Fail. 1994;1:71–80.
Cameron VA, Aitken GD, Ellmers LJ, Kennedy MA, Espiner EA. The sites of gene expression of atrial, brain, and C-type natriuretic peptides in mouse fetal development: temporal changes in embryos and placenta. Endocrinology. 1996;137:817–24.
Larsen TH, Saetersdal T. Regional appearance of atrial natriuretic peptide in the ventricles of infarcted rat hearts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:309–14.
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975.
Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL Jr, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P, O’Connor CM. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–20.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52.
Seronde MF, Gayat E, Logeart D, Lassus J, Laribi S, Boukef R, Sibellas F, Launay JM, Manivet P, Sadoune M, Nouira S, Solal AC, Mebazaa A. Comparison of the diagnostic and prognostic values of B-type and atrial-type natriuretic peptides in acute heart failure. Int J Cardiol. 2013;168:3404–11.
Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol. 2008;52:997–1003.
de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–53.
Nakamura M, Tanaka F, Onoda T, Takahashi T, Sakuma M, Kawamura K, Tanno K, Ohsawa M, Itai K, Sakata K, Makita S, Iwate KENCO Study Groups. Gender-specific risk stratification with plasma B-type natriuretic peptide for future onset of congestive heart failure and mortality in the Japanese general population. Int J Cardiol. 2010;143:124–9.
Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, Watson C, O’Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74.
Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman MMath SA, Pennells L, Gao P, Burgess S, Freitag DF, Sweeting M, Wood AM, Cook NR, Judd S, Trompet S, Nambi V, Hecht Olsen H, Everett BM, Kee F, Ärnlöv J, Salomaa V, Levy D, Kauhanen J, Laukkanen JA, Kavousi M, Ninomiya T, Casas JP, Daniels LB, Lind L, Kistorp CN, Rosenberg J, Mueller T, Rubattu S, Panagiotakos DB, Franco OH, de Lemos JA, Luchner A, Kizer JR, Kiechl S, Salonen JT, Wannamethee G, de Boer RA, Nordestgaard BG, Andersson J, Jørgensen T, Melander O, Ballantyne CM, DeFilippi C, Ridker PM, Cushman M, Rosamond WD, Thompson SG, Gudnason V, Sattar N, Danesh J, Di Angelantonio E. Natriuretic peptides and integrated risk assessment for cardiovascular disease: individual-participant data meta-analysis of 40 prospective studies. Lancet Diabetes Endocrinol. 2016;4:840–9.
Ichiki T, Burnett J Jr. Atrial natriuretic peptide. Old but new therapeutic in cardiovascular diseases. Circ J. 2017;81:913–9.
Gong B, Wu Z, Li Z. Efficacy and safety of nesiritide in patients with decompensated heart failure: a meta-analysis of randomised trials. BMJ Open. 2016;6:e008545.
Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J, Investigators TRUE-AHF. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;376:1956–64.
Nomura F, Kurobe N, Mori Y, Hikita A, Kawai M, Suwa M, Okutani Y. Multicenter prospective investigation on efficacy and safety of carperitide as a first-line drug for acute heart failure syndrome with preserved blood pressure: COMPASS: Carperitide effects observed through monitoring dyspnea in acute decompensated heart failure study. Circ J. 2008;72:1777–86.
Rose RA. CD-NP, a chimeric natriuretic peptide for the treatment of heart failure. Curr Opin Investig Drugs. 2010;11:349–56.
Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002;106:920–6.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Volpe M, Tocci G, Battistoni A, Rubattu S. Angiotensin II receptor blocker nephrilysin inhibitor (ARNI): new avenues in cardiovascular therapy. High Blood Press Cardiovasc Prev. 2015;22:241–6.
Dickey DM, Yoder AR, Potter LR. A familial mutation renders atrial natriuretic peptide resistant to proteolytic degradation. J Biol Chem. 2009;284:19196–202.
Arora P, Wu C, Khan AM, Bloch DB, Davis-Dusenbery BN, Ghorbani A, Spagnolli E, Martinez A, Ryan A, Tainsh LT, Kim S, Rong J, Huan T, Freedman JE, Levy D, Miller KK, Hata A, Del Monte F, Vandenwijngaert S, Swinnen M, Janssens S, Holmes TM, Buys ES, Bloch KD, Newton-Cheh C, Wang TJ. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. 2013;123:3378–82.
Wu C, Arora P, Agha O, Hurst LA, Allen K, Nathan DI, Hu D, Jiramongkolchai P, Smith JG, Melander O, Trenson S, Janssens SP, Domian I, Wang TJ, Bloch KD, Buys ES, Bloch DB, Newton-Cheh C. Novel microRNA regulators of atrial natriuretic peptide production. Mol Cell Biol. 2016;36:1977–87.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Volpe, M., Rubattu, S. (2019). Natriuretic Peptides. In: Dorobantu, M., Mancia, G., Grassi, G., Voicu, V. (eds) Hypertension and Heart Failure. Updates in Hypertension and Cardiovascular Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-93320-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-93320-7_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93319-1
Online ISBN: 978-3-319-93320-7
eBook Packages: MedicineMedicine (R0)